Abstract
Aims: Mesenchymal stromal cells (MSCs) are heterogeneous cells from adult tissues that are able to differentiate in vitro into adipocytes, osteoblasts, or chondrocytes. Such cells are widely studied in regenerative medicine. However, the success of cellular therapy depends on the cell survival. Heme oxygenase-1 (HO-1, encoded by the Hmox1 gene), an enzyme converting heme to biliverdin, carbon monoxide, and Fe2+, is cytoprotective and can affect stem cell performance. Therefore, our study aimed at assessing whether Hmox1 is critical for survival and functions of murine bone marrow MSCs.
Results: Both MSC Hmox1+/+ and Hmox1−/− showed similar phenotype, differentiation capacities, and production of cytokines or growth factors. Hmox1+/+ and Hmox1−/− cells showed similar survival in response to 50 μmol/L hemin even in increased glucose concentration, conditions that were unfavorable for Hmox1−/− bone marrow-derived proangiogenic cells (BDMC). Hmox1+/+ MSCs but not fibroblasts retained low ROS levels even after prolonged incubation with 50 μmol/L hemin, although both cell types have a comparable Hmox1 expression and similarly increase its levels in response to hemin. MSCs Hmox1−/− treated with hemin efficiently induced expression of a vast panel of antioxidant genes, especially enzymes of the glutathione pathway.
Innovation and Conclusion: Hmox1 overexpression is a popular strategy to enhance viability and performance of MSCs after the transplantation. However, murine MSCs Hmox1−/− do not differ from wild-type MSCs in phenotype and functions. MSC Hmox1−/− show better resistance to hemin than fibroblasts and BDMCs and rapidly react to the stress by upregulation of quintessential genes in antioxidant response. Antioxid. Redox Signal. 29, 111–127.
Keywords: : stem cells, antioxidant gene response, heme, mesenchymal stem cell
Introduction
Mesenchymal stromal cells (MSCs), also known as mesenchymal stem cells, or multipotent stromal cells are a heterogeneous population of connective tissue cells that contains osteoblast and adipocyte progenitors, fibroblasts, and smooth muscle cells (5). In vitro criteria for human MSCs include adherence to the plastic in standard culture conditions, differentiation in vitro to adipocytes, osteoblasts, and chondrocytes (9). MSCs should express CD73, CD90, and CD105 markers but not CD45, CD34, CD14, CD11b, CD79α, CD19, and HLA-DR (9). MSCs were further identified, also in vivo, by CD271 and CD106 (human) (33), CD146 (44), nestin (34), Sca-1 (mouse) and PDGFRα (36), leptin receptor LepR (8, 67), or high expression of CXCL12 (41).
Innovation.
Enhancement of stem and progenitor cells antioxidant capacity is the aim of many studies focusing on the cellular therapies. Many of such strategies propose the overexpression of Hmox1 as a protection against cell stress. For the first time, this article shows that mesenchymal stromal cells (MSCs) lacking Hmox1 can more efficiently than other cells deal with oxidative stress induced with hemin, using the mechanism involving the upregulation of glutathione pathway. High resistance to stress and unique ability to activate antioxidant response suggest that MSC may not need additional protection by Hmox1 overexpression.
MSCs were shown to be immune evasive or immunomodulatory, depending on the microenvironment (2). The mechanism of immunosuppression is complex and involves many factors, that is, prostaglandin E2, nitric oxide, and TGFβ (39). Although MSCs are commonly believed to deal with oxidative stress efficiently (55), the biggest obstacle to the therapeutic use of MSCs is their poor survival and engraftment after the transplantation (11). Therefore, many studies focus on the enhancement of their antioxidant activity with overexpression of various genes, for example, Hmox1 (54, 63).
Heme oxygenase-1 (HO-1, encoded by the HMOX1 gene) is an enzyme degrading heme to carbon monoxide (CO), biliverdin, and Fe2+ ions. Due to its enzymatic activity, heme oxygenase-1 influences cell survival, resistance to the oxidative stress, and angiogenesis (10). We have recently shown that proangiogenic cells isolated from the bone marrow of Hmox1 knock-out mice present impaired proliferation, migration, and formation of capillaries (16). What is more, overexpression of heme oxygenase-1 can lead to the block of differentiation, that is, in myoblasts (27).
Rat MSCs transfected with the plasmid coding for human heme oxygenase-1 showed decreased apoptosis in hypoxia and higher resistance to H2O2 (54). In our hands, pig bone marrow-derived cells transduced with adenoviral vectors encoding heme oxygenase-1 (AdHO1) were characterized by better angiogenic activity in vitro and improved left ventricular ejection fraction 30 min after infarction in pigs (63). Treatment with cobalt protoporphyrin IX (CoPP), heme oxygenase-1 activator, enhanced proliferation of human mesenchymal stem cells and production of VEGF; whereas tin protoporphyrin IX (SnPP), heme oxygenase-1 inhibitor, had an opposite influence (20). Further, CoPP-treated MSCs accelerated wound healing in a xenogeneic model of diabetic mice (20).
Modulation of heme oxygenase-1 activity with SnPP in human MSCs affected their ability to inhibit T cell proliferation in vitro. Interestingly, the effect of SnPP was not observed in rat MSCs, and T cell proliferation was restored only when concomitant treatment of nitric oxide synthase 2 was used (7). Moreover, heme oxygenase inhibition decreased the ability of MSCs to induce Tr1 and Th3 regulatory cells and to elevate levels of IL-10 and TGFβ, respectively. MSCs preconditioned with a mixed lymphocyte reaction showed decreased HO-1 levels as well as immunomodulatory activity (37).
Finally, the effect of heme oxygenase-1 on MSC differentiation to adipocytes and osteoblasts was also studied. Abraham and co-workers showed in the series of publications that enhanced expression of HO-1 in MSCs results in improved differentiation to osteoblasts, whereas its inhibition promotes adipogenesis (3, 56–59). On the other hand, Zarjou et al. reported no differences in differentiation potential between MSC Hmox1+/+ and Hmox1−/− (66). Also in other studies, overexpression of heme oxygenase-1 in MSCs did not affect their differentiation (18, 68).
Data on the influence of heme oxygenase-1 on MSCs are often contradictory. Conjointly, copper or tin protoporphyrins were used in many studies to modulate HO-1 activity, although they were shown to have many heme oxygenase-independent effects in various cell types (17, 23). MSCs are essential for the proper function of stem cell niches in bone marrow, and lack of heme oxygenase-1 was shown to potently affect other bone marrow-derived cells, that is, pro-angiogenic cells (PACs) (16). Therefore, we decided to characterize murine bone marrow-derived MSCs lacking the functional Hmox1 gene, with the focus on their response to oxidative stress.
Results
Hmox1+/+ or Hmox1−/− bone marrow MSCs show similar phenotype and differentiation
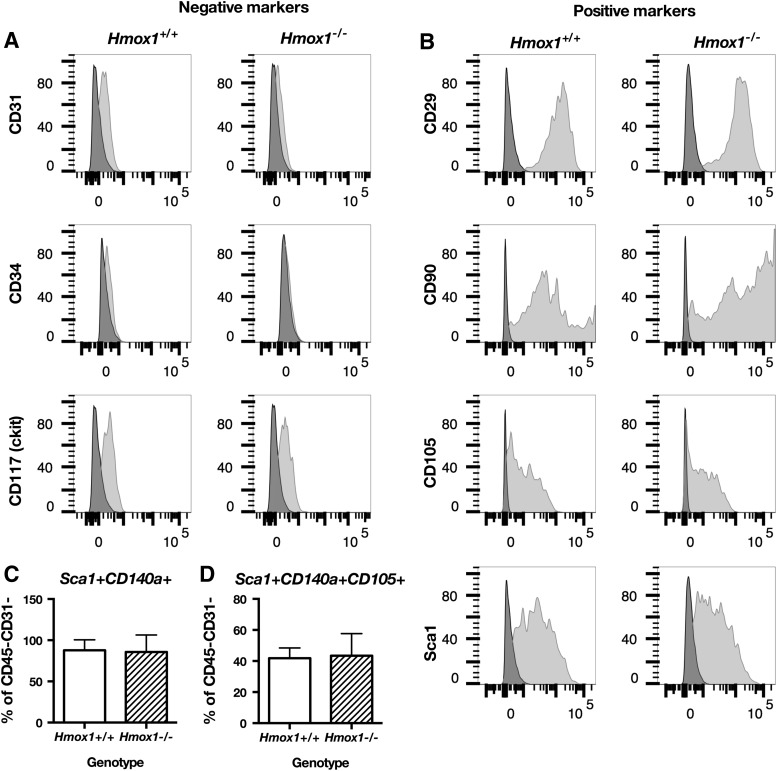
First, we compared the phenotypes of murine bone marrow stromal cells Hmox1+/+ or Hmox1−/− in culture by using flow cytometry. Regardless of the genotype, 60% of the cells in culture were CD45−CD31− (data not shown). Therefore, cells used for all the experiments were purified from the remaining CD45+ fraction with MACS sorting. Obtained cells lacked expression of endothelial markers CD31 and CD34, whereas CD117 (c-kit) was expressed only on a small sub-fraction of cells (Fig. 1A). Isolated MSCs expressed positive markers that were attributed to the mesenchymal stem/stromal cells, that is, CD29, CD90, CD105, Ly-6A/E (Sca-1) (Fig. 1B), and CD140a (PDGFRα) (Fig. 1C, D). Hmox1−/− bone marrow-derived PACs had impaired proliferation (16). However, the proliferation of MSC Hmox1+/+ or Hmox1−/− was similar, even when cells were grown under stress conditions in high glucose concentration (Fig. 2A).
FIG. 1.
MSC Hmox1+/+ or Hmox1−/− have similar phenotype. Expression of MSC positive markers CD29, CD90, CD105, and Sca-1 (A), MSC negative markers CD31, CD34, and ckit in Hmox1+/+ or Hmox1−/− MSCs (B). Phenotype of nonsorted bone marrow stromal cells in passage 4: fraction of CD45−CD31− cells in culture (C), fraction of Sca-1+CD140a+ cells within CD45−CD31− population (D), or Sca-1+CD140a+CD105+ cells within CD45−CD31− population in Hmox1+/+ or Hmox1−/− cells. (C, D, N = 9–10). MSCs, mesenchymal stromal cells.
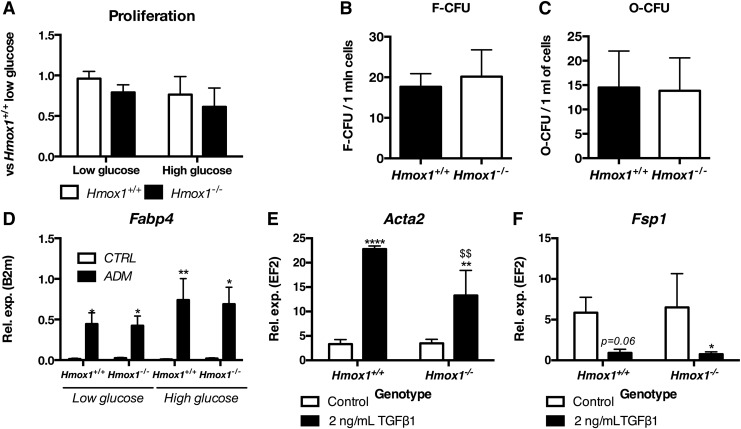
FIG. 2.
MSC Hmox1+/+ or Hmox1−/− show similar ability to differentiate to adipocytes, osteoblasts and smooth muscle cells. Proliferation of MSC Hmox1+/+ or Hmox1−/− in low or high glucose medium assessed with BrDU assay (A). Ability to form fibroblastoid (B) or osteoblast (C) colonies by bone marrow cells isolated from Hmox1+/+ or Hmox1−/− MSCs assessed with fibroblastoid or osteoblast colony-forming unit assay, respectively. Data shown as mean + SD, N = 3. Expression of Fabp4 in Hmox1+/+ or Hmox1−/− MSCs differentiated to adipocytes in low or high glucose conditions (D) Gene expression was assessed with qRT-PCR. Data are shown as mean + SD, N = 4–7. Expression of Acta2 (E), Fsp1 (F) measured with qRT-PCR in control and differentiated MSC Hmox1+/+ or Hmox1−/−. Data are shown as mean ± SD, *p < 0.05, **p < 0.01, ****p < 0.0001 control versus TGFb1; $$p < 0.01 Hmox1+/+ versus Hmox1−/−, N = 3. qRT-PCR, quantitative real-time PCR.
Further, bone marrow stromal cells isolated from long bones of mice Hmox1+/+ or Hmox1−/− formed similar numbers of colonies that consisted of fibroblastoid cells (Fig. 2B) or cells able to be differentiated to osteoblasts (Fig. 2C). MSCs were shown to differentiate into adipocytes, osteoblasts, and chondrocytes (6). Both Hmox1+/+ and Hmox1−/− MSCs differentiated into osteoblasts and adipocytes, which were evidenced with staining for osteopontin or Fabp4, respectively (Supplementary Fig. S1A, B; Supplementary Data are available online at www.liebertpub.com/ars).
Because HO-1 was suggested to play a crucial role in the regulation of MSC adipogenesis (59), we focused on the effects of Hmox1 knockout on the genes associated with lipid metabolism. Interestingly, differentiation of murine bone marrow MSCs to adipocytes induced similar changes in the gene expression in both Hmox1+/+ and Hmox1−/− cells (Supplementary Fig. S1C).
However, Hmox1+/+ MSCs were the only ones that increased expression of miR-21-5p, the microRNA that, via TGFβ signaling, regulates adipogenesis (26) (Supplementary Fig. S1D). Levels of other tested microRNAs associated with adipogenesis, namely miR-31-5p, miR-150-5p, miR-301a-5p, miR-378a-3p, or miR-378a-5p, remained unchanged in cells of both genotypes (Supplementary Fig. S1E–I). Further, basal expression of all tested microRNAs, including miR-21-5p, which increased during adipocyte differentiation only in Hmox1+/+ cells, was similar in MSC Hmox1+/+ and Hmox1−/− (data not shown).
Effects of changed expression of HMOX1 in human MSCs on the adipogenesis or osteogenesis were stronger when cells were cultured in high glucose concentration (3). Therefore, we tested markers of adipogenesis in MSC Hmox1+/+ or Hmox1−/− differentiated in low or high glucose concentration. Interestingly, adipogenic differentiation of murine bone marrow-derived Hmox1+/+ or Hmox1−/− MSCs did not change when cells were cultured under high glucose conditions. Both Hmox1+/+ and Hmox1−/− MSCs upregulated fatty acid-binding protein 4—a marker of adipocyte differentiation (Fig. 2D).
MSCs were shown to be precursors of fibroblasts and myofibroblasts and, therefore, contribute to the tumor stroma (35) or development of fibrosis (30). Heme oxygenase-1 can affect both tumor microenvironment (62) and kidney fibrosis (47). Therefore, we investigated whether the lack of Hmox1 gene in MSCs may influence their ability to form myofibroblasts. Hmox1+/+ and Hmox1−/− MSCs were differentiated to myofibroblasts with TGFβ1 treatment for 6 days. Cells changed their morphology (Supplementary Fig. S1J) and upregulated α-smooth muscle actin (Acta2) (Fig. 2E). Of note, upregulation of Acta2 was lower in myofibroblasts derived from Hmox1−/− cells.
Transcript levels of fibroblast-specific protein 1 (Fsp1) (Fig. 2F) tended to decrease in Hmox1+/+ and Hmox1−/− MSCs; however, this trend reached significance only in Hmox1−/− cells. To summarize, we show here that MSCs, regardless of the heme oxygenase-1, show similar phenotype and differentiation ability, even in stress conditions.
Lack of Hmox1 does not change MSCs immunomodulatory activity or production of cytokines
Akiyama et al. showed that MSCs injected intravenously can decrease numbers of circulating CD3+ and increase T cell apoptosis (1). Mougiakakos et al. suggested that inhibition of HO-1 activity can decrease MSCs ability to induce T regulatory cells (37). Therefore, to test the effect of complete knock-out of Hmox1 on immunomodulatory activity of MSCs, we injected wild-type C57Bl6 × FVB mice with MSC Hmox1+/+ or Hmox1−/− and analyzed apoptosis and activation of T cells.
Numbers of circulating CD3+ T cells did not change in mice injected with MSCs (Fig. 3A). However, numbers of apoptotic Annexin V+ CD3+ cells tended to increase after the MSC injection, but this trend did not reach statistical significance (Fig. 3B). Injection of MSC Hmox1+/+ or Hmox1−/− did not affect the numbers as well of activated CD3+CD4+CD25high T cells (Fig. 3C). In vitro, primary murine CD3+ T cells co-cultured with MSC Hmox1+/+ or Hmox1−/− showed a similar cell cycle (Supplementary Fig. S2A–C), percentage of Ki67+ cells (Supplementary Fig. S2D) and annexin V+ cells (Supplementary Fig. S2E).
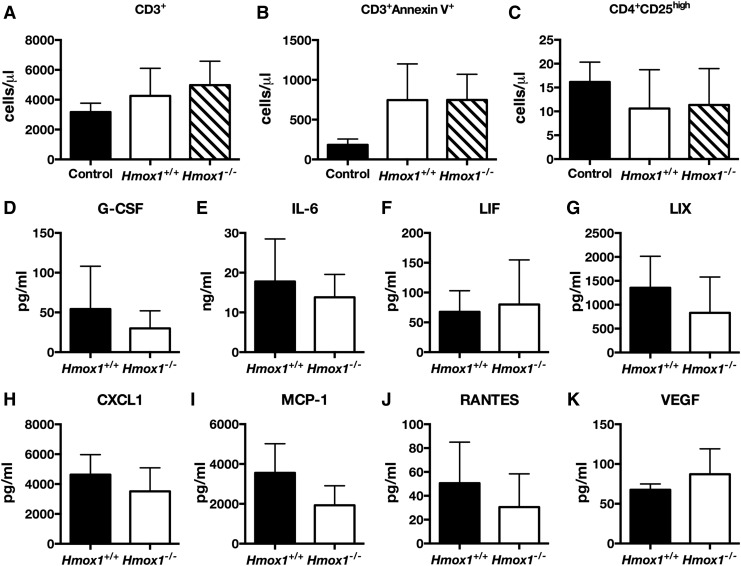
FIG. 3.
Lack of Hmox1 in MSC does not affect their immunomodulatory properties or secretory profile. Number of circulating CD3+ T cells (A), CD3+AnnexinV+ apoptotic T cells (B), or activated CD3+CD4+CD25high T cells (C) in control mice or injected i.v. with MSC Hmox1+/+ or Hmox1−/− (N = 3–4). Concentration of G-CSF (D), IL-6 (E), LIF (F), LIX (G), CXCL1 (H), MCP-1 (I), RANTES (J), and VEGF (K) in conditioned media from Hmox1+/+ or Hmox1−/−MSC assessed with multiplex assay on Luminex platform. Data are shown as mean ± SD, Mann–Whitney, N = 4.
Further, lack of the functional Hmox1 gene did not change the profile of cytokines and growth factors produced by MSC. Conditioned media from MSC Hmox1+/+ and Hmox1−/− contained similar amounts of G-CSF, IL-6, LIF, LIX, CXCL1, CXCL2, CCL5, and VEGF (Fig. 3D–K). Levels of eotaxin, GM-CSF, IGNγ, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-7, IL-9, IL-10, IL-12 p40, IL-12 p70, IL-13, IL-15, IL-17, MIP-1α, MIP-1β, M-CSF, MIP-2, MIG, and TNFα were under the threshold of detection. Therefore, we concluded that lack of the Hmox1 gene did not change the tested MSC secretome and did not affect the ability of MSCs to induce T cell death both in vitro and in vivo.
Hmox1+/+ and Hmox1−/− MSCs show high resistance to oxidative stress induced with H2O2 or hemin
Bone marrow-derived proangiogenic cells (BDMC) lacking Hmox1 gene were characterized by higher sensitivity to oxidative stress, that is, induced with hemin (16). Therefore, we decided to test whether it is also true for MSCs. Surprisingly, Hmox1+/+ and Hmox1−/− MSCs showed no difference in the viability when treated for 6 h with H2O2 or 50 μmol/L hemin in both low and high glucose conditions (Fig. 4A, B). Moreover, treatment with 10 ng/μl of TNFα for 24 h did not yield significant differences in the viability of MSCs with or without Hmox1 (Supplementary Fig. S3A, B).
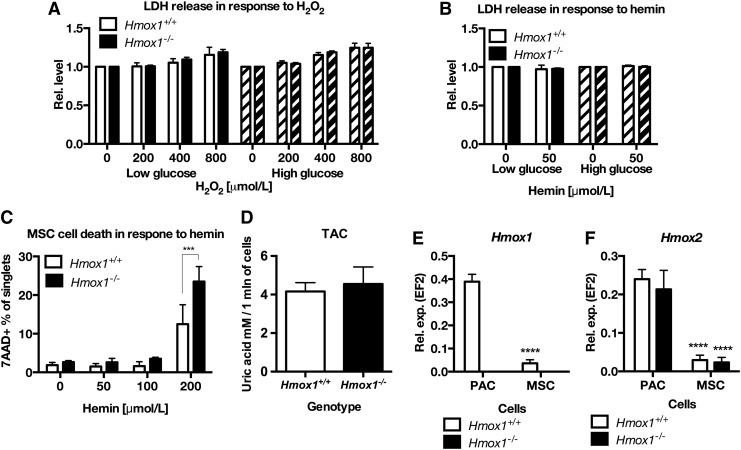
FIG. 4.
MSC, regardless of the low expression of both heme oxygenases 1 or 2, show high resistance to hemin or hydrogen peroxide. LDH release in MSC Hmox1+/+ or Hmox1−/− treated with H2O2 (A) or hemin (B) in low or high glucose medium. Cell death in MSC Hmox1+/+ or Hmox1−/− treated with increasing concentrations of hemin for 6 h, assessed with 7-AAD staining and flow cytometry (C). Data are shown as mean ± SD. ***p < 0.001 two-way ANOVA with Bonferroni post-test, N = 3. Total antioxidant capacity of conditioned media from MSC Hmox1+/+ or Hmox1−/−, measured with TAC kit (N = 4) (D). (E) Expression of Hmox1, (F) Hmox2 in murine bone marrow PAC and MSCs isolated from Hmox1+/+ or Hmox1−/− mice, ****p < 0.0001 MSC versus PAC; two-way ANOVA with Bonferroni post-test, N = 3. 7-AAD, 7-aminoactinomycin D; ANOVA, analysis of variance; LDH, lactate dehydrogenase; PAC, pro-angiogenic cell; TAC, total antioxidant capacity.
Subsequently, we checked the toxic concentrations of hemin for MSC Hmox1+/+ and Hmox1−/−. Surprisingly, hemin was more toxic for Hmox1−/− MSCs than for Hmox1+/+ cells not until at a concentration of 200 μmol/L (Fig. 4C). However, conditioned media from both MSC Hmox1+/+ and Hmox1−/− showed similar total antioxidant capacity assessed with total antioxidant capacity (TAC) (Fig. 4D), 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), or ferric-reducing antioxidant power (FRAP) assays (Supplementary Fig. S3C, D). The latter result suggests that the reason for the MSC resistance to oxidative stress is rather intrinsic.
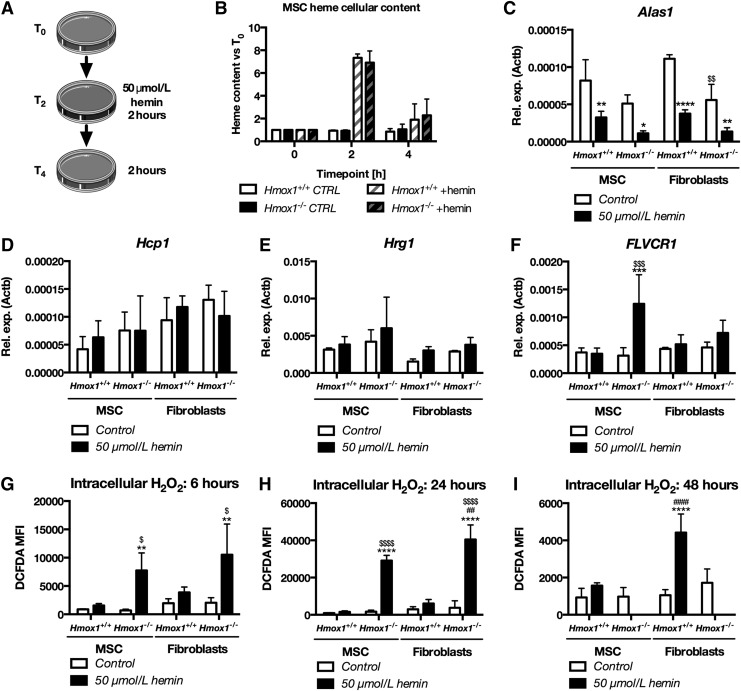
First, we supposed that low sensitivity of Hmox1+/+ and Hmox1−/− MSCs to hemin, especially in comparison to BDMC, could be related to the high expression of heme oxygenase-2. However, the expression of Hmox1 was lower in MSCs Hmox1+/+ than in PACs Hmox1+/+ (Fig. 4E). Similarly, the expression of Hmox2 was lower in MSCs than in PAC cells and similar in Hmox1+/+ and Hmox1−/− cells (Fig. 4F). Then, we hypothesized that low sensitivity of Hmox1−/− MSC cells could be caused by the low uptake of heme from the culture medium. Therefore, we analyzed heme uptake with two methods—direct measurement of heme in cells stimulated for 2 h with 50 μmol/L hemin, and then after 2 h in fresh medium (Fig. 5A), and with the measurement of tin protoporphyrin IX fluorescence.
FIG. 5.
Hemin increases intracellular hydrogen peroxide in Hmox1+/+ or Hmox1−/− and decreases Alas1 expression but does not affect levels of heme importers. Scheme of the experiment for the assessment of intracellular heme content (A). Heme cellular content measured with spectrophotometry in MSC Hmox1+/+ or Hmox1−/− stimulated for 2 h with hemin (T2) and then after 2 h in hemin-free medium (T4), N = 3 (B). Expression of FLVCR1 (C), Alas1 (D), Hmox1 (E), and Hmox2 (F) in Hmox1+/+ or Hmox1−/− MSCs or fibroblasts stimulated for 6 h with 50 μmol/L hemin. Levels of H2O2 measured with H2DCFDA after 6 (G), 24 (H), or 48 h (I) in Hmox1+/+ or Hmox1−/− MSCs or fibroblasts stimulated with hemin (50 μmol/L). Data are shown as mean ± SD, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 hemin-treated cells versus control; ##p < 0.01, ####p < 0.0001 MSC versus fibroblasts, $p < 0.05, $$p < 0.01, $$$p < 0.001, $$$$p < 0.0001 Hmox1+/+ versus Hmox1−/−. Two-way ANOVA with Bonferroni post-test, N = 3.
Hmox1+/+ and Hmox1−/− MSCs displayed comparable levels of cellular heme at all timepoints tested (Fig. 5B). Moreover, both cell genotypes showed comparable SnPP fluorescence proportional to the concentration of SnPP in the culture medium (Supplementary Fig. S4A). Therefore, higher resistance to hemin in comparison to PAC cannot be explained by the compensation of Hmox1 function by Hmox2 or by changed uptake of hemin.
Since bone marrow PACs are mostly of monocytic origin, and therefore distant from MSCs, we decided to further focus on the differences in stress response between MSCs and fibroblasts. Interestingly, mouse tail-tip fibroblasts had a phenotype similar to MSCs, that is, they expressed Sca1, CD106 and part of them showed positive for CD140a (Supplementary Fig. S5). Both fibroblasts and MSCs, regardless of Hmox1, were characterized by the similar expression of heme transporters Hcp1 (Slc46a1) and Hrg1 (Slc48a1) (Supplementary Fig. S4B, C). MSCs Hmox1−/− were the only ones to increase in response to hemin treatment and the expression of heme exporter FLVCR1 (Fig. 5C), which may protect them from the heme overload.
To further elucidate the effects of heme on MSCs, we analyzed genes involved in heme synthesis, of which Alas1 is regulated by heme. Treatment with hemin (50 μmol/L) decreased Alas1 expression in all treated cells. Therefore, we concluded that heme in the culture medium enters treated cells regardless of Hmox1 expression and affects known heme-regulated pathways. In Hmox1−/− fibroblasts, already control cells were characterized by lower Alas1 levels than Hmox1+/+ cells (Fig. 5D). Expression of Uros in hemin-treated Hmox1−/− fibroblasts was lower than in corresponding MSC cells (Supplementary Fig. S4D).
What is more, Hmox1−/− fibroblasts treated with hemin decreased Cpox expression whereas it remained unchanged in other cell types (Supplementary Fig. S4E). There were no differences in the expression of Hmbs, Alad, Ppox, and Fech (Supplementary Fig. S4E, F). Hmox1 was similarly upregulated in both Hmox1+/+ MSCs and fibroblasts (Fig. 5E). The expression of Hmox2 was not affected by hemin treatment and did not differ in any of the tested groups of cells (Fig. 5F). To sum up, MSCs Hmox1−/− show higher resistance to H2O2 and hemin than previously tested PACs (16). However, low sensitivity to hemin is not caused by changes in heme uptake or synthesis and it is not related to Hmox2.
Hemin increases cellular H2O2 in Hmox1−/− MSCs and fibroblasts
To assess the effects of hemin on oxidative stress in the MSCs Hmox1+/+ and Hmox1−/−, we analyzed the levels of cellular hydrogen peroxide by using H2DCFDA staining. Tail-tip fibroblasts isolated from the same Hmox1+/+ or Hmox1−/− mice were used as nonprogenitor internal control cells. After 6 h of incubation with hemin (50 μmol/L), levels of H2O2 were increased in Hmox1−/− MSCs and fibroblasts and higher than in respective wild-type cells (Fig. 5G). After a 24-h incubation period, levels of H2O2 remained low in Hmox1+/+ cells and were higher in Hmox1−/− fibroblasts than in Hmox1−/− MSCs (Fig. 5H). This suggests that murine MSCs might be equipped with an anti-oxidant protective mechanism, which works better than in fibroblasts.
Nevertheless, after 48 h of stimulation with hemin, both Hmox1−/− MSCs and Hmox1−/− fibroblasts were dead. Interestingly, at this late timepoint, levels of cellular H2O2 were higher in hemin-treated Hmox1+/+ fibroblasts than in Hmox1+/+ MSCs (Fig. 5I). Therefore, we concluded that both Hmox1+/+ and Hmox1−/− MSC cells showed higher resistance to hemin than respective tail-tip fibroblasts.
MSCs lacking Hmox1 efficiently induce antioxidant gene response
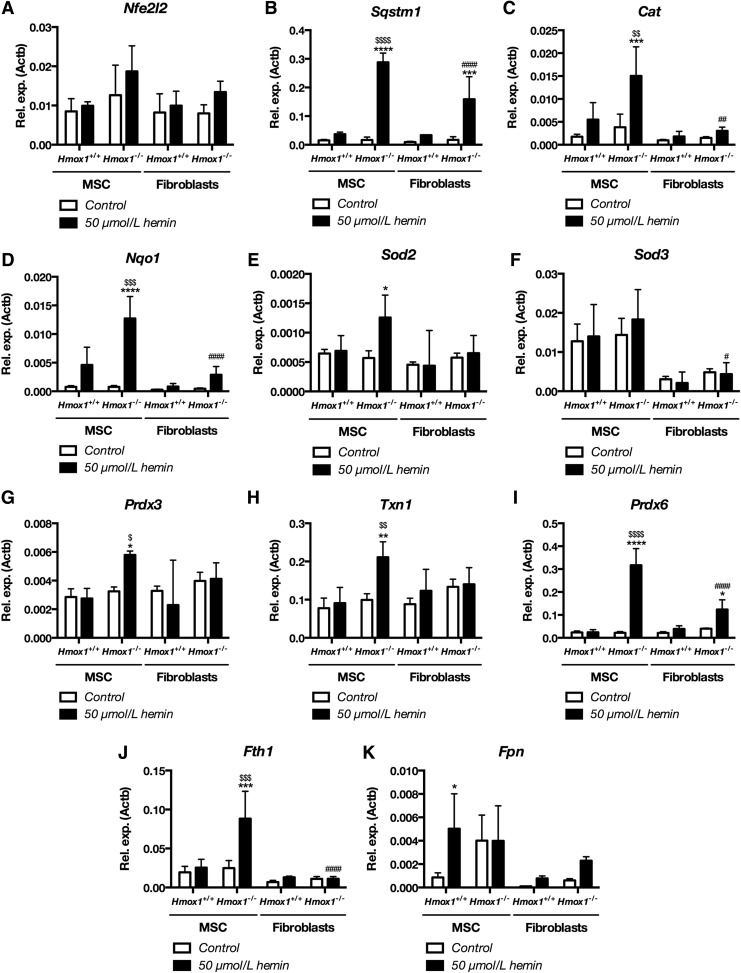
A lower concentration of H2O2 in MSC Hmox1−/− than Hmox1−/− fibroblasts after short incubation with hemin suggested that MSCs might more effectively respond to the pro-oxidative insult. Therefore, we evaluated the expression of a vast panel of antioxidant genes in MSCs and Hmox1+/+ or Hmox1−/− fibroblasts in response to 50 μmol/L hemin. Treatment with hemin did not change the expression of the major regulator of antioxidant gene response Nfe2l2 (Fig. 6A), which encodes for the Nrf2 transcription factor. Levels of Sqstm1, which is both target and regulator of Nrf2 (21), were increased in MSCs and Hmox1−/− fibroblasts but the upregulation was higher in MSCs (Fig. 6B).
FIG. 6.
MSC Hmox1+/+ show more efficient antioxidant response after treatment with hemin than fibroblasts Hmox1+/+. Expression of Nfe2l2 (A), Sqstm1 (B), Cat (C), Nqo1 (D), Sod2 (E), Sod3 (F), Prdx3 (G), Txn1 (H), Prdx6 (I), Fth1 (J), and Fpn (K) in Hmox1+/+ or Hmox1−/− MSCs or fibroblasts stimulated for 6 h with hemin (50 μmol/L). Data are shown as mean ± SD, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 hemin-treated cells versus control; #p < 0.05, ##p < 0.01, ####p < 0.0001 MSC versus fibroblasts, $p < 0.05, $$p < 0.01, $$$p < 0.001, $$$$p < 0.0001 Hmox1+/+ versus Hmox1−/−, Two-way ANOVA with Bonferroni post-test, N = 3.
Expression of Cat was the highest in hemin-treated MSC Hmox1−/−, the only ones to change Cat levels (Fig. 6C). Then, Hmox1−/− fibroblasts treated with hemin had lower expression of Nqo1 than Hmox1−/− MSCs, the only cells to upregulate Nqo1 and with its expression higher than Hmox1+/+ MSCs (Fig. 6D). Similarly, expression of Sod2 was changed only in Hmox1−/− MSC (Fig. 6E) whereas hemin-treated Hmox1−/− fibroblasts had lower Sod3 levels than corresponding MSC cells (Fig. 6F).
Further, expression of Prdx3 and Txn1 was enhanced with hemin only in Hmox1−/− MSC cells (Fig. 6G, H); whereas Prdx4, Prdx5, and Txnrd3 were not affected (Supplementary Fig. S6A–C). Interestingly, Prdx6, the only 1-Cys member of peroxiredoxin family (13), was potently upregulated in hemin-treated Hmox1−/− MSCs. Prdx6 expression was then higher in MSC Hmox1−/− than in all other cells (Fig. 6I).
MSCs Hmox1−/− were the only ones that upregulated ferritin heavy chain 1 (Fth1) expression in response to the hemin treatment (Fig. 6J). Levels of Fpn were, on the other hand, increased only in Hmox1+/+ MSCs (Fig. 6K). Hemin did not affect Nox4 expression in any cells (Supplementary Fig. S6D). Interestingly, only Hmox1−/− MSCs decreased Gpx1 expression in response to hemin (Supplementary Fig. S6E), whereas expression of Gpx3 and Gpx4 was unchanged (Supplementary Fig. S6F, G), and expression of Gpx8 was decreased in both MSC cell types (Supplementary Fig. S6H). On the other hand, control fibroblasts expressed lower levels of Gpx8 than respective MSCs (Supplementary Fig. S6H).
To summarize, both MSCs and fibroblasts Hmox1−/− upregulated Sqstm1 and Prdx6 in response to hemin. Moreover, the expression of both genes was higher in treated MSC Hmox1−/− than fibroblasts Hmox1−/−. MSC Hmox1−/− but not fibroblasts Hmox1−/− elevated levels of transcripts for Cat, Nqo1, Prdx3, Txn1, and Fth1, which confirmed their more efficient antioxidant response.
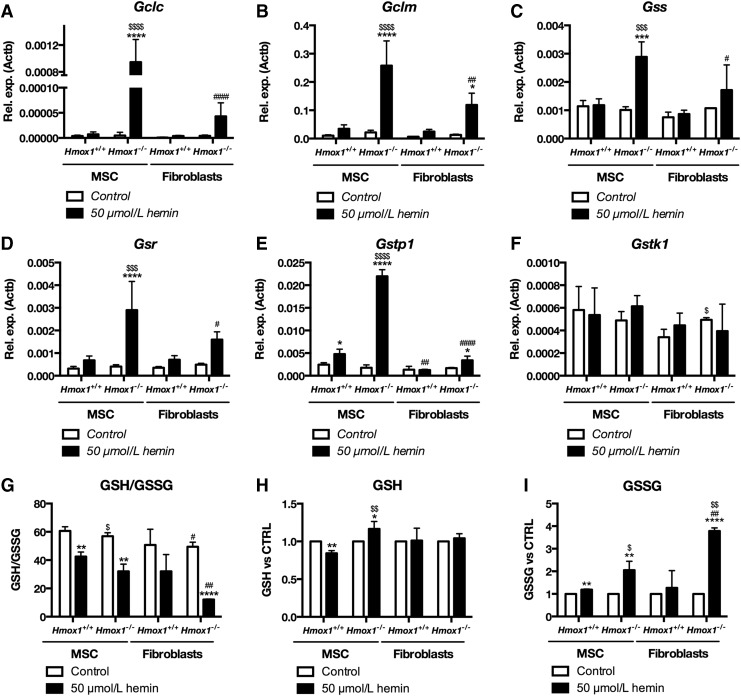
Importantly, MSC Hmox1−/− were the only cells to potently upregulate quintessential genes involved in the glutathione pathway, namely Gclc, Gclm, Gss, and Gsr. MSC Hmox1−/− also elevated Gstp1 (Fig. 7A–E), which forms a heterodimer with Prdx6 (13), also increased in hemin-treated MSC Hmox1−/−. Levels of Gstk1, another glutathione S-transferase, remained unchanged in all cell types (Fig. 7F). Levels of the upregulated glutathione pathway genes were higher in hemin-treated Hmox1−/− MSC cells than in hemin-treated Hmox1+/+ MSCs and Hmox1−/− fibroblasts. The latter ones increased expression only of Gclm and Gstp1 (Fig. 7B, F).
FIG. 7.
MSC Hmox1+/+ strongly induce expression of glutathione pathway enzymes and increase GSH levels after stimulation with hemin. Expression of Gclc (A), Gclm (B), Gss (C), Gsr (D), Gstp1 (E), and Gstk1 (F) in Hmox1+/+ or Hmox1−/− MSCs or fibroblasts stimulated for 6 h with hemin (50 μmol/L). Ratio of GSH to GSSG (G), changes in total GSH (H), and GSSG (I) in Hmox1+/+ or Hmox1−/− MSCs and fibroblasts stimulated for 6 h with hemin (50 μmol/L) and then kept in fresh medium for 2 h. Levels of GSH and GSSG were measured with GSH/GSSG-Glo™ Assay. Data are shown as mean ± SD, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 hemin-treated cells versus control; #p < 0.05, ##p < 0.01, ####p < 0.0001 MSC versus fibroblasts, $p < 0.05, $$p < 0.01, $$$p < 0.001, $$$$p < 0.0001 Hmox1+/+ versus Hmox1−/−, Two-way ANOVA with Bonferroni post-test, N = 3. GSH, reduced glutathione; GSSG, oxidized glutathione.
Finally, to functionally validate the unique influence of hemin on glutathione pathway genes in Hmox1−/− MSCs, we analyzed reduced glutathione (GSH) to oxidized glutathione (GSSG) ratio, changes in total GSH and GSSG in MSCs, and fibroblasts of both Hmox1+/+ and Hmox1−/− phenotypes. Cells were treated with hemin (50 μmol/L) for 6 h, like in gene expression experiments. However, we then incubated the cells for two more hours in hemin-free complete medium to allow for GSH recovery. The GSH/GSSG ratio that allows assessing cellular oxidative stress was decreased in both Hmox1+/+ and Hmox1−/− MSCs as well as in Hmox1−/− fibroblasts. Further, Hmox1−/− fibroblasts were characterized by lower GSH/GSSG ratio than Hmox1−/− MSC cells, which additionally had a slightly lower ratio than Hmox1+/+ MSCs (Fig. 7G).
Noteworthy, Hmox1−/− MSCs were the only ones that increased total GSH in response to hemin, whereas they caused a decrease in total GSH in Hmox1+/+ MSCs (Fig. 7H). Levels of GSSG were increased in Hmox1+/+ MSCs, Hmox1−/− MSCs, and Hmox1−/− fibroblasts. They were higher in both Hmox1−/− cell types than in their respective Hmox1+/+ counterparts. However, levels of GSSG increased more in Hmox1−/− fibroblasts than in Hmox1−/− MSCs (Fig. 7I).
MSCs isolated from Hmox1−/− mice showed, in comparison to fibroblasts and PAC, higher resistance to hemin. However, hemin still increased cellular concentration of H2O2 in both MSCs and fibroblasts Hmox1−/−. We show here for the first time that murine MSCs Hmox1−/− could better than mouse tail-tip fibroblasts induce antioxidant gene response, especially genes involved in the glutathione pathway. Importantly, changes in the glutathione pathway, observed on the mRNA level, were further validated functionally by the measurement of reduced and oxidized cellular glutathione. One may speculate that such a fast response to stress factors can, in part, contribute to the presence of only minor effects of Hmox1 knockout on other functions of murine bone marrow-derived MSCs.
Discussion
Murine bone marrow-derived MSCs isolated from Hmox1+/+ or Hmox1−/− mice showed similar phenotype, proliferation, and differentiation. In our hands, neither proliferation nor differentiation to primary lineages was affected by increased glucose concentrations in the culture media. However, we show here for the first time that in comparison to tail-tip fibroblasts, murine MSCs are more efficient in antioxidant response.
Available reports on the role of Hmox1 in MSC differentiation are often inconsistent, which may result from the use of cobalt or tin protoporphyrins to modulate Hmox1 activity or differences between mouse and human MSCs. Such species-dependent variations were reported, for example, for immunomodulatory activities of MSCs, which are regulated by nitric oxide synthase in murine cells and indoleamine-2,3-dioxygenase in human cells (51). We performed our experiments on murine bone marrow-derived MSCs isolated from wild-type or Hmox1−/− mice. Importantly, the unaffected phenotype of Hmox1−/− MSCs as well as no changes in the differentiation to adipocytes, osteoblasts, and chondrocytes were previously shown by Zarjou et al. (66), who also used cells isolated from Hmox1−/− mice.
On the other hand, Barbagallo et al. reported that expression of heme oxygenase-1 changes during the differentiation of human MSCs to osteoblasts (3) and treatment with osteogenic growth peptide increases HMOX1 expression in human bone marrow MSCs (56). Moreover, human MSCs stimulated with CoPP during the osteogenic differentiation had upregulated osteonectin, osteogenic growth peptide, and osteocalcin. CoPP decreased adipogenic differentiation of MSCs (3, 56), whereas downregulation of HMOX1 with siRNA resulted in enhanced adipogenesis.
Human bone marrow MSCs treated with epoxyeicosatrienoic acid displayed decreased levels of Bach1, a repressor of HMOX1 expression (52), and increased HMOX1 mRNA; whereas levels of PPARγ and C/EBPα, involved in adipogenesis, were decreased (57). Vanella et al. reported later on that inhibition of adipogenesis induced with CoPP and increase with tin mesoporphyrin could be linked to modulation of the canonical Wnt signaling (59).
Use of hemin, heme oxygenase-1 substrate, and potent inductor led to the opposite conclusions. Hemin increased adipogenesis in murine 3T3L1 preadipocytes and human bone marrow MSCs but also increased oxidative stress and induced DNA damage in murine preadipocytes (43). Importantly, all observed changes in gene expression induced with hemin could be reversed with antioxidant Tempol. Therefore, the authors concluded that oxidative stress is the key factor that regulates differentiation patterns in hemin-treated cells (43). Human bone marrow MSCs transduced with adenoviral vectors encoding for Hmox1 showed no changes in differentiation pattern but improved viability in hypoxia (18, 68). However, adenoviral vectors give only transient expression of the transgene, which was lost after 2 weeks of culture (18).
Primary rat osteoblasts showed reduced expression and activity of alkaline phosphatase and reduced osteocalcin and Runx2 expression levels when treated with hemin or when transduced with adenoviral vectors harboring the Hmox1 gene (32). Similar results were obtained with carbon monoxide releasing molecule CORM-2 or bilirubin or when Hmox1 expression was induced with prostaglandin J2 (PGJ2). Effects of hemin or PGJ2 could be reversed with heme oxygenase-1 inhibitor zinc protoporphyrin (32).
In another study, calcification of human smooth muscle cells was inhibited when cells were stimulated with heme (65). Importantly, the effect of heme and heme oxygenase-1 was mediated by ferritin (65). In our study, MSCs isolated from Hmox1−/− mice had basal levels of H2O2 similar to those of wild-type cells and potently elevated Fth1 in response to hemin. Further, Hmox1−/− MSCs could induce an efficient antioxidant response to hemin, which is a strong stress factor for cells lacking heme-degrading enzyme. One may speculate that relatively high resistance to oxidative stress observed in MSC Hmox1−/− can, at least in part, explain the observed lack of differences in the pattern of differentiation to basic lineages.
MSC Hmox1+/+ or Hmox1−/− tended to induce T cell apoptosis when injected in vivo. However, the change did not reach statistical significance. Both previous reports analyzing the role of Hmox1 in immunosuppressive activity of MSCs used SnPP to inhibit Hmox1 activity (7, 37) and human and rat (7) or human MSCs (37) and did not assess T cell apoptosis in vivo. Moreover, an in vivo experiment conducted on the role of Hmox1 in MSCs on the protection from graft rejection lacked the control group of animals treated with SnPP only, without MSCs (7). The role played by Hmox1 in human cells remained ambiguous, since Mougiakakos et al. failed to show the direct relation between Hmox1 levels and immunomodulatory activity of MSCs (37).
Surprisingly, both Hmox1+/+ and Hmox1−/− MSCs showed high resistance to H2O2 or, even more unexpectedly, to hemin, irrespective of the concentration of glucose in the medium. Cells devoid of the heme oxygenase-1, enzyme-degrading heme, were up to now shown to be highly sensitive to hemin (16). Free heme is toxic to the cells and increases oxidative stress that may lead to lipid peroxidation, DNA damage, and protein aggregation [reviewed in (28)].
In our study, we show for the first time that MSCs Hmox1−/− were resistant to hemin concentrations, which potently induced cell death in bone marrow PAC cells (16). Moreover, MSCs Hmox1−/− expressed lower basal levels of Hmox2 than PAC cells, and Hmox2 remained unaffected by hemin treatment. Subsequently, Hmox2-dependent heme degradation cannot be considered a rescue pathway. Finally, Hmox1+/+ MSCs were characterized by lower Hmox1 levels than PAC cells and had similar Hmox1 expression in comparison to fibroblasts.
We hypothesized that high MSC resistance to hemin could result from the low import of free heme. Interestingly, heme uptake in Hmox1+/+ and Hmox1−/− MSCs did not differ. The latter results were mirrored by the expression of Slc46a1 and Slc48a1—heme transporters. Expression of Slc48a1 was shown, however, to be regulated by Bach1 (61), which represses Hmox1 and responds to increased heme concentration. In our hands, hemin increased expression of heme exporter FLVCR1 in Hmox1−/− MSCs but not in Hmox1+/+ MSCs or fibroblasts, regardless of their genotype. Increased FLVCR1 was previously reported in kidneys of Hmox1−/− mice (48). Therefore, we might speculate that an increase in FLVCR1 can, at least in part, account for the MSC Hmox1−/− resistance to hemin.
As expected, hemin decreased expression of 5′-aminolevulinate synthase 1 in all tested cell types. Alas1 is a heme synthesis rate-limiting enzyme, whose levels are tightly regulated because of the presence of heme regulatory motif in its promoter (38). Other enzymes involved in the heme synthesis were mostly not changed and did not differ between Hmox1+/+ and Hmox1−/− cells. Regarding the iron metabolism, hemin-treated Hmox1+/+ MSCs upregulated ferroportin. Fpn expression did not change in MSC Hmox1−/− cells, but they showed a trend toward higher ferroportin levels than wild-type controls. On the other hand, Hmox1−/− but not Hmox1+/+ MSCs or any of fibroblast cells increased ferritin that captures labile iron and, therefore, protects cells from oxidative stress [reviewed in (28)].
Although 6 h of treatment with 50 μmol/L hemin did not increase cell death in Hmox1−/− MSCs, it elevated concentrations of cellular hydrogen peroxide. Levels of H2O2 were higher in Hmox1−/− fibroblasts than in Hmox1−/− MSCs also after 24 h of treatment with hemin and, importantly, in Hmox1+/+ fibroblasts than in Hmox1+/+ MSCs after 48 of culture. However, neither in fibroblasts nor in MSCs we found any differences in basal levels of H2O2.
Previously, higher H2O2 levels were reported in Hmox1−/− than in Hmox1+/+ iPS cells (31). Increased protein carbonylation and lipid peroxidation were also reported in livers and kidneys of Hmox1−/− mice (42). Basal intracellular ROS levels in human MSCs and fibroblasts were similar and lower than ROS in INS-1 insulinoma (55). Both MSCs and fibroblasts were characterized by similar levels of SOD1, SOD2, CAT, and GPX1 mRNA, and higher activities of catalase and glutathione peroxidase-1 than in INS-1 cells (55). Of note, in our study, the concentration of hemin, which was toxic for MSC Hmox1−/− cells, also caused some increase in cell death in Hmox1+/+ MSCs.
MSCs Hmox1−/− upregulated in response to hemin a set of genes involved in antioxidant defense, namely Sod2, Prdx3, Prdx6, Cat, Gclc, Gclm, Gss, Gsr, and Gstp1, all of which can be regulated by the Nrf2 transcription factor. Notably, hemin induced expression of enzymes involved in both synthesis and metabolism of glutathione. Bilirubin, which is rapidly formed by biliverdin reductase from biliverdin, a product of heme oxygenase activity, is a strong antioxidant (50) that has properties that are complementary to glutathione (46). Although glutathione has a much higher cellular concentration than bilirubin, it protects mainly hydrophilic proteins. On the other hand, lipophilic bilirubin can protect lipids. However, in our hands, MSC Hmox1−/− cells devoid of heme oxygenase-1 did not change the expression of heme oxygenase-2, another source of cellular biliverdin.
Increased expression of γ-glytamylcysteine ligase and glutathione synthetase leads to enhanced production of glutathione, whereas upregulated glutathione reductase restores GSH from GSSG. In our experimental setting, GSH to GSSG ratio, which is an indication of cell redox status, decreased in all tested cells treated with hemin. However, the decrease was much stronger in Hmox1−/− fibroblasts than in Hmox1−/− MSCs. Hmox1−/− fibroblasts were characterized by higher total GSSG levels, whereas Hmox1−/− MSCs were the only to increase total GSH.
Of note, HUVEC cells with long alleles of the HMOX1 promoter, and thus lower levels of HMOX1, treated with H2O2 had higher concentrations of total glutathione and GSSG, but lower GSH/GSSG ratio than cells with the short promoter (53). Further, hemin was shown to induce neuronal necroptosis, which was related to depletion of glutathione (29).
Increased expression of glutathione metabolism genes in Hmox1−/− MSCs was accompanied by the upregulation of peroxiredoxin-6. Prdx6 is the only 1-Cys peroxiredoxin that uses glutathione instead of thioredoxin, and works as a heterodimer with glutathione S-transferase π [reviewed in (13)], which was also upregulated in hemin-treated MSC Hmox1−/−. Peroxiredoxin-6 has double activity: peroxidase and phospholipase A2 (13). Interestingly, slightly higher levels of peroxiredoxin 6 were reported in human MSCs than in embryonic stem cells (22) and both peroxiredoxin-6 and glutathione S-transferase π but also peroxiredoxins 1 and 2 were highly abundant in human MSCs (60).
Expression of peroxiredoxin-6 was not changed in late passage MSCs in comparison to early passage MSCs, although aged and more senescent cells showed increased H2O2 concentration (19). In another study, aged MSCs were characterized rather by increased peroxiredoxin 5 expression (24). Surprisingly, hemin treatment, which is used to induce erythroid differentiation of K562 erythroleukemia cells, decreased peroxiredoxin-6 levels in K562 cells (25).
We put forward that increased expression of peroxiredoxin 6 and other antioxidant genes should be rather considered as a protective mechanism that allows cells to deal better with oxidative stress than BDMC (Supplementary Fig. S7). Although levels of H2O2 in fibroblasts and MSCs were similar, only the latter cells were able to upregulate peroxiredoxin 6 and its partner—glutathione S-transferase π.
MSCs isolated from Hmox1−/− mice were able to react to oxidative stress better than fibroblasts and recovered glutathione faster. One may speculate that MSCs can express lower levels of Keap1 or Nrf3, both of which can decrease activity of Nrf2 transcription factor. Nevertheless, our data show that cells such as MSCs are better equipped with the measures to deal with harsh conditions than other bone marrow-derived cells, especially proangiogenic cells. Further, we can speculate that certain cell types are less dependent on heme oxygenase-1, which is considered a crucial cytoprotective enzyme. Tested functions and differentiation potential of murine MSCs were mostly unaffected by the lack of Hmox1.
Materials and Methods
Animals
All procedures involving the use of animals were performed according to approved guidelines. Mice were maintained under the specific pathogen-free conditions, in individually ventilated cages, with full access to food and water. All animal experiments were approved by the Local Ethical Committee for Animal Research at the Jagiellonian University.
Isolation of MSCs
MSCs were isolated from femurs and tibia of C57Bl6 × FVB Hmox1+/+ or C57Bl6 × FVB Hmox1−/−. Mice were sacrificed with the overdose of ketamine/xylazine. Bones were resected under the sterile laminar flow hood and cut into small pieces (ca. 1 mm2) with a bone cutter. Then, bone chips were digested with 1 mg/mL type II collagenase (Gibco) for 90–120 min in 37°C in a rotary shaker (250 rpm). Released cells were washed once with PBS and resuspended in the growth medium [αMEM supplemented with 10% FBS (Lonza) and penicillin with streptomycin (Sigma-Aldrich, St. Louis, MO)].
Cells were seeded in six-well plates—bone marrow from one mouse per well. The medium was changed every 24 h in the first 3 days and every 2–3 days after that, and cells were passaged when confluent. Importantly, before the MACS sorting, cells were detached with short treatment with trypsin (2 min; Gibco) at room temperature to decrease the number of highly adherent macrophages in culture. After three passages, MSCs were further purified from the CD45+ fraction with MACS sorting.
MACS sorting of CD45− murine bone marrow stromal cells
Bone marrow-derived cells were detached with trypsin, washed with PBS, resuspended in AutoMACS running buffer (Miltenyi), and stained for 25 min with anti-mouse CD45 MicroBeads (Miltenyi) in 4°C. Then, the cells were washed with 1 mL of PBS, resuspended in AutoMACS Running Buffer, and separated on MACS MS columns (Miltenyi) or with AutoMACS (Miltenyi). Flow-through with CD45− cells was collected, and columns with CD45+ cells were discarded. Purified CD45− murine bone marrow MSCs were next counted and either used directly for the experiments or seeded for further culture (1.5–2.0 × 104/1 cm2) in αMEM complete medium (CM).
Isolation of murine fibroblasts
Murine adult tail fibroblasts were isolated from C57Bl6 × FVB Hmox1+/+ or C57Bl6 × FVB Hmox1−/− according to the previously published protocol (49) and cultured in DMEM (Lonza) medium supplemented with 10% FBS (Lonza) and penicillin with streptomycin (Sigma-Aldrich).
Isolation of murine bone marrow PAC
Murine bone marrow proangiogenic cells were isolated as described earlier (14, 16) and cultured in EGM2-MV medium (Lonza) with 10% FBS and penicillin with streptomycin (Sigma-Aldrich).
Analysis of fibroblast- or osteoblast colony-forming units
Cells isolated from the bone marrow were counted by using Türck solution (Merck) to lyse red blood cells. Then, 1 × 106 of bone marrow cells were seeded per well in six-well plates. Cells were cultured until colonies of fibroblastoid cells were formed. At that stage, part of the wells was fixed and stained with crystal violet and the colonies of fibroblastoid cells were counted. Another part of the cells was treated with osteogenic differentiation medium for the next 3 weeks, and then cells were stained with Alizarin Red S and positive colonies were counted.
MSCs phenotyping
MSCs cultured for three passages after the isolation were detached with trypsin, washed with PBS, and stained for 25 min in AutoMACS Running Buffer at 4°C with the following antibodies: anti-mouse CD45 (clone 30F-11; BD Biosciences), anti-mouse CD29 (clone HMβ1-1; BioLegend), anti-mouse CD31 (clone MEC13.3; BD Biosciences), anti-mouse CD34 (clone RAM34; BD Biosciences), anti-mouse CD90.2 (clone 30-H12; BioLegend), anti-mouse CD105 (clone MJ7/18; BioLegend), anti-mouse CD117 (c-kit) (clone 2B8; eBioscience), anti-mouse CD140a (clone APA5; eBioscience), and anti-mouse Ly-6A/E (Sca-1) (clone D7; eBioscience). The phenotype of the cells was assessed with BD LSR II or BD LSR Fortessa (Becton Dickinson).
MSCs: differentiation to osteoblasts
MSCs Hmox1+/+ or Hmox1−/− were differentiated to osteoblasts with the protocol by Zhu et al. (69). Briefly, 2.5 × 104 of sorted CD45− bone marrow stromal cells were seeded per 1 well of 24-well plates. Osteoblast differentiation was induced for 3 weeks with αMEM CM supplemented with 0.1 μmol/L dexamethasone, 10 mmol/L β-glycerol phosphate, and 50 μmol/L ascorbate-2-phosphate (all from Sigma-Aldrich) and verified with AlizarinRed S staining (Supplementary Fig. S1A), gene expression analysis, and immunofluorescent staining for osteopontin (clone EPR3688; Abcam). Control cells were cultured in αMEM CM. Low glucose differentiation or control medium contained 5 mmol/L glucose, and high glucose differentiation or control medium contained 33 mmol/L glucose.
MSCs: differentiation to adipocytes
MSCs Hmox1+/+ or Hmox1−/− were differentiated to adipocytes with the protocol by Zhu et al. (69). Briefly, 2.5 × 104 of sorted CD45− bone marrow stromal cells were seeded per 1 well of 24-well plates. Adipocyte differentiation was induced for 3 weeks with αMEM CM supplemented with 1.0 μmol/L dexamethasone, 50 μmol/L 3-isobutyl-1-methylxanthine (IBMX), and 10 ng/mL insulin (all from Sigma-Aldrich) and verified with OilRedO staining, gene expression analysis, and immunofluorescent staining for Fabp4 (clone EPR3579; Abcam). Control cells were cultured in αMEM CM. Low glucose differentiation or control medium contained 5 mmol/L glucose, and high glucose differentiation or control medium contained 33 mmol/L glucose.
MSCs: differentiation to myofibroblasts
Sorted CD45− bone marrow stromal cells were seeded in 24-well plates (2.5 × 104/well). Myofibroblast differentiation was induced for 6 days with αMEM CM supplemented with 2 ng/mL recombinant human TGFβ1 (Peprotech) and confirmed with gene expression analysis. Control cells were cultured in αMEM CM.
Analysis of MSC immunosuppressive activity in vivo
C57Bl6 × FVB mice were injected intravenously with 1 million of MSC Hmox1+/+ or Hmox1−/−, or saline (vehicle control) and sacrificed 24 h later. Numbers of circulating CD3+ T cells, CD3+AnnexinV+ apoptotic T cells, and CD3+CD4+CD25high activated T cells were assessed by flow cytometry on an LSR Fortessa cytometer (Becton Dickinson). Peripheral blood (PB) was collected in heparinized tubes. Red blood cells were lysed with ammonium chloride red blood cell lysis buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM EDTA). Obtained total nucleated cells were resuspended in autoMACS Running Buffer (Miltenyi).
Cells were then stained with anti-mouse CD3 (clone 17A2; BD Horizon), anti-mouse CD4 (clone RPA-T4; BD Horizon), anti-mouse CD25 (clone C37; BD Pharmingen), and anti-mouse CD45 (clone 30-F11; BD Pharmingen) antibodies. Annexin V+ cells were stained with TACS® Annexin V (AnV) kit (Trevigen) according to the manufacturer's instructions. The number of cells per 1 μL of PB was calculated based on the total leukocyte count (WBC, 103 cells/1 μL of PB) and the percentage of each population within the collected events. WBC was measured by using ABC Vet (scil animal care GmbH).
Analysis of MSC immunosuppressive activity in vitro
MSC Hmox1+/+ or Hmox1−/− were seeded on six-well plates. When MSCs reached 100% confluency, they were co-cultured for 24 h with 1 mln of primary mouse splenocytes in each well. After the co-culture, splenocytes were harvested and stained with anti-CD3-AlexaFluor 647 (clone 17A2; BD Pharmingen), anti-Ki67-AlexaFluor 488 (clone B56; BD Pharmingen), and DAPI for the analysis of cell proliferation or with anti-CD3-AlexaFluor 647 (clone 17A2; BD Pharmingen), and Anexin V-FITC (Trevigen) for the analysis of T cell apoptosis. Data are presented as percent of proliferating/apoptotic T cells within the population of CD3+ T cells.
Total antioxidant capacity assays
Total antioxidant capacity of conditioned media from MSC Hmox1+/+ or Hmox1−/− was measured with TAC Assay (Cell Biolabs) according to the manufacturer's protocol, or using ABTS, or FRAP method. ABTS assay was performed according to the previously published protocol (40) based on the (12) method. FRAP assay was based on the Benzie and Strain method (4).
Multiplex immunoassays
Levels of factors produced by MSC Hmox1+/+ or Hmox1−/− were measured with Milliplex® MAP Mouse Cytokine/Chemokine Bead Panel −32 Plex (Millipore) on Luminex FlexMap 3D platform (Millipore) and analyzed with Milliplex Analyst 3.4 software (Millipore).
Proliferation assay
The proliferation of MSCs was assessed with the BrdU method by using Cell Proliferation ELISA (Roche), according to the manufacturer's protocol. Low glucose medium contained 5 mmol/L glucose, and high glucose medium contained 33 mmol/L glucose. Cells were cultured in high or low glucose medium and BrdU labeling solution for 24 h.
Lactate dehydrogenase activity assay
Cytotoxicity of hemin or H2O2 in MSCs was evaluated with CytoTox 96® NonRadioactive Cytotoxicity Assay (Promega), according to the manufacturer's protocol. Low glucose medium contained 5 mmol/L glucose, and high glucose medium contained 33 mmol/L glucose. Cells were treated with hemin, H2O2, and/or high glucose for 6 h before the analysis.
7-aminoactinomycin D-based cell viability assay
The viability of MSCs stimulated for 6 h with high doses of hemin was assessed by 7-aminoactinomycin D (7-AAD) staining. Stimulated and control cells were detached with trypsin, washed with PBS, and resuspended in AutoMACS Running Buffer (Miltenyi). Then, cells were stained for 10 min with 7-AAD (BD Pharmingen) according to the manufacturer's protocol and analyzed on a BD LSR Fortessa cytometer.
Measurement of MSC viability in response to TNFα
MSC Hmox1+/+ or Hmox1−/− were incubated with 10 ng/mL TNFα for 24 h. Then, numbers of early and late apoptotic cells were assessed by flow cytometry by using the staining with Hoechst 33342 and 7-AAD according to the protocol by Schmid et al. (45).
Measurement of cellular H2O2 levels
Levels of cellular H2O2 were measured with H2DCFDA assay. Hmox1+/+ or Hmox1−/− MSCs and fibroblasts were stimulated for 6, 24, or 48 h with 50 μmol/L hemin (Frontiers Scientific). After each timepoint, the stimulated cells and nonstimulated controls were harvested with trypsin, washed with PBS, and stained for 30 min with 0.1 μmol/L H2DCFDA (Sigma-Aldrich) in PBS. Then, cells were washed twice with PBS, and DCFDA fluorescence was assessed with a BD LSR Fortessa cytometer (Becton Dickinson).
Heme cellular content assay
MSCs Hmox1+/+ or Hmox1−/− were cultured in 24-well plates. Heme content was assessed with the method by Foresti et al. (15) in nonstimulated cells (T0), after 2 h of stimulation with 50 μmol/L hemin (T2) and after 2 additional hours of culture in fresh αMEM CM (T4). Cells were lysed with 80% formic acid (POCH S.A.), and the lysate was transferred to clear plastic 96-well plates. Absorbance was measured at λ = 398 nm with a GENios microplate reader (Tecan).
SnPP binding assay
Cells stimulated with SnPP (Frontiers Scientific) show fluorescence in APC channel (λex = 640 nm, emission detected with 670/25 bandpass filter). Therefore, we stimulated Hmox1+/+ and Hmox1−/− MSCs with 10, 25, or 50 μmol/L SnPP for 6 h and analyzed their fluorescence with flow cytometry. Cells were detached with trypsin, washed, and resuspended in AutoMACS Running Buffer (Miltenyi). Flow cytometry analysis was performed on a BD LSR Fortessa cytometer (Becton Dickinson).
GSH/GSSG assay
Levels of total GSH, total GSSG, and GSH/GSSG ratio in MSCs and fibroblasts were assessed with GSH/GSSG-Glo™ Assay (Promega) according to the manufacturer's protocol. Then, 1.0 × 104 cells were seeded per well in 96-well plates. Cells were stimulated for 4 h with 50 μmol/L hemin in αMEM CM and cultured for 2 h in αMEM CM to let them recover GSH levels. Total GSH and total GSSG data are shown as a ratio to the control nonstimulated cells.
Analysis of gene expression
Total RNA was isolated by phenol-chloroform extraction, and it was reverse transcribed with the oligo(dT) primers and RevertAid Reverse transcriptase (Fermentas) or with the NCode™ VILO™ miRNA cDNA synthesis kit (Invitrogen). The expression of genes was assessed by quantitative real-time PCR (qRT-PCR), which was performed in the StepOnePlus system (Applied Biosystems, Foster City, CA) with the specific primers (Supplementary Tables S1 and S2), cDNA and SYBR Green Quantitative RT-PCR kit (Sigma-Aldrich), under conditions summarized in Supplementary Table S3.
Expression of lipid metabolism genes in Hmox1+/+ or Hmox1−/− MSCs differentiated to adipocytes was assessed with TaqMan® Array Mouse Lipid-Regulated Genes (Applied Biosystems) and TaqMan Universal PCR Master Mix (Applied Biosystems) with the PCR program described in Supplementary Table S3.
Statistical analysis
Statistical analysis of the data was performed with GraphPad Prism software. Results are expressed as mean ± SD unless otherwise stated. Statistical significance was accepted at p < 0.05. Data obtained in in vitro experiments were analyzed with Student's t-test when two groups of samples were used. In another case, we used one-way or two-way analysis of variance with Bonferroni post-test. The kind of statistical test applied to analyze given sets of data is provided in the description of figures.
Supplementary Material
Abbreviations Used
- 7-AAD
7-aminoactinomycin D
- ABTS
2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid)
- BDMC
bone marrow-derived proangiogenic cells
- CM
complete medium
- CoPP
cobalt protoporphyrin IX
- FRAP
ferric-reducing antioxidant power
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- HO-1
Heme oxygenase-1 (encoded by the HMOX1 gene)
- MSCs
mesenchymal stromal cells
- PAC
proangiogenic cell
- PB
peripheral blood
- PGJ2
prostaglandin J2
- qRT-PCR
quantitative real-time PCR
- SnPP
tin protoporphyrin IX
- TAC
total antioxidant capacity
Acknowledgments
This work was supported by the Polish National Science Centre (grants 2013/11/N/NZ3/00958, 2013/11/N/NZ1/02399, 2015/18/NZ3/00387), the European Union under the European Regional Development Fund Operational Programme Innovative Economy 2007–2013 (POIG-01.02.01-109/09), and the grant from the National Centre for Research and Development (STRATEGMED (2/269415/11/NCBR/2015). WNN was supported by the Foundation for Polish Science (FNP). The Faculty of Biochemistry, Biophysics and Biotechnology of Jagiellonian University is a partner of the Leading National Research Center (KNOW) that is supported by the Ministry of Science and Higher Education. Servier Medical Art image bank was used to prepare Figure 5A.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T, Cai T, Chen W, Sun L, and Shi S. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell 10: 544–555, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ankrum JA, Ong JF, and Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol 32: 252–260, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbagallo I, Vanella A, Peterson SJ, Kim DH, Tibullo D, Giallongo C, Vanella L, Parrinello N, Palumbo GA, Di Raimondo F, Abraham NG, and Asprinio D. Overexpression of heme oxygenase-1 increases human osteoblast stem cell differentiation. J Bone Miner Metab 28: 276–288, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benzie IF. and Strain JJ. Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol 299: 15–27, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Bianco P. “Mesenchymal” stem cells. Annu Rev Cell Dev Biol 30: 677–704, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Caplan AI. Mesenchymal stem cells. J Orthop Res 9: 641–650, 1991 [DOI] [PubMed] [Google Scholar]
- 7.Chabannes D, Hill M, Merieau E, Rossignol J, Brion R, Soulillou JP, Anegon I, and Cuturi MC. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood 110: 3691–3694, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Ding L, Saunders TL, Enikolopov G, and Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481: 457–462, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, and Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8: 315–317, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Dulak J, Deshane J, Jozkowicz A, and Agarwal A. Heme oxygenase-1 and carbon monoxide in vascular pathobiology: focus on angiogenesis. Circulation 117: 231–241, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eggenhofer E, Benseler V, Kroemer A, Popp FC, Geissler EK, Schlitt HJ, Baan CC, Dahlke MH, and Hoogduijn MJ. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol 3: 297, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem 37: 277–285, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Fisher AB. Peroxiredoxin 6: a bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. Antioxid Redox Signal 15: 831–844, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Florczyk U, Jazwa A, Maleszewska M, Mendel M, Szade K, Kozakowska M, Grochot-Przeczek A, Viscardi M, Czauderna S, Bukowska-Strakova K, Kotlinowski J, Jozkowicz A, Loboda A, and Dulak J. Nrf2 regulates angiogenesis: effect on endothelial cells, bone marrow-derived proangiogenic cells and hind limb ischemia. Antioxid Redox Signal 20: 1693–1708, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foresti R, Hoque M, Bains S, Green CJ, and Motterlini R. Haem and nitric oxide: synergism in the modulation of the endothelial haem oxygenase-1 pathway. Biochem J 372(Pt 2): 381–390, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grochot-Przeczek A, Kotlinowski J, Kozakowska M, Starowicz K, Jagodzinska J, Stachurska A, Volger OL, Bukowska-Strakova K, Florzyk U, Tertil M, Jazwa A, Szade K, Stepniewski J, Loboda A, Horrevoets AJ, Dulak J, and Jozkowicz A. Heme oxygenase-1 is required for angiogenic function of bone marrow-derived progenitor cells: role in therapeutic revascularization. Antioxid Redox Signal 20: 1677–1692, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grundemar L. and Ny L. Pitfalls using metalloporphyrins in carbon monoxide research. Trends Pharmacol Sci 18: 193–195, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Hamedi-Asl P, Halabian R, Bahmani P, Mohammadipour M, Mohammadzadeh M, Roushandeh AM, Jahanian-Najafabadi A, Kuwahara Y, and Roudkenar MH. Adenovirus-mediated expression of the HO-1 protein within MSCs decreased cytotoxicity and inhibited apoptosis induced by oxidative stresses. Cell Stress Chaperones 17: 181–190, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho P-J, Yen M-L, Tang B-C, Chen C-T, and Yen BL. H2O2 accumulation mediates differentiation capacity alteration, but not proliferative decline, in senescent human fetal mesenchymal stem cells. Antioxid Redox Signal 18: 1895–1905, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hou C, Shen L, Huang Q, Mi J, Wu Y, Yang M, Zeng W, Li L, Chen W, and Zhu C. The effect of heme oxygenase-1 complexed with collagen on MSC performance in the treatment of diabetic ischemic ulcer. Biomaterials 34: 112–120, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Jain A, Lamark T, Sjřttem E, Larsen KB, Awuh JA, Øvervatn A, McMahon M, Hayes JD, and Johansen T. P62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J Biol Chem 285: 22576–22591, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaishankar A, Barthelery M, Freeman WM, Salli U, Ritty TM, and Vrana KE. Human embryonic and mesenchymal stem cells express different nuclear proteomes. Stem Cells Dev 18: 793–802, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Jozkowicz A. and Dulak J. Effects of protoporphyrins on production of nitric oxide and expression of vascular endothelial growth factor in vascular smooth muscle cells and macrophages. Acta Biochim Pol 50: 69–79, 2003 [PubMed] [Google Scholar]
- 24.Kasper G, Mao L, Geissler S, Draycheva A, Trippens J, Kühnisch J, Tschirschmann M, Kaspar K, Perka C, Duda GN, and Klose J. Insights into mesenchymal stem cell aging: involvement of antioxidant defense and actin cytoskeleton. Stem Cells 27: 1288–1297, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Kim KB, Chae YC, Han A, Kang JY, Jung H, Park JW, Hahm JY, Kim S, and Seo SB. Negative regulation of peroxiredoxin 6 (Prdx 6) transcription by nuclear oncoprotein DEK during leukemia cell differentiation. Anim Cells Syst 18: 318–323, 2014 [Google Scholar]
- 26.Kim YJ, Hwang SJ, Bae YC, and Jung JS. MiR-21 regulates adipogenic differentiation through the modulation of TGF-beta signaling in mesenchymal stem cells derived from human adipose tissue. Stem Cells 27: 3093–3102, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Kozakowska M, Ciesla M, Stefanska A, Skrzypek K, Was H, Jazwa A, Grochot-Przeczek A, Kotlinowski J, Szymula A, Bartelik A, Mazam M, Yagensky O, Florczyk U, Lemke K, Zebzda A, Dyduch G, Nowak W, Szade K, Stepniewski J, Majka M, Derlacz R, Loboda A, Dulak J, and Jozkowicz A. Heme oxygenase-1 inhibits myoblast differentiation by targeting myomirs. Antioxid Redox Signal 16: 113–127, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar S. and Bandyopadhyay U. Free heme toxicity and its detoxification systems in human. Toxicol Lett 157: 175–188, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Laird MD, Wakade C, Alleyne CH, Jr, and Dhandapani KM. Hemin-induced necroptosis involves glutathione depletion in mouse astrocytes. Free Radic Biol Med 45: 1103–1114, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Lee CH, Shah B, Moioli EK, and Mao JJ. CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J Clin Invest 120: 3340–3349, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin CY, Peng CY, Huang TT, Wu ML, Lai YL, Peng DH, Chen PF, Chen HF, Yen BL, Wu KK, and Yet SF. Exacerbation of oxidative stress-induced cell death and differentiation in induced pluripotent stem cells lacking heme oxygenase-1. Stem Cells Dev 21: 1675–1687, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin TH, Tang CH, Hung SY, Liu SH, Lin YM, Fu WM, and Yang RS. Upregulation of heme oxygenase-1 inhibits the maturation and mineralization of osteoblasts. J Cell Physiol 222: 757–768, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Mabuchi Y, Morikawa S, Harada S, Niibe K, Suzuki S, Renault-Mihara F, Houlihan DD, Akazawa C, Okano H, and Matsuzaki Y. LNGFR(+)THY-1(+)VCAM-1(hi+) cells reveal functionally distinct subpopulations in mesenchymal stem cells, Stem Cell Reports 1: 152–165, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A, Enikolopov GN, and Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466: 829–834, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP, Ganesan S, Glod JW, and Banerjee D. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res 68: 4331–4339, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morikawa S, Mabuchi Y, Kubota Y, Nagai Y, Niibe K, Hiratsu E, Suzuki S, Miyauchi-Hara C, Nagoshi N, Sunabori T, Shimmura S, Miyawaki A, Nakagawa T, Suda T, Okano H, and Matsuzaki Y. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med 206: 2483–2496, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mougiakakos D, Jitschin R, Johansson CC, Okita R, Kiessling R, and Le Blanc K. The impact of inflammatory licensing on heme oxygenase-1-mediated induction of regulatory T cells by human mesenchymal stem cells. Blood 117: 4826–4835, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Munakata H. Role of the heme regulatory motif in the heme-mediated inhibition of mitochondrial import of 5-aminolevulinate synthase. J Biochem 136: 233–238, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Nauta AJ. and Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood 110: 3499–3506, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Nowak WN, Borys S, Kusińska K, Bukowska-Strakova K, Witek P, Koblik T, Józkowicz A, Małecki MT, and Dulak J. Number of circulating pro-angiogenic cells, growth factor and anti-oxidative gene profiles might be altered in type 2 diabetes with and without diabetic foot syndrome. J Diabetes Investig 5: 99–107, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Omatsu Y, Sugiyama T, Kohara H, Kondoh G, Fujii N, Kohno K, and Nagasawa T. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity 33: 387–399, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Poss KD. and Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci USA 94: 10919–10924, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puri N, Sodhi K, Haarstad M, Kim DH, Bohinc S, Foglio E, Favero G, and Abraham NG. Heme induced oxidative stress attenuates sirtuin1 and enhances adipogenesis in mesenchymal stem cells and mouse pre-adipocytes. J Cell Biochem 113: 1926–1935, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliagico E, Ferrari S, Robey PG, Riminucci M, and Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131: 324–336, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Schmid I, Uittenbogaart C, and Jamieson BD. Live-cell assay for detection of apoptosis by dual-laser flow cytometry using Hoechst 33342 and 7-amino-actinomycin D. Nat Protoc 2: 187–190, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Sedlak TW, Saleh M, Higginson DS, Paul BD, Juluri KR, and Snyder SH. Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proc Natl Acad Sci USA 106: 5171–5176, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stachurska A, Ciesla M, Kozakowska M, Wolffram S, Boesch-Saadatmandi C, Rimbach G, Jozkowicz A, Dulak J, and Loboda A. Cross-talk between microRNAs, nuclear factor E2-related factor 2, and heme oxygenase-1 in ochratoxin A-induced toxic effects in renal proximal tubular epithelial cells. Mol Nutr Food Res 57: 504–515, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Starzyński RR, Canonne Hergaux F, Lenartowicz M, Krzeptowski W, Willemetz A, Stys A, Bierla J, Pietrzak P, Dziaman T, and Lipinski P. Ferroportin expression in haem oxygenase 1-deficient mice. Biochem J 449: 69–78, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Stepniewski J, Kachamakova-Trojanowska N, Ogrocki D, Szopa M, Matlok M, Beilharz M, Dyduch G, Malecki MT, Jozkowicz A, and Dulak J. Induced pluripotent stem cells as a model for diabetes investigation. Sci Rep 5: 8597, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, and Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science 235: 1043–1046, 1987 [DOI] [PubMed] [Google Scholar]
- 51.Su J, Chen X, Huang Y, Li W, Li J, Cao K, Cao G, Zhang L, Li F, Roberts AI, Kang H, Yu P, Ren G, Ji W, Wang Y, and Shi Y. Phylogenetic distinction of iNOS and IDO function in mesenchymal stem cell-mediated immunosuppression in mammalian species 21: 388–396, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun J, Hoshino H, Takaku K, Nakajima O, Muto A, Suzuki H, Tashiro S, Takahashi S, Shibahara S, Alam J, Taketo MM, Yamamoto M, and Igarashi K. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase‐1 gene. EMBO J 21: 5216–5224, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taha H, Skrzypek K, Guevara I, Nigisch A, Mustafa S, Grochot-Przeczek A, Ferdek P, Was H, Kotlinoski J, Kozakowska M, Balcerczyk A, Muchova L, Vitek L, Weigel G, Dulak J, and Jozkowicz A. Role of heme oxygenase-1 in human endothelial cells: lesson from the promoter allelic variants. Arterioscler Thromb Vasc Biol 30: 1634–1641, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsubokawa T, Yagi K, Nakanishi C, Zuka M, Nohara A, Ino H, Fujino N, Konno T, Kawashiri MA, Ishibashi-Ueda H, Nagaya N, and Yamagishi M. Impact of anti-apoptotic and anti-oxidative effects of bone marrow mesenchymal stem cells with transient overexpression of heme oxygenase-1 on myocardial ischemia. Am J Physiol Heart Circ Physiol 298: H1320–H1329, 2010 [DOI] [PubMed] [Google Scholar]
- 55.Valle-Prieto A. and Conget PA. Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells Dev 19: 1885–1893, 2010 [DOI] [PubMed] [Google Scholar]
- 56.Vanella L, Kim DH, Asprinio D, Peterson SJ, Barbagallo I, Vanella A, Goldstein D, Ikehara S, Kappas A, and Abraham NG. HO-1 expression increases mesenchymal stem cell-derived osteoblasts but decreases adipocyte lineage. Bone 46: 236–243, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vanella L, Kim DH, Sodhi K, Barbagallo I, Burgess AP, Faick JR, Schwartzman ML, and Abraham NG. Crosstalk between EET and HO-1 downregulates Bach1 and adipogenic marker expression in mesenchymal stem cell derived adipocytes. Prostaglandins Other Lipid Mediat 96: 54–62, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vanella L, Sanford C, Kim DH, Abraham NG, and Ebraheim N. Oxidative stress and heme oxygenase-1 regulated human mesenchymal stem cells differentiation. Int J Hypertens 2012: 1–10, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vanella L, Sodhi K, Kim DH, Puri N, Maheshwari M, Hinds TD, Bellner L, Goldstein D, Peterson SJ, Shapiro JI, and Abraham NG. Increased heme-oxygenase 1 expression decreases adipocyte differentiation and lipid accumulation in mesenchymal stem cells via upregulation of the canonical Wnt signaling cascade. Stem Cell Res Ther 4: 28, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wagner W, Feldmann RE, Jr., Seckinger A, Maurer MH, Wein F, Blake J, Krause U, Kalenka A, Bürgers HF, Saffrich R, Wuchter P, Kuschinsky W, and Ho AD. The heterogeneity of human mesenchymal stem cell preparations—evidence from simultaneous analysis of proteomes and transcriptomes. Exp Hematol 34: 536–548, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Warnatz HJ, Schmidt D, Manke T, Piccini I, Sultan M, Borodina T, Balzereit D, Wruck W, Soldatov A, Vingron M, Lehrach H, and Yaspo ML. The BTB and CNC homology 1 (BACH1) target genes are involved in the oxidative stress response and in control of the cell cycle. J Biol Chem 286: 23521–23532, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Was H, Sokolowska M, Sierpniowska A, Dominik P, Skrzypek K, Lackowska B, Pratnicki A, Grochot-Przeczek A, Taha H, Kotlinowski J, Kozakowska M, Mazan A, Nowak W, Muchova L, Vitek L, Ratajska A, Dulak J, and Jozkowicz A. Effects of heme oxygenase-1 on induction and development of chemically induced squamous cell carcinoma in mice. Free Radic Biol Med 51: 1717–1726, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wojakowski W, Tendera M, Cybulski W, Zuba-Surma EK, Szade K, Florczyk U, Kozakowska M, Szymula A, Krzych L, Paslawska U, Pasławski R, Milewski K, Buszman P, Nabiałek E, Kuczmik W, Janiszewski A, Dziȩgiel P, Buszman PE, Józkowicz A, and Wojakowski W. Effects of intracoronary delivery of allogenic bone marrow-derived stem cells expressing heme oxygenase-1 on myocardial reperfusion injury. Thromb Haemost 108: 464–475, 2012 [DOI] [PubMed] [Google Scholar]
- 64. This reference has been deleted.
- 65.Zarjou A, Jeney V, Arosio P, Poli M, Antal-Szalmas P, Agarwal A, Balla G, and Balla J. Ferritin prevents calcification and osteoblastic differentiation of vascular smooth muscle cells. J Am Soc Nephrol 20: 1254–1263, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zarjou A, Kim J, Traylor AM, Sanders PW, Balla J, Agarwal A, and Curtis LM. Paracrine effects of mesenchymal stem cells in cisplatin-induced renal injury require heme oxygenase-1. Am J Physiol Renal Physiol 300: F254–F262, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou BO, Yue R, Murphy MM, Peyer JG, and Morrison SJ. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 15: 154–168, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou H, Ramiya VK, and Visner GA. Bone marrow stem cells as a vehicle for delivery of heme oxygenase-1 gene. Stem Cells Dev 15: 79–86, 2006 [DOI] [PubMed] [Google Scholar]
- 69.Zhu H, Guo ZK, Jiang XX, Li H, Wang XY, Yao HY, Zhang Y, and Mao N. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat Protoc 5: 550–560, 2010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.