Abstract
Objective
Recently, substantial revisions to DSM-IV criteria for autism spectrum disorders (ASD) have been proposed in efforts to increase diagnostic sensitivity and specificity. This study sought to evaluate the proposed DSM5 criteria using a sample of children with PDD and non-PDD diagnoses.
Methods
Study participants were obtained from three large datasets, resulting in 4,453 subjects with DSM-IV clinical diagnoses of Pervasive Developmental Disorder and 690 subjects with non-PDD diagnoses (e.g., language disorder, ADHD). Items from a parent-report measure of ASD symptoms (Autism Diagnostic Interview-Revised) and from a clinical observation instrument (Autism Diagnostic Observation Schedule) were matched to DSM-5 criteria and then used to evaluate the sensitivity and specificity of the proposed criteria when compared to clinical diagnoses.
Results
Based on parent-report data only, the proposed DSM-5 criteria identified 91% of children with DSM-IV PDD diagnoses. Sensitivity of DSM-5 criteria remained high in specific subgroups of children with ASD, including females and children under 4. Overall, specificity of DSM-5 ASD criteria was .53, while specificity of DSM-IV ranged from .24 (PDD-NOS criteria) to .53 (Autistic Disorder criteria). When evidence of abnormality was required from both parent-report and clinical observation, specificity of DSM-5 ASD criteria increased to .63.
Conclusions
Based on analyses of existing symptom data, results suggest that the majority of children with current DSM-IV-based PDD diagnoses will remain eligible for an ASD diagnosis under the proposed DSM-5 criteria. Compared to DSM-IV criteria for Asperger syndrome and PDD-NOS, specificity of DSM-5 ASD criteria is improved, particularly when abnormalities are evident from both parent report and clinical observation.
Introduction
The proposed changes to DSM-IV diagnostic criteria for pervasive developmental disorders (PDD) include: shifting from a multi-categorical model to a single diagnostic category of Autism Spectrum Disorder (ASD), replacing the three-domain model with a two-domain model, relaxing age of onset criteria, and adding symptoms not previously included in DSM-IV, such as sensory interests and aversions. Though these changes are based on empirical data (e.g. 1, 2), little is known about the sensitivity and specificity of the new criteria. In particular, it is unclear whether the revised criteria will inadvertently narrow the definition ofPDD. This is of major significance to families concerned that their affected children might not meet the new criteria for ASD, and therefore lose necessary services.
To date, various empirical studies have found support for a 2-domain ASD symptom model (3–5). In contrast to the original model, communication deficits are subsumed with social impairments. Mandy and colleagues (6) tested this model, including sensory behaviors aspart of the restricted and repetitive behavior criterion, and found this to be an excellent fitting model. In contrast, the original DSM-IV model did not meet statistical criteria for an acceptable fit. Though this work confirms the conceptual validity of the proposed changes to DSM-IV, it tells us little about the sensitivity of the new criteria.
Because of the newness of the proposed criteria, only a handful of studies have examined the DSM-5 criteria and all have examined slightly different versions of the criteria under consideration. McPartland and colleagues assessed the sensitivity and specificity of the proposed DSM-5 criteria by using the DSM-IV field trial checklist items and found DSM-5 to perform quite poorly. Using existing data from parent questionnaires, the Autism Diagnostic Interview-Revised (7), and the Autism Diagnostic Observation Schedule (8), Mattila et al. (9) examined an early draft of the criteria (2010) and found that only 46% of children with PDD diagnoses were identified as meeting ASD criteria. Notably, when the authors used criteria more similar to the current DSM-5 criteria), approximately 96% of children with PDD diagnoses were classified correctly.
The poor sensitivity of the early draft criteria, and the remarkable increase in sensitivity with the new draft, are likely explained by Mattila and colleagues’ stringent interpretation of the 2010 criteria. For example, sensitivity was improved when they required, “routines AND/OR rituals” instead of “routines AND rituals”. Furthermore, unlike the early draft, the improved model included “unusual sensory behaviors” and the removal of onset criteria of 36 months. This revision, which has been implemented in the latest DSM-5 draft, increased sensitivity, particularly in the “high-functioning” subgroup (i.e., full scale IQs ≥70).
In another study, Frazier et al. (10) mapped items from the Social Communication Questionnaire (11) and the Social Responsiveness Scale (12) to DSM-5 criteria and found 19% to 22% of children with DSM-IV PDD diagnoses did not meet the proposed criteria. Notably, these analyses were based on criteria from DSM Field Trial Phase 1, which required a greater number of symptomsthan the currently proposed criteria. When the authors required fewer symptoms within each criterion (as in the current DSM-5 proposal), sensitivity was comparable to DSM-IV and there was a slight improvement in specificity. This pattern of results was similar across many of the subgroups, such as in females, verbal youth, and multiplex families. Nevertheless, while Frazier et al.’s sample was large (n=14,744), the methodology of the study limits the interpretability of their findings. For example, analyses included items based on past behavior (“When she/he was 4 to 5, did she/he smile back if someone smiled at her/him?”), whereas proposed DSM-5 social communication criteria relate to current functioning and behavior.
Though an important focus of the proposed revisions, it is not yet clear that specificity will improve with the DSM-5 criteria. Frazier and colleagues’ recent analyses of the new criteria suggest improved specificity for DSM-5 criteria over DSM-IV (10), particularly with a relaxed version of DSM- 5 criteria using one less symptom per domain. However, these results were obtained from siblings of affected children, of which only about 30% had a caregiver-reported non-PDD diagnosis. Additional evidence from children with non-PDD diagnoses is necessary to make claims about DSM-5’s specificity.
The proposed change to a single ASD category, as well as the new requirement that there must be a history of restricted and repetitive behaviors, has led some to believe that DSM-5 will make it more difficult for some individuals with PDD to qualify for a diagnosis. Wing et al.’s comprehensive review of the proposed criteria articulates some of these concerns, explaining that DSM-5 could inadvertently exclude subgroups of affected people, including very young children and females, and those with diagnoses of Asperger Syndrome (13). The introduction of “Social Communication Disorder” (SCD) in DSM-5 raises additional concerns that children currently diagnosed with PDD will be misclassified with this disorder if they do not meet the DSM-5 restricted and repetitive behavior requirement.
In sum, from the existing empirical work, the sensitivity of the proposed DSM-5 criteria remains unclear. In addition, relatively little attention has been paid to questions about specificity. Thus, before the proposed diagnostic changes go forward, it is critical to make use of the recent availability of large and well-characterized samples of children with PDD and non-PDD diagnoses to attempt to shed light on these issues.
The current study sought to provide additional insights into DSM-5 sensitivity and specificity by assigning individual items from well-established autism diagnostic measures to the proposed criteria and then using symptom counts to estimate how many children with previous DSM-IV diagnoses of PDD or non-PDD (e.g., language disorder, Attention Deficit Hyperactivity Disorder) would meet DSM-5 ASD criteria. We apply these same methods to DSM-IV criteria. We also completed domain-specific analyses to examine whether any children with clinical diagnoses of PDD might meet criteria for DSM-5 Social Communication Disorder (SCD).
Methods
This study does not represent a field trial for DSM-5. It uses previously collected data to evaluate DSM-5 criteria in groups of children with DSM-IV clinical diagnoses.
Sample
Participant data were obtained from three sources; proband data from the Simons Simplex Collection, a genetic consortium study focusing on “simplex” ASD families, the Collaborative Programs of Excellence in Autism (subsequently referred to as “Collaborative Programs”), a multi-center study of ASD, and the University of Michigan Autism and Communication Disorders Center Databank (subsequently referred to as “University of Michigan”), which consists of research participants and clinical referrals for assessment of ASD. All samples have been previously described in detail (4,14,15). Institutional Review Board approval was obtained at each site, and written informed consent was obtained from participants’ legal guardians.
DSM-IV Diagnostic Confirmation
All study participants had previously undergonediagnostic testing that minimally included the Autism Diagnostic Interview-Revised, Autism Diagnostic Observation Schedule and cognitive or developmental testing. Clinical best-estimate diagnoses were determined by experienced clinicians (e.g., psychologists, psychiatrists) on the basis of all available information from the parent interview and child assessment.
Operationalizing of DSM Criteria
For the study analyses, we relied primarily on the Autism Diagnostic Interview-Revised, a 96-item, parent-report measure. It includes items assessing current as well as past behaviors and covers a wide range of ASD-related impairments (e.g.; the use of idiosyncratic language). We also used the Autism Diagnostic Observation Schedule, a clinician-based measure of ASD impairments. The Autism Diagnostic Interview-Revised and the Autism Diagnostic Observation Schedule are particularly well-suited for the current study because these measures include items based on current behavior and they take into account developmental level into their design. This is consistent with DSM-5 criteria, which operationalizes symptoms differently for individuals of different ages in order to account for the effect of development on ASD symptoms (19–22).
As a first step in our analyses, items from the Autism Diagnostic Interview-Revised and the Autism Diagnostic Observation Schedule were mapped on to DSM-5 criteria. Prior to assigning items to each criterion, samples were divided into age by language groups. Age groups for children under the age of 4 and over the age of 10 were created to be consistent with Autism Diagnostic Interview-Revised age-based routing rules. Children were assigned to language groups depending on which module of the Autism Diagnostic Observation Schedule they were administered. After consensus was reached among all study authors about item assignments for DSM-5 criteria, this process was repeated for DSM-IV criteria (item assignments areavailable in the online supplement).
For each item included in the DSM-IV and DSM-5 item maps, a score of 1, 2, or 3 on the item indicated the presence of a symptom, whereas a score of 0 indicated the absence of a symptom. DSM-IV and DSM-5 guidelines were then followed to determine whether each participant met or did not meet DSM-5 criteria for ASD and DSM-IV criteria for Autistic Disorder, Asperger Syndrome, and/or PDD-NOS. Initially we established “classifications” (e.g., met DSM-5 ASD vs. did not meet DSM-5 ASD, etc.) by extracting symptom information from only the Autism Diagnostic Interview-Revised. We then established classifications using information from both the Autism Diagnostic Interview-Revised and the Autism Diagnostic Observation Schedule (i.e., allowing evidence of symptoms to come from either parent report and/or direct observation). Unfortunately, it was not practical to attempt to establish classifications using only information from the Autism Diagnostic Observation Schedule, because there are no relevant items for certain sub-domains (e.g., see supplementary Tables 1 and 2). However, because there are adequate numbers of items on both instruments that assess DSM-5 criteria A1 and A2, we were able to examine sensitivity and specificity when symptoms in these domains were required from both measures.
To ensure that the creation of both DSM-5and DSM-IV item assignments agreed with other clinicians’ interpretations of the criteria, these were reviewed by 2 psychologists and 1 psychiatrist who were not otherwise involved in the design or execution of the current study. All have extensive experience with ASD diagnosis and the study instruments. As a result of their feedback, 2 items were re-assigned and 1 item was removed from DSM-5 criteria (for details, see supplementary Tables 1 and 2). Importantly, the majority of the study authors and the independent experts noted some overlap between DSM-5 criterion A1 and criterion A3. For example, whereas a poor quality social overture/initiation could be considered evidence of “abnormal social approach” (A1), it could also reflect “difficulties adjusting behavior to suit different social contexts” (A3). In general, however, the group agreed that items were easier to “map” onto DSM-5 criteria than DSM-IV criteria.
Statistical Analysis
Analyses were restricted to participants ages 2 to 17 for whom Autism Diagnostic Interview-Revised and Autism Diagnostic Observation Schedule data and DSM-IV clinical diagnoses were available. All statistical analyses were run using SPSS 17.0.
Analyses examined the sensitivity and specificity of proposed DSM-5ASD criteria and DSM-IV PDD criteria in the three samples, individually and combined, and in specific sub-groups of children (i.e., children with DSM-IV Asperger or PDD-NOS diagnoses, females, young children). For each clinical diagnosis of PDD, McNemar’s tests were used to compare the proportions of non-PDD children who, per their clinical best-estimate diagnosis, were correctly classified by DSM-5 compared to DSM-IV. Domain-specific analyses were also conducted to explore whether children who did not meet DSM-5 criteria for ASD might meet the proposed criteria for Social Communication Disorder (SCD).
Results
Demographic data and mean IQs for the study samples are displayed in Table 1. Participants ranged in age from 2 to 17:11. Participants represented a wide range of nonverbal and verbal ability; approximately 30% of the participants across all three samples had nonverbal IQs under 70. The majority of the participants were Caucasian males, but the samples had significantly different male to female ratios (University of Michigan PDD male to female ratio= 3.8:1; Collaborative Programs PDD ratio=5.3:1 and Simons Simplex Collection ratio= 6.7:1). The PDD sample from University of Michigan also had a lower nonverbal IQ (mean = 77.0, SD= 28.8) compared to the Collaborative Programs PDD sample (mean = 83.3, SD= 26.6) and the Simons Simplex Collection sample (mean = 84.8, SD= 26.1).
Table 1.
Sample Demographics
| UMACC All N=1465 |
ASD | UMACC Non-ASD N=527 |
CPEA All N=858 |
ASD | CPEA Non-ASD N=163 |
SSC All N=2130 |
ASD | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age | 6.6a,c | 3.5 | 7.3 | 3.7 | 6.4c,f | 3.6 | 5.8 | 3.4 | 9.4a,f | 3.5 |
| Verbal IQ | 66.2a | 33.9 | 84.2b | 26.7 | 68.4f | 29 | 74.4b | 26.2 | 77.7a,f | 31.4 |
| Nonverbal IQ | 77a,b | 28.8 | 86d | 26.2 | 83.3b | 26.6 | 80.3d | 24.1 | 84.8a | 26.1 |
|
|
||||||||||
| Male: Female Ratio | 3.8:1a | 2.0:1b | 5.3:1f | 2.1:1b | 6.7:1a,f | |||||
|
|
||||||||||
| Gender | N | % | N | % | N | % | N | % | N | % |
| Male | 1162 | 79.3 | 353 | 67.1 | 639 | 84.1 | 110 | 67.5 | 1854 | 87 |
| Female | 303 | 20.7 | 173 | 32.9 | 121 | 15.9 | 53 | 32.5 | 275 | 12.9 |
| Race | ||||||||||
| Caucasian | 621 | 80.4 | 316 | 73.8 | 721 | 86.6 | 144 | 88.3 | 1687 | 79.2 |
| African American | 59 | 7.6 | 57 | 13.3 | 23 | 2.8 | 4 | 2.5 | 76 | 3.6 |
| Asian | 20 | 2.6 | 7 | 1.6 | 15 | 1.8 | 1 | 0.6 | 79 | 3.7 |
| Biracial or Multi-racial | 47 | 6.1 | 29 | 6.8 | 63 | 7.6 | 12 | 7.4 | 169 | 7.9 |
| Other | 20 | 2.6 | 15 | 3.5 | 11 | 1.3 | 2 | 1.2 | 97 | 4.6 |
| Clinical Diagnosis | ||||||||||
| Autistic Disorder | 975 | 66.6 | - | 780 | 90.9 | - | 1466 | 68.8 | ||
| PDD-NOS | 465 | 31.7 | - | 78 | 9.1 | - | 428 | 20.1 | ||
| Asperger Syndrome | 25 | 1.7 | - | - | - | 236 | 11.1 | |||
Comparisons between All ASD samples (UMACC, CPEA, & SSC) and comparisons between Non-ASD samples (UMACC & CPEA). Comparisons were not completed for race and clinical diagnosis.
UMACC and SSC samples are significantly different from one another, p <.001
UMACC and CPEA samples are significantly different from one another, p < .001
UMACC and CPEA samples are significantly different from one another, p < .01
UMACC and CPEA samples are significantly different from one another, p < .05
SSC and CPEA samples are significantly different from one another, p < .001
SSC and CPEA samples are significantly different from one another, p < .05
DSM-IV PDD and DSM-5 ASD “Classifications” Compared to Best-Estimate Diagnoses
As outlined in Table 2, using parent-reported symptoms only, in children with a clinical best-estimate diagnosis of any PDD, sensitivity of proposed DSM-5 criteria ranged from .83 to .93. In every sample, sensitivity was highest for children with DSM-IV diagnoses of Autistic Disorder. Not surprisingly, given that it was the only sample in which participants’ initial eligibility was partially dependent on scores from the Autism Diagnostic Observation Schedule and Autism Diagnostic Interview-Revised, sensitivity was highest in the Simons Simplex Collection. Overall, sensitivity of DSM-5 criteria was similar to DSM-IV criteria (see Table 2).
Table 2.
Sensitivity and Specificity of DSM-5 and DSM-IV
| Clinical Diagnoses | CPEA | UMACC | SSC | Combined Samples | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| Autism n = 778 |
PDD or Asperger n = 78 |
All PDD Combined n = 856 |
Non-PDD n = 163 |
Autism n =974 |
PDD or Asperger n= 490 |
All PDD Combined n= 1463 |
Non-PDD n= 527 |
Autism n= 1466 |
PDD n= 428 |
Asperger n= 236 |
All PDD Combined n= 2130 |
All PDD Combined n = 4453 |
Non-PDD n = 690 |
|
|
| ||||||||||||||
| Sensitivity | Specificity | Sensitivity | Specificity | Sensitivity | Sensitivity | Specificity | ||||||||
|
| ||||||||||||||
| EXTRACTED FROM ADI-R ONLY | ||||||||||||||
| DSM-5 ASD Criteria – One Symptom1 | .94 | .77 | .93 | .63 | .95 | .78 | .89 | .49 | .93 | .83 | .94 | .91 | .91 | .53 |
| DSM-IV Autistic Disorder Criteria2 | .89 | .69 | .87 | .72 | .95 | .78 | .89 | .48 | .95 | .88 | .93 | .93 | .91 | .53 |
| DSM-IV Asperger Criteria3 | .97 | .86 | .96 | .51 | .99 | .90 | .96 | .30 | .99 | .92 | .99 | .97 | .97 | .34 |
| DSM-IV PDD-NOS Criteria4 | .99 | .92 | .98 | 36 | .99 | .93 | .97 | .20 | .99 | .96 | .98 | .98 | .98 | .24 |
| EXTRACTED FROM ADI-R AND ADOS | ||||||||||||||
| DSM-5 ASD Criteria – One Symptom5 | .99 | .94 | .99 | .50 | .99 | .93 | .97 | .28 | .99 | .99 | 1.0 | .99 | .99 | .33 |
| DSM-5 ASD Criteria – Two Symptoms6 | .85 | .51 | .82 | .78 | .86 | .70 | .80 | .62 | .97 | .87 | .95 | .95 | .88 | .66 |
| DSM-5 ASD Criteria – Two Symptoms, One Less Sub-Domain7 | .99 | .92 | .98 | .58 | .99 | .94 | .97 | .37 | .99 | .99 | .99 | .99 | .99 | .42 |
| DSM-IV Autistic Disorder Criteria8 | .98 | .84 | .96 | .46 | .99 | .90 | .96 | .29 | .99 | .98 | .97 | .99 | .97 | .33 |
| DSM-IV Asperger Criteria9 | .99 | .94 | .99 | .34 | .99 | .98 | .99 | .15 | .99 | .99 | 1.0 | .99 | .99 | .19 |
| DSM-IV PDD-NOS Criteria10 | 1.0 | .99 | .99 | .18 | 1.0 | .99 | .99 | .07 | 1.0 | 1.0 | 1.0 | 1.0 | .99 | .10 |
| EXTRACTED FROM ADI-R AND ADOS: SYMPTOMS REQUIRED FROM BOTH | ||||||||||||||
| DSM-5 ASD Criteria11 | .93 | .69 | .91 | .69 | .96 | .72 | .88 | .62 | .93 | .80 | .92 | .90 | .90 | .63 |
| DSM-IV Autistic Disorder Criteria12 | .93 | .62 | .90 | .94 | .96 | .76 | .89 | .57 | .96 | .89 | .92 | .94 | .92 | .61 |
| DSM-IV Asperger Criteria13 | .97 | .77 | .95 | .60 | .99 | .85 | .94 | .47 | .99 | .94 | .97 | .98 | .96 | .50 |
| DSM-IV PDD-NOS Criteria14 | .97 | .82 | .96 | .59 | .99 | .86 | .95 | .44 | .99 | .95 | .97 | .98 | .96 | .47 |
At least 1 ADI-R symptom from each A sub-domain and at least 1 ADI-R symptom from 2 or more B sub-domains
At least 1 ADI-R symptom from 2 or more A sub-domains, at least 1 ADI-R symptom from 1 or more B sub-domains, at least 1 ADI-R symptom from 1 or more C sub-domains, and having at least 1 ADI-R symptom in 6 or more sub-domains across A, B, and C
At least 1 ADI-R symptom from 2 or more A sub-domains and at least 1 ADI-R symptom from 1 or more C sub-domains
At least 1 ADI-R symptom from 2 or more A sub-domains and at least 1 ADI- R symptom from 1 or more B OR C sub-domains
At least 1 ADI-R or ADOS symptom from each A sub-domain and at least 1 ADI-R or ADOS symptom from 2 or more B sub-domains
At least 2 ADI-R or ADOS symptoms from each A sub-domain and at least 2 ADI-R or ADOS symptoms from 2 or more B sub-domains
At least 2 ADI-R or ADOS symptoms from each A sub-domain and at least 2 ADI-R or ADOS symptoms from 1 or more B sub-domains OR At least 2 ADI-R or ADOS symptoms from 2 or more A sub-domains and at least 2 ADI-R or ADOS symptoms from 2 or more B sub-domains (see Frazier et al., 2011)
At least 1 ADI-R or ADOS symptom from 2 or more A sub-domains, at least 1 ADI-R or ADOS symptom from 1 or more B sub-domains, at least 1 ADI-R or ADOS symptom from 1 or more C sub-domains, and having at least 1 ADI-R symptom in 6 or more sub-domains across A, B, and C
At least 1 ADI-R or ADOS symptom from 2 or more A sub-domains and at least 1 ADI-R or ADOS symptom from 1 or more C sub-domains
At least 1 ADI-R or ADOS symptom from 2 or more A sub-domains and at least 1 ADI- R or ADOS symptom from 1 or more B OR C sub-domains
At least 1 symptom (from ADI-R AND ADOS) from A1 and A2, at least 1 symptom (from either ADI-R OR ADOS) from A3, and at least 1 symptom (from either ADI-R OR ADOS) from 2 or more B sub-domains
At least 1 symptom (from ADI-R AND ADOS) from 2 or more A sub-domains, at least 1 symptom (from ADI-R AND ADOS) from 1 or more B sub-domains, at least 1 symptom (from either ADI-R OR ADOS) from 1 or more C sub-domains, and having at least 1 ADI-R or ADOS symptom in 6 or more sub-domains across A, B, and C
At least 1 symptom (from ADI-R AND ADOS) from 2 or more A sub-domains and at least 1 symptom (from either ADI-R OR ADOS) from 1 or more C sub-domains
At least 1 symptom (from ADI-R AND ADOS) from 2 or more A sub-domains and at least 1 symptom (from ADI-R AND ADOS) from 1 or more B sub-domains; OR at least 1 symptom (from ADI-R AND ADOS) from 2 or more A sub-domains and at least 1 symptom (from either ADI-R OR ADOS) from 1 or more C sub-domains
When examining specific DSM-IV PDD diagnostic groups separately, DSM-5 sensitivity in those with clinical diagnoses of Asperger Disorder or PDD-NOS ranged from .77 to .94 (see Table 2), while DSM-5 sensitivity in those with Autistic Disorder ranged from .93 to .94. Sensitivity of DSM-5 criteria was also examined within ASD phenotypic subgroups (based on sex, IQ and age). As shown in Table 3, sensitivity for females ranged from .88 to .93. For those in the “high-functioning” range of cognitive ability (nonverbal IQ> 70), DSM-5 sensitivity was between .86 and .91, while among those with nonverbal IQ≤ 70, sensitivity ranged between .93 and .97. In children under 4, sensitivity ranged between .90 and .98.
Table 3.
Sensitivity and Specificity of Proposed DSM-5 Criteria1 in Phenotypic Subgroups
| CPEA | CPEA | UMACC | UMACC | SSC | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DSM-IV Clinical Diagnosis of PDD | DSM-IV Clinical Diagnosis of Non-PDD | DSM-IV Clinical Diagnosis of PDD | DSM-IV Clinical Diagnosis of Non-PDD | DSM-IV Clinical Diagnosis of PDD | ||||||
| N | Sensitivity | N | Specificity | N | Sensitivity | N | Specificity | N | Sensitivity | |
|
| ||||||||||
| Males | 633 | .92 | 110 | .56 | 1158 | .89 | 350 | .48 | 1850 | .91 |
| Females | 121 | .91 | 53 | .76 | 303 | .88 | 173 | .51 | 276 | .93 |
| Over 10 years with fluent language | 147 | .90 | 26 | .77 | 194 | .81 | 99 | .57 | 622 | .89 |
| Under 4 years | 306 | .98 | 74 | .53 | 396 | .90 | 117 | .40 | - | - |
| NVIQ >70 | 571 | .91 | 103 | .68 | 843 | .86 | 374 | .54 | 1584 | .90 |
| NVIQ ≤ 70 | 280 | .97 | 60 | .53 | 574 | .93 | 140 | .37 | 542 | .95 |
At least 1 ADI-R symptom from each A sub-domain and at least 1 ADI-R symptom from 2 or more B sub-domains
Table 2 includes specificity values for the Collaborative Programs and University of Michigan samples (the Simons Simplex Collection was restricted to children with PDD). In the Collaborative Programs sample, using parent-reported items, DSM-IV specificity was as high as .72 for Autistic Disorder criteria and as low as .36 for Asperger Syndrome or PDD-NOS criteria. In the University of Michigan sample, DSM-IV specificity was .20 for PDD-NOS, .30 for Asperger Disorder and .48 for Autistic Disorder. In contrast, when DSM-5 ASD criteria were applied, specificity was .50 in the University of Michigan sample and .63 in the Collaborative Programs sample.
When evidence of impairments in social reciprocity and nonverbal behavior was required from both parent report and observation, specificity of the DSM-5 criteria improved (Table 2). This improvement was most clinically meaningful in the University of Michigan sample, of which approximately 36% had non-PDD diagnoses. In this group, DSM-5 specificity increased to .62. Specificity in the Collaborative Programs sample increased to .67 with the requirement that symptoms be evident on both instruments. On the other hand, this requirement led to a decrease in sensitivity across all groups but most strikingly for those with clinical diagnoses of PDD-NOS or Asperger Syndrome (see Table 2). As in Frazier et al.(10), requiring one less sub-domain from either domain, using either parent or clinical report, provided the best balance of sensitivity and specificity, though specificity remained low.
McNemar’s χ2 tests were used to investigate whether DSM-5’s proportion of correct classification of the non-PDD cases was significantly different than DSM-IV’s. Using items from the Autism Diagnostic Interview-Revised, the proportion of individuals with a non-PDD diagnosis that were correctly classified by DSM-5 but misclassified by DSM-IV as having PDD-NOS was significantly higher (p< .000) than the proportion that were misclassified by DSM-5 and accurately classified by DSM-IV (34.9% versus 5.9%). Misclassification by DSM-IV of a non-PDD as Asperger Disorder was also significantly higher (p< .000) than misclassification by DSM-5 (29% to 11%).
DSM-5 Domains
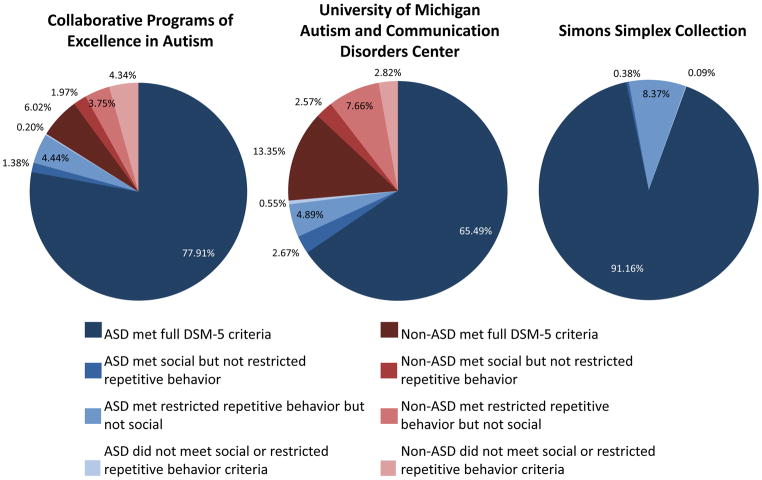
DSM-5 domains were examined individually to assess how many children might meet diagnosis for Social Communication Disorder and to better understand why some were “misclassified” when compared to their clinical diagnosis (see Figure 1). In the Simons Simplex Collection sample (N=2130), 8 subjects who had clinical diagnoses of PDD failed to meet DSM-5 criteria because they did not exhibit enough symptoms in the restricted and repetitive behavior domain, and a total of 178 did not meet criteria because they did not exhibit enough symptoms in the social communication domain. Similarly, in the Collaborative Programs sample; 14 subjects did not meet criteria in the DSM-5 restricted and repetitive behavior domain, while 45 did not meet DSM-5 criteria in the social communication domain. In the University of Michigan sample (N=1992), 53 children did not meet restricted and repetitive behavior criteria on DSM-5 domains, while 97 failed to meet DSM-5 criteria because they did not meet in the social communication domain. In total, 75 of 5,143 subjects met criteria in the social communication domain only.
Figure 1.
Discussion
This study explored the proposed DSM-5 criteria for ASD in three samples of children with DSM-IV PDD or non-PDD diagnoses. In these samples, the majority of children with clinical diagnoses of PDD met DSM-5 ASD criteria based on item scores from the Autism Diagnostic Interview-Revised and Autism Diagnostic Observation Schedule. Notably, application of DSM-5 criteria, demonstrated adequate sensitivity across all samples, as well as in phenotypic subgroups, including young children, females, and those denoted as cognitively “higher-functioning.” These results, together with those of Frazier et al.(10), provide support that the new criteria will likely be able to correctly classify a phenotypically wide range of children with ASD. What is more, the results of the current study provide evidence that the specificity of DSM-5 criteria is improved when compared to DSM-IV criteria for Asperger syndrome and PDD-NOS. Overall, the accuracy of non-spectrum classification made by DSM-5 was better compared to DSM-IV. Thus, though there is much room for improvement with respect to specificity, the proposed criteria appear to meet the stated goal of the DSM-5 committee to create criteria that better distinguishes ASD from non-spectrum disorders such as language disorders, intellectual disability, Attention Deficit Hyperactivity Disorder, and anxiety disorders. Our results further indicate that requiring evidence of clinician-observed social communication deficits, in addition to parent-reported deficits, can increase the specificity of the new criteria. However, the inevitable tradeoff between specificity and sensitivity occurred when evidence was required from both parent report and direct observation.
Given concerns that the restricted and repetitive behavior requirement might lead to reduced identification of children previously diagnosed with ASD under DSM-IV, and possible misclassification under Social Communication Disorder, we examined why some children with PDD failed to meet DSM-5. Interestingly, in all three samples, most children who failed to meet criteria did so because they did not demonstrate the required impairments in social and communication functioning, and not because they failed to meet the restricted and repetitive behavior criteria. In fact, few children failed to meet the restricted and repetitive behavior requirement in DSM-5. These results suggest that few children with ASD are likely to be misclassified with Social Communication Disorder and lend further support to the addition of the restricted and repetitive behavior criterion.
Finally, the process of matching individual items to criteria revealed potential challenges in the interpretation of DSM-5 criterion A3. In addition to the reduced number of items (especially on the Autism Diagnostic Observation Schedule) that could be applied to A3, it was also sometimes difficult to determine whether an item should be placed in A3 (“difficulties adjusting behavior to suit different social contexts”) or A1 (“abnormal social approach”). Though difficulty assigning specific items may have partially resulted from the fact that the study measures were based on DSM-IV criteria and therefore not designed to map directly onto DSM-5 criteria, it will be critical to ensure that the final wording of the DSM-5 criteria lends itself to being clearly and reliably interpreted by ASD diagnosticians.
Limitations
Replication of our findings in other samples (including adults), using both retrospective data analysis and prospective field-trial methodology, is needed. The composition of two of our samples may not be fully representative of children typically referred for assessment of ASD. Our study samples may represent extremes in terms of ASD phenotypes: on the one hand, clinical cases at the University of Michigan with complex presentations, and on the other, “clearer” cases of ASD in the Simons Simplex Collection.
The results obtained here may not reflect the new criteria’s true sensitivity and specificity. Using archival data and symptom counts is not comparable to clinical diagnosis. As the study instruments are largely based on DSM-IV criteria, it is likely that behaviors that might fit into DSM-5 criteria are not currently captured by these methods. In spite of the ADI-R’s breadth, analyses of existing data cannot begin to approximate a field trial. Conducting evaluations in “real time” and making determinations about whether a child meets DSM-5 criteria on the basis of all information gathered during that evaluation is the only way to assess the true sensitivity and specificity of DSM-5 criteria. Nevertheless, though in practice it would be inappropriate to make diagnoses solely on the basis of symptom counts, our use of these methods allows comparisons with other researchers’ analyses of DSM-5 criteria.
Conclusions
This study represents the most comprehensive assessment to date of the newly proposed DSM-5 ASD criteria. Based on symptom extraction from previously collected data, our findings indicate that the majority of children with DSM-IV PDD diagnoses would continue to be eligible for an ASD diagnosis under DSM-5. Additionally, these results further suggest that the revisions to the criteria, when applied to records of children with non-PDD diagnoses, yield fewer misclassifications. Our findings also contribute to literature that supports the use of both parent report and clinical observation for optimal classification accuracy.
Supplementary Material
Acknowledgments
This work was supported by R01MH081873-01A1 and RC1MH089721 to Catherine Lord, R01HD065277 to Somer Bishop, and a graduate fellowship from the Simons Foundation and a Dennis Weatherstone Predoctoral Fellowship to Vanessa Hus.
The authors are indebted to Drs. Mandy Steiman, Ph.D., Stephen Kanne, Ph.D., and Edwin Cook, M.D. The authors would also like to thank Nicole Saghy and Carrie Thomas for their assistance in the preparation of this manuscript.
We are grateful to all of the families who have consented to participation in the University of Michigan Databank and to those who participated in the Collaborative Programs for Excellence in Autism and Simons Simplex Collection. We must also extend our gratitude to the SFARI Simplex Collection sites, as well as the principal investigators (A. Beaudet, R. Bernier, J. Constantino, E. Cook, E. Fombonne, D. Geschwind, D. Grice, A. Klin, D. Ledbetter, C. Lord, C. Martin, D. Martin, R. Maxim, J. Miles, O. Ousley, B. Peterson, J. Piggot, C. Saulnier, M. State, W. Stone, J. Sutcliffe, C. Walsh, E. Wijsman). We appreciate obtaining access to phenotypic data on SFARI Base. Approved researchers can obtain a subset of the data described in this study at: https://base.sfari.org.
Footnotes
Conflict of Interest Statement: Dr. Lord receives royalties for the Autism Diagnostic Interview-Revised and the Autism Diagnostic Observation Schedule; Dr. Lord is a member of the DSM-5 Neurodevelopmental Disorders Committee. Other authors on this manuscript do not have any conflicts of interest (MH, SLB, AD, and VH).
References
- 1.Lord C, Petkova E, Hus VB, Gan W, Lu F, Martin DM, Ousley O, Guy L, Bernier R, Gerdts J, Algermissen M, Whitaker A, Sutcliffe JS, Warren Z, Klin A, Fombonne E, Steiman M, Miles J, Kanne S, Goin-Kochel RP, Peters SU, Cook EH, Guter S, Tjernagel J, Green-Snyder LA, Bishop S, Esler A, Gotham K, Luyster R, Miller F, Olsen J, Richler J, Risi S. A Multisite Study of the Clinical Diagnosis of Different Autism Spectrum Disorders. Arch Gen Psychiatry. 2011 doi: 10.1001/archgenpsychiatry.2011.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Billstedt E, Gillberg IC, Gillberg C. Autism in adults: Symptom patterns and early childhood predictors. Use of the DISCO in a community sample followed from childhood. Journal of Child Psychology And Psychiatry. 2007;48(11):1102–10. doi: 10.1111/j.1469-7610.2007.01774.x. [DOI] [PubMed] [Google Scholar]
- 3.Snow A, Lecavalier L. Sensitivity and specificity of the Modified Checklist for Autism in Toddlers and the Social Communication Questionnaire in preschoolers suspected of having pervasive developmental disorders. Autism. 2008;12(6):627. doi: 10.1177/1362361308097116. [DOI] [PubMed] [Google Scholar]
- 4.Gotham K, Risi S, Dawson G, Tager-Flusberg H, Joseph R, Carter A, Hepburn S, McMahon W, Rodier P, Hyman SL, Sigman M, Rogers S, Landa R, Spence MA, Osann K, Flodman P, Vokmar F, Hollander E, Buxbaum J, Pickles A, Lord C. A replication of the Autism Diagnostic Observation Schedule (ADOS) revised algorithms. Journal of Amer Academy of Child & Adolescent Psychiatry. 2008;47(6):642. doi: 10.1097/CHI.0b013e31816bffb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frazier TW, Youngstrom EA, Kubu CS, Sinclair L, Rezai A. Exploratory and confirmatory factor analysis of the Autism Diagnostic Interview-Revised. Journal of Autism and Developmental Disorders. 2008;38(3):474–80. doi: 10.1007/s10803-007-0415-z. [DOI] [PubMed] [Google Scholar]
- 6.Mandy WP, Charman T, Skuse DH. Testing the construct validity of proposed criteria for DSM-5 autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2012;51(1):41–50. doi: 10.1016/j.jaac.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 7.Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview-Revised (ADI-R) Los Angeles, California: Western Psychological Services; 2003. [Google Scholar]
- 8.Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule (ADOS) Los Angeles, California: Western Psychological Services; 1999. [Google Scholar]
- 9.Mattila M, Kielinen M, Linna S, Jussila K, Ebeling H, Bloigu R, Joseph RM, Moilanen I. Autism spectrum disorders according to DSM-IV-TR and comparison with DSM-5 draft criteria: An epidemiological study. Journal of the American Academy of Child & Adolescent Psychiatry. 2011;50(6):583–92. doi: 10.1016/j.jaac.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Frazier TW, Youngstrom EA, Speer L, Embacher R, Law P, Constantino J, Findling RL, Hardan AY, Eng C. Validation of proposed DSM-5 criteria for autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2012;51(1):28–40 e3. doi: 10.1016/j.jaac.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rutter M, Bailey A, Lord C. Social Communication Questionnaire (SCQ) Los Angeles, California: Western Psychological Serives; 2003. [Google Scholar]
- 12.Constantino JN, Gruber CP. Social Responsiveness Scale (SRS) Los Angeles, CA: Western Psychological Services; 2005. [Google Scholar]
- 13.Wing L, Gould J, Gillberg C. Autism spectrum disorders in the DSM-V: Better or worse than the DSM-IV? Research in developmental disabilities. 2011;32(2):768–73. doi: 10.1016/j.ridd.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Fischbach GD, Lord C. The Simons simplex collection: A resource for identification of autism genetic risk factors. Neuron. 2010;68(2):192–5. doi: 10.1016/j.neuron.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Gotham K, Risi S, Pickles A, Lord C. The Autism Diagnostic Observation Schedule: Revised algorithms for improved diagnostic validity. Journal of Autism and Developmental Disorders. 2007;37(4):613–27. doi: 10.1007/s10803-006-0280-1. [DOI] [PubMed] [Google Scholar]
- 16.Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The autism diagnostic observation schedule—generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–23. [PubMed] [Google Scholar]
- 17.Risi S, Lord C, Gotham K, Corsello C, Chrysler C, Szatmari P, Cook EH, Leventhal BL, Pickles A. Combining information from multiple sources in the diagnosis of autism spectrum disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45(9):1094–103. doi: 10.1097/01.chi.0000227880.42780.0e. [DOI] [PubMed] [Google Scholar]
- 18.Lord C, Rutter M, Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 19.Gotham K, Pickles A, Lord C. Standardizing ADOS Scores for a Measure of Severity in Autism Spectrum Disorders. Journal of Autism and Developmental Disorders. 2009;39(5):693–705. doi: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hus V, Pickles A, Cook EH, Risi S, Lord C. Using the Autism Diagnostic Interview—Revised to Increase Phenotypic Homogeneity in Genetic Studies of Autism. Biological Psychiatry. 2007;61(4):438–48. doi: 10.1016/j.biopsych.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 21.Bishop DVM, Maybery M, Wong D, Maley A, Hallmayer J. Characteristics of the broader phenotype in autism: A study of siblings using the children’s communication checklist-2. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2006;141B(2):117–22. doi: 10.1002/ajmg.b.30267. [DOI] [PubMed] [Google Scholar]
- 22.Joseph RM, Tager-Flusberg H, Lord C. Cognitive profiles and social-communicative functioning in children with autism spectrum disorder. Journal Of Child Psychology And Psychiatry, And Allied Disciplines. 2002;43(6):807. doi: 10.1111/1469-7610.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.