The World Health Organization (WHO) recently approved the first quantitative point-of-care (POC) HIV viral load assay for use in resource-limited settings.1 The Xpert® HIV-1 VL (Cepheid, Sunnyvale, US) requires 1 mL of plasma to measure a viral load (VL) result in 90 minutes on the GeneXpert® platform. The assay has been validated in several clinical settings,2,3 and detects virological failure (>1,000 copies/mL) with 94% sensitivity and 99% specificity.1 Current costs appear similar to existing laboratory VL technologies, with four module GeneXpert® platforms available at USD 17,000 and cartridges at USD 17 (excluding tax and shipping).2 WHO prequalification will allow the Xpert® HIV-1 VL to be procured through WHO tender processes, facilitating expanded access at lower costs, particularly in low- and middle-income countries (LMICs). This could be an important milestone for HIV programs, as POC VL testing has potential to fill gaps in VL coverage, and to change the way HIV care is provided, through more efficient, client-centred services.
As access to antiretroviral therapy (ART) increases, global demand for VL monitoring is estimated to grow from 7 million tests in 2013 to 15-30 million in 2018.4 Scale up is particularly challenging in Southern Africa, due to the lack of trained laboratory personnel, high costs of centralised laboratory infrastructure, and challenges with specimen transport and return of results.5 POC VL assays such as the Xpert® HIV-1 VL may allow decentralised VL testing that circumvents some of these problems. Automated systems allow operation by non-laboratory personnel, while near patient testing can eliminate the need for specimen transport. However, POC testing capacity will need to match patient loads, with clinic flows adapted to minimise turn-around times. Technological advances that further accelerate sample processing will be important, as will fingerprick and dried blood spot testing, which are currently not available on the Xpert® HIV-1 VL. Several other quantitative POC VL assays have been validated in decentralized clinics in Southern Africa, including the SAMBA I/II semi-Q (Diagnostics for the Real World Ltd., Cambridge, UK),6 Alere Q NAT (Alere, Waltham, MA, US),7 and Liat™ HIV Quant (Roche Diagnostics, Basel, Switzerland).8 While the widespread availability of the GeneXpert® platform for TB diagnostics may favour the introduction of the Xpert® HIV-1 VL, most machines are situated within centralized laboratories, reflecting the remaining challenges of implementing POC molecular diagnostics within existing care models.
The advent of POC VL technologies coincides with the development and rollout of new models of efficient, client-centred HIV care in LMICS.9 These ‘differentiated care services’ aim to increase health system capacity and quality, and have typically focused on providing ART to stable clients, through community adherence groups, decentralised ART pickup points, task shifting between healthcare professionals and lay workers, and reducing the frequency of client visits9. Integrating POC VL testing into these services may further improve and expand access to differentiated HIV care in several ways.
Firstly, rapid availability of POC results could allow quicker triage of clients into differentiated care pathways, while reducing the number of client visits. Routine laboratory-based VL testing requires two clinical contacts; one for blood draw and one to review results. In HIV services with frequent clinical visits, blood can be drawn at one visit and results reviewed at the next scheduled visit in 1-2 months’ time. In differentiated care services, where stable clients may have clinical visits only every 6 or 12 months,9 the interval between blood draw and review of results could be unacceptably long, unless extra visits are scheduled for VL assessment, or attempts are made to recall clients with abnormal results. Instead, POC VL testing could allow stable clients to have a VL drawn and reviewed in one clinical visit per year, with decentralised ART delivery in between (Figure 1). Resources could then be redirected to unstable clients, in whom POC testing may allow rapid identification of virological failure, earlier initiation of intensive adherence support, and ART regimen changes, if necessary. We are investigating a similar model of care in the STREAM study; a randomised trial of Xpert® HIV-1 VL testing, with results expected in late 2018.10
Figure 1.
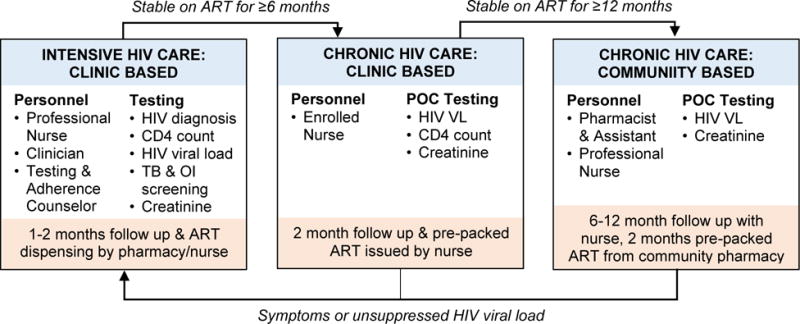
A conceptual model of differentiated HIV care and integrated point-of-care viral load testing, adapted from the STREAM study10.
ART antiretroviral therapy; TB Tuberculosis; OI opportunistic infection; VL viral load; POC point-of-care
Secondly, POC VL testing may be particularly useful in differentiated care for specific hard-to-reach populations. The relative portability of POC systems allows use in community outreach services, such as for people who inject drugs or rural populations.3 For clients who can only attend clinic sporadically, due to migrant labour or in conflict settings,9 the immediacy of POC results may empower clinicians and clients to manage ART appropriately, while minimising the need for frequent clinical contacts. Furthermore, in pregnant or breast-feeding women, faster identification and management of virological failure through POC testing may contribute to preventing mother-to-child transmission.
POC VL assays have potential to significantly expand VL coverage and improve differentiated care. However, successful integration into HIV services must take into account client needs, clinic flows, staff training, quality control, supply chain management, and maintenance of equipment at decentralised sites. Currently, there is no strong evidence to support roll out of POC VL testing. Therefore, while pre-qualification of the Xpert® HIV-1 VL is welcome, high quality implementation research coupled with cost-effectiveness studies should be prioritized, to determine whether POC VL testing can be successfully integrated into differentiated care.
Acknowledgments
The STREAM study is funded by the US National Institute for Health (NIH) (AI124719-01). Cepheid Inc. loaned the GeneXpert® instruments for this study at no cost. NIH and Cepheid had no role in the conception, drafting or submission of this commentary.
Footnotes
Declaration of interests
The authors are investigators on the Simplified TREAtment and Monitoring of HIV (STREAM) study, a randomised controlled trial of POC VL testing (using the Xpert® HIV-1 VL) and task shifting to nurses.
Authors’ contributions
JD conceived and drafted the manuscript. PD and NG critically revised the manuscript. JD, PD and NG all consented to publication.
References
- 1.World Health Organization. WHO prequalification of in vitro diagnostics. Product: Xpert® HIV-1 Viral Load with GeneXpert® Dx, GeneXpert® Infinity- 48, GeneXpert® Infinity-48s and GeneXpert® Infinity-80. 2017 http://www.who.int/diagnostics_laboratory/evaluations/pq-list/hiv-vrl/170720_final_pq_report_pqdx_0192_0193_0194_0195_070-00.pdf?ua=1 (accessed Aug 7, 2017).
- 2.Garrett NJ, Drain PK, Werner L, Samsunder N, Abdool Karim SS. Diagnostic Accuracy of the Point-of-Care Xpert HIV-1 Viral Load Assay in a South African HIV Clinic. J Acquir Immune Defic Syndr. 2016;72:e45–8. doi: 10.1097/QAI.0000000000000978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moyo S, Mohammed T, Wirth KE, et al. Point-of-Care Cepheid Xpert HIV-1 Viral Load Test in Rural African Communities Is Feasible and Reliable. J Clin Microbiol. 2016;54:3050–5. doi: 10.1128/JCM.01594-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. HIV Diagnostic Tests in Low and Middle-Income Countries: Forecasts of Global Demand for 2014–2018. Geneva: 2015. http://www.who.int/hiv/pub/amds/diagnostic-forecast2014-2018/en/ (accessed March 1, 2017). [Google Scholar]
- 5.Lecher S, Ellenberger D, Kim AA, et al. Scale-up of HIV Viral Load Monitoring--Seven Sub-Saharan African Countries. MMWR Morb Mortal Wkly Rep. 2015;64:1287–90. doi: 10.15585/mmwr.mm6446a3. [DOI] [PubMed] [Google Scholar]
- 6.Ritchie AV, Ushiro-Lumb I, Edemaga D, et al. SAMBA HIV semiquantitative test, a new point-of-care viral-load-monitoring assay for resource-limited settings. J Clin Microbiol. 2014;52:3377–83. doi: 10.1128/JCM.00593-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jani IV, Meggi B, Vubil A, et al. Evaluation of the whole-blood Alere Q NAT point-of-care RNA assay for HIV-1 viral load monitoring in a primary health care setting in Mozambique. J Clin Microbiol. 2016;54:2104–8. doi: 10.1128/JCM.00362-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott L, Gous N, Carmona S, Stevens W. Laboratory evaluation of the liat HIV quant (IQuum) whole-blood and plasma HIV-1 viral load assays for point-of-care testing in South Africa. J Clin Microbiol. 2015;53:1616–21. doi: 10.1128/JCM.03325-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimsrud A, Barnabas RV, Ehrenkranz P, Ford N. Evidence for scale up: the differentiated care research agenda. J Int AIDS Soc. 2017;20(S4):22024. doi: 10.7448/IAS.20.5.22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorward J, Garrett N, Quame-Amaglo J, et al. Protocol for a randomised controlled implementation trial of point-of-care viral load testing and task shifting: the Simplifying HIV TREAtment and Monitoring (STREAM) study. BMJ Open. 2017;7:e017507. doi: 10.1136/bmjopen-2017-017507. [DOI] [PMC free article] [PubMed] [Google Scholar]


