Abstract
Introduction
Multiple primary tumors (MPTs) are defined as two or more separate synchronous or metachronous neoplasms occurring in different sites in the same individual. These tumors differ in histology, as well as primary sites from which they arise. Risk factors associated with the occurrence of MPTs include germline alterations, exposure to prior cancer therapies, occupational hazards, and lifestyle and behavioral influences.
Case report
We present a case of a patient who was diagnosed with four metachronous primary tumors. In 2013, she was diagnosed with serous proliferations associated with psammomatous bodies of primary peritoneal origin (pT3NxM0). This was followed by invasive ductal carcinoma of the breast (stage pT2N0Mx, histological grade III/III) in 2014, melanoma (stage pT2bNxMx) in 2016 that further advanced to the lung and brain in 2017, and a low-grade lung carcinoid in 2017. To better understand the biology of this patient's MPTs, we performed next-generation sequencing (NGS) to assess for both somatic and germline alterations. The treatment course for this patient aims to target the tumor with the strongest prognostic value, namely her malignant melanoma, and has contributed favorably to the overall survival of this patient.
Conclusion
We report the clinical and genomic landscape of a patient with MPTs who had no identifiable unique somatic or germline mutations to explain her predilection to cancer. The treatment course and overall prognosis for this patient is important for understanding future cases with unrelated, metachronous MPTs, the occurrence of which cannot always be explained by underlying genetic mechanisms.
Keywords: Multiple primary tumors, Next-generation sequencing, Tumor mutational burden, Immunotherapy
Highlights
-
•
Multiple primaries are defined as more than one synchronous or metachronous cancers.
-
•
Next-generation sequencing can define the genetic landscape of multiple primaries.
-
•
Tumor mutational burden is a potential predictor of response to immunotherapy.
-
•
Management of multiple primaries is complex and requires multispecialty team care.
1. Introduction
Multiple primary tumors (MPTs) are defined as two or more separate synchronous or metachronous neoplasms of different primary disease site and histology. Due to an increase in cancer screening and prevention efforts, along with an improvement in overall life expectancy, there has been a consequent increase in the prevalence of MPTs. The incidence of MPTs is currently 2–17% (Vogt et al., 2017), and risk factors include germline alterations, exposure to prior cancer therapies, occupational hazards, and lifestyle influences (Vogt et al., 2017).
1.1. Case report
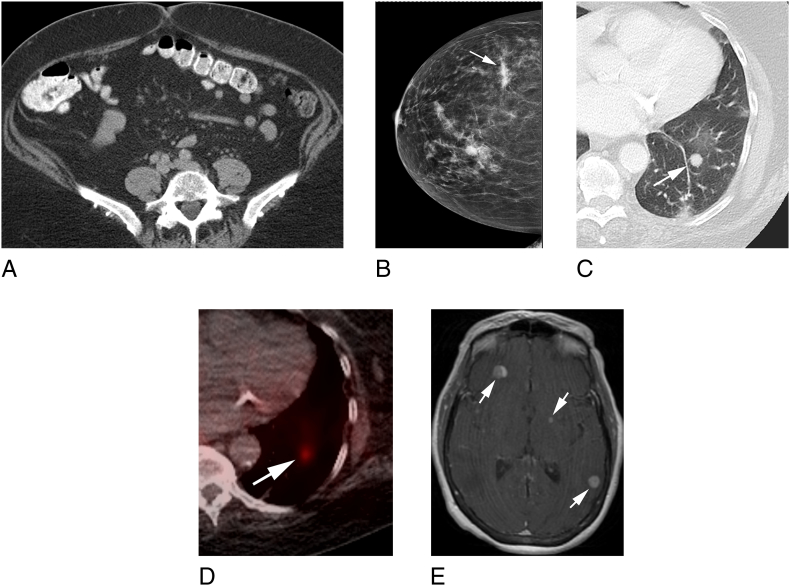
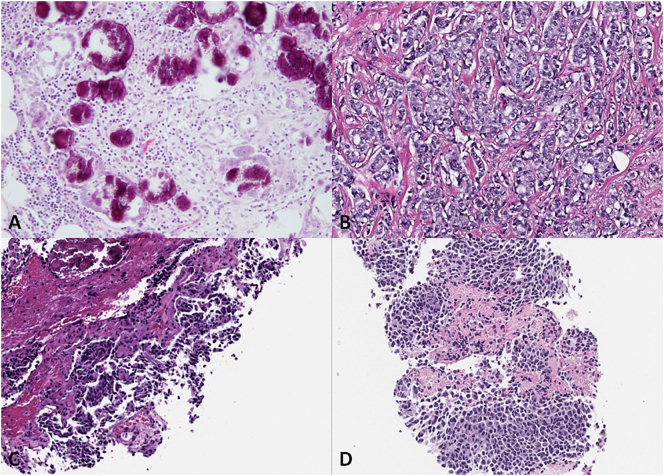
We present a 73-year-old woman with a medical history of Center of Disease Control (CDC) class 3 obesity (body mass index [BMI], 40 kg/m2) and a family history of bladder, colon and lung cancers. In January 2011, she presented with post-menopausal vaginal bleeding that was refractory to multiple dilatation and curettages and hormonal therapy. An endometrial biopsy revealed focal complex hyperplasia without atypia. Pelvic ultrasonography and a computed tomography (CT) scan of her abdomen revealed no significant pathology. In November 2012, she underwent a diagnostic total abdominal hysterectomy and bilateral salpingo-oophorectomy. Pathology revealed low-grade serous proliferation associated with psammomatous calcifications involving the uterine serosa and bilateral fallopian tubes with papillary tubal hyperplasia and associated psammomatous calcifications, consistent with a primary peritoneal origin. In May 2013, the patient reported ongoing abdominal pain, and an interval CT scan of her abdomen and pelvis (Fig. 1A) revealed subtle peritoneal nodularity, suspicious for peritoneal carcinomatosis as well as scattered subcentimeter pulmonary nodules at the lung bases. Omental biopsy revealed low-grade serous proliferation with psammomatous calcifications. In August 2013, she underwent a laproscopic omentectomy with multiple peritoneal biopsies, which confirmed a pT3NxM0 low-grade serous proliferation with psammomatous calcifications involving fibroadipose tissue in the omentum and peritoneal biopsy masses (Fig. 2A). No adjuvant systemic therapy was recommended, and the patient was monitored with interval cross-sectional imaging.
Fig. 1.
A Axial computed tomography (CT) images of the pelvis demonstrating small foci of soft tissue nodularity in the omentum (arrows) consistent with carcinomatosis. B. Right mammogram craniocaudal projection demonstrating an irregular high-density mass in the lateral breast (white arrow) consistent with biopsy-proven invasive ductal carcinoma. An equal density mass with indistinct margins is present 1.6 cm anterior to thr dominant mass, consistent with a biopsy-proven satellite lesion. Circumscribed masses with markers in place were benign. C. Axial CT images of the left chest demonstrating a 1.1-cm solid left lower lobe lung nodule (arrow). D. Axial fused images from a Gallium 68 Dotatate positron emission tomography (PET)/CT demonstrating increased tracer accumulation in the left lower lobe nodule (arrow) consistent with carcinoid tumor. E. Axial T1-weighted gadolinium-enhanced magnetic resonance images of the brain demonstrating bilateral enhancing brain metastases (arrows).
Fig. 2.
A Low-grade serous proliferation associated with psammomatous calcification involving omentum. Irregular to round glands of varying size haphazardly infiltrate dense fibrous stroma and are focally surrounded by clear spaces. The glands contain serous and mesothelial-type cells with moderate cytologic atypia. Although this proliferation would be considered non-invasive by classical criteria, some pathologists might characterize it as indeterminate due to the focal clefting or retraction associated with the epithelial clusters. Hematoxylin-eosin stain; original magnification ×200. B. Invasive ductal carcinoma of the breast, not otherwise specified. The tumor is moderately differentiated, with <10% tubule formation, intermediate nuclear grade and <8 mitotic figures per 10 high-power fields. Hematoxylin-eosin stain; original magnification ×200. C. Carcinoid tumor with spindle cell features involving the left lung. The tumor is comprised of round to spindle shaped cells with neuroendocrine differentiation supported by positive immunohistochemical staining for chromogranin; the Ki-67 proliferation index is <5% (not shown). No mitotic activity or necrosis is identified. Hematoxylin-eosin stain; original magnification ×200. D. Metastatic malignant melanoma involving the left lung. The tumor is composed of diffuse sheets of epithelioid cells with abundant eosinophilic cytoplasm, prominent vesicular nuclei, and macronucleoli. The diagnosis is supported by positive immunohistochemical staining for Melan-A and SOX10 and no immunoreactivity for chromogranin and synaptophysin (not shown). Hematoxylin-eosin stain; original magnification ×200.
In July 2014, a routine mammogram revealed an asymmetrical density in the right breast (Fig. 1B). A core needle biopsy of the right breast confirmed an invasive ductal carcinoma. She subsequently underwent a right lumpectomy and a sentinel lymph node biopsy. Pathology revealed invasive ductal carcinoma, pT2N0Mx, stage IIA (Fig. 2B). The tumor was estrogen receptor/progesterone receptor (ER/PR) positive and human epidermal growth factor receptor 2 negative. She was started on adjuvant anastrozole, an aromatase inhibitor, and remains without evidence of recurrent disease.
In May 2016, the patient presented with a bleeding ulcerated lesion on her back. A biopsy revealed an ulcerated malignant melanoma measuring 1.7 mm in thickness, with numerous mitoses. A wide resection identified an additional 0.4 mm melanoma, pT2bNxMx, stage IIB. The patient did not receive adjuvant systemic therapy.
In April 2017, surveillance imaging revealed a new 0.7-cm nodule in the left upper lobe, as well as increasing bilateral pulmonary nodularity at the lung bases, the largest nodule measuring 1.1 × 1.0 cm, initially 0.5 × 0.4 cm in 2013, concerning for possible metastatic disease (Fig. 1C). A biopsy of the dominant left lower lobe lung nodule revealed a carcinoid tumor with spindle cell features (Fig. 2C). The Ki67 proliferative index was <5%, and no mitoses or necrosis were identified, consistent with a low-grade primary carcinoid of the lung. Staging positron emission tomography revealed multiple bilateral pulmonary nodules without any extrathoracic foci of disease (Fig. 1D). As the patient was asymptomatic from her pulmonary carcinoid, she was placed under observation.
In July 2017, routine surveillance CT of the chest, abdomen, and pelvis revealed that a left lung nodule had increased in size. Given her history of MPTs, a repeat biopsy of the lung nodule was performed, revealing metastatic melanoma (Fig. 2D). In July 2017, she initiated systemic therapy with pembrolizumab. In August 2017, she noted persistent headaches and magnetic resonance imaging of the brain revealed innumerable brain metastases, presumed metastatic melanoma (Fig. 1E). Subsequently, immunotherapy was switched to ipilimumab and nivolumab given improved central nervous system response. As of March 2018, she remains on ipilimumab and nivolumab with ongoing clinical and radiologic response to therapy.
We performed next-generation sequencing (NGS) of all available tumor tissue to assess for somatic (Table 1) and germline alterations (Table 2) in the hopes of identifying an underlying etiology of her MPTs. We performed hybridization capture and deep-coverage NGS to detect somatic mutations in 468 somatic cancer-associated genes as well as 75 germline alterations. All mutations were called against the patient's matched normal sample, and the mean overall coverage of sequencing depth ranged from 472–860× (Cheng et al., 2015). No germline alterations were identified. Somatic alterations are listed in Table 1.
Table 1.
Next-generation sequencing MSK-IMPACT.
| Tissue | TMB | MSI score | Gene | Type | Alteration | Location |
|---|---|---|---|---|---|---|
| Melanoma (726X depth coverage) | 30.33 | 0.93 | NRAS | Missense | Q61L (c.182A>T) | Exon 3 |
| RAC1 | P29S (c.85C>T) | Exon 2 | ||||
| APC | E190K (c.568G>A) | Exon 6 | ||||
| ATRX | E1917K (c.5749G>A) | Exon 24 | ||||
| BARD1 | S541L (c.1622C>T) | Exion 7 | ||||
| BRD4 | V1089I (c.3265G>A) | Exon 15 | ||||
| CBL | I423N (c.1268T>A) | Exon 9 | ||||
| CDKN2A | P94L (c.281C>T) | Exon 2 | ||||
| CIC | S649F (c.1946C>T) | Exon 10 | ||||
| CIC | A650V (c.1949_1950delinsTT) | Exon 10 | ||||
| DAXX | L336S (c.1007T>C) | Exon 3 | ||||
| FLT4 | F1357L (c.4071C>A) | Exon 30 | ||||
| GLI1 | P879H (c.2636C>A) | Exon 12 | ||||
| GRIN2A | P1132L (c.3395C>T) | Exon 13 | ||||
| HLA-B | H221Y (c.661C>T) | Exon 4 | ||||
| IGF1 | G164R (c.490G>A) | Exon 4 | ||||
| KMT2B | P2258S (c.6772C>T) | Exon 28 | ||||
| MST1R | G591D (c.1772G>A) | Exon 5 | ||||
| PARK2 | P343S (c. 1027C>T) | Exon 9 | ||||
| PBRM1 | L254P (c.761T>C) | Exon 8 | ||||
| PDGFRA | A4978V (c. 1493C>T) | Exon 10 | ||||
| RBM10 | R111C (c.331C>T) | Exon 3 | ||||
| ROS1 | E1993K (c.5977G>A) | Exon 37 | ||||
| SYK | M392I (c.1176G>A) | Exon 9 | ||||
| TEK | L415F (c.1243C>T) | Exon 9 | ||||
| TP63 | R37Q (c.110G>A) | Exon 2 | ||||
| TEK | Non sense | W1004 (c.3011G>A) | Exon 19 | |||
| PBRM1 | Nonsense | R710 (c.2128C>T) | Exon 17 | |||
| TERT | Non-Coding | g.1295242_1295243delinsTT | Promoter | |||
| ARID5B | Frameshift Deletion | I497 (c.1481dupA) | Exon 10 | |||
| CDKN2A | Nonsense Mutation | R80 (c.238C>T) | Exon 2 | |||
| NCOR1 | Nonsense Mutation | Y266 (c.798T>A) | Exon 8 | |||
| SPOP | Nonstop Mutation | *375Qext*25 (c.1123T>C) | Exon 10 | |||
| ATRX | Splicing Mutation | c.6218-3C>T | Exon 8 | |||
| PPP2R1A | Splicing Mutation | X218splice (c.6521_652delinsAA) | Exon 6 | |||
| Carcinoid lung (860X depth coverage) | 0 | 0.25 | ARID1B | Intragenic | Deletion | Exons 5-6 |
| Breast (472X depth coverage) | 1.64 | 0.08 | PIK3CA | Missense | H1047R (c.3140A>G) | Exon 21 |
| KIT | Splicing Mutation | X829_Splice (c.2485-2A>T) | Exon 18 | |||
| MAPK3, NTHL1, TRAF7 | Whole gene | Amplification | 16p11.2 |
MSK-IMPACT, Memorial Sloan Kettering Cancer Center-Integrated Mutation Profiling of Actionable Cancer Targets; TMB, tumor mutational burden; MSI, microsatellite instability.
Table 2.
Investigational panel negative for germline mutations; 639× depth coverage. Tier 1 is composed of clinically actionable alterations. Tier 2 is composed of other genomic alterations in cancer-associated genes. Tier 3, not depicted, is composed of variants of unknown significance.
| Tier 1 | |||||
|---|---|---|---|---|---|
| APC | MLH1 | PMS2 | SDHC | TSC1 | |
| BAP1 | MSH2 | PTEN | SDHD | TSC2 | |
| BRCA1 | MSH6 | RB1 | SMAD3 | VHL | |
| CDH1 | MUTYH | RET | STK11 | WT1 | |
| FH | NF1 | SDHAF2 | TGFBR1 | ||
| FLCN | NF2 | SDHA | TGFBR2 | ||
| MEN1 | PALB2 | SDHB | TP53 | ||
| Tier 2 | |||||
|---|---|---|---|---|---|
| ALK | CHEK2 | JAK2 | NRAS | RAD51B | SUFU |
| ATM | DICER1 | KIT | PAX5 | RAD51C | TERT |
| BARD1 | EGFR | KRAS | PDGFRA | RAD51D | TMEM127 |
| BLM | EPCAM | MAX | PHOX2B | RECQL4 | |
| BMPR1A | FAM175A | MET | POLE | RUNX1 | |
| BRIP1 | GATA2 | MITF | PTCH1 | SMAD4 | |
| CDK4 | GREM1 | MRE11A | RAD50 | SMARCA4 | |
| CDKN2A | HRAS | NBN | RAD51 | SMARCB1 | |
We next assessed each individual malignancy for microsatellite instability and tumor mutational burden (TMB) (Table 1). Microsatellite instability assessment was performed via MSIsensor methodology (Niu et al., 2014). Evidence of microsatellite instability at 10% or greater of analyzable loci (MSIsensor score) is consistent with microsatellite instability and mismatch repair deficiency. There was no evidence of microsatellite instability in the tissue specimens sequenced. TMB, defined as the number of non-synonymous coding mutations per megabase of genome collected, was low for the breast and lung carcinoid specimen and highest in the melanoma specimen.
2. Discussion and conclusion
A diagnosis of four primary tumors in one patient is possibly suggestive of a germline or a de novo somatic alteration that may have predisposed this patient to the development of metachronous MPTs. Despite a significant familial history of cancer and extensive genetic sequencing, we did not observe any germline or unique somatic mutations linking the metachronous nature of her cancers (Table 1, Table 2). This case highlights the complexity and clinical management of patients with unrelated MPTs.
Genetic alterations, smoking, and alcohol are important factors associated with MPTs. While our patient denies a history of alcohol abuse or reported exposure to environmental toxins, our patient's CDC class 3 obesity is significant. Obesity has been associated with approximately 20% of all cancers (De Pergola and Silvestris, 2013). The International Agency for Research on Cancer and World Cancer Research Fund reports indicate that obesity is strongly associated with endometrial, esophageal adenocarcinoma, colorectal, postmenopausal breast, prostate, and renal cancers. This association has been attributed specifically to BMI, increase in weight, and amount of body fat (De Pergola and Silvestris, 2013). In a 10-year follow-up analysis prospective study in Sweden, patients with a BMI > 40 kg/m2 who received bariatric surgery with consequent 20-kg weight loss had a significantly lower cancer incidence compared with morbidly obese untreated patients (Sjöström et al., 2009). In addition, studies have shown that such patients are also at an increased risk for contralateral breast and endometrial cancers, which puts our patient at a greater risk for new primary tumors in the future (Trentham-Dietz et al., 2007; Dignam et al., 2003, 2006; Soerjomataram and Coebergh, 2009).
Understanding cancer associations is also significant in understanding MPTs. Reportedly, breast, prostate, colorectal, and gynecologic cancers are the most common types of primary cancers in patients with MPTs (Nemes and Nagy, 2018). Breast cancer represents 29% of multiple cancer prevalence reported in 2002 (Mariotto et al., 2007). A study by Raymond et al. aimed to report the most common primary tumors following breast cancer and overall survival of those women. Of >335,000 women diagnosed with breast cancer between 1973 and 2000, 40,068 women were diagnosed with one additional cancer, 3796 were diagnosed with two cancers, 351 with three cancers, and 31 with four cancers. The risk and incidence for secondary sites decreased with age, and was found to be much higher for women under the age of 50 (Raymond and Hogue, 2006).
Survival of patients with MPTs often depends on the site of primary and subsequent tumors. Patients with MPTs are found to have better survival than patients with a single primary (95% 5-year survival rate for metachronous MPTs versus 59% survival rate for single primary tumors) (Amer, 2014). In another study of 72 Chinese patients, the median survival for patients with metachronous versus synchronous MPTs was 17.3 years versus 3.8 years (Jiao et al., 2013).
In order to determine optimal treatment options for our patient, we assessed Microsatellite instability and TMB, known predictors of response to immunotherapy (Le et al., 2015; Goodman et al., 2017). In a recent study assessing TMB across multiple tumor types, TMB high was defined as >20 mutations/Mb; melanoma was found to have the third highest percentage of cases with a high TMB (Chalmers et al., 2017). In another study, of 151 patients who received immunotherapy, 88 patients with melanoma and high TMB proved to have better disease outcomes. In patients receiving anti–programmed death-1/programmed death ligand-1 (PD1/PD-L1) monotherapy, for those with intermediate-to-high versus low TMB, complete/partial response rates were 44% and 5%, respectively, and median progression-free survivals were 5.7 and 1.9 months, respectively (Goodman et al., 2017).
In cases such as this, determining whether treatment should be curative versus palliative, while also minimizing the toxicities of concurrent treatments, is an important goal. Assessing the tumor that most strongly affects overall survival is an approach that has guided our patient's treatment. Our patient's serous proliferations (psammomatous calcifications) and lung carcinoid are slow growing and her breast cancer is well controlled; therefore, her treatment strategy has been guided by the advanced nature of her melanoma. While immunotherapy appears to be an evidence-based approach that can improve our patient's survival, further research is needed to provide minimally toxic treatment options for patients with seemingly unrelated MPTs.
IRB approval
According to our institutional policies, this case report has obtained Institutional Review Board exemption.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Funding
Joshua K. Sabari received funding from NIH T32 CA009207; Vicky Makker is supported in part by MSK Cancer Center Support Grant P30CA008748.
Contributor Information
Madhuri Martin, Email: martinm1@mskcc.org.
Joshua K. Sabari, Email: sabarij@mskcc.org.
Gulisa Turashvili, Email: turashvg@mskcc.org.
Darragh F. Halpenny, Email: halpennd@mskcc.org.
Hira Rizvi, Email: rizvih@mskcc.org.
Natalie Shapnik, Email: shapnikn@mskcc.org.
Vicky Makker, Email: makkerv@mskcc.org.
References
- Amer H.M. Multiple neoplasms, single primaries, and patient survival. Cancer Manag. Res. 2014;6:119–134. doi: 10.2147/CMAR.S57378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D.T., Mitchell T.N., Zehir A., Shah R.H., Benayed R., Syed A. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J. Mol. Diagn. 2015;17:251–264. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers Z.R., Connelly C.F., Fabrizio D., Gay L., Ali S.M., Ennis R., Schrock A. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Gen. Med. 2017;9:1–34. doi: 10.1186/s13073-017-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pergola G., Silvestris F. Obesity as a major risk factor for cancer. J. Obes. 2013;2013:291546. doi: 10.1155/2013/291546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J.J., Wieand K., Johnson K.A., Fisher B., Xu L., Mamounas E.P. Obesity, tamoxifen use, and outcomes in women with estrogen receptor-positive early-stage breast cancer. J. Natl. Cancer Inst. 2003;95:1467–1476. doi: 10.1093/jnci/djg060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J.J., Wieand K., Johnson K.A., Raich P., Anderson S.J., Somkin C. Effects of obesity and race on prognosis in lymph node-negative, estrogen receptor-negative breast cancer. Breast Cancer Res. Treat. 2006;97:245–254. doi: 10.1007/s10549-005-9118-3. [DOI] [PubMed] [Google Scholar]
- Goodman A.M., Kato S., Bazhenova L., Patel S.P., Frampton G.M., Miller V. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol. Cancer Ther. 2017;16:2598–2608. doi: 10.1158/1535-7163.MCT-17-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao F., Yao L.J., Zhou J., Hu H., Wang L.W. Clinical features of multiple primary malignancies: a retrospective analysis of 72 Chinese patients. Cancer Causes Control. 2013;24:565–573. doi: 10.7314/apjcp.2014.15.1.331. [DOI] [PubMed] [Google Scholar]
- Le D.T., Uram J.N., Wang H., Bartlett B.R., Kemberling H., Eyring A.D. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariotto A.B., Rowland J.H., Ries L.A., Scoppa S., Feuer E.J. Multiple cancer prevalence: a growing challenge in long-term survivorship. Cancer Epidemiol. Biomark. Prev. 2007;16:566–571. doi: 10.1158/1055-9965.EPI-06-0782. [DOI] [PubMed] [Google Scholar]
- Nemes A., Nagy V. The impact of multiple primary neoplasms in daily practice - a systemic review of the literature. J. BUON. 2018;23:14–18. [PubMed] [Google Scholar]
- Niu B., Ye K., Zhang Q., Lu C., Xie M., McLellan M.D., Wendl M.C. MSIsensor: microsatellite instability detection using paired tumor-normal sequence data. Bioinformatics. 2014;30:1015–1016. doi: 10.1093/bioinformatics/btt755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond J.S., Hogue C.J. Multiple primary tumours in women following breast cancer, 1973–2000. Br. J. Cancer. 2006;94:1745–1750. doi: 10.1038/sj.bjc.6603172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström L., Gummesson A., Sjöström C.D., Narbro K., Peltonen M., Wedel H. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet. Oncol. 2009;10:653–662. doi: 10.1016/S1470-2045(09)70159-7. [DOI] [PubMed] [Google Scholar]
- Soerjomataram I., Coebergh J.W. Epidemiology of multiple primary cancers. Methods Mol. Biol. 2009;471:85–105. doi: 10.1007/978-1-59745-416-2_5. [DOI] [PubMed] [Google Scholar]
- Trentham-Dietz A., Newcomb P.A., Nichols H.B., Hampton J.M. Breast cancer risk factors and second primary malignancies among women with breast cancer. Breast Cancer Res. Treat. 2007;105:359–368. doi: 10.1007/s10549-006-9446-y. [DOI] [PubMed] [Google Scholar]
- Vogt A., Schmid S., Heinimann K., Frick H., Herrmann C., Cerny T. Multiple primary tumours: challenges and approaches, a review. ESMO Open. 2017;2 doi: 10.1136/esmoopen-2017-000172. [DOI] [PMC free article] [PubMed] [Google Scholar]