Abstract
Pharmacokinetic interactions between natural products (NPs) and conventional medications (prescription and nonprescription) are a longstanding but understudied problem in contemporary pharmacotherapy. Consequently, there are no established methods for selecting and prioritizing commercially available NPs to evaluate as precipitants of NP–drug interactions (NPDIs). As such, NPDI discovery remains largely a retrospective, bedside-to-bench process. This Recommended Approach, developed by the Center of Excellence for Natural Product Drug Interaction Research (NaPDI Center), describes a systematic method for selecting NPs to evaluate as precipitants of potential clinically significant pharmacokinetic NPDIs. Guided information-gathering tools were used to score, rank, and triage NPs from an initial list of 47 candidates. Triaging was based on the presence and/or absence of an NPDI identified in a clinical study (≥20% or <20% change in the object drug area under the concentration vs. time curve, respectively), as well as mechanistic and descriptive in vitro and clinical data. A qualitative decision-making tool, termed the fulcrum model, was developed and applied to 11 high-priority NPs for rigorous study of NPDI risk. Application of this approach produced a final list of five high-priority NPs, four of which are currently under investigation by the NaPDI Center.
Introduction
Natural products (NPs), which include botanical dietary supplements and foods, can precipitate clinically significant pharmacokinetic interactions with conventional drugs. These interactions can manifest as enhanced or reduced pharmacologic effect(s) of the object drug. Discovered ≥20 years ago, the pharmacokinetic interactions between St. John’s wort and cyclosporine (Barone et al., 2000; Breidenbach et al., 2000; Mai et al., 2000; Ruschitzka et al., 2000; Moschella and Jaber, 2001) and between grapefruit juice and felodipine (Bailey et al., 1989; Paine and Oberlies, 2007) are now textbook examples of clinically significant pharmacokinetic NP-drug interactions (NPDIs). Despite the clinical impact of these interactions, guidelines for systematically prioritizing commercially available NPs for NPDI investigations are nonexistent. As such, discovery of clinically significant NPDIs is left to chance and remains almost exclusively a bedside-to-bench process. This Recommended Approach, the first in a series of Recommended Approaches to be released by the Center of Excellence for Natural Product Drug Interaction Research (NaPDI Center), proposes a solution to this problem: a decision-making strategy for systematically identifying high-priority NPs that are likely to precipitate clinically significant pharmacokinetic NPDIs that warrant rigorous evaluation.
The need for development and widespread adoption of the aforementioned prospective strategy is evident. Historically, identification of clinically significant NPDIs has been driven by case reports of unexpected adverse drug reactions or loss of efficacy that were indicative of pharmacokinetic or pharmacodynamic perturbations of an object drug (Gardiner et al., 2008a). However, the value of these case reports for accurately identifying NPDIs is unclear. By one estimation, 68% of a representative sample of these case reports were inadequately documented such that determination of whether an NPDI occurred was not possible (Fugh-Berman and Ernst, 2001).
Adverse event reporting is a similarly flawed and inefficient method of NPDI discovery. A survey of NP consumers indicated that just 30% of users would report any adverse reaction to either a drug or an herbal remedy to their primary care physician, and only 6%–7% would report such an adverse reaction to their pharmacist (Barnes et al., 1998). In addition, 26% indicated they would report an adverse reaction to a conventional drug but would not report the same adverse reaction to an herbal remedy (Barnes et al., 1998). Adverse events with food have traditionally been reported more frequently to poison control centers than to the U.S. Food and Drug Administration (FDA), but the FDA now administers the reporting of dietary supplement-related adverse events and serious adverse events via MedWatch (Gardiner et al., 2008b; Frankos et al., 2010). Whether these reporting mechanisms lead to substantial advances in identification of NPDIs remains unclear. Given the inherent limitations of anecdotal case reports and postmarket serious adverse event reporting, a prospective and systematic research method for identifying high-risk NPs for NPDI studies is clearly needed.
The accompanying commentary introduces the premise, overarching goals, and objectives of the NaPDI Center and provides an anticipated list of Recommended Approaches to be released by the Center. These Recommended Approaches will present a coherent strategy for surmounting the unique challenges commonly encountered during the investigation of NPs as precipitants of NPDIs. This Recommended Approach, the first in the series, describes a systematic approach for identifying and prioritizing NPs that merit rigorous evaluation of NPDI risk.
Challenges and a Potential Solution to Current Practices
Current Regulatory Guidances for Evaluating Drug-Drug Interactions Are Not Sufficient for Evaluating NPDIs
The current draft regulatory guidances for evaluating drug-drug interactions (DDIs) recommend the following structured approach for testing a new chemical entity (NCE) as a pharmacokinetic DDI precipitant: 1) in vitro evaluation of the potency of the NCE as an inhibitor or inducer of a standard panel of major drug metabolizing enzymes and transporters; 2) simulation of in vivo interaction potential using static or dynamic models, the latter including physiologically based pharmacokinetic models; and, if necessary, and 3) evaluation of the DDI in human subjects (CHMP, 2012; CDER, 2017).
Although these guidances provide an essential framework for NPDIs, they are not fully suited for evaluating these events, partly due to the inherent complexity of NPs. The diversity and complexity of NP composition is underscored by the inclusiveness of the definition established by the National Center for Complementary and Integrative Health: “a large and diverse group of substances from a variety of sources . . . produced by marine organisms, bacteria, fungi, and plants,” which encompasses both “complex extracts from these producers, but also the isolated compounds derived from those extracts” (NCCIH 2017). The typical commercial formulation of an NP is usually a complex botanical mixture consisting of a prodigious assemblage of phytoconstituents from multiple plant species and/or organs (Freedman et al., 2011; Alolga et al., 2015). Predictably, these mixtures often vary significantly in composition depending on the sourcing and processing, thus complicating the selection of a single product, formulation, or constituent for NPDI studies (Ross et al., 2000; Vandermolen et al., 2013; VanderMolen et al., 2014; Raclariu et al., 2017; Raman et al., 2017).
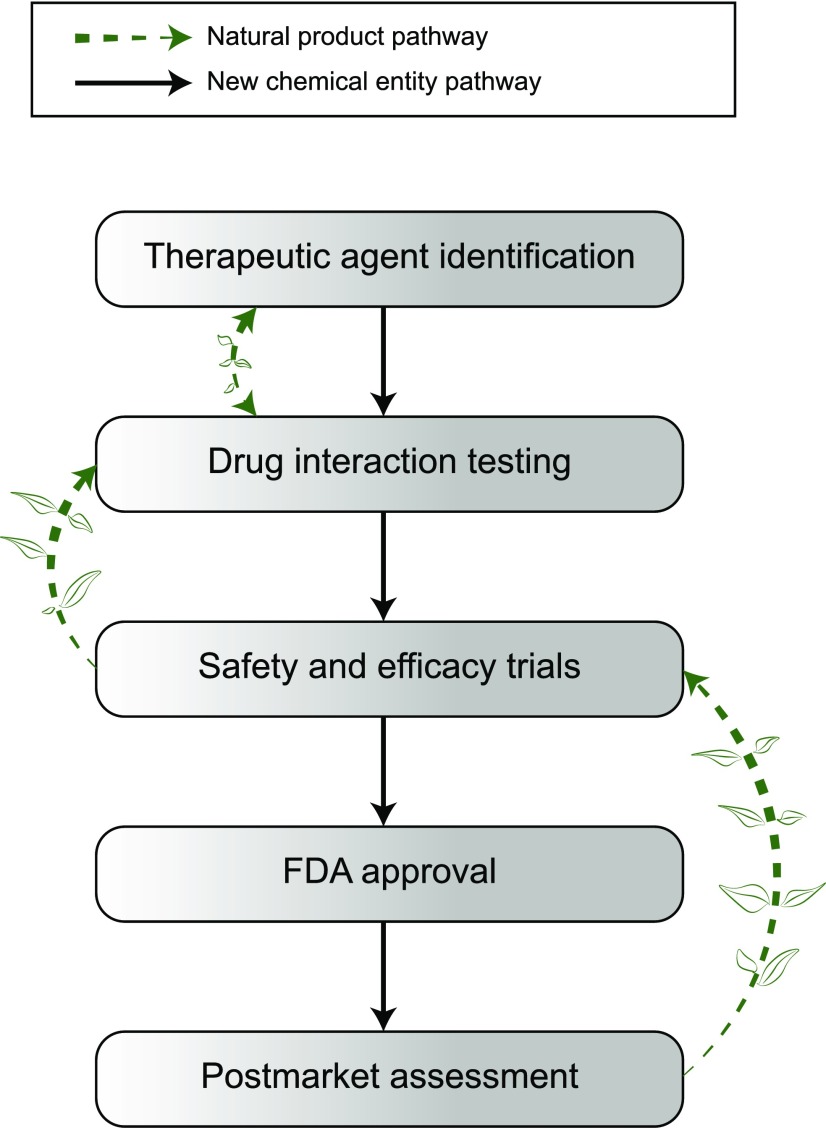
Basic experimental hurdles also preclude NPs from being evaluated in accordance with regulatory DDI guidelines. For example, authentic analytical standards do not always exist for quantification of the NP constituents or associated metabolites in human biologic matrices, and well-designed human pharmacokinetic studies of NP constituents and metabolites have not been routinely conducted. The complex stereochemistry of botanical constituents introduces additional challenges. Collectively, these experimental impediments have historically precluded development of a systematic approach for selecting NPs to study as potential precipitants of NPDIs. Thus, assessing and predicting the drug interaction liability of individual NP constituents requires a strategic adaptation and/or inversion of the DDI assessment process for NCEs (Fig. 1).
Fig. 1.
Pathways to drug interaction testing for new chemical entities and natural products: comparison of drug interaction identification processes for new chemical entities (NCEs) (solid arrows) versus natural products (dashed arrows). Drug interaction testing for NCEs is an early step during preclinical assessment, which includes predicting pharmacokinetic drug-drug interactions using in vitro data and static or dynamic models to guide the need for clinical assessment. In contrast, drug interaction testing for natural products is not required and is typically conducted after case reports of unexpected adverse drug reactions or an unexpected loss of efficacy has been reported in humans.
Recommended Approach for Identifying and Selecting NPs as Precipitants of Pharmacokinetic NPDIs
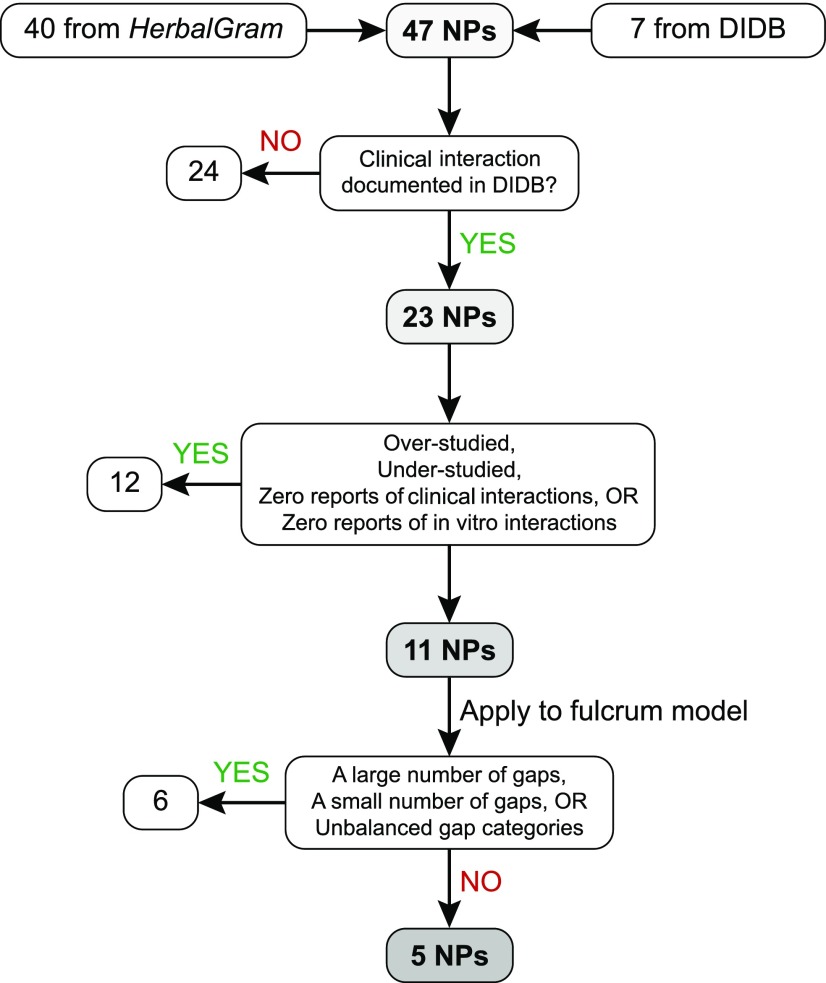
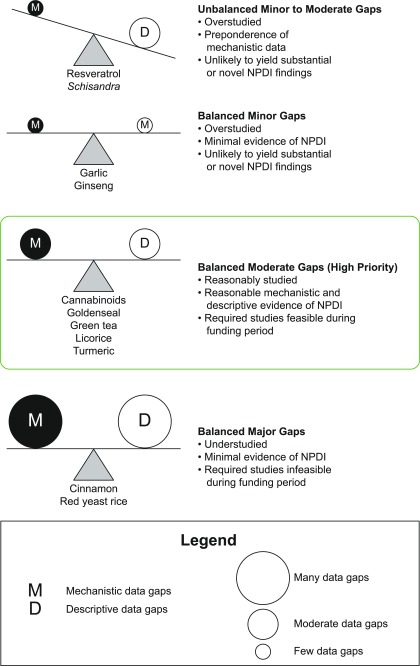
The NaPDI Center, in consultation with the National Center for Complementary and Integrative Health, developed a systematic approach to select high-priority NPs for investigation as precipitants of clinically significant pharmacokinetic NPDIs (Fig. 2). The Center’s Pharmacology Core developed the fulcrum model (Fig. 3), a decision-making tool that is the crux of this approach. By facilitating a balanced evaluation of mechanistic and descriptive in vitro and clinical data, the fulcrum model enabled visual identification of the final high-priority NPs. Currently, four of the remaining five high-priority NPs are under investigation by the NaPDI Center. Although the following strategy was developed with a focus on pharmacokinetic NPDIs involving the North American NP market, the approach is generalizable, and the accompanying tools also may be adapted to pharmacodynamic NPDIs.
Fig. 2.
Workflow for identifying natural products as high-risk precipitants of pharmacokinetic natural product–drug interactions (NPDIs). An initial list of natural products (NPs) was gathered from HerbalGram and the University of Washington Drug Interaction Database. A series of elimination steps were used to triage 42 of these NPs, leaving five for advancement to NPDI studies by the NaPDI Center.
Fig. 3.
The NaPDI fulcrum model: balancing evidence in natural product–drug interaction prediction. A qualitative, conceptual decision tool, termed the fulcrum model, was developed to facilitate selection of the final list of high-priority natural products for drug interaction liability testing by the NaPDI Center. The magnitude of evidence gaps in mechanistic (“M”) and descriptive (“D”) data categories were balanced against each other. Natural products for which moderate levels of evidence gaps balanced each other were prioritized over those that had too few gaps (small circles), many gaps (large circles), and/or unbalanced gaps.
Phase I: Screening of Candidate NPs.
An initial list of 47 candidate NPs (Table 1) was compiled from two sources: the 40 top-selling botanical NPs reported by HerbalGram (Smith, 2015) and seven from the University of Washington’s Drug Interaction Database (DIDB), which houses the largest manually curated collection of in vitro and in vivo data related to drug interactions in humans (http://www.druginteractioninfo.org/). Only human data were evaluated for the information gathering step due to the well-established species differences in common interaction targets (e.g., drug-metabolizing enzymes and transporters) (Baillie and Rettie, 2011).
TABLE 1.
Initial list of 47 candidate natural products to study as precipitants of pharmacokinetic natural product–drug interactions
Candidates 1–40 were obtained from the 2015 HerbalGram report of the top 40 herbal products by sales (Smith, 2015). Candidates without a sales rank were obtained from the University of Washington Drug Interaction Database (https://www.druginteractioninfo.org/).
| Rank | Natural Product | Rank | Natural Product |
|---|---|---|---|
| 1 | Horehound | 25 | Chia seed/chia oil |
| 2 | Cranberry | 26 | Turmeric |
| 3 | Echinacea | 27 | Maca |
| 4 | Black cohosh | 28 | Fenugreek |
| 5 | Flaxseed/flaxseed oil | 29 | Isoflavones |
| 6 | Valerian | 30 | Ginseng |
| 7 | Yohimbe | 31 | St. John’s wort |
| 8 | Bioflavonoid complex | 32 | Green tea |
| 9 | Saw palmetto | 33 | Fennel |
| 10 | Ginger | 34 | Horsetail |
| 11 | Aloe vera | 35 | Tribulus |
| 12 | Milk thistle | 36 | White kidney bean |
| 13 | Garlic | 37 | Evening primrose oil |
| 14 | Cinnamon | 38 | Kelp |
| 15 | Rhodiola | 39 | Gymnema |
| 16 | Horny goat weed | 40 | Grass |
| 17 | Ginkgo | — | Berberine |
| 18 | Plant sterols | — | Cannabinoids |
| 19 | Red yeast rice | — | Feverfew |
| 20 | Elderberry | — | Glycyrrhizin |
| 21 | Guarana | — | Goldenseal |
| 22 | Coconut oil | — | Shisandra chinensis |
| 23 | Senna | — | Resveratrol |
| 24 | Ivy leaf |
DIDB query strategy.
The DIDB searches were conducted for each of the 47 initial candidates using the Therapeutic Class Queries tool, with the key words “herbal medications” as “precipitants,” and the condition as “in vivo.” The “Overall Effect” column of the resulting table was filtered using the term “20% effect” (i.e., ≥20% change in the object drug area under the concentration versus time curve, or AUC) to identify NPs that could potentially precipitate a clinically significant pharmacokinetic NDPI. When common names from the HerbalGram sales report did not coincide with those listed in the DIDB (e.g., horny goat weed, feverfew, grass), the Latin or scientific name was used to query the DIDB. Rather than names of specific extracts or formulations, the broadest possible terms were used in queries.
Scoring.
NPs for which no in vivo interaction data existed in the DIDB were triaged (n = 24). An information-gathering form was subsequently used to compile query results for the 23 remaining NPs (Table 2). This form tabulated counts of the presence of an in vivo interaction (≥20% increase or decrease in object drug AUC), absence of an in vivo interaction (<20% increase or decrease in object drug AUC), and in vitro targets (i.e., drug metabolizing enzymes, transporters, nuclear receptors) for which data were collated in the DIDB.
TABLE 2.
Precipitant natural product candidates advanced to phase II
Twenty-three products, with entries listed by descending priority level.
| Natural Product | Presence of In Vivo Interaction (count)a | Absence of In Vivo Interaction (count)b | Total In Vivo Interactions (count) | Total In Vitro Targets (count)c | Priority Level |
|---|---|---|---|---|---|
| Cannabinoids | 9 | 7 | 16 | 11 | High |
| Ginseng | 5 | 3 | 8 | 5 | High |
| Green tea | 5 | 5 | 10 | 13 | High |
| Berberine (from goldenseal) | 5 | 3 | 8 | 12 | High |
| Resveratrol | 5 | 0 | 5 | 25 | High |
| Garlic | 4 | 9 | 13 | 5 | High |
| Glycyrrhizin (from licorice) | 3 | 1 | 4 | 14 | High |
| Goldenseal | 2 | 2 | 4 | 3 | High |
| Cinnamon | 1 | 0 | 1 | 2 | High |
| Red yeast rice | 1 | 1 | 2 | 1 | High |
| Turmeric | 1 | 0 | 1 | 3 | High |
| Schisandra chinensis extract | 1 | 0 | 1 | 1 | High |
| Ginkgo | 8 | 32 | 40 | 21 | Intermediate |
| Echinacea | 4 | 15 | 19 | 9 | Intermediate |
| Cranberry (juice) | 2 | 10 | 12 | 4 | Intermediate |
| Black cohosh | 1 | 5 | 6 | 4 | Intermediate |
| St. John’s wort | 50 | 27 | 77 | 12 | Low |
| Milk thistle (including silymarin and silibinin) | 31 | 17 | 48 | 54 | Low |
| Evening primrose oil | 1 | 0 | 1 | 0 | Low |
| Echinacea (extract combination) | 0 | 1 | 1 | 1 | Low |
| Valerian | 0 | 6 | 6 | 7 | Low |
| Saw palmetto | 0 | 6 | 6 | 6 | Low |
| Ginger | 0 | 3 | 3 | 2 | Low |
Reports indicating ≥20% change in object drug AUC.
Reports indicating <20% change in object drug AUC.
Reports of in vitro enzyme-, transporter-, or nuclear receptor-mediated interactions (inhibition, induction, or activation). Data were extracted from the University of Washington Drug Interaction Database (https://www.druginteractioninfo.org/) and tabulated.
Phase II: Identifying Low-, Intermediate-, and High-Priority NPs.
The 23 remaining NPs were binned into one of three priority levels—low, intermediate, or high—to triage NPs that were unlikely to precipitate interactions, or for which interactions were markedly understudied or overstudied. A low priority was assigned if the DIDB query returned any of the following:
very high counts of the presence of an in vivo interaction, indicating that the NP was overstudied or well-characterized as an NPDI precipitant (e.g., St. John’s wort, milk thistle);
counts of exclusively the absence of an in vivo interaction, indicating that the NP was understudied or had a low interaction liability (e.g., saw palmetto, valerian); or
counts of either the presence or absence of an in vivo interaction but no counts of an in vitro interaction, again indicating that the NP was understudied or had a low interaction liability (e.g., evening primrose oil).
An intermediate priority was assigned if the query returned a ≥3:1 ratio of counts of the absence of an in vivo interaction relative to counts of the presence of an in vivo interaction (e.g., ginkgo, black cohosh). Based on these criteria, a high priority was assigned to the remaining 11 NPs: cannabinoids, cinnamon, garlic, ginseng, goldenseal, green tea, licorice, red yeast rice, resveratrol, Schisandra spp., and turmeric.
Phase III: Gap Analysis.
Data mining.
For each of the 11 high-priority NPs identified in phase II, a systematic primary literature search and gap analysis was conducted by the NaPDI Center’s Pharmacology Core, which is composed of experts in the areas of NPDIs and DDIs. Gaps were identified by evaluating the primary literature and reputable websites (e.g., the DIDB) to determine which of the following mechanistic or descriptive elements were missing or understudied: names and structures of known NP constituents, potential enzyme and/or transporter target(s) of NPDI-precipitating constituents, human pharmacokinetic studies, and current liquid chromatography with tandem mass spectrometry bioanalytical methods. The gap analysis was précised into an executive summary (Supplemental Table 1). Brief summaries of each section of the gap analysis are provided below.
Known NP constituents. The first section of the gap analysis consisted of profiling constituents within NPs and determining whether these constituents had been evaluated for NPDI liability. Constituents containing functional groups with known potential to trigger time-dependent inhibition of the cytochrome P450 isoforms were flagged, especially if these constituents had shown NPDI potential (Table 3). Substructures associated prominently with time-dependent inhibition, including alkylamines and methylene dioxyphenyls, the metabolism of which can lead to “quasi irreversible” metabolite-intermediate complexes that are known to feature in DDIs (Grimm et al., 2009; Orr et al., 2012), were reported in constituents of many NPs, including those in goldenseal and Schisandra spp. Catechols, olefins, acetylenes, and α,β-unsaturated Michael acceptors, which may give rise to reactive intermediates that could impact CYP function (Kalgutkar et al., 2005) also were identified.
Potential enzyme and/or transporter target(s) and essential experimental systems. The second section of the gap analysis consisted of an evaluation of the strength of NPDI evidence for each constituent identified in the first section. Detailed categories of essential experimental systems, including panels of key drug metabolizing enzymes, transporters, and nuclear receptors, were defined by the Pharmacology Core (Supplemental Table 1, section 2.1). Next, experimental data for any potential targets within these categories were compiled (section 2.2). These data included details of experimental systems, NP source, probe substrate(s) used to test the NPDI, the form of the NP (e.g., extracts and/or as isolated constituents), enzyme/transporter/receptor target(s), induction or inhibition parameter (e.g., Ki, IC50, Emax), and the data source. As the function, expression, and tissue distribution of key drug metabolizing enzymes and transporters exhibit known interspecies differences (Baillie and Rettie, 2011), only data from human-derived systems were included in this analysis. Missing elements were summarized as key gaps in the executive summary.
Human pharmacokinetic NPDI studies. The third section of the gap analysis consisted of the following data extracted from any report of an in vivo pharmacokinetic study for each constituent of the NP: formulation and route of NP administration, object drug(s), description of the study participants, pharmacokinetic outcome(s), and reference(s). These data were evaluated for gaps, such as unstudied major constituents, unknown pharmacokinetic end points, and unstudied interaction targets.
Bioanalytical methods. The fourth section of the gap analysis consisted of reports of liquid chromatography with tandem mass spectrometry (LC-MS/MS)–based bioanalytical method(s) for quantifying NP constituents in human biologic matrices, including microsomes, hepatocytes, plasma, and urine. If a large number of LC-MS/MS methods were available for a given NP (e.g., forensic methods for analysis of cannabinoids), the most recent reports (typically within the last 5 years) were recorded. Data elements collected from each report included the NP constituent(s), the biologic matrix analyzed, any other pertinent data such as lower limits of detection and the reference(s). If methods for some constituents were not found, this gap was noted in the executive summary.
Executive summary. Members of the Pharmacology Core compiled the gap analysis for each NP into an executive summary as a bulleted list.
TABLE 3.
Structural alerts for constituents in select natural products
| Constituent(s)/Natural Product | Structural Alert | Alert Substructure |
|---|---|---|
| Flavonoids, phenylpropanoids/Echinacea Glycyrrhizin, glycyrrhizinic acid/licorice | Catechols | 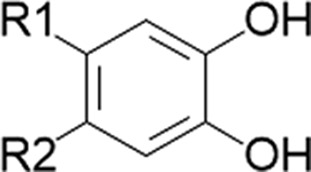 |
| Isoquinoline alkaloids/goldenseal Terpenoids/cinnamon Curcuminoids/turmeric | Masked catechol |
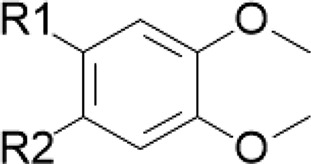 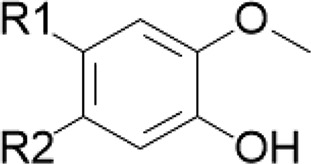
|
| Isoquinoline alkaloids/goldenseal Shizandrins/Schisandra spp. Gomisins/Schisandra spp. | Methylene dioxyphenyl | 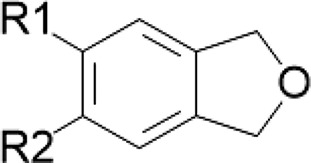 |
| Cycloartenol/black cohosh | Subterminal olefin | 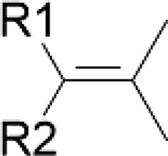 |
| Polyacetylenes/Echinacea | Terminal and subterminal acetylenes |
 
|
| Terpenoids/cinnamon Diallyl di- and trisulfides/garlic | Terminal olefin | 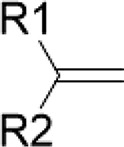 |
| Cinnamaldehyde/cinnamon | α,β-Unsaturated aldehyde | 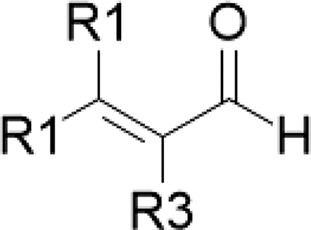 |
| Curcuminoids/turmeric | α,β-Unsaturated ketone | 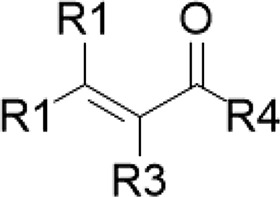 |
Application of the fulcrum model.
Mechanistic and descriptive data gaps from each executive summary were used to populate the fulcrum model (Fig. 3). This qualitative, conceptual decision-making tool was developed to facilitate identification of the final high-priority NPs. For this final triage, NPs with a large number of gaps were eliminated because completing the required in vitro and clinical studies during the 5-year funding period was not feasible. Conversely, NPs with a small number of gaps were triaged because additional experiments were unlikely to yield novel information.
Finally, NPs with unbalanced gap categories were eliminated because 1) the existing evidence could not adequately guide future experiments or 2) at least one of the complementary categories of evidence was not sufficient to substantiate the other. Thus, NPs that balanced the fulcrum with a moderate quantity of gaps in each category were prioritized. A final list of five high-priority NPs emerged from application of this fulcrum model: cannabinoids, goldenseal, green tea, licorice, and turmeric. The first four NPs are currently under evaluation by the NaPDI Center (Kellogg et al., 2017; Tian et al., 2018).
Summary
This NaPDI Center Recommended Approach provides one possible solution to the long-standing question of how to identify high-priority NPs for NPDI studies. The major labor-intensive aspect of this approach is data extraction from both the primary literature and a curated data base. In the future, this process could be partially automated with appropriate data base querying methods (Wu et al., 2014). This Recommended Approach also suggests categories of evidence gaps that should be considered essential when evaluating NPs and their individual constituents as potential NPDI precipitants.
Application of this Recommended Approach identified five popularly consumed NPs for which the existing evidence is sufficient to guide further investigation and currently warrants reasonable suspicion of clinically significant NPDI liability. Four of these NPs are now the subjects of targeted interaction projects, which are designed to fill essential scientific gaps related to NPDI potential and, if warranted, conduct clinical pharmacokinetic NPDI studies.
Acknowledgments
We thank Dr. Isabelle Ragueneau-Majlessi and Dr. Jingjing Yu for expert consultation and support regarding the use and querying of the University of Washington Drug Interaction Database, and Kazuya Ishida, Gabriela Patilea-Vrana, and Muhammad Farooq (Department of Pharmaceutics, University of Washington) for assisting with curation of data from the primary literature. M.F.P. dedicates this article to Dr. David P. Paine.
Abbreviations
- AUC
area under the concentration versus time curve
- DDI
drug-drug interaction
- DIDB
Drug Interaction Database
- FDA
U.S. Food and Drug Administration
- LC-MS/MS
liquid chromatography with tandem mass spectrometry
- NaPDI Center
Center of Excellence for Natural Product Drug Interaction Research
- NCE
new chemical entity
- NP
natural product
- NPDI
natural product–drug interaction
Authorship Contributions
Participated in research design: González-Peréz, Lin, McCune, Paine, Rettie, Shen, Unadkat.
Conducted experiments: Johnson, González-Peréz, Lin, Paine, Rettie, Tian, Unadkat.
Wrote or contributed to the writing of the manuscript: Johnson, González-Peréz, Lin, McCune, Paine, Rettie, Shen, Tian, Unadkat.
Footnotes
This work was supported by the National Institutes of Health National Center for Complementary and Integrative Health [U54 AT008909], National Institute on Drug Abuse [P01 DA032507] (to J.D.U.), National Cancer Institute [R01 CA182963] (to J.S.M.), and National Institute of General Medical Sciences [R01 GM077482] (to M.F.P.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
References
- Alolga RN, Fan Y, Zhang G, Li J, Zhao YJ, Lelu Kakila J, Chen Y, Li P, Qi LW. (2015) Pharmacokinetics of a multicomponent herbal preparation in healthy Chinese and African volunteers. Sci Rep 5:12961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DG, Spence JD, Edgar B, Bayliff CD, Arnold JM. (1989) Ethanol enhances the hemodynamic effects of felodipine. Clin Invest Med 12:357–362. [PubMed] [Google Scholar]
- Baillie TA, Rettie AE. (2011) Role of biotransformation in drug-induced toxicity: influence of intra- and inter-species differences in drug metabolism. Drug Metab Pharmacokinet 26:15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes J, Mills SY, Abbot NC, Willoughby M, Ernst E. (1998) Different standards for reporting ADRs to herbal remedies and conventional OTC medicines: face-to-face interviews with 515 users of herbal remedies. Br J Clin Pharmacol 45:496–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone GW, Gurley BJ, Ketel BL, Lightfoot ML, Abul-Ezz SR. (2000) Drug interaction between St. John’s wort and cyclosporine. Ann Pharmacother 34:1013–1016. [DOI] [PubMed] [Google Scholar]
- Breidenbach T, Kliem V, Burg M, Radermacher J, Hoffmann MW, Klempnauer J. (2000) Profound drop of cyclosporin A whole blood trough levels caused by St. John’s wort (Hypericum perforatum). Transplantation 69:2229–2230. [DOI] [PubMed] [Google Scholar]
- Center for Drug Evaluation and Research (CDER) (2017) Guidance for Industry: Clinical Drug Interaction Studies—Study Design, Data Analysis, and Clinical Implications [Draft Guidance], U.S. Department of Health and Human Services, Food and Drug Administration, Rockville, MD: https://www.fda.gov/downloads/drugs/guidances/ucm292362.pdf. [Google Scholar]
- Committee for Human Medicinal Products (CHMP) (2012) Guideline on the Investigation of Drug Interactions [Final], European Medicines Agency, London: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/07/WC500129606.pdf. [Google Scholar]
- Frankos VH, Street DA, O’Neill RK. (2010) FDA regulation of dietary supplements and requirements regarding adverse event reporting. Clin Pharmacol Ther 87:239–244. [DOI] [PubMed] [Google Scholar]
- Freedman ND, Curto TM, Morishima C, Seeff LB, Goodman ZD, Wright EC, Sinha R, Everhart JE, HALT-C Trial Group (2011) Silymarin use and liver disease progression in the hepatitis C antiviral long-term treatment against cirrhosis trial. Aliment Pharmacol Ther 33:127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugh-Berman A, Ernst E. (2001) Herb-drug interactions: review and assessment of report reliability. Br J Clin Pharmacol 52:587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner P, Phillips R, Shaughnessy AF. (2008a) Herbal and dietary supplement--drug interactions in patients with chronic illnesses. Am Fam Physician 77:73–78. [PubMed] [Google Scholar]
- Gardiner P, Sarma DN, Low Dog T, Barrett ML, Chavez ML, Ko R, Mahady GB, Marles RJ, Pellicore LS, Giancaspro GI. (2008b) The state of dietary supplement adverse event reporting in the United States. Pharmacoepidemiol Drug Saf 17:962–970. [DOI] [PubMed] [Google Scholar]
- Grimm SW, Einolf HJ, Hall SD, He K, Lim HK, Ling KH, Lu C, Nomeir AA, Seibert E, Skordos KW, et al. (2009) The conduct of in vitro studies to address time-dependent inhibition of drug-metabolizing enzymes: a perspective of the pharmaceutical research and manufacturers of America. Drug Metab Dispos 37:1355–1370. [DOI] [PubMed] [Google Scholar]
- Kalgutkar AS, Gardner I, Obach RS, Shaffer CL, Callegari E, Henne KR, Mutlib AE, Dalvie DK, Lee JS, Nakai Y, et al. (2005) A comprehensive listing of bioactivation pathways of organic functional groups. Curr Drug Metab 6:161–225. [DOI] [PubMed] [Google Scholar]
- Kellogg JJ, Graf TN, Paine MF, McCune JS, Kvalheim OM, Oberlies NH, Cech NB. (2017) Comparison of metabolomics approaches for evaluating the variability of complex botanical preparations: green tea (Camellia sinensis) as a case study. J Nat Prod 80:1457–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg JJ, Wallace ED, Graf TN, Oberlies NH, Cech NB. (2017) J Pharm Biomed Anal 145:604–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai I, Krüger H, Budde K, Johne A, Brockmöller J, Neumayer HH, Roots I. (2000) Hazardous pharmacokinetic interaction of Saint John’s wort (Hypericum perforatum) with the immunosuppressant cyclosporin. Int J Clin Pharmacol Ther 38:500–502. [DOI] [PubMed] [Google Scholar]
- Moschella C, Jaber BL. (2001) Interaction between cyclosporine and Hypericum perforatum (St. John’s wort) after organ transplantation. Am J Kidney Dis 38:1105–1107. [DOI] [PubMed] [Google Scholar]
- National Center for Complementary and Integrative Health (NCCIH) 2017. Natural products research—information for researchers. U.S. Department of Health & Human Services, Bethesda, MD: https://nccih.nih.gov/grants/naturalproducts [Google Scholar]
- Orr ST, Ripp SL, Ballard TE, Henderson JL, Scott DO, Obach RS, Sun H, Kalgutkar AS. (2012) Mechanism-based inactivation (MBI) of cytochrome P450 enzymes: structure-activity relationships and discovery strategies to mitigate drug-drug interaction risks. J Med Chem 55:4896–4933. [DOI] [PubMed] [Google Scholar]
- Paine MF, Oberlies NH. (2007) Clinical relevance of the small intestine as an organ of drug elimination: drug-fruit juice interactions. Expert Opin Drug Metab Toxicol 3:67–80. [DOI] [PubMed] [Google Scholar]
- Raclariu AC, Paltinean R, Vlase L, Labarre A, Manzanilla V, Ichim MC, Crisan G, Brysting AK, de Boer H. (2017) Comparative authentication of Hypericum perforatum herbal products using DNA metabarcoding, TLC and HPLC-MS. Sci Rep 7:1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman V, Bussmann RW, Khan IA. (2017) Which bay leaf is in your spice rack? A quality control study. Planta Med 83:1058–1067. [DOI] [PubMed] [Google Scholar]
- Ross SA, Ziska DS, Zhao K, ElSohly MA. (2000) Variance of common flavonoids by brand of grapefruit juice. Fitoterapia 71:154–161. [DOI] [PubMed] [Google Scholar]
- Ruschitzka F, Meier PJ, Turina M, Lüscher TF, Noll G. (2000) Acute heart transplant rejection due to Saint John’s wort. Lancet 355:548–549. [DOI] [PubMed] [Google Scholar]
- Smith T, Lynch ME, Johnson J, Kawa K, Bauman H, Blumenthal M. (2015) Herbal dietary supplement sales in US increase 6.8% in 2014. HerbalGram 107:52–59. http://cms.herbalgram.org/herbalgram/issue107/hg107-mktrpt-2014hmr.html [Google Scholar]
- Tian DD, Kellogg JJ, Okut N, Oberlies NH, Cech NB, Shen DD, McCune JS, Paine MF. (2018) Identification of intestinal UDP-glucuronosyltransferase inhibitors in green tea (Camellia sinensis) using a biochemometric approach: application to raloxifene as a test drug via in vitro to in vivo extrapolation. Drug Metab Dispos 46:552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderMolen KM, Ainslie GR, Paine MF, Oberlies NH. (2014) Labeled content of two furanocoumarins in dietary supplements correlates with neither actual content nor CYP3A inhibitory activity. J Pharm Biomed Anal 98:260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermolen KM, Cech NB, Paine MF, Oberlies NH. (2013) Rapid quantitation of furanocoumarins and flavonoids in grapefruit juice using ultra-performance liquid chromatography. Phytochem Anal 24:654–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HY, Chiang CW, Li L. (2014) Text mining for drug-drug interaction. Methods Mol Biol 1159:47–75. [DOI] [PMC free article] [PubMed] [Google Scholar]