Significance
Strategy-switching flexibility is a critical executive function necessary for living in an ever-evolving environment, and this ability is often impaired in attentional deficit and hyperactivity disorder, schizophrenia, and early Parkinson’s disease. To date, the underlying brain circuitry and receptor mechanisms are not entirely clear. The results of the present study suggest the essential role of a specific projection from prelimbic cortex to nucleus accumbens (NAc) D2 medium spiny neurons as well as NAc dopamine and presynaptic dopamine receptors of this projection in controlling the strategy-switching flexibility. These findings promote a better understanding of circuitry and neurobiology of strategy-switching flexibility and could contribute to identifying novel therapeutic targets for patients suffering from strategy-switching inflexibility.
Keywords: task-switching, strategy abandoning, prelimbic cortex, nucleus accumbens, dopamine receptors
Abstract
The ability to abandon old strategies and adopt new ones is essential for survival in a constantly changing environment. While previous studies suggest the importance of the prefrontal cortex and some subcortical areas in the generation of strategy-switching flexibility, the fine neural circuitry and receptor mechanisms involved are not fully understood. In this study, we showed that optogenetic excitation and inhibition of the prelimbic cortex–nucleus accumbens (NAc) pathway in the mouse respectively enhances and suppresses strategy-switching ability in a cross-modal spatial-egocentric task. This ability is dependent on an intact dopaminergic tone in the NAc, as local dopamine denervation impaired the performance of the animal in the switching of tasks. In addition, based on a brain-slice preparation obtained from Drd2-EGFP BAC transgenic mice, we demonstrated direct innervation of D2 receptor-expressing medium spiny neurons (D2-MSNs) in the NAc by prelimbic cortical neurons, which is under the regulation by presynaptic dopamine receptors. While presynaptic D1-type receptor activation enhances the glutamatergic transmission from the prelimbic cortex to D2-MSNs, D2-type receptor activation suppresses this synaptic connection. Furthermore, manipulation of this pathway by optogenetic activation or administration of a D1-type agonist or a D2-type antagonist could restore impaired task-switching flexibility in mice with local NAc dopamine depletion; this restoration is consistent with the effects of knocking down the expression of specific dopamine receptors in the pathway. Our results point to a critical role of a specific prelimbic cortex–NAc subpathway in mediating strategy abandoning, allowing the switching from one strategy to another in problem solving.
In an ever-evolving environment, an individual has to keep developing new strategies to replace old ones for survival. The ability to switch strategies to adapt to the new surroundings, a type of behavioral flexibility, is impaired in some disorders including attentional deficit and hyperactivity disorder (ADHD), schizophrenia, and early Parkinson’s disease (1–3). In the past few decades, a growing body of studies has demonstrated that the frontal cortex mediates learning flexibility in addition to other cognitive functions (4–8). However, according to experimental investigations in both primates (9) and rodents (4), different subregions of the frontal cortex may process different types or different levels of cognitive and behavioral information, to achieve behavioral flexibility under different contexts. The prelimbic–infralimbic area is one of the best-studied subregions in the prefrontal cortex. Inactivation of this area has been reported to ruin behavior flexibility including cross-modal shift (e.g., place vs. response discrimination) but not intramodal shift (10). Other behavioral flexibility mediated by the prelimbic–infralimbic area includes extradimensional attentional set-shifting (e.g., odor vs. texture), match-to-sample and nonmatch-to-sample shift, and paired-associate learning (5, 10–13). To decipher the neurobiological basis of behavioral flexibility, it is necessary to refine the neural circuits and mechanisms that underlie different types or aspects of learning flexibility.
One of the major targets of the prefrontal cortex is the nucleus accumbens (NAc) (14), a ventral analog to the dorsal striatum with respect to cytoarchitectonic and chemoarchitectonic features, although distinct functions are believed to be carried out in this region (15, 16). Based on differences in the expression of neuroactive substances as well as inputs and outputs, the NAc is divided into shell and core subregions (16). There is evidence showing that inactivation of the NAc core impairs strategy-switching flexibility (17), implying the importance of this subregion for this cognitive function. Throughout the shell and core subregions, around 95% of NAc neurons are medium spiny projection neurons (MSNs) that express either dopamine D1 receptors (D1-MSNs) or D2 receptors (D2-MSNs). These two subpopulations are suggested to be exclusive from a young age (18). In contrast to the canonical understanding that these two types of neurons are separately involved in striatomesencephalic and striatopallidal pathways, some early studies suggested that D1-MSNs project to both the ventral tegmental area (VTA) in the midbrain and the ventral pallidum (VP) and that D2-MSNs preferentially project to the VP (19, 20). This finding has been refined by a recent study demonstrating that virtually 50% of VP neurons are under the innervation of D1-MSNs from the NAc core (21). Despite this, D2-MSNs, but not D1-MSNs, are suggested to be involved in the learning flexibility (22).
Dopaminergic innervation from the VTA represents a crucial control over behavioral flexibility executed by the prefrontal cortex and the medial striatum (23–25). The NAc also receives dopaminergic input from the VTA and, in addition to its known involvement in various learning and memory-associated behaviors (26–28), this mesolimbic dopamine pathway has been reported to regulate some forms of behavior flexibility (22, 29–31). However, there are contradictory conclusions regarding the types of dopamine receptors involved in the behavior flexibility (22, 31). Dopamine receptors are not expressed only by MSNs in the NAc. Physiological evidence indicates that they are also functionally present in the cortical terminals, including those from the prelimbic cortex (PrL), to regulate the glutamate release into the NAc (32–35). Thus, the presynaptic dopamine receptors, in addition to their postsynaptic counterparts, could act as important sites for mediating functions of dopamine. However, the available reports in the literature are still controversial regarding which dopamine receptor type, D1 or D2, mediates the presynaptic modulation of PrL–NAc glutamatergic transmission (32–35). Moreover, these studies did not take into account D1-MSNs and D2-MSNs being functionally segregated populations.
In this study, we elucidated the functional circuitry that links the roles of the prefrontal cortex and the NAc in behavioral flexibility by examining the involvement of PrL–NAc projection and NAc dopamine receptors in a spatial-egocentric strategy-switching task. A multidisciplinary approach was adopted. Our results unveiled a critical role of a PrL–NAc subpathway in mediating the strategy abandoning necessary for task-switching flexibility.
Results
PrL–NAc Pathway Contributes to Switching but Not Learning of a Strategy.
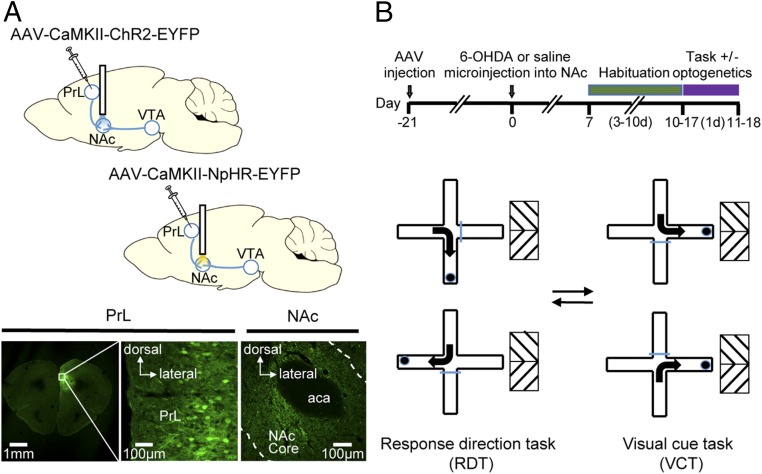
To determine the participation of the prefrontal cortex–NAc projection in cognitive flexibility, we made use of optogenetic manipulation to functionally activate or inhibit this pathway and examined the effects on the learning and switching of strategy to get a food reward. Adeno-associated viruses (AAVs) encoding channelrhodopsin (ChR2)-EYFP or halorhodopsin (NpHR)-EYFP under the control of the CaMKII promoter were injected into the PrL, and optical cannula were implanted targeting the NAc (Fig. 1A, Upper). EYFP-positive terminals were found in the NAc, including the core region, suggesting the presence of a direct projection from the PrL to the NAc (Fig. 1A, Lower), which was consistent with results in patch-clamp recording experiments (see below). Indeed, the expression of functional ChR2 and NpHR in the PrL–NAc pathway responsive to light stimulation were verified in brain-slice preparations (Fig. S1). The experimental design is shown in the schematic in Fig. 1B, Upper. The behavioral paradigms involve a response-direction task (RDT) and a visual cue task (VCT) based on a four-arm cross-maze and the switching of these tasks (Fig. 1B, Lower). These spatial-egocentric tasks evaluate the ability of the animals to learn particular rules for rewards and their flexibility in switching to a new strategy by abandoning a previously learned one (22, 35), which was determined by the accumulated number of trials needed to reach the criterion as well as the number of different types of errors, including perseverative, regressive, and never-reinforced errors (for details see Materials and Methods).
Fig. 1.
Schematics of the optogenetic manipulation of the PrL–NAc pathway and behavioral tasks. (A, Upper) AAV-CaMKII-ChR2-EYFP and AAV-CaMKII-NpHR-EYFP were injected into the PrL of test animals, and an optical cannula was implanted directly above the NAc delivering 470 nm or 590 nm light. (Lower) Postmortem histological examination confirmed the expression of EYFP in soma of PrL neurons as well as in terminal-like structures in the NAc core region. aca, anterior commissure, anterior part. (B, Upper) The design and time-line of experiments. (Lower) In the RDT the animal is required to turn against its turn bias, and in the VCT the animal is required to turn to the arm associated with a visual cue. In task-switching test, the subject needs to abandon the previously learned rule and adopt another rule. The blue horizontal bar indicates a closed door in the corresponding paradigm.
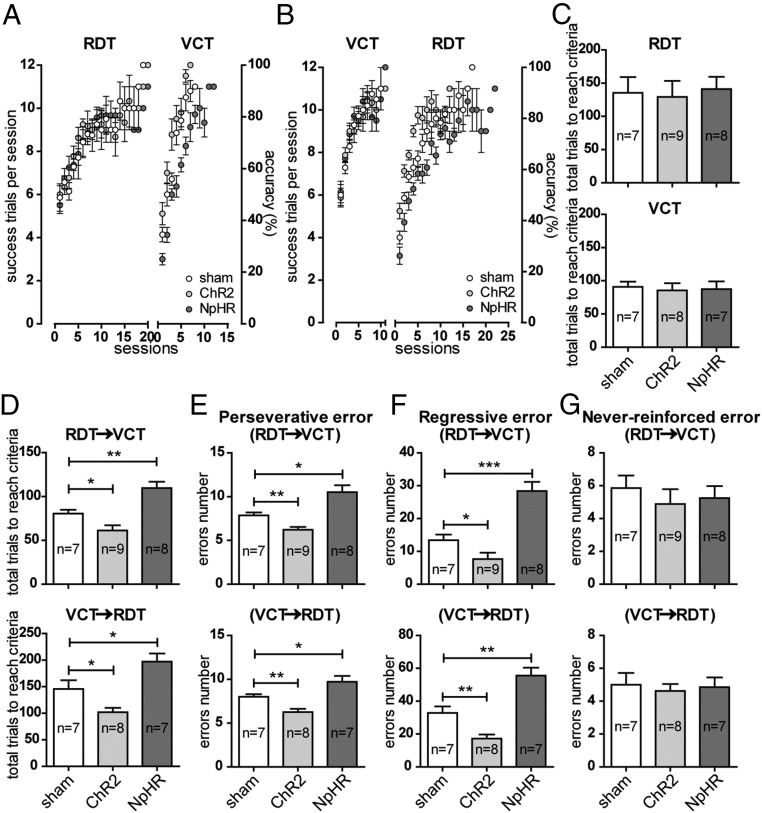
In the mice with the expression of ChR2 in the PrL neurons, optogenetic activation of their terminals in the NAc did not affect the performance in task acquisition in both the RDT (total number of trials needed to reach the criterion: sham, 135.4 ± 23.7, n = 7; ChR2, 129.3 ± 24.1, n = 9; P > 0.05) and the VCT (total number of trials needed to reach the criterion: sham, 90.9 ± 7.8, n = 7; ChR2, 85.5 ± 11.0, n = 8; P > 0.05) tests compared with respective sham groups. The numbers of successful trials in consecutive sections (and the corresponding accuracy, in percentage) are shown in Fig. 2 A and B, and the total number of trials needed to reach the criterion is shown in Fig. 2C. Optogenetic inhibition of the terminals through NpHR activation also had no influence on learning (total number of trials needed to reach the criterion: in RDT, NpHR, 141.0 ± 18.5, n = 8; P > 0.05; in VCT, NpHR, 87.4 ± 11.6, n = 7; P > 0.05, both compared with their respective sham groups) (Fig. 2 A–C). These data suggested that the PrL–NAc pathway is not required for learning these two strategies.
Fig. 2.
The PrL–NAc pathway contributes to task-switching flexibility. (A–D) Learning of the RDT (A) and VCT (B) tests by the mice, which could reach the criterion within 1 d. For the RDT, data were pooled from seven, nine, and eight animals for the sham, ChR2, and NpHR groups, respectively. For the VCT, seven, eight, and seven animals were used in the sham, ChR2, and NpHR groups, respectively. Compared with the sham group, ChR2 or NpHR activation of the PrL–NAc projection did not affect the total number of trials needed to reach the criterion in either the RDT (A and C) or the VCT (B and C). On the other hand, in both the RDT–VCT and VCT–RDT switching paradigms, activation of the PrL–NAc pathway in the ChR2 group significantly reduced the total number of trials needed to reach the criterion, and the number of trials needed to reach the criterion was significantly increased when this pathway was inhibited in the NpHR group (D). (E and F) The perseverative errors (E) and regressive errors (F) committed exhibited a pattern of change similar to the total number of trials needed to reach the criterion in both switching paradigms. (G) Never-reinforced errors did not change with either stimulation or suppression of the PrL–NAc projection. *P < 0.05; **P < 0.01; ***P < 0.001, unpaired t test. Data are presented as mean ± SEM.
We next examined the ability of the animals in switching to learn the second strategy, after learning the first strategy in previous training sessions. A 1-h break was introduced between the two tasks. In the RDT-to-VCT switching paradigm, optogenetic activation of the PrL–NAc pathway significantly reduced the total number of trials needed to reach the criterion compared with the sham group (sham, 80.6 ± 4.3, n = 7; ChR2, 61.3 ± 5.8, n = 9; P < 0.05) (Fig. 2D). A similar result was found in VCT-to-RDT switching (sham, 145.7 ± 16.4, n = 7; ChR2, 102.0 ± 8.2, n = 8; P < 0.05) (Fig. 2D). On the other hand, when this pathway was inhibited optogenetically, the total number of trials needed to reach the criterion was significantly increased in both RDT-to-VCT switching (NpHR, 109.5 ± 7.3, n = 8; P < 0.01 compared with sham) and VCT-to-RDT switching (NpHR, 197.1 ± 15.5, n = 7; P < 0.05 compared with sham) (Fig. 2D). In addition to collecting data on the number of trials needed to reach the criterion, we classified error types to assess if impairments in shifting were due to deficits in the suppression of old modes of responding (perseverative errors), in exploring novel strategies (never-reinforced errors), or in the maintenance of novel strategies once perseveration has ceased (regressive errors). Analysis of these three subtypes of errors committed during strategy switching revealed that both perseverative errors and regressive errors (Fig. 2 E and F) were significantly altered and paralleled the changes in the total number of trials needed to reach the criterion. In contrast, there were no significant differences in the number of never-reinforced errors (Fig. 2G). The significant difference in perseverative and regressive errors from optogenetically manipulated groups suggest the PrL–NAc pathway is necessary for successful disengagement from a previously relevant strategy.
NAc Dopamine Depletion Impairs Strategy-Switching Flexibility.
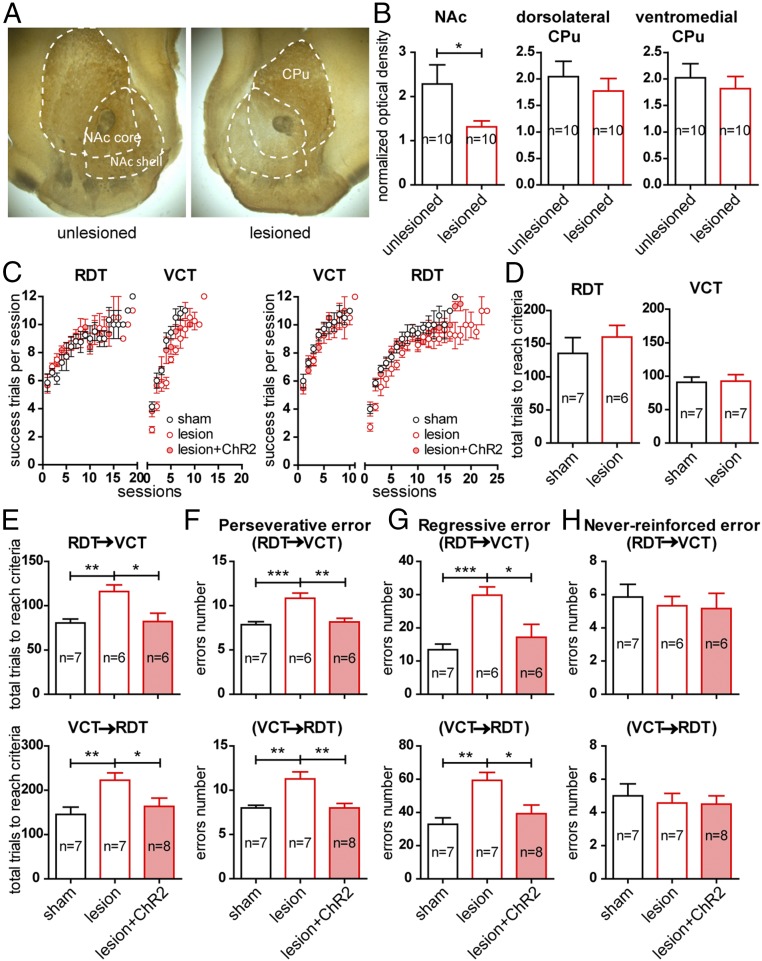
To examine if accumbal dopamine also plays a role in the same strategy-switching task, stereotaxic injection of 6-hydroxydopamine hydrochloride (6-OHDA) into the NAc together with i.p. injection of desipramine were performed to selectively damage dopaminergic terminals. Tyrosine hydroxylase (TH) immunostaining in the slices confirmed that NAc TH-containing fibers were reduced 2 wk following 6-OHDA lesion (Fig. 3A). The normalized optical density of the NAc from the lesioned side was significantly lower than that of the unlesioned side (unlesioned, 2.29 ± 0.43; lesioned, 1.31 ± 0.13; n = 10 mice, P < 0.05) (Fig. 3B), which suggested that dopamine was partially but significantly depleted. In contrast, there was no change in the optical density of TH signals in the dorsolateral striatum (unlesioned, 2.04 ± 0.29; lesioned, 1.77 ± 0.24; n = 10 mice, P > 0.05) (Fig. 3B) and ventromedial striatum (unlesioned, 2.02 ± 0.26; lesioned, 1.82 ± 0.23; n = 10 mice, P > 0.05) (Fig. 3B). The partial depletion of dopamine was also confirmed by measurement of in vivo dopamine levels in the NAc by standard HPLC (unlesioned, 12.09 ± 1.21 ng/mg; lesioned, 6.09 ± 0.76 ng/mg; n = 3 mice, P < 0.05). To verify that unilateral depletion of dopamine would not impose any asymmetry in motor ability, e.g., in executing turning movements, we quantified the locomotor behaviors of the animals in the open-field arena and also the turn bias in the T-maze. We found that no rotational behaviors were induced by the unilateral NAc dopamine depletion. There were no differences between sham and lesioned mice in general mobility, rotation ratio (Fig. S2 A and B), and turn bias in the T-maze (Fig. S2C).
Fig. 3.
Focal dopamine depletion in the NAc impairs strategy-switching flexibility, and the impairment can be rescued by optogenetic activation of the PrL–NAc pathway. (A) A typical section showing that TH immunoreactivity was reduced in the NAc core and shell regions 2 wk after focal injection of 6-OHDA into the NAc. CPu, caudate putamen. (B) Normalized data of optical density in the NAc (Left), dorsolateral CPu (Middle), and ventromedial CPu (Right) from 10 animals. *P < 0.05, paired t test. (C) Tracking of success trials in RDT and VCT learning as well as their switching in the sham, 6-OHDA lesioned, and lesioned+ChR2 groups. (D–H) Pooled data suggest that NAc dopamine depletion did not affect the ability of the animals to acquire the RDT and the VCT (D). However, in the RDT–VCT and VCT–RDT switching tests, NAc dopamine depletion resulted in a significantly higher number of trials before reaching the criterion (E), accompanied by increases in perseverative errors (F) and regressive errors (G), but not in never-reinforced errors (H). Optogenetic activation of ChR2 of the PrL–NAc pathway in the lesioned mice restored switching performance to that in the sham group. *P < 0.05; **P < 0.01; ***P < 0.001, unpaired t test. Data are presented as mean ± SEM.
Under the condition of local dopamine depletion in the NAc, the performances of the sham groups and lesioned groups were comparable in both the RDT and VCT. Fig. 3C shows their performance in successive sessions. In the RDT, the total number of trials needed to reach the criterion was 135.4 ± 23.7, n = 7, in the sham group and was 160.0 ± 17.7, n = 6, in the lesioned group (P > 0.05) (Fig. 3D). For the VCT, the total number of trials needed to reach the criterion was 90.9 ± 7.8, n = 7, in the sham group and was 92.6 ± 9.7, n = 7, in the lesioned group (P > 0.05) (Fig. 3D). These data suggested that acquisition of the tasks was not impaired by NAc dopamine depletion. However, in the mice switching from the RDT to the VCT, the performance of the lesioned group was degraded (Fig. 3C), and a significantly larger number of the trials was required to reach the criterion (sham, 80.6 ± 4.3, n = 7; lesion, 116.0 ± 7.4, n = 6; P < 0.01) (Fig. 3E). Likewise, lesioned mice undergoing VCT-to-RDT switching needed more trials to reach the criterion (sham, 145.7 ± 16.4, n = 7; lesion, 222.9 ± 16.5, n = 7; P < 0.01) (Fig. 3 C and E). These results indicate that NAc dopamine depletion could impair strategy-switching flexibility. Again, these impairments were contributed by perseverative errors and regressive errors. In both RDT–VCT and VCT–RDT switching, the numbers of these errors were significantly higher in the lesioned groups than in the sham groups (Fig. 3 F and G), while the numbers of never-reinforced errors remained unaffected (Fig. 3H).
Impairment in Task-Switching Flexibility Can Be Rescued by Optogenetic Activation of the PrL–NAc Pathway.
The previous data imply that the PrL–NAc projection plays a significant role in mediating task-switching flexibility by facilitating the abandoning of an old strategy. This ability is also dependent on intact dopamine innervation within the NAc. We next asked whether impaired task-switching flexibility under NAc dopamine depletion could be rescued by enhancing the PrL–NAc pathway. Indeed, while optogenetic activation of the PrL–NAc pathway in the dopamine-depleted mice did not affect the learning of the RDT and VCT per se (Fig. S3A), this paradigm reduced the total number of trials needed to reach the criterion in both RDT-to-VCT switching experiments and VCT-to-RDT switching experiments to levels comparable to those of the respective sham groups (RDT-to-VCT: sham, 80.6 ± 4.3, n = 7; lesion, 116.0 ± 7.4, n = 6; lesion+ChR2, 82.0 ± 9.5, n = 6; P < 0.05 between lesion and lesion+ChR2; P > 0.05 between sham and lesion+ChR2; VCT-to-RDT: sham, 145.7 ± 16.4, n = 7; lesion, 222.9 ± 16.5, n = 7; lesion+ChR2, 163.5 ± 18.8, n = 8; P < 0.05 between lesion and lesion+ChR2; P > 0.05 between sham and lesion+ChR2) (Fig. 3E). The improvements in task-switching performance with optogenetic activation of prelimbic terminals in the dopamine-denervated NAc were accompanied by similar alterations in perseverative errors and regressive errors (Fig. 3 F and G) but not in never-reinforced errors (Fig. 3H).
PrL Innervates D2-MSNs in the Core Subregion of the NAc.
Our data strongly suggest an interaction between the PrL–NAc pathway and the dopaminergic input from midbrain in the NAc in task-switching flexibility. An obvious question to address is whether dopamine released in the NAc directly modulates the PrL–NAc glutamatergic transmission. Among the MSNs in NAc, there is strong evidence showing that D2-MSNs in NAc, rather than D1-MSNs, are crucial in learning flexibility (22, 36), but the underlying mechanism has yet to be explored. Thus, as a first step in deciphering the neural circuitry, we focused on the role of the projection from the PrL to the D2-MSNs. In this regard, we first had to confirm that the PrL does innervate D2-MSNs in the NAc. To achieve this, we established an in vitro preparation that would allow us to study the modulation of the synaptic connection by electrophysiological and pharmacological approaches and also via an optogenetic stimulation paradigm.
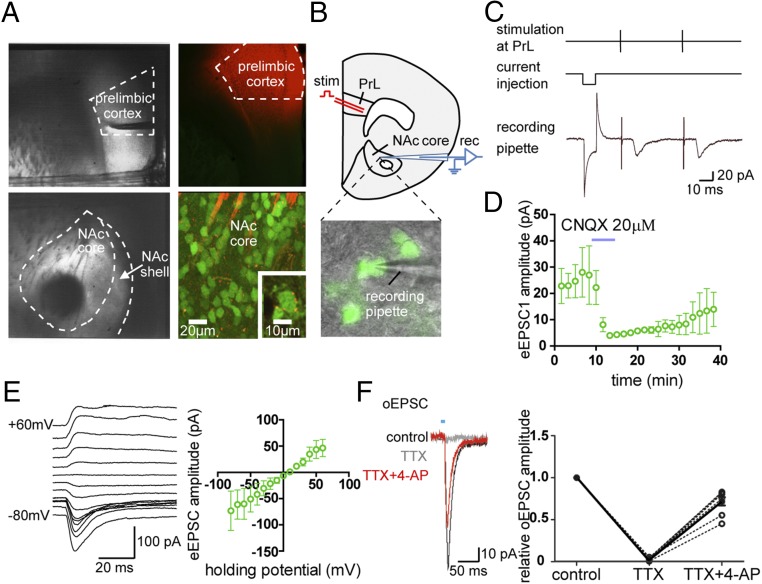
We employed Drd2-EGFP BAC transgenic mice that allow visualization of D2 receptor-expressing neurons (37). As described in detail in Materials and Methods, brain slices from the Drd2-EGFP BAC transgenic mice were cut at an oblique angle such that the projection from the PrL to the core region of the NAc was preserved and could be identified under the microscope (Fig. 4A). In some animals, we injected DiI dye into the PrL 3 wk before they were killed for brain-slice preparation. As shown in Fig. 4A, fibers labeled with DiI were found running through the NAc. More importantly, DiI-labeled terminal-like structures were found to surround fluorescent soma and nonfluorescent soma in the core region of the NAc, suggesting that the PrL sends projections to D2-MSNs and putative D1-MSNs in the NAc. To confirm the functional integrity of these projections in our preparation, we made whole-cell patch-clamp recordings from visually identified D2-MSNs in the brain slice (Fig. 4B). Nonfluorescent putative D1-MSNs were also recorded as a comparison. As expected, excitatory postsynaptic currents (EPSCs) could be evoked in these neurons following focal electrical stimulation in the PrL. The distributions of the effective stimulation and the recording sites are summarized in Fig. S4A. A typical example of EPSCs recorded from a D2-MSN is shown in Fig. 4C in which paired stimuli in the PrL elicited robust EPSCs with constant latency. In four D2-MSNs tested, the evoked EPSCs (eEPSCs) could be largely blocked by cyanquixaline (CNQX) (20 µM), indicating that the eEPSCs were mediated by AMPA receptors (Fig. 4D). The current–voltage relationship of the eEPSC as shown in Fig. 4E indicated that Na+ is the main ion mediating the synaptic current. Among our samples, 81.0% of D2-MSNs and 88.0% of putative D1-MSNs in core subregion responded to PrL stimulation. D2-MSNs and putative D1-MSNs had similar eEPSC amplitude, latency, and paired-pulse ratio (Fig. S4B). The fact that the evoked synaptic event was monosynaptic rather than multisynaptic in nature was demonstrated by experiments based on optogenetic stimulation of PrL terminals in NAc expressing ChR2 (Fig. S1B). Under this condition, the application of blue light could directly evoke optical EPSCs (oEPSCs) in D2-MSNs. While perfusion of TTX abolished oEPSCs, the addition of the K-channel blocker 4-aminopyridine (4-AP) could largely restore them (Fig. 4F). Together, these results confirmed that an excitatory glutamatergic projection is sent from the PrL to innervate NAc D2-MSNs directly.
Fig. 4.
The PrL sends glutamatergic projections to D2-MSNs in the NAc core subregion. (A, Left) The PrL and the NAc could be clearly identified under the microscope in the in vitro brain-slice preparation. (Right) In brain slices prepared from Drd2-EGFP BAC transgenic mice injected with DiI into the PrL 3 wk before mice were killed, DiI-labeled fibers were found running through the NAc core and surrounding fluorescent somata representing D2-MSNs (Inset). (B) Whole-cell recordings were made from identified D2-MSNs while focal stimulation was delivered in the PrL. (C and D) A typical example showing EPSCs in response to paired stimuli (C), which could be blocked by superfusion of the AMPA receptor blocker CNQX (D); n = 4. The response to a 5-mV current injection pulse was recorded to monitor the serial resistance. (E) Current–voltage plot of eEPSCs (n = 4). (F) oEPSCs recorded in an NAc neuron with optical stimulation of ChR2-expressed prelimbic terminals in the NAc were completely abolished by TTX (1 µM) (Left) and were rescued by further 4-AP (100 µM) perfusion (Right). This phenomenon was observed consistently in eight NAc neurons. Data are presented as mean ± SEM.
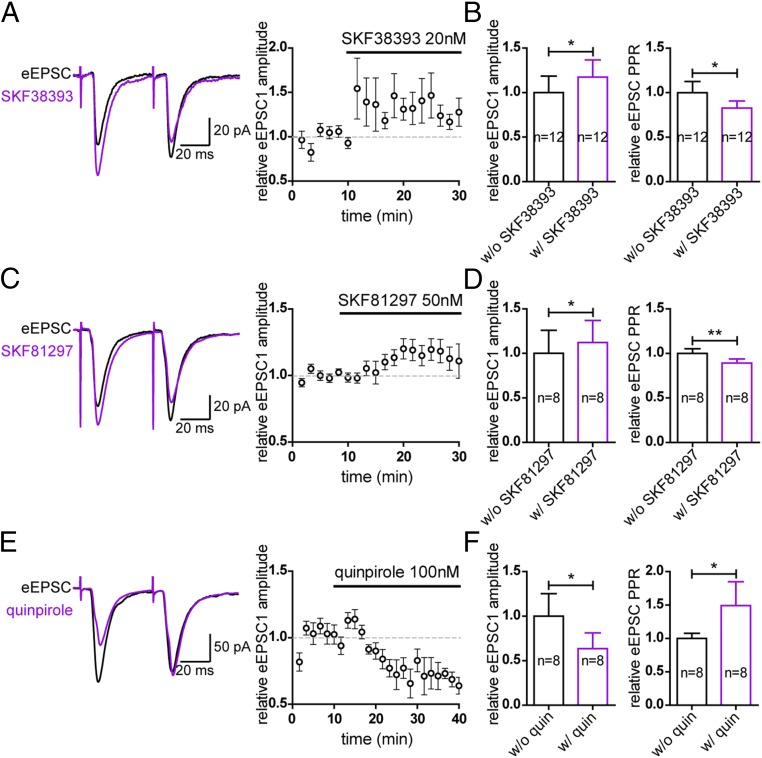
Presynaptic D1-Type Receptors and D2-Type Receptors Respectively Facilitate and Suppress PrL–NAc D2-MSN Transmission.
To investigate the potential modulatory role of dopamine and its receptors on D2 MSN-associated PrL–NAc projections, in our brain-slice preparation we examined the effects of specific D1-type and D2-type receptor agonists on membrane excitability as well as synaptic transmission. In these neurons, despite the expression of D2 receptors, the D2-type receptor agonist quinpirole (100 nM) did not induce a consistent change in membrane current. Thus, the membrane excitability was not acutely modulated by the postsynaptic D2-type receptors, at least not with 100 nM quinpirole. To study the effects on the synaptic transmission, paired electrical stimulation with a 50-ms interval was delivered to the PrL. The amplitudes of the eEPSCs recorded from D2-MSNs were measured, and the paired-pulse ratios (PPRs) were also determined. The changes in PPR are inversely correlated with the changes in synaptic release probability of presynaptic terminals and thus can indicate if there is presynaptic modulation and in what direction (38, 39). We found that 20 nM of the D1-type agonist SKF38393 induced an increase in normalized eEPSC amplitude (without SKF38393: 1.00 ± 0.18; with SKF38393: 1.18 ± 0.19; n = 12, P < 0.05) (Fig. 5 A and B) with a concomitant decrease in normalized PPR (without SKF38393: 1.00 ± 0.12; with SKF38393: 0.83 ± 0.08; n = 12, P < 0.05) (Fig. 5 A and B). These changes suggest that presynaptic D1-type receptors are involved in enhancing D2 MSN-associated PrL–NAc transmission. The full D1-type receptor agonist SKF81297 (50 nM) also exerted a similar effect on normalized eEPSC amplitude (without SKF81297: 1.00 ± 0.26; with SKF81297: 1.12 ± 0.25; n = 8, P < 0.05) (Fig. 5 C and D) and normalized PPR (without SKF81297: 1.00 ± 0.05; with SKF81297: 0.89 ± 0.05; n = 8, P < 0.01) (Fig. 5 C and D). Intriguingly, the D2-type receptor agonist quinpirole exerted opposite effects on this pathway. As shown in Fig. 5 E and F, quinpirole at 100 nM significantly decreased the normalized eEPSC amplitude (without quinpirole: 1.00 ± 0.25; with quinpirole: 0.63 ± 0.18; n = 8, P < 0.05), which was accompanied by an increase in normalized PPR (without quinpirole: 1.00 ± 0.08; with quinpirole: 1.49 ± 0.35; n = 8, P < 0.05). Consistently, in the oEPSCs, which have been shown to be monosynaptically activated (Fig. 4F), the D1-type and D2-type receptor agonists exerted effects similar to those they exerted in the eEPSCs (Fig. S5). These changes suggest that presynaptic D2-type receptors suppress D2 MSN-associated PrL–NAc transmission. Through reducing excitatory glutamatergic drive, this presynaptic D2-type receptor activation should lead to reduced excitability of D2-MSNs. Together, these results demonstrated that both D1-type receptors and D2-type receptors contribute to the acute modulation of excitatory neurotransmission from the PrL to NAc D2-MSNs. Furthermore, both D1-type and D2-type receptors can be expressed presynaptically, but they modulate the transmission in an opposite manner. Since activation of presynaptic D1-type receptors, but not D2-type receptors, can enhance neurotransmission from the PrL to NAc D2-MSNs, dopamine release from a VTA projection targeting these receptors may play a crucial role in facilitating task-switching ability.
Fig. 5.
Presynaptic D1-type and D2-type receptors modulate PrL–NAc transmission in an opposite manner. (A) Paired stimuli with a 50-ms interval delivered to the PrL eEPSCs recorded from identified D2-MSNs. Application of the D1-type receptor partial agonist SKF38393 increased the amplitude of the eEPSCs while decreasing the PPR. (Left) A typical example. (Right) The pooled time course of the effect of SKF38393 on the eEPSC amplitude. (B) Summary of the population changes in amplitude and PPR. (C and D) The full D1-type receptor agonist SKF81297 exerted an effect similar to that of SKF38393 on the eEPSCs. (E and F) In contrast, superfusion of the D2-type receptor agonist quinpirole decreased the amplitude of eEPSCs with a concomitant increase in the PPR. *P < 0.05; **P < 0.01, paired t test. Data are presented as mean ± SEM.
Opposite Roles of NAc D1-Type Receptors and D2-Type Receptors in Modulating Task-Switching Flexibility.
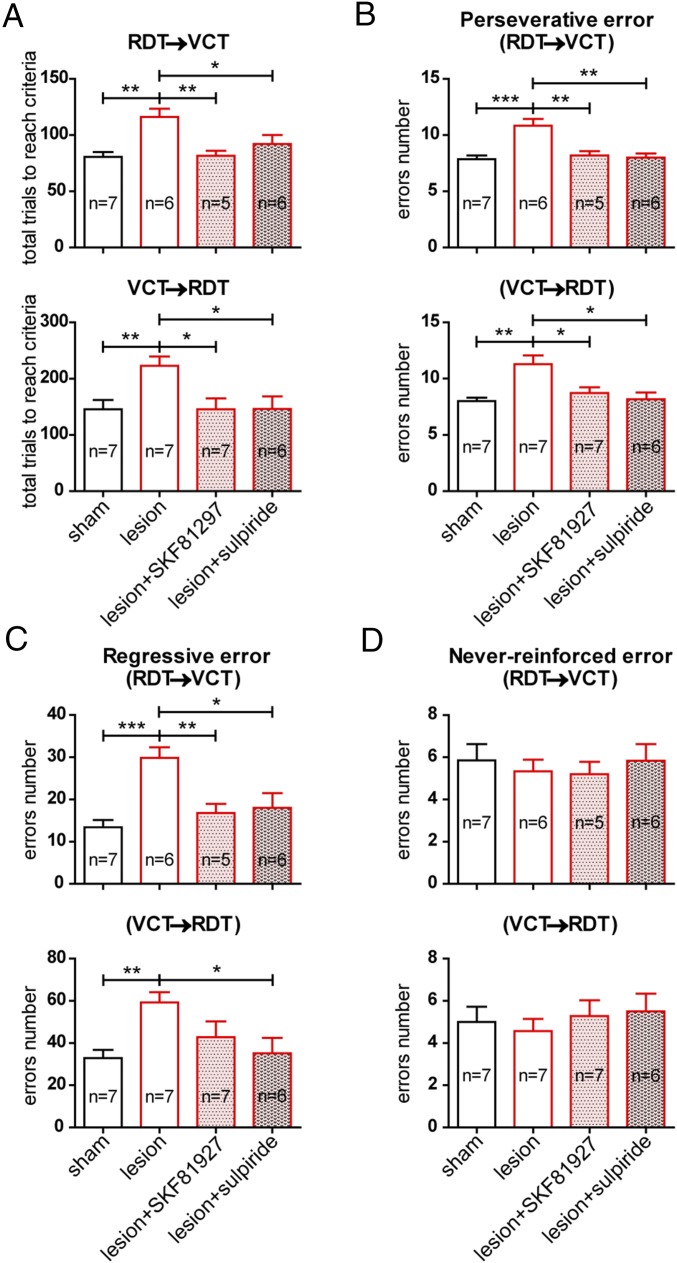
The role of the PrL projection onto NAc D2-MSNs in mediating task-switching ability is supported by pharmacological evidence. In view of the facilitating action of D1-type receptors and the suppressing action of D2-type receptors on this pathway, we speculated that activation of the D1-type receptors or blockade of the D2-type receptors is beneficial in alleviating the cognitive impairment induced by dopamine depletion. To test this hypothesis, we implanted an infusion cannula into the NAc core in dopamine-depleted mice (Materials and Methods) and infused the D1-type receptor agonist SKF81297 or the D2-type receptor antagonist sulpiride into the NAc 10 min before behavioral training. Compared with the lesion group, the pharmacological infusion did not affect the acquisition of the RDT and VCT tasks per se (Fig. S3A) but restored the task-switching performance in both RDT-to-VCT switching tasks (total number of trials needed to reach the criterion: sham, 80.6 ± 4.3, n = 7; lesion, 116.0 ± 7.4, n = 6; lesion+SKF81297, 81.6 ± 4.5, n = 5; lesion+sulpiride, 92.0 ± 8.0, n = 6; P < 0.01 between lesion and lesion+SKF81297; P < 0.05 between lesion and lesion+sulpiride; P > 0.05 between sham and lesion+SKF81297 or lesion+sulpiride) (Fig. 6A) and VCT-to-RDT switching tasks (total number of trials needed to reach the criterion: sham, 145.7 ± 16.4, n = 7; lesion, 222.9 ± 16.5, n = 7; lesion+ SKF81297, 145.7 ± 19.3, n = 7; lesion+sulpiride, 146.0 ± 22.7, n = 6; P < 0.05 between lesion and lesion+SKF81297 or lesion+sulpiride; P > 0.05 between sham and lesion+SKF81297 or lesion+sulpiride) (Fig. 6A). Again, the improvements in task-switching performance were accompanied by parallel reduced perseverative errors (Fig. 6B) and regressive errors (Fig. 6C) but not never-reinforced errors (Fig. 6D), indicating the beneficial effect of pharmacological treatments on restoring task-switching ability.
Fig. 6.
D1-type receptor activation and D2-type receptor suppression ameliorate dopamine depletion-induced task-switching inflexibility. (A) In both the RDT–VCT and VCT–RDT switching paradigms, pretreatment with the D1-type agonist SKF81297 or the D2-type antagonist sulpiride restored the increased total number of trials needed to reach the criterion caused by dopamine lesion in the NAc. (B–D) The effects of the drugs are also reflected in the number of perseverative errors (B) and regressive errors (C) committed but not in the number of never-reinforced errors (D). In A–D, sham and lesion groups as shown in Fig. 3 E–H are included here for comparisons. *P < 0.05; **P < 0.01; ***P < 0.001, unpaired t test. Data are presented as mean ± SEM.
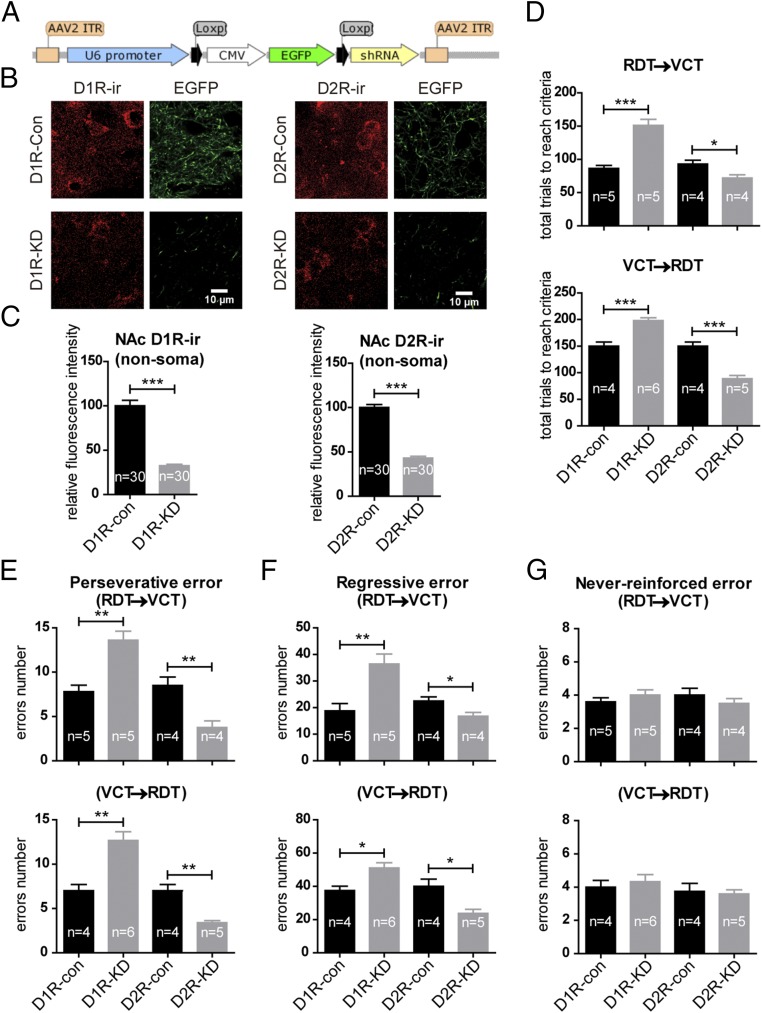
To further support the roles of presynaptic dopamine receptors of the PrL–NAc pathway in task-switching ability, we adopted a strategy based on the viral knockdown of these receptors. The strategy for the production of the specific viruses (cre-dependent D1R-AAV-shRNA and D2R-AAV-shRNA) is summarized in Fig. S6 and Fig. 7A. The PrL was infected with AAV-hSyn-iCre virus injected stereotaxically for 10 d followed by injection of D1R-AAV-shRNA or D2R-AAV-shRNA at the same site; control mice received injections of AAV-shRNAs without AAV-hSyn-iCre virus. After 21 d of recovery, this strategy was validated to suppress the expression of D1 and D2 receptors by the PrL neurons, including the presynaptic receptors at their projection terminals in the NAc (Fig. 7 B and C). The other injected animals were tested for their ability in initial learning as well as in task switching. We found that animals with knockdown of either dopamine receptor learned the tasks at the same pace as control animals (Fig. S3B). However, in task-switching tests, animals with D1R knockdown required more trials to reach the criterion due to more perseverative and regressive errors (Fig. 7 D–G). In contrast, the mice with D2R knockdown committed fewer of these types of errors and therefore needed fewer total trials to reach the criterion (Fig. 7 D–G).
Fig. 7.
Knockdown of D1 receptors and D2 receptors in the PrL respectively impairs and improves task-switching flexibility. (A) Schematic of the construction of cre-dependent shRNA. (B) Typical images showing that 3 wk after AAV-shRNA injection (control) or AAV-shRNA injection in combination with prior AAV-hSyn-Cre injection (knockdown), D1R immunoreactivity (D1R-ir) or D2R immunoreactivity (D1R-ir) in the NAc was reduced in D1R-knockdown (D1R-KD) or D2R-knockdown (D2R-KD) groups, respectively, compared with the corresponding control (Con) groups. These reductions were in parallel with decreased EGFP fibers in the NAc due to the excision of the EGFP reporter by cre-recombinase present in the knockdown groups. (C) Quantification of the fluorescence intensity of D1R immunoreactivity or D2R immunoreactivity from the NAc nonsoma area revealed a significant reduction in the D1R-knockdown or D2R-knockdown groups compared with the respective controls. Each group was quantified from 30 randomly chosen regions of interest from two mice. (D) In both the RDT–VCT and the VCT–RDT switching paradigms, mice with D1R knockdown and D2R knockdown in PrL neurons respectively increased and decreased the total number of trials needed to reach the criterion compared with the respective controls. (E and F) In these animals, the number of perseverative errors (E) and regressive errors (F) committed changed in parallel with the total number of trials needed to reach the criterion. (G) Never-reinforced errors were not altered following the knockdown of either receptor. *P < 0.05; **P < 0.01; ***P < 0.001, unpaired t test. Data are presented as mean ± SEM.
Discussion
The involvement of the prefrontal cortex, including its subregions, and also the subcortical NAc in strategy-switching flexibility has been pursued and confirmed in separate studies (4, 5, 22, 36, 40, 41). It is known that the prefrontal cortex is connected with the NAc. Trials using pharmacological inactivation and disconnection lesions further suggested that the prefrontal cortex connections with the mediodorsal nuclei of the thalamus and NAc core mediate strategy switching by inhibiting perseveration (42). Here we demonstrated optogenetically that activating or inhibiting the terminals of neurons of the PrL in the NAc core could respectively enhance or suppress strategy-switching flexibility. Thus, our results extend the previous understanding of the role of prefrontal cortex–subcortical area connections, and in particular the contribution of the PrL–NAc pathway, in the expression of cognitive flexibility.
At the same time, accumulating evidence has long supported a critical control by dopamine of strategy-switching flexibility. In the prefrontal cortex or its subregion, the infusion of dopamine receptor agonists or antagonists differentially alters strategy-switching flexibility (23, 24). The dopaminergic innervation of the prefrontal cortex arises from the VTA that also sends projections to the NAc. In the present study, we observed that local depletion of dopamine in the NAc induced an impairment in switching different strategies (egocentric response-based vs. visual cue-based) to get a food reward, demonstrating the causal role of NAc dopamine on this type of cognitive flexibility. Thus, the interconnections among the VTA, prefrontal cortex, and the NAc together constitute an important circuitry that mediates this aspect of behavioral flexibility. Indeed, in diseases such as ADHD, schizophrenia, and early Parkinson’s disease that are accompanied by impaired behavior flexibility (1–3, 43, 44), changes in the dopamine system (e.g., dopamine and its transporters and receptors) in the NAc are often detected or suggested (45–52).
By taking advantage of the Drd2-EGFP BAC transgenic mice, we further dissected the circuitry between the prefrontal cortex and ventral striatum by confirming that both D2-MSNs and putative D1-MSNs in the NAc receive input from the PrL, as this notion has remained inconclusive from previous studies (53). While the role of innervation from the PrL to D1-MSNs is currently unknown, we provide evidence from electrophysiological and optogenetic approaches in brain slices that the glutamatergic connection to D2-MSNs is under tight regulation by presynaptic D1-type and D2-type receptors, which enhance and suppresses the pathway, respectively. Although contributions by other dopamine receptors, including postsynaptic dopamine receptors on NAc MSNs (22), could not be excluded, observations from our in vivo and in vitro experiments together implicate key roles of these presynaptic dopamine receptors in the NAc in mediating task-switching flexibility. Thus, local dopamine depletion demonstrated not only the importance of an intact dopamine tone in this region but also that this deficit could be restored by the local administration of a D1-type agonist and a D2-type antagonist, as is consistent with their facilitating actions determined in in vitro experiments. On the other hand, although knockdown of D1 and D2 receptors via the AAV-shRNAs is not confined to the terminal regions of the PrL–NAc pathway, the fact that these manipulations resulted in suppression and facilitation of task-switching ability similar to that observed after administration of a D1-type agonist and a D2-type antagonist into the NAc is also consistent with the roles of the presynaptic dopamine receptors located in NAc.
It has been argued that cross-modal shift facilitated by the prelimbic–infralimbic cortex represents higher-order processing compared with intramodel shift because a new strategy is required to solve a task (9). We have shown in our study that the PrL–NAc projection, probably to the D2-MSNs, is critical in facilitating the abandoning of the old strategy but has minimal role in the learning of a new strategy. This notion is supported by the observation that the deficit in behavioral flexibility could be contributed by perseverative errors and regressive errors (23, 39), both implying a choice associated with the previously acquired but now incorrect or inefficient strategy. The key difference in the two types of errors is when the errors occur in the choice sequence. Perseverative errors are responses in which a previously reinforced strategy continues to be used despite a switch in the category rule and termination of positive feedback. Regressive errors are trials in which mice identify the newly reinforced response choice but then are unable to maintain this new response set and instead revert to the previously reinforced strategy. On the other hand, the never-reinforced errors, which can be interpreted as an index of how quickly animals are able to parse out an ineffective strategy and explore new response set, were unaffected in all manipulations in the present study. Our results are in line with a previous finding by Floresco et al. (17) that functional inactivation of NAc core neurons by the infusion of GABA agonists did not impair initial learning of strategies but disrupted the ability to shift to a different strategy. However, in their study, regressive errors, but not perseverative errors, were impaired after NAc inactivation, which may reflect the consequence of a different interventional strategy. Combined together, these findings strongly indicate that the neuroplasticity process underlying the learning of a new task is a function distinct from that of task switching and probably is mediated by a different mechanism or circuit. Similar conclusions about the dissociation between the learning of a strategy and strategy switching have also been indicated in studies of the rodent medial prefrontal cortex (10, 11) and the primate lateral prefrontal cortex (54). However, we point out the lack of impact of optogenetic manipulation of prelimbic input on learning could also be due to the methodology in our study, i.e., unilateral rather than bilateral manipulations. On the other hand, it is possible that the activation of opsins during the learning phase could have a carryover effect on the strategy-switching phase, although we gave a 1-h break between the two phases. Other than methodology concerns, it is of particular interest to ascertain the specific functional neural circuitries that facilitate different components of strategy switching.
In conclusion, we demonstrated the critical involvement of PrL–NAc projections, in particular to D2-MSNs, and presynaptic dopamine receptors of this synapse in strategy-switching flexibility. Interestingly, perseverative types of errors are committed more by human patients with ADHD or schizophrenia who show deficits in cognitive flexibility (3, 55). Findings in this study may contribute to the development of novel therapeutic strategies for the impaired behavioral flexibility observed in ADHD, schizophrenia, and early Parkinson’s disease patients.
Materials and Methods
Animals, chemicals, stereotaxic surgery for in vivo studies, optogenetic stimulation, in vivo drug infusion, local dopamine denervation, immunohistochemical studies, brain-slice preparation, whole-cell recording, and the construction of AAV-shRNAs are described in SI Materials and Methods. All animals were handled in strict accordance with the guidelines by The Chinese University of Hong Kong on animal ethics. All animal procedures were approved by the Chinese University of Hong Kong Animal Experimentation Ethics Committee.
Strategy Acquisition and Switching.
Strategy acquisition and switching in goal-directed behavior were tested with an RDT and a VCT and using a custom-built four-arm cross-maze, as described in other studies (22, 56). The four-arm cross-maze was made of a clear plastic wall with a gray floor and placed 90 cm above the floor of the room. Each arm was 25 cm long and 5 cm wide, and the center platform was 5 × 5 cm. The position of a mouse was detected by a video camera (C615; Logitech) suspended over the maze and was analyzed by the Any-Maze software (version 4.70; Stoelting Co.).
Habituation and turn bias.
Animals were food-restricted to maintain about 85% of the original ad libitum weight from the beginning of behavioral task, which was started with habituation. The complete test consisted of several components including habituation, turn bias, RDT, VCT, and their switching. For animal treated with 6-OHDA injection, habituation training started 1 wk after surgery. Before each day’s habituation, mice were handled for 10 min. On the first habituation phase, three reward pellets were placed in each of the arms of the cross-maze (two in the food well at the arm end and one down the length of the arm). The mice were allowed to navigate freely and consume the food pellets for 15 min. If a mouse consumed all 12 pellets within 15 min, it was removed from the maze and placed in the holding cage. After the maze was rebaited with eight additional pellets in the food well at the arm end, the mouse was placed back in the center of the maze and was allowed to consume all the pellets. On the second habituation phase, the procedure was almost the same, except that whenever the mouse traversed the entire length of an arm and consumed the two food pellets in the food well, it was picked up and placed at the entrance of a different arm, habituating the animal to repeat handling after consuming the food reward. On the third habituation phase, each arm was baited with only two pellets in the food well. A piece of black-and-white striped cardboard (10 cm wide × 50 cm long × 0.5 cm thick, as a visual cue) was placed outside and adjacent to one arm. After consuming all eight pellets, the mouse was placed in the holding cage, the visual cue was moved to a different arm, and the food was rebaited, until the mouse had consumed eight food pellets four times within 15 min. All mice finished the habituation training within 10 d (averaged 5 d, range 3–10 d).
Immediately after maze habituation, the turn bias was assessed in a T-maze (blocking one arm’s entry). No food was provided in this procedure. Mice were put in the stem arm and could turn 90° left or right when entering the center platform. After choosing an arm and reaching its end, the mouse was picked up, placed in the stem arm, and allowed to make the next choice. The direction being turned four or more times over seven trials was considered the mouse’s turn bias.
RDT and VCT.
In the RDT, mice were required always to turn in the opposite direction of their turn bias to receive a food pellet, regardless of the location of the visual cue. In the VCT, mice were trained to enter the arm indicated by the visual cue. Twelve consecutive trials were set as one session. The starting arm for each trial and the position of the visual cue were determined pseudorandomly such that it occurred in each arm with equal frequency for every consecutive set of 12 trials. Each task continued until the mouse reached the acquisition criterion of more than 10 correct choices in two consecutive sessions. Accuracy was calculated as the percentage of correct choices per session, and the total number of trials needed to reach the criterion was also recorded.
RDT–VCT shifting.
After successful acquisition of RDT task, the mouse was placed back in the holding cage for 1 h and then was shifted to the VCT. Errors committed during the set shift determined the animal’s ability to abandon a previously learned strategy and acquire a new one. Perseverative errors were defined as the accumulated number of egocentric errors when a mouse entered the incorrect arm on three or more trials per block of four trials that required it to enter the arm indicated by the visual cue, which was always opposite to the turning direction required in the previous RDT. After the first time a mouse made fewer than three perseverative errors in a block, subsequent errors were no longer counted as perseverative errors because at this point the mouse started to choose an alternative strategy at least half of the time. Instead, the stem arm was randomly selected in the following trials, and the subsequent errors following the previous RDT rule were counted as regressive errors. The third type of error, never-reinforced errors, was scored when a mouse entered the incorrect arm during trials in which the visual cue was placed in the same arm as previous RDT.
VCT–RDT shifting.
Animals for this set of experiment were initially trained on the VCT, followed by testing on the RDT after a 1-h rest in the holding cage. All other aspects were as described above, and the three subtypes of errors were evaluated in the same way.
Data Analysis and Statistics.
Imaging data were analyzed using ImageJ (NIH). Patch-clamp recording data were analyzed using Clampfit 10.2 (Molecular Devices). GraphPad Prism 7 was used for performing statistics and graphically depicting the population data. Normal distributions were assumed for all datasets in the present study. Population data in the main text are presented as mean ± SEM. Error bars in the figures represented the SEM. An unpaired t test was performed on comparisons of two groups of independent samples, and a paired t test was used for comparing paired data. One-way ANOVA was used to compare multiple independent groups. Statistical significance was preset at P < 0.05.
Supplementary Material
Acknowledgments
We thank Leo Yan, Curtis Wong, Howard Chan, and Ada Fu for their assistance in some of the experiments and Prof. Savio Chan of the Feinberg School of Medicine, Northwestern University for generous support of this study. This work was supported by Hong Kong Research Grants Council (HKRGC)-General Research Fund Grants 14111715 (to W.-H.Y. and Y.K.) and 14107616 (to Y.K. and W.-H.Y.), HKRGC-Collaborative Research Fund Grants C6003-14G (to N.Y.I. and W.-H.Y.) and C2012-15G (to Y.K.), and Area of Excellence Grant AoE/M-604/16 (to N.Y.I. and W.-H.Y.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1717106115/-/DCSupplemental.
References
- 1.Pantelis C, et al. Comparison of set-shifting ability in patients with chronic schizophrenia and frontal lobe damage. Schizophr Res. 1999;37:251–270. doi: 10.1016/s0920-9964(98)00156-x. [DOI] [PubMed] [Google Scholar]
- 2.Cools R, Barker RA, Sahakian BJ, Robbins TW. Mechanisms of cognitive set flexibility in Parkinson’s disease. Brain. 2001;124:2503–2512. doi: 10.1093/brain/124.12.2503. [DOI] [PubMed] [Google Scholar]
- 3.Reeve WV, Schandler SL. Frontal lobe functioning in adolescents with attention deficit hyperactivity disorder. Adolescence. 2001;36:749–765. [PubMed] [Google Scholar]
- 4.Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann N Y Acad Sci. 2007;1121:355–375. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- 5.Ragozzino ME, Kim J, Hassert D, Minniti N, Kiang C. The contribution of the rat prelimbic-infralimbic areas to different forms of task switching. Behav Neurosci. 2003;117:1054–1065. doi: 10.1037/0735-7044.117.5.1054. [DOI] [PubMed] [Google Scholar]
- 6.Rowe JB, Toni I, Josephs O, Frackowiak RS, Passingham RE. The prefrontal cortex: Response selection or maintenance within working memory? Science. 2000;288:1656–1660. doi: 10.1126/science.288.5471.1656. [DOI] [PubMed] [Google Scholar]
- 7.Surmeier DJ. Dopamine and working memory mechanisms in prefrontal cortex. J Physiol. 2007;581:885. doi: 10.1113/jphysiol.2007.134502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seniów J. Executive dysfunctions and frontal syndromes. Front Neurol Neurosci. 2012;30:50–53. doi: 10.1159/000333407. [DOI] [PubMed] [Google Scholar]
- 9.Wise SP, Murray EA, Gerfen CR. The frontal cortex-basal ganglia system in primates. Crit Rev Neurobiol. 1996;10:317–356. doi: 10.1615/critrevneurobiol.v10.i3-4.30. [DOI] [PubMed] [Google Scholar]
- 10.Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci. 1999;19:4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dias R, Aggleton JP. Effects of selective excitotoxic prefrontal lesions on acquisition of nonmatching- and matching-to-place in the T-maze in the rat: Differential involvement of the prelimbic-infralimbic and anterior cingulate cortices in providing behavioural flexibility. Eur J Neurosci. 2000;12:4457–4466. doi: 10.1046/j.0953-816x.2000.01323.x. [DOI] [PubMed] [Google Scholar]
- 13.Kesner RP, Ragozzino ME. The role of the prefrontal cortex in object-place learning: A test of the attribute specificity model. Behav Brain Res. 2003;146:159–165. doi: 10.1016/j.bbr.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 14.Gorelova N, Yang CR. The course of neural projection from the prefrontal cortex to the nucleus accumbens in the rat. Neuroscience. 1997;76:689–706. doi: 10.1016/s0306-4522(96)00380-6. [DOI] [PubMed] [Google Scholar]
- 15.Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Groenewegen HJ, Trimble M. The ventral striatum as an interface between the limbic and motor systems. CNS Spectr. 2007;12:887–892. doi: 10.1017/s1092852900015650. [DOI] [PubMed] [Google Scholar]
- 17.Floresco SB, Ghods-Sharifi S, Vexelman C, Magyar O. Dissociable roles for the nucleus accumbens core and shell in regulating set shifting. J Neurosci. 2006;26:2449–2457. doi: 10.1523/JNEUROSCI.4431-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Z, et al. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50:443–452. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Robertson GS, Jian M. D1 and D2 dopamine receptors differentially increase Fos-like immunoreactivity in accumbal projections to the ventral pallidum and midbrain. Neuroscience. 1995;64:1019–1034. doi: 10.1016/0306-4522(94)00426-6. [DOI] [PubMed] [Google Scholar]
- 20.Lu XY, Ghasemzadeh MB, Kalivas PW. Expression of D1 receptor, D2 receptor, substance P and enkephalin messenger RNAs in the neurons projecting from the nucleus accumbens. Neuroscience. 1998;82:767–780. doi: 10.1016/s0306-4522(97)00327-8. [DOI] [PubMed] [Google Scholar]
- 21.Kupchik YM, et al. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat Neurosci. 2015;18:1230–1232. doi: 10.1038/nn.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yawata S, Yamaguchi T, Danjo T, Hikida T, Nakanishi S. Pathway-specific control of reward learning and its flexibility via selective dopamine receptors in the nucleus accumbens. Proc Natl Acad Sci USA. 2012;109:12764–12769. doi: 10.1073/pnas.1210797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ragozzino ME. The effects of dopamine D(1) receptor blockade in the prelimbic-infralimbic areas on behavioral flexibility. Learn Mem. 2002;9:18–28. doi: 10.1101/lm.45802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MT. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology. 2006;31:297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- 25.Kehagia AA, Murray GK, Robbins TW. Learning and cognitive flexibility: Frontostriatal function and monoaminergic modulation. Curr Opin Neurobiol. 2010;20:199–204. doi: 10.1016/j.conb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Parkinson JA, et al. Nucleus accumbens dopamine depletion impairs both acquisition and performance of appetitive Pavlovian approach behaviour: Implications for mesoaccumbens dopamine function. Behav Brain Res. 2002;137:149–163. doi: 10.1016/s0166-4328(02)00291-7. [DOI] [PubMed] [Google Scholar]
- 27.Tran AH, et al. Dopamine D1 receptors involved in locomotor activity and accumbens neural responses to prediction of reward associated with place. Proc Natl Acad Sci USA. 2005;102:2117–2122. doi: 10.1073/pnas.0409726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phillips AG, Vacca G, Ahn S. A top-down perspective on dopamine, motivation and memory. Pharmacol Biochem Behav. 2008;90:236–249. doi: 10.1016/j.pbb.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Taghzouti K, Simon H, Louilot A, Herman JP, Le Moal M. Behavioral study after local injection of 6-hydroxydopamine into the nucleus accumbens in the rat. Brain Res. 1985;344:9–20. doi: 10.1016/0006-8993(85)91184-9. [DOI] [PubMed] [Google Scholar]
- 30.Dalley JW, et al. Nucleus accumbens dopamine and discriminated approach learning: Interactive effects of 6-hydroxydopamine lesions and systemic apomorphine administration. Psychopharmacology (Berl) 2002;161:425–433. doi: 10.1007/s00213-002-1078-2. [DOI] [PubMed] [Google Scholar]
- 31.Haluk DM, Floresco SB. Ventral striatal dopamine modulation of different forms of behavioral flexibility. Neuropsychopharmacology. 2009;34:2041–2052. doi: 10.1038/npp.2009.21. [DOI] [PubMed] [Google Scholar]
- 32.O’Donnell P, Grace AA. Tonic D2-mediated attenuation of cortical excitation in nucleus accumbens neurons recorded in vitro. Brain Res. 1994;634:105–112. doi: 10.1016/0006-8993(94)90263-1. [DOI] [PubMed] [Google Scholar]
- 33.Harvey J, Lacey MG. Endogenous and exogenous dopamine depress EPSCs in rat nucleus accumbens in vitro via D1 receptors activation. J Physiol. 1996;492:143–154. doi: 10.1113/jphysiol.1996.sp021296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicola SM, Kombian SB, Malenka RC. Psychostimulants depress excitatory synaptic transmission in the nucleus accumbens via presynaptic D1-like dopamine receptors. J Neurosci. 1996;16:1591–1604. doi: 10.1523/JNEUROSCI.16-05-01591.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci. 2005;8:805–812. doi: 10.1038/nn1471. [DOI] [PubMed] [Google Scholar]
- 36.Macpherson T, et al. Nucleus accumbens dopamine D2-receptor expressing neurons control behavioral flexibility in a place discrimination task in the IntelliCage. Learn Mem. 2016;23:359–364. doi: 10.1101/lm.042507.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan CS, et al. Strain-specific regulation of striatal phenotype in Drd2-eGFP BAC transgenic mice. J Neurosci. 2012;32:9124–9132. doi: 10.1523/JNEUROSCI.0229-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 39.Branco T, Staras K. The probability of neurotransmitter release: Variability and feedback control at single synapses. Nat Rev Neurosci. 2009;10:373–383. doi: 10.1038/nrn2634. [DOI] [PubMed] [Google Scholar]
- 40.Oualian C, Gisquet-Verrier P. The differential involvement of the prelimbic and infralimbic cortices in response conflict affects behavioral flexibility in rats trained in a new automated strategy-switching task. Learn Mem. 2010;17:654–668. doi: 10.1101/lm.1858010. [DOI] [PubMed] [Google Scholar]
- 41.Nakanishi S, Hikida T, Yawata S. Distinct dopaminergic control of the direct and indirect pathways in reward-based and avoidance learning behaviors. Neuroscience. 2014;282:49–59. doi: 10.1016/j.neuroscience.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 42.Block AE, Dhanji H, Thompson-Tardif SF, Floresco SB. Thalamic-prefrontal cortical-ventral striatal circuitry mediates dissociable components of strategy set shifting. Cereb Cortex. 2007;17:1625–1636. doi: 10.1093/cercor/bhl073. [DOI] [PubMed] [Google Scholar]
- 43.Thoma P, Wiebel B, Daum I. Response inhibition and cognitive flexibility in schizophrenia with and without comorbid substance use disorder. Schizophr Res. 2007;92:168–180. doi: 10.1016/j.schres.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 44.Skogli EW, Egeland J, Andersen PN, Hovik KT, Øie M. Few differences in hot and cold executive functions in children and adolescents with combined and inattentive subtypes of ADHD. Child Neuropsychol. 2014;20:162–181. doi: 10.1080/09297049.2012.753998. [DOI] [PubMed] [Google Scholar]
- 45.Farley IJ, Price KS, Hornykiewicz O. Dopamine in thelimbic regions of the human brain: Normal and abnormal. Adv Biochem Psychopharmacol. 1977;16:57–64. [PubMed] [Google Scholar]
- 46.Owen F, et al. Increased dopamine-receptor sensitivity in schizophrenia. Lancet. 1978;2:223–226. doi: 10.1016/s0140-6736(78)91740-3. [DOI] [PubMed] [Google Scholar]
- 47.Price KS, Farley IJ, Hornykiewicz O. Neurochemistry of Parkinson’s disease: Relation between striatal and limbic dopamine. Adv Biochem Psychopharmacol. 1978;19:293–300. [PubMed] [Google Scholar]
- 48.Mackay AV, et al. Increased brain dopamine and dopamine receptors in schizophrenia. Arch Gen Psychiatry. 1982;39:991–997. doi: 10.1001/archpsyc.1982.04290090001001. [DOI] [PubMed] [Google Scholar]
- 49.Memo M, Kleinman JE, Hanbauer I. Coupling of dopamine D1 recognition sites with adenylate cyclase in nuclei accumbens and caudatus of schizophrenics. Science. 1983;221:1304–1307. doi: 10.1126/science.6310753. [DOI] [PubMed] [Google Scholar]
- 50.Gray JA, et al. The role of mesolimbic dopaminergic and retrohippocampal afferents to the nucleus accumbens in latent inhibition: Implications for schizophrenia. Behav Brain Res. 1995;71:19–31. doi: 10.1016/0166-4328(95)00154-9. [DOI] [PubMed] [Google Scholar]
- 51.Du Y, et al. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. Proc Natl Acad Sci USA. 2001;98:14669–14674. doi: 10.1073/pnas.251341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Volkow ND, et al. Evaluating dopamine reward pathway in ADHD: Clinical implications. JAMA. 2009;302:1084–1091. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wall NR, De La Parra M, Callaway EM, Kreitzer AC. Differential innervation of direct- and indirect-pathway striatal projection neurons. Neuron. 2013;79:347–360. doi: 10.1016/j.neuron.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dias R, Robbins TW, Roberts AC. Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin Card Sort Test: Restriction to novel situations and independence from “on-line” processing. J Neurosci. 1997;17:9285–9297. doi: 10.1523/JNEUROSCI.17-23-09285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schirmbeck F, et al. Stable cognitive deficits in schizophrenia patients with comorbid obsessive-compulsive symptoms: A 12-month longitudinal study. Schizophr Bull. 2013;39:1261–1271. doi: 10.1093/schbul/sbs123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ragozzino ME, Ragozzino KE, Mizumori SJ, Kesner RP. Role of the dorsomedial striatum in behavioral flexibility for response and visual cue discrimination learning. Behav Neurosci. 2002;116:105–115. doi: 10.1037//0735-7044.116.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.