Significance
It has been known for some time that ATM and RAD3-related kinase (ATR) phosphorylates and activates checkpoint kinase 1 (CHK1) in response to DNA damage. However, how CHK1 is targeted to chromatin and how it is released has not been fully deciphered. Previously we demonstrated that CHK1 K63-linked ubiquitination contributes to its chromatin association and activation. The study presented here highlights the involvement of ubiquitin-specific protease 3 (USP3) in removing the K63-linked ubiquitin chain, thus allowing not only the release of CHK1 from chromatin but also the access of its substrates. Together our studies have revealed a stepwise regulation of CHK1 in DNA damage response.
Keywords: CHK1, deubiquitination, genome stability, USP3, DNA damage response
Abstract
Checkpoint kinase 1 (CHK1), a Ser/Thr protein kinase, is modified by the K63-linked ubiquitin chain in response to genotoxic stress, which promotes its nuclear localization, chromatin association, and activation. Interestingly, this bulky modification is linked to a critical residue, K132, at the kinase active site. It is unclear how this modification affects the kinase activity and how it is removed to enable the release of CHK1 from chromatin. Herein, we show that the K63-linked ubiquitin chain at CHK1’s K132 residue has an inhibitory effect on the kinase activity. Furthermore, we demonstrate that this modification can be removed by ubiquitin-specific protease 3 (USP3), a deubiquitinating enzyme that targets K63-linked ubiquitin chains. Wild-type USP3, but not the catalytically defective or nuclear localization sequence-deficient mutants, reduced CHK1 K63-linked ubiquitination. Conversely, USP3 knockdown elevated K63-linked ubiquitination of the kinase, leading to prolonged CHK1 chromatin association and phosphorylation. Paradoxically, by removing the bulky ubiquitin chain at the active site, USP3 also increased the accessibility of CHK1 to its substrates. Thus, our findings on the dual roles of USP3 (namely, one to release CHK1 from the chromatin and the other to open up the active site) provide further insights into the regulation of CHK1 following DNA damage.
Genomic instability is a prominent feature of many human cancers, resulting mostly from oncogene activation or tumor-suppressor inactivation. The DNA damage response (DDR) and cell-cycle checkpoints are evolutionarily conserved cellular mechanisms for safeguarding the genome’s integrity. ATR–CHK1 and ATM–CHK2 are the two main signaling axes in the DDR network (1). Checkpoint kinase 1 (CHK1), a Ser/Thr protein kinase, is activated by ssDNA generated at the stalled replication fork or in the double-strand break-repair process (2, 3). Upon UV exposure or replication stress, CHK1 is activated through phosphorylation of its S317 and S345 residues by the upstream kinase ATR (4). Once activated, CHK1 phosphorylates an array of downstream substrates to modulate various cellular processes after DNA damage (5, 6). For example, CHK1 was shown to maintain cell survival after treatment with hydroxyurea (HU), a ribonucleotide reductase inhibitor (7). In addition, CHK1 functions in the spindle checkpoint to safeguard genome stability, as it was found to phosphorylate Aurora B and promote its activity to enhance the kinetochore localization of budding uninhibited by benzymidazol 1-related (BUBR1) kinase after paclitaxel treatment (8).
In addition to the ATR-mediated phosphorylation of CHK1, other modifications to regulate its stability, activity, and cellular localization have been reported. Camptothecin treatment promotes CHK1 degradation through cullin 1- and cullin 4-dependent polyubiquitination (9). Protein kinase B (AKT) phosphorylation of CHK1 preferentially retains the enzyme in the cytoplasm (10). Recently, we demonstrated that the B cell translocation gene 3 (BTG3) promotes CHK1 K63-linked ubiquitination by the ubiquitin E3 ligase CRL4Cdt2, which contributes to its nuclear localization, chromatin association, and activation to maintain cell survival and the mitotic checkpoint (11). Others have reported that, upon phosphorylation and activation, CHK1 is released from chromatin to phosphorylate its downstream targets (12). However, the exact mechanism underlying its release has not been fully elucidated. On the basis of other reports as well as our own investigation, we reasoned that a deubiquitinase (DUB) may play a role in removing CHK1 K63-linked ubiquitination to modulate the chromatin association and activation of CHK1 after DNA damage.
DUBs such as BRCA1/BRCA2-containing complex subunit 36 (BRCC36), ubiquitin-specific protease 3 (USP3), otubains (OTUB1 and OTUB2), cylindromatosis D lysine 63 deubiquitinase (CYLD), and A20 have been reported to be involved in the deubiquitination of K63-linked ubiquitin chains. For example, BRCC36, USP3, OTUB1, and OTUB2 function in the DDR and DNA-repair pathway (13–16). Furthermore, BRCC36 and USP3 can associate with chromatin and deubiquitinate histone proteins (17, 18). In this study, we demonstrate that USP3 counteracts BTG3-promoted CHK1 K63-linked ubiquitination to regulate CHK1 chromatin association and activation in the maintenance of cell survival and genome stability.
Results
USP3 Negatively Regulates CHK1 Phosphorylation After DNA Damage.
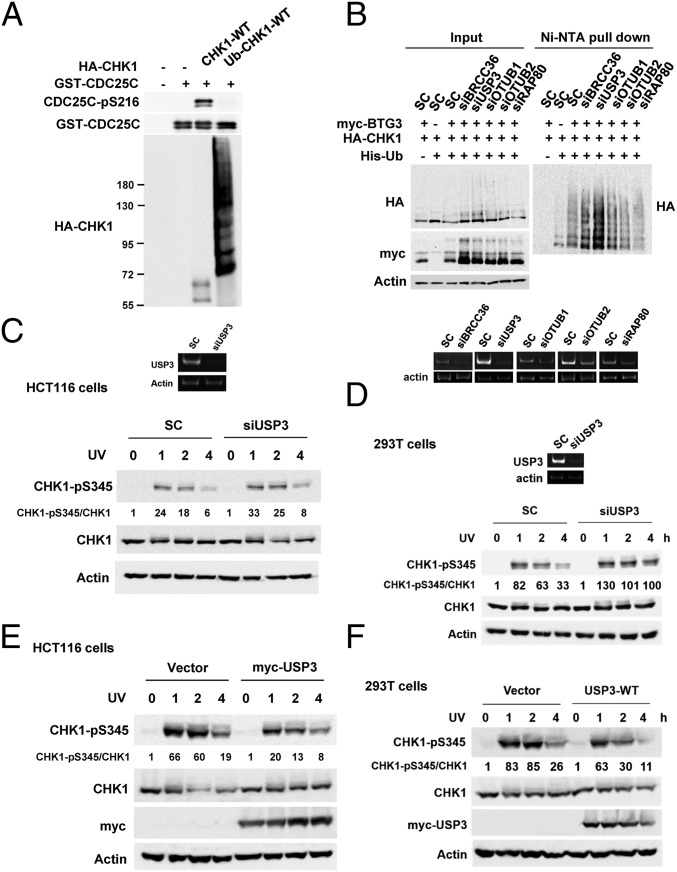
Previously, we had demonstrated that the CRL4Cdt2 E3 ubiquitin ligase complex ubiquitinates CHK1 at the K132 residue, which promotes CHK1 chromatin association (11). As K132 is a critical residue at the kinase catalytic site (19), we postulated that the polyubiquitin chain linked to the residue may render CHK1 inactive by impeding substrate binding. To verify this hypothesis, kinase assays were performed with either unmodified or ubiquitinated CHK1. The results showed that BTG3 promoted CHK1 ubiquitination, but the facilitation of CHK1 chromatin association inadvertently caused inactivation of its kinase activity (Fig. 1A). Thus, the modification would need to be removed to allow full activation of the kinase.
Fig. 1.
USP3 regulates BTG3-promoted CHK1 ubiquitination. (A) The ubiquitinated CHK1 lost its catalytic activity. The in vitro kinase assay was performed with unmodified CHK1 or ubiquitinated CHK1 immunoprecipitated from 293T cells coexpressing or not coexpressing BTG3, using GST-Cdc25C200–256 as the substrate. (B) USP3 down-regulation increased BTG3-promoted CHK1 ubiquitination. RT-PCR was used to confirm the knockdown. SC, scramble control siRNA. (C and D) Down-regulation of USP3 increased UV-induced CHK1 activation (pS345) in HCT116 (C) and 293T (D) cells. (E and F) UV-induced CHK1 S345 phosphorylation was diminished by USP3 overexpression in HCT116 (E) and 293T (F) cells.
To identify which DUB may be involved in the regulation of BTG3-promoted CHK1 ubiquitination, we investigated the effects of depletion of a series of DUBs (BRCC36, USP3, OTUB1, and OTUB2) and an adaptor (RAP80) using in vivo ubiquitination assays (11). Down-regulation of RAP80 did not impact the modification (Fig. 1B), thus excluding the involvement of RAP80-containing DUB complexes such as MERIT40 (20). The results also indicated that down-regulation of USP3, but not of the other DUBs, by siRNA dramatically enhanced BTG3-promoted CHK1 ubiquitination (Fig. 1 B and C), suggesting that USP3 may play a role in CHK1 deubiquitination and activation. Indeed, phosphorylation of CHK1’s S345 residue after UV irradiation was enhanced by USP3 knockdown in HCT116 and 293T cells (Fig. 1 C and D), possibly as a result of increased chromatin association. The result was also confirmed by another USP3 siRNA (siUSP3-4) that targets a different sequence, thus excluding off-target effects (SI Appendix, Fig. S1). By contrast, overexpression of USP3 significantly decreased S345 phosphorylation after UV treatment (Fig. 1 E and F). These results suggest that USP3 plays a role in regulating CHK1 phosphorylation by the upstream kinases after DNA damage, possibly by modulating the chromatin-association process.
USP3 Interacts with CHK1.
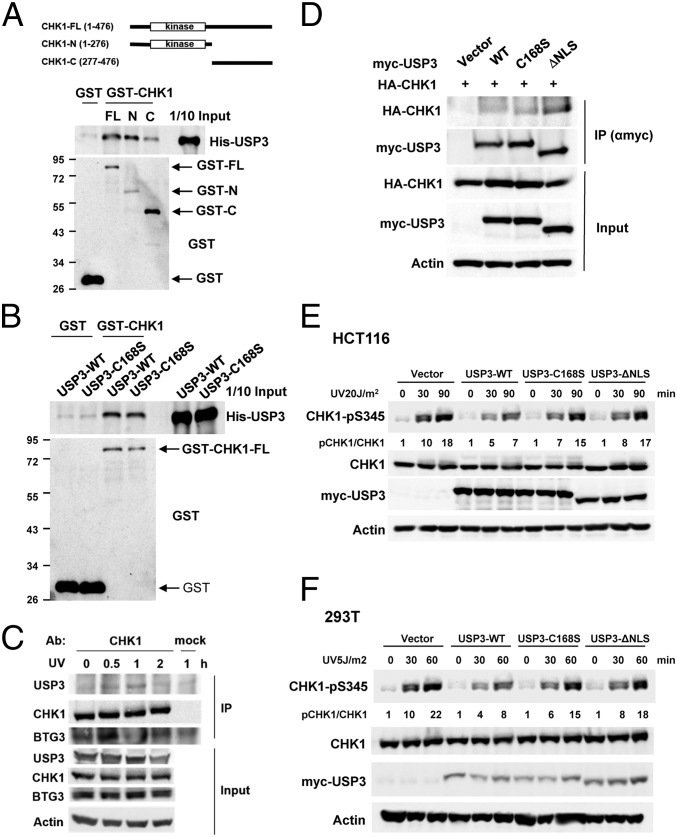
Next, we tested whether there is direct interaction between USP3 and CHK1. GST pull-down assays were performed in vitro with recombinant CHK1 proteins (GST-CHK1-FL-His, GST-CHK1-N-His, and GST-CHK1-C-His) and either recombinant His-USP3 WT or the catalytically defective C168S mutant. The results showed that USP3 binds preferentially to the N-terminal domain of CHK1 (amino acids 1–276), and both WT USP3 and the C168S-mutant bind CHK1-FL in vitro (Fig. 2 A and B). In cells, coimmunoprecipitation of USP3 and CHK1 was observed in UV-irradiated HCT116 cells (Fig. 2C). Notably, CHK1 interacted with USP3 at a later time than with BTG3, suggesting that these events may be sequentially regulated. Furthermore, ectopically expressed USP3 mutants lacking either the catalytic activity (C168S) or the putative nuclear localization sequence (ΔNLS) also coimmunoprecipitated with CHK1 (Fig. 2D). Despite the interaction, the C169S and ΔNLS mutants were impaired in their ability to ameliorate CHK1 S345 phosphorylation in HCT116 and 293T cells (Fig. 2 E and F), suggesting that the catalytic activity and nuclear localization are required for USP3 to impact CHK1 phosphorylation. The increased cytoplasmic localization of ΔNLS was confirmed by confocal microscopy (SI Appendix, Fig. S2).
Fig. 2.
USP3 interacts with CHK1. (A) An in vitro GST pull-down assay showed that the recombinant His-USP3 interacted preferentially with the N-terminal domain of CHK1 (N). FL and C, full-length and the C-terminal domain of CHK1, respectively. (B) WT USP3 and the DUB activity mutant C168S interacted directly with CHK1, as assessed by an in vitro GST pull-down assay. (C) The in vivo interaction between USP3 and CHK1 was enhanced after UV treatment in HCT116 cells. Coimmunoprecipitation was conducted with anti-CHK1 antibody. (D) DUB activity and the nuclear localization sequence were not required for the interaction with CHK1. (E and F) WT USP3, but not the C168S or ΔNLS mutant, dampened CHK1 S345 phosphorylation after UV treatment in HCT116 (E) and 293T (F) cells.
USP3 Mediates CHK1 Deubiquitination.
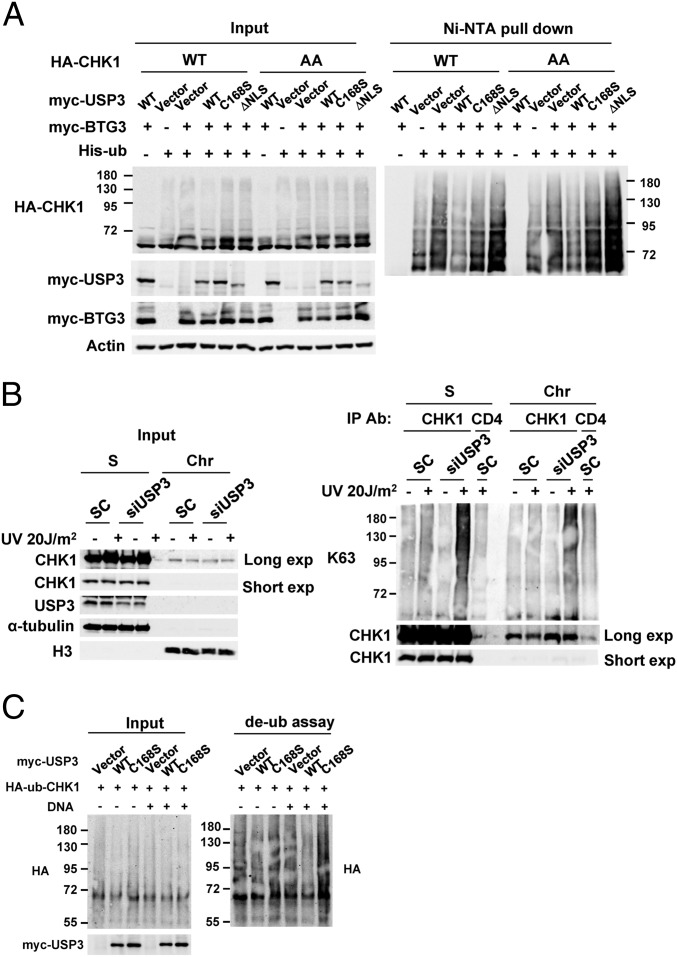
To further confirm that USP3 can affect CHK1 ubiquitination, CHK1 was coexpressed with myc-BTG3, His-ubiquitin, and either the WT or the mutants of USP3 and then was subjected to a nickel-nitrilotriacetic acid-agarose (Ni-NTA) bead pull-down assay. The results indicated that coexpression with WT USP3, but not with the mutants, decreased BTG3-promoted CHK1 ubiquitination (Fig. 3A). In addition, WT USP3 also dampened ubiquitination of the CHK1-317/345AA mutant, suggesting that USP3-mediated CHK1 deubiquitination was independent of its phosphorylation by ATR (Fig. 3A). To verify the existence of CHK1 deubiquitination in cells, we immunoprecipitated endogenous CHK1 from control or USP3-knockdown cells that had been treated or not treated with UV and then probed it with an antibody specific for K63-linked ubiquitin chains. The results showed that USP3 depletion enhanced UV-induced CHK1 ubiquitination (Fig. 3B). Conversely, USP3 overexpression reduced endogenous CHK1 ubiquitination (SI Appendix, Fig. S3). In vitro, WT USP3, but not the C168S mutant, could deubiquitinate purified ubiquitinated CHK1 preferentially in the presence of DNA, suggesting that USP3 most likely mediates CHK1 deubiquitination directly (Fig. 3C). Although it is unclear whether the binding of DNA ends activates USP3, a nonspecific DNA-binding activity was detected in USP3 in vitro (SI Appendix, Fig. S4).
Fig. 3.
USP3 regulates CHK1 ubiquitination through its catalytic activity and nuclear localization. (A) BTG3-promoted CHK1 ubiquitination was decreased by its coexpression with WT USP3 but not with the C168S or ΔNLS mutants. In vivo ubiquitination was performed in 293T cells transfected with the indicated constructs, and the products were pulled down using Ni-NTA beads. AA, CHK1 mutant with Ser317 and Ser345 mutated to Ala. (B) K63-linked ubiquitination of endogenous CHK1 was increased by USP3 knockdown in HCT116 cells. Ubiquitination of CHK1 in soluble (S) and chromatin (Chr)-enriched fractions was analyzed by immunoprecipitation with anti-CHK1 antibody, followed by Western blotting using K63-linked specific ubiquitin antibody. (C) WT USP3, but not the C168S mutant, deubiquitinated CHK1 in vitro. The in vitro deubiquitination assay was performed by coincubating the purified ubiquitinated CHK1 and immunoprecipitated USP3 in the presence or absence of DNA.
Nuclear Localization of CHK1 Is Enhanced After UV Irradiation in USP3-Depleted Cells.
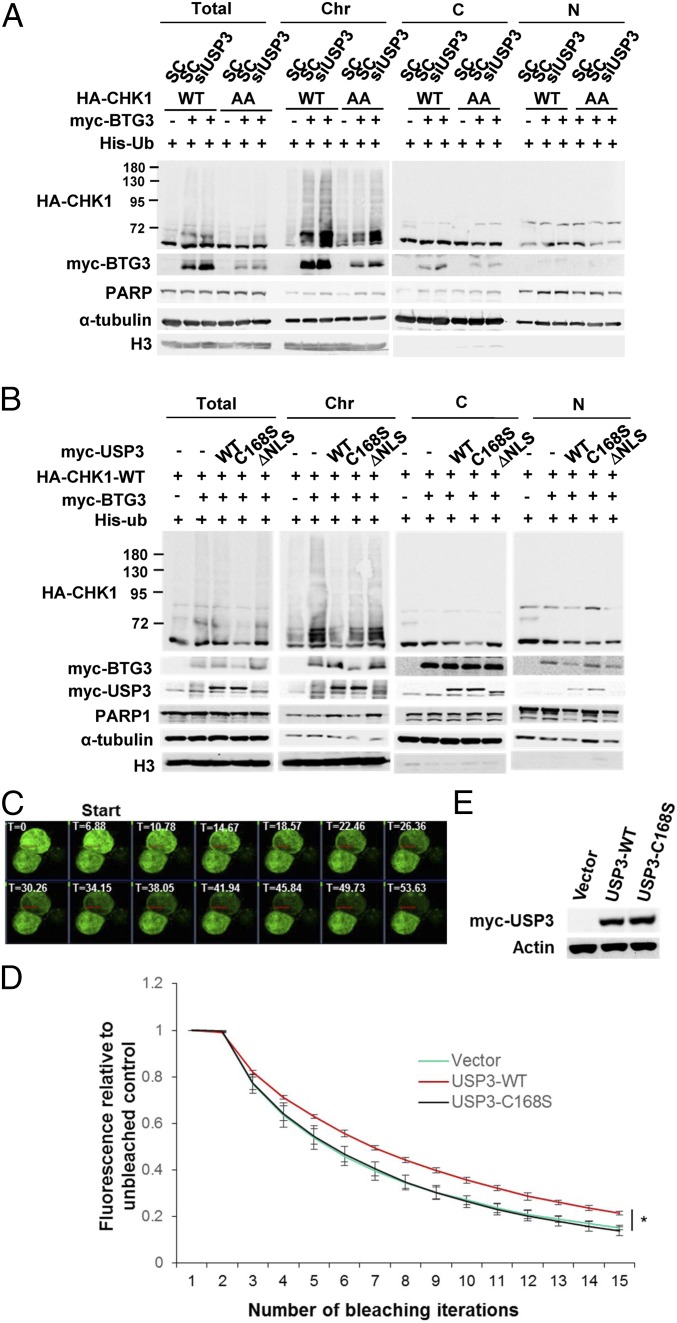
Our earlier studies indicated that K63-linked ubiquitination of CHK1 promotes its chromatin association (11). It was therefore functionally relevant to ask if USP3, acting as a DUB, could regulate CHK1 chromatin association. Cell fractionation was used to determine the subcellular localization of WT CHK1 or the CHK1-317/345AA mutant in USP3-knockdown cells. The results indicated that down-regulation of USP3 increased CHK1 chromatin binding irrespective of the kinase’s phosphorylation at S317 and S345 (Fig. 4A). In addition, coexpression with WT USP3, but not with the C168S or ΔNLS mutant, decreased CHK1 chromatin association (Fig. 4B). These results suggest that USP3 may deubiquitinate CHK1 on chromatin, and this in turn may regulate CHK1 chromatin association.
Fig. 4.
USP3 regulates BTG3-promoted CHK1 chromatin association. (A) USP3 depletion increased ubiquitinated CHK1 or the CHK1-317/345AA mutant in the chromatin fraction of transfected 293T cells. PARP1, α-tubulin, and histone H3 were used as nuclear soluble (N), cytosolic (C), and chromatin (Chr) markers, respectively. (B) CHK1 chromatin association was decreased by its coexpression with WT USP3 but not by coexpression with the C168S or ΔNLS mutant. Fractionation was performed as described for A. (C–E) Redistribution or mobility of ubiquitinated CHK1 in the nucleus was reduced by USP3. FLIP was conducted in HCT116 cells coexpressing BTG3, GFP-CHK1, and either WT USP3 or the C168S mutant. Red rectangles in C mark the area used for data acquisition and analysis. Fluorescence was bleached with an increasing number of hits. An adjacent unbleached cell served as the control. T, time in seconds. The mean ± SD from three experiments is shown in D, and the relative expression of WT USP3 and C168S is displayed in E.
To further explore the mechanistic regulation of chromatin association by USP3, we used fluorescence loss in photobleaching (FLIP) microscopy to compare the rate of redistribution or mobility of ubiquitinated CHK1 (CHK1 coexpressed with BTG3) in the presence of WT USP3 or the C168S mutant. Our data showed that USP3 in fact reduced the mobility of nuclear CHK1, suggesting that, once the ubiquitin chain is removed, the redistribution of CHK1 to other chromatin sites for activation becomes less likely (Fig. 4 C–E).
To determine if the above-observed effects on chromatin association indeed have an impact on CHK1 activation, phosphorylation of CHK1’s S345 residue (pS345) was determined in the chromatin fraction. The results showed that USP3 knockdown prolonged chromatin-associated S345 phosphorylation (Fig. 5A). Similar to CHK1 pS345, CHK1 pS317 foci were increased and appeared to be more sustained in USP3-depleted cells, as observed by immunofluorescent microscopy (Fig. 5B and SI Appendix, Fig. S5). These results suggest that USP3 may facilitate CHK1 chromatin release after DNA damage-induced phosphorylation. In support of this, USP3 down-regulation preferentially retained CHK1 in the nucleus after UV damage, as demonstrated by confocal microscopy (Fig. 5 C and D). Collectively, our data implicate a role of USP3 in facilitating CHK1 release from chromatin after its activation.
Fig. 5.
USP3 regulates the phosphorylation of chromatin-bound CHK1 after UV treatment. (A) CHK1 S345 phosphorylation was increased in the chromatin fraction by USP3 knockdown in 293T cells. Cells were collected at the indicated time after UV irradiation (15 J/m2). Cell fractionation was done as described in Fig. 4A. (B) CHK1 pS317 foci were more prominent and sustained by depletion of USP3 in HCT116 cells, as assessed by confocal microscopy. Staining of pS317 is shown in SI Appendix, Fig. S5. The mean ± SD from three experiments is shown. UT, untreated. (C) USP3 depletion promoted CHK1 nuclear retention after UV treatment, as assessed by confocal microscopy. (D) The intensity of CHK1 was quantified, and the cell population with increased CHK1 nuclear presence was determined. Data are shown as the mean ± SD from three independent experiments.
USP3 Knockdown Promotes Cell Survival After Replication Blockage.
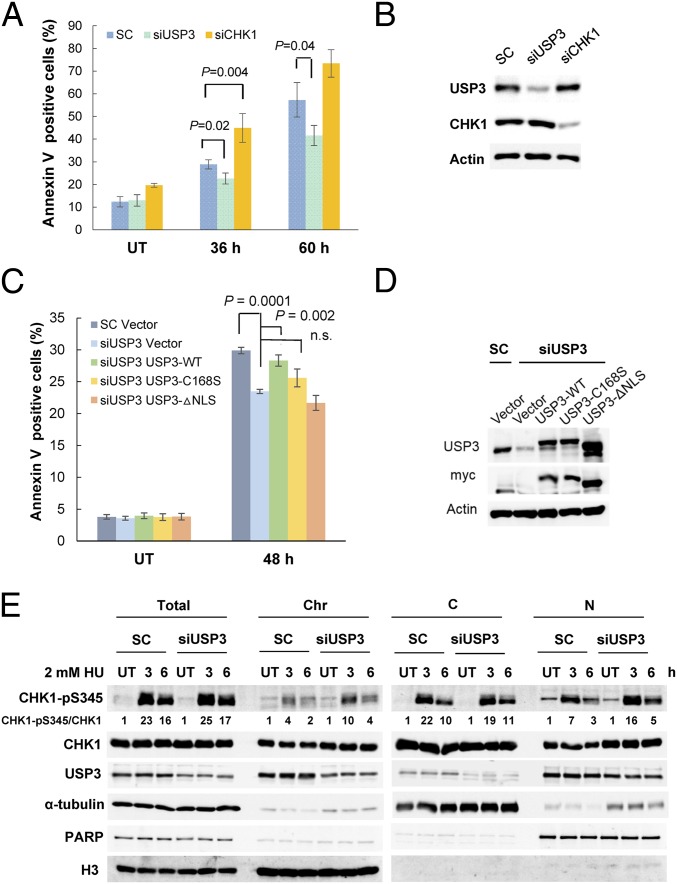
Based on the existing understanding of the role of CHK1 in replication stress, we next examined the survival of USP3-knockdown cells after HU treatment. In contrast to CHK1-depleted cells, which displayed increased cell death, USP3–down-regulated cells showed reduced apoptosis (Fig. 6 A and B). Furthermore, cell death could be restored through the reexpression of siRNA-resistant WT USP3, but not of C168S or ΔNLS, indicating that the catalytic activity and nuclear localization of USP3 are required for this effect (Fig. 6 C and D).
Fig. 6.
USP3 down-regulation promotes cell survival after replication stress. (A) HU-induced apoptosis was reduced in USP3-knockdown cells. Flow cytometry analysis was conducted with siRNA-transfected HCT116 cells with or without 2 mM HU pretreatment for the indicated times. CHK1 knockdown was used as a positive control. The mean ± SD from three independent experiments is shown. (B) Immunoblots confirming the knockdown. (C) WT USP3, but not the C168S or ΔNLS mutants, resensitized the USP3-depleted cells to HU-induced apoptosis. The mean ± SD from three independent experiments is shown. (D) Western blots showing knockdown of USP3 and levels of reexpressed constructs. (E) CHK1 pS345 was increased in the nuclear and chromatin fractions along with total CHK1 in USP3-knockdown cells. Cell fractionation was performed with siRNA-transfected 293T cells with or without HU pretreatment.
To determine if USP3 knockdown could affect the CHK1 phosphorylation induced by HU treatment, CHK1 pS345 was assessed in different cellular fractions. The results showed that USP3 depletion increased CHK1 pS345 concomitantly with the CHK1 protein levels in the chromatin and nuclear soluble fractions after HU treatment (Fig. 6E). Cumulatively, these results suggest that USP3 modulates cell survival after replication stress by regulating CHK1 ubiquitination and chromatin association.
In addition to siRNA-mediated knockdown of USP3 expression, we also attempted to knock out USP3 in HCT116 cells using the CRISPR-Cas9 system (SI Appendix, Fig. S6A). As a result, several clones with null USP3 expression were obtained (SI Appendix, Fig. S6B). Two of the USP3-null clones, clone 3 and clone 8, were analyzed further for their response to HU and UV. Consistent with cells with USP3 knockdown, these cells were resistant to HU-induced cell death compared with the parental cells (SI Appendix, Fig. S6C), and they also had more prominent and sustained CHK1 pS317 foci after UV treatment (SI Appendix, Fig. S6 D and E), thus validating our observations with the knockdown approaches.
USP3 Down-Regulation Enhances Genome Stability.
BTG3-promoted CHK1 activation has been implicated in the spindle assembly checkpoint by ensuring proper kinetochore attachment of the mitotic spindle (11), possibly through the regulation of Aurora B–BUBR1 signaling (8). To explore the possibility that USP3, by modulating CHK1 function, also participates in safeguarding mitotic progression, we examined kinetochore attachment in USP3–down-regulated cells by confocal microscopy. Contrary to CHK1 depletion, USP3 knockdown decreased the populations of misaligned and lagging chromosomes in HeLa cells (SI Appendix, Fig. S7 A–D). In addition, paclitaxel-induced CHK1 activity, represented by Cdc25C S216 kinase activity, was enhanced in USP3-knockdown cells (SI Appendix, Fig. S7 E and F), supporting the notion that USP3 could modulate CHK1 activity at the mitotic checkpoint and in the maintenance of genome stability.
Discussion
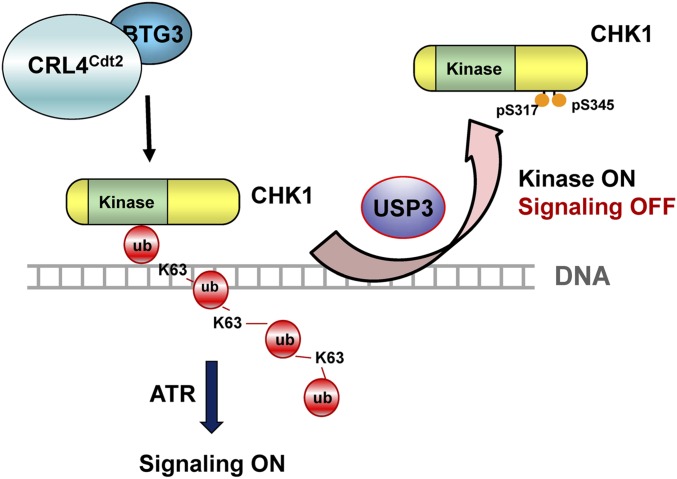
Following our earlier uncovering of the K63-linked ubiquitin modification of CHK1 in the DDR, we took a further step to determine how this modification is removed and its impact on checkpoint signaling. Our data showed that the K63-linked ubiquitin chain in CHK1 is removed mainly by the deubiquitinating enzyme USP3. Failure to do so, e.g., in USP3-depleted cells, resulted in prolonged CHK1 chromatin association and increased CHK1 pS345. These results thus implicate a possible role of USP3 in CHK1 release from chromatin and termination of the upstream (or further) signaling in checkpoint recovery (Fig. 7).
Fig. 7.
Model depicting the role of USP3 in the regulation of CHK1 in the DDR. Upon DNA damage, USP3 binds with CHK1 on chromatin, likely through the K63-linked ubiquitin chain on CHK1. By removing the ubiquitin chain from CHK1, USP3 releases CHK1 from chromatin, preventing its further phosphorylation and activation by upstream kinases. Concordantly, USP3 also renders CHK1 more accessible to its substrates by removing the ubiquitin chain that blocks the kinase active site.
Induction of the DDR and Delay of S-Phase Progression by USP3 Depletion.
It has been reported that USP3 plays a role in H2A/H2B deubiquitination and that knockdown of USP3 increases the DDR, such as CHK1 pS345 p and γ-H2AX foci, and the delay of S-phase progression (18). It was suggested that H2A/H2B deubiquitination by USP3 may have an impact on the recruitment of regulatory factors with ubiquitin-binding domains in DNA-damage signaling, or it may affect other histone modifications, although the mechanism was not fully elucidated. Our results presented herein suggest another possible explanation. In this scenario, elevated CHK1 K63-linked ubiquitination in USP3-depleted cells enhances CHK1 chromatin association and activation, leading to prolonged checkpoint arrest and a delay in S-phase progression. Our results might also provide an explanation, at least in part, for the observed lymphopenia and cellular aging in USP3-deficient mice (21). It is possible that the persistent CHK1 activation and cell-cycle arrest in response to spontaneous DNA damage pose an unwelcoming effect on cell proliferation. By identifying a USP3 target (viz., CHK1), our study sheds light on the involvement of USP3 in the DDR, especially in checkpoint recovery. In that respect, one obvious unresolved issue is whether USP3 activity is regulated by the DDR and, if so, the mechanism involved. Foreseeably, premature activation of USP3 or its overexpression may cause a defective DDR. Our in vitro deubiquitination assay suggested that the full activity of USP3 requires the presence of DNA ends (Fig. 3C). It is unclear if USP3 requires direct binding to the DNA ends for activation. On the other hand, it may also possibly undergo protein modification. These details remain to be determined.
Temporal and Spatial Regulation of CHK1 Ubiquitination and Deubiquitination.
Similar to phosphorylation and dephosphorylation, cycles of ubiquitination and deubiquitination provide additional on/off switches to regulate protein activation. For example, growth factors stimulate the E3 ligase TNF receptor-associated factor 6 (TRAF6) to promote AKT K63-linked ubiquitination, membrane localization, and activation (22). CYLD, a deubiquitinating enzyme, removes the ubiquitin chain of AKT to turn off its activation (23, 24). Previously, we had shown that BTG3 promotes CHK1 K63-linked ubiquitination, chromatin localization, and activation (11). In this study, we demonstrate that the DUB USP3 removes the ubiquitin chain on CHK1 to control its release from chromatin. This conclusion is based on the observation that the knockdown of USP3 did not significantly affect the level of CHK1 on chromatin in the initial phase of the DDR to UV treatment but promoted the accumulation of CHK1 pS345 on chromatin (Fig. 5A). In conjunction with the observation that CHK1 interacted with USP3 at a later time than with BTG3 (Fig. 2C), two possibilities can be envisioned. One is that since CHK1 and BTG3 reside mainly in the cytoplasm before DNA damage, nuclear USP3 would be able to interact with CHK1 only when the enzyme is ubiquitinated (as prompted by DNA damage and BTG3) (11) and translocated to the nucleus. The other scenario is that once CHK1 is ubiquitinated, it can then be recognized by USP3 through the ubiquitin chain. Of note, recognition of the C-terminal diglycine of unanchored ubiquitin is required for optimal catalytic activation by USP5, which the protease achieves through its zinc-finger ubiquitin-binding domain (25), which USP3 similarly possesses. Recruitment of USP3 to the damage sites through binding to the ubiquitinated histone variant H2AX may provide an additional means to gauge the activity when the enzyme is localized to DNA ends. It is fully possible that these scenarios coexist in a sequentially and spatially regulated manner.
Aside from USP3, our data suggest that other DUBs may be involved in the regulation. Our cell-survival assays (Fig. 6 A and B) indicated that depletion of USP3 had an effect opposite that of CHK1 knockdown, suggesting that USP3 down-regulation may enhance CHK1 function. How can CHK1 then act as a kinase with the active site blocked by the ubiquitin chain? One explanation would be that during its association with or disassociation from chromatin or upon its redistribution CHK1 could be spontaneously deubiquitinated or targeted by another DUB. Further investigation would provide a fuller picture of the regulatory process involved.
USP3 Nuclear Localization.
During the course of our studies, we also observed that DNA damage, such as that created by UV, causes nuclear accumulation of USP3 (Fig. 5C), the mechanism of which is still unclear. We do not believe that increased protein expression is the cause, as the overall protein levels were not significantly altered after UV treatment (Figs. 2C and 6C). Our data from the in vivo ubiquitination assay also indicated that BTG3 does not promote ubiquitination of the USP3 protein (Fig. 3A), thus excluding a mechanism similar to the one used by CHK1. One possibility is that this is through binding of USP3 to its ubiquitinated substrates, such as H2A and H2AX (14), in addition to CHK1. Once the substrates are deubiquitinated, the dynamic distribution of USP3 would then be reestablished. In relation to this, an active nuclear export sequence that is dependent on the exportin CRM1 has been identified in USP3 (26), and we have identified and tested herein at least one of the NLSs. It should be noted that deletion of amino acids 129–149 in our ΔNLS construct did not render USP3 completely cytoplasmic (Fig. 4B and SI Appendix, Fig. S2), suggesting that other, yet to be identified NLSs may also be at play.
In summary, we have identified a DUB involved in the regulation of CHK1, as well as its underlying mechanisms and potential impacts on both cell survival under stress and genome stability. So far, not many CHK1 modulatory proteins have been identified. As CHK1 is increasingly becoming a therapeutic target (27), our findings here, apart from deciphering a fundamental pathway, would certainly provide insights into the strategic approach for targeting CHK1.
Materials and Methods
Detailed protocols regarding cell treatment, immunoblotting, immunoprecipitation, GST pull-down, cell fractionation, in vitro and in vivo deubiquitination, flow cytometry, immunofluorescence confocal microscopy, kinase assay, and EMSA and information regarding expression vectors, siRNA, and HCT116 USP3-knockout cells can be found in SI Appendix, SI Materials and Methods.
Antibodies.
The antibodies used were anti-CHK1 (A300-162A) and anti-USP3 (A301-540A) from Bethyl Laboratories; anti-CHK1 pS345 (no. 2348), anti-CDC25C pS216 (no. 4901), and anti-CDC2 pY15 (no. 9111) from Cell Signaling Technology; anti-H3 pS10 (no. 06-570), anti-H3 (no. 07-690), and anti–K63-linked ubiquitin (no. 05-1308) from Millipore; anti-actin (A2066) and anti–α-tubulin (T6074) from Sigma-Aldrich; anti-CHK1 (sc8408), anti-Myc (sc40 and sc789), anti-GST (sc138), anti–CENP-B (sc22788), anti-CDC2 (SC54), and anti-CD4 (sc1140) from Santa Cruz Biotechnology; anti-BUBR1 (K0169-3) from MBL International; anti-PARP1 (1835238) from Roche; anti-HA from LTK; and anti-USP3 (GTX128238) from GeneTex. The BTG3 antibody has been described previously (28).
Fluorescence Loss in Photobleaching Microscopy.
HCT116 cells seeded in a confocal dish (200350; SPL Life Sciences) were transiently transfected with GFP-tagged CHK1 and myc-USP3, WT USP3, or C168S. To induce the DDR, the cells were irradiated with UV light at 1 h before photobleaching and fluorescence capture (Zeiss LSM510). For FLIP microscopy, a 10 × 120 μm area in the nucleus was bleached continuously for 50 iterations using a 405-nm laser operating at 100% power. The loss of fluorescence was analyzed in a nearby region (indicated by the rectangle in Fig. 4C).
Statistical Analysis.
Data are presented as the mean ± SD from at least three independent experiments. Student’s t test was conducted to analyze the data, and a difference was considered significant if P < 0.05.
Supplementary Material
Acknowledgments
This work was funded by Academia Sinica and the Ministry of Science and Technology, Taiwan.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1719856115/-/DCSupplemental.
References
- 1.Ciccia A, Elledge SJ. The DNA damage response: Making it safe to play with knives. Mol Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001;15:2177–2196. doi: 10.1101/gad.914401. [DOI] [PubMed] [Google Scholar]
- 3.Myers JS, Cortez D. Rapid activation of ATR by ionizing radiation requires ATM and Mre11. J Biol Chem. 2006;281:9346–9350. doi: 10.1074/jbc.M513265200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao H, Piwnica-Worms H. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol Cell Biol. 2001;21:4129–4139. doi: 10.1128/MCB.21.13.4129-4139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Latif C, den Elzen NR, O’Connell MJ. DNA damage checkpoint maintenance through sustained Chk1 activity. J Cell Sci. 2004;117:3489–3498. doi: 10.1242/jcs.01204. [DOI] [PubMed] [Google Scholar]
- 6.Meuth M. Chk1 suppressed cell death. Cell Div. 2010;5:21. doi: 10.1186/1747-1028-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez R, Meuth M. Chk1 and p21 cooperate to prevent apoptosis during DNA replication fork stress. Mol Biol Cell. 2006;17:402–412. doi: 10.1091/mbc.E05-07-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zachos G, et al. Chk1 is required for spindle checkpoint function. Dev Cell. 2007;12:247–260. doi: 10.1016/j.devcel.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang YW, et al. Genotoxic stress targets human Chk1 for degradation by the ubiquitin-proteasome pathway. Mol Cell. 2005;19:607–618. doi: 10.1016/j.molcel.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 10.Puc J, et al. Lack of PTEN sequesters CHK1 and initiates genetic instability. Cancer Cell. 2005;7:193–204. doi: 10.1016/j.ccr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Cheng YC, Lin TY, Shieh SY. Candidate tumor suppressor BTG3 maintains genomic stability by promoting Lys63-linked ubiquitination and activation of the checkpoint kinase CHK1. Proc Natl Acad Sci USA. 2013;110:5993–5998. doi: 10.1073/pnas.1220635110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smits VA, Reaper PM, Jackson SP. Rapid PIKK-dependent release of Chk1 from chromatin promotes the DNA-damage checkpoint response. Curr Biol. 2006;16:150–159. doi: 10.1016/j.cub.2005.11.066. [DOI] [PubMed] [Google Scholar]
- 13.Kato K, et al. Fine-tuning of DNA damage-dependent ubiquitination by OTUB2 supports the DNA repair pathway choice. Mol Cell. 2014;53:617–630. doi: 10.1016/j.molcel.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 14.Sharma N, et al. USP3 counteracts RNF168 via deubiquitinating H2A and γH2AX at lysine 13 and 15. Cell Cycle. 2014;13:106–114. doi: 10.4161/cc.26814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun XX, Challagundla KB, Dai MS. Positive regulation of p53 stability and activity by the deubiquitinating enzyme Otubain 1. EMBO J. 2012;31:576–592. doi: 10.1038/emboj.2011.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang B, Elledge SJ. Ubc13/Rnf8 ubiquitin ligases control foci formation of the Rap80/Abraxas/Brca1/Brcc36 complex in response to DNA damage. Proc Natl Acad Sci USA. 2007;104:20759–20763. doi: 10.1073/pnas.0710061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng L, Wang J, Chen J. The Lys63-specific deubiquitinating enzyme BRCC36 is regulated by two scaffold proteins localizing in different subcellular compartments. J Biol Chem. 2010;285:30982–30988. doi: 10.1074/jbc.M110.135392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicassio F, et al. Human USP3 is a chromatin modifier required for S phase progression and genome stability. Curr Biol. 2007;17:1972–1977. doi: 10.1016/j.cub.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 19.Chen P, et al. The 1.7 a crystal structure of human cell cycle checkpoint kinase Chk1: Implications for Chk1 regulation. Cell. 2000;100:681–692. doi: 10.1016/s0092-8674(00)80704-7. [DOI] [PubMed] [Google Scholar]
- 20.Shao G, et al. MERIT40 controls BRCA1-Rap80 complex integrity and recruitment to DNA double-strand breaks. Genes Dev. 2009;23:740–754. doi: 10.1101/gad.1739609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lancini C, et al. Tight regulation of ubiquitin-mediated DNA damage response by USP3 preserves the functional integrity of hematopoietic stem cells. J Exp Med. 2014;211:1759–1777. doi: 10.1084/jem.20131436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang WL, et al. The E3 ligase TRAF6 regulates Akt ubiquitination and activation. Science. 2009;325:1134–1138. doi: 10.1126/science.1175065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim JH, et al. CYLD negatively regulates transforming growth factor-β-signalling via deubiquitinating Akt. Nat Commun. 2012;3:771. doi: 10.1038/ncomms1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang WL, et al. Cycles of ubiquitination and deubiquitination critically regulate growth factor-mediated activation of Akt signaling. Sci Signal. 2013;6:ra3. doi: 10.1126/scisignal.2003197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reyes-Turcu FE, et al. The ubiquitin binding domain ZnF UBP recognizes the C-terminal diglycine motif of unanchored ubiquitin. Cell. 2006;124:1197–1208. doi: 10.1016/j.cell.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 26.García-Santisteban I, Bañuelos S, Rodríguez JA. A global survey of CRM1-dependent nuclear export sequences in the human deubiquitinase family. Biochem J. 2012;441:209–217. doi: 10.1042/BJ20111300. [DOI] [PubMed] [Google Scholar]
- 27.Manic G, Obrist F, Sistigu A, Vitale I. Trial Watch: Targeting ATM-CHK2 and ATR-CHK1 pathways for anticancer therapy. Mol Cell Oncol. 2015;2:e1012976. doi: 10.1080/23723556.2015.1012976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ou YH, et al. The candidate tumor suppressor BTG3 is a transcriptional target of p53 that inhibits E2F1. EMBO J. 2007;26:3968–3980. doi: 10.1038/sj.emboj.7601825. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.