Significance
In agricultural crops, plants are cultivated in close proximity, exposed to mutual shading. Photosensory receptors perceive this light environment and initiate growth responses that adjust the position of plant organs within the canopy to maximize light capture. During the early hours under shade, these responses are mediated by elevated levels of the hormone auxin. Here we show that under prolonged shade, the PHYTOCHROME INTERACTING FACTOR 4 light-signaling transcription factor, selected auxin receptors, their upstream regulatory miRNA, and downstream transcriptional regulators change their abundance to enhance growth responses, while auxin levels return to those observed before shade. Thus, in the presence of persistent shade, plants elicit a system-level rearrangement of auxin signaling, which sustains growth without increased auxin.
Keywords: phytochrome B, PIF4, Aux/IAA, auxin receptor, MIR393
Abstract
Light cues from neighboring vegetation rapidly initiate plant shade-avoidance responses. Despite our detailed knowledge of the early steps of this response, the molecular events under prolonged shade are largely unclear. Here we show that persistent neighbor cues reinforce growth responses in addition to promoting auxin-responsive gene expression in Arabidopsis and soybean. However, while the elevation of auxin levels is well established as an early event, in Arabidopsis, the response to prolonged shade occurs when auxin levels have declined to the prestimulation values. Remarkably, the sustained low activity of phytochrome B under prolonged shade led to (i) decreased levels of PHYTOCHROME INTERACTING FACTOR 4 (PIF4) in the cotyledons (the organs that supply auxin) along with increased levels in the vascular tissues of the stem, (ii) elevated expression of the PIF4 targets INDOLE-3-ACETIC ACID 19 (IAA19) and IAA29, which in turn reduced the expression of the growth-repressive IAA17 regulator, (iii) reduced abundance of AUXIN RESPONSE FACTOR 6, (iv) reduced expression of MIR393 and increased abundance of its targets, the auxin receptors, and (v) elevated auxin signaling as indicated by molecular markers. Mathematical and genetic analyses support the physiological role of this system-level rearrangement. We propose that prolonged shade rewires the connectivity between light and auxin signaling to sustain shade avoidance without enhanced auxin levels.
Canopy shade entails a stronger reduction of red and blue than far-red and green wavelengths, due to selective absorption by photosynthetic pigments. These cues are sensed via a reduction in the activity of photosensory receptors, mainly phytochrome B (phyB) and cryptochrome 1 (cry1), consequently initiating shade-avoidance responses such as the promotion of stem and petiole elongation (1–3). Light-driven changes in canopy architecture can significantly impact yield of agricultural crops (4).
The phyB triggers the degradation and/or interferes with the specific DNA binding capacity of PHYTOCHROME INTERACTING FACTOR 3 (PIF3), PIF4, PIF5, and PIF7 (5, 6). Low red/far-red ratios reduce phyB activity and, as such, increase the activities of PIF3 (7), PIF4, PIF5 (8), and PIF7 (9). Low blue light levels reduce cry1 activity and also increase the abundance of at least PIF4 and PIF5, particularly when combined with low red/far-red ratios (10, 11). Consequently, in the presence of neighbor signals, PIFs show increased binding to the promoters of several of their targets, including the YUCCA (YUC) genes, activating their expression (9, 12, 13). Since YUC genes encode a rate-limiting enzyme in the synthesis of auxin, low red/far-red ratios lead to increased auxin levels in the cotyledons (14). Auxin is then transported from the cotyledons that perceive the neighbor signals to the growth-limiting epidermal tissue of the stem (15–17) and from the leaf lamina to the petioles (18, 19) to promote growth.
Auxin is perceived by the nuclear TRANSPORT INHIBITOR RESPONSE 1/AUXIN SIGNALING F-BOX (TIR1/AFBs) proteins, which effect ubiquitination and degradation of the coreceptor AUXIN/INDOLE-3-ACETIC ACID (Aux/IAA) transcriptional regulators (20). Aux/IAAs are active repressors of the AUXIN RESPONSE FACTOR (ARF) transcription factors, which mediate auxin-regulated gene expression upon Aux/IAA degradation (21). Aux/IAA genes are, in addition, direct targets of ARFs, and their expression increases in response to auxin. The dynamic consequences of the dual action of auxin on Aux/IAA transcription and Aux/IAA stability are a focus of intense analysis (22–24). The microRNA MIR393 negatively regulates TIR1, AFB2, and AFB3 transcript and protein accumulation in response to environmental stimuli (21, 25, 26).
Shade-avoidance responses can persist for weeks (27), and enhanced expression of auxin-related genes can be observed beyond the first day after the initiation of neighbor signals (14, 16, 28). However, the early activation of YUC genes and auxin synthesis can be severely reduced after several hours of exposure to low red/far-red ratios (29, 30), suggesting that different mechanisms might operate later on. Here we show that prolonged shade evokes a system-level commitment to shade avoidance that involves changes in PIF4, MIR393, TIR1, AFB2, and ARF6 abundance and the relative expression of different Aux/IAA genes.
Results
Commitment to Shade Avoidance Under Prolonged Shade.
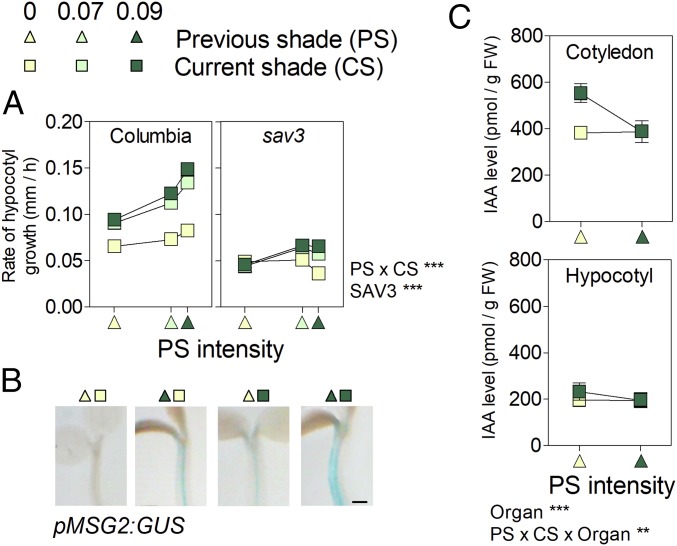
Three-day-old Arabidopsis plants were transferred to simulated shade (low red/far-red, low irradiance) or left as controls. The following day, half of the plants of each group were transferred to the opposite condition, to obtain plants subjected to all possible combinations of current shade (CS) and previous shade (PS). Growth promotion was strongly reinforced when plants had been subjected to intense PS (Fig. 1A; note significant PS × CS interaction). The impact of PS was observed for a further 9 h, but with a decreasing magnitude (SI Appendix, Fig. S1). Both CS and PS treatments also led to increased GUS staining in the hypocotyl of pMASSUGU 2 (MSG2)/IAA19:GUS reporter line (Fig. 1B). Therefore, prolonged shade not only maintains, it actually enhances the shade-avoidance responses. This is not a feature restricted to young (recently deetiolated) Arabidopsis seedlings, as stem and petiole growth and the expression of auxin-related genes such as Aux/IAAs (including MSG2/IAA19) and SMALL AUXIN UP-REGULATED (SAUR)-like were also maximal in glasshouse-grown soybean plants exposed to both PS and CS (SI Appendix, Figs. S2 and S3 and Table S1).
Fig. 1.
Persistent shade avoidance in the absence of elevated auxin levels in Arabidopsis. Shade intensity (based on the inverse of photosensory receptor activity estimated from the light spectra) is 0 for simulated sunlight, 0.07 for weak shade, and 0.09 for deep shade. (A) Columbia wild-type and sav3 hypocotyl growth rate during day 4 (0 h to 3 h after the beginning of the photoperiod) as affected by CS (day 4) and PS (day 3, abscissas). Means ± SE (smaller than the symbols) are from SI Appendix, Table S2. (B) Representative images of GUS stained seedlings bearing the pMSG2/IAA19:GUS transgene. (Scale bar = 200 µm.) (C) Auxin levels on day 4 (3 h after the beginning of the photoperiod). Means ± SE of four biological replicates. **P < 0.01; ***P < 0.001.
Persistent Shade Avoidance Takes Place Without Elevated Auxin Levels.
Both, CS and PS hypocotyl growth responses were impaired in the sav3 mutant (Fig. 1A), compromised in auxin synthesis. The slope of the relationship between growth rate under intense shade (CS = 0.09) and PS was significantly reduced (P < 0.05) in sav3, indicating that persistent response to shade is dependent on the levels of bioactive auxin. We harvested seedlings 3 h after the beginning of day 4 for auxin determination. In agreement with previous reports (14), auxin levels were higher in plants recently transferred to shade, compared with control seedlings subjected to simulated sunlight. However, in plants that had been exposed to intense PS, auxin content was similar to that of seedlings exposed to simulated sunlight (Fig. 1C). These results show that auxin accumulation is only transiently induced by shade (29, 30) and demonstrate that this pattern is PS-specific and not caused by the seedling age. From these findings, we conclude that increased growth under prolonged shade depends on auxin, but it is not mediated by elevated auxin levels.
Prolonged Shade Maintains Low phyB Activity.
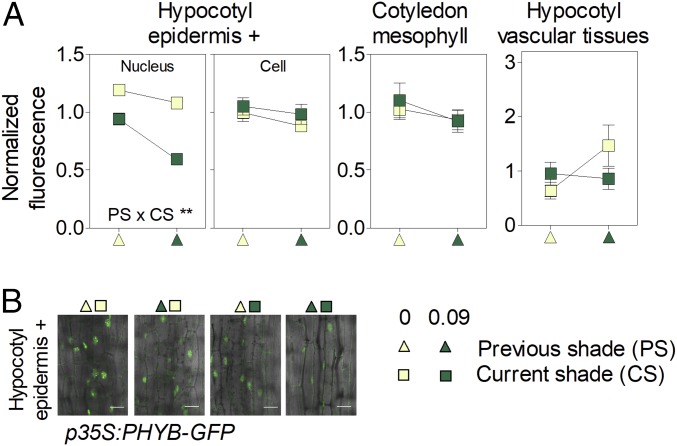
The transient nature of the enhanced auxin levels could be due to partial recovery of phyB activity under persistent shade (31). However, PHYB expression (SI Appendix, Fig. S4A) and total cell fluorescence of p35S:PHYB-GFP reporter line (a proxy for phyB abundance; Fig. 2A) were not significantly affected by simulated sunlight or shade conditions. The nuclear abundance of phyB remained unaffected by prolonged shade in the cotyledons and vascular tissues of the hypocotyl and decreased with prolonged shade in epidermal cells of the hypocotyl (Fig. 2; the kinetics is shown in SI Appendix, Fig. S5A). Since the low red/far-red ratios of shade establish low proportions of active phyB (Pfr), and nuclear levels of phyB were either unaffected or lowered, phyB activity remains low or even decreases under prolonged shade. In the wild type, hypocotyl growth under intense shade (CS = 0.09) was accelerated by increasing PS intensity (P < 0.0001; note positive slope in Fig. 1A), but this response was absent in the phyB mutant (where prolonged shade inhibited rather than promoting growth, P < 0.05; SI Appendix, Table S2). This indicates that prolonged shade enhanced hypocotyl growth by maintaining low phyB activity.
Fig. 2.
Dynamics of phyB under persistent shade. Shown are cell and nuclear fluorescence driven by the p35S:PHYB-GFP transgene in mesophyll cells of the cotyledons, the epidermal and subepidermal cells of the hypocotyl (epidermis +), and the vascular tissues of the hypocotyl as affected by CS and PS. (A) Means ± SE (whenever larger than the symbols) of at least 24 seedlings. **P < 0.01. (B) Representative confocal images of the epidermal and subepidermal cells of the hypocotyl. (Scale bar = 25 µm.)
Prolonged Shade Modifies the PIF4 Profile.
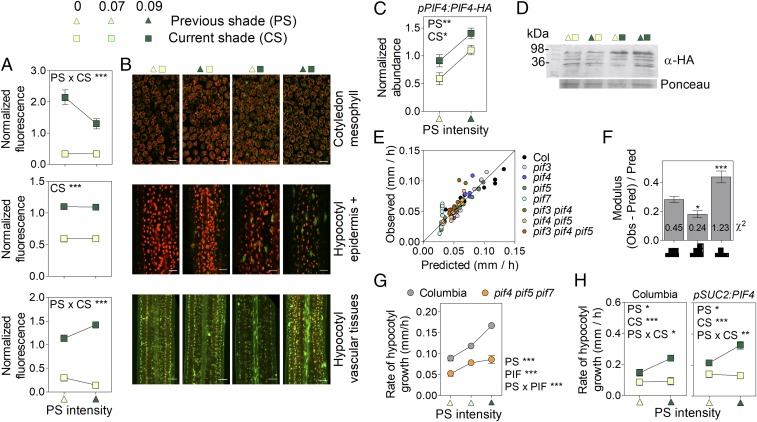
Among phyB targets, PIF4 has a large effect on shade avoidance. PIF4 expression was not significantly affected by shade (SI Appendix, Fig. S4 B and C). To describe the dynamics of the PIF4 protein, we generated lines bearing the pPIF4:PIF4-GFP transgene. Confocal microscopy analyses revealed that nuclear abundance of the PIF4 protein decreased in mesophyll cells in response to prolonged shade, with much lower nuclear GFP levels observed in cotyledons of plants subjected to prolonged shade than in those exposed to a first shade event (Fig. 3 A and B). Since YUC8 and YUC9 are direct targets of PIF4 (12), this reduction in PIF4 protein abundance could account for the decrease in auxin levels observed under prolonged shade (Fig. 1C). By contrast, under prolonged shade, PIF4 levels remained high in epidermal/subepidermal cells of the hypocotyl (Fig. 3 A and B; kinetics are shown in SI Appendix, Fig. S5B) and specifically overaccumulated in the vascular tissues of the hypocotyl (Fig. 3 A and B). In protein blots of entire seedlings, PIF4-HA increased additively in response to CS and PS (Fig. 3 C and D), suggesting that PIF4 in the stem vascular tissues makes a significant contribution to the overall pool of the protein, consistent with the pattern of PIF4 gene expression in seedlings (SI Appendix, Fig. S4C). Transcriptional activity of PIF4 also depends on the abundance of negative regulators such as HFR1 (32) and ELF3 (33, 34), but stability of these proteins was not affected by PS (SI Appendix, Fig. S6), despite the strong response of HFR1 to CS (35).
Fig. 3.
Persistent shade increases PIF4 levels to promote growth. Shown are effects of CS and PS on (A and B) nuclear fluorescence driven by the pPIF4P:PIF4-GFP transgene in mesophyll cells of the cotyledons, the epidermal and subepidermal cells of the hypocotyl (epidermis +), and the vascular tissues of the hypocotyl and on (C and D) PIF4 abundance in protein blots. (In B, scale bar = 25 μm. For representative confocal images, the GFP signal is green, while red represents autofluorescence of chlorophyll. In D, a representative protein blot is shown.) (E) Observed hypocotyl growth (SI Appendix, Table S2) vs. values predicted by a model based on PIF4 levels increased by CS and PS (i.e., overincreasing under persistent shade). (F) Goodness-of-fit of the model [as denoted by low observed (Obs)/predicted (Pred) Obs ratios and the χ2 values (numbers on bars)] when considering either stably increased, overincreasing, or decreasing PIF activity (represented in abscissa). (G) The pif4 pif5 pif7 mutant has significantly reduced response to PS. (H) Expression of PIF4 in vascular tissues promotes hypocotyl growth in Arabidopsis (both lines in the Columbia wild-type background). Means ± SE (whenever larger than the symbols) of (A and G) 20 or (H) 15 seedlings or (C) 3 biological replicates. *P < 0.05; **P < 0.01; ***P < 0.001.
Under prolonged shade, the abundance of PIF4 remained elevated in the epidermis of hypocotyl tissues, continued to increase (i.e., it was overelevated) in vascular tissues, and decreased in the cotyledons. We therefore investigated which one of these three temporal patterns shows the best correlation with growth. The model based on a transient increase in PIF activity showed poor goodness of fit compared with the model based on elevated or overelevated PIF activity under persistent shade (Fig. 3 E and F). This result suggests that the commitment to shade avoidance requires persistent PIF activity. To test this prediction, we compared the effects of PS intensity in the wild type and the pif4 pif5 pif7 mutant exposed to intense CS. The significantly lower slope of the mutant confirms the prediction of the model (Fig. 3G). The model based on overelevated PIF activity outperformed that based on stably elevated activity (Fig. 3F), suggesting that the overaccumulated PIF4 observed in vascular tissues could be important. To test this hypothesis independently, we analyzed whether overaccumulation of PIF4 in vascular tissues promotes growth. Expression of PIF4 in phloem companion cells, by using the SUC2 promoter, was, per se, sufficient to promote hypocotyl growth in transgenic seedlings (Fig. 3H), confirming the prediction of the model.
Prolonged Shade Reshapes Auxin Perception and Signaling.
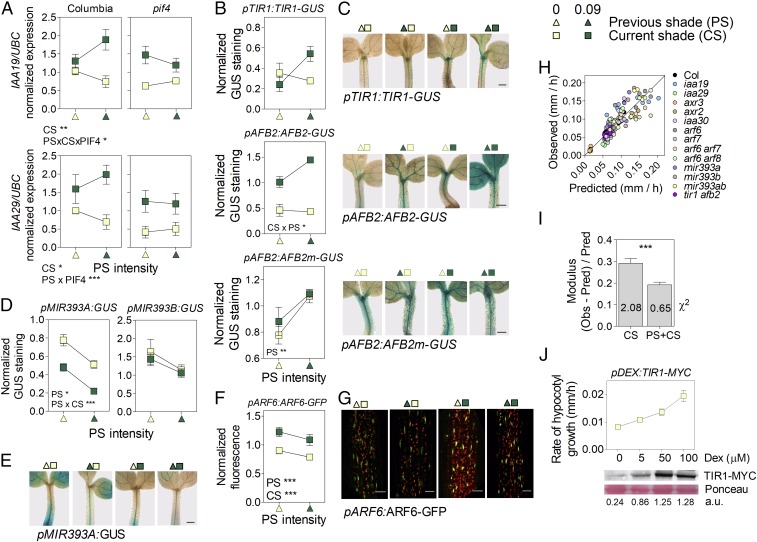
Since growth under prolonged shade depends on auxin but is not mediated by enhanced auxin levels, we investigated the levels of auxin perception and signaling components. The MSG2/IAA19 and IAA29 genes are direct targets of PIF4 (12) expressed in vascular tissues of the hypocotyl (36, 37), and prolonged shade enhanced their expression in a PIF4-dependent manner (Fig. 4A, see also Fig. 1B). Prolonged shade also enhanced the abundance of the AFB2 and TIR1 auxin receptor proteins in the hypocotyl (Fig. 4 B and C and SI Appendix, Fig. S8). This effect correlated with a reduction in the expression of MIR393 (Fig. 4 D and E and SI Appendix, Fig. S8), which is a negative regulator of auxin receptor genes (25, 26). The GUS signal, driven by a mutant pAFB2:AFB2m-GUS transgene resistant to MIR393, showed distorted responses (Fig. 4 B and C). Finally, CS and PS both slightly induced ARF6 expression (SI Appendix, Fig. S7 and Table S1), although ARF6 protein levels were smaller under PS, probably due to an effect of PS on ARF6 stability (Fig. 4 F and G).
Fig. 4.
Persistent shade modifies auxin perception and signaling to promote growth. Effects of (A) CS and PS on MSG2/IAA19 and IAA29 expression in Columbia wild type and pif4, (B and C) GUS staining driven by the pAFB2:AFB2-GUS, pTIR1:TIR1-GUS, or pAFB2:AFB2m-GUS transgenes, (D and E) GUS staining driven by the pMIR393a:GUS transgene, and (F and G) fluorescence driven by the pARF6:ARF6-GFP transgene. (In C and E, scale bar = 200 μm. In G, scale bar = 35 μm.) (H) Observed hypocotyl growth (SI Appendix, Table S2) vs. values predicted by a model based on MSG2/IAA19, IAA29, ARF6, AFB2, and TIR1 levels as affected by CS and PS. (I) Goodness-of-fit of the models [as denoted by low Obs-pred/Obs ratios and the χ2 values (numbers on bars)] when considering either CS alone or CS and PS. (J) Enhanced expression of TIR1 by Dexamethasone induction is enough to promote hypocotyl growth. Means ± SE of (A, D, and F) five and (B) seven biological replicates or (J) 15 seedlings, and (C, E, and G) representative images. *P < 0.05; **P < 0.01; ***P < 0.001.
Changes in Auxin Perception/Signaling Sustain Growth Promotion.
The slope of the growth response to PS in seedlings exposed to intense CS was significantly reduced in the tir1 afb2 mutant compared with the wild type (growth rate/PS, 1.07 ± 0.08 and 1.69 ± 0.04, respectively; P < 0.0001), indicating that the commitment to shade avoidance requires auxin perception. To assess the physiological significance of the temporal dynamics of auxin perception and signaling components, we modeled growth as a function of the levels of AFB2 plus TIR1, MSG2/IAA19, IAA29, and ARF6 as established by CS (i.e., the values established by CS with PS = 0) or the combination of CS and PS, including several auxin-related mutants in the analysis. The model where dynamics of these components was modified according to CS and PS showed better goodness of fit (Fig. 4 H and I), suggesting that the effects of PS on auxin perception/signaling are important for the commitment to shade avoidance. If the growth promotion induced by prolonged shade is at least partially mediated by enhanced levels of auxin receptors, increasing the levels of TIR1 should, per se, promote hypocotyl growth. The enhanced growth observed in a TIR1 inducible line in the presence of inductor and the absence of shade (CS = 0, PS = 0) confirms this prediction of the model (Fig. 4J).
Persistent Shade Enhances Sensitivity to Auxin.
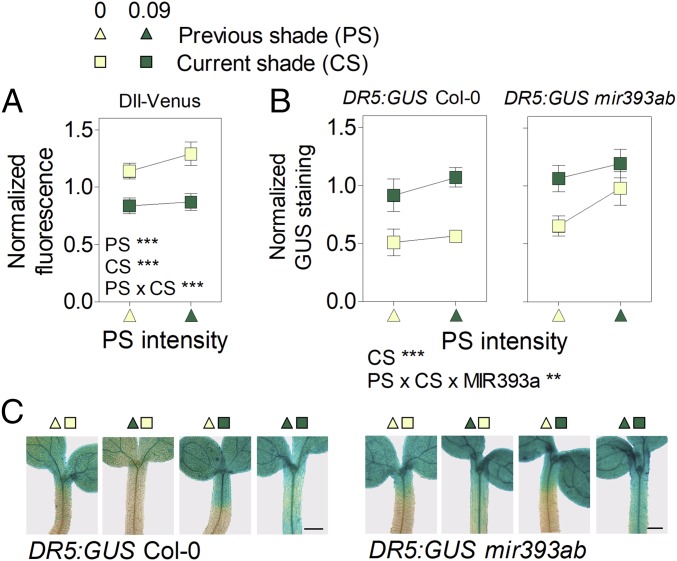
The model also predicts that persistent shade should enhance the sensitivity to auxin, and we tested this possibility with molecular markers. We observed reduced DII fluorescence in the epidermal cells of the hypocotyl (Fig. 5A) and increased GUS staining driven by DR5:GUS (Fig. 5B) in the hypocotyl in seedlings exposed to persistent shade compared with the light controls. The seedlings transferred from simulated shade to light showed relatively high DII and low DR5:GUS activities, indicating that the patterns observed under persistent shade are not the confounding consequence of lack of time to revert the effects of the high auxin levels experienced earlier under shade. Since light controls and seedlings exposed to persistent shade have similar levels of endogenous auxin (Fig. 1C), the differences in the activity of the DII and DR5:GUS markers can be attributed to enhanced sensitivity to auxin. Conversely, the reduced DII and high DR5:GUS staining observed in seedlings recently transferred to shade can be attributed to high auxin levels (Fig. 1C). In the mir393ab background, DR5:GUS levels failed to decrease normally upon transfer from simulated shade to sunlight (Fig. 5 B and C), confirming the importance of MIR393 in the control of sensitivity to auxin under fluctuating shade/light conditions.
Fig. 5.
Persistent shade increases auxin signaling despite the reduced auxin levels. (A) DII fluorescence in epidermal and subepidermal tissues of the hypocotyl. (B and C) GUS staining driven by DR5:GUS in the hypocotyl of seedlings of the Columbia wild type and of the mir393ab mutant background. (In C, scale bar = 200 μm.) Means ± SE of (A) 40 or (B) 8 biological replicates, and (C) representative images. **P < 0.01; ***P < 0.001.
MSG2/IAA19 and IAA29 Can Act to Promote Auxin Signaling.
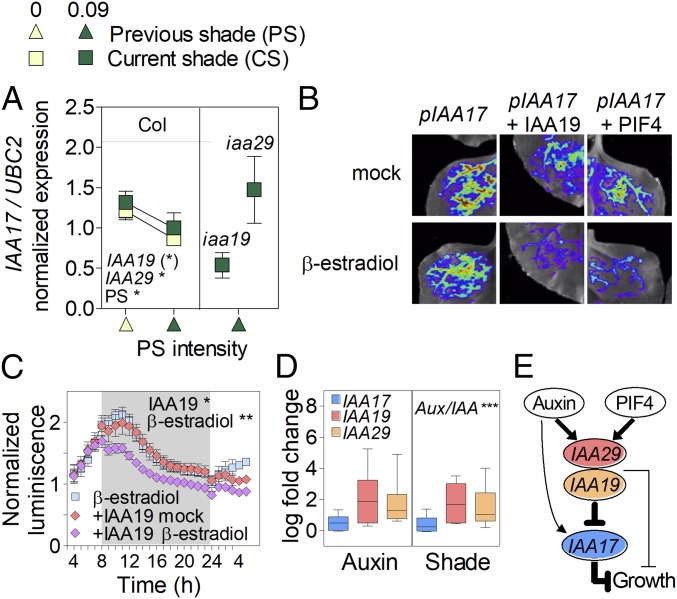
The mutant analysis revealed that MSG2/IAA19, IAA29, and AXR2/IAA7 promoted and AXR3/IAA17 inhibited hypocotyl growth (SI Appendix, Table S2). Given that hypocotyl growth under PS depends on auxin and Aux/IAA proteins repress auxin responses (20, 21), we propose that some Aux/IAA could indirectly promote growth, by suppressing auxin-induced expression of a subset of Aux/IAA genes with stronger growth inhibitory function. In favor of this interpretation, MSG2/IAA19 and IAA29 inhibited the expression of AXR3/IAA17 (Fig. 6A; based on gain- and loss-of-function mutants for IAA19 and IAA29, respectively). We also performed transactivation experiments by transiently coexpressing the pIAA17:LUC reporter together with a β-estradiol inducible IAA19 effector cassette in Nicotiana benthamiana leaves. The β-estradiol induction of the IAA19 effector decreased pIAA17 activity in this system, providing independent support to the idea that IAA19 can repress the expression of IAA17 (Fig. 6 B and C). Noteworthy is that PIF4 enhances the expression of IAA19 and IAA29 (Fig. 4A), and β-estradiol induction of the PIF4 effector also reduced pIAA17 activity (Fig. 6B). A metaanalysis showed that MSG2/IAA19 and IAA29 expression is more significantly induced by both shade and auxin in Arabidopsis than the expression of AXR3/IAA17 (Fig. 6D). Similarly, in soybean, four genes with similarity to MSG2/IAA19 showed increased expression in response to CS and PS (cluster 1) whereas AXR3/IAA17 (cluster 3) only showed a modest response (SI Appendix, Table S1). The weak promotion of AXR3/IAA17 by shade or auxin is consistent with the strong induction of IAA19 and IAA29 by these stimuli.
Fig. 6.
MSG2/IAA19 and IAA29 negatively regulate the strong growth inhibitor AXR3/IAA17 under prolonged shade. (A) Expression of IAA17 in the iaa19/msg2 gain of function and the iaa29 loss of function mutants compared with the Columbia wild type. Means ± SE of three biological replicates. (*) P < 0.06; *P < 0.05. (B and C) The β-estradiol induction of IAA19 or PIF4 effectors inhibits the activity of pIAA17:LUC reporter when transiently expressed in Nicotiana benthamiana leaves: (B) in vivo imaging and (C) time course of pIAA17:LUC activity in response to the β-estradiol−induced IAA19 effector. Median ± SE of 12 biological replicates. *P < 0.05; **P < 0.01. (D) Metaanalysis showing that neighbor signals and auxin induce MSG2/IAA19 and IAA29 expression to higher levels than expression of IAA17. Shown is fold change relative to controls without auxin or shade. The significance of the differences among Aux/IAA genes is indicated. ***P < 0.001. (E) Incoherent feed-forward loop involved in the promotion of growth by IAA19 and IAA29. Thicker lines indicate stronger positive (→) or negative (┤) regulations.
Discussion
The promotion of stem growth can be induced by neighbor signals within minutes (38–40) and persist for weeks (27). Analyses of plants of the same age subjected to different PS conditions exposed a further temporal dimension of the shade-avoidance syndrome, where prolonged shade reinforces the growth response and its auxin-related gene expression signature, both in Arabidopsis (Figs. 1B and 4A) and soybean (SI Appendix, Fig. S3 and Table S1).
The commitment to shade avoidance under prolonged shade requires auxin but is not mediated by sustained auxin accumulation (Fig. 1 A and C). We cannot rule out that prolonged shade has localized effects on auxin abundance that escaped our detection, but it is clear that the early auxin burst observed in response to shade does not accompany the response to prolonged shade. Previous studies based on pharmacology or expression of auxin-related genes suggested enhanced auxin sensitivity in response to neighbor signals (29, 30, 41, 42). Here we show that prolonged shade (i) increases the sensitivity to auxin as revealed by low DII fluorescence and high DR5:GUS staining (Fig. 5 B and C) in the absence of high auxin levels (Fig. 1C) and (ii) modifies auxin perception/signaling at multiple levels, by enhancing the abundance of AFB2 and TIR1, inducing MSG2/IAA19 and IAA29 expression, and reducing ARF6 abundance (Fig. 4 A–F). Mathematical modeling suggests a contribution of increased AFB2, TIR1, MSG2/IAA19, and IAA29 and reduced ARF6 to the promotion of growth under prolonged shade (Fig. 4 H and I and SI Appendix, Table S2). The role of TIR1 and AFB2 in the commitment to shade avoidance was confirmed by the reduced response to PS in tir1 afb2 and the growth promotion caused in the absence of shade by increased TIR1 expression in an inducible line (Fig. 4J).
We propose that the growth promotion by MSG2/IAA19 and IAA29 observed here (SI Appendix, Table S2) is indirect, via an incoherent feed-forward loop where they inhibit AXR3/IAA17 expression (Fig. 6E). In fact, AXR3/IAA17 is a strong growth inhibitor (SI Appendix, Table S2), and its expression is inhibited by MSG2/IAA19 and IAA29 (Fig. 6 A–C). Based on gene expression patterns, MSG2/IAA19 and IAA29 had been proposed to promote growth early in the morning (43); yet, these IAAs can antagonize auxin action in other contexts (18, 44). The observed growth inhibition by ARF6 (SI Appendix, Table S2) provides experimental support to the predictions of mathematical modeling indicating that increased ARF levels can reduce auxin responses in some contexts (23).
The phyB-PIF4 module and MIR393 linked the prolonged-shade cue to growth via changes in auxin sensitivity. The activity of phyB did not recover under prolonged shade (Fig. 2 A and B and SI Appendix, Figs. S4A and S5A). Persistent shade acted mainly by establishing and maintaining low levels of phyB activity (the response was fully distorted in the phyB mutant). In all tissues examined, we observed a rapid increment of the fluorescence driven by the pPIF4:PIF4-GFP transgene in response to shade (8). Prolonged shade maintained elevated levels of PIF4 in the epidermal and subepidermal tissues of the hypocotyl, and enhanced the accumulation of PIF4 in the hypocotyl vascular tissues (Fig. 3 A and B and SI Appendix, Fig. S5B). These high PIF4 levels would directly activate the expression of MSG2/IAA19 and IAA29 in the hypocotyl (Figs. 1B and 4A). However, prolonged shade led to a subsequent decrease in nuclear PIF4 levels in the cotyledon mesophyll cells, which can be linked to the decay in auxin levels. The reduction in MIR393 expression with prolonged shade (Fig. 4 D and E) could account for the increased accumulation of TIR1 and AFB2 (25, 26) (Fig. 4 B and C).
We conclude that sustained shade avoidance under prolonged cues from neighboring vegetation proceeds in two distinct temporal phases. During an initial triggering phase, PIFs bind to the promoters of auxin synthesis genes and generate a burst of auxin that promotes growth. This phase appears important to break the growth inertia. Later, the commitment phase is characterized by a system-level shift of the growth-controlling network which leads to a stronger physiological response, without the elevated levels of auxin observed during the initiation state. The initial phase triggered at the cotyledons gives place to a phase apparently dominated by hypocotyl events. Reduced levels of nuclear phyB, MIR393, IAA17/AXR3, and ARF6, together with the accumulation of PIF4, AFB2, TIR1, MSG2/IAA19, and IAA29, are characteristic of the second phase.
Materials and Methods
Plant materials, generation of transgenic plants, and shade intensities are described in SI Appendix, SI Materials and Methods. The details and procedures for the analyses of gene expression (RNA-seq, qRT-PCR, metaanalysis), growth rates, confocal microscopy, IAA abundance, protein blots, GUS activity (staining and fluorometric), dexamethasone induction, agroinfiltration, and transient activation assays are provided in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dolf Weijers (Wageningen University), Vinicius Galvao (University of Lausanne), Jason W. Reed (University of North Carolina), Mark Estelle (University of California, San Diego), Franck Vazquez (Botanical Institute Basel), Seth J. Davis (University of York), Chuanyou Li (Institute of Genetics and Developmental Biology), and the Arabidopsis Biological Resource Center for seed samples. Supported by the Argentine Agencia Nacional de Promoción Científica y Tecnológica (Grant PICT-2015-1796), Alexander von Humboldt Foundation, Universidad de Buenos Aires (Grant 20020100100437), and Fundación Rene Baron.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1721110115/-/DCSupplemental.
References
- 1.Fraser DP, Hayes S, Franklin KA. Photoreceptor crosstalk in shade avoidance. Curr Opin Plant Biol. 2016;33:1–7. doi: 10.1016/j.pbi.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Casal JJ. Photoreceptor signaling networks in plant responses to shade. Annu Rev Plant Biol. 2013;64:403–427. doi: 10.1146/annurev-arplant-050312-120221. [DOI] [PubMed] [Google Scholar]
- 3.Ballaré CL, Pierik R. The shade-avoidance syndrome: Multiple signals and ecological consequences. Plant Cell Environ. 2017;40:2530–2543. doi: 10.1111/pce.12914. [DOI] [PubMed] [Google Scholar]
- 4.López Pereira M, Sadras VO, Batista W, Casal JJ, Hall AJ. Light-mediated self-organization of sunflower stands increases oil yield in the field. Proc Natl Acad Sci USA. 2017;114:7975–7980. doi: 10.1073/pnas.1618990114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leivar P, Quail PH. PIFs: Pivotal components in a cellular signaling hub. Trends Plant Sci. 2011;16:19–28. doi: 10.1016/j.tplants.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee N, Choi G. Phytochrome-interacting factor from Arabidopsis to liverwort. Curr Opin Plant Biol. 2017;35:54–60. doi: 10.1016/j.pbi.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Leivar P, Monte E, Cohn MM, Quail PH. Phytochrome signaling in green Arabidopsis seedlings: Impact assessment of a mutually negative phyB-PIF feedback loop. Mol Plant. 2012;5:734–749. doi: 10.1093/mp/sss031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lorrain S, Allen T, Duek PD, Whitelam GC, Fankhauser C. Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J. 2008;53:312–323. doi: 10.1111/j.1365-313X.2007.03341.x. [DOI] [PubMed] [Google Scholar]
- 9.Li L, et al. Linking photoreceptor excitation to changes in plant architecture. Genes Dev. 2012;26:785–790. doi: 10.1101/gad.187849.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pedmale UV, et al. Cryptochromes interact directly with PIFs to control plant growth in limiting blue light. Cell. 2016;164:233–245. doi: 10.1016/j.cell.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Wit M, et al. Integration of phytochrome and cryptochrome signals determines plant growth during competition for light. Curr Biol. 2016;26:3320–3326. doi: 10.1016/j.cub.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 12.Hornitschek P, et al. Phytochrome interacting factors 4 and 5 control seedling growth in changing light conditions by directly controlling auxin signaling. Plant J. 2012;71:699–711. doi: 10.1111/j.1365-313X.2012.05033.x. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, et al. A quartet of PIF bHLH factors provides a transcriptionally centered signaling hub that regulates seedling morphogenesis through differential expression-patterning of shared target genes in Arabidopsis. PLoS Genet. 2013;9:e1003244. doi: 10.1371/journal.pgen.1003244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tao Y, et al. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell. 2008;133:164–176. doi: 10.1016/j.cell.2008.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Procko C, Crenshaw CM, Ljung K, Noel JP, Chory J. Cotyledon-generated auxin is required for shade-induced hypocotyl growth in Brassica rapa. Plant Physiol. 2014;165:1285–1301. doi: 10.1104/pp.114.241844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keuskamp DH, Pollmann S, Voesenek LACJ, Peeters AJM, Pierik R. Auxin transport through PIN-FORMED 3 (PIN3) controls shade avoidance and fitness during competition. Proc Natl Acad Sci USA. 2010;107:22740–22744. doi: 10.1073/pnas.1013457108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Procko C, et al. The epidermis coordinates auxin-induced stem growth in response to shade. Genes Dev. 2016;30:1529–1541. doi: 10.1101/gad.283234.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michaud O, Fiorucci AS, Xenarios I, Fankhauser C. Local auxin production underlies a spatially restricted neighbor-detection response in Arabidopsis. Proc Natl Acad Sci USA. 2017;114:7444–7449. doi: 10.1073/pnas.1702276114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pantazopoulou CK, et al. Neighbor detection at the leaf tip adaptively regulates upward leaf movement through spatial auxin dynamics. Proc Natl Acad Sci USA. 2017;114:7450–7455. doi: 10.1073/pnas.1702275114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lavy M, Estelle M. Mechanisms of auxin signaling. Development. 2016;143:3226–3229. doi: 10.1242/dev.131870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weijers D, Wagner D. Transcriptional responses to the auxin hormone. Annu Rev Plant Biol. 2016;67:539–574. doi: 10.1146/annurev-arplant-043015-112122. [DOI] [PubMed] [Google Scholar]
- 22.Middleton AM, King JR, Bennett MJ, Owen MR. Mathematical modelling of the Aux/IAA negative feedback loop. Bull Math Biol. 2010;72:1383–1407. doi: 10.1007/s11538-009-9497-4. [DOI] [PubMed] [Google Scholar]
- 23.Vernoux T, et al. The auxin signalling network translates dynamic input into robust patterning at the shoot apex. Mol Syst Biol. 2011;7:508. doi: 10.1038/msb.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winkler M, et al. Variation in auxin sensing guides AUX/IAA transcriptional repressor ubiquitylation and destruction. Nat Commun. 2017;8:15706. doi: 10.1038/ncomms15706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navarro L, et al. A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science. 2006;312:436–439. doi: 10.1126/science.1126088. [DOI] [PubMed] [Google Scholar]
- 26.Si-Ammour A, et al. miR393 and secondary siRNAs regulate expression of the TIR1/AFB2 auxin receptor clade and auxin-related development of Arabidopsis leaves. Plant Physiol. 2011;157:683–691. doi: 10.1104/pp.111.180083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan DC, Smith H. The relationship between phytochrome-photoequilibrium and development in light grown Chenopodium album L. Planta. 1978;142:187–193. doi: 10.1007/BF00388211. [DOI] [PubMed] [Google Scholar]
- 28.Das D, St Onge KR, Voesenek LA, Pierik R, Sasidharan R. Ethylene- and shade-induced hypocotyl elongation share transcriptome patterns and functional regulators. Plant Physiol. 2016;172:718–733. doi: 10.1104/pp.16.00725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bou-Torrent J, et al. Plant proximity perception dynamically modulates hormone levels and sensitivity in Arabidopsis. J Exp Bot. 2014;65:2937–2947. doi: 10.1093/jxb/eru083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Wit M, Ljung K, Fankhauser C. Contrasting growth responses in lamina and petiole during neighbor detection depend on differential auxin responsiveness rather than different auxin levels. New Phytol. 2015;208:198–209. doi: 10.1111/nph.13449. [DOI] [PubMed] [Google Scholar]
- 31.Klose C, et al. Systematic analysis of how phytochrome B dimerization determines its specificity. Nat Plants. 2015;1:15090. doi: 10.1038/nplants.2015.90. [DOI] [PubMed] [Google Scholar]
- 32.Hornitschek P, Lorrain S, Zoete V, Michielin O, Fankhauser C. Inhibition of the shade avoidance response by formation of non-DNA binding bHLH heterodimers. EMBO J. 2009;28:3893–3902. doi: 10.1038/emboj.2009.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nusinow DA, et al. The ELF4-ELF3-LUX complex links the circadian clock to diurnal control of hypocotyl growth. Nature. 2011;475:398–402. doi: 10.1038/nature10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nieto C, López-Salmerón V, Davière J-M, Prat S. ELF3-PIF4 interaction regulates plant growth independently of the evening complex. Curr Biol. 2015;25:187–193. doi: 10.1016/j.cub.2014.10.070. [DOI] [PubMed] [Google Scholar]
- 35.Pacín M, Semmoloni M, Legris M, Finlayson SA, Casal JJ. Convergence of CONSTITUTIVE PHOTOMORPHOGENESIS 1 and PHYTOCHROME INTERACTING FACTOR signalling during shade avoidance. New Phytol. 2016;211:967–979. doi: 10.1111/nph.13965. [DOI] [PubMed] [Google Scholar]
- 36.Tatematsu K, et al. MASSUGU2 encodes Aux/IAA19, an auxin-regulated protein that functions together with the transcriptional activator NPH4/ARF7 to regulate differential growth responses of hypocotyl and formation of lateral roots in Arabidopsis thaliana. Plant Cell. 2004;16:379–393. doi: 10.1105/tpc.018630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krogan NT, Yin X, Ckurshumova W, Berleth T. Distinct subclades of Aux/IAA genes are direct targets of ARF5/MP transcriptional regulation. New Phytol. 2014;204:474–483. doi: 10.1111/nph.12994. [DOI] [PubMed] [Google Scholar]
- 38.Morgan D, Smith H. Simulated sunflecks have large, rapid effects on plant stem extension. Nature. 1978;273:534–536. [Google Scholar]
- 39.Morgan DC, O’Brien T, Smith H. Rapid photomodulation of stem extension in light-grown Sinapis alba L.: Studies on kinetics, site of perception and photoreceptor. Planta. 1980;150:95–101. doi: 10.1007/BF00582351. [DOI] [PubMed] [Google Scholar]
- 40.Cole B, Kay SA, Chory J. Automated analysis of hypocotyl growth dynamics during shade avoidance in Arabidopsis. Plant J. 2011;65:991–1000. doi: 10.1111/j.1365-313X.2010.04476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hersch M, et al. Light intensity modulates the regulatory network of the shade avoidance response in Arabidopsis. Proc Natl Acad Sci USA. 2014;111:6515–6520. doi: 10.1073/pnas.1320355111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krishna Reddy S, Finlayson SA. Phytochrome B promotes branching in Arabidopsis by suppressing auxin signaling. Plant Physiol. 2014;164:1542–1550. doi: 10.1104/pp.113.234021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kunihiro A, et al. Phytochrome-interacting factor 4 and 5 (PIF4 and PIF5) activate the homeobox ATHB2 and auxin-inducible IAA29 genes in the coincidence mechanism underlying photoperiodic control of plant growth of Arabidopsis thaliana. Plant Cell Physiol. 2011;52:1315–1329. doi: 10.1093/pcp/pcr076. [DOI] [PubMed] [Google Scholar]
- 44.Sun J, Qi L, Li Y, Zhai Q, Li C. PIF4 and PIF5 transcription factors link blue light and auxin to regulate the phototropic response in Arabidopsis. Plant Cell. 2013;25:2102–2114. doi: 10.1105/tpc.113.112417. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.