Significance
Many animals, including humans, can generate identical twins from a single egg. We perfected a method by which a frog (Xenopus) egg cut in half along the dorsal–ventral (back to belly) axis at the 4,000-cell stage produced twins at high frequency. The large wound generated by bisection healed within an hour, juxtaposing cells that would normally form the most dorsal and ventral tissues in the intact embryo. Tracing the fate of microinjected cells showed that the dorsal Spemann organizer was formed 90° away from its original location in bisected embryos. A new gradient of dorsal–ventral signaling was generated by this displacement, explaining the regeneration of the missing half. The experiments help explain twinning in a classic model system.
Keywords: regeneration, morphogens, gradients, Chordin, monozygotic twins
Abstract
The formation of identical twins from a single egg has fascinated developmental biologists for a very long time. Previous work had shown that Xenopus blastulae bisected along the dorsal–ventral (D-V) midline (i.e., the sagittal plane) could generate twins but at very low frequencies. Here, we have improved this method by using an eyelash knife and changing saline solutions, reaching frequencies of twinning of 50% or more. This allowed mechanistic analysis of the twinning process. We unexpectedly observed that the epidermis of the resulting twins was asymmetrically pigmented at the tailbud stage of regenerating tadpoles. This pigment was entirely of maternal (oocyte) origin. Bisecting the embryo generated a large wound, which closed from all directions within 60 minutes, bringing cells normally fated to become Spemann organizer in direct contact with predicted ventral-most cells. Lineage-tracing analyses at the four-cell stage showed that in regenerating embryos midline tissues originated from the dorsal half, while the epidermis was entirely of ventral origin. Labeling of D-V segments at the 16-cell stage showed that the more pigmented epidermis originated from the ventral-most cells, while the less-pigmented epidermis arose from the adjoining ventral segment. This suggested a displacement of the organizer by 90°. Studies with the marker Chordin and phospho-Smad1/5/8 showed that in half embryos a new D-V gradient is intercalated at the site of the missing half. The displacement of self-organizing morphogen gradients uncovered here may help us understand not only twin formation in amphibians, but also rare cases of polyembryony.
Animal embryos have a remarkable capacity of self-organization that has fascinated biologists since the beginning of experimental embryology. In 1891, Hans Driesch separated the first two blastomeres of a sea urchin embryo and found that each one was able to self-organize and give rise to complete, although smaller, embryos (1). Later, it was found that each of the first four blastomeres of a sea urchin could form normal larvae (2). In 1895, Thomas Hunt Morgan removed one of the two blastomeres of a frog embryo and generated a complete embryo from half an egg (3). In amphibians it was possible, by constriction with a hair loop at the two-cell stage, to obtain two embryos of half size but normal proportions from the same egg (4, 5). Duplicated embryos were also obtained by partially sectioning insect eggs (6), indicating that the intriguing self-organizing ability of developmental systems is widespread. These experiments showed that when an embryo is damaged, it tends to self-regulate toward formation of the whole.
Spemann showed that at the start of gastrulation the newt embryo could be subdivided by constriction into a dorsal and ventral fragment, generating a dorsal half embryo scaled to normal proportions and a ventral half embryo consisting of ventral tissues devoid of an axis, while sagittal constriction did not form twins (7). Xenopus is the model organism for these studies in modern times. It has many advantages, such as a large number of eggs produced throughout the year. A key experimental advantage is a cortical rotation in the fertilized egg (8) that results in the displacement of the maternal oocyte pigment, forming a dorsal crescent, which reliably marks the dorsal (back) side of the embryo in regularly cleaving embryos (9). The opposite, darker side of the embryo gives rise to the ventral (belly) side. The displacement of egg cytoplasmic determinants along microtubules toward the dorsal side triggers an early Wnt signal (10), which is responsible for localizing the subsequent formation of the Spemann organizer signaling center in the marginal zone at the gastrula stage. The Spemann organizer is a tissue that secretes a mixture of growth factor antagonists, such as Chordin, Noggin, Follistatin, Cerberus, Frzb1, and Dickkopf, which are able to induce embryonic cell differentiation (11). Diffusion of these antagonists establishes a dorsal–ventral (D-V) gradient of bone morphogenetic protein (BMP) activity, and a ventral center secreting other molecules, such as BMP4/7, Sizzled, and Tolloid is formed at the side of maximal BMP signaling (12, 13).
In Xenopus, dorsal- and ventral-half embryos can be generated by simply cutting the embryo with forceps or a surgical blade (14). The ventral half lacks an organizer and develops as a mass of ventral tissues with very high BMP signaling (12). Embryos bisected sagittally at the gastrula stage fail to form twins. In 2006, we reported that identical twins could self-organize after sagittal bisection of Xenopus embryos (using forceps or a metal knife) at the 4,000-cell blastula stage, before Spemann’s organizer is formed (15). Although remarkable, this regeneration occurred only at very low frequency (about 1%), precluding meaningful mechanistic analyses. The mystery of how the embryo achieves this ultimate regeneration of an entire missing body half has remained an enduring puzzle.
In the present study, we improved the bisection technique of blastula embryos, resulting in increased frequency of twinning after sagittal bisection, obtaining 50% or more twinning. This made the regeneration process amenable to analysis. We unexpectedly noted that in properly regenerating twins the maternal pigment of the egg was concentrated on one side of the tadpole epidermis in preference to the other. Normally, the epidermis on the left and right sides of the embryo have equal pigmentation, which is entirely of maternal (egg) origin in Xenopus. Investigation of this asymmetry led to the finding that, following healing of the bisected blastula, the organizer is formed at a distance from the place where it would normally develop. This displacement in the position of the organizer explains how the missing half of the embryo is regenerated.
Results
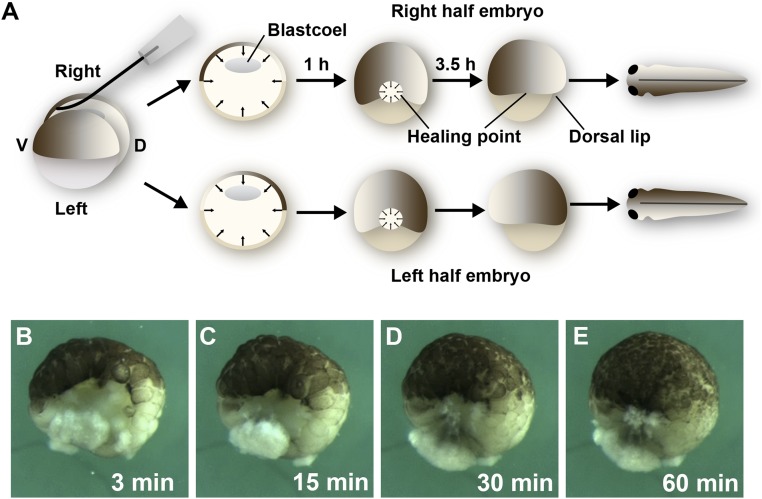
We noted a higher frequency of identical twins by bisecting Xenopus laevis stage 8 blastulae (16) along the sagittal plane (dividing the dorsal crescent) when an eyelash (from Y.M.) was used to cut the embryo instead of using metal instruments (Fig. 1A and SI Appendix, Movie S1). The large vegetal cells could still be separated using forceps, but the initial use of the eyelash knife greatly improved regeneration. Other improvements were provided by performing the surgery in 0.3× modified Barth solution (MBS) (17), allowing the half embryos to heal for 60 min, and transferring to 0.1× MBS for further culture. Using this improved technique up to 50% of twins, complete with development of two eyes (and more rarely of one eye), was observed. After bisection, cells rapidly migrate from all directions to close the large gap left by the bisection wound, which becomes smaller and smaller until 60 min later only a small healing point remains (Fig. 1 B–E and SI Appendix, Movie S2). This healing process results in half embryos in which the most dorsal region becomes directly juxtaposed to the cells that would become the most-ventral region during the course of normal development.
Fig. 1.
Sagittal bisection of Xenopus blastulae with an eyelash knife generates twins. (A) Diagram showing that bisection by an eyelash knife (SI Appendix, Movie S1). Bisection leaves a large wound that heals by tissue movements from all directions, closing the gap within 1 h, and leaving only a small healing point where the dorsal- and ventral-most cells become juxtaposed. Dorsal lip formation starts 3.5 h later and pigment asymmetry can be observed 1 d later in the epidermis of tailbud tadpoles. (B–E) Still images from SI Appendix, Movie S2 showing the process of convergence of blastula cell sheets toward a central healing point at which the original dorsal and ventral tissues become juxtaposed. Note that cell divisions continue in the blastula as the healing process is completed, but cell division near the wound proceeds at the same rate as in the rest of the embryo. The 1.3× objective lens of Zeiss stereomicroscope, which was connected to a DCR-PC350 Sony Handicam, was used for images.
Pigment Asymmetry in the Epidermis of Xenopus Twins.
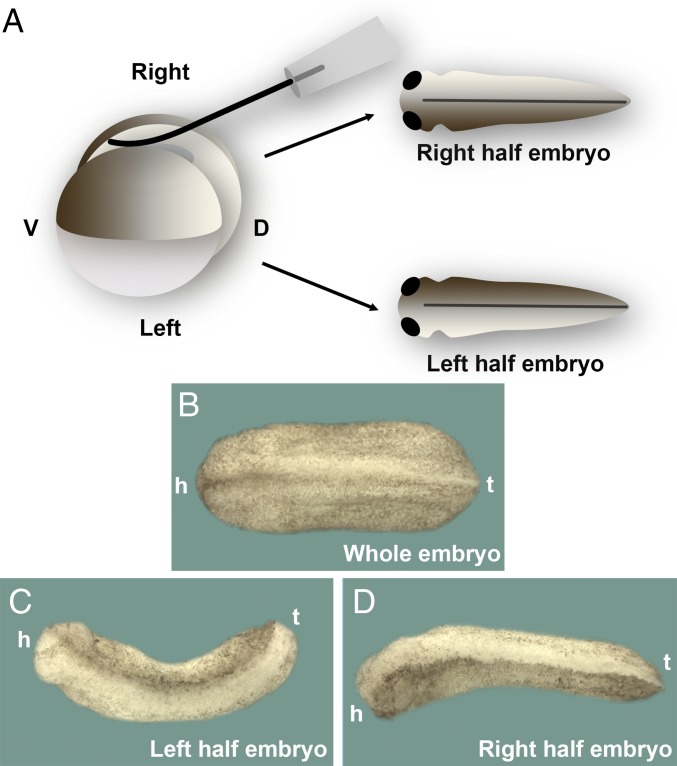
We were surprised to find that a striking asymmetry in epidermal pigmentation was observed in most twins that healed properly (Fig. 2A). The pigmentation during the first 2 d of development in Xenopus embryos is entirely derived from the cortical pigment granules provided by the oocyte, and the pigment is uniformly distributed on both sides of the wild-type tailbud tadpole (Fig. 2B). When twins are formed, a darker pigmentation was found at either the left or right side of the half embryos (Fig. 2 C and D; note that both half embryos derived from same egg developed complete axes with two eyes). This unexpected observation provided the starting point of the following investigations on the developmental mechanics by which the missing half of the embryo is regenerated.
Fig. 2.
Xenopus embryos bisected sagittally develop into twins with an intriguing asymmetric epidermal pigmentation. (A) Experimental diagram. Half embryos were cultured in the same dish until tailbud stage. (B) Whole embryo; note that pigmentation of the epidermis is uniform on both sides. All of this pigment is inherited maternally from the oocyte. h, head; t, tail. (C and D) Phenotype of left and right half embryos from the same egg. Note that one side of epidermis is strikingly more pigmented than the other (n = 204 half embryos with pigment asymmetry). The search for the cause of this asymmetry was the starting point for the investigations reported here. The 40× digital magnification zoom of Axio Zoom V.16 Stereo Zoom Zeiss was used for images.
The Entire Epidermis Is Derived from Ventral Lineages.
We investigated the regeneration process by lineage tracing of dorsal or ventral halves of the embryos. This was achieved by microinjection of a mixture of GFP or RFP mRNAs consisting of soluble, membrane-targeted, and histone H2B fluorescent protein fusions into the two ventral or dorsal blastomeres of regularly cleaving embryos (9). Embryos were cultured until early tailbud (stage 22), embedded in low-melting agarose, and serially sectioned with a Vibratome (Fig. 3A). We naïvely presumed that the less-pigmented epidermis would be dorsal in origin, but found otherwise.
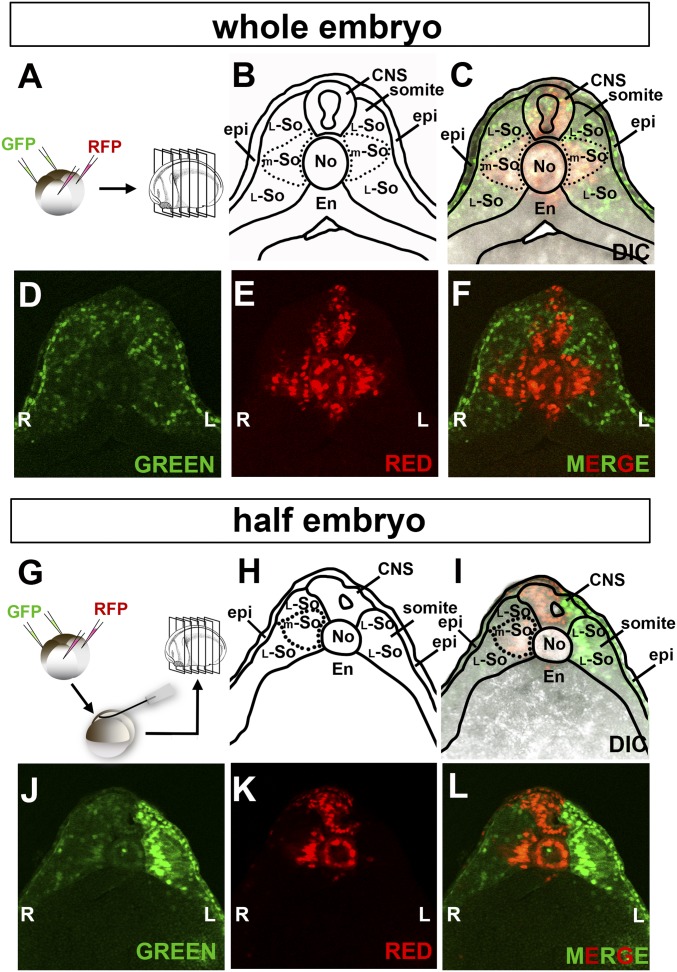
Fig. 3.
Fate of dorsal and ventral cells marked at the four-cell embryos: dorsal cells give rise to the midline even in half embryos. (A) Diagram of lineage tracing in whole embryos. A mixture of GFP mRNAs was microinjected into the two ventral blastomeres while RFP mRNAs were injected into dorsal ones, embryos cultured until tailbud, and sectioned with a Vibratome. (B–F) Sections showing that the entire epidermis (except for a few cells in the dorsal midline) and most of the lateral somite was derived from the ventral side (in green), while midline trunk organs, such as the CNS, notochord, dorsal endoderm, and medial somite were derived from the dorsal side (in red) (n = 28). (G) Experimental diagram of lineage tracing in half embryos bisected at midblastula. (H–L) Section of a right-side half embryo showing that tissues in the dorsal midline derived from the dorsal side, while the epidermis and lateral somite were still of ventral origin (n = 20). The 5× objective lens of LSM880 Zeiss confocal microscope was used for images. En, endoderm; epi, epidermis; L, left; L-So, lateral somite; m-So, medial somite; No, notochord; R, right.
In the whole embryo, the dorsal blastomeres give rise to the notochord, dorsal endoderm, and most of the CNS in the trunk region (Fig. 3 A–F). Part of the somite lying next to the notochord was also found to be of dorsal origin, and we designated this region the medial somite. The rest of somite was of ventral origin. We were surprised by this but should not have been, because in the chicken embryo it has long been known that the medial somite derives from regions close to Hensen’s node and the lateral somite from more posterior (i.e., ventral) regions in the primitive streak (18, 19). Lineage studies at neurula stages (Fig. 4) revealed that in Xenopus the medial somite is formed as a result of convergence and extension movements in the embryo by which cells of lateral origin move toward the midline above and below the medial somite. In fact, the entire somite was found to have an exquisite fate map according to the D-V origin of its cells during cleavage. At the 16-cell stage individual D-V segments can be labeled by four injections (SI Appendix, Fig. S1A). These segments are designated 1–4 (for example, segment 1 is the one that gives rise to dorsal blastomeres A1, B1, C1, and D1 at the 32-cell stage) (20). Labeling each segment in different colors at the 16-cell stage showed that the Xenopus somites (and presumably the presomitic mesoderm from which they derive) had highly organized cell origins (SI Appendix, Fig. S1 B–I). Perhaps more importantly for the present analysis, the epidermis was found to be mostly of ventral origin (Figs. 3D and 4 C and D).
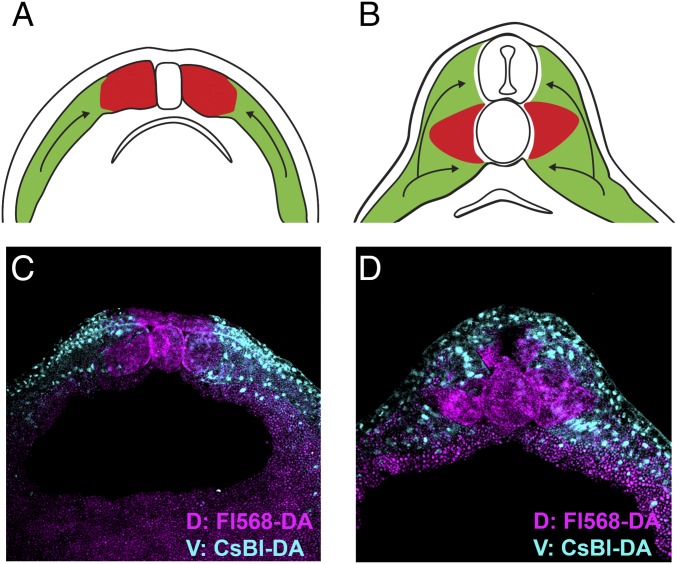
Fig. 4.
The origin of medial somite in Xenopus. The medial region of the Xenopus somite is normally derived from the dorsal half of the embryo while more lateral regions of the somite migrate over and under it during movements of convergence and extension in the tailbud embryo. Embryos were lineage-traced by injecting the two dorsal or two ventral blastomeres at the four-cell stage and sectioned at neurula or tailbud stages. (A and B) Diagram showing that the region of the somite next to the notochord originates from dorsal cells (in red), and during development is surrounded by converging more lateral cells originating from the ventral half of the embryo (in green). (C) Stage 16 neurula embryo before CNS closure labeled ventrally with Cascade blue dextran amine (CsBl-DA) and dorsally with fluorescent Alexa 568 dextran amine (F1568-DA) at the four-cell stage. Note that the medial somite, notochord, and neural plate are of dorsal origin (n = 3). (D) Xenopus sibling sectioned at tailbud stage 22 showing that the medial somite, ventral spinal cord, notochord and dorsal endoderm are of dorsal origin, while the rest of the somite and all of the epidermis are ventral (n = 2). Images were acquired with the 5× objective lens of LSM880 Zeiss confocal microscope. D, dorsal; V, ventral.
In bisected embryos, the dorsal region gave rise to the trunk midline, while the epidermis derived from the ventral lineages, which was also the case in the whole embryo (Fig. 3 G–L). Given that the dorsal-most and ventral-most tissues of the blastula become juxtaposed during healing, this suggested that profound rearrangements in cell fate must take place during twinning. As shown in SI Appendix, Fig. S2 and the corresponding SI Appendix, Movie S3, while the healing point is located precisely at the intersection of dorsal and ventral tissues, the dorsal blastopore lip forms at a distance of about 90° within the dorsal region (SI Appendix, Fig. S2D′, arrowhead). Gastrulation directs involution starting from the dorsal lip, and by the end of the process both the left and right epidermis were derived from the ventral side in half embryos (SI Appendix, Fig. S2F′ and Movie S3). This indicated that although the original dorsal-most tissue was located at the healing point, the site of formation of the Spemann organizer was displaced. A similar indication was provided by the fact that the neural plate was mostly of dorsal origin and that its midline, under which the axial mesoderm involutes, is flanked by dorsal tissues (SI Appendix, Fig. S3 and Movie S4).
In conclusion, these experiments in which four-cell embryos were lineage-traced in the dorsal and ventral halves suggested a displacement of the Spemann’s organizer in sagittally bisected embryos.
Origin of the Pigmented and Less-Pigmented Epidermis.
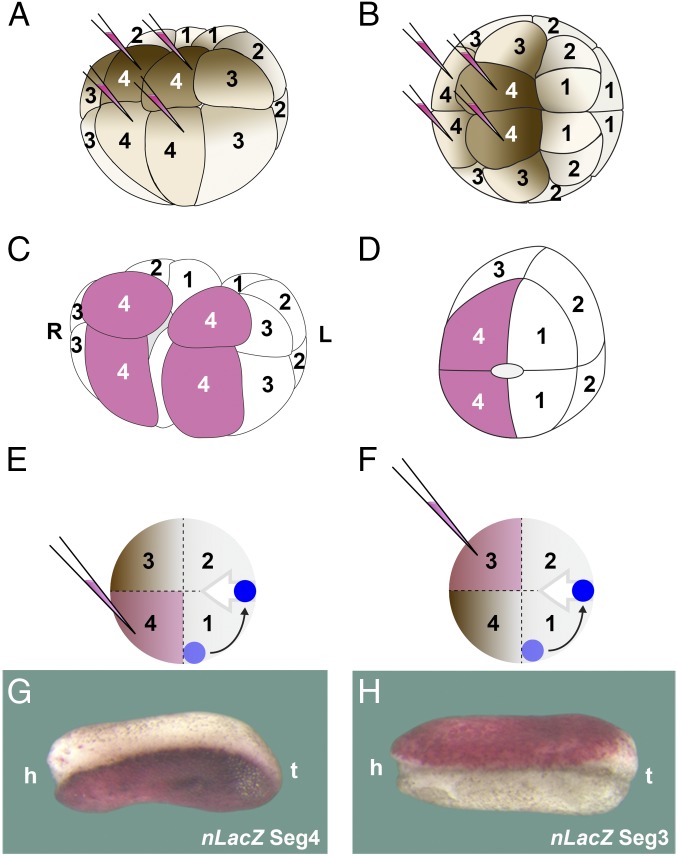
We next traced the origin of the intriguing epidermal difference in pigmentation in regenerating twins. At the 16-cell stage the embryo can be subdivided into four segments via four injections of lineage tracer (Fig. 5 A and B). We labeled each of the four 16-cell segments in this way, which were then sagittally bisected at stage 8 (Fig. 5 C and D). We observed that the more pigmented epidermis invariably derived from segment 4, which is the most ventral one (n = 36) (Fig. 5 E and G). The less-pigmented epidermis of the twins, which we had speculated might arise from the dorsal side, instead originated from descendants of segment 3 (Fig. 5 F and H). Segment 3 is the second-most ventral one and is less pigmented than segment 4 (Fig. 5 A and B). These results indicate that the involuting axial mesodermal cells separate the border of segment 3 and 4. This implies that the organizer, which should have been formed in segment 1 at the region now juxtaposed to the ventral-most cells, was now formed at a distance of the healing point, displaced about by 90° within the dorsal half of the embryo, as indicated in the diagrams in Fig. 5 E and F.
Fig. 5.
Experiment showing that the maternal pigment asymmetry in twins results from the splitting of ventral segments 3 and 4 during gastrulation; this suggests that Spemman organizer formation is displaced about 90° from its original site in the intact embryo. (A and B) Diagram indicating that segment 4 can be labeled by four injections of nLacZ mRNA at 16-cell stage in ventral and dorsal views; note that segment 3 is less pigmented than segment 4. All segments were analyzed in this way. (C) Sagittal bisection splits the embryo in two halves, allowing one to follow the fate of each segment (in the case shown here the ventral-most segment 4). (D) Diagram of a half embryo showing that during the healing process, segment 4 is juxtaposed to the dorsal-most segment 1. The healing scar is outlined in the center of half embryo. (E and F) Diagram indicating how a displacement of organizer formation (in blue) would result in the splitting of segments 3 and 4 by the involuting mesoderm (gray arrow). (G) In half embryos, the more pigmented side is derived from the segment 4 after regeneration (n = 36). (H) In bisected twins, the less pigmented epidermis is derived from segment 3 (n = 24). Note that the epidermis without lineage tracer is lighter in G than in H; both half embryos are from the same clutch and therefore the pigmentation of the epidermis is comparable. The diagrams in D–F and the embryos in G and H all correspond to right half embryos. The 40× digital magnification zoom of Axio Zoom V.16 Stereo Zoom Zeiss was used for images. h, head; L, left; R, right t, tail.
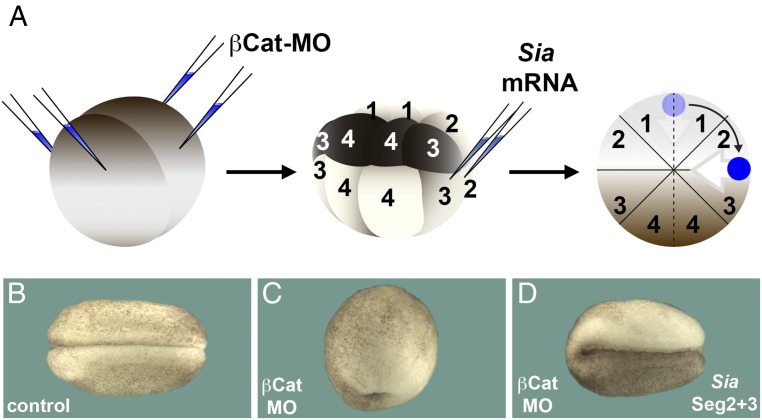
Organizer formation does not occur at an invariable place. To illustrate that displacement of the organizer can indeed cause pigment asymmetry in the Xenopus embryo, we used an experimental approach in which the position of organizer can be manipulated at will. Embryos microinjected with β-catenin morpholino (βCat MO) do not form an organizer for lack of the early Wnt/βCat signal in the dorsal crescent, resulting in radially ventralized embryos lacking all dorsal structures (21, 22). Microinjection of mRNA encoding the early Wnt target transcription factor Siamois can restore organizer formation at any site of injection along the marginal zone of βCat-depleted embryos (23, 24) (Fig. 6A). When Siamois mRNA was microinjected into the lateral marginal zone 90° from the site at which the organizer would have been normally formed (border between segment 2 and 3), the asymmetric pigmentation of the epidermis found in bisected twins was mimicked (Fig. 6 B–D).
Fig. 6.
Displacement of organizer formation can lead to pigment asymmetry in whole embryos. (A) Diagram of experiment in which organizer formation is blocked by βCat antisense MO and subsequently a new organizer displaced by 90° (dark blue) is induced by injecting Siamois mRNA. Gray arrow indicates the direction of gastrulation. (B) Control embryo; note uniform pigmentation of the epidermis (n = 46). (C) Embryo injected with βCat MO showing a ventralized phenotype lacking all axial structures due to loss of Spemann’s organizer (n = 50). (D) Embryo injected with βCat MO and rescued by injection of Siamois mRNA into the vegetal tier of segments 2 and 3 injected embryo at the 16-cell stage (n = 62, of which 60 showed pigment asymmetry). Note that the pigmentation of the epidermis is asymmetrical after inducing a new organizer displaced by 90° from its original site. The 40× digital magnification zoom of Axio Zoom V.16 Stereo Zoom Zeiss was used for images.
Taken together, these experiments indicate that in sagittally bisected embryos the organizer is not formed at its original site at the junction of segment 1 and 4, but is instead displaced by about 90° to the segment 1 and 2 border. Presumably this displacement is caused by signals emanating from the ventral-most region, which was experimentally juxtaposed to the dorsal-most region.
The Dorsal and Ventral Signaling Centers Are Displaced in Regenerating Twins.
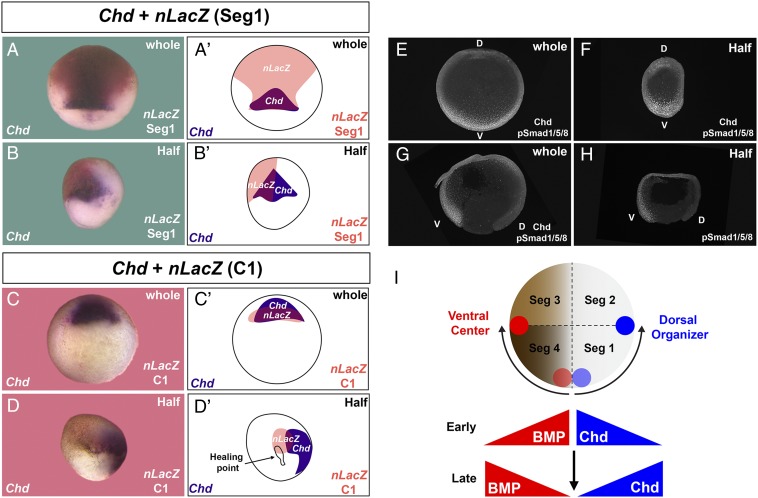
To investigate further whether the Spemann organizer formation is displaced after healing of half embryos, segment 1 was lineage labeled with nLacZ mRNA at the 16-cell stage, cultured until gastrula, and embryos analyzed by in situ hybridization for the organizer marker chordin (chd). The domain of expression of chd was included within the segment 1 lineage in the whole embryo, but in sagittally bisected half embryos chd expression was found in segment 1 and in adjoining uninjected regions corresponding to segment 2 (Fig. 7 A–B′). Similarly, labeling of the dorsal C1 blastomere at 32-cell stage showed lineage tracing overlapping with chd in the whole embryo but displaced from the C1 lineage (and the healing point scar) in regenerating embryos (Fig. 7 C–D′).
Fig. 7.
Formation of the organizer and the ventral center was displaced in half embryos arising by sagittal bisection. (A and A′) The organizer marker chd was expressed within the progeny of segment 1 (n = 65). Embryos were lineage labeled at 16-cell and cultured until gastrula. (B and B′) In half embryos the chd-expressing region only partially overlapped with segment 1, indicating a displacement of the organizer from its normal location (n = 101, 9 independent experiments). (C and C′) The progeny of blastomere C1 at the 32-cell stage also overlapped with chd (n = 4). (D and D′) In half embryos the chd+ organizer region was displaced at a distance from the original dorsal C1 region (n = 13); in this particular embryo the healing point at which the original dorsal- and ventral-most cells were juxtaposed was still visible, underscoring the extent of the displacement. (E) Staining with antiphospho-Smad1/5/8 antibody in whole embryos showing the ventral BMP gradient (n = 2). (F) The ventral center (high BMP signaling) was regenerated in half embryos; staining with Chd antibody (weaker because the much stronger pSmad1/5/8 signal required stopping the staining) indicated that both centers were formed opposite each other (n = 2). (G) Phospho-Smad1/5/8 signaling in sagittal sections of stage 12 gastrula embryos (n = 6). (H) Regenerated half embryo showing that the ventral (V) center was formed directly opposite the dorsal (D) blastopore lip (n = 4). Right-side half embryos are shown in B, D, and H, while a left-side half embryo is shown in F. (I) Hypothetical diagram indicating how the dorsal organizer and ventral center are displaced in opposite directions in half embryos. The original dorsal center region (light blue) is displaced 90° to the boundary between segment 1 and 2 (dark blue). The ventral center is also displaced from the region in segment 4 next to the healing point (light red) to the boundary between segments 3 and 4 (dark red). Early after healing at blastula the prospective regions of high BMP and high Chd signaling are juxtaposed in the half embryo. However, by the gastrula stage the dorsal and ventral centers are formed at a distance from their original location by the intercalation of a new D-V morphogen gradient. The 5× objective lens of LSM880 Zeiss confocal microscope was used for A–D′ and the 40× digital magnification zoom of Axio Zoom V.16 Stereo Zoom Zeiss for E–H.
We also examined the formation of the BMP signaling gradient, which is maximal in the ventral side and can be followed by nuclear phospho-Smad1/5/8 (12, 25). A phospho-Smad1/5/8 gradient was formed both in whole embryos and half embryos on the ventral side opposite the dorsal lip (Fig. 7 E–H). Because in the wild-type embryo the ventral center is formed in segment 4 and the dorsal center in segment 1 (Fig. 5D), these two regions would have been expected to lie next each other in the regenerating twin. Instead, what was found was that the new dorsal and ventral centers were formed 180° from each other.
These results imply that the position of both the dorsal and ventral centers was respecified in the regenerating embryo (Fig. 7I). In the half embryo, organizer formation was displaced by 90°, which corresponds to 45° in the whole embryo. At early stages after healing, cells fated to develop into the highest BMP or Chd expressing cells become artificially juxtaposed, but the entire gradient is displaced so that at late stages both the dorsal organizer and ventral center are displaced 90° in opposite directions, as indicated in the diagram in Fig. 7I. The molecular pathways that affect the relocation of the D-V gradient remain unknown and a topic for future investigation.
Discussion
Twinning in Xenopus.
We improved the frequency of twinning in the model system X. laevis by bisecting embryos at the midblastula stage with an eyelash knife and changing culture conditions. The embryo rapidly closes the bisection gap, with cells migrating from all of the directions toward a small healing point. These movements bring the prospective ventral-most and dorsal-most cells into close juxtaposition, with important developmental consequences. The yield of well-proportioned half embryos could be increased to about 80% by successively selecting half embryos that had healed well by 60 min after bisection, those in which the dorsal blastopore formed at a distance from the healing point, and those that had asymmetric pigmentation at neurula stage 13.
Spemann was able to obtain twins by constriction with a baby hair loop of the fertilized egg before first division of the newt embryo (7). Many years later, we are able to obtain twins by bisecting the embryo sagittally at midblastula (15). However, once the blastopore lip has appeared the embryo is no longer able to regenerate the missing half, even though dorsal halves can heal and form a well-proportioned embryo (while ventral halves lack an axis and differentiate into ventral tissues) (14). The difference in regeneration potential between midblastula and gastrula may be accounted by the time required for repatterning of the D-V axis, which resets a proper balance of BMPs and Wnts and their antagonists. At midblastula the Spemann organizer is not yet formed. Between the time of bisection and the appearance of the dorsal blastopore lip in the half embryo, about 4.5 h elapse, during which the future position of the Spemann organizer is displaced in the half embryo.
The key observation that prompted our subsequent analysis was that in regenerated half embryos the maternal pigmentation was stronger on one side, instead of being uniform as in the intact embryo (Fig. 2). Lineage-tracing studies showed that the entire epidermis derived from the ventral side. The lighter part of the twinned embryo derived from segment 3, while the darker half derived from segment 4 (Fig. 5). These and other experiments indicated that in twin embryos the Spemann organizer was formed about 90° from its original position. It still forms within the dorsal side, which is primed by βCat for dorsal development (10, 22). The ventral center, which is marked by maximal BMP activity and nuclear phospho-Smad1/5/8, was also displaced 90° in the opposite direction. As summarized in Fig. 7I, healing of the embryo juxtaposes early on—before the Spemann organizer is formed—regions of prospective high and low BMP signaling. The D-V gradient is respecified, so that a new gradient is intercalated before the start of gastrulation, leading to the separation of 180° between the new dorsal organizer and the ventral centers.
Possible Molecular Mechanisms.
The findings reported here are embryological in nature, and do not address the molecular pathways by which the D-V gradient is respecified. The Spemann organizer requires low BMP signaling for its formation, while the opposite is true for the ventral center (11, 26). It is conceivable that as BMP levels start rising in the ventral side, they displace the formation of the Spemann organizer. The role of BMP signaling in this reprograming could be tested, for example, by injecting BMP-MOs (14) or dominant-negative BMP receptors (27) into segment 4 (the predicted result being that the organizer would not be displaced). In the reciprocal reaction, once the organizer is formed, it will secrete BMP and Wnt inhibitors that will dampen BMP signals. The Chd–BMP pathway is regulated by the metalloproteinase Tolloid and its inhibitor Sizzled (12, 26), and it can be expected that these molecules might play a role as well.
While BMP signaling is likely to be an important player in twinning, other pathways are likely to be involved. The Nodal-related molecules Xnr5 and -6 are some of the earliest growth factor genes activated zygotically in the dorsal endoderm of the Xenopus embryo (28). Xenopus nodals are required for the induction of the organizer genes by the Nieuwkoop center, in combination with the dorsal βCat signal (29). In the chick embryo, removal of the hypoblast causes multiple embryonic axes to form in the overlying epiblast. The chick hypoblast secretes Cerberus, a multifunctional antagonist of Nodal, Wnt, and BMP signaling, and this twinning can be prevented by inhibiting Nodal signaling (30). Another important player is xWnt8, which has a major role in promoting ventral development in Xenopus (31) and is turned on transcriptionally by BMP signaling (32). Wnt signaling reinforces BMP and Nodal/TGF-β signaling by decreasing inhibitory phosphorylations in Smads mediated by glycogen synthase kinase 3 (33, 34). Unraveling the relative roles of this network of intertwined D-V patterning signals will require much work. The present results concern only the cellular mechanisms by which the patterning gradient, by displacing the positioning of the Spemann organizer, results in the induction of the missing half of the embryo.
The recent completion of the X. laevis genome has greatly increased the value of this model organism for gene discovery (35). We have performed preliminary RNA-seq analyses with pools of regenerating sagittally bisected embryos, but these did not reveal reproducible changes in up-regulated or down-regulated transcripts. Analyses of individual regenerating half embryos are under way, but it may well be that the D-V system is respecified by repositioning signaling centers rather than by changes in the transcription of any particular regeneration gene.
Twinning in Other Animals: Polyembryony.
Animal stem cells have the property of self-organizing into complex organoids (36, 37). The ultimate example of regulatory development is twinning, in which an entire body is formed. In humans monozygotic twins occur in 3 of 1,000 live births, the majority of which are thought to result from spontaneous splitting of the inner cell mass of the blastocyst (38). In the chick, the early blastoderm, which consists of 20,000–60,000 cells, can be sliced into four fragments, with each one giving rise to an embryo (39). The chick embryo has active signaling mechanisms that prevent formation of multiple embryos (30, 40). Interestingly, the chick organizer, called Hensen’s node, is not defined by a fixed population of cells; blastoderm cells are continuously entering and leaving the Chd-expressing node and have inductive properties only while residing within the node (30). Thus, organizer formation is not predetermined.
The most extreme examples of self-organization are provided by species with obligate polyembryony, in which a single zygote gives rise to multiple individuals every generation. In mammals, the nine-banded armadillo gives rise to identical quadruplets sharing a common amniotic cavity (41). The parasitic wasp Copidosoma floridanum lays a single egg into the fertilized egg of a moth, and develops into up to 2,000 monozygotic embryos in the nutritious environment of the caterpillar (42). Unlike other insects, this wasp embryo hatches from its chorion, proliferates, and self-organizes into hundreds of morulae consisting of round undifferentiated cells that subsequently gastrulate as the caterpillar molts. The most extreme example of making many from one is provided by the tapeworm Echinococcus granulosus. It produces eggs in the intestine of dogs. A single egg can colonize secondary hosts such as sheep or humans, forming a hydatid cyst in liver and other organs. Each cyst can develop up to a million tapeworm heads that develop into adults when ingested by canids, or into additional cysts if accidentally dispersed in the human body (43). It appears likely that these extreme adaptations will involve the self-organization of morphogen gradients, as is the case described here in Xenopus.
Conclusion.
The formation of twins has interested experimental embryologists since the beginning of the discipline (7). Studying the amphibian embryo, we found an unexpected asymmetry in the maternal pigmentation of the epidermis in twin tadpoles. Cell lineage studies indicated that the dorsal organizer and the ventral center are displaced in opposite directions from their expected site of appearance in the embryo. A new D-V gradient is generated, leading to the regeneration of the missing half of the body through the remarkable inductive activity of Spemann’s organizer.
Materials and Methods
Embryo Manipulations and mRNA Synthesis.
X. laevis were purchased from Nasco and kept on a diet of minced calf liver. All animal experiments were approved by the University of California, Los Angeles animal research review board. Embryos were generated through in vitro fertilization, those with clear D-V polarity (9) selected at the two- and four-cell stages, cultured in 0.1× Marc’s modified Ringer’s (MMR) (44), and staged according to Nieuwkoop and Faber (16). For in vitro mRNA synthesis, pCS2-nLacZ, pCS2-EGFP-Flag, pCS2-RFP-HA, pCS2-mGFP (containing a Gap43 membrane signal), pCS2-mRFP, pCS2-H2B-GFP, and pCS2-H2B-RFP were linearized with NotI and transcribed with SP6 RNA polymerase using the Ambion mMessage mMachine kit; pRN3-Siamois was linearized with Sfi1 and transcribed with T3 RNA polymerase (24). To obtain ventralized embryos, a total of 24 ng of antisense MO against Xenopus βCat (21) was injected four times marginally at the two-cell stage.
Lineage-Tracer Injections and Whole-Mount in Situ Hybridization.
Synthetic mRNAs used for lineage tracing were injected into each blastomere (four injections were required to label an entire segment at 16-cell) in 4 nL containing the following amounts: 400 pg of nLacZ; 333 pg of soluble GFP or RFP; 133 pg of membrane-targeted GFP or RFP; or 6.7 pg of histone H2B-GFP or H2B-RFP. Fluorescent dyes conjugated to dextran amine (10 kb, all from Fisher Scientific) were injected into each blastomere as follows: 1.3 ng of Alexa Flour 568; 2.5 ng of Rhodamine, Cascade blue or Alexa Fluor 647; or 5 ng of Fluorescein (F-DA). All embryos injected with fluorescent lineage tracers were cultured in the dark until the stages indicated. To relocalize the Spemann’s organizer in ventralized embryos, 50 pg of Siamois mRNA were microinjected into two blastomeres of the lateral marginal zone at the 16-cell stage. To prepare an in situ hybridization probe, a pBlueScript-xChd (pBS-59) was linearized with EcoR1 and transcribed with T7 RNA polymerase. In situ hybridizations were performed as described (24). After hybridization, whole-mount z-stack images were acquired with an Axio Zoom.V16 Stereo Zoom Zeiss microscope and in-focus images stacked using the Zen 2.3 pro Zeiss software.
Generation of Sagittally Bisected Twins.
Midblastula embryos (stage 8) were dechorionated manually and bisected in 0.3× MBS saline (17). The animal and marginal regions of embryos were cut along the midline of the dorsal crescent with an eyelash knife, and the vegetal cells then fully separated with fine forceps (SI Appendix, Movie S1). Half embryos were washed free of dead cells with a pulled glass pipette, transferred within 3 min into fresh 2% agar-coated plates, placed cut face up in depressions previously made with a heated Pasteur pipette (their position being marked on the outside of the plastic Petri dish with a felt-tip pen), and allowed to heal for 60 min in 0.3× MBS solution (17). The regenerating half embryos were then transferred into fresh agar-coated plates (with depressions) and cultured in 0.1× MBS until they reached gastrula or tailbud stage. Embryos that did not heal completely within 60 min were discarded. The frequency of twins can be increased by selecting those embryos in which the dorsal lip is formed 90° away from the healing point at gastrula, and those that show pigment asymmetry at neurula stage 13.
Vibratome Sections.
Lineage-traced whole or half embryos were fixed in 0.5× MEMFA (44) overnight at 4 °C. After washing in PBS, single embryos or halves were transferred into 5 mL of 6% (wt/vol) NuSieve GTG (Lonza) low-melting agarose in PBS in a glass vial (Fisher #03-339-25B) at 60 °C, and immediately embedded in an plastic mold (Electron Microscopy Sciences #62352-07). Samples were oriented head down with forceps under the dissection microscope until the agarose solidified. After incubating for 10 min floating on ice water, the excess agarose was trimmed with a Vibratome blade (Electron Microscope Sciences #71990), the embedded sample was removed from the mold, attached with super glue to a specimen block (Leica #39053747), and serially sectioned with a Vibratome (Lancer Vibratome Series 1000) at 100 µm. Sections were picked up from PBS with a cut pipette tip, placed on clean microscope slides, excess PBS removed, and mounted in antifade reagent (Molecular Probes #P36934). Images were acquired with an LSM880 Zeiss confocal microscope equipped with four lasers.
LacZ Staining.
Whole or half embryos were fixed with 0.5 × MEMFA for only 20 min and washed in PBS for 10 min at room temperature four times. Embryos were stained in Red-Gal solution [8 µL of 0.5M K3Fe(CN)6, 8 µL of 0.5 M K4Fe(CN)6, 4 µL of 0.5 M MgCl2, 25 µL of 0.12 M Red-Gal, in 1 mL of PBS final volume] for 6 h at room temperature. After staining, samples were washed for 5 min at room temperature twice and fixed again overnight in MEMFA.
X. laevis Immunostaining.
The embryos were collected and fixed in MEMFA at stage 12 and bisected with a surgical blade. The Wallingford immunostaining process was followed (45), and only those embryos resulting in a clean horizontal or sagittal cut were imaged. A rabbit polyclonal antiphospho-Smad1/5/8 (Cell Signaling #9511L; 1:100) was used to identify the BMP gradient. An anti-Chd rabbit polyclonal antiserum raised against the amino terminus of Xenopus Chd was affinity-purified, as described previously (12), and used at 1:100. Cy3-conjugated goat anti-rabbit antibody (from Jackson Immunoresearch) was used at 1:1,000 as the secondary antibody. Stacked images of whole and half embryos were acquired with an Axio Zoom.V16 Stereo Zoom Zeiss microscope with Apotome function and stacked images reconstructed using the Zen 2.3 pro Zeiss software.
Movies.
SI Appendix, Movie S1 was taken with a DCR-PC350 Sony Handicam and edited using iMovie software. Time-lapse images for SI Appendix, Movie S2 were taken in a Leica stereo microscope using an AxioCam HRc Zeiss camera controlled by Zeiss Axiovision 4.6 and assembled with Fiji software. SI Appendix, Movies S3 and S4 were taken with an inverted Zeiss Observer Z.1 microscope with fluorescence filters controlled by Axiovision 4.6 software.
Supplementary Material
Acknowledgments
We thank Sir John Gurdon for the Siamois plasmid; John Wallingford for histone H2B-GFP and H2B-RFP; the staff of the Advanced Xenopus Imaging Workshop at the Woods Hole Marine Biology Laboratory for training Y.M.; and Yi Ding, Maggie Bui, Alyssa Dsouza, Gabriele Colozza, and Nydia Tejeda for critical reading of the manuscript. Y.M. was partially supported by a fellowship from the Japan Society for the Promotion of Science. This work was supported by Norman Sprague Endowment and the Howard Hughes Medical Institute, of which E.M.D.R. is an Investigator.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1802749115/-/DCSupplemental.
References
- 1.Driesch H. Entwicklungsmechanische Studien: I. Der Werthe der beiden ersten Furchungszellen in der Echinogdermenentwicklung. Experimentelle Erzeugung von Theilund Doppelbildungen. Zeitschrift für wissenschaftliche Zoologie. 1891;53:160–184. [Google Scholar]
- 2.Hörstadius S, Wolsky A. Studien über die Determination der Bilateralsymmetrie des jungen Seeigelkeimes. Wilhelm Roux Arch Entwickl Mech Org. 1936;135:69–113. doi: 10.1007/BF02569029. [DOI] [PubMed] [Google Scholar]
- 3.Morgan TH. Half embryos and whole embryos from one of the first two blastomeres. Anat Anz. 1895;10:623–638. [Google Scholar]
- 4.Herlitzka A. Sullo sviluppo di embrioni completi da blastomeri isolati di uova di tritone (Molge cristata) Archiv fürEntwicklungsmechanik der Organismen. 1897;8:824–834. [Google Scholar]
- 5.Spemann H. Entwickelungsphysiologische Studien am Triton-Ei. Arch f Entw Mech Org. 1901;12:224–264. [Google Scholar]
- 6.Krause G, Sander K. Ooplasmic reaction system in insect embryogenesis. Adv Morphog. 1962;2:259–303. [Google Scholar]
- 7.Spemann H. Embryonic Development and Induction. Yale Univ Press; New Haven, CT: 1938. [Google Scholar]
- 8.Harland R, Gerhart J. Formation and function of Spemann’s organizer. Annu Rev Cell Dev Biol. 1997;13:611–667. doi: 10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]
- 9.Klein SL. The first cleavage furrow demarcates the dorsal-ventral axis in Xenopus embryos. Dev Biol. 1987;120:299–304. doi: 10.1016/0012-1606(87)90127-8. [DOI] [PubMed] [Google Scholar]
- 10.Larabell CA, et al. Establishment of the dorso-ventral axis in Xenopus embryos is presaged by early asymmetries in β-catenin that are modulated by the Wnt signaling pathway. J Cell Biol. 1997;136:1123–1136. doi: 10.1083/jcb.136.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Robertis EM, Kuroda H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol. 2004;20:285–308. doi: 10.1146/annurev.cellbio.20.011403.154124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plouhinec J-L, Zakin L, Moriyama Y, De Robertis EM. Chordin forms a self-organizing morphogen gradient in the extracellular space between ectoderm and mesoderm in the Xenopus embryo. Proc Natl Acad Sci USA. 2013;110:20372–20379. doi: 10.1073/pnas.1319745110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Robertis EM, Moriyama Y. The chordin morphogenetic pathway. Curr Top Dev Biol. 2016;116:231–245. doi: 10.1016/bs.ctdb.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Reversade B, De Robertis EM. Regulation of ADMP and BMP2/4/7 at opposite embryonic poles generates a self-regulating morphogenetic field. Cell. 2005;123:1147–1160. doi: 10.1016/j.cell.2005.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Robertis EM. Spemann’s organizer and self-regulation in amphibian embryos. Nat Rev Mol Cell Biol. 2006;7:296–302. doi: 10.1038/nrm1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nieuwkoop PD, Faber J. 1967. Normal Table of Xenopus laevis (Daudin): A Systematical and Chronological Survey of the Development from the Fertilized Egg till the End of Metamorphosis (Garland Publishing, New York), (republished in 1994)
- 17.Gurdon JB. Injected nuclei in frog oocytes: Fate, enlargement, and chromatin dispersal. J Embryol Exp Morphol. 1976;36:523–540. [PubMed] [Google Scholar]
- 18.Bellairs R. 1986. The tail bud and cessation of segmentation in the chick embryo. Somites in Developing Embryos, NATO Series A, eds Bellairs R, Ede D, Lash J (Plenum Press, New York), pp 161–178.
- 19.Selleck MAJ, Stern CD. Fate mapping and cell lineage analysis of Hensen’s node in the chick embryo. Development. 1991;112:615–626. doi: 10.1242/dev.112.2.615. [DOI] [PubMed] [Google Scholar]
- 20.Dale L, Slack JMW. Fate map for the 32-cell stage of Xenopus laevis. Development. 1987;99:527–551. doi: 10.1242/dev.99.4.527. [DOI] [PubMed] [Google Scholar]
- 21.Heasman J, Kofron M, Wylie C. β-Catenin signaling activity dissected in the early Xenopus embryo: A novel antisense approach. Dev Biol. 2000;222:124–134. doi: 10.1006/dbio.2000.9720. [DOI] [PubMed] [Google Scholar]
- 22.Hikasa H, Sokol SY. Wnt signaling in vertebrate axis specification. Cold Spring Harb Perspect Biol. 2013;5:a007955. doi: 10.1101/cshperspect.a007955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kessler DS. Siamois is required for formation of Spemann’s organizer. Proc Natl Acad Sci USA. 1997;94:13017–13022. doi: 10.1073/pnas.94.24.13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding Y, et al. Spemann organizer transcriptome induction by early beta-catenin, Wnt, Nodal, and Siamois signals in Xenopus laevis. Proc Natl Acad Sci USA. 2017;114:E3081–E3090. doi: 10.1073/pnas.1700766114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tucker JA, Mintzer KA, Mullins MC. The BMP signaling gradient patterns dorsoventral tissues in a temporally progressive manner along the anteroposterior axis. Dev Cell. 2008;14:108–119. doi: 10.1016/j.devcel.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Robertis EM, Moriyama Y, Colozza G. Generation of animal form by the Chordin/Tolloid/BMP gradient: 100 years after D’Arcy Thompson. Dev Growth Differ. 2017;59:580–592. doi: 10.1111/dgd.12388. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki A, et al. A truncated bone morphogenetic protein receptor affects dorsal-ventral patterning in the early Xenopus embryo. Proc Natl Acad Sci USA. 1994;91:10255–10259. doi: 10.1073/pnas.91.22.10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi S, et al. Two novel nodal-related genes initiate early inductive events in Xenopus Nieuwkoop center. Development. 2000;127:5319–5329. doi: 10.1242/dev.127.24.5319. [DOI] [PubMed] [Google Scholar]
- 29.Agius E, Oelgeschläger M, Wessely O, Kemp C, De Robertis EM. Endodermal Nodal-related signals and mesoderm induction in Xenopus. Development. 2000;127:1173–1183. doi: 10.1242/dev.127.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bertocchini F, Stern CD. The hypoblast of the chick embryo positions the primitive streak by antagonizing nodal signaling. Dev Cell. 2002;3:735–744. doi: 10.1016/s1534-5807(02)00318-0. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura Y, de Paiva Alves E, Veenstra GJC, Hoppler S. Tissue- and stage-specific Wnt target gene expression is controlled subsequent to β-catenin recruitment to cis-regulatory modules. Development. 2016;143:1914–1925. doi: 10.1242/dev.131664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoppler S, Moon RT. BMP-2/-4 and Wnt-8 cooperatively pattern the Xenopus mesoderm. Mech Dev. 1998;71:119–129. doi: 10.1016/s0925-4773(98)00004-5. [DOI] [PubMed] [Google Scholar]
- 33.Fuentealba LC, et al. Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell. 2007;131:980–993. doi: 10.1016/j.cell.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Demagny H, Araki T, De Robertis EM. The tumor suppressor Smad4/DPC4 is regulated by phosphorylations that integrate FGF, Wnt, and TGF-β signaling. Cell Rep. 2014;9:688–700. doi: 10.1016/j.celrep.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 35.Session AM, et al. Genome evolution in the allotetraploid frog Xenopus laevis. Nature. 2016;538:336–343. doi: 10.1038/nature19840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holtfreter J. Neural differentiation of ectoderm through exposure to saline solution. J Exp Zool. 1944;95:307–343. [Google Scholar]
- 37.Sasai Y. Cytosystems dynamics in self-organization of tissue architecture. Nature. 2013;493:318–326. doi: 10.1038/nature11859. [DOI] [PubMed] [Google Scholar]
- 38.Sadler TW. Langman’s Medical Embryology. 13th Ed Wolters Kluwer Health; Philadelphia: 2015. [Google Scholar]
- 39.Spratt NT, Haas H. Integrative mechanisms in development of the early chick blastoderm. I. Regulative potentiality of separated parts. J Exp Zool. 1960;145:97–137. [Google Scholar]
- 40.Joubin K, Stern CD. Molecular interactions continuously define the organizer during the cell movements of gastrulation. Cell. 1999;98:559–571. doi: 10.1016/s0092-8674(00)80044-6. [DOI] [PubMed] [Google Scholar]
- 41.Carter AM. Classics revisited: Miguel Fernández on germ layer inversion and specific polyembryony in armadillos. Placenta. 2018;61:55–60. doi: 10.1016/j.placenta.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 42.Zhurov V, Terzin T, Grbić M. Early blastomere determines embryo proliferation and caste fate in a polyembryonic wasp. Nature. 2004;432:764–769. doi: 10.1038/nature03171. [DOI] [PubMed] [Google Scholar]
- 43.Craig SF, Slobodkin LB, Wray GA, Biermann CH. The paradox of polyembryony: A review of the cases and a hypothesis for its evolution. Evol Ecol. 1997;11:127–143. [Google Scholar]
- 44.Sive HL, Grainger RM, Harland RM. Early Development of Xenopus laevis: A Laboratory Manual. Cold Spring Harbor Lab Press; Cold Spring Harbor, NY: 2000. [Google Scholar]
- 45.Lee C, Kieserman E, Gray RS, Park TJ, Wallingford J. Whole-mount fluorescence immunocytochemistry on Xenopus embryos. CSH Protoc. 2008;2008:t4957. doi: 10.1101/pdb.prot4957. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.