Significance
As a ligand-activated transcription factor, farnesoid X receptor (FXR) is abundantly expressed in the liver and small intestine, where it plays an important role in the maintenance of bile acid, lipid, and glucose homeostasis. Increasing evidence is emerging that FXR may be a critical regulatory factor in renal physiology and pathophysiology. The present study demonstrates an essential role of FXR in promoting cell survival of medullary collecting duct cells under hypertonic stress and identifies FXR-induced TonEBP expression and activation as an underlying mechanism. This work provides an insight into the role of FXR in renal urine concentration.
Keywords: bile acid receptor, NFAT5, hypertonicity, osmoprotection, cell viability
Abstract
Hypertonicity in renal medulla is critical for the kidney to produce concentrated urine. Renal medullary cells have to survive high medullary osmolarity during antidiuresis. Previous study reported that farnesoid X receptor (FXR), a nuclear receptor transcription factor activated by endogenous bile acids, increases urine concentrating ability by up-regulating aquaporin 2 expression in medullary collecting duct cells (MCDs). However, whether FXR is also involved in the maintenance of cell survival of MCDs under dehydration condition and hypertonic stress remains largely unknown. In the present study, we demonstrate that 24-hours water restriction selectively up-regulated renal medullary expression of FXR with little MCD apoptosis in wild-type mice. In contrast, water deprivation caused a massive apoptosis of MCDs in both global FXR gene-deficient mice and collecting duct-specific FXR knockout mice. In vitro studies showed that hypertonicity significantly increased FXR and tonicity response enhancer binding protein (TonEBP) expression in mIMCD3 cell line and primary cultured MCDs. Activation and overexpression of FXR markedly increased cell viability and decreased cell apoptosis under hyperosmotic conditions. In addition, FXR can increase gene expression and nuclear translocation of TonEBP. We conclude that FXR protects MCDs from hypertonicity-induced cell injury very likely via increasing TonEBP expression and nuclear translocation. This study provides insights into the molecular mechanism by which FXR enhances urine concentration via maintaining cell viability of MCDs under hyperosmotic condition.
Whole-body water homeostasis is critical for human health and depends on the balance between water intake stimulated by thirst and water excretion controlled mainly by the kidneys. Each day, an adult human produces ∼1.5 L of urine despite 180 L of fluid filtered through glomerular basement membrane. Approximately, 90% of glomerular filtrate is constitutively reabsorbed in the proximal tubules and descending loop of Henle. The remaining filtered water is reabsorbed in renal medullary collecting duct cells (MCDs), where the water transport is tightly regulated by arginine vasopressin (AVP), a circulating hormone also known as antidiuretic hormone (ADH) (1).
Under dehydration condition, AVP is produced by the hypothalamus and released from the neurohypophysis into the blood in response to increased plasma osmolarity or reduced blood volume. It promotes water reabsorption in MCDs by increasing gene expression and apical membrane targeting of aquaporin 2 (AQP2) mainly through the AVP-V2R-PKA pathway (2). However, AVP also increases sodium chloride (NaCl) reabsorption in the thick ascending limb (TAL) and distal convolute tubule and urea permeability in the inner MCDs to establish a hyperosmotic state in renal medulla, which is essential for the generation of a concentrated urine (3).
Renal medulla is a unique tissue in which residing cells including MCDs are exposed to the harsh hypertonic and hypoxic environment and have to survive significant rises in NaCl and urea concentrations during antidiuresis. Multiple osmoprotective mechanisms have been reported to be important in maintaining the survival of renal medullary cells under hypertonic stress (4–7). Among them, it is generally believed that the transcription factor tonicity-responsive enhancer binding protein (TonEBP) and its target osmoprotective genes including aldose reductase (AR) and heat shock protein 70 (HSP70) represent the most important protective mechanism.
Farnesoid X receptor (FXR), a member of the nuclear receptor superfamily, is a transcription factor activated by endogenous bile acids. In addition to the liver and small intestine where FXR plays an important role in bile acid, glucose, and lipid metabolism, the kidney also exhibits highly abundant FXR expression, especially in renal collecting duct cells (8). It has been previously reported that activation of FXR increases urine concentration by up-regulating the expression of AQP2, one of its direct target genes, in MCDs (9). However, whether FXR is also involved in the maintenance of cell survival of MCDs under hypertonic stress remains largely unknown. The present studies aimed to investigate whether FXR plays an important role in regulating cell survival of MCDs under hyperosmotic stress in vitro and in vivo. The underlying mechanism by which FXR affects cell viability of MCDs exposed to hypertonic condition was also examined.
Results
Water Restriction Selectively Up-Regulated FXR Expression in Mouse Renal Medulla.
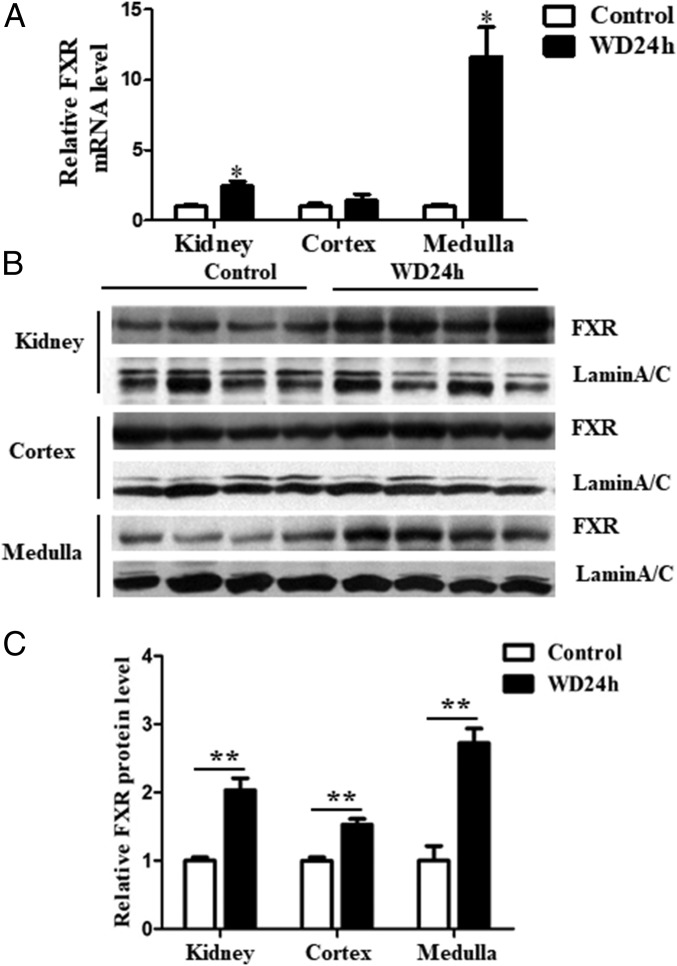
Wild-type (WT) C57BL/6 mice were subjected to water deprivation (WD) for 24 h. Compared with the mice with free access to water, water-deprived mice exhibited ∼10-fold and ∼2-fold increase in FXR expression in renal medulla at mRNA and protein level, respectively (Fig. 1). In contrast to renal medulla, cortical expression of FXR at both mRNA and protein level remained unaltered (Fig. 1). Because renal medullas of dehydrated mice are hypertonic, these results suggest FXR may be regulated by hypertonicity and plays an important role in urine concentration.
Fig. 1.
Water restriction selectively induced FXR expression in renal medullas of C57BL/6 mice. Male C57BL/6 mice were water-deprived for 24 h. (A) FXR mRNA expression was selectively induced in renal medulla. FXR mRNA levels were analyzed by qRT-PCR, *P < 0.05 vs. control (n = 4). (B) FXR protein expression was significantly increased in renal medulla as assessed by Western blot. Lamin A/C was used as protein loading control. (C) Statistical analysis of Western blot shown in B, **P < 0.01 vs. control (n = 4).
Hypertonicity Induced FXR Expression in Cultured MCDs.
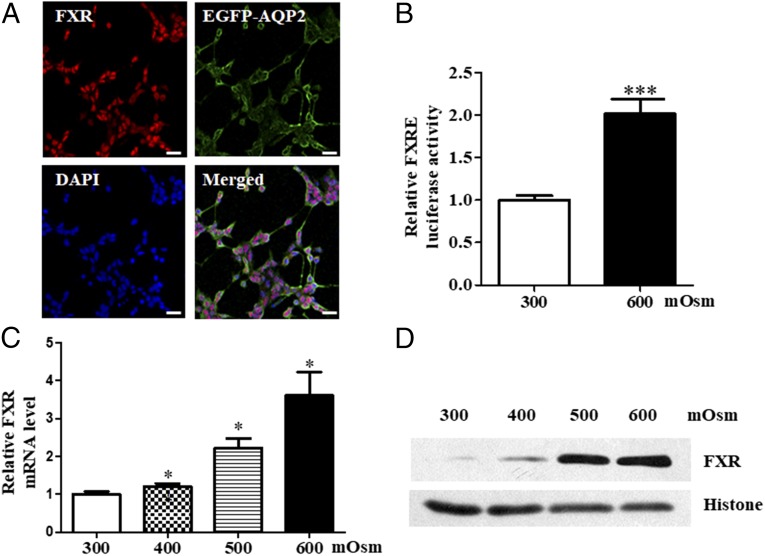
Since FXR was mainly expressed in MCDs (9), we first determined whether hyperosmolarity can induce FXR expression. mIMCD3 cell line and primary cultured mouse MCDs were exposed to hypertonicity. As shown in Fig. 2, FXR was constitutively and functionally expressed in mIMCD3 cells (Fig. 2 A and B), where its expression was significantly induced by hypertonicity at mRNA (Fig. 2C) and protein levels (Fig. 2D). Similarly, in primary cultured mouse MCD cells, FXR was constitutively expressed (SI Appendix, Fig. S1) and increased hypertonicity significantly up-regulated FXR expression at mRNA (SI Appendix, Fig. S2A) and protein (SI Appendix, Fig. S2 B and C) levels. Compared with the levels of FXR at 800 mOsm, FXR protein expression at 900 mOsm was markedly reduced, which might be due to reduced cell viability of MCD cells treated with 900 mOsm (SI Appendix, Fig. S2B).
Fig. 2.
Hypertonicity-induced FXR expression in mouse MCDs. (A) Constitutive expression of FXR in mouse inner MCDs (mIMCD3). Cultured mIMCD3 cells were transfected with an EGFP-AQP2 expression vector and then exposed to hypertonic stress (600 mOsm) for 6 h. Note that FXR was mainly expressed in the nucleus (red) and AQP2 was predominantly located in cell membrane (green). (Scale bar, 25 uM.) (B) Luciferase reporter assay demonstrating that hypertonicity at 600 mOsm significantly induced FXR transcription activity. ***P < 0.001 vs. 300 mOsm (n = 12). (C) Induction of FXR mRNA expression by hypertonicity in a dose-dependent manner. Cultured mIMCD3 cells were incubated with hypertonic solutions for 6 h. *P < 0.05 vs. 300 mOsm (n = 4). (D) Western blot assay showing that hypertonicity significantly induced FXR protein expression in mIMCD3 cells. Cells were exposed to various hypertonic stress (400, 500, and 600 mOsm) for 6 h.
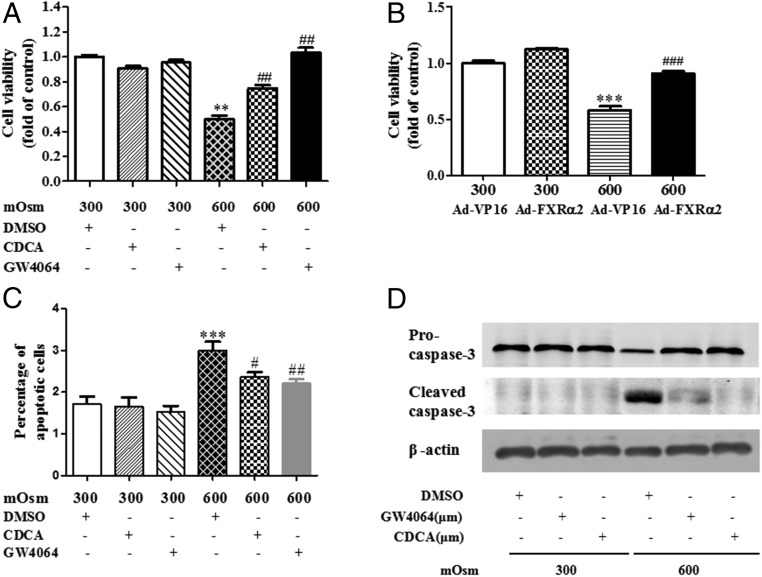
FXR Activation and Overexpression Protected mIMCD3 Cells from Hypertonicity-Induced Cell Death.
To investigate the role of FXR in cell survival under hypertonic stress, mIMCD3 cells were treated with an endogenous agonist (CDCA) and a synthetic agonist (GW4064) of FXR or infected with an adenovirus expressing FXRα2 (9). Cells were treated with either isotonic (300 mOsm) or hyperosmotic medium (600 mOsm), and cell viability was determined by the MTT assay. FXR activation by both CDCA and GW4064 significantly reduced cell death induced by hypertonic stress, with little effect on cell viability under the isotonic condition (Fig. 3A). Similarly, overexpression of FXR with Ad-FXRα2 markedly promoted cell survival under hyperosmotic challenge (Fig. 3B). Flow cytometry analysis was further performed to examine hypertonicity-induced apoptosis of mIMCD3 cells. FXR activation by CDCA and GW4064 significantly reduced hypertonicity (600 mOsm)-induced cell apoptosis (Fig. 3C). Consistently, CDCA and GW4064 treatment blocked hypertonicity-induced cleavage of procaspase-3 (Fig. 3D). These findings suggest that FXR may promote cell survival of MCDs under dehydration state possibly by blocking caspase-3 activation-associated apoptosis.
Fig. 3.
FXR protected mIMCD3 cells from hypertonic stress-induced cell apoptosis. The mIMCD3 cells were subjected to hypertonicity (600 mOsm) for 6 h in the presence or absence of FXR selective agonist GW4064 and CDCA. (A) MTT cell viability analysis showing that hypertonicity-induced cell death was significantly attenuated by GW4064 and CDCA treatment. **P < 0.01 vs. 300 mOsm+DMSO, ##P < 0.01 vs. 600 mOsm+DMSO (n = 10). (B) Overexpression of FXR-ameliorated mIMCD3 cell death under hypertonic stress. Cells were infected with Ad-FXRα2 or Ad-VP16. After 36 h of infection, the cells were challenged with hypertonic stress (600 mOsm) for 6 h. Cell viability was determined by the MTT assay. ***P < 0.001 vs. 300 mOsm+Ad-VP16, ###P < 0.001 vs. 600 mOsm+Ad-VP16 (n = 6). (C) FXR activation blocked hypertonicity-induced apoptosis of mIMCD3 cells. The mIMCD3 were treated with isotonic (300 mOsm) or hypertonic medium (600 mOsm) for 6 h, and apoptotic cells were analyzed by the flow cytometry. Treatment of cells with GW4064 (2.5 μM) and CDCA (50 μM) markedly reduced hypertonicity-induced cell apoptosis. ***P < 0.001 vs. 300 mOsm+DMSO, and #P < 0.05 and ##P < 0.01 vs. 600 mOsm+DMSO (n = 3). (D) FXR activation with GW4064 and CDCA markedly reduced hypertonicity-induced activation of the caspase-3 apoptotic cascade. Note that protein expression of the apoptotic marker, cleaved caspase-3, was markedly attenuated by GW4064 and CDCA. The results are representative of three independent experiments.
FXR Gene Deficiency Potentiated MCD Cell Apoptosis in Dehydrated Mice in Vivo.
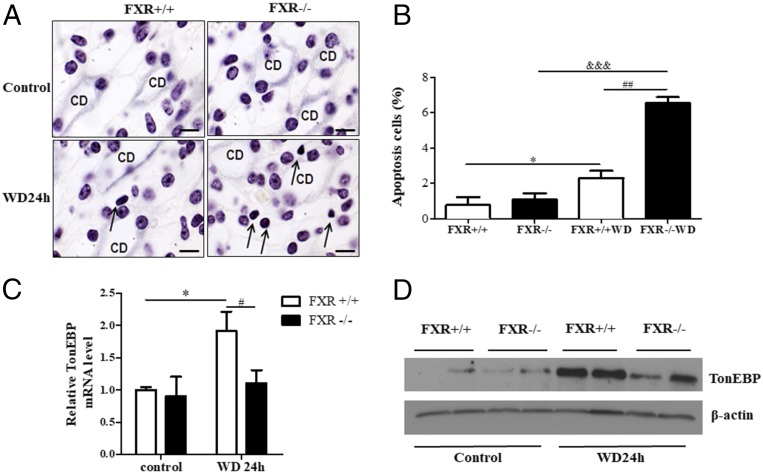
To address whether FXR promotes the survival of MCDs under dehydrated condition, WT, global FXR gene-deficient mice (FXR−/−), and collecting duct-specific FXR gene knockout mice (CD-FXR−/−) were generated and were deprived of water for 24 h. As shown in Fig. 4 A and B, the TUNEL assay demonstrated that water restriction induced a small amount apoptosis of MCDs in WT mice but resulted in a massive apoptosis in FXR−/− mice. FXR−/− mice exhibited much more apoptotic cells than WT mice after 24-h water restriction. The majority of the apoptotic cells were the MCDs (Fig. 4 A and B). To further confirm the role of collecting duct FXR, collecting duct-specific FXR gene knockout mice (CD-FXR−/−) were generated by crossing FXR flox/flox mice with AQP2-Cre mice as previously reported (10–13) and were subjected to 24-h WD. Compared with WT mice, CD-FXR−/− mice also displayed a significant increase in apoptotic cells of medullary collecting ducts after water restriction (SI Appendix, Fig. S3). Together, these findings clearly demonstrate that FXR can protect MCDs from hypertonicity-induced injury and promote their survival under hypertonic stress.
Fig. 4.
FXR deficiency accelerated MCD cell apoptosis and attenuated TonEBP expression in dehydrated mice. C57BL/6 mice were water-deprived for 24 h. Apoptotic cells were identified as dark brown staining by the TUNEL assay. Mice with free access to water were used as control. (A) TUNEL assay demonstrating increased apoptotic cells were observed in the kidneys of FXR−/− mice with 24-h water restriction. Immunohistochemical staining of AQP2 (light blue) was used to indicate renal-collecting ducts. Twenty-four-hour WD barely induced apoptosis of renal medullary cells in WT mice (FXR+/+) but resulted in a massive apoptosis in FXR−/− mice (arrows). (Scale bar, 10 uM.) (B) Quantitative analysis of apoptotic cells in renal medullas of FXR+/+ and FXR−/− mice. *P < 0.05 vs. FXR+/+ control mice, ##P < 0.01 vs. FXR+/+ with WD, &&&P < 0.001 vs. FXR−/− control mice (n = 3). (C and D) WD failed to induce TonEBP expression at mRNA (C) and protein level (D) in the kidneys of FXR−/− mice. FXR+/+ and FXR−/− mice were water-deprived for 24 h. TonEBP mRNA and protein expression was measured by real-time PCR and immunoblot, respectively. *P < 0.05 vs. FXR+/+ control mice, #P < 0.05 vs. FXR−/− with WD (n = 6). CD, collecting duct.
FXR Gene Knockout Mice Exhibited Attenuated Renal TonEBP Expression After 24-h Water Restriction.
Tonicity-responsive enhancer binding protein (TonEBP) is a transcription factor promoting cellular accumulation of organic osmolytes in the hypertonic renal medulla by stimulating expression of its target genes including aldose reductase (AR) and heat shock protein 70 (HSP70) (7). To elucidate the underlying mechanism by which FXR protects MCDs from hypertonic stress, renal expression of TonEBP and its target genes AR and HSP70 was determined. As expected, 24-h dehydration markedly increased renal TonEBP expression in WT mice at both mRNA (Fig. 4C) and protein level (Fig. 4D). However, in FXR−/− mice, 24-h water restriction just slightly induced TonEBP mRNA and protein expression (Fig. 4 C and D). These results suggest that FXR may exert osmoprotective effect on MCDs via increasing TonEBP expression and transcriptional activity in renal medulla.
FXR Induced TonEBP Expression and Nuclear Translocation in Cultured MCDs.
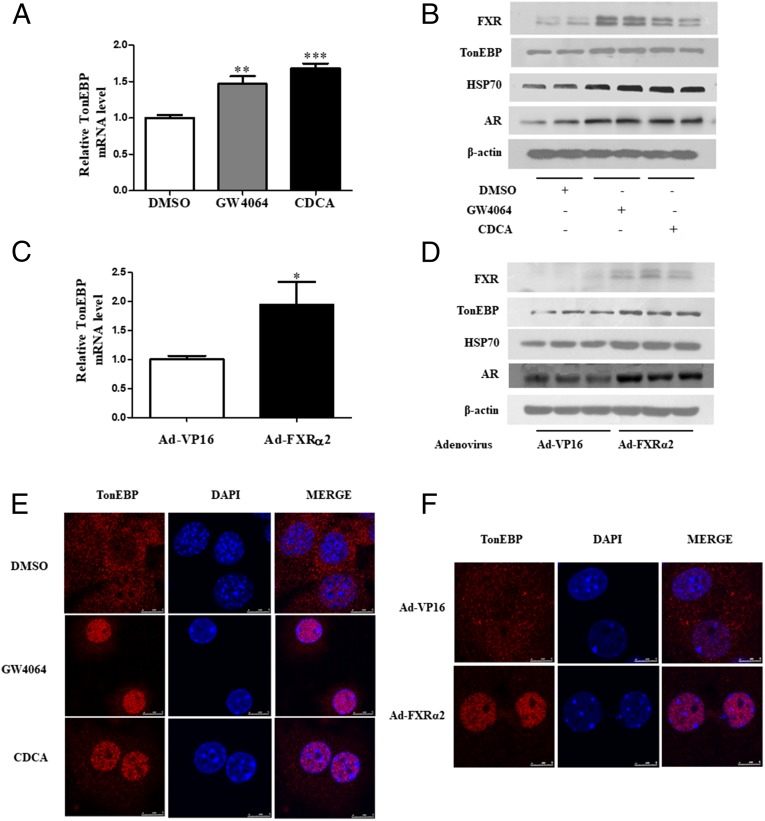
To confirm the effect of FXR on TonEBP expression and activity, primary cultured mouse MCDs were treated with two FXR agonists or infected with Ad-FXRα2. As shown in Fig. 5 A and B, CDCA and GW4064 treatment markedly up-regulated TonEBP mRNA and protein expression. FXR activation also induced protein expression of FXR and TonEBP target genes including AR and Hsp70 (Fig. 5B). Similarly, overexpression of FXRα2 via an adenovirus-based approach increased TonEBP expression at both mRNA and protein levels (Fig. 5 C and D). Expression of FXR as well as AR and Hsp70 was also significantly induced (Fig. 5D). These findings demonstrate that FXR can promote TonEBP expression at both mRNA and protein level.
Fig. 5.
FXR up-regulated TonEBP expression and nuclear translocation in primary cultured mouse IMCD cells. Primary MCD cells were treated with GW4064 (2.5 μM) and CDCA (50 μM) for 6 h or infected with Ad-FXRα2. (A) Real-time PCR analysis demonstrating mRNA expression of TonEBP in primary MCD cells isolated from WT mice. **P < 0.01, ***P < 0.001 vs. DMSO (n = 4). (B) Western blot assay showing that treatment of MCDs with GW4064 and CDCA markedly induced protein expression of FXR, TonEBP, and osmoprotective genes HSP70 and AR. (C) Quantitative PCR analysis showing that overexpression of FXR significantly up-regulated TonEBP mRNA levels in MCDs. *P < 0.05 vs. Ad-VP16 (n = 8). (D) Immunoblot assay demonstrating overexpression of FXR markedly induced FXR, TonEBP, HSP70, and AR expression. (E) FXR activation promoted nuclear translocation of TonEBP. Primary MCDs were treated with GW4064 and CDCA for 6 h. Confocal immunofluorescence was performed to detect TonEBP (red) nuclear translocation. The nucleus was visualized by staining with DAPI (blue). (Scale bar, 5 uM.) (F) FXR overexpression with Ad-FXRα2 markedly facilitated nuclear translocation of TonEBP. Cells infected with a control virus (Ad-VP16) had little effect on TonEBP nuclear localization. (Scale bar, 5 uM.)
It is clear that transcriptional activity of TonEBP depends on its nuclear translocation (14, 15). To further test whether FXR can increase TonEBP transcriptional activity, primary MCDs were treated with the FXR agonist CDCA and GW4064 or overexpressed with FXRα2. Both FXR activation and overexpression markedly promoted TonEBP nuclear translocation (Fig. 5 E and F). Importantly, in primary cultured MCDs from FXR−/− mice, Ad-FXRα2 infection not only rescued FXR expression, but also caused a significant translocation of TonEBP from the cytoplasm to the nucleus (SI Appendix, Fig. S4). Together, these findings demonstrate that FXR indeed increases TonEBP activity by enhancing its nuclear translocation.
Discussion
This study demonstrates a critical role of FXR in the survival of MCDs under dehydration condition. Twenty-four-hour water restriction selectively up-regulates renal medullary expression of FXR with little MCD apoptosis in WT mice. In contrast, WD causes a massive apoptosis of MCDs in both global FXR gene-deficient mice and collecting duct-specific FXR knockout mice. In cultured MCDs, activation and overexpression of FXR markedly promote cell viability and increase TonEBP expression and activity under hyperosmotic stress. FXR may represent a factor protecting the MCDs from hypertonic stress-induced injury via increasing TonEBP activity, thereby maintaining the ability of renal medulla to concentrate urine under hydration status.
FXR belongs to the superfamily of nuclear receptor transcription factors and can be activated by many naturally occurred endogenous bile acids (16). It is abundantly expressed in the tissues with active cholesterol metabolism including the liver, intestine, and adrenal gland, where FXR plays an important role in bile acid biosynthesis and transport, cholesterol metabolism, and glucocorticoid production (17–19). It has been previously reported that FXR is also highly expressed in the kidney and is critical for maintaining renal function (9). Disruption of FXR gene causes a significant defect in urine concentrating ability, resulting in a polyuria phenotype in mice. In addition, FXR dysfunction is associated with a worsened renal injury in diabetic mice (20). The present study further demonstrates that FXR gene deficiency significantly increases cell death of MCDs under hypertonic conditions, while FXR activation and overexpression markedly reduces hypertonicity-induced apoptosis of MCDs. These findings provide evidence that FXR is a key factor in promoting cell survival of MCDs in hypertonic renal medulla, thereby maintaining renal urine concentrating ability during antidiuresis.
TonEBP, also called nuclear factor of activated T-cell 5 (NFAT5), is a transcription factor critical for osmoadaptive response of renal medullary cells to hyperosmolar stress (14). It exerts an osmoprotective effect on renal medullary cells mainly via up-regulating gene transcription of many of its target genes including AR, HSP70, and aquaporins (AQPs) (15). Genetically modified animals with deficient TonEBP activity in the kidney suffer from severe medullary atrophy in association with cell death, demonstrating that TonEBP is essential for the survival of the renal medullary cells (7). Although it is well accepted that TonEBP is a key transcription factor in osmoprotection, mechanisms involved in TonEBP expression and activation remain largely unknown. In the present study, we found that renal TonEBP expression was significantly reduced in FXR−/− mice with WD, while FXR activation markedly up-regulated expression of TonEBP and its target genes. These findings raise a possibility that FXR may be a transcription factor important for driving TonEBP expression. However, the underlying mechanism warrants further investigation.
The present study also provides clear evidence that FXR can promote nuclear translocation of TonEBP protein in MCDs. It is well known that nuclear import of TonEBP is essential for its transcriptional activity. Multiple mechanisms have been reported to contribute to the nucleocytoplasmic shuttling of TonEBP protein, which requires the presence of the nuclear export signal and nuclear localization signal at its N terminus (21). The signaling cascades of Fyn, a shrinkage-activated tyrosine kinase, and p38, a subgroup of the mitogen-activated kinases, have been reported to be major signaling pathways for hypertonic activation of TonEBP (22). In contrast, transcriptional coactivator with PDZ-binding motif (TAZ) can suppress TonEBP activity through tyrosine phosphorylation (23). We report here that FXR activation and overexpression can dramatically diminish cytoplasmic TonEBP levels and increase its nuclear localization, providing a mechanism for TonEBP transactivation. Currently, the mechanism by which FXR drives nuclear import of TonEBP is not clear. One possibility worth addressing is that FXR may form a transcriptional complex with TonEBP, which increases both TonEBP nuclear translocation and its transactivation. Given a ubiquitous distribution pattern of both FXR and TonEBP, they may act in concert in regulating many other biological processes than those involved in the response to hypertonicity in renal medulla.
Similar to TonEBP, FXR may also represent a hypertonicity-responsive gene. Twenty-four-hour water restriction selectively up-regulated FXR expression in renal medulla at both mRNA and protein levels. Similarly, hypertonic stress significantly induced FXR mRNA and protein levels and its transcriptional activity in cultured MCDs. These findings demonstrate that FXR gene is transcriptionally regulated in response to hypertonicity. However, it is currently unclear whether there is a hypertonicity-responsive element in the promoter region of FXR gene. It is also uncertain whether FXR is under the transcriptional regulation of TonEBP. Addressing these important issues may significantly advance our knowledge in understanding the mechanism by which FXR is regulated and the importance of FXR in renal physiology.
In summary, the present studies demonstrate that dehydration and hypertonicity increase FXR expression and its transcriptional activity in the MCDs. FXR gene deficiency significantly potentiates MCD death in mice with water restriction. FXR activation and overexpression markedly promote cell survival of cultured MCDs. FXR-driven TonEBP expression and nuclear translocation may contribute to its osmoprotective role in renal medulla.
Materials and Methods
Male C57BL/6 WT mice (8-10 wk old) were purchased from the Experimental Animal Center at Peking University Health Science Center. FXR knockout mice were purchased from the Jackson Laboratory. Renal collecting duct-specific FXR gene knockout mice were generated by crossing FXRflox/flox mice with an aquaporin 2 gene promoter driven-Cre transgenic mouse and validated as previously described (10–13). Eight- to 10-week-old male animals used in the study were housed on a daily 12-h light/black cycle under controlled temperature and humidity in the animal facility of Peking University Health Science Center. All animals had free access to food. Mice were allowed free access to water or subjected to water restriction for 24 h. After water restriction, mice were killed and perfused with cold PBS. The left kidneys were fixed and paraffin-embedded, while the right kidneys were used to dissect renal cortex and medulla. The use of animals and the study protocols were reviewed and approved by the Animal Care and Use Review Committee of Dalian Medical University.
Chemicals and reagents, methods of cell viability assay, real-time PCR, Western blot, immunostaining, flow cytometry, primary culture of mouse IMCD cells, and luciferase assay, TUNEL assay, immunofluorescence staining, and statistical analyses are described in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Frank Gonzalez for providing FXRflox/flox mice. This work was supported by the National Natural Science Foundation of China Grants 81570636, 81722010 (to X.Z.), 81390351, and 91639201 (to Y.G.); Dalian High-level Talent Innovation Support Program 2016RD13; and Peacock Plan of Shenzhen city Grant KQTD20140630100746562. J.Å.G. was supported by Robert A. Welch Foundation Grant E-0004.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1803945115/-/DCSupplemental.
References
- 1.Knepper MA, Kwon TH, Nielsen S. Molecular physiology of water balance. N Engl J Med. 2015;372:1349–1358. doi: 10.1056/NEJMra1404726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y, Wei Y, Zheng F, Guan Y, Zhang X. Prostaglandin E2 in the regulation of water transport in renal collecting ducts. Int J Mol Sci. 2017;18:E2539. doi: 10.3390/ijms18122539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachmann S, Mutig K. Regulation of renal Na-(K)-Cl cotransporters by vasopressin. Pflugers Arch. 2017;469:889–897. doi: 10.1007/s00424-017-2002-2. [DOI] [PubMed] [Google Scholar]
- 4.Hao CM, et al. Dehydration activates an NF-kappaB-driven, COX2-dependent survival mechanism in renal medullary interstitial cells. J Clin Invest. 2000;106:973–982. doi: 10.1172/JCI9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han Q, et al. AMPK potentiates hypertonicity-induced apoptosis by suppressing NFκB/COX-2 in medullary interstitial cells. J Am Soc Nephrol. 2011;22:1897–1911. doi: 10.1681/ASN.2010080822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hao CM, Redha R, Morrow J, Breyer MD. Peroxisome proliferator-activated receptor delta activation promotes cell survival following hypertonic stress. J Biol Chem. 2002;277:21341–21345. doi: 10.1074/jbc.M200695200. [DOI] [PubMed] [Google Scholar]
- 7.Lee SD, et al. TonEBP stimulates multiple cellular pathways for adaptation to hypertonic stress: Organic osmolyte-dependent and -independent pathways. Am J Physiol Renal Physiol. 2011;300:F707–F715. doi: 10.1152/ajprenal.00227.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang XY, Wang B, Guan YF. Nuclear receptor regulation of aquaporin-2 in the kidney. Int J Mol Sci. 2016;17:E1105. doi: 10.3390/ijms17071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, et al. Farnesoid X receptor (FXR) gene deficiency impairs urine concentration in mice. Proc Natl Acad Sci USA. 2014;111:2277–2282. doi: 10.1073/pnas.1323977111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson RD, et al. Expression of an AQP2 Cre recombinase transgene in kidney and male reproductive system of transgenic mice. Am J Physiol. 1998;275:C216–C226. doi: 10.1152/ajpcell.1998.275.1.C216. [DOI] [PubMed] [Google Scholar]
- 11.Sinal CJ, et al. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 12.Guan Y, et al. Thiazolidinediones expand body fluid volume through PPARgamma stimulation of ENaC-mediated renal salt absorption. Nat Med. 2005;11:861–866. doi: 10.1038/nm1278. [DOI] [PubMed] [Google Scholar]
- 13.Gao M, et al. Disruption of prostaglandin E2 receptor EP4 impairs urinary concentration via decreasing aquaporin 2 in renal collecting ducts. Proc Natl Acad Sci USA. 2015;112:8397–8402. doi: 10.1073/pnas.1509565112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferraris JD, Burg MB. Tonicity-dependent regulation of osmoprotective genes in mammalian cells. Contrib Nephrol. 2006;152:125–141. doi: 10.1159/000096320. [DOI] [PubMed] [Google Scholar]
- 15.Burg MB, Ferraris JD, Dmitrieva NI. Cellular response to hyperosmotic stresses. Physiol Rev. 2007;87:1441–1474. doi: 10.1152/physrev.00056.2006. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro H, Kolodziejczyk AA, Halstuch D, Elinav E. Bile acids in glucose metabolism in health and disease. J Exp Med. 2018;215:383–396. doi: 10.1084/jem.20171965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inagaki T, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Hoekstra M, et al. FXR agonist GW4064 increases plasma glucocorticoid levels in C57BL/6 mice. Mol Cell Endocrinol. 2012;362:69–75. doi: 10.1016/j.mce.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Houten SM, Volle DH, Cummins CL, Mangelsdorf DJ, Auwerx J. In vivo imaging of farnesoid X receptor activity reveals the ileum as the primary bile acid signaling tissue. Mol Endocrinol. 2007;21:1312–1323. doi: 10.1210/me.2007-0113. [DOI] [PubMed] [Google Scholar]
- 20.Herman-Edelstein M, Weinstein T, Levi M. Bile acid receptors and the kidney. Curr Opin Nephrol Hypertens. 2018;27:56–62. doi: 10.1097/MNH.0000000000000374. [DOI] [PubMed] [Google Scholar]
- 21.Cheung CY, Ko BC. NFAT5 in cellular adaptation to hypertonic stress–Regulations and functional significance. J Mol Signal. 2013;8:5. doi: 10.1186/1750-2187-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ko BC, et al. Fyn and p38 signaling are both required for maximal hypertonic activation of the osmotic response element-binding protein/tonicity-responsive enhancer-binding protein (OREBP/TonEBP) J Biol Chem. 2002;277:46085–46092. doi: 10.1074/jbc.M208138200. [DOI] [PubMed] [Google Scholar]
- 23.Jang EJ, et al. TAZ suppresses NFAT5 activity through tyrosine phosphorylation. Mol Cell Biol. 2012;32:4925–4932. doi: 10.1128/MCB.00392-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.