Selective Inhibitors Enable Fundamental Physiological Studies
A selective inhibitor is a drug that inhibits the function of one type of protein more than others. This precise block of function can reveal the physiological role of the targeted endogenous protein. In PNAS, Koster et al. (1) describe the design and characterization of inhibitors that distinguish between closely related CLC chloride channel proteins. This is a boon to studies of CLC physiological function. In this Commentary, we discuss why selective inhibitors represent a scientific breakthrough, how these well-behaved inhibitors evolved from problematic CLC pharmacophores, and what this work portends for the field.
The Importance of Being Druggable
Selective inhibitors of protein function are uniquely valuable tools for studying physiological function. Before the era of molecular biology, inhibitors were the primary, if not only, means of testing the functional significance of proteins. Now, full genomes are sequenced, and a dizzying array of genetic means are available to distinguish protein functions. However, selective inhibitors remain as valuable as ever. Genetic manipulation of protein function is of course phenomenally useful. However, genetic manipulation takes time to implement and always perturbs endogenous physiology. Pharmacology remains irreplaceable as a means of manipulating endogenous function within the time frame of a physiological experiment. As evidence for this claim, the physiological function of proteins with known inhibitors are generally well understood, but the functions of those without inhibitors often remain mysterious.
A selective inhibitor for a protein can enable rigorous testing of squishy physiological hypotheses. Examples of selective inhibitors leading to scientific breakthroughs abound. A classic example is how the selective inhibition of the Na+/K+ ATPase by the cardiac glycoside ouabain revealed that sodium and potassium gradients are established by the same ion pump. Soon after, selective inhibition of sodium currents by the paralytic poison tetrodotoxin provided strong evidence that sodium and potassium currents flow through separate channels. These studies relied on thoughtful application of selective inhibitors within the time frame of a physiological experiment. Such protocols are especially powerful for studying ion channel signaling, where homeostatic responses readily remodel electrical currents to mask genetic manipulations. One beautiful recent example is the use of a recently discovered voltage-gated potassium channel inhibitor to reveal the channel’s role in electrical signaling (2). As in many other studies, a physiological role of an ionic current was not apparent in genetic knockdown experiments but was revealed by application of an inhibitor within experiments. A similarly improved understanding of the roles played by CLC channel subtypes is now within reach, thanks to the discovery of the selective inhibitors reported by Koster et al.
Selective CLC Inhibitors Are Needed
The many members of the mammalian CLC family of Cl− channels and transporters have been cloned for some time. One might think that the molecular responsibilities, physiological properties, and mechanistic quirks of Cl− channels would surface. However, we are still largely at a loss. Based on associations of gene mutations with disease, we know CLC channels are important to our physiology (for review, see ref. 3). However, these findings are largely based on association with genetic perturbations, and compensation for loss of function is commonplace among ion channels and transporters. Genetic knockout studies aimed at pinpointing precise mechanistic roles played by CLC proteins have also resulted in unexpected phenotypes, such as a missing hippocampus (4). This leaves a nagging apprehension that undetected compensation may have exaggerated or occluded results.
As nicely summarized by Koster et al. (1), genetic results in humans and mice have indicated that the human CLC subtypes Ka and Kb serve functionally distinct physiological roles and inhibitors that select between them could be used to treat hyponatremia or hypertension, respectively. Well-behaved inhibitors that distinguish between CLC-Ka and Kb are needed for the distinct physiological roles of these channels to be validated.
The Rogues' Gallery of CLC Inhibitors
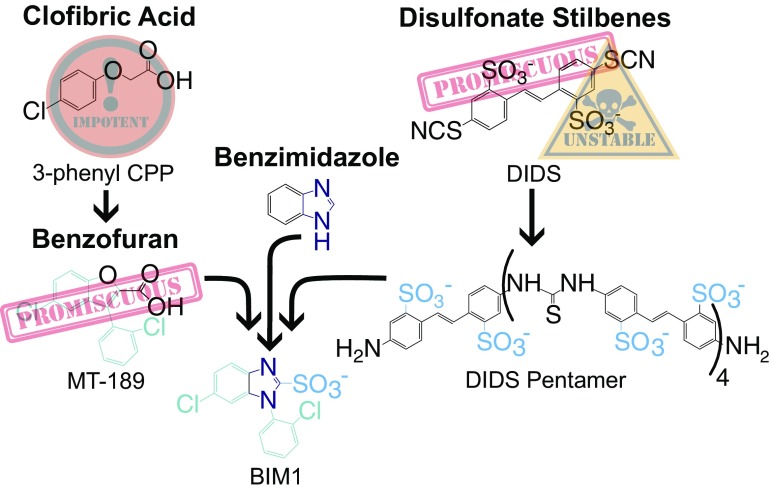
Hiring a motorcycle gang for security can have disastrous results, as can conducting a physiology experiment with nonselective, unstable drugs. Historically, CLC inhibitors have been a troubled lot plagued by promiscuity, instability, and bizarre behavior (Fig. 1). Employment of this band of miscreant molecules to inhibit Cl− channels in animal models has led to some dreadful conclusions. The need for better CLC drugs is highlighted by survey of some of the pharmacology CLC physiologists have had to work with.
Fig. 1.
Miscreant CLC drugs were tamed with a new scaffold.
The Stilbenes: Unstable and Reactive.
Disulfonate stilbene derivatives have been pervasive in the Cl− flux field; these compounds have been used for over four decades. 4,4’-Diisothiocyanatostilbene-2,2’-disulfonic acid (DIDS) is perhaps the most widely used stilbene inhibitor. Originally intended as a covalent modifier of anion transporters, the observation that blocking effects were reversible led to its use as a conventional inhibitor. DIDS has enabled fundamental discoveries and also embodies the problems plaguing CLC pharmacology. Careful use of DIDS provided strong evidence that CLC channels were functional dimers of identical protein subunits. In many other cases, interpretations of studies with DIDS are limited by its instability and promiscuity. DIDS acts on at least three different CLC channel subtypes, as well as ATP-sensitive K+ channels, P-type ATPases, and many other proteins. To make matters messier, DIDS hydrolyzes to form oligomers that have increased potencies against CLCs. Interestingly, the DIDS oligomers have the greatest selectivity, 100-fold, of any known compound for CLC-Ka over CLC-Kb (5).
Clofibric Acids: Low Affinity with a Wee Bit of Selectivity.
A series of clofibric acid-derived compounds have led to a structural understanding of one type of CLC inhibition. Although generally ornery, poorly selective, and low affinity (at best around 100 µM), some clofibric acid derivatives could weakly distinguish between CLC-Ka and CLC-Kb. A neutral asparagine on CLC-Ka that was replaced by a negatively charged aspartate on CLC-Kb seemed to be a key interacting residue as it could influence the subtype selective preferences of an inhibitor (6).
Benzofurans: More Potent.
Covalently constraining the flexible backbone of clofibric acid was an astute synthetic maneuver that created benzofurans that inhibit CLCs when applied at low micromolar concentrations (7). When these molecules were discovered, an accompanying pharmocophore model and binding site were proposed. No gains in selectivity over their clofibric acid ancestors were achieved, but some could weakly discriminate. MT-189, for example, has a threefold preference for CLC-Ka over Kb, a glimmer of hope.
Assembling Redeeming Qualities into a Reliable, Selective Inhibitor
While all of these CLC inhibitors were problematic, each class contained members with desirable attributes: some were selective, efficacious, or stable. At Stanford, Merritt Maduke’s physiology laboratory and Justin Du Bois’s chemistry laboratory teamed up to synthesize the useful features of several CLC inhibitors into one Voltron-like super drug. The design was to create a subtype-selective, chemically stable CLC inhibitor using a chemical architecture more compatible with synthetic modification. Their design conserved the 3D form of the benzofuran scaffold and swapped in a benzimidazole, which is much more synthetically accessible to derivatization. This change facilitated synthesis of a sequence of 19 derivatives to evaluate the structure–activity relationships and define the pharmacophore underlying this new class of inhibitors. To enhance selectivity, they included a sulfonate in most of these, as sulfonates seemed key to the impressive selectivity of stilbene oligomers (5). Remarkably, this strategy worked, resulting in BIM1: a molecule with a >20-fold preference for CLC-Ka over CLC-Kb.
Koster et al. produce a compelling explanation for the enhanced selectivity of the BIM1 molecule (1). Guided by computational docking to a homology model, they were able to reverse BIM1 CLC homolog selectivity by merely switching a single residue, the same that imbued selectivity on clofibric acids. Koster et al. go on to explore the impact of other predicted key interactions in their structural hypothesis for binding. By careful assessment of chemical structure–activity relationships, they came to the unexpected conclusion that the selectivity of BIM1 was not induced by the strategic addition of the sulfonate in their design strategy, but rather by the reduced hydrophobicity of the new benzimidazole scaffold. Thus, as is so often the case, both design and serendipity played key roles in this breakthrough.
Expect Civilized Inhibitors to Illuminate CLC Physiology
How will the new BIM class of inhibitors impact our understanding of CLC function? Most certainly in unexpected ways, but we can hazard a few guesses as to where the science may lead.
Most immediately, these inhibitors could be deployed to stringently test hypotheses concerning the physiological roles of CLC-Ka. Specifically BIM1 seems well suited to test proposal that CLC-Ka drives renal water reabsorption via Cl− flux across the thin ascending limb of the kidney (8). This hypothesis currently rests on circumstantial and genetic evidence, and highly selective drugs could settle whether or not CLC-Ka inhibition is a promising avenue for pharmaceutical intervention in hyponatremia.
After a bit of molecular tinkering, derivatives of these BIM inhibitors could serve as molecular probes of CLC localization. When labeled with fluorophores or isotopes, extracellular inhibitors have proven valuable for identifying when and where their protein targets are functional at the cell surface. The BIM inhibitors bind extracellularly and the negatively charged sulfonated derivatives presumably do not cross cell membranes, making them ideal to guide reporters to CLC proteins on the cell surface. The Du Bois laboratory has proven adept at synthesizing a variety of creative sodium channel tracers for live imaging by derivatizing the selective sodium channel inhibitor saxitoxin (9, 10). The docking model, structure–activity relationship, and modular synthetic routes developed by Koster et al. should enable synthesis of labeled tracers to identify the locations of CLCs in live tissue.
In the big picture, the synthetic route to BIM inhibitors developed by Koster et al. opens the door to large-scale medicinal chemistry approaches to further improve the specificity, affinity, and additional characteristics of this class of inhibitors. The BIM scaffold may have potential to specifically target other CLC homologs. The wide variety of research tools that could be developed from the BIM class of CLC inhibitors has the potential to enable experiments that greatly advance our understanding of fundamental CLC physiology. Characterizing the basic physiology of proteins is a limiting step in validating drug targets for pharmaceutical exploitation. With a recipe for stable, selective, and reliable CLC inhibitors in hand, we expect this newly available precision pharmacology to usher in an era of gratifyingly rigorous hypothesis testing.
Acknowledgments
We acknowledge the many scientists whose findings we mention but did not properly cite due to space limitations. We thank Georgeann Sack for thoughtful critiques and editing. R.J.S. and J.T.S. were supported by National Institutes of Health Grants R01NS096317, R01HL128537, and T32GM007377.
Footnotes
The authors declare no conflict of interest.
See companion article on page E4900.
References
- 1.Koster AK, et al. A selective class of inhibitors for the CLC-Ka chloride ion channel. Proc Natl Acad Sci USA. 2018;115:E4900–E4909. doi: 10.1073/pnas.1720584115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu PW, Bean BP. Kv2 channel regulation of action potential repolarization and firing patterns in superior cervical ganglion neurons and hippocampal CA1 pyramidal neurons. J Neurosci. 2014;34:4991–5002. doi: 10.1523/JNEUROSCI.1925-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stauber T, Weinert S, Jentsch TJ. Cell biology and physiology of CLC chloride channels and transporters. Compr Physiol. 2012;2:1701–1744. doi: 10.1002/cphy.c110038. [DOI] [PubMed] [Google Scholar]
- 4.Stobrawa SM, et al. Disruption of ClC-3, a chloride channel expressed on synaptic vesicles, leads to a loss of the hippocampus. Neuron. 2001;29:185–196. doi: 10.1016/s0896-6273(01)00189-1. [DOI] [PubMed] [Google Scholar]
- 5.Matulef K, et al. Discovery of potent CLC chloride channel inhibitors. ACS Chem Biol. 2008;3:419–428. doi: 10.1021/cb800083a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Picollo A, et al. Molecular determinants of differential pore blocking of kidney CLC-K chloride channels. EMBO Rep. 2004;5:584–589. doi: 10.1038/sj.embor.7400169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liantonio A, et al. Molecular switch for CLC-K Cl− channel block/activation: Optimal pharmacophoric requirements towards high-affinity ligands. Proc Natl Acad Sci USA. 2008;105:1369–1373. doi: 10.1073/pnas.0708977105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liantonio A, et al. In-vivo administration of CLC-K kidney chloride channels inhibitors increases water diuresis in rats: A new drug target for hypertension? J Hypertens. 2012;30:153–167. doi: 10.1097/HJH.0b013e32834d9eb9. [DOI] [PubMed] [Google Scholar]
- 9.Ondrus AE, et al. Fluorescent saxitoxins for live cell imaging of single voltage-gated sodium ion channels beyond the optical diffraction limit. Chem Biol. 2012;19:902–912. doi: 10.1016/j.chembiol.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoehne A, et al. A 18F-labeled saxitoxin derivative for in vivo PET-MR imaging of voltage-gated sodium channel expression following nerve injury. J Am Chem Soc. 2013;135:18012–18015. doi: 10.1021/ja408300e. [DOI] [PMC free article] [PubMed] [Google Scholar]