Significance
Leaf traits, such as photosynthetic capacity, nitrogen concentration, and leaf mass per area, strongly affect plant growth and nutrient cycles. Understanding relationships among leaf traits is, therefore, a fundamental challenge in plant biology, crop science, and ecology. Different groups of leaves exhibit distinct relationships among pairs of traits. For example, photosynthetic capacity per unit leaf area increases strongly with leaf mass per area from sun to shade within species, but these same traits are only weakly related across global species. Our analysis suggests that divergent trait relationships can be understood by partitioning leaf mass into photosynthetic and structural support components. Our paper clarifies the causes of relationships among traits and why those relationships differ among different groups of plants.
Keywords: functional traits, tropical forests, leaf mass per area, plant functional types, leaf longevity
Abstract
Understanding variation in leaf functional traits—including rates of photosynthesis and respiration and concentrations of nitrogen and phosphorus—is a fundamental challenge in plant ecophysiology. When expressed per unit leaf area, these traits typically increase with leaf mass per area (LMA) within species but are roughly independent of LMA across the global flora. LMA is determined by mass components with different biological functions, including photosynthetic mass that largely determines metabolic rates and contains most nitrogen and phosphorus, and structural mass that affects toughness and leaf lifespan (LL). A possible explanation for the contrasting trait relationships is that most LMA variation within species is associated with variation in photosynthetic mass, whereas most LMA variation across the global flora is associated with variation in structural mass. This hypothesis leads to the predictions that (i) gas exchange rates and nutrient concentrations per unit leaf area should increase strongly with LMA across species assemblages with low LL variance but should increase weakly with LMA across species assemblages with high LL variance and that (ii) controlling for LL variation should increase the strength of the above LMA relationships. We present analyses of intra- and interspecific trait variation from three tropical forest sites and interspecific analyses within functional groups in a global dataset that are consistent with the above predictions. Our analysis suggests that the qualitatively different trait relationships exhibited by different leaf assemblages can be understood by considering the degree to which photosynthetic and structural mass components contribute to LMA variation in a given assemblage.
Leaf traits related to the carbon and nutrient economies of plants represent important aspects of plant functional diversity (1–3). Understanding the drivers of trait variation and relationships among traits is essential for our basic understanding of plant ecology (4, 5) and agricultural production (6, 7) and for accurately representing plant functional diversity in global carbon-climate models (8, 9). Strong correlations among leaf mass per area (LMA), leaf lifespan (LL), and mass-normalized values of four leaf traits related to photosynthesis and metabolism—light-saturated net photosynthetic rate (Amax), dark respiration rate (Rdark), nitrogen concentration (N), and phosphorus concentration (P)—have been interpreted as evidence for a mass-based global “leaf economics spectrum” ranging from low-cost (low LMA) short-lived leaves with fast photosynthetic returns to high-cost (high LMA) long-lived leaves with slow photosynthetic returns (2). However, strong correlations among some pairs of traits may result from mass normalization itself (10), and there is currently no consensus on how to interpret statistical relationships among some leaf traits (11–14).
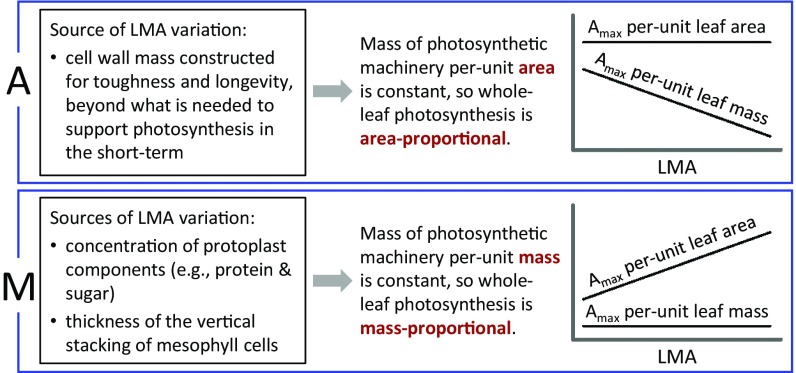
An important observation that can help clarify the underlying causes of leaf trait relationships is that inter- and intraspecific patterns of trait variation are often fundamentally different (13, 15). For example, in contrast to interspecific global patterns (where area-normalized Amax, Rdark, N, and P are only weakly related to LMA) (2, 11), area-normalized Amax, Rdark, N, and P often increase within species as LMA increases from shade to sun (15–19). This contrast between interspecific global patterns and intraspecific canopy gradients suggests the presence of multiple biological drivers. Variation in LMA integrates variation in the mass per area of multiple tissues and functions, including metabolically active mesophyll cytoplasm that largely determines photosynthetic capacity, as well as structural components (e.g., cellulose) that contribute to leaf toughness (20–23). These different sources of LMA variation can lead to divergent trait relationships (Fig. 1) and may help reconcile opposing inter- and intraspecific patterns.
Fig. 1.
Two extreme cases showing how contrasting sources of variation in leaf mass per area (LMA) (20–23) can lead to different relationships between photosynthetic capacity (Amax) and LMA. In case A (Upper), the source of LMA variation affects structural toughness and leaf lifespan but does not directly affect gas exchange (whole-leaf Amax). Therefore, whole-leaf Amax increases with leaf area but not leaf mass. This “area-proportional” trait variation (patterns in Upper Right) is characteristic of the global flora (11). In case M (Lower), the sources of LMA variation directly affect whole-leaf Amax. Therefore, whole-leaf Amax increases with leaf mass but not leaf area. This “mass-proportional” trait variation (patterns in Lower Right) is characteristic of intraspecific canopy gradients from sun to shade (15–19). An explanation of trait mass vs. area proportionality is in the text. The linear relationships shown at far right are idealized; real relationships may be nonlinear and would include scatter due to LMA-independent sources of variation.
In this paper, we seek a general understanding of the drivers of leaf trait variation across light gradients within species, among species, and among functional groups. In doing so, we present (i) analyses of intra- and interspecific leaf trait variation at three tropical forest sites (wet and dry sites in Panama and a wet site in Ecuador), (ii) analyses of interspecific patterns within functional groups in the Glopnet global leaf traits database (2), and (iii) a conceptual model that explains LMA variation in terms of two primary components—photosynthetic capacity and leaf toughness. The conceptual model—which is introduced in Fig. 1 and is discussed in more detail later in this paper—provides an explanation for divergent patterns in trait variation within species, among species, and among functional groups.
Mass vs. Area Proportionality of Leaf Traits
A useful starting point for understanding trait relationships is to recognize that the gas exchange rates (e.g., Amax or Rdark) or nutrient amounts (e.g., N or P) associated with an entire leaf (the “whole-leaf trait amount”) can depend on leaf mass, leaf area, or some combination of mass and area (11). Leaves with large mass also tend to have large area, but the correlation between mass and area is imperfect due to variation among leaves in lamina thickness and tissue density. The resulting variation in LMA allows the variance in size-dependent traits (e.g., Amax, Rdark, N, and P) to be partitioned into mass- vs. area-proportional fractions (11).
A trait is defined as being purely area proportional in a given assemblage of leaves if the whole-leaf trait amount increases in proportion to leaf area but is uncorrelated with leaf mass after controlling for variation in leaf area. Area-proportional traits are uncorrelated with LMA when expressed per unit area and are negatively correlated with LMA when expressed per unit mass (case A in Fig. 1). Conversely, a trait is defined as being purely mass proportional if the whole-leaf trait amount increases in proportion to leaf mass but is uncorrelated with leaf area after controlling for variation in leaf mass. Mass-proportional traits are uncorrelated with LMA when expressed per unit mass and are positively correlated with LMA when expressed per unit area (case M in Fig. 1).
In both area- and mass-proportional cases, normalizing by the variable (mass or area) that controls the whole-leaf trait amount results in normalized trait values that are statistically independent of mass, area, and LMA (Fig. 1), because in this case, normalization “explains” (or “accounts for”) any size-dependent variation in the trait. In contrast, normalizing by the unrelated variable (i.e., normalizing an area-proportional trait by mass or normalizing a mass-proportional trait by area) results in normalized trait values that are either negatively or positively correlated with LMA (Fig. 1), because in this case, the whole-leaf trait amount is being divided by an unrelated quantity that is either the numerator (mass) or denominator (area) of LMA. Here, “unrelated quantity” refers specifically to whole-leaf trait amounts and is not meant to imply that only mass or area is biologically meaningful in a given leaf assemblage (e.g., even in cases where traits are purely area proportional, leaf mass is still an important quantity related to the economics of investment and return) (14). We use the concepts of mass and area proportionality not to advocate for how traits should be normalized, but rather to provide an analytical framework for quantifying and understanding trait variation in different leaf assemblages.
Quantifying Trait Mass vs. Area Proportionality Within and Among Tropical Tree Species
Here, we extend a previously developed framework for quantifying mass vs. area proportionality across species (11) to quantify both inter- and intraspecific variation in Amax, Rdark, N, and P at tropical forest sites in Panama and Ecuador. In doing so, we clarify how trait variation differs within vs. among species before discussing our conceptual model (Fig. 1) in greater detail and then using the conceptual model to understand differences in trait variation among functional groups in a global dataset.
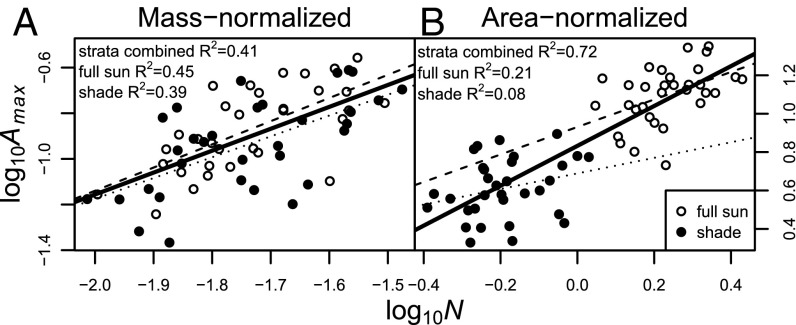
Contrasting patterns of trait variation within vs. among species are illustrated by relationships between Amax and N for sun and shade leaves of 32 plant species in Panama (Fig. 2 and Table S1). As our analyses below show, interspecific variation in these traits is primarily area proportional (i.e., across species, whole-leaf trait amounts are better predicted by leaf area than mass), whereas intraspecific variation in these traits is primarily mass proportional (i.e., within species, whole-leaf trait amounts are better predicted by leaf mass than area). Following the same logic as described above for relationships between size-dependent traits and LMA, correlations between two size-dependent traits (e.g., between Amax and N) are stronger when the traits are normalized by the variable (either mass or area) that does not control the whole-leaf trait amount. For example, within species, sun leaves tend to have greater area-normalized Amax and N than shade leaves (Fig. 2B), because sun leaves have higher LMA than shade leaves (Fig. S1) and because intraspecific variation in Amax and N is primarily mass proportional (results are shown below). Thus, when Amax and N are mass normalized, sun and shade leaves occupy a single envelope of points (Fig. 2A), whereas area normalization results in two separate envelopes and a stronger correlation for the combined sun–shade dataset (Fig. 2B). This strong correlation is simply driven by the separation of sun (high LMA) and shade (low LMA) leaves. Similarly, among leaves at a given light level, the stronger interspecific correlations obtained from mass normalization (Fig. 2A) compared with area normalization (Fig. 2B) are also the result of LMA variation, with high values of Amax/mass and N/mass corresponding to low LMA and vice versa (11, 12, 14). The key point here is not to suggest that only one normalizer is valid in a given situation but simply to clarify how mass vs. area proportionality combines with normalization to influence trait relationships.
Fig. 2.
Mass-normalized (A) and area-normalized (B) relationships between maximum net photosynthetic rate (Amax) and nitrogen concentration (N) for full sun upper canopy (white circles and dashed lines), shaded understory (black circles and dotted lines), and all leaves combined (solid lines). Each point represents 1 of 32 species for which canopy and understory traits were measured in Panama (wet and dry sites yielded similar results and are pooled). Among species within each canopy layer, traits are primarily area proportional (Fig. 3, white bars), and therefore, mass-normalized relationships are stronger (higher R2 values for sun and shade leaves in A compared with B). In contrast, within species, traits are primarily mass proportional (Fig. 3, gray bars), and therefore, area-normalized relationships are stronger for the pooled sun–shade dataset (higher R2 for strata combined in B compared with A). An explanation of trait mass vs. area proportionality and their effects on trait correlations is in the text. Amax units are micromoles per second per gram (mass based) and micromoles per second per square meter (area based), and N units are grams per gram (mass based) and grams per square meter (area based).
We now describe our statistical framework for quantifying mass proportionality within and among species. Inferences about mass and area proportionality are interconvertible (e.g., strong mass proportionality implies weak area proportionality and vice versa). Consider the simpler problem of quantifying interspecific mass proportionality, and assume that the whole-leaf amount of trait i in a species j leaf (Xij) depends on its area (Areaj) and mass (Massj) as
where ai is a fitted constant; b1i and b2i quantify how trait i depends on leaf area and mass, respectively, across species; and εij is a log-normally distributed random variable (assumed to have a median of one in our analysis) that represents both measurement error and interspecific variation that is not explained by leaf area or mass (e.g., variation in photosynthetic capacity due to effects of internal leaf structure on CO2 diffusion) (23). The sum of the estimates for b1i and b2i is typically close to one (Table S2), and therefore, the above expression can be approximated as
| [1] |
Eq. 1 leads to simple expressions for area- and mass-normalized trait values (XAij and XMij, respectively) that can be analyzed with widely available area- and mass-normalized trait data. The area- and mass-normalized forms, obtained by dividing Eq. 1 by area and mass, respectively, are
| [2] |
| [3] |
Eqs. 1–3 yield identical estimates for bi, which is an index of mass proportionality across species (e.g., if bi = 0, then trait i is purely area proportional, whereas if bi = 1, then trait i is purely mass proportional). (Eqs. 1–3 also yield identical estimates for ai, the value of XAij and XMij if LMA = 1, but we focus our attention on parameter b, because parameter a is not relevant to our main questions.) These expressions may be log transformed and analyzed using ordinary least squares (OLS) regression, which yields very similar estimates of mass proportionality as other methods described in ref. 11 (Fig. S2).
To extend Eq. 1 to estimate mass proportionality both within and among species, we assume that the total amount of trait i in leaf k of species j (Xijk) depends on species j’s mean leaf area and mass ( and , respectively) and also, on leaf-level departures from these means due to intracanopy plasticity (24):
| [4] |
Parameters bi and wi quantify mass proportionality of trait i among and within species, respectively. For example, if trait i is strongly mass proportional within species (wi near one), then intraspecific variation in leaf mass (differences between Massjk and ) would strongly affect the whole-leaf trait amount (Xijk), whereas intraspecific variation in leaf area (differences between Areajk and ) would have little effect on Xijk.
Because Eq. 4 relies on whole-leaf trait values that are not commonly reported, it is useful to derive alternative forms that can be fit to widely available normalized trait data. Noting that the mean of LMAjk across all leaves k in species j, , is approximately equal to , we can approximate Eq. 4 in area- and mass-normalized forms in terms of interspecific variation in mean LMA and intraspecific LMA variation (i.e., differences between LMAjk and ) due to intracanopy plasticity (24):
| [5] |
| [6] |
Eqs. 5 and 6 yield identical estimates of inter- and intraspecific mass proportionality (bi and wi, respectively), and they can be log transformed and analyzed using OLS regression. As in the simpler interspecific model (Eqs. 1–3), the error term () represents not only measurement error but also, trait variation within or among species that is unrelated to LMA (and therefore, not explained by our model).
We fit Eqs. 5 and 6 to data from Panama (dry and wet sites combined, because both sites yielded similar results), where two values (sun and shade leaves) were available for Amax (44 species), Rdark (34 species), N (32 species), and P (32 species), and Ecuador, where two values (sun and shade leaves) were available for N for each of 67 species.
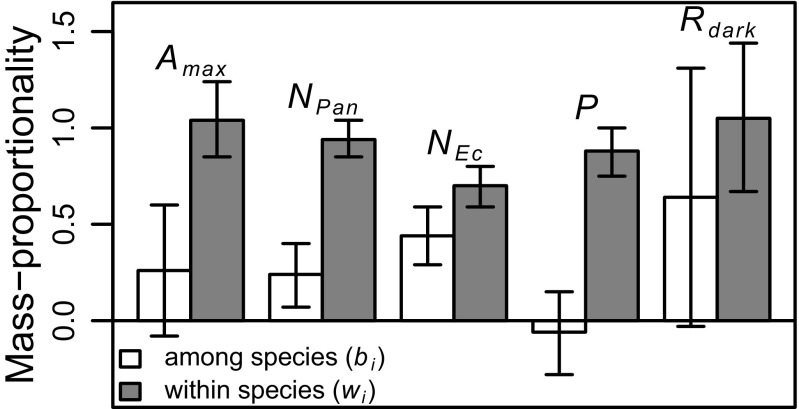
Intraspecific trait variation at the Panama and Ecuador sites was primarily mass proportional (wi > 0.7 in all cases) (Fig. 3), whereas interspecific trait variation was primarily area proportional (bi < 0.5 in four of five cases) (Fig. 3). The one exception to interspecific area proportionality (bi = 0.64 for Rdark) has large uncertainty (Fig. 3). Strong interspecific area proportionality for Amax, N, and P is also apparent across tropical tree species in the Glopnet global database (Table S3) (no estimate for Rdark due to small sample size). In addition to these results based on Eqs. 5 and 6, strong intraspecific mass proportionality is also implied by an alternative statistical approach (Fig. S3) and by visual inspection of the raw data themselves, because area-normalized trait values tend to be higher for sun leaves (high LMA) than shade leaves (low LMA), whereas there is no systematic tendency for mass-normalized trait values to increase or decrease across canopy layers (Fig. S4). In summary, strong interspecific area proportionality across tropical plant species emerges from analyses of multiple datasets (Panama, Ecuador, and Glopnet) (Table S3), and strong intraspecific mass proportionality seems to be a robust pattern that does not depend on the particular assumptions used to derive Eqs. 5 and 6.
Fig. 3.
Mass proportionality among (white bars) and within (gray bars) species for four leaf traits (maximum net photosynthetic rate is Amax, dark respiration rate is Rdark, nitrogen concentration is N, and phosphorus concentration is P) from Panama (subscript “Pan”: dry and wet sites combined) and one trait (N) from Ecuador (subscript “Ec”). Parameters bi and wi (Eqs. 5 and 6) are zero and one, respectively, for traits that are purely area or mass proportional. Among species, trait variation is primarily area proportional (bi < 0.5 in most cases). In contrast, within species, trait variation is primarily mass proportional (wi > 0.7). An explanation of trait mass vs. area proportionality is in the text. Error bars are 95% CIs. Sample sizes are in Table S3.
Multiple Drivers of Variation in Leaf Mass per Area
Why are traits related to photosynthesis and metabolism (Amax, Rdark, N, and P) primarily mass proportional within species (Fig. 3, gray bars) but primarily area proportional across tropical species (Fig. 3, white bars and Table S3) and across the global flora (11)? We can gain insight into the causes of these divergent patterns by recognizing that LMA is a composite trait, with density and volume components for multiple types of tissues and chemical compounds (20). Nearly all LMA components may be considered to contribute to a leaf’s lifetime photosynthesis in some way. However, some structural and chemical components that strongly affect leaf toughness (e.g., cellulose mass per leaf volume) (21, 22)—and thus, the lifespan over which photosynthetic dividends are returned (2, 25)—have little direct effect on photosynthetic capacity and metabolism (Fig. 1). These structural LMA components may even decrease photosynthetic capacity (e.g., due to the negative effects of cell wall thickness on mesophyll conductance) (26–28). Thus, to understand the causes of covariation between Amax (as well as Rdark, N, and P) and LMA in a given leaf assemblage, it is important to consider the degree to which LMA variation is driven by variation in the mass per area of components that directly contribute to photosynthetic capacity (e.g., cytoplasm in palisade mesophyll) vs. those that do not (e.g., cellulose).
Conceiving of LMA variation in terms of the mass per area of photosynthetic vs. structural components provides a simple explanation for contrasting intra- and interspecific leaf trait relationships. Shipley et al. (29) used a similar conceptual model to understand interspecific trait covariance in the global flora. We suggest that this conceptual framework also provides a simple explanation for divergent patterns in trait variation within vs. among tropical species (Fig. 3) as well as among different plant functional groups (see below). Our conceptual model posits that variation among leaves in the mass per area of photosynthetic machinery leads to mass proportionality of Amax and related traits (because in this case, variation in LMA is associated with variation in Amax per area), whereas variation among leaves in the mass per area of structural components that contribute to toughness and LL but not photosynthetic capacity leads to area proportionality (because in this case, variation in LMA is independent of variation in Amax per area). The degree of mass vs. area proportionality in a given leaf assemblage should thus reflect the balance between these different drivers of LMA variation.
Consider the expected contributions of photosynthetic vs. structural leaf mass to LMA variation in interspecific assemblages with highly variable LL (e.g., the global flora and tropical forest communities, where LL often varies by a factor of five or more) (Fig. S1) (21, 30). In these cases, we expect much LMA variation to be associated with variation in the mass per area of structural and chemical components that contribute to toughness and LL (20–22, 31, 32). Thus, Amax and related traits should be largely area proportional, because much of the variation in LMA should be unrelated to the mass of photosynthetic machinery per leaf area (case A in Fig. 1). Mass-normalized values of these area-proportional traits will exhibit strong negative correlations with LMA and strong positive correlations with each other for reasons described above and in refs. 11 and 12.
In contrast to interspecific assemblages in which LL increases with LMA, LL often decreases within species as LMA increases from shade to sun (Fig. S1) (33, 34). This negative intraspecific association between LL and LMA may be explained by differences in optimal LL across light environments [e.g., due to differences in the costs/benefits of deploying new leaves vs. retaining existing ones (35)] and is unlikely to reflect a direct causal relationship between LMA and LL. Rather, intraspecific variation in LMA across light gradients is associated with variation in photosynthetic capacity (16, 19, 20, 36, 37). In this case, we expect Amax and related traits to be strongly mass proportional, because much of LMA variation is a direct consequence of variation in the mass of photosynthetic machinery per leaf area (case M in Fig. 1).
Unlike the above examples, some other leaf assemblages may resemble neither of the hypothetical cases illustrated in Fig. 1. For example, in temperate deciduous forests with modest (e.g., twofold) variation in LL, interspecific variation in both photosynthetic capacity and toughness likely contributes to LMA variation (38) and thus, should exhibit an intermediate degree of mass proportionality.
The above conceptual model of LMA variation in terms of the mass per area of photosynthetic and structural components has important limitations. First, interpreting LMA in terms of mass per area components does not explicitly account for leaf anatomy or density (e.g., cellulose mass per volume is a better predictor of leaf toughness and LL than cellulose mass per area in at least some cases) (21, 22). Second, some LMA components cannot easily be partitioned into photosynthetic vs. structural functions. For example, thick cell walls enhance leaf toughness and can increase LL (26), but at least some cell wall mass is required for biomechanical support (e.g., for internal leaf structure and optimal leaf display) and water transport that enable photosynthesis (23, 39, 40). Despite these limitations, interpreting LMA in terms of photosynthetic and structural components is useful for understanding divergent patterns in trait variation within vs. among tropical species (see above) as well as among different plant functional groups (see below).
Understanding Differences in Trait Mass Proportionality Among Plant Functional Groups
Conceiving of LMA variation as arising from variation in photosynthetic and structural components leads to testable predictions regarding patterns of trait variation in different functional groups of species. As explained above and in Fig. 1, interspecific mass proportionality of Amax, Rdark, N, and P should be relatively low within assemblages in which LL is highly variable and strongly associated with LMA variation. In such assemblages, much of the LMA variation among species should be due to structural leaf mass that contributes little to whole-leaf values of Amax, Rdark, N, and P; thus, these whole-leaf trait amounts should be roughly proportional to leaf area rather than mass. Conversely, interspecific mass proportionality should be relatively high within assemblages in which LL varies little and is weakly associated with LMA, because in these cases, much of the variation in LMA should be directly related to the mass of photosynthetic machinery per unit leaf area. The above general predictions can be translated into two quantitative predictions that can be tested by comparing patterns of trait variation across different functional groups: first, the degree of interspecific mass proportionality of Amax, Rdark, N, and P should increase as the strength of the LL–LMA relationship decreases; second, statistically controlling for variation in LL should increase the estimated degree of mass proportionality of Amax, Rdark, N, and P, because as LL variation decreases, the fraction of LMA variation associated with photosynthetic and metabolic traits should increase.
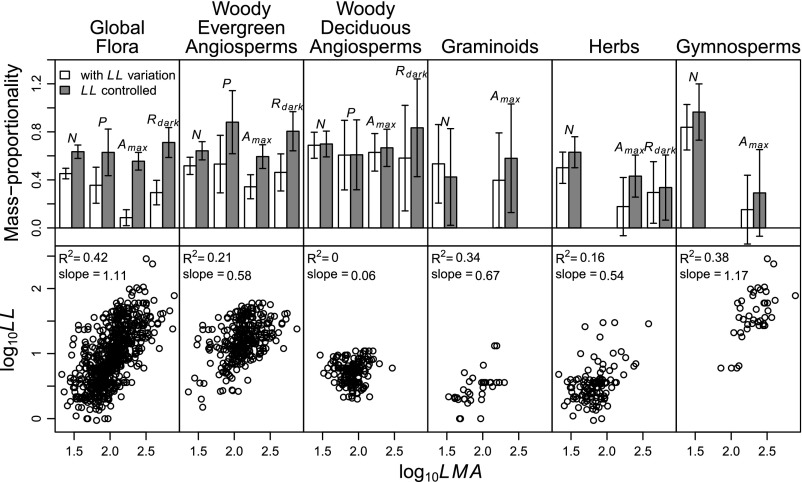
To evaluate the first prediction (interspecific mass proportionality should increase as the strength of the LL–LMA relationship decreases), we used Eqs. 2 and 3 to estimate interspecific mass proportionality (bi) of Amax, Rdark, N, and P for different functional groups in the Glopnet global dataset (Fig. 4, white bars and Table S4). Results for Amax strongly support the predicted increase in mass proportionality with decreasing strength of the LL–LMA relationship (Fig. S5), and results for Rdark, N, and P are also generally supportive, despite the presence of a few outliers and high uncertainty in some estimates (Fig. S5).
Fig. 4.
(Upper) Mass proportionality among species within Glopnet functional groups tends to be greater when controlling for leaf lifespan (LL) variation (Eq. 7) (gray bars) vs. when LL variation is not accounted for (Eq. 2) (white bars). Error bars are 95% CIs. (Lower) LL (months) vs. leaf mass per area (LMA; grams per square meter). R2 and slopes are from log10 regressions. Analyses were restricted to Glopnet records with a reported LL value, so that both estimates (white and gray bars) use the same set of records. Sample sizes (n ≥ 25) are in Table S4.
To evaluate the second prediction (interspecific mass proportionality should increase after controlling for LL variation), we modified Eqs. 2 and 3 to statistically control for LL when estimating interspecific mass proportionality (bi):
| [7] |
| [8] |
Applying Eqs. 7 and 8 (which yield identical results) to Glopnet functional groups suggests that mass proportionality is indeed greater among leaves of equal LL than among leaves of variable LL. Specifically, Eqs. 7 and 8 yield estimates of bi that tend to be greater than those from Eqs. 2 and 3 (Fig. 4, compare gray and white bars and Table S4), particularly for groups with strong LL–LMA relationships (e.g., the global flora, woody evergreen angiosperms, and gymnosperms).
Discussion
It is widely recognized that LMA variation is associated with multiple tissues and functions, including metabolically active mesophyll that determines photosynthetic capacity, as well as structural and chemical components that contribute to leaf toughness (20–23). Here, we have shown that interpreting LMA variation in terms of photosynthetic and structural components leads to a simple, unified explanation for divergent patterns in trait variation within species, among species, and among functional groups. In leaf assemblages where LMA variation is driven primarily by structural components that contribute to leaf toughness and leaf lifespan (LL) but not photosynthetic capacity, per unit area values of photosynthetic and metabolic traits (e.g., Amax, Rdark, N, and P) should be largely independent of LMA (case A in Fig. 1). Such cases include the global flora and the functionally diverse tropical forest communities studied here. In contrast, in leaf assemblages where LMA variation is primarily caused by variation in leaf photosynthetic capacity, per unit area values of Amax and related traits should increase with LMA (case M in Fig. 1). Such cases include intraspecific sun–shade comparisons (16, 19, 20, 36, 37). Interspecific assemblages with moderate variation in LL (e.g., temperate deciduous trees) are somewhere between the extreme cases illustrated in Fig. 1.
Observed patterns in trait variation within and among species and functional groups are largely consistent with the above interpretation of LMA variation in terms of photosynthetic and structural components. This conceptual model also clarifies why whole-leaf trait values (e.g., the rate of gas exchange of an entire leaf or the amount of nutrient in an entire leaf) are better predicted by leaf area than mass in some cases (area proportionality) and better predicted by leaf mass than area in other cases (mass proportionality). Our conceptual model and supporting evidence underscore the futility of advocating for either mass or area normalization of leaf traits as a general principle. Our analyses show that traits are not, as a general rule, either mass or area proportional, and our conceptual model explains why this is so.
Future efforts should focus on moving beyond the conceptual model presented here to provide a more mechanistic framework for representing leaf functional variation in terms of measurable anatomical and chemical traits. Recent efforts to decompose interspecific LMA variation into anatomical components (41) and to quantify interspecific tradeoffs related to leaf nitrogen allocation and cell wall thickness (26) could be extended to study intraspecific variation. Simple models based on leaf density and lamina thickness (both of which contribute to LMA variation within and among the tropical species that we studied) fail to capture the main patterns in the datasets analyzed here (Table S5). Leaf density and cellulose mass per leaf volume are good predictors of leaf toughness and LL in some previous studies (21, 22, 34) and in some subsets of our data, but they are poor predictors of LL for sun leaves at the speciose wet Panama site (Table S5). This inconsistency may reflect the variety of factors, including photosynthetic capacity and self-shading, that affect optimal LL (35, 42). Developing a quantitative, mechanistic model for leaf trait variation within and among species may require integrating optimal LL theory (35) with anatomical tradeoffs, such as the increase in leaf toughness and the decline in mesophyll conductance (and thus, photosynthetic capacity) as cell wall thickness increases (26–28).
Materials and Methods
Trait Data Sources.
The Panama data (Table S1) include leaves sampled at two different canopy positions (“canopy”: full sun at the top of the canopy; and “understory”: well-shaded, sampled within 2 m of the forest floor) from plant species (including trees, treelets, lianas, vines, epiphytes, and hemiepiphytes) within the reach of a canopy crane at two sites: Parque Natural Metropolitano (PNM) and Bosque Protector San Lorenzo (SL). The “dry” PNM site is a semideciduous coastal Pacific forest with a 5-mo dry season from December to April and 1,740 mm of annual rainfall (43). The PNM crane is 40 m tall with a 51-m-long boom. The “wet” SL site is an evergreen Caribbean coastal forest with 3,100 mm of annual rainfall (43). The SL crane is 52 m tall with a 54-m-long boom. Methods for quantifying traits at the Panama sites are described in SI Materials and Methods.
The Ecuador data (44, 45) (SI Materials and Methods) are from the Yasuní Forest Dynamics Plot in a mature terra firme forest in Yasuní National Park and Biosphere Reserve in Amazonian Ecuador, with 3,081 mm of annual rainfall (46). Full sun canopy leaves from Ecuador were collected from sapling and adult trees growing at least partially in forest gaps and clearings with branches that could be clipped from the ground with a pole pruner.
The Glopnet dataset, available from ref. 2, includes ∼2,500 records with one or more of the following six traits: Amax, Rdark, N, P, LMA, and LL. Eighty-three percent of species in Glopnet are represented by a single record, and therefore, our analyses of Glopnet data primarily represent interspecific variation. Most records are missing data for one or more traits, which restricts the sample size for a given analysis. Analyses of Glopnet tropical trees (Table S3) used records where growth form was reported as “tree” and biome was reported as “tropical rainforest” or “tropical seasonal forest.” Analyses of Glopnet functional groups (Fig. 4 and Fig. S5) used taxonomy, leaf habit (evergreen or deciduous), and growth form data reported in Glopnet to partition species into functional groups: gymnosperms, graminoids (families Poaceae, Cyperaceae, and Juncaceae), herbaceous plants (excluding graminoids), evergreen woody angiosperms (shrubs and trees), and deciduous woody angiosperms (shrubs and trees).
Analysis.
Results reported in the text are based on OLS regression analysis of log10-transformed versions of Eqs. 2, 5, and 7 (area-normalized forms). These analyses are normalization independent, and therefore, fitting Eqs. 3, 6, and 8 (mass-normalized forms) yields equivalent results. Not all analyses could be performed for all datasets. For example, Eq. 7 could not be fit to the Ecuador dataset, which lacks LL, and we did not attempt to fit Eq. 5 to Glopnet data, which primarily reports interspecific variation for sun leaves. Additional analyses were performed for comparison with the text OLS analyses as described in SI Materials and Methods.
Supplementary Material
Acknowledgments
For data collected in Panama, we thank Milton Garcia for assistance in the field and the Andrew W. Mellon Foundation and the Smithsonian Tropical Research Institute for funding. For data collected in Ecuador, N.J.B.K. thanks Laura Williams, Adam Martin, Pablo Alvia, and Tania Aftandilians for assistance with trait collection; Renato Valencia and the funding sources for the Yasuní National Park Forest Dynamics Plot (the Andrew W. Mellon Foundation, the Smithsonian Tropical Research Institute, Aarhus University, and Pontificia Universidad Católica del Ecuador Grant 13 373); and the Ecuadorian Ministry of the Environment for approving the work (permit 002–015–IC–FLO–PNY-DPAO). S.W.P. thanks the Carbon Mitigation Initiative at the Princeton Environmental Institute at Princeton University for funding.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1803989115/-/DCSupplemental.
References
- 1.Reich PB, Walters MB, Ellsworth DS. From tropics to tundra: Global convergence in plant functioning. Proc Natl Acad Sci USA. 1997;94:13730–13734. doi: 10.1073/pnas.94.25.13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wright IJ, et al. The worldwide leaf economics spectrum. Nature. 2004;428:821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
- 3.Díaz S, Cabido M. Vive la différence: Plant functional diversity matters to ecosystem processes. Trends Ecol Evol. 2001;16:646–655. [Google Scholar]
- 4.Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ. Plant ecological strategies: Some leading dimensions of variation between species. Annu Rev Ecol Syst. 2002;33:125–159. [Google Scholar]
- 5.Reich PB. The world-wide “fast-slow” plant economics spectrum: A traits manifesto. J Ecol. 2014;102:275–301. [Google Scholar]
- 6.Nunes-Nesi A, et al. Natural genetic variation for morphological and molecular determinants of plant growth and yield. J Exp Bot. 2016;67:2989–3001. doi: 10.1093/jxb/erw124. [DOI] [PubMed] [Google Scholar]
- 7.Tomeo NJ, Rosenthal DM. Variable mesophyll conductance among soybean cultivars sets a tradeoff between photosynthesis and water-use-efficiency. Plant Physiol. 2017;174:241–257. doi: 10.1104/pp.16.01940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakschewski B, et al. Resilience of Amazon forests emerges from plant trait diversity. Nat Clim Chang. 2016;6:1032–1036. [Google Scholar]
- 9.Scheiter S, Langan L, Higgins SI. Next-generation dynamic global vegetation models: Learning from community ecology. New Phytol. 2013;198:957–969. doi: 10.1111/nph.12210. [DOI] [PubMed] [Google Scholar]
- 10.Field C, Mooney H. The photosynthesis-nitrogen relationship in wild plants. In: Givnish TJ, editor. On the Economy of Plant Form and Function: Proceedings of the Sixth Maria Moors Cabot Symposium, Evolutionary Constraints on Primary Productivity, Adaptive Patterns of Energy Capture in Plants, Harvard Forest, August 1983. Cambridge Univ Press; Cambridge, UK: 1986. pp. 25–55. [Google Scholar]
- 11.Osnas JLD, Lichstein JW, Reich PB, Pacala SW. Global leaf trait relationships: Mass, area, and the leaf economics spectrum. Science. 2013;340:741–744. doi: 10.1126/science.1231574. [DOI] [PubMed] [Google Scholar]
- 12.Lloyd J, Bloomfield K, Domingues TF, Farquhar GD. Photosynthetically relevant foliar traits correlating better on a mass vs an area basis: Of ecophysiological relevance or just a case of mathematical imperatives and statistical quicksand? New Phytol. 2013;199:311–321. doi: 10.1111/nph.12281. [DOI] [PubMed] [Google Scholar]
- 13.Poorter H, Lambers H, Evans JR. Trait correlation networks: A whole-plant perspective on the recently criticized leaf economic spectrum. New Phytol. 2014;201:378–382. doi: 10.1111/nph.12547. [DOI] [PubMed] [Google Scholar]
- 14.Westoby M, Reich PB, Wright IJ. Understanding ecological variation across species: Area-based vs mass-based expression of leaf traits. New Phytol. 2013;199:322–323. doi: 10.1111/nph.12345. [DOI] [PubMed] [Google Scholar]
- 15.Evans JR, Poorter H. Photosynthetic acclimation of plants to growth irradiance: The relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ. 2001;24:755–767. [Google Scholar]
- 16.Reich PB, Walters MB. Photosynthesis-nitrogen relations in Amazonian tree species. II. Variation in nitrogen vis-a-vis specific leaf area influences mass- and area-based expressions. Oecologia. 1994;97:73–81. doi: 10.1007/BF00317910. [DOI] [PubMed] [Google Scholar]
- 17.Niinemets Ü, Keenan TF, Hallik L. A worldwide analysis of within-canopy variations in leaf structural, chemical and physiological traits across plant functional types. New Phytol. 2015;205:973–993. doi: 10.1111/nph.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thornton PE, Zimmermann NE. An improved canopy integration scheme for a land surface model with prognostic canopy structure. J Clim. 2007;20:3902–3923. [Google Scholar]
- 19.Ellsworth DS, Reich PB. Canopy structure and vertical patterns of photosynthesis and related leaf traits in a deciduous forest. Oecologia. 1993;96:169–178. doi: 10.1007/BF00317729. [DOI] [PubMed] [Google Scholar]
- 20.Poorter H, Niinemets U, Poorter L, Wright IJ, Villar R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009;182:565–588. doi: 10.1111/j.1469-8137.2009.02830.x. [DOI] [PubMed] [Google Scholar]
- 21.Kitajima K, et al. How cellulose-based leaf toughness and lamina density contribute to long leaf lifespans of shade-tolerant species. New Phytol. 2012;195:640–652. doi: 10.1111/j.1469-8137.2012.04203.x. [DOI] [PubMed] [Google Scholar]
- 22.Kitajima K, Wright SJ, Westbrook JW. Leaf cellulose density as the key determinant of inter- and intra-specific variation in leaf fracture toughness in a species-rich tropical forest. Interface Focus. 2016;6:20150100. doi: 10.1098/rsfs.2015.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niinemets U, Sack L. Structural determinants of leaf light-harvesting capacity and photosynthetic potentials. Prog Bot. 2006;67:383–419. [Google Scholar]
- 24.Sack L, Melcher PJ, Liu WH, Middleton E, Pardee T. How strong is intracanopy leaf plasticity in temperate deciduous trees? Am J Bot. 2006;93:829–839. doi: 10.3732/ajb.93.6.829. [DOI] [PubMed] [Google Scholar]
- 25.Westoby M, Warton D, Reich PB. The time value of leaf area. Am Nat. 2000;155:649–656. doi: 10.1086/303346. [DOI] [PubMed] [Google Scholar]
- 26.Onoda Y, et al. Physiological and structural tradeoffs underlying the leaf economics spectrum. New Phytol. 2017;214:1447–1463. doi: 10.1111/nph.14496. [DOI] [PubMed] [Google Scholar]
- 27.Terashima I, Hanba YT, Tholen D, Niinemets Ü. Leaf functional anatomy in relation to photosynthesis. Plant Physiol. 2011;155:108–116. doi: 10.1104/pp.110.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evans JR, Kaldenhoff R, Genty B, Terashima I. Resistances along the CO2 diffusion pathway inside leaves. J Exp Bot. 2009;60:2235–2248. doi: 10.1093/jxb/erp117. [DOI] [PubMed] [Google Scholar]
- 29.Shipley B, Lechowicz MJ, Wright I, Reich PB. Fundamental trade-offs generating the worldwide leaf economics spectrum. Ecology. 2006;87:535–541. doi: 10.1890/05-1051. [DOI] [PubMed] [Google Scholar]
- 30.Reich PB, et al. Generality of leaf trait relationships: A test across six biomes. Ecology. 1999;80:1955–1969. [Google Scholar]
- 31.Onoda Y, Richards L, Westoby M. The importance of leaf cuticle for carbon economy and mechanical strength. New Phytol. 2012;196:441–447. doi: 10.1111/j.1469-8137.2012.04263.x. [DOI] [PubMed] [Google Scholar]
- 32.Villar R, Ruiz-Robleto J, Ubera JL, Poorter H. Exploring variation in leaf mass per area (LMA) from leaf to cell: An anatomical analysis of 26 woody species. Am J Bot. 2013;100:1969–1980. doi: 10.3732/ajb.1200562. [DOI] [PubMed] [Google Scholar]
- 33.Lusk CH, Reich PB, Montgomery RA, Ackerly DD, Cavender-Bares J. Why are evergreen leaves so contrary about shade? Trends Ecol Evol. 2008;23:299–303. doi: 10.1016/j.tree.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Russo SE, Kitajima K. The ecophysiology of leaf lifespan in tropical forests: Adaptive and plastic responses to environmental heterogeneity. In: Goldstein G, Santiago LS, editors. Tropical Tree Physiology. Springer; Berlin: 2016. pp. 357–383. [Google Scholar]
- 35.Kikuzawa K. A cost-benefit analysis of leaf habit and leaf longevity of trees and their geographical pattern. Am Nat. 1991;138:1250–1263. [Google Scholar]
- 36.Jackson LWR. Effect of shade on leaf structure of deciduous tree species. Ecology. 1967;48:498–499. [Google Scholar]
- 37.Terashima I, Miyazawa SI, Hanba YT. Why are sun leaves thicker than shade leaves? Consideration based on analyses of CO2 diffusion in the leaf. J Plant Res. 2001;114:93–105. [Google Scholar]
- 38.Koike T. Leaf structure and photosynthetic performance as related to the forest succession of deciduous broad‐leaved trees. Plant Species Biol. 1988;3:77–87. [Google Scholar]
- 39.Sack L, Scoffoni C. Leaf venation: Structure, function, development, evolution, ecology and applications in the past, present and future. New Phytol. 2013;198:983–1000. doi: 10.1111/nph.12253. [DOI] [PubMed] [Google Scholar]
- 40.Niinemets U, Portsmuth A, Tobias M. Leaf shape and venation pattern alter the support investments within leaf lamina in temperate species: A neglected source of leaf physiological differentiation? Funct Ecol. 2007;21:28–40. [Google Scholar]
- 41.John GP, et al. The anatomical and compositional basis of leaf mass per area. Ecol Lett. 2017;20:412–425. doi: 10.1111/ele.12739. [DOI] [PubMed] [Google Scholar]
- 42.Ackerly DD, Bazzaz FA. Leaf dynamics, self-shading and carbon gain in seedlings of a tropical pioneer tree. Oecologia. 1995;101:289–298. doi: 10.1007/BF00328814. [DOI] [PubMed] [Google Scholar]
- 43.Wright SJ, et al. Tropical canopy biology program, Republic of Panama. In: Basset Y, Horlyck V, Wright SJ, editors. Studying Forest Canopies from Above: The International Canopy Crane Network. Smithsonian Tropical Research Institute and the United Nations Environmental Programme; Panama City, Panama: 2003. pp. 137–155. [Google Scholar]
- 44.Kraft NJ, Valencia R, Ackerly DD. Functional traits and niche-based tree community assembly in an Amazonian forest. Science. 2008;322:580–582. doi: 10.1126/science.1160662. [DOI] [PubMed] [Google Scholar]
- 45.Kraft NJB, Ackerly DD. Functional trait and phylogenetic tests of community assembly across spatial scales in an Amazonian forest. Ecol Monogr. 2010;80:401–422. [Google Scholar]
- 46.Valencia R, et al. Yasuní forest dynamics plot, Ecuador. In: Losos EC, Leigh EG, editors. Tropical Forest Diversity and Dynamism: Findings from a Large-Scale Plot Network. Univ Chicago Press; Chicago: 2004. pp. 609–628. [Google Scholar]
- 47.Wright SJ, et al. Functional traits and the growth-mortality trade-off in tropical trees. Ecology. 2010;91:3664–3674. doi: 10.1890/09-2335.1. [DOI] [PubMed] [Google Scholar]
- 48.Westbrook JW, et al. What makes a leaf tough? Patterns of correlated evolution between leaf toughness traits and demographic rates among 197 shade-tolerant woody species in a neotropical forest. Am Nat. 2011;177:800–811. doi: 10.1086/659963. [DOI] [PubMed] [Google Scholar]
- 49.Kitajima K, Mulkey S, Wright S. Decline of photosynthetic capacity with leaf age in relation to leaf longevities for five tropical canopy tree species. Am J Bot. 1997;84:702–708. [PubMed] [Google Scholar]
- 50.Kraft NJB. 2008. Functional trait and phylogenetic-based tests of community assembly in a neotropical forest. PhD dissertation (University of California, Berkeley, CA)
- 51.Bolker BM. 2017. bbmle: Tools for General Maximum Likelihood Estimation, version 1.0.20.
- 52.R Core Team 2017. R: A language and environment for statistical computing (R Foundation for Statistical Computing, Vienna)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.