Abstract
Long-acting muscarinic antagonists (LAMAs), along with long-acting β2-agonists (LABAs), are the mainstay for treatment of patients with COPD. Glycopyrrolate, or glycopyrronium bromide, like other LAMAs, inhibits parasympathetic nerve impulses by selectively blocking the binding of acetylcholine to muscarinic receptors. Glycopyrrolate is unusual in that it preferentially binds to M3 over M2 muscarinic receptors, thereby specifically targeting the primary muscarinic receptor responsible for bronchoconstriction occurring in COPD. Inhaled glycopyrrolate is slowly absorbed from the lungs and rapidly eliminated from the bloodstream, most likely by renal excretion in its unmetabolized form, limiting the potential for systemic adverse events. Inhaled glycopyrrolate is a fast-acting, efficacious treatment option for patients with moderate–severe COPD. It improves lung function, reduces the risk of exacerbations, and alleviates the symptoms of breathlessness, which in turn may explain the improvement seen in patients’ quality of life. Inhaled formulations containing glycopyrrolate are well tolerated, and despite being an anticholinergic, few cardiovascular-related events have been reported. Inhaled glycopyrrolate is thus of value as both monotherapy and in combination with other classes of medication for maintenance treatment of COPD. This review covers the mechanism of action of inhaled glycopyrrolate, including its pharmacokinetic, pharmacodynamic, and safety profiles, and effects on mucus secretion. It also discusses the use of inhaled glycopyrrolate in the treatment of COPD, as monotherapy and in fixed-dose combinations with LABAs and inhaled corticosteroid–LABAs, including a triple therapy recently approved in Europe.
Keywords: glycopyrronium bromide, long-acting muscarinicantagonist, anticholinergic, bronchodilator
Plain language summary
Patients with COPD have narrowed airways and cannot fully empty their lungs, which together can make breathing uncomfortable. Doctors often prescribe an inhaler containing drugs that widen the airways or reduce inflammation in the lungs, making it easier for patients with COPD to breathe. Patients who do not show enough benefit from treatment with one drug alone may be given two or more drugs, which can be combined into one inhaler. One drug used to widen the airways in patients with COPD is glycopyrrolate (also referred to as glycopyrronium bromide). Glycopyrrolate can be used to treat COPD on its own, as well as in combination with other drugs. In this article, we discuss the evidence for how glycopyrrolate works in the body, how glycopyrrolate enters and leaves the body, and the effectiveness and side effects of glycopyrrolate when used to treat patients with COPD in clinical trials alone and combined in one inhaler with another airway-widening drug with or without a drug used to reduce inflammation in the lungs.
Introduction
Long-acting muscarinic antagonists (LAMAs) or long-acting β2-agonists (LABAs), alone or in combination, are the mainstay for the maintenance treatment of patients with COPD.1,2 In the 1980s, inhaled glycopyrrolate, also known as glycopyrronium bromide, was found to be a long-acting bronchodilator3,4 and improved pulmonary function after exercise in patients with asthma,5 although inhaled glycopyrrolate is not currently licensed for use in asthma.6 Inhaled glycopyrrolate, a rapid-onset LAMA, is now US Food and Drug Administration (FDA)- and European Medicines Agency (EMA)-approved for maintenance treatment of patients with COPD.1,6,7 This review covers the mechanism of action of inhaled glycopyrrolate, its pharmacokinetic (PK) and pharmacodynamic (PD) profiles, safety profile, effects on mucus secretion, and use in the treatment of COPD as monotherapy and in fixed-dose combinations (FDCs) with LABAs and inhaled corticosteroid (ICS)–LABAs.
Pharmacokinetics and pharmacodynamics of inhaled glycopyrrolate
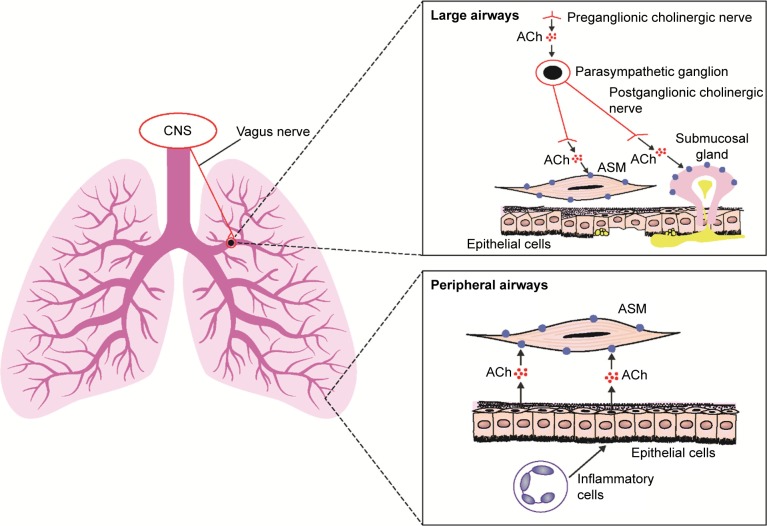
Preganglionic parasympathetic nerves innervate the airways via the vagus nerve (Figure 1).8 At parasympathetic ganglia, preganglionic nerves synapse with postganglionic nerves.8 Acetylcholine (ACh) is a neurotransmitter that is released during parasympathetic nerve impulses and acts by binding to and activating muscarinic receptors and nicotinic receptors.8,9 There are five muscarinic receptor subtypes, referred to as M1–M5. 9
Figure 1.
Role of ACh and muscarinic receptors in the lung.
Notes: Data from these studies.11,17 In large airways, ACh released from cholinergic nerves activates M3 receptors on ASM, causing bronchoconstriction. In peripheral airways, where cholinergic nerves are absent, inflammatory cells stimulate epithelial cells to release ACh, activating M3 receptors on ASM and causing bronchoconstriction. M3 muscarinic receptors are shown as solid blue circles. For simplicity, other types of muscarinic receptor are not shown.
Abbreviations: ACh, acetylcholine; ASM, airway smooth muscle; CNS, central nervous system.
M1 receptors are highly expressed in the peripheral airways, whereas M2 and M3 receptors predominate in the larger airways.8 M3 receptors are the muscarinic receptors primarily responsible for ACh-induced bronchoconstriction,8 as they activate phospholipase C, which produces inositol 1,4,5-triphosphate and diacylglycerol, leading to intracellular calcium release.9 Anticholinergics block parasympathetic nerve impulses by selectively preventing ACh from binding to muscarinic receptors.8,10 These drugs inhibit bronchoconstriction in peripheral airways by antagonizing the effects on airway smooth muscle cells of ACh released by epithelial cells; this release is stimulated by inflammatory cells.11
The anticholinergic effects of inhaled glycopyrrolate are primarily limited to the airways, thereby reducing the likelihood of systemic adverse events (AEs).12 Inhaled glycopyrrolate has a bioavailability of 57%, with 53% absorbed via the lungs.12 Based on its ability to inhibit methacholine-induced calcium release (half-life [t½] 6.1±2.1 minutes), inhaled glycopyrrolate has a rapid onset of action.13 Inhaled glycopyrrolate is long lasting in the body, with a terminal elimination-phase t½ of 52.5 hours following inhalation (vs t½ of 6.2 hours following intravenous administration).12 Population PK modeling has shown that inhaled glycopyrrolate is absorbed slowly, predominantly unchanged, from the lungs.12 It has a slow-phase absorption t½ of 3.5 days, accounting for 79% of drug absorption.12 Furthermore, inhaled glycopyrrolate is eliminated rapidly from the bloodstream.12 Intravenous glycopyrrolate is excreted, mainly in its unmetabolized form, by the kidney.14,15 Metabolism is less important for the elimination of this drug from the body.14,15 Renal excretion is also likely to be important for the elimination of glycopyrrolate systematically absorbed from the lung.15 However, no inhaled glycopyrrolate dose adjustments are required for patients with mild–moderate renal impairment, and those with severe renal impairment may be given inhaled glycopyrrolate if the benefits are judged to outweigh the risks.6
Pharmacokinetic and pharmacodynamic profiles of inhaled glycopyrrolate vs other LAMAs
Available anticholinergic drugs bind to all muscarinic ACh receptors present in the airways (ie, M1 – M3). 8,16 Importantly, M3 receptors are the primary therapeutic target for bronchodilation, with antagonism at M2 autoreceptors tending to attenuate bronchodilator effects.17 The PK and PD profiles of glycopyrrolate have been compared with other LAMAs in various preclinical studies, although not all of these studies tested the inhaled delivery of glycopyrrolate (Table 1).13,16,18,19 Glycopyrrolate is the only anticholinergic to date to show higher relative affinity for M3 than M2 receptors, although its absolute binding affinity for these receptors is lower than that of aclidinium and tiotropium.13,16 Glycopyrrolate, aclidinium, tiotropium, and ipratropium (a short-acting muscarinic antagonist) all dissociate more rapidly from M2 than M3 receptors, with all four drugs showing similar ratios of dissociation t½.13,16 In addition, glycopyrrolate has a shorter absolute dissociation time at both M2 and M3 receptors than at aclidinium and tiotropium.16 An ex vivo study examining muscarinic receptor binding in the rat lung showed receptor binding lasted 24 hours for glycopyrrolate and tiotropium, whereas ipratropium binding was observed at 2 hours, but not at 12 hours.18
Table 1.
Preclinical studies comparing the pharmacological profile of glycopyrrolate with other LAMAs and ipratropium
| Study | Characteristic | Glycopyrrolate | Aclidinium | Tiotropium | Ipratropium |
|---|---|---|---|---|---|
| In vitro calcium assay13 | Equilibrium binding constant, mean ± SE: | – | – | ||
| M2 | 8.70±0.04 | 10.05±0.03 | |||
| M3 | 9.59±0.05 | 10.37±0.04 | |||
| Drug binding t½ (minutes) at M3 vs M2 receptors (kinetic selectivity ratio) | 11.4 vs 1.07 (10.7) | – | 46.2 vs 10.8 (4.3) | – | |
|
| |||||
| In vitro study of recombinant human receptors16 | Muscarinic receptor-binding affinity (Ki, nM), mean ± SE: | ||||
| M2 | 1.77±0.06 | 0.14±0.04 | 0.13±0.04 | 1.12±0.13 | |
| M3 | 0.52±0.04 | 0.14±0.02 | 0.19±0.04 | 1.24±0.08 | |
|
| |||||
| Ex vivo binding in rat lungs18 | Binding to muscarinic receptors in lung | Binding lasted 24 hours | – | Binding lasted 24 hours | Binding observed at 2 hours, but not at 12 hours |
|
| |||||
| In vitro study of recombinant human receptors16 | M3 vs M2 receptors, dissociation t½ in hours (kinetic selectivity ratio) | 8.1 vs 1.1 (7.3) | 29.2 vs 4.7 (6.2) | 62.2 vs 15.1 (4.1) | 0.5 vs 0.1 (5.9) |
|
| |||||
| In vivo study in guinea pigs16 | Onset of action (hours) postadministration | 2 | 2 | 4 | 2 |
|
| |||||
| Ex vivo study in human airways19 | Onset of action of 1 µM dose, minutes ± SEM | 3.4a±0.4 | 6.4±0.5 | 8.4±1.1 | – |
|
| |||||
| In vitro study in guinea pig trachea16 | Duration of action (t½ offset of electrically stimulated contractions) at M3 receptors | >8 hours | >8 hours | >8 hours | 42 minutes |
|
| |||||
| In vivo study in guinea pigs16 | Duration of bronchodilator action, in hours (t½ offset of maximal inhibitory effect) | 13 | 29 | 64 | 8 |
|
| |||||
| In vivo rat salivation study16 | ED50 (µg/kg) for inhibition of salivation | 0.74 | 38 | 0.88 | – |
|
| |||||
| In vitro human plasma study16 | Hydrolysis t½ in plasma (hours) | 6 | 0.04 | 1.6 | 33 |
Notes:
P<0.05 vs both aclidinium and tiotropium at equal concentrations. “–” indicates data not available.
Abbreviations: ED50, dose required to inhibit salivation in 50% of rats; Ki, antagonist dissociation constant; LAMAs, long-acting muscarinic antagonists; SE, standard error; SEM, standard error of the mean; t½, half-life.
In an in vivo study on guinea pigs, glycopyrrolate, ipratropium, and aclidinium had a similar onset of action, which was more rapid than tiotropium.16 However, in isolated human airways, glycopyrrolate had significantly more rapid onset of action than both aclidinium and tiotropium.19 Glycopyrrolate had a similar duration of action at M3 receptors to that of aclidinium and tiotropium in vitro, but had a shorter duration of action than tiotropium and aclidinium in vivo in guinea pig studies.16 Sykes et al proposed that drug molecules, once dissociated from their target receptors, may not be able to diffuse away from the receptor environment and thus are likely to rebind to localized receptors.13 The authors suggested that this may explain why some LAMAs have a long duration of action, despite rapid dissociation rates.13 In an in vitro study, aclidinium was shown to have lower stability than tiotropium, which in turn had lower stability than glycopyrrolate in rat, guinea pig, and human plasma.16 This indicates that glycopyrrolate undergoes a slower rate of hydrolysis, which may result in a propensity to cause anticholinergic AEs in patients with COPD.16 These findings are in agreement with a previous study that compared the plasma stability of aclidinium with tiotropium and ipratropium, with aclidinium found to be the least stable of the three bronchodilators evaluated.20 Furthermore, a lower dose of glycopyrrolate and tiotropium than aclidinium is required to inhibit salivation effectively in rats, possibly because of the slower rate of hydrolysis in the blood.16
Inhaled glycopyrrolate: effects on mucus secretion and mucociliary clearance in COPD
Mucus is secreted by submucosal mucus glands and goblet cells in the bronchi, and excessive mucus secretion is a feature of chronic bronchitis and COPD.21 M1 and M3 receptors are expressed at a 1:2 ratio in submucosal glands.22 The M3 receptor is involved primarily in mediating mucus secretion, whereas water and electrolyte secretion are likely to be regulated by M3 and M1 receptors in combination.22 LABAs are known to enhance mucociliary clearance in patients with COPD,23 probably via an effect on ciliary beat frequency,24 and there are numerous theories regarding the alteration of mucus production by LAMAs.25 However, the results from clinical trials testing these theories are conflicting.25 In an open-label, non-placebo-controlled trial in 22 patients with COPD, tiotropium reduced cough symptoms and nasal clearance times.26 The authors concluded that this effect may result from inhibition of mucus hypersecretion and an increase in mucociliary clearance in the airways.26 However, this supposition is not supported by other studies: in a randomized, double-blind trial, Hasani et al failed to find an effect of tiotropium on mucociliary clearance in 34 patients with COPD,27 whereas Meyer et al reported that tiotropium treatment slowed mucociliary clearance in their randomized, open-label, crossover study in 24 patients.28 Furthermore, in a double-blind, crossover study, ipratropium was actually found to decrease cough clearance of secretions in patients with COPD.29
Oral glycopyrrolate is known to reduce drooling in children, and glycopyrrolate injections can reduce preoperative salivation, as well as respiratory secretions during end-of-life care.30,31 However, on review of published literature cited in PubMed, no studies could be found that examined the role of inhaled glycopyrrolate in mucus secretion. There also appear to be limited published data examining the effect of glycopyrrolate on mucociliary clearance,25 and hence, there is a need for further study in this area.
The effect of inhaled glycopyrrolate on cognition
Glycopyrrolate is a water-soluble, highly polar quaternary ammonium compound, which limits its passage across lipid membranes such as the blood–brain barrier; therefore, glycopyrrolate does not exhibit any central nervous system activity.32 Inhaled glycopyrrolate is thus unlikely to have a significant effect on cognition in patients with COPD, although there is a lack of evidence to support this.
Clinical evidence for the use of inhaled glycopyrrolate in patients with COPD
In its inhaled form, glycopyrrolate and other approved LAMAs are used for the management of COPD.1,6,33–37 Glycopyrrolate is available as a monotherapy via a dry-powder inhaler (DPI)6 and was approved by the FDA in 2017 as a nebulized monotherapy (Table 2).38 Inhaled glycopyrrolate is also available in an FDC with formoterol, delivered using co-suspension delivery technology in a pressurized metered dose inhaler (pMDI),34 and in a DPI FDC with indacaterol.35,36 Inhaled glycopyrrolate has been approved in Europe as a triple FDC with formoterol and beclomethasone, delivered via a pMDI.39
Table 2.
Available glycopyrrolate and other LAMA formulations
| Formulation | Brand name | Delivery method | Regions approved | Approved dosage |
|---|---|---|---|---|
|
Glycopyrrolate formulations
| ||||
| Monotherapy6,33 | Seebri Neohaler | DPI | US | 15.6 µg BID |
| Seebri Breezhaler | DPI | Europe | 50 µg QD (glycopyrronium moiety in capsule) | |
|
| ||||
| Monotherapy78 | Lonhala Magnair | Nebulized | US | 25 µg BID |
|
| ||||
| Glycopyrrolate–formoterol34 | Bevespi Aerosphere | pMDI | US | 18/9.6 µg BID (as glycopyrrolate–formoterol fumarate) |
|
| ||||
| Glycopyrrolate–indacaterol35,36 | Utibron Neohaler | DPI | US | 15.6/27.5 µg BID |
| Ultibro Breezhaler | DPI | Europe | 50/110 µg QD (in capsule, as glycopyrronium/indacaterol) | |
|
| ||||
| Glycopyrrolate–beclomethasone–formoterol37 | Trimbow | pMDI | Europe | 12.5/100/6 µg BID (metered dose, as glycopyrronium bromide–beclomethasone dipropionate–formoterol fumarate dihydrate) |
|
| ||||
|
Tiotropium formulations
| ||||
| Monotherapy79,80 | Spiriva HandiHaler | DPI | US | 18 µg QD (as tiotropium bromide) |
| Spiriva Respimat | SMI | US | 5 µg QD (as tiotropium bromide) | |
|
| ||||
| Tiotropium–olodaterol81,82 | Stiolto Respimat | SMI | US | 5/5 µg QD |
| Spiolto Respimat | SMI | Europe | 5/5 µg QD (delivered dose, as tiotropium bromide monohydrate–olodaterol hydrochloride) | |
|
| ||||
|
Umeclidinium formulations
| ||||
| Monotherapy83,84 | Incruse Ellipta | DPI | US | 62.5 µg QD |
| Incruse Ellipta | DPI | Europe | 62.5 µg QD (predispensed dose, as umeclidinium) | |
|
| ||||
| Umeclidinium–vilanterol85,86 | Anoro Ellipta | DPI | US | 62.5/25 µg QD |
| Anoro Ellipta | DPI | Europe | 62.5/25 µg QD (predispensed dose, as umeclidinium–vilanterol trifenatate) | |
|
| ||||
| Umeclidinium–fluticasone furoate–vilanterol87 | Trelegy Ellipta | DPI | US | 62.5/100/25 µg QD (as umeclidinium–fluticasone furoate–vilanterol) |
|
| ||||
|
Aclidinium formulations
| ||||
| Monotherapy88–90 | Eklira Genuair | DPI | Europe | 400 µg BID (as aclidinium bromide) |
| Bretaris Genuair | DPI | Europe | 400 µg BID (as aclidinium bromide) | |
| Tudorza Pressair | DPI | US | 400 µg BID (as aclidinium bromide) | |
|
| ||||
| Aclidinium–formoterol91 | Duaklir Genuair | DPI | Europe | 400/12 µg BID (as aclidinium bromide–formoterol fumarate dihydrate) |
|
| ||||
|
Revefenacin formulation
| ||||
| Monotherapy92 | TBCa | Nebulized | Completed Phase III trials | NA |
Note:
Theravance Biopharma and Mylan submitted a new drug application for revefenacin use in patients with COPD in November 2017 and were waiting for a response at the time of submission of this review article.93
Abbreviations: BID, bis in die (twice daily); DPI, dry-powder inhaler; LAMA, long-acting muscarinic antagonist; NA, not applicable; pMDI, pressurized metered dose inhaler; QD, quaque die (once daily); SMI, soft-mist inhaler; TBC, to be confirmed.
Efficacy of inhaled glycopyrrolate monotherapy
Onset of action
In a randomized, double-blind study to determine the most appropriate dose of inhaled glycopyrrolate monotherapy (delivered via a DPI) for patients with moderate–severe COPD, glycopyrrolate 50 µg once daily (QD) provided significant bronchodilation over 24 hours.40 However, the efficacy of glycopyrrolate 50 µg QD was not significantly different from the same total daily dose administered twice daily (BID).40 The randomized GLOW trials were conducted to evaluate the use of 50 µg QD of inhaled glycopyrrolate for treating patients with moderate–severe COPD.41–46 The GLOW1 study showed that glycopyrrolate 50 µg QD had a rapid onset of bronchodilation in patients with COPD, with a significant improvement from baseline in lung function vs placebo as early as 5 minutes after treatment (P<0.001).41 Supporting the preclinical data, inhaled glycopyrrolate has been shown to have faster onset of action than tiotropium in several clinical trials. In the randomized GLOW2 study, glycopyrrolate 50 µg QD resulted in significantly more rapid bronchodilation than tiotropium 18 µg QD treatment at all time points from 5 minutes to 4 hours after the first dose on day 1 (P<0.01).42 Similarly, in the randomized GLOW5 study, glycopyrrolate 50 µg QD resulted in significantly more rapid improvement in lung function than tiotropium 18 µg QD treatment at 5 and 15 minutes after first dose.45 Additionally, in a post hoc analysis of the randomized SPRING study in patients with moderate–severe COPD, glycopyrrolate 50 µg QD resulted in a significantly greater improvement in lung function than tiotropium 18 µg QD at 5 minutes, 15 minutes, and 1 hour after the first dose on day 1 (P=0.015, P=0.026, and P=0.014, respectively).47 In a further study in patients with moderate–severe COPD, single doses of both glycopyrrolate (50 µg) and aclidinium (400 µg) had more rapid onset of action than tiotropium (18 µg), resulting in greater levels of bronchodilation 90 minutes after treatment, although the authors concluded that faster onset of action seen in the clinic may not be relevant for patients who are undergoing long-term treatment for chronic disease.19
Lung function and other efficacy end points
The efficacy of inhaled glycopyrrolate monotherapy (via a DPI) has been investigated in patients with COPD in numerous clinical studies, including the GLOW study series and the GEM studies (Table 3).38,41–52 Two network meta-analyses have also been conducted to compare the efficacy of glycopyrrolate with other LAMAs.53,54 The first (including 21 trials) compared glycopyrrolate 50 µg QD with aclidinium 400 µg BID, tiotropium 18 µg QD (HandiHaler), and tiotropium 5 µg QD (Respimat) in patients with moderate–severe COPD.53 After 24 weeks, glycopyrrolate 50 µg treatment resulted in a similar improvement in lung function compared with aclidinium 400 µg, tiotropium 18 µg, and tiotropium 5 µg.53 At the same time point, glycopyrrolate 50 µg treatment resulted in similar improvements in St George’s Respiratory Questionnaire (SGRQ) scores from baseline compared with aclidinium 400 µg and tiotropium 18 µg, and a greater improvement compared with tiotropium 5 µg.53 Improvements in breathlessness symptoms were similar for all treatments.53
Table 3.
Clinical evidence of the efficacy of glycopyrrolate monotherapy in patients with COPD
| Clinical trial | Treatment duration | Treatments | Key findings | ||||
|---|---|---|---|---|---|---|---|
| GLOW trials: double-blind, randomized, placebo-controlled studies in patients with moderate–severe COPD | |||||||
| Phase III GLOW141 | 26 weeks | Glycopyrrolate 50 µg QD (n=552), placebo (n=270) | Glycopyrrolate significantly increased trough FEV1 vs placebo at week 12 (LSM treatment difference 0.108 L, P<0.001), increase maintained at week 26 (LSM treatment difference 0.113 L, P<0.001) Significant improvements in dyspnea (TDI focal score 1.84 vs 0.80 points, P<0.001) and time to first moderate/severe exacerbation (P=0.023) vs placebo at week 26 |
||||
| Phase III GLOW242 | 52 weeks | Glycopyrrolate 50 µg QD (n=529), placebo (n=269), open-label tiotropium 18 µg QD (n=268) | Glycopyrrolate significantly increased trough FEV1 vs placebo at week 12 (LSM treatment difference 97 mL, P<0.001) Glycopyrrolate significantly increased trough FEV1 vs tiotropium at week 26 (LSM treatment difference 50 mL, P<0.01), while trough FEV1 was similar at weeks 12 and 52 (LSM treatment difference 14 and 19 mL, respectively) Significant improvements in dyspnea at week 26 (TDI focal score 2.13 vs 1.32, P=0.002) and risk of exacerbations at week 52 (P=0.001) vs placebo |
||||
| Phase III GLOW3 crossover43 | 3 weeks | Glycopyrrolate 50 µg QD, then placebo (n=55); placebo, then glycopyrrolate 50 µg QD (n=53) | Glycopyrrolate significantly improved submaximal exercise endurance time vs placebo on day 1 (P<0.001), and this improvement increased at day 21 (P<0.001) | ||||
| Phase III GLOW545 | 12 weeks | Glycopyrrolate 50 µg QD (n=327), tiotropium 18 µg QD (n=330) | Glycopyrrolate was noninferior to tiotropium for trough FEV1 at week 12 (LSM treatment difference 0 L, P<0.001) Glycopyrrolate had a significantly faster onset of action on day 1 than tiotropium (postdose FEV1 at 0- to 4-hour time points, all P<0.001) |
||||
| GLOW746 | 26 weeks | Glycopyrrolate 50 µg QD (n=306), placebo (n=154) | Glycopyrrolate significantly improved trough FEV1 vs placebo at week 12 (LSM treatment difference 141 mL, P<0.001) Glycopyrrolate significantly improved SGRQ scores (LSM treatment difference −4.92 points, P<0.001) and dyspnea severity (LSM treatment difference 1 point, P<0.001) at week 26 vs placebo |
||||
| SPRING trial: randomized, blinded study in patients with moderate–severe COPD | |||||||
| SPRING crossover47 | 4 weeks | Glycopyrrolate 50 µg QD, then tiotropium 18 µg QD; tiotropium 18 µg QD, then glycopyrrolate 50 µg QD (n=126) | On day 1, glycopyrrolate 50 µg treatment significantly improved FEV1 AUC0–4 after the first dose compared with tiotropium 18 µg (LSM treatment difference 0.030 L, P=0.025) Improvements in morning symptoms of COPD were similar between treatments |
||||
| GEM trials: Phase III, double-blind, randomized, placebo-controlled studies in patients with COPD and moderate–severe airflow limitation | |||||||
| GEM148 | 12 weeks | Glycopyrrolate 15.6 µg BID (n=222), placebo (n=219) | Glycopyrrolate significantly improved FEV1 AUC0–12 vs placebo at week 12 (LSM treatment difference 0.139 L, P<0.001) Glycopyrrolate significantly improved TDI (LSM treatment difference 0.92 points, P=0.003) and SGRQ scores (LSM treatment difference −2.8 points, P=0.016) vs placebo at week 12 |
||||
| GEM249 | 12 weeks | Glycopyrrolate 15.6 µg BID (n=216), placebo (n=216) | Glycopyrrolate significantly improved FEV1 AUC0–12 vs placebo at day 1 (LSM treatment difference 0.119 L, P<0.001), improvement maintained at week 12 (LSM treatment difference 0.123 L, P<0.001) Glycopyrrolate significantly improved SGRQ scores (LSM treatment difference −5.2 points, P<0.001) vs placebo at week 12 |
||||
| GOLDEN trials: Phase III, randomized studies of nebulized glycopyrrolate in patients with moderate–very severe COPD | |||||||
| Double-blind, placebo-controlled GOLDEN 3 and GOLDEN 438 | 12 weeks | Glycopyrrolate 25 µg BID (n=431), glycopyrrolate 50 µg BID (n=432), placebo (n=430) | At week 12, treatment with glycopyrrolate 25 and 50 µg BID resulted in significant and clinically important improvements in trough FEV1 vs placebo (GOLDEN 3 [LSM difference 0.105 and 0.126 L, respectively] and GOLDEN 4 [LSM difference 0.084 and 0.082 L, respectively], all P≤0.0001) At week 12, glycopyrrolate 25 and 50 µg BID significantly improved trough FVC vs placebo (GOLDEN 3 [LSM difference 0.149 and 0.167 L, respectively; both P<0.001], GOLDEN 4 [LSM difference 0.130 and 0.113 L, respectively; P<0.01]) At week 12/end of study, glycopyrrolate 25 and 50 µg BID significantly increased SGRQ scores vs placebo (GOLDEN 3 [LSM difference −3.072, P<0.05 and −1.848 points, P=NS, respectively]; GOLDEN 4 [LSM difference −3.585 and −3.557 points, respectively; P<0.01]) |
||||
| Open-label, active-controlled GOLDEN 551 | 48 weeks | Glycopyrrolate 50 µg BID (n=621), tiotropium 18 µg QD (n=466) | Glycopyrrolate treatment improved trough FEV1 from baseline, which was maintained until week 48 (LSM change from baseline at week 48 0.069 L) Change from baseline in FEV1 at 48 weeks did not differ significantly between glycopyrrolate and tiotropium |
||||
| Randomized, double-blind studies of glycopyrrolate delivered using co-suspension delivery technology in patients with moderate–severe COPDa | |||||||
| Chronic-dosing, Phase II, crossover trial52 | 2 weeks | Glycopyrrolate 18, 9, 4.6, 2.4, 1.2, 0.6 µg BID (n=64, 64, 62, 64, 57, 59, respectively), open-label tiotropium 18 µg QD (n=62), placebo BID (n=62) | After 14 days’ treatment, glycopyrrolate at doses 18, 9, 4.6, and 2.4 µg BID resulted in significant and clinically relevant improvements in FEV1 AUC0–12 compared with placebo (LSM difference from baseline 126–158 mL, P<0.0001) Glycopyrrolate 18 µg BID was noninferior to open-label tiotropium 18 µg QD for peak change in FEV1 on day 1 (LSM change from baseline 0.231 vs 0.270 L) and morning predose trough FEV1 on day 14 (LSM change from baseline 0.089 vs 0.126 L) and was the most appropriate dose for further evaluation |
||||
| Chronic-dosing, Phase IIB, incomplete block, crossover trial57 | 7 days | Glycopyrrolate (as glycopyrronium) 28.8, 14.4, 7.2, 3.6 µg BID (n=192), placebo BID (n=48), open-label ipratropium 34 µg QID (n=48)b | At day 7, all glycopyrrolate doses were superior to placebo for improvement from baseline in FEV1 AUC0–12 (LSM treatment differences vs placebo 0.121–0.191 L, all P<0.0001) All glycopyrrolate doses were noninferior to ipratropium (data not shown) Glycopyrrolate 28.8 and 14.4 µg were the most effective doses for improving all secondary efficacy end points |
||||
Notes:
Phase II studies included, as Phase III trial data have not yet been published for this product;
each patient received three treatments of two doses of glycopyrrolate and either placebo or ipratropium.
Abbreviations: AUC, area under curve; BID, bis in die (twice daily); FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; LSM, least squares mean; NS, not significant; QD, quaque die (once daily); QID, quater in die (four times daily); SGRQ, St George’s Respiratory Questionnaire; TDI, Transition Dyspnea Index.
The second network meta-analysis (including 24 trials) compared the efficacy of glycopyrrolate 50 µg QD, tiotropium 18 µg QD, aclidinium 400 µg BID, and umeclidinium 62.5 µg QD in patients with COPD.54 At weeks 12 and 24, all LAMA treatments evaluated resulted in clinically relevant (>100 mL) improvements in trough forced expiratory volume in 1 second (FEV1) compared with placebo.54 Glycopyrrolate 50 µg treatment resulted in the greatest improvement over placebo in lung function at week 24 (FEV1 difference 135.8 mL).54 For all LAMAs vs placebo, improvements were seen from baseline in 24-week SGRQ and Transition Dyspnea Index (TDI) scores.54 Glycopyrrolate 50 µg QD treatment did not reach the minimal clinically important difference (MCID) in SGRQ score of 4 units compared with placebo, but did reach the MCID of ≥1 for TDI focal score.54 However, aclidinium 400 µg and umeclidinium 62.5 µg treatments reached MCID for both SGRQ and TDI focal scores compared with placebo, whereas tiotropium did not reach MCID for either of the two measures.54
For patients with moderate–severe COPD, clinical trial data for treatment with inhaled glycopyrrolate as a monotherapy indicate that glycopyrrolate 50 µg QD (the EMA-approved dose) improves lung function and health-related quality of life (HRQoL), decreases the severity of breathlessness and the risk of exacerbations, and improves morning symptoms.41,42,45,47,51 Furthermore, glycopyrrolate 50 µg QD was found to be noninferior to tiotropium in its ability to increase airflow to the lungs.45 In a short-term, crossover trial (3-week treatment periods), glycopyrrolate also significantly improved patients’ abilities to exercise vs placebo.43 In Phase III trials of glycopyrrolate 15.6 µg BID (the FDA-approved dose) in patients with moderate–severe COPD, there were also significant improvements in FEV1 scores from baseline, TDI focal scores, and SGRQ scores vs placebo.48,49 In two Phase III trials of the nebulized form of glycopyrrolate, 50 µg BID treatment resulted in significant and clinically important increases from baseline in lung function and SGRQ scores vs placebo.38 Finally, in two studies in patients with moderate–severe COPD, glycopyrrolate was delivered by pMDI using innovative co-suspension delivery technology, which allows aerosol delivery of micronized drug suspended with microsized, phospholipid-based porous particles.52,55–57 In these studies, patients treated with glycopyrrolate (doses ≥2.4 µg BID) showed clinically relevant, significant improvements in lung function from baseline vs placebo.52,57 Furthermore, the highest glycopyrrolate dose tested in the first study (18 µg BID) was found to be noninferior to tiotropium 18 µg QD for improving lung function.52 In the second study, all glycopyrrolate doses (delivered as glycopyrronium 28.8, 14.4, 7.2, and 3.6 µg BID) were found to be noninferior to ipratropium 34 µg 4 times daily.57 Based on these studies, 18 µg (equivalent to 14.4 µg glycopyrronium) was selected as the optimal glycopyrrolate dose for the glycopyrrolate–formoterol combination studies.52,57
Efficacy of inhaled glycopyrrolate combinations
Combining a LAMA with a LABA can increase efficacy, as they have distinctly different mechanisms of action (ie, target different receptors). In addition, LABAs modify the release of ACh, leading to amplification of bronchial smooth muscle relaxation induced by the LAMA.58 Moreover, LABAs act on presynaptic β2-receptors in the efferent cholinergic pathway, resulting in the inhibition of cholinergic transmission.59
In 2016, glycopyrrolate–formoterol pMDI 18/9.6 µg BID was licensed by the FDA “for the long-term maintenance treatment of airflow obstruction in patients with COPD”.34 Several randomized controlled trials have examined the efficacy of glycopyrrolate–formoterol 18/9.6 µg BID (Table 4).60,61 Five studies (PINNACLE-1, PINNACLE-2, PT003011, PT003012, and PINNACLE-3) showed that (as expected) glycopyrrolate–formoterol 18/9.6 µg BID significantly improved lung function compared with individual components and placebo.60–62 In addition, glycopyrrolate–formoterol 18/9.6 µg BID was at least as efficacious as open-label tiotropium 18 µg QD.61,62 Furthermore, the efficacy of glycopyrrolate–formoterol 18/9.6 µg BID was maintained over the 1-year treatment period.60,61 A post hoc analysis of PINNACLE-1 and -2 indicated that glycopyrrolate–formoterol improved FEV1 independently of baseline symptom severity, although high baseline symptom severity was significantly correlated with greater improvement in health outcomes after treatment.63
Table 4.
Recent clinical evidence (2015 to present) of efficacy of glycopyrrolate combinations in patients with COPD
| Clinical trial | Treatment duration | Treatments | Key findings | |
|---|---|---|---|---|
| Glycopyrrolate–formoterol FDC delivered using co-suspension delivery technology via a pMDI | ||||
| Phase III, randomized, double-blind, PINNACLE-1 and -2 studies in moderate–very severe COPD60 | 24 weeks | Glycopyrrolate–formoterol 18/9.6 µg BID (n=1,039), glycopyrrolate 18 µg BID (n=891), formoterol 9.6 µg BID (n=891), placebo BID (n=444), open-label tiotropium 18 µg (PINNACLE-1 only, QD; n=453) | At week 24, the change in predose trough FEV1 for glycopyrrolate–formoterol was significantly greater than for glycopyrrolate, formoterol, and placebo (LSM difference in change from baseline vs comparators, PINNACLE-1, 0.059–0.150 L, P<0.0001; PINNACLE-2, 0.054–0.103 L, P<0.001) Change in predose trough FEV1 for glycopyrrolate–formoterol did not differ significantly from tiotropium in PINNACLE-1, but did in PINNACLE-2 (LSM difference in change from baseline 0.021, P=NS and 0.103, P<0.001, respectively) | |
| Phase III, randomized, double-blind trial (PINNACLE-3 [a PINNACLE-1 and -2 extension study])61 | 28 weeks | Glycopyrrolate–formoterol 18/9.6 µg BID (n=290), glycopyrrolate 18 µg BID (n=218), formoterol 9.6 µg BID (n=213), open-label tiotropium 18 µg QD (n=171) | At week 52, the change in predose trough FEV1 for glycopyrrolate–formoterol was significantly greater than that for all other treatments (LSM difference in change from baseline 0.025–0.065 L, P≤0.0117) | |
| Two Phase IIIB, double-blind, crossover studies (PT003011 and PT003012) in moderate–very severe COPD62 | 4 weeks | Glycopyrrolate–formoterol 18/9.6 µg BID (n=115), placebo (n=112), open-label tiotropium 5 µg QD (PT003011 only, n=73) | By day 29, glycopyrrolate–formoterol had significantly improved FEV1 AUC0–24 vs placebo (LSM treatment difference, PT003011, 0.265 L; PT003012, 0.249 L; both P<0.0001) In PT003011, glycopyrrolate–formoterol treatment resulted in significantly improved FEV1 AUC0–12 vs tiotropium (LSM treatment difference 0.080 L, P=0.0001) FEV1 AUC12–14 improvements with glycopyrrolate–formoterol were also greater than for placebo (LSM treatment difference for FEV1 AUC0–12, PT003011 0.251 and PT003012 0.255; for AUC0–24, PT003011 0.277 and PT003012 0.242) |
|
| Glycopyrrolate–indacaterol FDC via a DPI | ||||
| Randomized, double-blind, noninferiority FLAME trial in patients with high exacerbation risk67 | 52 weeks | Glycopyrrolate–indacaterol 50/110 µg QD (n=1,680), fluticasone propionate–salmeterol 500/50 µg BID (n=1,682) | The glycopyrrolate–indacaterol combination was found to be superior to fluticasone propionate–salmeterol in reducing annual COPD exacerbations (P=0.003) and increasing time to first exacerbation (P<0.001) | |
| Randomized, double-blind, LANTERN study in moderate–severe COPD with a history of exacerbations68 | 26 weeks | Glycopyrrolate–indacaterol 50/110 µg QD (n=372), fluticasone propionate–salmeterol 500/50 µg BID (n=372) | At week 26, glycopyrrolate–indacaterol was significantly superior at improving trough FEV1 to fluticasone propionate–salmeterol (LSM treatment difference 0.075, P<0.001) At week 26, the glycopyrrolate–indacaterol group showed a significantly greater improvement in FEV1 AUC0–4 than the fluticasone propionate–salmeterol group (LSM treatment difference 0.122 L, P<0.001) Glycopyrrolate–indacaterol treatment reduced moderate/severe exacerbations to a significantly greater extent than fluticasone propionate–salmeterol (P=0.048) |
|
| Post hoc analysis of pooled ILLUMINATE and LANTERN trials examining symptomatic (GOLD B and D)a patients with moderate–severe COPD72 | 26 weeks | Glycopyrrolate–indacaterol 50/110 µg QD (n=630), fluticasone propionate–salmeterol 500/50 µg BID (n=633) | At week 26, glycopyrrolate–indacaterol significantly improved lung function from baseline vs fluticasone propionate–salmeterol in symptomatic patients (LSM treatment difference in predose trough FEV1 in GOLD B and D patients 0.10 and 0.08 L, respectively [both P<0.0001]; LSM treatment difference in FEV1 AUC0–12 0.14 L [P<0.0001] in GOLD B and 0.11 L [P<0.005] in GOLD D) | |
| Two randomized, double-blind, crossover studies (A2349 and A2350) in patients with moderate–severe COPD71 | 12 weeks | Glycopyrrolate–indacaterol 15.6/27.5 µg BID, umeclidinium–vilanterol 62.5/25 µg QD (A2349, n=357; A2350, n=355) | Noninferiority of glycopyrrolate–indacaterol vs umeclidinium–vilanterol for change from baseline in FEV1 AUC0–24 at week 12 was not met (lower boundary of 97.5% one-sided CI for glycopyrrolate–indacaterol below prespecified margin of −20 mL in both studies; LSM between-treatment differences −11.5 mL, 95% CI −26.9 to 3.8 [A2349] and −18.2 mL, 95% CI −34.2 to 2.3 [A2350]) Both glycopyrrolate–indacaterol and umeclidinium–vilanterol improved FEV1 AUC12–24 (208 and 203 mL [A2349], 163 and 154 mL [A2350]) and trough FEV1 levels from baseline at week 12 (189 and 201 mL [A2349], 168 and 177 mL [A2350]) |
|
| Randomized, double-blind, crossover, MOVE study in patients with moderate–severe COPD73 | 21 days | Glycopyrrolate–indacaterol 50/110 µg QD, then placebo; placebo, then glycopyrrolate–indacaterol 50/110 µg QD (n=194) | Glycopyrrolate–indacaterol significantly improved peak IC levels (LSM treatment difference 0.202 L, P<0.0001) and activity-related energy used (P=0.040) vs placebo after 21 days’ treatment | |
| Randomized, multicenter, blinded, QUANTIFY study in moderate–severe COPD66 | 26 weeks | Glycopyrrolate–indacaterol 50/110 µg QD (n=476), tiotropium 18 µg QD + formoterol 12 µg BID (n=458) | At week 26, glycopyrrolate–indacaterol was found to be noninferior to tiotropium + formoterol in improving SGRQ scores (LSM treatment difference −0.69) At week 26, significantly more patients using glycopyrrolate–indacaterol vs tiotropium + formoterol achieved a clinically relevant improvement in TDI score (49.6% vs 42.4%, P=0.033) and significantly improved predose FEV1 (LSM treatment difference 0.068 L, P<0.001) and FVC (LSM treatment difference 0.074 L, P<0.01) |
|
| Randomized, open-label, crossover, FAVOR study in symptomatic (GOLD B or D)a patients who had previously received tiotropium therapy65 | 4 weeks | Open-label glycopyrrolate–indacaterol 50/110 µg QD, then tiotropium 18 µg QD (n=43); tiotropium 18 µg QD, then open-label glycopyrrolate–indacaterol 50/110 µg QD (n=45) | Glycopyrrolate–indacaterol significantly increased FEV1 1 hour postdose vs tiotropium after 4 weeks’ treatment (treatment difference 0.081 L, P=0.0017) | |
| Pooled analysis of randomized, double-blind, parallel-group, FLIGHT1 and FLIGHT2 studies in moderate–severe COPD69 | 12 weeks | Glycopyrrolate–indacaterol 15.6/27.5 µg BID (n=260), indacaterol 27.5 µg BID (n=260), glycopyrrolate 15.6 µg BID (n=261), placebo (n=261) | Glycopyrrolate–indacaterol improved FEV1 AUC0–12 significantly more than individual components (LSM treatment difference 0.103 L vs indacaterol and 0.088 L vs glycopyrrolate, respectively; both P<0.001) and placebo (LSM treatment difference 0.246 L, P<0.001) Compared with individual components and placebo, glycopyrrolate–indacaterol produced significantly greater and more clinically meaningful improvements in TDI total score (LSM treatment difference 0.78 points vs indacaterol, 0.73 points vs glycopyrrolate, and 1.64 points vs placebo; all P<0.001) and SGRQ score (LSM treatment difference −1.7 points vs indacaterol [P<0.05], −1.5 points vs glycopyrrolate [P<0.05], −5.0 points vs placebo [P<0.001]) |
|
| Randomized, multicenter, double-blind, parallel-group, FLIGHT3 study in moderate–severe COPD70 | 52 weeks | Glycopyrrolate–indacaterol 15.6/27.5 µg BID (n=204), glycopyrrolate–indacaterol 31.2/27.5 µg BID (n=204), indacaterol 75 µg QD (n=207) | Improvements in predose trough FEV1 were greater with glycopyrrolate–indacaterol 15.6/27.5 µg and 31.2/27.5 µg (LSM treatment difference 0.080 L and 0.079 L, respectively), and improvements in 1-hour postdose FEV1 were greater with glycopyrrolate–indacaterol 15.6/27.5 µg (LSM treatment difference 0.108 L) than indacaterol alone at week 52 | |
| Glycopyrrolate via DPI plus FDC fluticasone propionate–salmeterol via DPI | ||||
| Randomized, blinded, GLISTEN study in moderate–severe COPD74 | 12 weeks | Glycopyrrolate 50 µg QD + fluticasone propionate–salmeterol 500/50 µg BID (n=258), tiotropium 18 µg QD + fluticasone propionate–salmeterol 500/50 µg BID (n=258), placebo + fluticasone propionate–salmeterol 500/50 µg BID (n=257) | At week 12, glycopyrrolate + fluticasone propionate–salmeterol significantly improved trough FEV1 from baseline vs placebo + fluticasone propionate–salmeterol (LSM treatment difference 0.101 L, P<0.001) At week 12, glycopyrrolate + fluticasone propionate–salmeterol was noninferior to tiotropium + fluticasone propionate–salmeterol (LSM treatment difference −0.007 L) At week 12, glycopyrrolate + fluticasone propionate–salmeterol significantly improved SGRQ total scores from baseline vs placebo + fluticasone propionate–salmeterol (LSM treatment difference −2.15, P=0.02) |
|
| Extrafine glycopyrrolate–beclomethasone–formoterol FDC via pMDI | ||||
| Randomized, double-blind, active-comparator, TRILOGY study in patients with symptomatic COPD75 | 52 weeks | 2-week open-label run-in period, beclomethasone–formoterol 100/6 µg; patients then randomly assigned to beclomethasone–formoterol 100/6 µg (n=681) or stepped up to glycopyrrolate–beclomethasone–formoterol 12.5/100/6 µg (n=687) | At week 26, triple FDC significantly improved predose (treatment difference 0.081 L) and 2-hour postdose FEV1 (treatment difference 0.117 L) from baseline vs the dual ICS–LABA (both P<0.001) At week 52, patients in the triple FDC group experienced 23% fewer moderate/severe exacerbations than those in the dual ICS–LABA FDC group |
|
| Randomized, double-blind, TRINITY study in patients with symptomatic COPD76 | 52 weeks | 2-week open-label tiotropium 18 µg QD, then open-label tiotropium µg QD (n=1,075), glycopyrrolate–beclomethasone–formoterol 12.5/100/6 µg BID (n=1,078), beclomethasone–formoterol 100/6 µg BID + tiotropium 18 µg QD (n=538) | Glycopyrrolate–beclomethasone–formoterol was superior to tiotropium in reducing the rate of exacerbations (P=0.0025) and week 52 predose FEV1 (treatment difference 0.061 L, P<0.0001) Glycopyrrolate–beclomethasone–formoterol had a similar efficacy to beclomethasone–formoterol + tiotropium in terms of risk of exacerbations (P=0.890) and was noninferior for improvement in predose FEV1 at week 52 (treatment difference −0.003 L, P=0.85) |
|
Note:
GOLD B and D defined according to the 2013 GOLD guidelines (FAVOR), the 2010 GOLD guidelines (ILLUMINATE), or the 2009 GOLD guidelines (LANTERN).
Abbreviations: AUC, area under curve; BID, bis in die (twice daily); DPI, dry-powder inhaler; FDC, fixed-dose combination; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Pulmonary Disease; LSM, least squares mean; IC, inspiratory capacity; ICS-LABA, inhaled corticosteroid-long-acting β2-agonist; NS, not significant; pMDI, pressurized metered-dose inhaler; QD, quaque die (once daily); SGRQ, St George’s Respiratory Questionnaire; TDI, Transition Dyspnea Index.
Inhaled glycopyrrolate has also been combined with indacaterol for maintenance therapy in patients with COPD. Glycopyrrolate–indacaterol DPI was approved for use by the FDA in 2015 (at 15.6/27.5 µg BID)36 and by the EMA in 2013 (at 50/110 µg QD).35 Glycopyrrolate–indacaterol 50/110 µg QD significantly improved lung function compared with individual components (P<0.001)64 and tiotropium 18 µg QD (P=0.0017).65 The glycopyrrolate–indacaterol combination was also preferred to tiotropium by both patients (P=0.00004) and physicians (P<0.0001).65 Furthermore, glycopyrrolate–indacaterol 50/110 µg QD significantly increased lung function, and a significantly greater proportion of patients achieved a clinically relevant improvement in dyspnea compared with tiotropium 18 µg QD plus formoterol 12 µg BID.66 In two clinical trials, glycopyrrolate–indacaterol 50/110 µg QD was more effective at reducing COPD exacerbations than the ICS–LABA combination fluticasone propionate–salmeterol 500/50 µg BID.67,68 Additionally, glycopyrrolate–indacaterol 50/110 µg QD was found to be noninferior to tiotropium 18 µg QD plus formoterol 12 µg BID in improving HRQoL.66
The 15.6/27.5 µg BID formulation of glycopyrrolate–indacaterol DPI has been shown to perform favorably in improving lung function when compared with its individual components69,70 and provide clinically meaningful improvements in breathlessness and HRQoL.69 Two crossover studies of glycopyrrolate–indacaterol 15.6/27.5 µg BID vs umeclidinium–vilanterol 62.5/25 µg QD did not meet their primary efficacy end point of noninferiority of change from baseline in FEV1 area under the curve from 0 to 24 hours at week 12. The authors concluded that although glycopyrrolate–indacaterol was not non-inferior to umeclidinium–vilanterol, the differences in improvement in lung function between the combinations were not clinically relevant.71 Several clinical trials have indicated that glycopyrrolate–indacaterol is effective at improving lung function in patients with moderate–severe COPD,68,69,71–73 and patients with COPD and a high risk of exacerbations.67 One of these trials also demonstrated that glycopyrrolate–indacaterol decreases hyperinflation and improves patients’ ability to undertake daily physical activity.73
Glycopyrrolate also showed potential as a COPD treatment when added to the ICS–LABA combination fluticasone propionate–salmeterol.74 After 12 weeks’ treatment, glycopyrrolate 50 µg QD plus fluticasone propionate–salmeterol 500/50 µg BID significantly improved lung function when compared with placebo plus fluticasone propionate–salmeterol, and was noninferior to tiotropium (18 µg QD) plus fluticasone propionate–salmeterol.74
An inhaled triple FDC, glycopyrrolate–beclomethasone–formoterol (12.5/100/6 µg BID pMDI) was approved for use by the EMA in 2017 for the maintenance treatment of patients with COPD.39 Two long-term trials have demonstrated that this triple FDC is efficacious in this patient group.75,76 In the first trial, the triple FDC was significantly more effective at improving lung function vs beclomethasone–formoterol at week 26.75 The triple FDC also decreased the risk of exacerbations from baseline to a greater extent than beclomethasone–formoterol by week 52.75 The second trial provided evidence that this triple FDC was significantly superior to tiotropium in reducing the exacerbation rate and improving lung function from baseline.76
Safety of inhaled glycopyrrolate monotherapy
Several trials have shown that glycopyrrolate 50 µg QD DPI is well tolerated,41–47 with a similar overall incidence of AEs to tiotropium 18 µg QD.42,44,45,47 In a study comparing glycopyrrolate 50 µg QD with open-label tiotropium 18 µg QD, the incidences of AEs and serious AEs were similar between groups; AEs with incidence >10% were worsening COPD (24% [glycopyrrolate] vs 33% [tiotropium]) and nasopharyngitis (31% vs 33%, respectively).44 Glycopyrrolate also had an acceptable safety profile at 15.6 µg BID,48–50 with AE incidence after 52 weeks’ treatment similar to that seen after treatment with indacaterol 75 µg QD.50 Furthermore, nebulized glycopyrrolate (50 µg BID) was also shown to have an acceptable safety profile over 48 weeks’ treatment compared with tiotropium 18 µg QD.51 Treatment discontinuation due to treatment-emergent AEs was higher for nebulized glycopyrrolate than for tiotropium (10% vs 3%, respectively); the authors suggested several reasons for this, including the open-label nature of the trial.51 In another study, nebulized glycopyrrolate was well tolerated at 25 and 50 µg BID, as measured by the incidences of AEs and cardiovascular (CV) events.38 Finally, in two chronic-dosing trials of glycopyrrolate pMDI delivered using co-suspension delivery technology, glycopyrrolate had an acceptable safety profile at all doses evaluated, with no unexpected safety findings reported.52,57
Newly prescribed LAMAs and LABAs have been associated with a greater risk of CV events compared with nonuse in patients with COPD (adjusted OR 1.14 [95% CI 1.01–1.28, P=0.03] and 1.31 [95% CI 1.12–1.52, P<0.001] for LAMAs and LABAs, respectively).77 However, the studies discussed in this review provide no evidence for an increased risk of CV events with glycopyrrolate monotherapy vs tiotropium or indacaterol monotherapy.45,50 Furthermore, in one of the trials investigating nebulized glycopyrrolate, fewer patients treated with glycopyrrolate experienced major CV AEs than those treated with tiotropium (0.5% vs 1.7%).51
Safety of inhaled glycopyrrolate combinations
The FDC glycopyrrolate–formoterol is designed to minimize the risk of AEs associated with high doses of LAMA or LABA monotherapies. In its Phase III clinical development program, glycopyrrolate–formoterol pMDI 18/9.6 µg BID showed a safety profile consistent with that of the individual components (PINNACLE-1 and -2), placebo (PINNACLE-1 and -2, PT003011, and PT003012), and open-label tiotropium 18 µg QD (PINNACLE-1 and PT003011).60–62
Furthermore, several clinical trials have indicated that the glycopyrrolate–indacaterol DPI combination at both EMA- and FDA-approved doses (50/110 µg QD and 15.6/27.5 µg QD, respectively) has good tolerability and an acceptable safety profile in patients with moderate–severe COPD.65,67–69,71–73 A trial by Ferguson et al showed that the risk of CV events was similar with the glycopyrrolate–indacaterol FDC to with indacaterol alone.70
Safety end points have also been assessed in triple FDC therapy regimens that incorporate glycopyrrolate. For example, in a study comparing glycopyrrolate plus fluticasone propionate–salmeterol with the ICS–LABA combination administered with either tiotropium 18 µg QD or placebo, the authors reported no significant differences between the number of AEs or severe AEs in any of the treatment groups evaluated.74 In a study comparing the glycopyrrolate–beclomethasone–formoterol FDC with both tiotropium and beclomethasone–formoterol plus tiotropium, AE incidence was similar among treatment groups.76 A further study comparing glycopyrrolate–beclomethasone–formoterol with beclomethasone–formoterol also reported similar rates of AEs between treatment groups, although one serious treatment-emergent AE (atrial fibrillation) occurred in a patient from the triple-FDC group.75
Conclusion
Inhaled glycopyrrolate is a fast-acting, efficacious treatment option for patients with moderate–severe COPD and is available in a variety of doses. It improves lung function, reduces the risk of exacerbations, and alleviates the symptoms of breathlessness, which in turn may explain the improvement seen in patients’ QoL. Glycopyrrolate has comparable effects on lung function to tiotropium, and glycopyrrolate and aclidinium showed more rapid onset of action than tiotropium. Formulations containing inhaled glycopyrrolate are well tolerated and, despite being an anticholinergic, few CV-related events have been reported, with glycopyrrolate showing a similar safety profile to tiotropium. Inhaled glycopyrrolate is thus of value both as monotherapy and for optimizing bronchodilation when used in FDCs with LABAs and ICS–LABAs for maintenance treatment of COPD.
Acknowledgments
We thank Johan Karlberg, MD, PhD, of the Clinical Trial Magnifier Newsletter for some of the information in this manuscript. Medical writing support was provided by Carly Hayes, PhD, of Core (London, UK) and editorial support was provided by Maryam Vahdat, PGDip of Core (London, UK), which was in accordance with Good Publication Practice (GPP3) guidelines and funded by AstraZeneca LP (Wilmington, DE, USA). AstraZeneca LP reviewed the manuscript for medical accuracy prior to submission.
Footnotes
Author contributions
DPT and NJG made substantial contributions to the conception of this article, drafted, and critically revised it for important intellectual content; provided final approval of the version to be published; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved.
Disclosure
DPT serves on advisory boards for AstraZeneca, Sunovion, Mylan, and Theravance/Innoviva, and as a speaker for AstraZeneca, Boehringer-Ingelheim, and Sunovion. NJG has no conflicts of interest.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. Bethesda, MD: GOLD; 2017. [Google Scholar]
- 2.Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179–191. doi: 10.7326/0003-4819-155-3-201108020-00008. [DOI] [PubMed] [Google Scholar]
- 3.Schroeckenstein DC, Bush RK, Chervinsky P, Busse WW. Twelve-hour bronchodilation in asthma with a single aerosol dose of the anticholinergic compound glycopyrrolate. J Allergy Clin Immunol. 1988;82(1):115–119. doi: 10.1016/0091-6749(88)90060-7. [DOI] [PubMed] [Google Scholar]
- 4.Walker FB, Kaiser DL, Kowal MB, Suratt PM. Prolonged effect of inhaled glycopyrrolate in asthma. Chest. 1987;91(1):49–51. doi: 10.1378/chest.91.1.49. [DOI] [PubMed] [Google Scholar]
- 5.Johnson BE, Suratt PM, Gal TJ, Wilhoit SC. Effect of inhaled glycopyrrolate and atropine in asthma: precipitated by exercise and cold air inhalation. Chest. 1984;85(3):325–328. doi: 10.1378/chest.85.3.325. [DOI] [PubMed] [Google Scholar]
- 6.Food US, Administration Drug. Seebri Neohaler [prescribing Information] 2015. [Accessed March 20, 2018]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207923lbl.pdf.
- 7.European Medicines Agency European public assessment report: Seebri Breezhaler. 2012. [Accessed March 20, 2018]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002430/WC500133771.pdf.
- 8.Cazzola M, Page CP, Calzetta L, Matera MG. Pharmacology and therapeutics of bronchodilators. Pharmacol Rev. 2012;64(3):450–504. doi: 10.1124/pr.111.004580. [DOI] [PubMed] [Google Scholar]
- 9.Racké K, Matthiesen S. The airway cholinergic system: physiology and pharmacology. Pulm Pharmacol Ther. 2004;17(4):181–198. doi: 10.1016/j.pupt.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Franko BV, Lunsford CD. Derivatives of 3-pyrrolidinols – III: the chemistry, pharmacology, and toxicology of some N-substituted-3-pyrrolidyl α-substituted phenylacetates. J Med Pharm Chem. 1960;2:523–540. doi: 10.1021/jm50012a004. [DOI] [PubMed] [Google Scholar]
- 11.Barnes PJ. Anticholinergics. In: Celli BR, editor. Pharmacotherapy in Chronic Obstructive Pulmonary Disease. New York: Marcel Dekker; 2004. pp. 201–216. [Google Scholar]
- 12.Bartels C, Looby M, Sechaud R, Kaiser G. Determination of the pharmacokinetics of glycopyrronium in the lung using a population pharmacokinetic modelling approach. Br J Clin Pharmacol. 2013;76(6):868–879. doi: 10.1111/bcp.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sykes DA, Dowling MR, Leighton-Davies J, et al. The influence of receptor kinetics on the onset and duration of action and the therapeutic index of NVA237 and tiotropium. J Pharmacol Exp Ther. 2012;343(2):520–528. doi: 10.1124/jpet.112.194456. [DOI] [PubMed] [Google Scholar]
- 14.Kaltiala E, Penttilä A, Vapaatalo H, Larmi T. The fate of intravenous (3H) glycopyrrolate in man. J Pharm Pharmacol. 1974;26(5):352–354. doi: 10.1111/j.2042-7158.1974.tb09287.x. [DOI] [PubMed] [Google Scholar]
- 15.Sechaud R, Renard D, Zhang-Auberson L, de la Motte S, Drollmann A, Kaiser G. Pharmacokinetics of multiple inhaled NVA237 doses in patients with chronic obstructive pulmonary disease (COPD) Int J Clin Pharmacol Ther. 2012;50(2):118–128. doi: 10.5414/cp201612. [DOI] [PubMed] [Google Scholar]
- 16.Gavaldà A, Ramos I, Carcasona C, et al. The in vitro and in vivo profile of aclidinium bromide in comparison with glycopyrronium bromide. Pulm Pharmacol Ther. 2014;28(2):114–121. doi: 10.1016/j.pupt.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Hansel TT, Barnes PJ. Tiotropium bromide: a novel once-daily anti-cholinergic bronchodilator for the treatment of COPD. Drugs Today (Barc) 2002;38(9):585–600. doi: 10.1358/dot.2002.38.9.696535. [DOI] [PubMed] [Google Scholar]
- 18.Ogoda M, Niiya R, Koshika T, Yamada S. Comparative characterization of lung muscarinic receptor binding after intratracheal administration of tiotropium, ipratropium, and glycopyrrolate. J Pharmacol Sci. 2011;115(3):374–382. doi: 10.1254/jphs.10311fp. [DOI] [PubMed] [Google Scholar]
- 19.Rogliani P, Calzetta L, Ora J, et al. Pharmacological assessment of the onset of action of aclidinium and glycopyrronium versus tiotropium in COPD patients and human isolated bronchi. Eur J Pharmacol. 2015;761:383–390. doi: 10.1016/j.ejphar.2015.04.042. [DOI] [PubMed] [Google Scholar]
- 20.Sentellas S, Ramos I, Alberti J, et al. Aclidinium bromide, a new, long-acting, inhaled muscarinic antagonist: in vitro plasma inactivation and pharmacological activity of its main metabolites. Eur J Pharm Sci. 2010;39(5):283–290. doi: 10.1016/j.ejps.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Ramos FL, Krahnke JS, Kim V. Clinical issues of mucus accumulation in COPD. Int J Chron Obstruct Pulmon Dis. 2014;9:139–150. doi: 10.2147/COPD.S38938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gosens R, Zaagsma J, Meurs H, Halayko AJ. Muscarinic receptor signaling in the pathophysiology of asthma and COPD. Respir Res. 2006;7:73. doi: 10.1186/1465-9921-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melloni B, Germouty J. The influence of a new β-agonist: formoterol on mucociliary function. Rev Mal Respir. 1992;9(5):503–507. French. [PubMed] [Google Scholar]
- 24.Lindberg S, Khan R, Runer T. The effects of formoterol, a long-acting β2-adrenoceptor agonist, on mucociliary activity. Eur J Pharmacol. 1995;285(3):275–280. doi: 10.1016/0014-2999(95)00416-i. [DOI] [PubMed] [Google Scholar]
- 25.Restrepo RD. Inhaled adrenergics and anticholinergics in obstructive lung disease: do they enhance mucociliary clearance? Respir Care. 2007;52(9):1159–1173. [PubMed] [Google Scholar]
- 26.Tagaya E, Yagi O, Sato A, et al. Effect of tiotropium on mucus hypersecretion and airway clearance in patients with COPD. Pulm Pharmacol Ther. 2016;39:81–84. doi: 10.1016/j.pupt.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Hasani A, Toms N, Agnew JE, Sarno M, Harrison AJ, Dilworth P. The effect of inhaled tiotropium bromide on lung mucociliary clearance in patients with COPD. Chest. 2004;125(5):1726–1734. doi: 10.1378/chest.125.5.1726. [DOI] [PubMed] [Google Scholar]
- 28.Meyer T, Reitmeir P, Brand P, et al. Effects of formoterol and tiotropium bromide on mucus clearance in patients with COPD. Respir Med. 2011;105(6):900–906. doi: 10.1016/j.rmed.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Bennett WD, Chapman WF, Mascarella JM. The acute effect of ipratropium bromide bronchodilator therapy on cough clearance in COPD. Chest. 1993;103(2):488–495. doi: 10.1378/chest.103.2.488. [DOI] [PubMed] [Google Scholar]
- 30.Hugel H, Ellershaw J, Gambles M. Respiratory tract secretions in the dying patient: a comparison between glycopyrronium and hyoscine hydrobromide. J Palliat Med. 2006;9(2):279–284. doi: 10.1089/jpm.2006.9.279. [DOI] [PubMed] [Google Scholar]
- 31.US Food and Drug Administration Medical review: glycopyrrolate. 2009. [Accessed March 20, 2018]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022571Orig1s000MedR.pdf.
- 32.Mirakhur RK, Dundee JW, Jones CJ. Evaluation of the anticholinergic actions of glycopyrronium bromide. Br J Clin Pharmacol. 1978;5(1):77–84. doi: 10.1111/j.1365-2125.1978.tb01601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.European Medicines Agency Seebri Breezhaler [summary of product characteristics] 2012. [Accessed March 20, 2018]. Available from: https://www.medicines.org.uk/emc/medicine/27138.
- 34.AstraZeneca Pharmaceuticals LP. Bevespi Aerosphere [prescribing information] 2016. [Accessed March 20, 2018]. Available from: https://www.azpicentral.com/bevespi/bevespi_pi.pdf.
- 35.European Medicines Agency Ultibro Breezhaler [summary of product characteristics] 2016. [Accessed March 20, 2018]. Available from: https://www.medicines.org.uk/emc/medicine/29533.
- 36.US Food and Drug Administration Utibron Neohaler [prescribing information] 2017. [Accessed March 20, 2018]. Available from: https://www.accessdata.fda.gov/drug-satfda_docs/label/2015/207930s000lbl.pdf.
- 37.European Medicines Agency Trimbow [summary of product characteristics] 2017. [Accessed March 20, 2018]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004257/WC500233163.pdf.
- 38.Kerwin E, Donohue JF, Goodin T, Tosiello R, Wheeler A, Ferguson GT. Efficacy and safety of glycopyrrolate/eFlow CS (nebulized glycopyrrolate) in moderate-to-very-severe COPD: Results from the glycopyrrolate for obstructive lung disease via electronic nebulizer (GOLDEN) 3 and 4 randomized controlled trials. Respir Med. 2017;132:238–250. doi: 10.1016/j.rmed.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 39.European Medicines Agency 2017. [Accessed March 20, 2018]. Trimbow [initial authorization] Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/004257/WC500228080.pdf.
- 40.Arievich H, Overend T, Renard D, et al. A novel model-based approach for dose determination of glycopyrronium bromide in COPD. BMC Pulm Med. 2012;12:74. doi: 10.1186/1471-2466-12-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D’Urzo A, Ferguson GT, van Noord JA, et al. Efficacy and safety of once-daily NVA237 in patients with moderate-to-severe COPD: the GLOW1 trial. Respir Res. 2011;12:156. doi: 10.1186/1465-9921-12-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kerwin E, Hébert J, Gallagher N, et al. Efficacy and safety of NVA237 versus placebo and tiotropium in patients with COPD: the GLOW2 study. Eur Respir J. 2012;40(5):1106–1114. doi: 10.1183/09031936.00040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beeh KM, Singh D, Di Scala L, Drollmann A. Once-daily NVA237 improves exercise tolerance from the first dose in patients with COPD: the GLOW3 trial. Int J Chron Obstruct Pulmon Dis. 2012;7:503–513. doi: 10.2147/COPD.S32451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sekiya M, Kawayama T, Fukuchi Y, et al. Safety and efficacy of NVA237 once daily in Japanese patients: the GLOW4 trial. Eur Respir J. 2012;40(Suppl 56):P2013. [Google Scholar]
- 45.Chapman KR, Beeh KM, Beier J, et al. A blinded evaluation of the efficacy and safety of glycopyrronium, a once-daily long-acting muscarinic antagonist, versus tiotropium, in patients with COPD: the GLOW5 study. BMC Pulm Med. 2014;14:4. doi: 10.1186/1471-2466-14-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang C, Sun T, Huang Y, et al. Efficacy and safety of once-daily glycopyrronium in predominantly Chinese patients with moderate-to-severe chronic obstructive pulmonary disease: the GLOW7 study. Int J Chron Obstruct Pulmon Dis. 2015;10:57–68. doi: 10.2147/COPD.S72650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marin JM, Beeh KM, Clemens A, et al. Early bronchodilator action of glycopyrronium versus tiotropium in moderate-to-severe COPD patients: a cross-over blinded randomized study (Symptoms and Pulmonary Function in the Morning) Int J Chron Obstruct Pulmon Dis. 2016;11:1425–1434. doi: 10.2147/COPD.S106127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.LaForce C, Feldman G, Spangenthal S, et al. Efficacy and safety of twice-daily glycopyrrolate in patients with stable, symptomatic COPD with moderate-to-severe airflow limitation: the GEM1 study. Int J Chron Obstruct Pulmon Dis. 2016;11:1233–1243. doi: 10.2147/COPD.S100445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kerwin E, Siler TM, Korenblat P, et al. Efficacy and safety of twice-daily glycopyrrolate versus placebo in patients with COPD: the GEM2 study. Chronic Obstr Pulm Dis. 2016;3(2):549–559. doi: 10.15326/jcopdf.3.2.2015.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mahler DA, Gifford AH, Satti A, et al. Long-term safety of glycopyrrolate: a randomized study in patients with moderate-to-severe COPD (GEM3) Respir Med. 2016;115:39–45. doi: 10.1016/j.rmed.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 51.Ferguson GT, Goodin T, Tosiello R, Wheeler A, Kerwin E. Long-term safety of glycopyrrolate/eFlow CS in moderate-to-very-severe COPD: results from the Glycopyrrolate for Obstructive Lung Disease via Electronic Nebulizer (GOLDEN) 5 randomized study. Respir Med. 2017;132:251–260. doi: 10.1016/j.rmed.2017.08.020. [DOI] [PubMed] [Google Scholar]
- 52.Fabbri LM, Kerwin EM, Spangenthal S, et al. Dose-response to inhaled glycopyrrolate delivered with a novel Co-Suspension Delivery Technology metered dose inhaler (MDI) in patients with moderate-to-severe COPD. Respir Res. 2016;17(1):109. doi: 10.1186/s12931-016-0426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karabis A, Lindner L, Mocarski M, Huisman E, Greening A. Comparative efficacy of aclidinium versus glycopyrronium and tiotropium, as maintenance treatment of moderate to severe COPD patients: a systematic review and network meta-analysis. Int J Chron Obstruct Pulmon Dis. 2013;8:405–423. doi: 10.2147/COPD.S48967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ismaila AS, Huisman EL, Punekar YS, Karabis A. Comparative efficacy of long-acting muscarinic antagonist monotherapies in COPD: a systematic review and network meta-analysis. Int J Chron Obstruct Pulmon Dis. 2015;10:2495–2517. doi: 10.2147/COPD.S92412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lechuga-Ballesteros D, Noga B, Vehring R, Cummings RH, Dwivedi SK. Novel cosuspension metered-dose inhalers for the combination therapy of chronic obstructive pulmonary disease and asthma. Future Med Chem. 2011;3(13):1703–1718. doi: 10.4155/fmc.11.133. [DOI] [PubMed] [Google Scholar]
- 56.Vehring R, Lechuga-Ballesteros D, Joshi V, Noga B, Dwivedi SK. Cosuspensions of microcrystals and engineered microparticles for uniform and efficient delivery of respiratory therapeutics from pressurized metered dose inhalers. Langmuir. 2012;28(42):15015–15023. doi: 10.1021/la302281n. [DOI] [PubMed] [Google Scholar]
- 57.Kerwin EM, Spangenthal S, Kollar C, Rose E, Reisner C. A phase IIB randomized, chronic-dosing, incomplete block, cross-over study of glycopyrronium, delivered via metered dose inhaler, compared with a placebo and an active control in patients with moderate-to-severe COPD. Respir Res. 2018;19(1):38. doi: 10.1186/s12931-018-0739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cazzola M, Matera MG. The effective treatment of COPD: anticholinergics and what else? Drug Discov Today Ther Strateg. 2006;3(3):277–286. [Google Scholar]
- 59.Barisione G, Baroffio M, Crimi E, Brusasco V. Beta-adrenergic agonists. Pharmaceuticals (Basel) 2010;3(4):1016–1044. doi: 10.3390/ph3041016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martinez FJ, Rabe KF, Ferguson GT, et al. Efficacy and safety of glycopyrrolate/formoterol metered dose inhaler formulated using Co-Suspension Delivery Technology in patients with COPD. Chest. 2017;151(2):340–357. doi: 10.1016/j.chest.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 61.Hanania NA, Tashkin DP, Kerwin EM, et al. Long-term safety and efficacy of glycopyrrolate/formoterol metered dose inhaler using novel Co-Suspension Delivery Technology in patients with chronic obstructive pulmonary disease. Respir Med. 2017;126:105–115. doi: 10.1016/j.rmed.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 62.Reisner C, Gottschlich G, Fakih F, et al. 24-h bronchodilation and inspiratory capacity improvements with glycopyrrolate/formoterol fumarate via co-suspension delivery technology in COPD. Respir Res. 2017;18:157. doi: 10.1186/s12931-017-0636-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martinez FJ, Fabbri LM, Ferguson GT, et al. Baseline symptom score impact on benefits of glycopyrrolate/formoterol metered dose inhaler in COPD. Chest. 2017;152(6):1169–1178. doi: 10.1016/j.chest.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 64.Bateman ED, Ferguson GT, Barnes N, et al. Dual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE study. Eur Respir J. 2013;42(6):1484–1494. doi: 10.1183/09031936.00200212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kardos P, Hagedorn-Peinz I. The impact of indacaterol/glycopyrronium fixed-dose combination versus tiotropium monotherapy on lung function and treatment preference: a randomized crossover study: the FAVOR study. Int J Chron Obstruct Pulmon Dis. 2018;13:69–77. doi: 10.2147/COPD.S146189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buhl R, Gessner C, Schuermann W, et al. Efficacy and safety of once-daily QVA149 compared with the free combination of once-daily tiotropium plus twice-daily formoterol in patients with moderate-to-severe COPD (QUANTIFY): a randomised, non-inferiority study. Thorax. 2015;70(4):311–319. doi: 10.1136/thoraxjnl-2014-206345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374(23):2222–2234. doi: 10.1056/NEJMoa1516385. [DOI] [PubMed] [Google Scholar]
- 68.Zhong N, Wang C, Zhou X, et al. LANTERN: a randomized study of QVA149 versus salmeterol/fluticasone combination in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:1015–1026. doi: 10.2147/COPD.S84436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mahler DA, Kerwin E, Ayers T, et al. FLIGHT1 and FLIGHT2: efficacy and safety of QVA149 (indacaterol/glycopyrrolate) versus its monocomponents and placebo in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192(9):1068–1079. doi: 10.1164/rccm.201505-1048OC. [DOI] [PubMed] [Google Scholar]
- 70.Ferguson GT, Taylor AF, Thach C, et al. Long-term maintenance bronchodilation with indacaterol/glycopyrrolate versus indacaterol in moderate-to-severe COPD patients: the FLIGHT 3 study. Chronic Obstr Pulm Dis. 2016;3(4):716–728. doi: 10.15326/jcopdf.3.4.2016.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kerwin E, Ferguson GT, Sanjar S, et al. Dual bronchodilation with indacaterol maleate/glycopyrronium bromide compared with umeclidinium bromide/vilanterol in patients with moderate-to-severe COPD: results from two randomized, controlled, cross-over studies. Lung. 2017;195(6):739–747. doi: 10.1007/s00408-017-0055-9. [DOI] [PubMed] [Google Scholar]
- 72.Vogelmeier C, Zhong N, Humphries MJ, et al. Indacaterol/glycopyrronium in symptomatic patients with COPD (GOLD B and GOLD D) versus salmeterol/fluticasone: ILLUMINATE/LANTERN pooled analysis. Int J Chron Obstruct Pulmon Dis. 2016;11:3189–3197. doi: 10.2147/COPD.S116786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Watz H, Mailänder C, Baier M, Kirsten A. Effects of indacaterol/glycopyrronium (QVA149) on lung hyperinflation and physical activity in patients with moderate to severe COPD: a randomised, placebo-controlled, crossover study (the MOVE study) BMC Pulm Med. 2016;16(1):95. doi: 10.1186/s12890-016-0256-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Frith PA, Thompson PJ, Ratnavadivel R, et al. Glycopyrronium once-daily significantly improves lung function and health status when combined with salmeterol/fluticasone in patients with COPD: the GLISTEN study, a randomised controlled trial. Thorax. 2015;70(6):519–527. doi: 10.1136/thoraxjnl-2014-206670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singh D, Papi A, Corradi M, et al. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting β2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial. Lancet. 2016;388(10048):963–973. doi: 10.1016/S0140-6736(16)31354-X. [DOI] [PubMed] [Google Scholar]
- 76.Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet. 2017;389(10082):1919–1929. doi: 10.1016/S0140-6736(17)30188-5. [DOI] [PubMed] [Google Scholar]
- 77.Gershon A, Croxford R, Calzavara A, et al. Cardiovascular safety of inhaled long-acting bronchodilators in individuals with chronic obstructive pulmonary disease. JAMA Intern Med. 2013;173(13):1175–1185. doi: 10.1001/jamainternmed.2013.1016. [DOI] [PubMed] [Google Scholar]
- 78.Food US, Administration Drug. 2017. [Accessed March 20, 2018]. Lonhala Magnair [prescribing information] Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208437lbl.pdf.
- 79.Boehringer Ingelheim 2016. [Accessed March 20, 2018]. Spiriva HandiHaler [prescribing information] Available from: http://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Spiriva/Spiriva.pdf.
- 80.Boehringer Ingelheim Spiriva Respimat [prescribing information] 2017. [Accessed March 20, 2018]. Available from: http://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Spiriva%20Respimat/spirivarespimat.pdf.
- 81.Boehringer Ingelheim Stiolto Respimat [prescribing information] 2016. [Accessed March 20, 2018]. Available from: http://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Stiolto%20Respimat/stiolto.pdf.
- 82.European Medicines Agency 2015. [Accessed March 20, 2018]. Spiolto Respimat [summary of product characteristics] Available from: https://www.medicines.org.uk/emc/medicine/30495.
- 83.European Medicines Agency Incruse [summary of product characteristics] 2017. [Accessed March 20, 2018]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002809/WC500167430.pdf.
- 84.GlaxoSmithKline Incruse Ellipta [prescribing information] 2017. [Accessed March 20, 2018]. Available from: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Incruse_Ellipta/pdf/INCRUSE-ELLIPTA-PI-PIL.PDF.
- 85.European Medicines Agency Anoro [summary of product characteristics] 2017. [Accessed March 20, 2018]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002751/WC500168424.pdf.
- 86.GlaxoSmithKline Anoro Ellipta [prescribing information] 2013. [Accessed March 20, 2018]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/203975s000lbl.pdf.
- 87.GlaxoSmithKline Trelegy Ellipta [prescribing information] 2017. [Accessed March 20, 2018]. Available from: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Trelegy/pdf/TRELEGY-PI-MG-IFU.PDF.
- 88.European Medicines Agency Eklira Genuair [summary of product characteristics] 2017. [Accessed March 20, 2018]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002211/WC500132661.pdf.
- 89.AstraZeneca Pharmaceuticals LP. Tudorza Pressair [prescribing information] 2012. [Accessed March 20, 2018]. Available from: http://www.azpicentral.com/tudorza/pi_tudorza.pdf.
- 90.European Medicines Agency Bretaris Genuair [summary of product characteristics] 2017. [Accessed March 20, 2018]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002706/WC500132732.pdf.
- 91.European Medicines Agency Duaklir Genuair [summary of product characteristics] 2017. [Accessed March 20, 2018]. Available from: https://ec.europa.eu/health/documents/community-register/2014/20141119130022/anx_130022_en.pdf.
- 92.Theravance Biopharma Revefenacin peak inspiratory flow rate (PIFR) study in COPD. [Accessed March 20, 2018]. Available from: https://clinicaltrials.gov/ct2/show/NCT03095456. NLM identifier: NCT03095456.
- 93.Theravance BioPharma Theravance Biopharma and Mylan submit new drug application to FDA for revefenacin (TD-4208) in adults with chronic obstructive pulmonary disease. 2017. [Accessed March 20, 2018]. Available from: http://investor.theravance.com/news-releases/news-release-details/theravance-biopharma-and-mylan-submit-new-drug-application-fda.