Abstract
The interaction between HLA class II peptide complexes on antigen-presenting cells and CD4+ T cells is of fundamental importance for anti-cancer and anti-pathogen immunity as well as for the maintenance of immunological tolerance. To study CD4+ T cell reactivities, detailed knowledge of the presented peptides is necessary. In recent years, dramatic advances in the characterization of membranal and soluble HLA class I peptidomes could be observed. However, the same is not true for HLA class II peptidomes, where only few studies identify more than hundred peptides. Here we describe a mass spectrometry-based workflow for the characterization of membranal and soluble HLA class II DR and DQ peptidomes. Using this workflow, we identified a total of 8595 and 3727 HLA class II peptides from Maver-1 and DOHH2 cells, respectively. Based on this data, a motif-based binding predictor was developed and compared to NetMHCIIpan 3.1. We then applied the workflow to human plasma, resulting in the identification of between 34 and 152 HLA-DR and between 100 and 180 HLA-DQ peptides, respectively. Finally, we implemented a data-independent acquisition workflow to increase reproducibility and sensitivity of HLA class II peptidome characterizations.
Keywords: Biomarker, DIA, HLA class II, HLA peptidomics, immunopeptidomics
1. Introduction
Human leukocyte antigen (HLA) class II complexes present peptides on the surface of professional antigen presenting cells (APC). Displayed peptides originate mainly from endosomal degradation of endogenous and exogenous proteins and have a variable length of around 15 amino acids [1]. The presented HLA-peptide complexes can maintain tolerance or activate CD4+ T cells, depending on secondary signals. Activated CD4+ T cells can detect pathologic events, such as malignant transformation or infection with pathogens, and induce an appropriate immune response [2, 3]. CD4+ T cells further maintain immunological tolerance by differentiating into regulatory T cells [2]. If the fine balance between pro- and anti-inflammatory signals is broken, autoimmunity can develop [4]. Besides membrane-bound HLA class II complexes, a soluble form of the HLA class II complexes (sHLA-II) has been detected in serum as well as in various other body fluids [5, 6]. The function of these sHLA-II complexes remains to be determined, but a role in the induction and maintenance of immunological tolerance has been proposed [7, 8].
In recent years, it was also observed that CD4+ T cells can play an important role in cancer immunotherapy [9]. Traditionally, CD8+ T cells were assumed to be responsible for rejecting established tumors, and were therefore selected as the major target for vaccination strategies. However, innovative strategies are required to translate the promising preclinical observations into clinical success [10]. In mouse models, induction of CD4+ T cell responses has recently been suggested to improve therapeutic efficacy [11]. Similarly, two clinical studies evaluating personalized neo-epitope vaccination strategies in patients with melanoma observed a pronounced induction of an immunological response only after stimulation of CD4+ T cells [12, 13]. Both groups recognized that currently the development of vaccines inducing CD4+ T cell responses is prevented by the fact that HLA class II peptide binding prediction is not sufficiently established. One possibility to improve the peptide binding prediction is the definition of HLA-specific motifs in high resolution. However, for HLA class II, only very few studies demonstrate the confident identification of hundreds of HLA class II peptides [14–18]. As a consequence, only very few HLA class II-specific motifs have been characterized sufficiently to allow peptide binding prediction [19].
In this work, we investigated a workflow for the purification and identification of HLA class II DR and DQ peptides. We established and tested the workflow using Maver-1 and DOHH2 cells resulting in the identification of 6075 DR and 2577 DQ peptides for Maver-1 and 3238 DR and 540 DQ peptides for DOHH2 cells, respectively. Based on the identified peptides, we generated a motif-based binding predictor and compared its performance to NetMHCIIpan 3.1. We then applied the workflow to the purification and identification of sHLA-II peptides from human plasma resulting in the identification of between 34 and 152 HLA-DR and 100 and 180 HLA-DQ peptides, respectively. In an attempt to increase sensitivity and reproducibility of HLA class II peptidome characterizations, we established a data-independent acquisition (DIA) workflow, which we apply to the analysis of HLA class II peptidomes from cell lines.
2. Materials and Methods
Cell lines and Antibodies
The human mantle cell lymphoma cell line MAVER-1 was a kind gift of Prof Alberto Zamo [20]. The human follicular lymphoma cell line DOHH-2 was obtained from the DSMZ, (Braunschweig). Cell lines were cultivated in RPMI 1640 medium (Gibco) with 10% heat-inactivated FCS (Gibco). HLA typing was performed by the MVZ (Martinsried, Germany) [Supporting Information Table 1]. The L243 hybridoma cell line (HB-55) and the W6/32 hybridoma cell line (HB-95) were obtained from ATCC, the SPVL3 hybridoma cell line was a kind gift of Prof Dr Hergen Spits. Hybridoma cells were cultivated in CD Hybridoma medium (Gibco) with 2 mM glutamine (Ultraglutamine I, Lonza), at 37°C and 5% CO2. The L243 [21–23], SPVL3 [24–29] and W6/32 antibodies were purified from the respective hybridoma supernatants using Protein-A Sepharose and subsequently coupled to AminoLink Plus Coupling Resin (Thermo Fisher Scientific) following the manufacturer`s instructions.
Cell lysis and human plasma
Cells were lysed as previously described [30]. Plasma from donors was generated by drawing blood with EDTA tubes (S-Monovette 9 ml K3E, Sarstedt). Tubes were readily inverted, incubated for 30 min at RT and centrifuged at 2’000g for 10 min. Plasma was transferred into new vials, snap-frozen and stored at -80°C. After thawing, plasma was cleared by centrifugation at 3’345g for 5 min, 4°C prior to HLA purification. All donors were from the Department of Nephrology (Tenon Hospital Paris, France) and provided written informed consent for usage of plasma and clinical data for scientific purposes. Donors one and two were healthy volunteers, donors three and four were patients suffering from the rare kidney disease membranous nephropathy. This project was approved by the IRB/Ethics committee CPP Ile-de-France IV, Hôpital Saint-Louis, Paris (ethic vote 2013/03/14).
Volunteers were HLA typed by NGS technologies resulting in an unambiguous annotation of the A, B, C, DR and DQ alleles [Supporting Information Table 1].
Affinity purification of HLA complexes and enrichment of HLA-bound peptides from cell lines and plasma
HLA class II complexes were purified from lysate or 2.5-3 ml of plasma using L243 antibody-coupled resin for HLA class II DR and SPVL3 antibody-coupled resin for HLA class II DQ complexes. Resin was incubated for 2 h at 4°C, then washed once with 10 ml of lysis buffer, buffer A (150 mM NaCl, 20 mM Tris, pH 7.4), buffer B (400 mM NaCl, 20 mM Tris, pH 7.4), buffer A again and buffer C (20 mM Tris, pH 8.0). Peptide-HLA complexes were eluted with 10% acetic acid. Peptides were separated from protein by elution from C18 Macro SpinColums (Harvard Apparatus) with 25% ACN, 0.1% TFA, and subsequently dried and stored at -20°C. HLA class I complexes were purified from plasma as described previously [31].
DDA analysis of HLA peptides by UHPLC MS
Purified dried HLA class II peptides were resuspended in 3% ACN, 0.1% formic acid and iRT peptides (Biognosys, Schlieren) were added to all of the samples. Peptides were analyzed by liquid chromatography–tandem mass spectrometry (LC-MS/MS) using a Q Exactive Mass Spectrometer fitted with an EASY-nLC 1000 (both Thermo Fisher Scientific). Peptides were resolved with an Acclaim PepMap RSLC C18, 50 μm x 150 mm, 2 μm analytical column (Thermo Fisher Scientific) at a flow rate of 0.3 μL/min by running a linear gradient from 0% to 30% ACN over 120 min. All buffers contained 0.1% formic acid. MS spectra were recorded in full ion scan mode from 380–2’000 m/z, with a resolution of 70’000 and a maximum injection time of 80 ms. MS/MS were recorded at a resolution of 17’500 and a maximum injection time of 240 ms. The ten most intense masses with charges of two to five were selected for HCD fragmentation. Dynamic exclusion was set to 10 seconds.
Resulting spectra were processed and analyzed using the Proteome Discoverer software (Thermo Fisher Scientific, Version 1.4.1.14). MS/MS data was searched against a database consisting of the human (93’250 entries) and the bovine (24’339 entries) reference proteomes downloaded from the UniProt homepage on the 7th of July 2017, spiked with the 11 iRT peptide sequences from the iRT kit. The following analysis settings were used with SEQUEST: i) No-Enzyme (Unspecific), ii) precursor mass tolerance 7 ppm, iii) fragment mass tolerance 0.02 Da, iv) one variable modification (oxidation of methionine). False discovery rates were calculated with the Percolator plug-in and filtered at 1% FDR. HLA class I peptides were analyzed as described [31].
Gibbs clustering of HLA class II peptides
HLA peptides were analyzed using the GibbsCluster-2.0 Server [32]. HLA class II peptides with a length of at least 9 amino acids were clustered using the predefined “MHC class II ligands” parameters.
Ovalbumin feeding of Maver-1 cells
Sterile-filtered Ovalbumin stock solution (10 mg/ml Ovalbumin in PBS) was added to Maver-1 cells 48h prior to cell lysis and again 6h before lysis at a final concentration of 0.16mg/ml medium. Maver-1 cells were lysed and HLA class II peptides were isolated and analyzed as described above.
HLA class I binding prediction of identified peptides
Peptides with a length between 8 and 11 amino acids were subjected to HLA class I binding prediction analysis using NetMHCpan 3.0 [33]. Each peptide was assigned the minimal predicted peptide rank (%). Peptides were annotated as being predicted to bind if the recalculated rank was below 2 % for at least one of the alleles of the cell line.
Matrix-based scoring of peptide sequences and peptide-HLA class II binding prediction
Data from Ooi et al. [18] and from Clement et al. [17] were the basis for the calculation of position-specific scoring matrices (PSSM). Peptides identified from mono-allelic cells, or from cells expressing DRB1*15:01 and DRB5*01:01 were submitted to GibbsCluster-2.0 Server [32] analysis using the standard parameters for MHC class II including preference for hydrophobic AAs at P1. Core sequences identified from the clustering analysis of the DRB1*01:01, DRB1*15:01 and DRB5*01:01 clusters were submitted to Seq2Logo 2.0 server [34], using the following parameters: Logo type: Kullback-Leibler, Clustering method: Hobohm1, Threshold for clustering: 0.63, Weight on prior: 200, Information content: Bits, in the advanced settings, Blossum62 matrix and respective background frequencies were enabled. The position-specific scoring matrix (PSSM) was downloaded after calculation of the sequence logo. Scoring and binding prediction was performed essentially as described [19], applying a sliding window of nine amino acids to each peptide sequence. Core regions (i.e. the region binding to the cognate HLA class II allele through anchor residues) were defined by the region with the highest score. A ROC curve analysis of peptides identified from cell lines positive for a given allele and of all 15mers calculated for the human reference proteome (93’250 protein entries) downloaded from the UniProt homepage on 07th July 2017 was used to calculate score cutoff values defining predicted binding.
Prediction of peptide-HLA class II binding with the NetMHCIIpan 3.1 server [33] was performed keeping all standard parameters, selecting the HLA class II allele and submitting peptide sequences with at least nine amino acids. The threshold for binding was defined as a rank below 10%.
Spectral library generation
Libraries were generated essentially as described [35] using SpectraST (Version 5.0.0) [36]. Libraries were based on the peptides identified from SEQUEST with a 1% FDR performing retention time alignment with Spectrast2irt_alignment.py. The consensus spectrast.splib file was converted to a transition list using spectrast2tsv.py with the following settings: spectrast2tsv.py -l 250,2000 -s b,y -x 1,2 -o 3 -n 6 -g -17.03,-18.01 -p 0.05 -d -e -w windows.txt -k openswath –a output.csv input.sptxt.
DIA analysis was performed as in [35] with small modifications: The DIA method consisted of a survey scan from 380-2000 m/z at a resolution of 70’000. Equally spaced DIA windows of 30 m/z [Supporting Information Table 2] were recorded at a resolution of 35’000 in profile mode with fixed first mass set to 200 m/z and an NCE of 27. Raw files were centroided, converted to the mz5 format using msconvert (ProteoWizzard) and analyzed by Skyline (version 3.6.0.10162) [37] [Supporting Information Table 3]. Transition lists were pasted into Skyline, a retention time calibrator based on the iRT kit was created and decoys were added (decoy generation method: Reverse Sequence). Samples were imported into Skyline and peaks were filtered for an mProphet q-value below 0.01.
3. Results
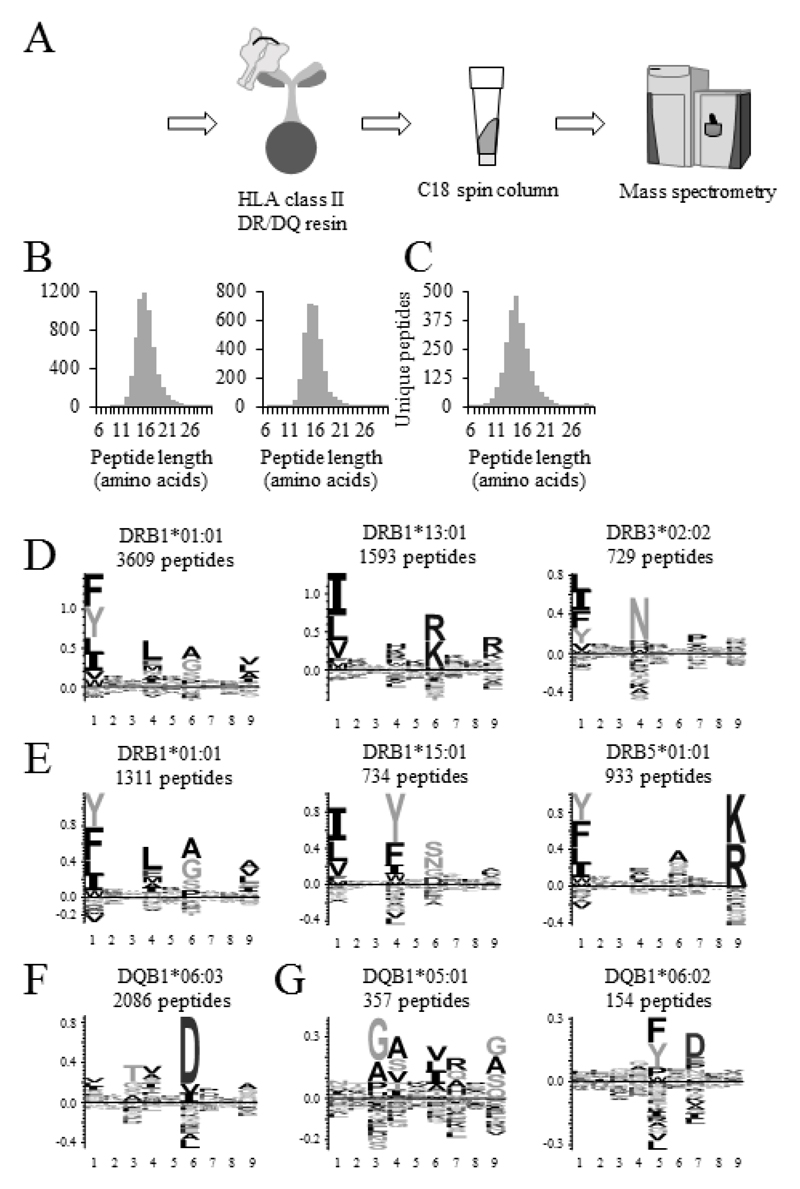
HLA class II peptidomes of the human mantle cell lymphoma cell line Maver-1 and the human follicular lymphoma cell line DOHH2 were characterized following affinity purification of DR or DQ complexes with anti-HLA class II DR antibody L243 or anti-HLA class II DQ antibody SPVL3 as outlined in Figure 1A. Both B cell lymphoma cell lines were HLA typed resulting in an unambiguous annotation of their DR and DQ alleles [Supporting Information Table 1]. Purified peptides were analyzed on a Q Exactive mass spectrometer, applying a 1% FDR on peptide identifications. HLA class II DR purifications resulted in the identification of a total of 6075 peptides from Maver-1 and 3238 peptides from DOHH2 cells [Supporting Information Table 4 a and b for a list of all peptide identifications]. The peptide length distributions of the DR peptides for both cell lines centered around 15 amino acids with the majority of peptides ranging between 12 and 22 amino acids [Figure 1B], consistent with previous reports on HLA class II peptide length [14, 15]. HLA class II DQ purifications resulted in the identification of a total of 2577 peptides from Maver-1 and 540 peptides from DOHH2 cells [Supporting Information Table 4 c and d for a list of all peptide identifications] with a length distribution comparable to DR peptides [Figure 1C].
Figure 1. HLA class II DR and DQ peptidome analysis of Maver-1 and DOHH2 cells.
(A) Schematic representation of the purification of HLA class II peptides. HLA class II complexes are purified from cell lysates and plasma using either L243-conjugated resin (HLA DR) or SPVL3-conjugated resin (HLA DQ). After acid elution of HLA class II complexes, peptides are separated from proteins by stepwise elution from C18 resin and analyzed by LC-MS/MS. (B, C) Length distribution of identified peptides from HLA class II purifications. The number of peptides between 6 and 30 amino acids is plotted in relation to their length. Length distributions for HLA class II DR peptides (B) and for HLA class II DQ peptides (C) are shown. Left panel Maver-1, right panel DOHH2, respectively (D-G) Definition of HLA-specific motifs from HLA class II DR and DQ purifications. HLA class II-specific motifs were created from all unique peptide sequences with a minimum length of 9 amino acids isolated from Maver-1 (D, F) or DOHH2 cells (E, G) using the GibbsCluster-2.0 Server.
We then evaluated if it was possible to extract the HLA-specific motifs for DR1*01:01, DR1*13:01, and DR3*02:02 from the Maver-1 DR peptidome. Gibbs cluster reported HLA-specific motifs only for two of the three DR alleles [see Supporting Information Figure 1]. Similarly, NetMHCIIpan 3.1 was able to identify putative DRB1*01:01-derived peptides, but did not report sequence logos with defined anchor residues at P4, P6, or P9 for DRB1*13:01 and DRB3*02:02 [see Supporting Information Figure 1]. Only after subtracting all putative DRB1*01:01 peptides, Gibbs cluster analysis of remaining peptides resulted in two distinct motifs, thereby finally revealing the three HLA-DR-specific motifs of Maver-1 in high resolution [Figure 1D and Supporting Information Figure 1]. Comparison of the data with the SYFPEITHI database [38] confirmed the annotation to respective alleles. Gibbs cluster also only revealed two of the three HLA DR-specific motifs of DOHH2 cells, while NetMHCIIpan 3.1 reported comparable core sequences to bind to all three alleles [see Supporting Information Figure 2]. Based on the DR1, DR15 and DR51-specific motifs obtained from the data reported by Ooi and colleagues [18], it was possible to assign the identified sequences to one of the three alleles, respectively [Figure 1E and Supporting Information Figure 2]. Gibbs cluster results for DQ revealed one and two clusters for Maver-1 [Figure 1F] and DOHH2 [Figure 1G], respectively.
In the past, we found high-resolution murine MHC class II-specific motifs to be very useful in predicting which regions of a protein can be presented by APCs [19]. To test if this was also the case for the human HLA class II-specific motifs, we were feeding ovalbumin to Maver-1 cells prior to analysis of the HLA DR peptidome. In total 21 peptide sequences clustering around three cores were identified [Supporting Information Table 5]. The three identified core sequences had further the highest and second highest score of all possible ovalbumin 9mer sequences. In contrast, NetMHCIIpan 3.1 predicted the binding of only one of the three cores to either DRB1*01:01 and DRB1*13:01 and reported two, five, and ten peptide sequences of ovalbumin to have a better rank score as compared to the identified core sequences.
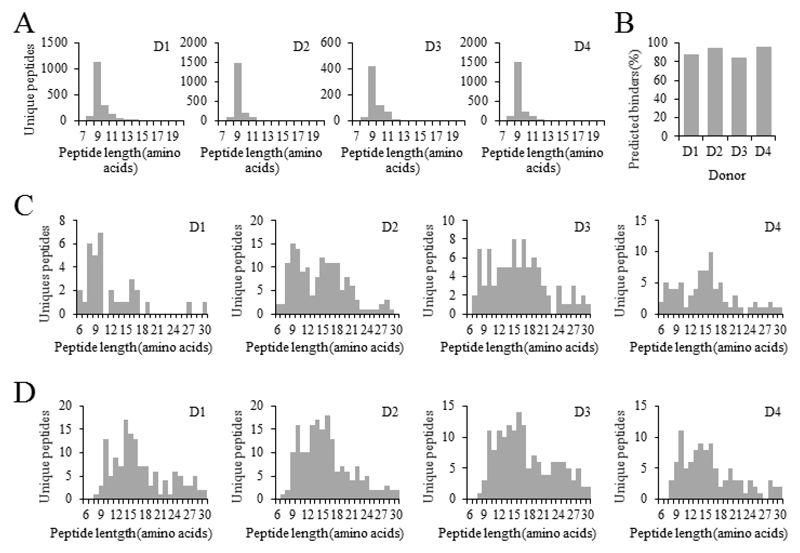
We further investigated on the possibility to elute peptides from sHLA-II complexes present in circulation [6], in analogy to our previous report on sHLA-class I peptides isolated from serum and plasma [30, 31]. Based on the low blood concentration of HLA class II complexes, we expected to be able to sequence very few peptides. Therefore, we first validated the compatibility of the obtained plasma preparations from four donors by purifying their sHLA-I complexes [Supporting Information Table 1 for the HLA typing of the four donors]. Reassuringly, results were comparable with our previous findings, resulting in between 702 and 2084 peptide sequences from 2.5-3 ml of plasma [Figure 2A]. Moreover, the quality of HLA class I preparations was high, with between 87.9 and 96.6% of all identified 8-11mers being predicted to bind the respective HLA alleles of the donors by NetMHCpan 3.0 [Figure 2B].
Figure 2. Analysis of the soluble HLA class I and class II complexes from plasma.
(A) Length distribution of sHLA-I peptides isolated from 4 donors (D1 to D4). The number of peptides between 7 and 20 amino acids is plotted in relation to their length. (B) NetMHCpan 3.0 binding prediction of 8 to 11-mers identified from plasma. Peptides were annotated as being predicted to bind if the rank received from NetMHCpan was below 2% for at least one of the alleles of the respective donor. (C, D) Length distribution of sHLA-II peptides isolated from plasma. sHLA-II DR peptides (C) or sHLA-II DQ peptides (D) were isolated from 2.5-3 mL of plasma from four donors (D1 to D4). The number of peptides between 6 and 30 amino acids is plotted in relation to their length.
From 2.5-3 ml of plasma of the four donors, 34, 152, 91, and 77 putative HLA DR-derived peptide sequences were identified [Supporting Information Table 6 for a complete list of peptide identifications]. Peptide length distributions presented in Figure 2C demonstrate enrichment of longer peptide sequences as compared to class I. After removing obvious contaminants such as Keratin, Fibrinogen and other commonly identified sequences, we found 26 high-quality peptides from 14 proteins [Table 1]. Of these, five were previously identified from human thymus samples by Collado and colleagues [39], 8 were identified from monocyte-derived dendritic cells by Ciudad and colleagues [40], seven were identified from Maver-1 cells, and three from DOHH2 cells. Further, HLA DR alleles of the donors were typically in line with the sample from which the same peptide sequence was previously identified [Table 1].
Table I. Putative HLA DR-derived peptide sequences identified from plasma of four donors (D1-D4).
| Uniprot Accession | Protein Name | Gene Name | Aligned Peptide Sequence | D1 D2 D3 D4 | Collado1 | Ciudad2 | Maver-1 | DOHH2 |
|---|---|---|---|---|---|---|---|---|
| P01023 | Alpha-2-macroglobulin | A2M | SSKFQVDNNNRLL | X | Donor A, B (DRB1*03:01) | |||
| P01833 | Polymeric immunoglobulin receptor | PIGR |
ASVDSGSSEEQGGSSRA –SVDSGSSEEQGGSSRA |
X X |
||||
| P02808 | Statherin | STATH |
DSSEEKFL– DSSEEKFLR |
X X |
||||
| P04406 | Glyceraldehyde-3-phosphate dehydrogenase | GAPDH |
GKVKVGVNGFGRIGR– GKVKVGVNGFGRIGRL |
X X X |
X | |||
| P05186 | Alkaline phosphatase, tissue-nonspecific isozyme | ALPL | AHNNYQAQSAVPLRHE | X | t1 (DRB1*01:01) | |||
| P06280 | Alpha-galactosidase A | GLA | GPRSYTIAVASLGKG | X | Donor D (DRB1*01:01) | X | X | |
| P07339 | Cathepsin D | CTSD | YTVFDRDNNRVGFAEAAR | X X | X | |||
| P07355 | Annexin A2 | ANXA2 | RDALNIETAIKTKG | X | Donor D (DRB1*01:01) | X | ||
| P11279 | Lysosome-associated membrane glycoprotein 1 | LAMP1 | LNTILPDARDPAFK | X | Donor A, B (DRB1*03:01) | |||
| P17936 | Insulin-like growth factor-binding protein 3 | IGFBP3 |
HSKIIIIKKGHAKD– – HSKIIIIKKGHAKDSQ |
X X X |
t4 (DRB1*03:01) | |||
| P21333 | Filamin-A | FLNA |
EETVITVDTKAAGKGK –ETVITVDTKAAGKGK |
X X |
Donor A (DRB1*03:01, DRB1*11:01) | |||
| P61769 | Beta-2-microglobulin | B2M |
RTPKIQVYSRHPAENGK –TPKIQVYSRHPAE– – – –TPKIQVYSRHPAEN– – –TPKIQVYSRHPAENGK |
X X X X |
t5 (DRB1*11:04) |
Donor B (DRB1*01:01) |
X X X X |
|
| Q02413 | Desmoglein-1 | DSG1 |
VATDLDTGRPSTTVR– VATDLDTGRPSTTVRY |
X X |
||||
| Q9H3G5 | Probable serine carboxypeptidase CPVL | CPVL |
AGKYVPAIAHLIH– AGKYVPAIAHLIHS – –KYVPAIAHLIH– – –KYVPAIAHLIHS |
X X X X |
t1 (DRB1*01:01) t2 (DRB1*01:01) |
Donor G (DRB1*07:01, DRB1*15:01) Multiple Donors |
X |
After HLA DQ purification, 180, 100, 154, and 137 peptide sequences could be identified from plasma of the four donors, respectively [Supporting Information Table 7 for a complete list of peptide identifications]. Peptide length distributions presented in Figure 2D demonstrate enrichment of peptides with an average length of around 15 amino acids. After removing most obvious contaminants, 34 peptide sequences from 16 proteins remained [Table 2] of which 16 were previously identified by Bergseng and colleagues [14], indicating that they can be presented on DQ complexes.
Table II. Putative HLA DQ-derived peptide sequences identified from plasma of four donors (D1-D4).
| Uniprot Accession | Protein Name | Gene Name | Aligned Peptide Sequence | D1 D2 D3 D4 | Bergseng1 |
|---|---|---|---|---|---|
| O15127 | Secretory carrier-associated membrane protein 2 | SCAMP2 |
TFHRAASSAAQGAFQGN – –HRAASSAAQGAFQGN |
X X |
X |
| O95810 | Caveolae-associated protein 2 | CAVIN2 |
TKIVSVERREKIK– – – TKIVSVERREKIKKSL |
X X X |
|
| RPPGFSPFR | X X | ||||
| P01127 | Platelet-derived growth factor subunit B | PDGFB |
HTHDKTALKETLG– HTHDKTALKETLGA |
X X X |
|
| P01903 | HLA class II histocompatibility antigen, DR alpha chain | HLA- DRA |
ASFEAQGALANIAVDK– ASFEAQGALANIAVDKA – –SFEAQGALANIAVDK– – – –EAQGALANIAVDK– – – –EAQGALANIAVDKA |
X X X X X X X |
X X X X X |
| TGVSETVFLPREDH | X | X | |||
| P04114 | Apolipoprotein B-100 | APOB |
KTTKQSFDLSVKAQYKKNKHRHS –TTKQSFDLSVKAQYKKNKHRHS |
X X |
|
| P04233 | HLA class II histocompatibility antigen gamma chain | CD74 | KPPKPVSKMRMATPLLMQA | X | X |
| P04406 | Glyceraldehyde-3-phosphate dehydrogenase | GAPDH |
GKVKVGVNGFGRIGR– GKVKVGVNGFGRIGRL |
X X X |
|
| P05019-2 | Isoform 2 of Insulin-like growth factor I | IGF1 |
HTDMPKTQKEVHLKNASRGSAGNKN– – – HTDMPKTQKEVHLKNASRGSAGNKNY– – HTDMPKTQKEVHLKNASRGSAGNKNYRM |
X X X X X X |
|
| P07339 | Cathepsin D | CTSD | YTVFDRDNNRVGFAEAAR | X X X | X |
| P10124 | Serglycin | SRGN | SLDRNLPSDSQDLGQHGLEED | X | X |
| P16109 | P-selectin | SELP |
HLGTYGVFTNAA– – – – – HLGTYGVFTNAAFDPSP |
X X X |
|
| P21796 | Voltage-dependent anion-selective channel protein 1 | VDAC1 | AGGHKLGLGLEFQA | X | X |
| P30443 | HLA class I histocompatibility antigen, A-1 alpha chain | HLA-A |
EDLRSWTAADMAAQ– EDLRSWTAADMAAQI |
X X |
X |
| P30460 | HLA class I histocompatibility antigen, B-8 alpha chain | HLA-B |
EDLRSWTAADTAAQ– – EDLRSWTAADTAAQI– EDLRSWTAADTAAQIT |
X X X |
X X X |
| P45880 | Voltage-dependent anion-selective channel protein 2 | VDAC2 | AGGHKVGLALELEA | X | X |
| Q05682 | Caldesmon | CALD1 |
STHQAAIVSKIDSRLE – –HQAAIVSKIDSRLE |
X X X X X X X X |
Peptide sequence identified by Bergseng et al. [14]
To increase the sensitivity and reproducibility of HLA class II peptidome analyses, an attractive strategy might be the application of data-independent acquisition (DIA) workflows as this significantly increased the sensitivity and reproducibility of HLA class I peptidome analyses [35]. We therefore designed a DIA setup [Supporting Information Table 2] with 21 windows between 380 and 1010 m/z with a fixed size of 30 Da, the area in which most peptides with charge state 2, 3, 4 and 5 were present [Figure 3A]. Applying DR- and DQ-specific spectral libraries for Maver-1 and DOHH2 cells to DIA analyses, more than 90% of the library peptides could be recovered with Skyline [Figure 3B and Supporting Information Table 3 for Skyline settings]. The DIA analyses resulted in the identification of a total of 5939 DR peptides and 2505 DQ peptides for Maver-1 cells with overlaps between triplicates of 88.52% and 94.85%, respectively [Figure 3C left panels]. For the DOHH2 cell line, 3157 DR peptides and 481 DQ peptides were identified, with overlaps of 93.19% and 91.06% between triplicates [Figure 3C right panels]. We then evaluated on the sensitivity of the workflow by purifying HLA class II DR peptides from Maver-1 lysate corresponding to 100, 25, 10, 5, and 1 million cells. The DIA workflow increased the identification rate in all samples, with the strongest effect observed for samples with lower input [Figure 3D]. Using the DIA workflow, up to an average of 1552 peptides could be identified from 1 million cells, more than 4 fold the number of identifications as from DDA samples.
Figure 3. DIA analysis of HLA DR and DQ peptidomes of Maver-1 and DOHH2 cells.
(A) Precursor mass distribution from a triplicate DDA analysis of the Maver-1 HLA DR peptidome. The m/z distributions of all peptides with charge state 2 (z=2, solid black), 3 (z=3, solid grey), 4 (z=4, dotted grey) and 5 (z=5, dotted black) were plotted. (B) Library generation and DIA analysis of Maver-1 and DOHH2 cells. Spectral libraries were created using SpectraST based on SEQUEST results filtered to 1% FDR with Percolator from triplicate DR and DQ DDA analyses of Maver-1 and DOHH2 cells. DR and DQ peptidomes from Maver-1 and DOHH2 cells were acquired in DIA mode in triplicates and analysed using Skyline. The graph displays the number of unique entries in the spectral libraries (light grey) and the number of peptides identified in a triplicate DIA analysis with an mProphet q-value below 0.01 (dark grey). (C) Reproducibility of Maver-1 and DOHH2 DIA peptidome analysis. Triplicate DIA DR and DQ peptidome analyses of Maver-1 and DOHH2 cells were analysed using Skyline and filtered to 1% FDR with mProphet. Euler-Venn diagrams display the overlap of peptide identifications between replicates. (D) Comparison of sensitivity between DDA and DIA with varying input cell numbers. DR peptidomes were isolated from Maver-1 lysate corresponding to 1, 5, 10, 25 and 100 million Maver-1 cells. Samples were acquired in either DDA or DIA mode in triplicates. DDA data was analyzed with SEQUEST and results filtered for 1% FDR with Percolator. DIA data was analyzed with Skyline using the Maver-1 DR library described in (B) and the FDR was estimated with mProphet and set to 1% at peptide precursor level. The graph shows the means and corresponding standard deviations of unique peptides identified in DDA mode (dark grey) and DIA mode (light grey) for the different input amounts.
4. Discussion
The definition of HLA-specific motifs in high resolution is important as they facilitate evaluation and prediction if a given peptide can be presented by certain HLA complexes. Such strategies have been implemented for the design of cancer vaccines [11–13] and for prediction of immunogenicity of biologic drugs [41]. For HLA class I, large databases of HLA-specific motifs have been collected and are publicly available [42, 43]. In contrast, few HLA class II-specific motifs have been defined in high resolution, including DRB1*01:01, DRB1*15:01, DRB1*08:01, DRB1*10:01, DRB5*01:01 [17, 18, 44, 45]. One difficulty relates to the nature of the class II peptides, which feature a central core and overlapping ends. This obviously complicates deconvolution of binding motifs if more than two alleles are present, especially when motifs are similar (such as DRB1*01:01 and DRB5*01:01). This is exemplified by the fact that Gibbs cluster failed to reveal the three allele-specific motifs expected for Maver-1 and DOHH2 cells, while it usually separates three to five distinct clusters for HLA class I data [30]. This is even more problematic for HLA DQ, where the only available dataset with acceptable number of peptides was only recently presented [14]. The DQ-specific motifs reported so far are however not very specific, and do not feature the typical setup of anchor residues at position P1, P4, P6, P9, typically observed for DR alleles, thereby raising doubt on their quality and validity. If Gibbs cluster was able to identify the real motifs from our HLA DQ preparations is very difficult to evaluate due to the scarcity of HLA-DQ data. Our cell lines did not share any of the HLA-DQB alleles with the ones studied by Bergseng and colleagues [14], while, the reported motifs are comparable as such that they are less defined in terms of their anchor residues. In the future, it will be important to study more mono-allelic cell lines, reanalyze existing data with specialized bioinformatics strategies and evaluate the influence of different DQB1-DQA1 combinations, as it is believed that DQ cis- and trans-pairing can occur [46].
The pipeline for the identification of thousands of high confidence peptides eluted from HLA DR and DQ complexes was subsequently applied to the study of blood-soluble HLA-II complexes resulting in the detection of between 34 and 180 sequences. Due to the low concentration of class II complexes as compared to class I complexes, the low number of identifications is not surprising. However, we believe that we indeed were able to detect eluted peptides, as some of the peptide sequences have been previously detected from cultured cells and from thymus lysates (Table 1 and Table 2). In the future, with more sensitive mass spectrometers it is very well possible that higher numbers of peptides can be identified which might serve as biomarkers for inflammatory diseases such as arthritis, multiple sclerosis or inflammatory bowel disease.
Finally, we implemented DIA of HLA class II peptidomes following initial work on HLA class I. We could demonstrate that also for HLA class II, DIA methods increase the sensitivity and reproducibility of the resulting peptidomes significantly. DIA strategies will allow to identify and quantify disease relevant antigen in scarce clinical samples such as bronchoalveolar lavage fluid [47], or similar biopsy material [39, 40], with the goal to develop tolerance inducing therapeutic strategies.
In summary, we isolated and identified human HLA class II DR and DQ peptides leading to the precise definition of HLA-specific motifs allowing the prediction of peptide presentation on respective alleles. We further investigated on the presence of sHLA-II complexes in blood and could for the first time demonstrate that the complexes are still associated with peptide ligands. We finally established a DIA methodology resulting in the reliable and sensitive definition of HLA class II peptidomes, probably applicable to a range of biological questions. Given the importance of HLA class II peptides in modulating immune responses, e.g. by cancer vaccines [11], and in autoimmune condition [7], it is imperative to expand current efforts to the definition of additional HLA class II-specific motifs, allowing the development of refined binding prediction tools and of personalized therapeutic strategies.
Supplementary Material
Significance of the study.
Peptides displayed on HLA class II complexes on the surface of antigen presenting cells play a central role in the activation of CD4+ T cells and as such are of fundamental importance for anti-cancer and anti-pathogen immunity as well as for the maintenance of immunological tolerance. Recent studies evaluating cancer vaccination strategies observed a pronounced response only upon stimulation of CD4+ T cells. However, development of novel personalized anti-cancer vaccines targeting CD4+ T cells is hampered by the fact that HLA class II peptide binding prediction is not sufficiently established. In this study, we present strategies for the identification of HLA class II peptides from cell lines for the development of an HLA allele-specific motif-based binding predictor. Such tools may facilitate design of more efficacious cancer vaccines. We further investigate on peptides presented on blood-soluble HLA class II complexes (sHLA-II). While their function is believed to contribute to immunological tolerance, it was unknown if they are still bound to cognate antigen, or if the peptide is rapidly lost. Identifying sHLA-II peptides may allow to study their function and may allow to identify biomarkers of HLA class II-associated diseases such as multiple sclerosis or rheumatoid arthritis.
Acknowledgements
We thank Camilla Bacci (Philogen S.p.A) for providing W6/32 antibody, Tina Frauknecht (Philochem AG) for providing L243 and SPVL3 antibody and Chiara Libbra (Philogen S.p.A) for support with the implementation of SOPs.
Funding: This work was supported financially by ETH Zürich, the Swiss National Science Foundation, the European Union’s Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 305309 (PRIAT) and no. 305608 (EURenOmics) and by the European Research Council (ERC advanced grant “ZAUBERKUGEL” to DN and “OSAI” to PR).
Abbreviations
- HLA
human leukocyte antigen
- DDA
data-dependent acquisition
- DIA
data-independent acquisition
Footnotes
Conflicts of Interest: Dario Neri is co-founder of Philogen, shareholder and member of the board. Tim Fugmann and Danilo Ritz are employees of Philochem AG. Emiliano Sani is an employee of Philogen S.p.A. The authors declare no additional conflict of interest.
References
- [1].Unanue ER, Turk V, Neefjes J. Variations in MHC Class II Antigen Processing and Presentation in Health and Disease. Annu Rev Immunol. 2016;34:265–297. doi: 10.1146/annurev-immunol-041015-055420. [DOI] [PubMed] [Google Scholar]
- [2].Adamopoulou E, Tenzer S, Hillen N, Klug P, Rota IA, Tietz S, Gebhardt M, Stevanovic S, Schild H, Tolosa E, Melms A, et al. Exploring the MHC-peptide matrix of central tolerance in the human thymus. Nat Commun. 2013;4:2039. doi: 10.1038/ncomms3039. [DOI] [PubMed] [Google Scholar]
- [3].Haabeth OA, Tveita AA, Fauskanger M, Schjesvold F, Lorvik KB, Hofgaard PO, Omholt H, Munthe LA, Dembic Z, Corthay A, Bogen B. How Do CD4(+) T Cells Detect and Eliminate Tumor Cells That Either Lack or Express MHC Class II Molecules? Front Immunol. 2014;5:174. doi: 10.3389/fimmu.2014.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Muller-Hilke B. HLA class II and autoimmunity: epitope selection vs differential expression. Acta Histochem. 2009;111:379–381. doi: 10.1016/j.acthis.2008.11.011. [DOI] [PubMed] [Google Scholar]
- [5].Aultman D, Adamashvili I, Yaturu K, Langford M, Gelder F, Gautreaux M, Ghali GE, McDonald J. Soluble HLA in human body fluids. Hum Immunol. 1999;60:239–244. doi: 10.1016/s0198-8859(98)00122-0. [DOI] [PubMed] [Google Scholar]
- [6].Jendro M, Goronzy JJ, Weyand CM. Structural and functional characterization of HLA-DR molecules circulating in the serum. Autoimmunity. 1991;8:289–296. doi: 10.3109/08916939109007636. [DOI] [PubMed] [Google Scholar]
- [7].Clemente-Casares X, Blanco J, Ambalavanan P, Yamanouchi J, Singha S, Fandos C, Tsai S, Wang J, Garabatos N, Izquierdo C, Agrawal S, et al. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature. 2016;530:434–440. doi: 10.1038/nature16962. [DOI] [PubMed] [Google Scholar]
- [8].Bakela K, Kountourakis N, Aivaliotis M, Athanassakis I. Soluble MHC-II proteins promote suppressive activity in CD4+ T cells. Immunology. 2015;144:158–169. doi: 10.1111/imm.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, Wunderlich JR, Somerville RP, Hogan K, Hinrichs CS, Parkhurst MR, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–645. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].van der Burg SH, Arens R, Ossendorp F, van Hall T, Melief CJ. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat Rev Cancer. 2016;16:219–233. doi: 10.1038/nrc.2016.16. [DOI] [PubMed] [Google Scholar]
- [11].Kreiter S, Vormehr M, van de Roemer N, Diken M, Lower M, Diekmann J, Boegel S, Schrors B, Vascotto F, Castle JC, Tadmor AD, et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature. 2015;520:692–696. doi: 10.1038/nature14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Lower M, Bukur V, Tadmor AD, Luxemburger U, Schrors B, Omokoko T, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547:222–226. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- [13].Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, Zhang W, Luoma A, Giobbie-Hurder A, Peter L, Chen C, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547:217–221. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bergseng E, Dorum S, Arntzen MO, Nielsen M, Nygard S, Buus S, de Souza GA, Sollid LM. Different binding motifs of the celiac disease-associated HLA molecules DQ2.5, DQ2.2, and DQ7.5 revealed by relative quantitative proteomics of endogenous peptide repertoires. Immunogenetics. 2015;67:73–84. doi: 10.1007/s00251-014-0819-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mommen GP, Marino F, Meiring HD, Poelen MC, van Gaans-van den Brink JA, Mohammed S, Heck AJ, van Els CA. Sampling From the Proteome to the Human Leukocyte Antigen-DR (HLA-DR) Ligandome Proceeds Via High Specificity. Mol Cell Proteomics. 2016;15:1412–1423. doi: 10.1074/mcp.M115.055780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sofron A, Ritz D, Neri D, Fugmann T. High-resolution analysis of the murine MHC class II immunopeptidome. Eur J Immunol. 2016;46:319–328. doi: 10.1002/eji.201545930. [DOI] [PubMed] [Google Scholar]
- [17].Clement CC, Becerra A, Yin L, Zolla V, Huang L, Merlin S, Follenzi A, Shaffer SA, Stern LJ, Santambrogio L. The Dendritic Cell Major Histocompatibility Complex II (MHC II) Peptidome Derives from a Variety of Processing Pathways and Includes Peptides with a Broad Spectrum of HLA-DM Sensitivity. J Biol Chem. 2016;291:5576–5595. doi: 10.1074/jbc.M115.655738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ooi JD, Petersen J, Tan YH, Huynh M, Willett ZJ, Ramarathinam SH, Eggenhuizen PJ, Loh KL, Watson KA, Gan PY, Alikhan MA, et al. Dominant protection from HLA-linked autoimmunity by antigen-specific regulatory T cells. Nature. 2017;545:243–247. doi: 10.1038/nature22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fugmann T, Sofron A, Ritz D, Bootz F, Neri D. The MHC Class II Immunopeptidome of Lymph Nodes in Health and in Chemically Induced Colitis. J Immunol. 2017;198:1357–1364. doi: 10.4049/jimmunol.1601157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zamo A, Ott G, Katzenberger T, Adam P, Parolini C, Scarpa A, Lestani M, Menestrina F, Chilosi M. Establishment of the MAVER-1 cell line, a model for leukemic and aggressive mantle cell lymphoma. Haematologica. 2006;91:40–47. [PubMed] [Google Scholar]
- [21].Lampson LA, Levy R. Two populations of Ia-like molecules on a human B cell line. J Immunol. 1980;125:293–299. [PubMed] [Google Scholar]
- [22].Robbins PA, Evans EL, Ding AH, Warner NL, Brodsky FM. Monoclonal antibodies that distinguish between class II antigens (HLA-DP, DQ, and DR) in 14 haplotypes. Hum Immunol. 1987;18:301–313. doi: 10.1016/0198-8859(87)90077-2. [DOI] [PubMed] [Google Scholar]
- [23].Gorga JC, Horejsi V, Johnson DR, Raghupathy R, Strominger JL. Purification and characterization of class II histocompatibility antigens from a homozygous human B cell line. J Biol Chem. 1987;262:16087–16094. [PubMed] [Google Scholar]
- [24].Spits H, Borst J, Giphart M, Coligan J, Terhorst C, De Vries JE. HLA-DC antigens can serve as recognition elements for human cytotoxic T lymphocytes. Eur J Immunol. 1984;14:299–304. doi: 10.1002/eji.1830140404. [DOI] [PubMed] [Google Scholar]
- [25].Bontrop RE, Schreuder GM, Mikulski EM, van Miltenburg RT, Giphart MJ. Polymorphisms within the HLA-DR4 haplotypes. Various DQ subtypes detected with monoclonal antibodies. Tissue Antigens. 1986;27:22–31. [PubMed] [Google Scholar]
- [26].Peretti M, Villard J, Barras E, Zufferey M, Reith W. Expression of the three human major histocompatibility complex class II isotypes exhibits a differential dependence on the transcription factor RFXAP. Mol Cell Biol. 2001;21:5699–5709. doi: 10.1128/MCB.21.17.5699-5709.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mahon NG, Madden BP, Caforio AL, Elliott PM, Haven AJ, Keogh BE, Davies MJ, McKenna WJ. Immunohistologic evidence of myocardial disease in apparently healthy relatives of patients with dilated cardiomyopathy. J Am Coll Cardiol. 2002;39:455–462. doi: 10.1016/s0735-1097(01)01762-4. [DOI] [PubMed] [Google Scholar]
- [28].Long HM, Haigh TA, Gudgeon NH, Leen AM, Tsang CW, Brooks J, Landais E, Houssaint E, Lee SP, Rickinson AB, Taylor GS. CD4+ T-cell responses to Epstein-Barr virus (EBV) latent-cycle antigens and the recognition of EBV-transformed lymphoblastoid cell lines. J Virol. 2005;79:4896–4907. doi: 10.1128/JVI.79.8.4896-4907.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gudgeon NH, Taylor GS, Long HM, Haigh TA, Rickinson AB. Regression of Epstein-Barr virus-induced B-cell transformation in vitro involves virus-specific CD8+ T cells as the principal effectors and a novel CD4+ T-cell reactivity. J Virol. 2005;79:5477–5488. doi: 10.1128/JVI.79.9.5477-5488.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ritz D, Gloger A, Weide B, Garbe C, Neri D, Fugmann T. High-sensitivity HLA class I peptidome analysis enables a precise definition of peptide motifs and the identification of peptides from cell lines and patients' sera. Proteomics. 2016;16:1570–1580. doi: 10.1002/pmic.201500445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ritz D, Gloger A, Neri D, Fugmann T. Purification of soluble HLA class I complexes from human serum or plasma deliver high quality immuno peptidomes required for biomarker discovery. Proteomics. 2017;17 doi: 10.1002/pmic.201600364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Andreatta M, Alvarez B, Nielsen M. GibbsCluster: unsupervised clustering and alignment of peptide sequences. Nucleic Acids Res. 2017 doi: 10.1093/nar/gkx248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nielsen M, Andreatta M. NetMHCpan-3.0; improved prediction of binding to MHC class I molecules integrating information from multiple receptor and peptide length datasets. Genome Med. 2016;8:33. doi: 10.1186/s13073-016-0288-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Thomsen MC, Nielsen M. Seq2Logo: a method for construction and visualization of amino acid binding motifs and sequence profiles including sequence weighting, pseudo counts and two-sided representation of amino acid enrichment and depletion. Nucleic Acids Res. 2012;40:W281–287. doi: 10.1093/nar/gks469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ritz D, Kinzi J, Neri D, Fugmann T. Data Independent Acquisition of HLA Class I Peptidomes on the Q Exactive Mass Spectrometer Platform. Proteomics. 2017 doi: 10.1002/pmic.201700177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lam H, Deutsch EW, Eddes JS, Eng JK, King N, Stein SE, Aebersold R. Development and validation of a spectral library searching method for peptide identification from MS/MS. Proteomics. 2007;7:655–667. doi: 10.1002/pmic.200600625. [DOI] [PubMed] [Google Scholar]
- [37].MacLean B, Tomazela DM, Shulman N, Chambers M, Finney GL, Frewen B, Kern R, Tabb DL, Liebler DC, MacCoss MJ. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics. 2010;26:966–968. doi: 10.1093/bioinformatics/btq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanovic S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics. 1999;50:213–219. doi: 10.1007/s002510050595. [DOI] [PubMed] [Google Scholar]
- [39].Collado JA, Alvarez I, Ciudad MT, Espinosa G, Canals F, Pujol-Borrell R, Carrascal M, Abian J, Jaraquemada D. Composition of the HLA-DR-associated human thymus peptidome. Eur J Immunol. 2013;43:2273–2282. doi: 10.1002/eji.201243280. [DOI] [PubMed] [Google Scholar]
- [40].Ciudad MT, Sorvillo N, van Alphen FP, Catalan D, Meijer AB, Voorberg J, Jaraquemada D. Analysis of the HLA-DR peptidome from human dendritic cells reveals high affinity repertoires and nonconventional pathways of peptide generation. J Leukoc Biol. 2017;101:15–27. doi: 10.1189/jlb.6HI0216-069R. [DOI] [PubMed] [Google Scholar]
- [41].Hamze M, Meunier S, Karle A, Gdoura A, Goudet A, Szely N, Pallardy M, Carbonnel F, Spindeldreher S, Mariette X, Miceli-Richard C, et al. Characterization of CD4 T Cell Epitopes of Infliximab and Rituximab Identified from Healthy Donors. Front Immunol. 2017;8:500. doi: 10.3389/fimmu.2017.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bassani-Sternberg M, Chong C, Guillaume P, Solleder M, Pak H, Gannon PO, Kandalaft LE, Coukos G, Gfeller D. Deciphering HLA-I motifs across HLA peptidomes improves neo-antigen predictions and identifies allostery regulating HLA specificity. PLoS Comput Biol. 2017;13:e1005725. doi: 10.1371/journal.pcbi.1005725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Rapin N, Hoof I, Lund O, Nielsen M. MHC motif viewer. Immunogenetics. 2008;60:759–765. doi: 10.1007/s00251-008-0330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Muixi L, Gay M, Munoz-Torres PM, Guitart C, Cedano J, Abian J, Alvarez I, Jaraquemada D. The peptide-binding motif of HLA-DR8 shares important structural features with other type 1 diabetes-associated alleles. Genes Immun. 2011;12:504–512. doi: 10.1038/gene.2011.26. [DOI] [PubMed] [Google Scholar]
- [45].Scholz EM, Marcilla M, Daura X, Arribas-Layton D, James EA, Alvarez I. Human Leukocyte Antigen (HLA)-DRB1*15:01 and HLA-DRB5*01:01 Present Complementary Peptide Repertoires. Front Immunol. 2017;8:984. doi: 10.3389/fimmu.2017.00984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Charron DJ, Lotteau V, Turmel P. Hybrid HLA-DC antigens provide molecular evidence for gene trans-complementation. Nature. 1984;312:157–159. doi: 10.1038/312157a0. [DOI] [PubMed] [Google Scholar]
- [47].Heyder T, Kohler M, Tarasova NK, Haag S, Rutishauser D, Rivera NV, Sandin C, Mia S, Malmstrom V, Wheelock AM, Wahlstrom J, et al. Approach for Identifying Human Leukocyte Antigen (HLA)-DR Bound Peptides from Scarce Clinical Samples. Mol Cell Proteomics. 2016;15:3017–3029. doi: 10.1074/mcp.M116.060764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.