Abstract
Circadian rhythms are present in most leaving organisms and these rhythms are not just a consequence of the day/night fluctuation, but rather they are generated by endogenous biological clocks with a periodicity of about 24 hrs. In mammals, the master pacemaker of circadian rhythms is localized in the suprachiasmatic nuclei of the hypothalamus (SCN). The SCN controls circadian rhythms in peripheral organs. The retina also contains circadian clocks which regulate many aspects of retinal physiology, independently of the SCN. Emerging experimental evidence indicates that the retinal circadian clocks also affect ocular health and a few studies have now demonstrated that disruption of retinal clocks may contribute to the development of retinal diseases. Our study indicates that in mice lacking the clock gene Bmal1 photoreceptor viability during aging is significantly reduced. Bmal1 knock-out mice at 8-9 months of age have 20-30% less nuclei in the outer nuclear layer. No differences were observed in the other retinal layers. Our study suggests that the retinal circadian clock is an important modulator of photoreceptor health.
XX.1 Introduction
Circadian rhythms have been observed in animals, plants, fungi and even cyanobacteria. In mammals, including humans, the master pacemaker controlling 24-hour rhythms is localized in the suprachiasmatic nuclei of the hypothalamus (SCN). The SCN is responsible for orchestrating circadian clocks in peripheral organs to regulate physiological functions such as behavior, sleep, body temperature, blood pressure and hormone release (Herzog and Tosini 2001). Accumulating evidence indicates that dysfunction of the circadian rhythms due to genetic mutations or environmental factors (i.e., jet-lag or shift work) may contribute to the development of many serious diseases, including cancer and type-2 diabetes (Evans and Davidson 2013).
The retinal circadian clock was the first extra-SCN circadian oscillator to be discovered in mammals (Tosini and Menaker 1996). The molecular clockwork mechanism of the retinal clock is similar to what has been reported for the SCN (Tosini et al. 2008), albeit it appears that the retinal clock is less robust (Ruan et al. 2012; Jaeger et al. 2015). Several studies have also established that many aspects of retinal physiology and function are under the control of retinal circadian clocks (see McMahon et al. 2014 for a review) and new experimental evidence suggests that other ocular structures (e.g., cornea, retinal pigment epithelium) also possess circadian clocks that control important physiological functions (Yoo et al. 2005; Baba et al. 2010; Baba et al. 2015, Buhr et al. 2015). Interestingly, as seen in the SCN, it appears that the neural retina communicates the photic information to the other ocular structures via humoral signals (e.g., melatonin and dopamine, Ruan et al. 2008, Baba et al. 2015) since most of these ocular structures are not capable of direct light transduction (Baba et al. 2010).
XX.2 The Retinal Circadian Clock and Ocular health
Similar to molecular circadian clock in SCN, the retinal clock also consists of auto-regulatory transcriptional/translational negative feedback loops involving several clock genes and their protein products which generate approximately 24 hours cycle. The primary core loop involves two basic helix-loop-helix-PAS domain transcription factors, BMAL1 and CLOCK, which heterodimerize and bind to E-box elements in promoter region to enhance transcription of Period 1 and 2, and Cryptochrome 1and 2. The protein products, PERIOD and CRYPTOCHROME together then inhibit their own transcription by blocking CLOCK/BMAL1-mediated transactivation (see Tosini et al. 2008, Figure 1 for a schematic illustration). The second feedback loop involves the negative and positive transactivation of five other genes, Rev-erb α, β and Ror α, β, c via REV-ERB/ROR response element (RRE) promoter elements in promoter regions. REV-ERB as a negative element inhibits Clock and Bmal1 transcription whereas ROR as a positive element promotes Clock and Bmal1 transcription. The transcriptions of Rev-erbs and Rors are regulated via E-box elements in their promoter regions. REV-ERBs and RORs compete for binding to RRE in the Bmal1 promoter regions to regulate rhythmic expressions of Bmal1. These intertwining oscillation signals also regulate transcription of other clock controlled genes via E-box or RRE elements, and the products of these genes serve as circadian clock outputs (Takahashi et al. 2008). In the mouse, clock genes are rhythmically expressed in the different retinal layers (Hiragaki et al. 2014). In the photoreceptor layer only the cones appear to express all the circadian clock proteins (Lui et al. 2012).
Fig. 1.
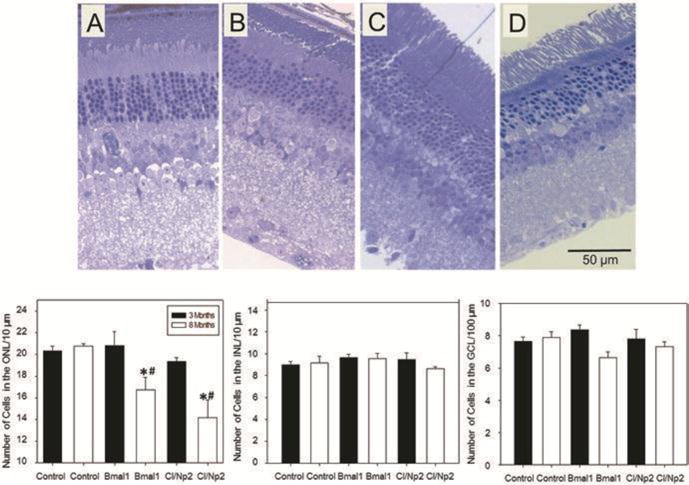
Morphometric analysis of retinae obtained from young (3 months old, black bars) and old (8 months old, white bars) of control, Bmal1−/− and Clock−/−/Npas2−/− (Cl/Np2) mice. The number of cells in the ONL of 8 months old Bmal1 (B) and Clock/Npas2 KOs (D) were significantly lower than the number of cells in 3 months old mice (A, C) of the same genotype (One-way ANOVA following post hoc test * p< 0.05) and age matched control group (# p<0.05). No differences in the number of cells were observed in the INL and/or GCL (n= 3-4). The number of cells in the ONL of young Bmal1 and Clock/Npas2 KO mice was not different from the number of cells in control mice of the same genotype (C57BL/6). The microphotographs in A, B, C and D represent a typical example of a section obtained from 3 months old Bmal1 KO (A), 8 months old Bmal1 KO (B), 3 months old Clock/Npas2 KO (C) and 8 months old Clock/Npas2 KO (D).
Emerging evidence suggests that retinal circadian clocks and their output signals contribute to retinal disease and pathology, as well as normal retinal function. For example, diabetic retinopathy is associated with reduced clock gene expression in the retina (Busik et al. 2009), circadian disruption recapitulates diabetic retinopathy in mice (Bhatwadekar et al. 2013), and removal of Period2 induces dysfunction in the retinal microvasculature (Jadhav et al. 2016).
Trophic signaling by the retinal clock and its outputs seem to play a role in the regulation of eye growth and refractive errors (reviewed in: Stone et al. 2013). A recent study has also reported that mice lacking Period1 and Period2 show significant alteration in the distribution of cone photoreceptors (Ait-Hmyed et al. 2013) and mice lacking Rev-erb α show a significant alteration in photoreceptor response to light (Ait-Hmyed et al. 2016).
Finally, it is worthwhile mentioning that the retinal clock influences the susceptibility of photoreceptors to light induced damage (Organisciak et al. 2000) and recent genomic studies have also implicated the clock genes Rev-erbα and Rora in retinal functioning (Mollema et al. 2011) and age-related macular degeneration (Jun et al. 2011).
XX.3 Bmal1 and retinal cell viability
As previously mentioned, Bmal1 gene (also known as Arntl) is a key component of the mammalian circadian clock. Bmal1 knock-out mice (Bmal1−/−) do not show any circadian rhythmicity (Bunger et al. 2000) and develop several pathologies (Kondrakov et al. 2006). Bmal1−/− mice show premature aging and their lifespan is significantly reduced (about 9 months) (Kondratov et al. 2006). In the mouse retina, Bmal1 is expressed in many cell types, (Ruan et al. 2008), but within the photoreceptor layer BMAL1 was only detected in the cones (Liu et al. 2012). Storch et al. (2007) reported that many genes (more than a thousand) show a daily rhythm in mouse retina, but a large fraction of these genes are no longer rhythmically expressed or have reduced amplitude in Bmal1−/− mice. In Bmal1−/− mice, the day/night (circadian) changes in the amplitude of the photopic b-wave are no longer present (Storch et al. 2007). The same result has been also obtained from the mice lacking Bmal1 only in the retina (Chx10-Cre-ArntlloxP/loxP mice; Storch et al. 2007), thus indicating that retinal Bmal1 is required for the circadian rhythm in visual processing. Interestingly, the photoreceptors of these mice (2-3 months) appear to be normal and unaffected by the lack of Bmal1 (Storch et al. 2007). Additional studies have reported that in mice lacking the Bmal1 gene there is a significant increase in the rate of cataract development and corneal inflammation during aging (Kondratov et al. 2006).
Previous studies have shown that the effects of circadian disruption become evident during the aging process (Baba et al. 2009; Musiek et al. 2013). Hence we decided to investigate whether removal of the Bmal1 gene affects retinal cell viability during aging. Eyes from Bmal1−/− and control mice at two different ages (3 months and 7-8 months) were obtained and then the morphometric analysis of the retina was performed according to a well-established method in our laboratory (see Baba et al. 2009 for details). As expected, and previously reported by Storch et al. (2007), young Bmal1−/− did not show any significant variation in the number of cells in the outer nuclear layer or in any other retinal layers (Figure 1) whereas in older Bmal1−/− mice we observed a significant reduction in the number of photoreceptor cell nuclei (about 20-30%) with respect to control. No changes were detected in the other retinal layers (Figure 1). Previous studies have shown that Bmal1 can interact with a large number of genes (more than 1000) and therefore the phenotypes observed in Bmal1 KOs may or may not be the consequence of a dysfunctional circadian clock (Rey et al. 2011). Thus we decided to investigate the retinal morphometry in another mouse model in which the circadian clock has been disabled. A previous investigation reported that Clock/Npas2 KO mice do not have a functional circadian clock (DeBruyne et al. 2007, Musiek et al. 2013). Eyes from young (3months) and old (9 months) Clock/Npas2 KO mice were obtained from Dr. David Weaver’s laboratory (University of Massachusetts Medical School) and the retinal morphometry was investigated using the same method mentioned for Bmal1−/− mice retinas. As shown in Figure 1, Clock/Npas2 KO mice showed an almost identical phenotype as Bmal1−/− animals. The fact that almost identical results were obtained in Bmal1 and Clock/Npas2 KOs indicates that the reduced photoreceptor viability observed in Bmal1−/− is likely due lack of a functional circadian clock in these cells and not to a possible pleiotropic effect of Bmal1. Our preliminary data indicate that dysfunctions of circadian clock genes may affect the photoreceptor cell viability during aging.
XX.4 Conclusions
The circadian clock is responsible for a wide variety of physiological functions and a large number of studies have now demonstrated that retinal circadian clocks regulate many functions in the eye. Experimental evidence also suggests that dysfunction of the retinal clocks may promote ocular diseases. In addition, our preliminary data indicate that genetic removal of clock genes affects photoreceptor viability during aging. Further studies are required to fully understand the role of circadian clock and its associated gene product in the regulation of retinal function and health.
Acknowledgments
Research in the author’s laboratories is supported by NIH grants GM116760 to KB, EY018640 to CR, R01EY004864 and P30EY006360 to PMI, EY022216, EY026291 to GT, and an unrestricted departmental grant from Research to Prevent Blindness (Emory Department of Ophthalmology).
References
- Ait-Hmyed HO, Felder-Schmittbuhl MP, Garcia-Garrido M, et al. Mice lacking Period 1 and Period 2 circadian clock genes exhibit blue cone photoreceptor defects. Eur J Neurosci. 2013;37:1048–60. doi: 10.1111/ejn.12103. [DOI] [PubMed] [Google Scholar]
- Ait-Hmyed HO, Acar N, Savier E, et al. Rev-Erbα modulates retinal visual processing and behavioral responses to light. FASEB J. 2016 doi: 10.1096/fj.201600414R. pii: fj.201600414R. [DOI] [PubMed] [Google Scholar]
- Baba K, Pozdeyev N, Mazzoni F, et al. Melatonin modulates visual function and cell viability in the mouse retina via the MT1 melatonin receptor. Proc Natl Acad Sci U S A. 2009;106:15043–15048. doi: 10.1073/pnas.0904400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba K, Sengupta A, Tosini M, et al. Circadian regulation of the PERIOD 2::LUCIFERASE bioluminescence rhythm in the mouse retinal pigment epithelium-choroid. Mol Vis. 2010;16:2605–2611. [PMC free article] [PubMed] [Google Scholar]
- Baba K, Davidson AJ, Tosini G. Melatonin Entrains PER2::LUC Bioluminescence Circadian Rhythm in the Mouse Cornea. Invest Ophthalmol Vis Sci. 2015;56:4753–4758. doi: 10.1167/iovs.15-17124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatwadekar AD, Yan Y, Qi X, et al. Per2 mutation recapitulates the vascular phenotype of diabetes in the retina and bone marrow. Diabetes. 2013;62:273–282. doi: 10.2337/db12-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhr ED, Yue WW, Ren X, et al. Neuropsin (OPN5)-mediated photoentrainment of local circadian oscillators in mammalian retina and cornea. Proc Natl Acad Sci U S A. 2015;112:13093–8. doi: 10.1073/pnas.1516259112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunger MK, Wilsbacher LD, Moran SM, et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busik JV, Tikhonenko M, Bhatwadekar A, et al. Diabetic retinopathy is associated with bone marrow neuropathy and a depressed peripheral clock. Journal of Experimental Medicine. 2009;206:2897–2906. doi: 10.1084/jem.20090889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBruyne JP, Weaver DR, Reppert SM. CLOCK and NPAS2 have overlapping roles in the suprachiasmatic circadian clock. Nat Neurosci. 2007;10:543–5. doi: 10.1038/nn1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JA, Davidson AJ. Health consequences of circadian disruption in humans and animal models. Prog Mol Biol Transl Sci. 2013;119:283–323. doi: 10.1016/B978-0-12-396971-2.00010-5. [DOI] [PubMed] [Google Scholar]
- Herzog ED, Tosini G. The mammalian circadian clock shop. Semin Cell Dev Biol. 2001;4:295–303. doi: 10.1006/scdb.2001.0257. [DOI] [PubMed] [Google Scholar]
- Hiragaki S, Baba K, Coulson E, et al. Melatonin signaling modulates clock genes expression in the mouse retina. PLoS One. 2014;9:e106819. doi: 10.1371/journal.pone.0106819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav V, Luo Q, M Dominguez J, 2nd, et al. Per2-Mediated Vascular Dysfunction Is Caused by the Upregulation of the Connective Tissue Growth Factor (CTGF) PLoS One. 2016;9:e0163367. doi: 10.1371/journal.pone.0163367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger C, Sandu C, Malan A, et al. Circadian organization of the rodent retina involves strongly coupled, layer-specific oscillators. FASEB J. 2015;4:1493–504. doi: 10.1096/fj.14-261214. [DOI] [PubMed] [Google Scholar]
- Jun G, Nicolaou M, Morriso MA, et al. Influence of ROBO1 and RORA on risk of age-related macular degeneration reveals genetically distinct phenotypes in disease pathophysiology. PLoS One. 2011;6:e25775. doi: 10.1371/journal.pone.0025775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratov RV, Kondratova AA, Gorbacheva VY, et al. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006;20:1868–73. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhang Z, Ribelayga CP. Heterogeneous expression of the core circadian clock proteins among neuronal cell types in mouse retina. PLoS One. 2012;11:e50602. doi: 10.1371/journal.pone.0050602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon DG, Iuvone PM, Tosini G. Circadian organization of the mammalian retina: From gene regulation to physiology and diseases. Prog Retin Eye Res. 2014;39C:58–76. doi: 10.1016/j.preteyeres.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollema NJ, Yuan Y, Jelcick AS, et al. Nuclear receptor Rev-erb alpha (Nr1d1) functions in concert with Nr2e3 to regulate transcriptional networks in the retina. PLoS One. 2011;6:e17494. doi: 10.1371/journal.pone.0017494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek ES, Lim MM, Yang G, et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J Clin Invest. 2013;123:5389–400. doi: 10.1172/JCI70317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organisciak DT, Darrow RM, Barsalou L, et al. Circadian-dependent retinal light damage in rats. Invest Ophthalmol Vis Sci. 2000;41:3694–3701. [PubMed] [Google Scholar]
- Rey G, Cesbron F, Rougemont J, et al. Genome-wide and phase-specific DNA-binding rhythms of BMAL1 control circadian output functions in mouse liver. PLoS Biol. 2011;9:e1000595. doi: 10.1371/journal.pbio.1000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan GX, Allen GC, Yamazaki S, et al. An autonomous circadian clock in the inner mouse retina regulated by dopamine and GABA. PLoS Biol. 2008;6:e249. doi: 10.1371/journal.pbio.0060249. Ken this os not the right paper. The right one is the 2006 PNAS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan GX, Gamble KL, Risner ML, et al. Divergent roles of clock genes in retinal and suprachiasmatic nucleus circadian oscillators. PLoS One. 2012;6:e3898. doi: 10.1371/journal.pone.0038985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone RA, Pardue MT, Iuvone PM, et al. Pharmacology of myopia and potential role for intrinsic retinal circadian rhythms. Exp Eye Res. 2013;114:35–47. doi: 10.1016/j.exer.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch KF, Paz C, Signorovitch J, et al. Intrinsic circadian clock of the mammalian retina: importance for retinal processing of visual information. Cell. 2007;130:730–741. doi: 10.1016/j.cell.2007.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi JS, Hong HK, Ko CH, et al. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;10:764–75. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosini G, Menaker M. Circadian rhythms in cultured mammalian retina. Science. 1996;272:419–421. doi: 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- Tosini G, Pozdeyev N, Sakamoto K, et al. The circadian clock system in the mammalian retina. Bioessays. 2008;30:624–33. doi: 10.1002/bies.20777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SH, Ko CH, Lowrey PL. A noncanonical E-box enhancer drives mouse Period2 circadian oscillations in vivo. Proc Natl Acad Sci U S A. 2005;102:2608–13. doi: 10.1073/pnas.0409763102. [DOI] [PMC free article] [PubMed] [Google Scholar]


