Abstract
Background and Purpose
Tubulo‐interstitial fibrosis is the final pathway in the progression of chronic kidney disease (CKD) to kidney failure. The renin‐angiotensin system (RAS) plays a major role in CKD progression. Hence, we determined the efficacy of novel RAS inhibitors isolated from Poria cocos against renal fibrosis.
Experimental Approach
Effects of three novel tetracyclic triterpenoid compounds, poricoic acid ZC (PZC), poricoic acid ZD (PZD) and poricoic acid ZE (PZE), were investigated on TGFβ1‐ and angiotensin II (AngII)‐treated HK‐2 cells and unilateral ureteral obstruction (UUO) in mice. Immunofluorescence staining, quantitative real‐time PCR, siRNA, co‐immunoprecipitation and Western blot analyses were used to evaluate expression of key molecules in RAS, Wnt/β‐catenin and TGFβ/Smad pathways.
Key Results
Addition of the above compounds to culture media and their administration to UUO mice: (i) significantly attenuated epithelial‐to‐mesenchymal transition and extracellular matrix production in TGFβ1‐ and AngII‐treated HK‐2 cells and UUO mice by inhibiting Wnt/β‐catenin pathway activation and Smad3 phosphorylation; (ii) selectively inhibited Smad3 phosphorylation by blocking the interaction of TGFBR1 with Smad3; and (iii) specifically inhibited Smad3 activation. PZC and PZD showed a strong inhibitory effect on all RAS components, and PZE showed a strong inhibitory effect on renin. Furthermore, the secolanostane tetracyclic triterpenoids, PZC and PZD, showed a stronger inhibitory effect than the lanostane tetracyclic triterpenoid PZE. Therefore, compounds with secolanostance skeleton showed stronger bioactivity than those with lanostance skeleton.
Conclusion and Implications
The secolanostane tetracyclic triterpenoids effectively blocked RAS by simultaneously targeting multiple RAS components and lanostane tetracyclic triterpenoids inhibited renin and protected against tubulo‐interstitial fibrosis.
Abbreviations
- ACE
angiotensin‐converting enzyme
- ACEI
angiotensin‐converting enzyme inhibitor
- AGT
angiotensinogen
- AngII
angiotensin II
- ARB
angiotensin II type 1 receptor blocker
- AT1 receptor
angiotensin II type 1 receptor
- CKD
chronic kidney disease
- DEPT
distortionless enhancement by polarization transfer
- ECM
extracellular matrix
- EMT
epithelial‐to‐mesenchymal transition
- FSP1
fibroblast‐specific protein 1
- LEF
lymphoid enhancer‐binding factor
- NOESY
nuclear overhauser effect spectroscopy
- PAI‐1
plasminogen activator inhibitor‐1
- PZC
poricoic acid ZC
- PZD
poricoic acid ZD
- PZE
poricoic acid ZE
- qRT‐PCR
quantitative real‐time PCR
- RAS
renin‐angiotensin system
- SLPC
surface layer of Poria cocos
- TCF
T‐cell factor
- TGFBR
TGFβ receptor
- TIF
tubulo‐interstitial fibrosis
- UUO
unilateral ureteral obstruction
- α‐SMA
α smooth muscle actin
Introduction
Many natural products are widely applied as an alternative to standard pharmacological compounds for treatment of chronic kidney disease (CKD) (Zhao, 2013; Zhong et al., 2013, 2015; Tian et al., 2014; Wang et al., 2017b). Poria cocos (Schw.) Wolf (Poliporaceae), is a well‐known medicinal mushroom that parasitizes the roots of pine trees, and is widely used in Asia and North America (Wang et al., 2013). The sclerotia of P. cocos, also known as ‘Fu Ling’, ‘tuckahoes’ or ‘Indian bread’ (Zhao et al., 2013a), exhibits various biological activities such as anti‐tumour, anti‐inflammatory, antioxidant and lipid‐lowering effects (Wang et al., 2013; Miao et al., 2016). Our previous studies demonstrated that the surface layer of P. cocos (SLPC) enhances diuresis and is highly effective as a treatment of CKD and hyperlipidaemia (Zhao et al., 2012b; 2013b,c; Feng et al., 2013; Miao et al., 2015).
Tubulo‐interstitial fibrosis (TIF), one of the common features and mediators of CKD, is associated with hypertension, inflammation, activation of the renin‐angiotensin system (RAS), and TGF‐β/Smad and Wnt/β‐catenin signalling pathways (Kobori et al., 2007; Chen et al., 2016, 2017a,b; Meng et al., 2016). All of the RAS components are expressed in kidney tissues, and numerous studies have demonstrated the critical role of the pathological activation of the intrarenal RAS in the pathogenesis of hypertension, renal injury and cardiovascular disease (Zhou et al., 2015). There is mounting evidence pointing to marked activation of the intrarenal RAS following kidney injury (Kobori et al., 2007; D'Agati et al., 2016). Intrarenal http://www.guidetoimmunopharmacology.org/GRAC/LigandDisplayForward?ligandId=2504 (AngII) is formed by multiple independent mechanisms. Proximal tubular angiotensinogen (AGT), collecting duct http://www.guidetoimmunopharmacology.org/GRAC/ObjectDisplayForward?objectId=2413 and http://www.guidetoimmunopharmacology.org/GRAC/ObjectDisplayForward?objectId=34 (AT1 receptors) are positively augmented by intrarenal AngII. Therefore, http://www.guidetoimmunopharmacology.org/GRAC/ObjectDisplayForward?objectId=1613 inhibitors (ACEI) and AT1 receptor blockers (ARBs) could effectively reduce blood pressure in hypertensive patients and have been recommended as standard treatments of hypertension, cardiovascular disease and CKD (Yang and Xu, 2017). They have been demonstrated to decrease proteinuria and retard CKD progression. However, the AngII and aldosterone levels can rise with chronic ACEI or ARB administration, which is known as AngII and aldosterone escape. A reactive rise in renin levels occurs when mineralocorticoid receptor antagonists or a renin inhibitor are used. Chronic elevations in AngII and aldosterone results in the acceleration of heart failure and renal disease (Siragy and Carey, 2010). These studies demonstrated that ACEIs and ARBs cannot fully block the RAS cascade. For this reason, novel RAS inhibitors capable of simultaneously targeting multiple RAS components will be more effective for prevention and treatment of hypertension, cardiovascular disease and CKD.
TGFβ, which is the primary driver of fibrosis, induces TIF via activation of both Smad‐based and non‐Smad‐based signalling pathways (Meng et al., 2016). Hence, inhibition of http://www.guidetoimmunopharmacology.org/GRAC/LigandDisplayForward?ligandId=5060 or its downstream signalling could substantially alleviate TIF in various kidney diseases. AngII stimulates TGFβ expression in the kidney by complex mechanisms (Meng et al., 2016).
The http://www.guidetoimmunopharmacology.org/GRAC/FamilyDisplayForward?familyId=964/http://www.guidetoimmunopharmacology.org/GRAC/LigandDisplayForward?ligandId=5371 pathway is an evolutionarily conserved developmental signalling system that plays an important role in organ development and tissue homeostasis. In the presence of Wnts, β‐catenin is stabilized and translocated into the nucleus, where it binds to T‐cell factor (TCF)/lymphoid enhancer‐binding factor (LEF) gene family of transcription factors and forms a complex by recruiting the transcriptional co‐activator cAMP response element‐binding protein to transactivate its target genes (Spranger et al., 2015). Wnt/β‐catenin signalling is activated in various kidney diseases such as obstructive nephropathy, diabetic nephropathy and polycystic kidney disease (Edeling et al., 2016; Zhou and Liu, 2016). In a previous study it was demonstrated that all RAS genes contain TCF/LEF binding sites in their promoter regions and are regulated by the Wnt/β‐catenin pathway (Zhou et al., 2015). In the kidney, β‐catenin is an important regulator controlling the expression of the intrarenal RAS. The RAS, TGF‐β/Smad, Wnt/β‐catenin signalling and TIF are intimately linked as they form a vicious cycle in which the RAS provokes TIF by triggering TGF‐β/Smad and Wnt/β‐catenin signalling. By limiting renal blood flow, TIF elevates arterial blood pressure and activates the RAS which, in turn, amplifies the activation of the TGF‐β/Smad and Wnt/β‐catenin signalling pathways (Yang and Xu, 2017). Accordingly, blockade of these signalling pathways may attenuate kidney injury and reduce TIF in CKD. Therefore, simultaneous blockade of RAS activation and inhibition of TGF‐β/Smad and Wnt/β‐catenin signalling may represent an effective therapy for CKD.
Using cultured human proximal tubular epithelial cells (HK‐2) and mice with unilateral ureteral obstruction (UUO), the present study was designed to test the hypothesis that the novel tetracyclic triterpenoid compounds, poricoic acid ZC (PZC), poricoic acid ZD (PZD) and poricoic acid ZE (PZE) (Figure 1A), can alleviate TIF by inhibiting the activation of the RAS, TGF‐β/Smad and Wnt/β‐catenin pathways.
Figure 1.
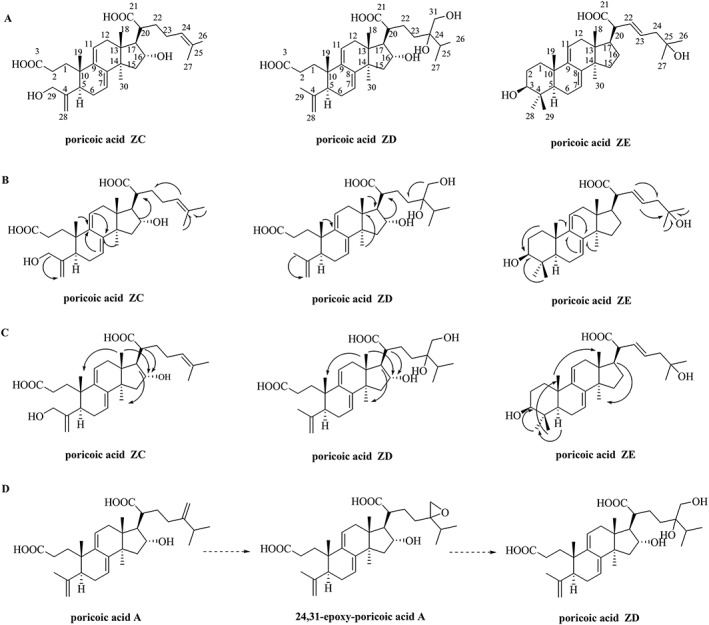
Three novel tetracyclic triterpenoids from the SLPC. (A) Chemical structures of compounds PZC, PZD and PZE. (B) Key HMBC correlations (from H to C) for compounds PZC, PZD and PZE. (C) Key NOESY correlations for compounds PZC, PZD and PZE. (D) Plausible biosynthetic pathway of compound PZD.
Methods
Materials, extraction and isolation
SLPC were collected in Kunming (Yunnan Province, China) in July 2016. Pulverized dried SLPC (20 kg) was extracted three times with 95% EtOH (100 L). The solvent was removed under reduced pressure. The resulting residue (804 g) was subsequently dissolved in water and extracted with petroleum ether, EtOAC and n‐BuOH for three times respectively.
The EtOAC fraction (438 g) was fractionated by MCI gel CHP 20P eluted with a MeOH/H2O mixture providing seven subfractions (FLP1‐7). Subfraction FLP4 (39 g) was subjected to the MPLC/ODS column and eluted with a MeOH/H2O mixture; five fractions were obtained. Fraction FLP4‐2 was subjected to reversed‐phase HPLC (C18, column size: 20 × 250 mm) eluted with the mobile phase MeOH: H2O = 70:30, resulting in the separation of PZC (8 mg). Fraction FLP4‐4 was subjected to reversed‐phase HPLC eluted with the mobile phase MeOH: H2O = 75:25, resulting in the separation of PZD (6 mg). Subfraction FLP5 (14 g) was purified by Sephadex LH‐20 using methyl alcohol as a mobile phase and was then purified over reversed‐phase HPLC (mobile phase: MeOH‐H2O, 85:15) to obtain PZE (4 mg). To treat the UUO mice, pulverized dried SLPC (1000 kg) was extracted three times in 95% EtOH by use of a heating reflux method. PZC, PZD and PZE were obtained using the above‐mentioned procedures.
Animal experiments
All animal care and experimental procedures were approved by the Ethics Committee for Animal Experiments of the Northwest University (No. SYXK2010‐004). Male BALB/c mice (6–8 weeks old and weighing 18–22 g) were purchased from the Animal Centre of Xi'an Jiaotong University (Xi'an, Shaanxi). Mice were provided with food and water ad libitum and housed in plastic cages (≤5 mice per cage) placed in a specific pathogen‐free air‐conditioned vivarium with 40–70% humidity at 22 ± 2°C and 12 h light/12 h dark cycle. They were acclimatized to their housing environment for 7 days prior to experimentation and to the experimental room for 1 h before experiments. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015).
Animals were housed 4 to a plastic cage with conventional bedding and provided with food and water ad libitum under a temperature (22 ± 1.5°C) and 12 h day/night cycle. UUO was performed according to an established protocol described previously (Klahr and Morrissey, 2002). Following anaesthesia with sodium pentobarbital by intraperitoneal injection (3%, 10 ml·kg−1), the left ureter was ligated twice using 4‐0 nylon surgical sutures below the lower pole of the kidney. Mice were randomly divided into five groups: (i) sham operated group (n = 6); (ii) placebo‐treated UUO group (n = 6); (iii) UUO + PZC (10 mg·kg−1) group (n = 6); (iv) UUO + PZD (10 mg·kg−1) group (n = 6); and (v) UUO + PZE (10 mg·kg−1) group (n = 6). The mice in the treatment groups were administered PZC, PZD and PZE (10 mg·kg−1·day−1) by gastric gavage for 7 days. Under general anaesthesia, the animals were killed at day 7 after UUO.
Cell culture and treatment
HK‐2 cells purchased from the China Centre for Type Culture Collection were used to test the activity of PZC, PZD and PZE. HK‐2 cells were cultured in DMEM‐F12 with 10% FBS (GIBCO, Carlsbad, CA, USA) at 37°C under 5% CO2. HK‐2 cells were treated with the recombinant human TGFβ1 protein (R&D system, USA) at a dose of 2.5 ng·mL−1 and the recombinant human AngII protein (R&D system) at 1.0 μM. The concentrations of PZC, PZD and PZE were 10 μM in this study. The AT1 receptor blocker http://www.guidetoimmunopharmacology.org/GRAC/LigandDisplayForward?ligandId=590 (1 mM, Selleck Chemicals, Huston, USA) was used as a reference drug.
Toxicity of PZC, PZD and PZE
The ability of cells to maintain or recover viability after treatment with each of the agents was determined. Viability can be distinguished from the all‐or‐nothing states of life and death by use of a quantifiable index between 0 and 1 (or 0% and 100%). Cell viability was performed as described previously (Wang et al., 2018). Cell viability was measured by the cell counting kit‐8 assay kit (EnoGene, China). The cells (1 × 104) were treated with different concentrations of PZC, PZD and PZE (0, 1, 10, 50 and 100 μM) for 24 h in 96‐well plates. CCK‐8 reagent (10 μL) was added into fresh medium (100 μL) per well. After 3 h incubation, the absorbance was measured at 450 nm on a microplate reader (Thermo, New York, USA). The measurement of cell viability at each concentration was repeated for six times.
Knockdown of Smad3 by siRNA
Smad3 siRNA or control siRNA was transfected into HK‐2 cells. The siRNA targeting human Smad3 (5′‐CCGCAUGAGCUUCGUCAAATT‐3′) and negative control (5′‐UUCUCCGAACGUGUCACGUTT‐3′) was synthesized by Sangon (Guangzhou, China). Cells were cultured in six‐well plates and transfected with 3 μL of 10 μM siRNA per well by using 9 μL lipofectamine RNAiMAX (Invitrogen, USA). After a 48 h incubation, HK‐2 cells were treated and then collected for quantitative real‐time PCR (qRT‐PCR) and Western blot analyses.
Gene expression studies by quantitative real‐time PCR
The mRNA expression was performed by qRT‐PCR analysis (Chen et al., 2017c). Total RNA were extracted by using a High Pure RNA Isolation Kit (Tokyo, Japan) and reverse transcribed by a Transcriptor First Strand cDNA Synthesis Kit, according to the manufacturer's instructions (Roche, Germany). The PCR reaction mixture in a 20 μL volume contained 10 μL of SYBR® Premix Ex Taq™ II (Takara Bio, Japan), 1.0 μL of reverse transcription product, 0.4 μL of sense and antisense primer sets and 8.2 μL of double distilled water. The housekeeping gene β‐actin was used as an internal standard. Table 1 presents the primers used to amplify the genes.
Table 1.
Primers for real‐time RT‐PCR (5′➔3′) of EMT and RAS genes
| Gene | Species | Forward | Reverse | Product size (bp) |
|---|---|---|---|---|
| Collagen I | Homo sapiens | TGTGCCACTCTGACTGGAAG | CGCCATACTCGAACTGGAATC | 228 |
| Fibronectin | Homo sapiens | CCACAGTGGAGTATGTGGTTAG | CAGTCCTTTAGGGCGATCAAT | 104 |
| α‐SMA | Homo sapiens | GATGGTGGGAATGGGACAAA | GCCATGTTCTATCGGGTACTTC | 94 |
| Vimentin | Homo sapiens | GATTCACTCCCTCTGGTTGATAC | GTCATCGTGATGCTGAGAAGT | 108 |
| E‐cadherin | Homo sapiens | CTTCTGCTGATCCTGTCTGATG | TGCTGTGAAGGGAGATGTATTG | 144 |
| AGT | Homo sapiens | GATGTTGCTGCTGAGAAGATTG | AGTGGACGTAGGTGTTGAAAG | 118 |
| Renin | Homo sapiens | CTCTACACTGCCTGTGTGTATC | CACTGACTGTCCCTGTTGAATA | 109 |
| ACE | Homo sapiens | GGTGGTGTGGAACGAGTATG | CAGGGTGTGGTTGGCTATTT | 112 |
| AT1 | Homo sapiens | GGAAACAGCTTGGTGGTGATAG | GCATAAGTCAGCCAGTGCTAAA | 96 |
| β‐actin | Homo sapiens | ACAAGCCACAAGATTACAAG | ATCAGCAG TCTCATTCCAA | 92 |
Western blot analysis
Total proteins of cultured HK‐2 cells were extracted by using RIPA. Protein concentration was measured by using Pierce™ BCA Protein Assay Kit (23227, Thermo Scientific, USA). Twenty or 30 μg of total protein was fractionated on 8–12% Tris‐glycine resolving gel and then transferred to a 0.45 μm PVDF membrane (10600023, Amersham™ Hybond™, GE Healthcare, USA). The membrane was washed three times with 1 × TBS with 0.1% Tween‐20 (TBST). After incubation with 5% of non‐fat milk blocking buffer, the membrane was incubated overnight at 4°C in primary antibody. The membrane was washed in TBST three times and then incubated in goat anti‐rabbit (1:5000, ab6721, Abcam, USA), goat anti‐mouse (1:5000, A21010, Abbkine, USA) or rabbit anti‐goat (1:5000, A21110, Abbkine) secondary antibodies for 2 h. The membrane was visualized by ECL detection reagent (RPN2232, GE Healthcare, USA). Images were acquired using Tanon 6600 Luminescent Imaging Workstation (Tanon Science & Technology Co., Ltd. Shanghai, China) and quantified by ImageJ software (version 1.48v, NIH, Bethesda, MD, USA). The densities of bands were normalized to α‐tubulin expression level. Each protein was repeated three times in the study.
The following primary antibodies were employed (dilution): collagen I (1:5000, ab34710, Abcam), fibronectin (1:1000, ab2413, Abcam), α smooth muscle actin (α‐SMA, 1:300, ab7817, Abcam), E‐cadherin (1:2000), vimentin (1;2000), AGT (AGT, 1:200, sc‐7419, Santa Cruz Biotechnology, CA, USA), renin (1:2000, 14291‐1‐AP, Proteintech, China), ACE (1:1000, sc‐23908, Santa Cruz Biotechnology), AT1 receptor (1:800, ab124505, Abcam), http://www.guidetoimmunopharmacology.org/GRAC/LigandDisplayForward?ligandId=3672 (1:1000, ab85060, Abcam), dephosphorylated active β‐catenin (1:2000, 05‐665, Millipore, USA), β‐catenin (1:1000, 610154, BD Transduction Laboratories, USA), Snail1 (1:1000, ab180714, Abcam), Twist (1:2000, ab50581, Abcam), http://www.guidetoimmunopharmacology.org/GRAC/ObjectDisplayForward?objectId=1631 (1:400, ab5706, Abcam), plasminogen activator inhibitor‐1 (PAI‐1, 1;5000, 612024, BD, USA), fibroblast‐specific protein 1 (FSP1, 1:1000, ab197896, Abcam), http://www.guidetoimmunopharmacology.org/GRAC/ObjectDisplayForward?objectId=1795 (TGFBR2, 1:2000, ab186838, Abcam), http://www.guidetoimmunopharmacology.org/GRAC/ObjectDisplayForward?objectId=1788 (TGFBR1, 1:1000, ab31013, Abcam), p‐Smad2 (1:2000, 3180, CST, USA), Smad2 (1:2000, 5339, CST, USA), p‐Smad3 (1:2000, 9520, CST, USA), Smad3 (1:2000, 9523, CST, USA), Smad4 (38454, CST, USA), Smad7 (1:500, sc‐365846, Santa Cruz, USA) and http://www.guidetoimmunopharmacology.org/GRAC/FamilyDisplayForward?familyId=858 (Proteintech Company, China).
Histological analysis
All kidney tissues were excised, fixed in 4% formaldehyde and embedded in paraffin. Kidney tissue sections (5 μm) were obtained and used for histological analysis. Haematoxylin–eosin (H&E) and Masson's trichrome staining were performed according to the standard H&E and Masson's trichrome protocol (Zhang et al., 2015). All histological data were obtained in a blinded manner by two independent observers.
Immunofluorescence staining
HK‐2 cells were cultured on coverslips and fixed with 4% paraformaldehyde for 10 min at 4°C. After being blocked with normal goat serum for 30 min, HK‐2 cells were incubated with primary antibodies against vimentin (1:200), AT1 receptor (1:200) and p‐Smad3 (1:200) at 4°C for overnight. The secondary antibodies Alexa Fluor® 488 or 594‐conjugated goat anti‐rabbit IgG H&L were incubated with 2 h at room temperature. DAPI was used to detect nuclear localization. The stained HK‐2 cells were mounted with 80% glycerol/PBS for subsequent examination by a laser‐scanning confocal microscope (FV1000, Olympus, Japan) using FV10‐ASW 4.0 VIEW.
Co‐immunoprecipitation analysis
The interactions within the TGFβ/Smad signalling pathway were determined by co‐immunoprecipitation. To avoid any interference caused by antibodies present in the cell lysates, lysates were pretreated with protein A/G (Immunoprecipitation Starter Pack, GE Healthcare, USA) for 1 h at 4°C. The supernatant was transferred into a new eppendorf tube and incubated with anti‐AngII (1:100), TGFBR1 (1:100), anti‐Smad2 (1:100) and anti‐Smad3 (1:100) antibody overnight at 4°C to couple antigen with antibody. Then, to precipitate the immune complexes, the protein A/G plus was immune precipitated overnight at 4°C. The precipitated complexes were washed with lysis buffer three times and boiled for 3 min in sample buffer followed by immunoblotting with ACE, AT1 receptor, TGFBR1, TGFBR2, p‐Smad2, p‐Smad3, Smad2 and Smad3.
Statistical analysis
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). The number of replicates was six per group for each data set, and results are presented as mean ± SD unless stated otherwise. GraphPad Prism (GraphPad software, San Diego, CA, USA) was used for statistical analysis of the data. Statistical analysis for multiple groups was performed by one‐way ANOVA followed by post hoc test when F achieved P < 0.05 and there was no significant variance in homogeneity. Some results were normalized to control to avoid unwanted sources of variation. P < 0.05 was considered statistically significant.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a,b,c).
Results
Structure elucidation of the new compounds (PZC, PZD and PZE) isolated
Compound PZC was isolated from SLPC as a yellow and amorphous powder. Its molecular structure, C30H44O6 (m/z 523.25, 9° of unsaturation), was identified based on analysis of its HRESI/MS, 13C NMR and distortionless enhancement by polarization transfer (DEPT) spectra. The IR spectrum showed absorptions for OH (3435 cm−1), C=O (1707 cm−1) and C=C (1639 cm−1) functional groups. The 1H NMR spectrum presents five signals at δH 1.09 (3H, s, Me‐19), 1.14 (3H, s, Me‐18), 1.46 (3H, s, Me‐30), 1.56 (3H, s, Me‐27) and 1.58 (3H, s, Me‐26), which were assigned to five methyl groups (Table 2). Furthermore, one‐terminal olefin proton signals at δH 5.61 (1H, br s, H‐28) and 5.23 (1H, br s, H‐28), one oxymethine hydrogen at δH 4.52 (1H, m, H‐16), suggested that compound PZC belonged to the group of 3,4‐seco‐lanostan‐7,9(11)‐diene type triterpenes. The 13C NMR data showed the resonances for 30 carbon atoms, whose substitution patterns were revealed from the DEPT and heteronuclear single‐quantum coherence experiments as five methyls, nine methylenes, seven methines and nine quaternary carbons (Table 2). According to the 13C NMR spectrum, there were the typical signals of four olefinic carbons at δC 118.6, 142.6, 138.0 and 121.2 corresponding to C‐7, C‐8, C‐9 and C‐11, respectively, one oxymethine carbon at δC 76.9 corresponding to C‐16, two carboxylic carbons at δC 177.1 and 179.1 corresponding to C‐3 and C‐21. The NMR data of PZC resembled those of the known compound poricoic acid B with the exception of an additional oxymethylene signal at δC 65.8 [δH 4.41 (2H, s)] (Alexander et al., 2017a) and the disappearance of a methyl group signal. A substitution with CH2OH at position 29 can be deduced by heteronuclear multiple bond correlation (HMBC) cross peaks from H‐5 and H3‐28 to C‐29 and from H2‐29 to C‐5 and C‐28 (Figure 1B). The relative stereochemistry of compound PZC was assessed by its nuclear overhauser effect spectroscopy (NOESY) spectrum (Figure 1C). The NOESY cross peaks of H‐16/H‐18 and H‐16/H‐20 suggested the α‐orientation assignments of OH‐16. Therefore, this compound was elucidated as (20R)‐16α,29‐dihydroxy‐3,4‐seco‐lanosta‐4(28),7,9(11),24(25)‐tetraene‐3,21‐dioic acid (PZC).
Table 2.
13C NMR and 1H NMR spectroscopic data of compounds PZC, PZD and PZE
| PZC | PZD | PZE | ||||
|---|---|---|---|---|---|---|
| Position | 13C | 1H | 13C | 1H | 13C | 1H |
| 1 | 36.8 (t) | 2.14, 1.91 | 36.7 (t) | 2.11 | 36.7 (t) | 1.44, 1.92 |
| 2 | 30.8 (t) | 2.49, 2.54 | 30.6 (t) | 2.52, 2.47 | 29.0 (t) | 1.80, 1.94 |
| 3 | 177.1 (s) | – | 177.1 (s) | – | 78.4 (d) | 3.45 (m) |
| 4 | 154.5 (s) | – | 149.6 (s) | – | 39.7 (s) | – |
| 5 | 47.2 (d) | 2.45 | 51.0 (d) | 2.31 (s) | 50.1 (d) | 3.61 (s) |
| 6 | 29.8 (t) | 2.20, 2.60 | 28.9 (t) | 2.53, 2.03 | 23.9 (t) | 2.14 |
| 7 | 118.6 (d) | 5.24 (s) | 118.3 (d) | 5.24 (s) | 121.8 (d) | 5.61 (d, 5.6) |
| 8 | 142.6 (s) | – | 142.3 (s) | – | 143.1 (s) | – |
| 9 | 138.0 (s) | – | 137.9 (s) | – | 146.9 (s) | – |
| 10 | 39.9 (s) | – | 39.2 (s) | – | 38.2 (s) | – |
| 11 | 121.2 (d) | 5.32 (s) | 120.7 (d) | 5.30 (br s) | 117.0 (d) | 5.37 (d, 5.9) |
| 12 | 37.5 (t) | 2.45, 2.65 | 37.5 (t) | 2.45, 2.65 | 36.2 (t) | 2.37, 2.52 |
| 13 | 46.2 (s) | – | 46.9 (s) | – | 44.6 (s) | – |
| 14 | 49.7 (s) | – | 49.7 (s) | – | 50.8 (s) | – |
| 15 | 44.3 (t) | 2.39, 1.79 | 44.1 (t) | 2.34, 1.77 | 31.9 (t) | 1.62, 1.33 |
| 16 | 76.9 (d) | 4.52 (m) | 76.6 (d) | 4.46 (m) | 27.4 (t) | 1.38, 2.10 |
| 17 | 58.2 (d) | 2.82 (dd, 11.1, 6.0) | 57.8 (d) | 2.88 (t) | 48.2 (d) | 2.54 |
| 18 | 18.9 (q) | 1.14 (s) | 18.7 (q) | 1.03 (s) | 16.7 (q) | 0.99 (s) |
| 19 | 22.6 (q) | 1.09 (s) | 22.6 (q) | 1.00 (s) | 23.4 (q) | 1.08 (s) |
| 20 | 48.8 (d) | 2.90 | 49.4 (d) | 2.90 | 49.9 (d) | 2.70 (dd, 10.8, 3.2) |
| 21 | 179.1 (s) | – | 179.2 (s) | – | 178.3 (s) | – |
| 22 | 33.5 (t) | 2.32, 2.48 | 27.1 (t) | 2.43, 2.76 | 142.8 (d) | 6.19 (dd, 14.2, 7.6) |
| 23 | 27.6 (t) | 2.30, 2.43 | 33.5 (t) | 2.28 | 124.2 (d) | 6.14 (d, 15.5) |
| 24 | 125.6 (d) | 5.30 | 76.2 (s) | – | 36.3 (t) | 2.63 |
| 25 | 132.0 (s) | – | 33.7 (d) | 2.24 | 70.0 (s) | – |
| 26 | 26.4 (q) | 1.58 (s) | 17.9 (q) | 1.15 (s) | 31.1 (q) | 1.50 (s) |
| 27 | 18.2 (q) | 1.56 (s) | 17.8 (q) | 1.14 (s) | 30.9 (q) | 1.50 (s) |
| 28 | 109.5 (t) | 5.61 (s), 5.23 (s) | 112.5 (t) | 4.79 (br s), 4.73 (br s) | 29.2 (q) | 1.14 (s) |
| 29 | 65.8 (t) | 4.41 (s) | 22.7 (q) | 1.70 (s) | 16.9 (q) | 1.08 (s) |
| 30 | 25.4 (q) | 1.46 (s) | 25.2 (q) | 1.47 (s) | 26.2 (q) | 1.23 (s) |
| 31 | 36.8 (t) | 2.14, 1.91 | 66.3 (t) | 4.00 (q, 10.9) | 36.7 (t) | 1.44, 1.92 |
Compound PZD was isolated as a white powder. Its molecular structure was identified as C31H48O7 based on the negative HR‐ESI‐MS (m/z 531.3329). The IR spectrum indicated the presence of OH (3430 cm−1), C=O (1711 cm−1) and C=C (1640 cm−1) functionalities. The 1H NMR spectrum presents six signals at δH 1.00 (3H, s, Me‐19), 1.03 (3H, s, Me‐18), 1.14 (3H, s, Me‐27), 1.15 (3H, s, Me‐26), 1.47 (3H, s, Me‐30) and 1.70 (3H, s, Me‐29), which were assigned to six methyl groups (Table 2). Furthermore, one terminal olefin proton signals at δH 4.79 (1H, br s, H‐28) and 4.73 (1H, br s, H‐28), one oxymethine hydrogen at δH 4.46 (1H, m, H‐16), suggested that compound PZD belonged to the group of 3,4‐seco‐lanostan‐7,9(11)‐diene type triterpenes. The 13C NMR data showed the resonances for 31 carbon atoms, including six methyls, nine methylenes, seven methines and nine quaternary carbons (Table 2). According to the 13C NMR spectrum, there were the typical signals of four olefinic carbons at δC 118.3, 142.3, 137.9 and 120.7 corresponding to C‐7, C‐8, C‐9 and C‐11, respectively, one oxymethine carbon at δC 76.6 corresponding to C‐16, two carboxylic carbons at δC 177.1 and 179.2 corresponding to C‐3 and C‐21. The hydroxyl group was located at C‐31 on the basis of the downfield shifts of H‐29 at δ 4.00 (s, 2H), which was supported by the correlation between δH 4.00 (s, 2H) and δC 33.5 (C‐23), 33.7(C‐25) in the HMBC experiment (Figure 1B). The relative stereochemistry of compound PZD was assessed by its NOESY spectrum. The NOESY cross peaks of H‐16/H‐18 and H‐16/H‐20 suggested the α‐orientation assignments of OH‐16 (Figure 1C). Therefore, compound PZD was identified as (20R)‐16α,24,31‐trihydroxy‐3,4‐seco‐lanosta‐4(28),7,9(11)‐triene‐3,21‐dioic acid (PZD).
Compound PZE was obtained as a white, amorphous powder. Its molecular structure was identified as C30H46O4, based on 13C NMR data and a [M + Na]+ peak at m/z 493.3266 determined by HRESIMS. The IR spectrum indicated the presence of OH, C=O and C=C functional groups, based on the absorptions at 3425, 1722 and 1639 cm−1 respectively. The 1H NMR spectrum presents six signals at δH 0.99 (3H, s, Me‐18), 1.05 (3H, s, Me‐29), 1.08 (3H, s, Me‐19), 1.14 (3H, s, Me‐28), 1.23 (3H, s, Me‐30) and 1.50 (6H, s, Me‐26, Me‐27), which were assigned to seven methyl groups (Table 2). The 13C and DEPT data suggested the presence of 30 carbons, including a carboxylic carbon at δC 178.3, four olefinic carbons at δC 142.8 (C‐22), 124.2 (C‐23), 121.8 (C‐7) and 117.0 (C‐11). The HMBC correlations from the H‐17 (δH 2.54) to C‐22, the H‐20 (δH 2.70) to C‐23, the H‐22 (δH 6.19) to C‐24, C‐25 and the H‐23 (δH 6.14) to C‐25 suggested that two olefinic carbons are at C‐22 and C‐23 (Table 2). The tertiary hydroxyl group at δC 70.0 is connected to C‐25 on the basis of the correlation between H‐23 (δH 6.14), H‐26 (δH 1.50) and H‐27 (δH 1.50) to C‐25 in the HMBC experiment (Figure 1B). Interactions between H‐3 (δH 3.45) and Me‐29 (δH 1.08) revealed that they were on the same side of the molecule, while the NOESY between Me‐18 (δH 0.99) and H‐19 (δH 1.08) suggested that Me‐18 and H‐19 were on the same side (Figure 1C). On the basis of the analyses described above, the compound was identified as (20R)‐3β,25‐dihydroxy‐7,9(11),22,23‐triene‐21‐oic acid (PZE).
Toxicity of PZC, PZD and PZE in vitro and in vivo
To assess the toxicity of PZC, PZD and PZE and select the optimal concentrations of PZC, PZD and PZE, a dose‐response on HK‐2 cells was performed at 0, 1, 10, 50 and 100 μM concentrations. The cell viability values for PZC were 1.00 ± 0.03, 1.01 ± 0.03, 0.99 ± 0.05, 0.99 ± 0.05 and 0.99 ± 0.06. The cell viability values for PZD were 1.00 ± 0.04, 0.99 ± 0.04, 0.99 ± 0.06, 0.99 ± 0.07 and 1.01 ± 0.05. The cell viability values of PZE were 1.00 ± 0.04, 0.99 ± 0.05, 1.00 ± 0.07, 1.00 ± 0.09 and 1.00 ± 0.07. Treatment with PZC, PZD and PZE did not show a proliferative or cytotoxic effect on HK‐2 cells at concentrations of 1, 10, 50 and 100 μM. The effects of 1, 10, 50 and 100 μM of each of the three compounds were determined on α‐SMA expression in TGFβ1‐treated HK‐2 cells. At 10 μM, each of these compounds showed strong anti‐fibrotic bioactivity, and this concentration was subsequently used for all in vitro experiments (data not shown). A toxicity study in mice indicated that mice administered, by oral gavage, doses of PZC, PZD and PZE up to 800 mg·kg−1 did not die or develop an obvious adverse event during the 7 day observation period.
PZC, PZD and PZE block epithelial‐to‐mesenchymal transition (EMT)
We initially examined the inhibitory effects of PZC, PZD and PZE on EMT and the production of extracellular matrix (ECM) in TGFβ1‐treated HK‐2 cells. Vimentin is an important marker of fibroblasts, and immunofluorescent staining showed vimentin protein was significantly up‐regulated in TGFβ1‐treated HK‐2 cells and this effect was reversed after PZC, PZD and PZE treatment (Figure 2A). Losartan, a classical ARB, also showed an inhibitory effect on vimentin expression in TGFβ1‐treated HK‐2 cells. In addition, immunofluorescent staining revealed that PZC, PZD or PZE treatment had no effect on vimentin protein expression in HK‐2 cells (Figure 2A). These results demonstrate that these compounds had no effect on HK‐2 cells in the absence of TGFβ1 stimulation. HK‐2 cells subjected to TGFβ1 or AngII stimulation exhibited a down‐regulation of the epithelial marker E‐cadherin and up‐regulation of mesenchymal markers including collagen I, fibronectin and α‐SMA (Figure 2B, E), which were attenuated after PZC, PZD and PZE treatment.
Figure 2.
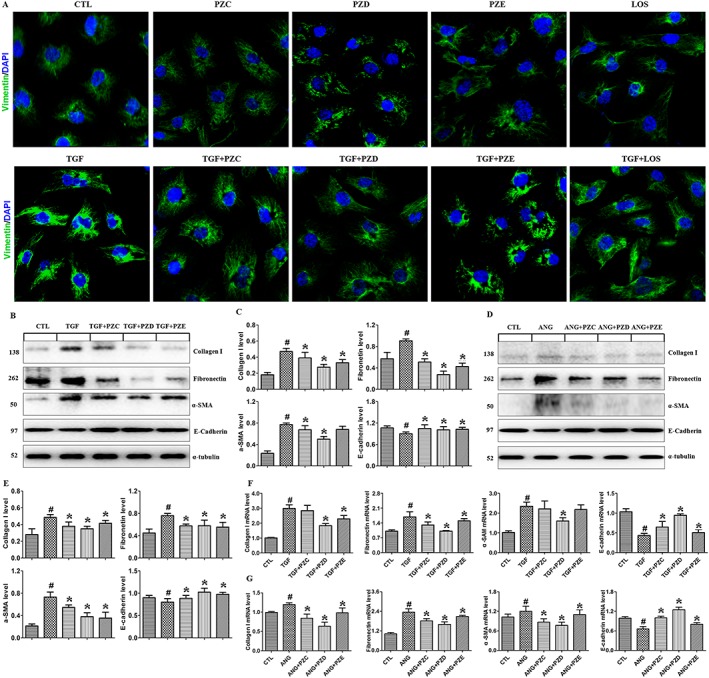
PZC, PZD and PZE reduce EMT and enhance podocyte marker in the TGFβ1‐ or AngII‐induced HK‐2 cells and podocytes. HK‐2 cells or podocytes were cultured in a serum‐fasted medium and treated with TGFβ1 or AngII (n = 6). (A) HK‐2 cells stimulated by TGFβ1 were stained with primary antibody against vimentin. The in situ expression of vimentin was analysed by Laser scanning confocal microscopes. Representative immunofluorescent staining images of TGFβ1‐induced vimentin expression in the different groups. (B) Protein expression and (C) quantitative analysis in the HK‐2 cells induced by TGFβ1. (D) Protein expression and (E) quantitative analysis in the HK‐2 cells induced by AngII. qRT‐PCR analysis of the mRNA expression of genes encoding collagen I, fibronectin, α‐SMA and E‐cadherin in the HK‐2 cells stimulated by TGFβ1 (F) or AngII (G). # P < 0.05 compared with control group (n = 6); * P < 0.05, compared with TGFβ1‐ or AngII‐ stimulated group (n = 6).
In addition, we determined the inhibitory effect of PZC, PZD and PZE on the TGFβ1‐ and AngII‐induced expression of ECM genes including collagen I, fibronectin, α‐SMA and E‐cadherin in HK‐2 cells. Except for PZC, which did not significantly alter collagen I and α‐SMA expression, and PZE, which did not significantly alter α‐SMA expression, collectively exposure to these agents significantly reduced collagen I, fibronectin and α‐SMA and restored E‐cadherin mRNA expression in HK‐2 cells (Figure 2F, G). Although all three compounds had beneficial effects, PZC and PZD showed stronger bioactivities than PZE. Furthermore, PZD was more bioactive compared with PZC. These results indicate that the structures of these compounds have an important effect on their bioactivities.
PZC, PZD and PZE suppress TGFβ1‐ and AngII‐induced RAS activation
To investigate the underlying mechanism by which the three novel compounds inhibit EMT production, Western blot analyses were performed to detect RAS components including AGT, renin, ACE and AT1 receptors. TGFβ1 and AngII stimulation up‐regulated the protein expression of all of the RAS components in HK‐2 cells. Except for PZE which did not affect ACE and AT1 receptors, PZC, PZD and PZE treatment significantly down‐regulated the protein expression of RAS components in HK‐2 cells (Figure 3A–D). Additionally, the significant upregulation of AGT, renin, ACE and AT1 receptor mRNA expression were inhibited in TGFβ1‐ or AngII‐stimulated HK‐2 cells (Figure 3E, F). Immunofluorescent staining also showed that PZC and PZD treatment had a significant inhibitory effect on the expression of AT1 receptors in TGFβ1‐stimulated HK‐2 cells (Figure 3G). The inhibitory effects of PZC and PZD on AT1 receptor expression in TGFβ1‐stimulated HK‐2 cells were similar to the effects of losartan, while PZE did not have significant effect on AT1 receptor expression. Immunofluorescent staining also showed that PZC, PZD or PZE alone had no effect on AT1 receptor protein expression (Figure 3G). The results indicate that these compounds do not affect HK‐2 cells in the absence of TGFβ1 stimulation. So secolanostane tetracyclic triterpenoid compounds served as simultaneous inhibitors of multiple targets within the RAS, whereas PZE only has a strong inhibitory effect on AGT and renin in HK‐2 cells. Hence, lanostane tetracyclic triterpenoid compounds are considered to be renin‐specific inhibitors.
Figure 3.
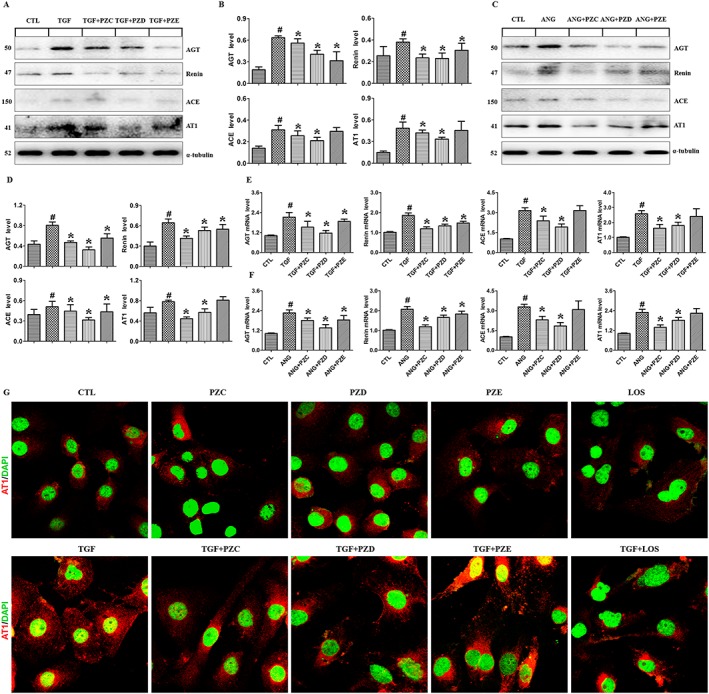
PZC, PZD and PZE suppress the activation of RAS in the TGFβ1‐ or AngII‐stimulated HK‐2 cells and podocytes. (A) Protein expression and (B) quantitative analysis of AGT, renin, ACE and AT1 receptors in the HK‐2 cells induced by TGFβ1. (C) Protein expression and (D) quantitative analysis of RAS components in the HK‐2 cells induced by AngII. qRT‐PCR analysis of the mRNA expression of genes encoding AGT, renin, ACE and AT1 receptors in the HK‐2 cells induced by TGFβ1 (E) or AngII (F). (G) HK‐2 cells stimulated by TGFβ1 were stained with primary antibody against AT1 receptors. The in situ expression of AT1 receptors was analysed by Laser scanning confocal microscopes. Representative immunofluorescent staining images of TGFβ1‐ stimulated increase in AT1 receptor expression in the different groups. # P < 0.05 compared with control group (n = 6); * P < 0.05 compared with TGFβ1‐ or AngII‐ stimulated group (n = 6). LOS, losartan.
PZC, PZD and PZE suppress the up‐regulation of Wnt/β‐catenin signalling
To evaluate the effects of PZC, PZD and PZE on the Wnt/β‐catenin signalling in HK‐2 cells, Western blot analysis was used to detect Wnt‐1 and β‐catenin as well as its downstream mediators, which include Snail1, Twist, MMP7, PAI‐1 and FSP1, in TGFβ1‐ and AngII‐stimulated HK‐2 cells. The addition of TGFβ1 and AngII up‐regulated the expression of Wnt‐1, β‐catenin and its target proteins in HK‐2 cells. PZC, PZD and PZE significantly decreased the expressions of these proteins in HK‐2 cells (Figure 4A–D). Immunofluorescent staining showed that TGFβ1 up‐regulated β‐catenin protein expression in HK‐2 cells and PZC, PZD and PZE treatment significantly down‐regulated the active β‐catenin protein expression in TGFβ1‐stimulated HK‐2 cells (Figure 4G). Losartan also showed an inhibitory effect on β‐catenin expression. In addition, immunofluorescent staining showed that PZC, PZD or PZE alone had no effect on β‐catenin protein expression in HK‐2 cells (Figure 4G). Interestingly, PZD showed a stronger inhibitory effect than the other two compounds. Taken together, these findings demonstrate the inhibitory effects of the three compounds on the Wnt/β‐catenin pathway in HK‐2 cells.
Figure 4.
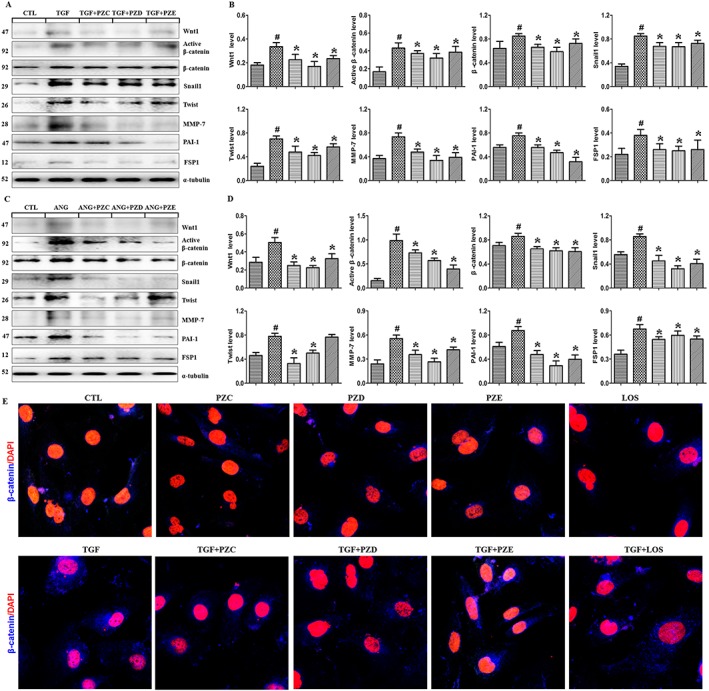
PZC, PZD and PZE inhibited the up‐regulation of Wnt1/β‐catenin signalling in the TGFβ1‐ or AngII‐stimulated HK‐2 cells and podocytes. (A) Protein expression and (B) quantitative analysis of Wnt1, active β‐catenin, β‐catenin and its target proteins Snail1, Twist, MMP7, PAI‐1 and FSP1 in the HK‐2 cells induced by TGFβ1. (C) Protein expression and (D) quantitative analysis of Wnt‐1/β‐catenin signalling in the HK‐2 cells induced by AngII. (E) HK‐2 cells stimulated by TGFβ1 were stained with primary antibody against β‐catenin. The in situ expression of β‐catenin was analysed. Representative immunofluorescent staining images of TGFβ1‐induced β‐catenin expression in the different groups. # P < 0.05 compared with control group (n = 6); * P < 0.05 compared with TGFβ1‐ or AngII‐ stimulated group (n = 6).
PZC, PZD and PZE selectively inhibit Smad3 phosphorylation
To further explore the mechanism underlying the anti‐fibrotic effect of PZC, PZD and PZE, we examined their effects on the activation of TGFβ/Smad signalling in TGFβ1‐ and AngII‐treated HK‐2 cells. TGFβ1 and AngII significantly increased the expression of TGFBR2 but not TGFBR1 in HK‐2 cells and podocytes. PZD and PZE significantly inhibited the up‐regulation of TGFBR2 in the TGFβ1‐treated HK‐2 cells (Figure 5A, B). However, PZC and PZD significantly inhibited the up‐regulation of TGFBR2 in the AngII‐treated HK‐2 cells (Figure 5C, D). TGFβ1 and AngII significantly induced phosphorylation of Smad2 and Smad3 in both HK‐2 cells (Figure 5A–D). PZC, PZD and PZE attenuated TGFβ1‐ and AngII‐induced Smad3 phosphorylation but did not affect Smad2 phosphorylation. To confirm the specific effect of PZC, PZD and PZE on Smad3, we further examined protein expression of other Smads including Smad4 and Smad7. PZC, PZD and PZE did not affect the protein expression of Smad4 and Smad7 (Figure 5A–D). Immunofluorescent staining showed that PZC, PZD and PZE treatment had a significant inhibitory effect on the p‐Smad3 in the TGFβ1‐stimulated HK‐2 cells (Figure 5E). Immunofluorescent staining also showed that PZC, PZD and PZE had no effect on p‐Smad3 protein expression in untreated HK‐2 cells (Figure 5E). These results demonstrate that these compounds do not affect HK‐2 cells in the absence of TGFβ1 stimulation. Taken together, these data indicate that PZC, PZD and PZE selectively inhibit Smad3 phosphorylation. Interestingly, PZC and PZD showed a stronger inhibitory effect on TGFBR1 and Smad3 than PZE.
Figure 5.
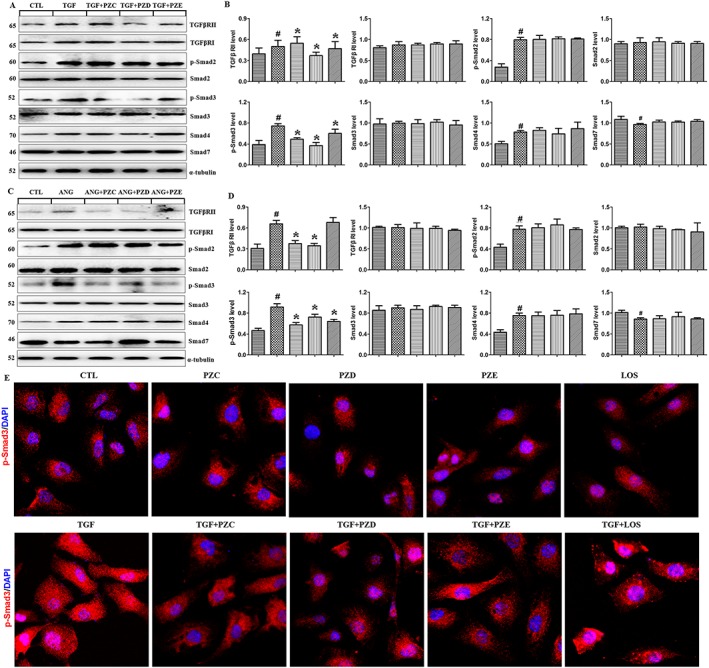
PZC, PZD and PZE suppressed the up‐regulation of TGFβ/Smad signalling in the TGFβ1‐ or AngII‐stimulated HK‐2 cells and podocytes. (A) Protein expression and (B) quantitative analysis of TGFβ/Smad signalling in the HK‐2 cells induced by TGFβ1. (C) Protein expression and (D) quantitative analysis of TGFβ/Smad signalling in the HK‐2 cells induced by AngII. (E) HK‐2 cells stimulated by TGF‐β1 were stained with primary antibody against p‐Smad3. The in situ expression of p‐Smad3 was analysed by Laser scanning confocal microscopes. Representative immunofluorescent staining images of TGF‐β1‐induced p‐Smad3 expression in the different groups. # P < 0.05 compared with control group (n = 6); * P < 0.05 compared with TGFβ1‐ or AngII‐stimulated group (n = 6).
PZC, PZD and PZE selectively suppress the interaction of p‐Smad3 with TGFBR1
To further test whether PZC, PZD and PZE specifically affect Smad3 phosphorylation, we knocked down the Smad3 expression at the cellular level using RNA interference in TGFβ1‐stimulated HK‐2 cells. Western blot analysis showed that Smad3 protein in knocked‐down cells was significantly lower compared with that in normal cells and scrambled siRNA‐infected cells (Figure 6A). Furthermore, compared with Smad3 siRNA in the absence of TGFβ1 stimulation, Smad3 protein expression did not change after treatment with three compounds. The results indicate that the three compounds had no effects on Smad3 protein expression without TGFβ1 stimulation and had no influence on the process of RNA interference (Figure 6B). Due to the suppression of Smad3 expression by siRNA, the inhibitory effects of PZC, PZD and PZE on the up‐regulation of collagen I, fibronectin, α‐SMA and E‐cadherin were abolished (Figure 6C, D). These data further demonstrate that PZC, PZD and PZE exert anti‐fibrotic effects via specific effects on the Smad3 phosphorylation pathway. Therefore, PZC, PZD and PZE can serve as potent inhibitors of the TGFβ1/Smad3 pathway.
Figure 6.
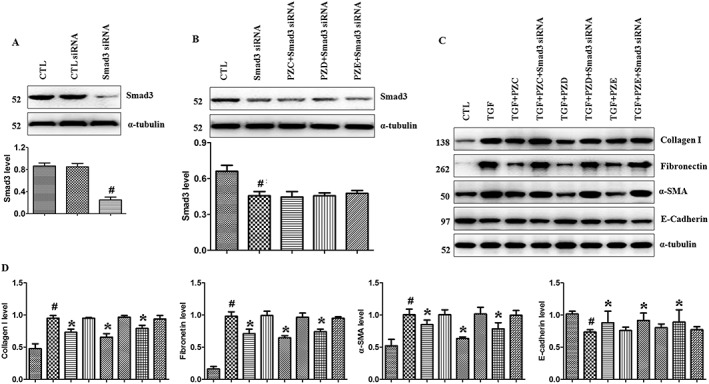
PZC, PZD and PZE effects on Smad3 siRNA during TGFβ1‐stimulated tubular EMT in HK‐2 cells. (A) Knockdown of Smad3 in the TGFβ1‐stimulated HK‐2 cells. HK‐2 cells were infected with Smad3‐specific siRNA or scrambled siRNA. Smad3 protein expression was examined by Western blot analysis. # P < 0.05 compared with control (CTL) siRNA group. (B) After being infected with Smad3‐specific siRNA or scrambled siRNA, HK‐2 cells were treated with PZC, PZD and PZE without treatment with TGFβ1. Smad3 protein expression was examined by Western blot analysis. ## P < 0.01 compared with CTL group. (C) After being infected with Smad3‐specific siRNA or scrambled siRNA, TGFβ1‐stimulated HK‐2 cells were treated with PZC, PZD and PZE. Protein expression of collagen I, fibronectin, α‐SMA and E‐cadherin were detected by Western blot analysis. (D) Quantitative analysis of collagen I, fibronectin, α‐SMA and E‐cadherin expression in the HK‐2 cells induced by TGFβ1. # P < 0.05 compared with control group (n = 6); * P < 0.05 compared with TGFβ1‐induced group (n = 6).
Upon TGFβ1 stimulation, TGFBR2 phosphorylates TGFBR1, which binds to Smad2/3 and phosphorylates it. To test whether PZC, PZD and PZE affect the TGFBR1‐Smad3 interaction, co‐immunoprecipitation was carried out in TGFβ1‐stimulated HK‐2 cells. As shown in Figure 7, the interactions of TGFBR1 with p‐Smad2, Smad2, p‐Smad3 and Smad3 were enhanced in TGF‐β1‐stimulated HK‐2 cells. PZC, PZD and PZE treatment significantly inhibited the interaction of p‐Smad3 with TGFBR1 and the interaction of Smad3 with TGFBR1 but did not affect the interaction of p‐Smad2 with TGFBR1 and the interaction of Smad2 with TGFBR1. These results indicate that three compounds inhibited Smad3 phosphorylation by selectively blocking the Smad3‐TGFBR1 interaction.
Figure 7.
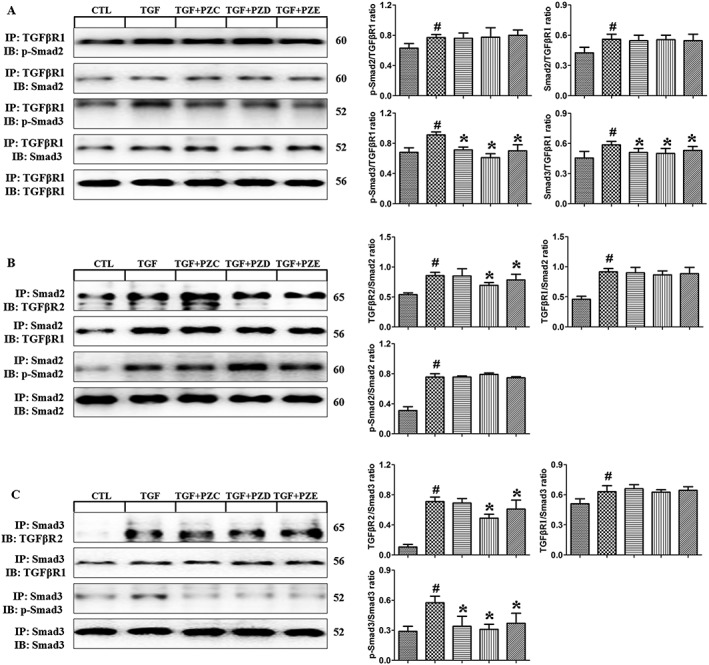
PZC, PZD and PZE block the interaction between TGFβ receptors and Smad in HK‐2 cells. (A) Cell lysates were immunoprecipitated with anti‐TGFBR1, followed by immunoblotting using antibodies against p‐Smad2, Smad2, p‐Smad3, Smad3 and TGFBR1. (B) Cell lysates were immunoprecipitated with anti‐Smad2, followed by immunoblotting using antibodies against TGFBR2, TGFBR1, p‐Smad2 and Smad2 (n = 6). (C) Cell lysates were immunoprecipitated with anti‐Smad3, followed by immunoblotting using antibodies against TGFBR2, TGFBR1, p‐Smad3 and Smad3. # P < 0.05 compared with control group (n = 6); * P < 0.05 compared with TGF β1‐stimulated group (n = 6). IP, immunoprecipitation; IB, immunoblotting.
PZC, PZD and PZE selectively inhibit Smad3 phosphorylation, RAS activation and Wnt/β‐catenin signalling in UUO mice
To confirm the specific effect of PZC, PZD and PZE on p‐Smad3 and RAS activation and Wnt/β‐catenin signalling pathway, we further examined their effects in UUO mice. In the preliminary study, PZC, PZD and PZE were given after the operation by oral gavage, 5, 10, 20 or 40 mg·kg−1 once every day for seven continuous days. Mice were killed on day 7. RT‐PCR and Western blot analyses showed that 10 mg·kg−1 of PZC, PZD and PZE significantly inhibited the α‐SMA expression at both the mRNA (Figure 8A) and protein (Figure 8B) levels. Therefore, 10 mg·kg−1 of PZC, PZD and PZE were used in the following experiments.
Figure 8.
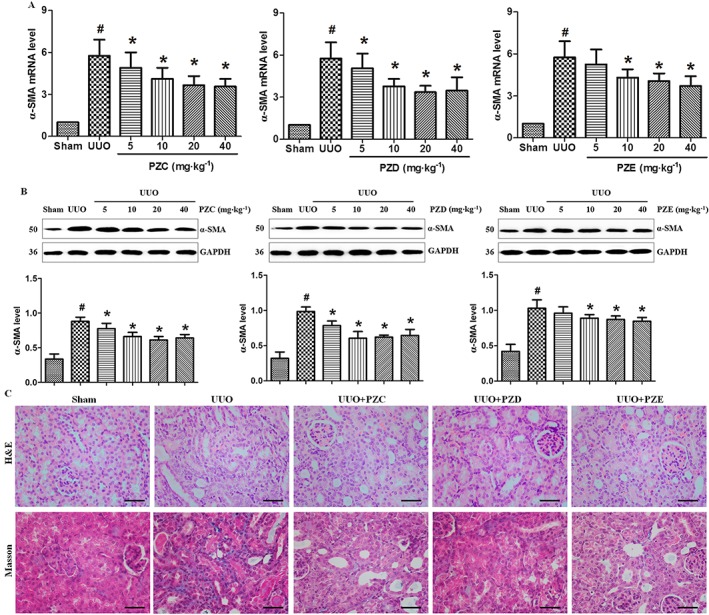
Intervention effects of PZC, PZD and PZE in UUO mice. (A) The inhibitory effects of different doses of PZC, PZD and PZE on α‐SMA mRNA expression in UUO mice. # P < 0.05 compared with sham group (n = 6). * P < 0.05 compared with UUO group (n = 6). (B) The different doses of PZC, PZD and PZE inhibitory effects on α‐SMA protein expression in UUO mice. (C) Representative micrographs showed kidney injury in different groups. Paraffin sections were used for H&E and Masson trichrome staining. Scale bar, 50 μm.
H&E and Masson's trichrome stainings exhibited severe interstitial inflammation and fibrosis in UUO mice. Treatment with PZC, PZD and PZE significantly attenuated inflammatory cell infiltration and renal fibrosis (Figure 8C). Treatment with PZC, PZD and PZE significantly suppressed the up‐regulation of collagen I, fibronectin and α‐SMA and down‐regulation of E‐cadherin in UUO mice (Figure 9A, B). Treatment with PZC and PZD significantly inhibited the expression of AGT, renin, ACE and AT1 receptors, but treatment with PZE only significantly inhibited AGT and renin in UUO mice (Figure 9C, D).
Figure 9.
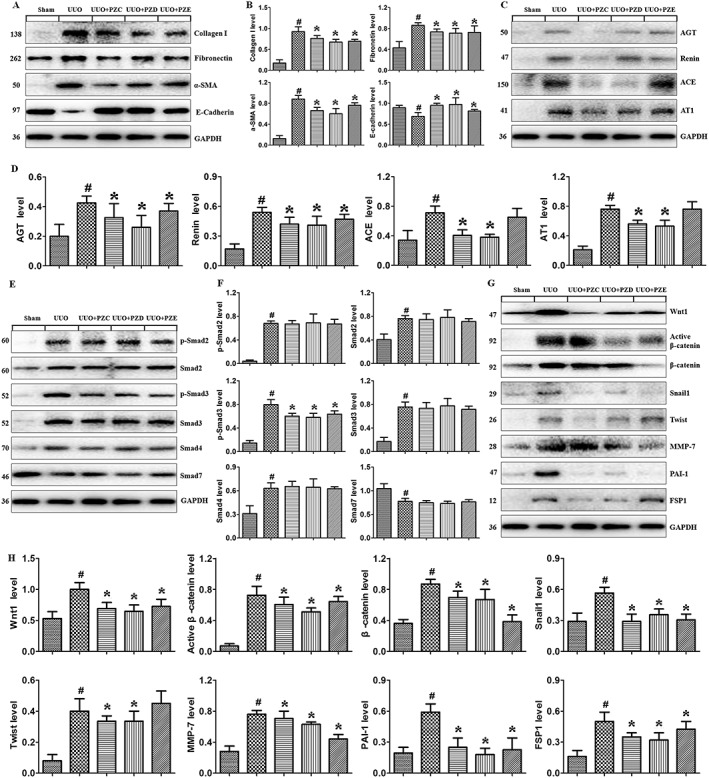
Intervention effects of PZC, PZD and PZE in UUO mice. (A, B) Western blot analyses and quantitative data show protein expression of collagen I, fibronectin, α‐SMA and E‐cadherin in UUO mice and the different treatment groups as indicated. (C, D) Western blot analyses and quantitative data show protein expression of AGT, renin, ACE and AT1 receptors in UUO mice and the different treatment groups as indicated. (E, F) Western blot analyses and quantitative data show protein expression of p‐Smad2, Smad2, p‐Smad3, Smad3, Smad4 and Smad7 in UUO mice and the different treatment groups as indicated. (G, H) Western blot analyses and quantitative data show protein expression of Wnt1, active β‐catenin, β‐catenin, Snail1, Twist, MMP7, PAI‐1 and FSP1 in UUO mice and the different treatment groups as indicated. # P < 0.05 compared with sham group (n = 6). * P < 0.05 compared with UUO group (n = 6).
To confirm the specific inhibitory effect of PZC, PZD and PZE on p‐Smad3, we further examined the expression of other Smads. PZC, PZD and PZE did not affect the protein expression of p‐Smad2, Smad2, Smad3, Smad4 and Smad7 in UUO mice but significantly inhibited p‐Smad3 expression (Figure 9E, F), suggesting that treatment with PZC, PZD and PZE selectively inhibited Smad3 phosphorylation in the TGFβ/Smad signalling pathway. Next, we tested the effect of PZC, PZD and PZE on the Wnt/β‐catenin signalling pathway. Treatment with PZC, PZD and PZE significantly inhibited the expression of Wnt‐1 and β‐catenin as well as its target proteins, which include Snail1, Twist, MMP‐7, PAI‐1 and FSP1 in UUO mice (Figure 9G, H). Taken together, these in vivo data indicate that PZC, PZD and PZE selectively inhibit Smad3 phosphorylation and suppress the activation of the RAS and Wnt/β‐catenin signalling pathway in UUO mice, which are consistent with the in vitro results.
Structure–function relationship and plausible biogenetic pathway of the new compounds
Our bioactivity and action mechanism analyses found that PZC and PZD showed a stronger bioactivity than PZE. This result indicates that compounds with a secolanostance skeleton have a stronger bioactivity than compounds with a lanostance skeleton. The renoprotective effect of these compounds is closely associated with the first ring in the tetracyclic triterpenoids. Our study suggested that only a slight variation of the first ring (from intact‐ring structure to opening‐ring structure) had a remarkable impact on the bioactivity. Increasing the number of carboxyl groups may be associated with the renoprotective effect. PZD showed a strong bioactivity compared with PZC. The results indicate that the removal of the OH group from the C‐29 position and addition of the OH group at C‐24 and C‐31 position caused a significant increase in bioactivity. The OH group in the side chain was important for the renoprotective effect, and the presence of more than one OH group in the side chain increased renoprotective activity. This observation was important for understanding the renoprotective biological activity of triterpenoids, which are contained in many fungi.
In this study, we isolated three novel triterpenoid compounds (PZC, PZD and PZE) from SLPC. The compound PZD showed a characteristic hydroxy substitution on the terminal methyl group and the CH2OH group on position 31 as well as the hydroxy on position 24, identified for the first time in triterpenoids isolated from SLPC. Moreover, the unsaturated double bond on positions 22 and 23 of the compound PZE was also found in triterpenoids isolated from SLPC for the first time. The 24,31‐epoxy‐poricoic acid A might act as an intermediate in the biosynthetic pathway of poricoic acid A to compound PZD (Figure 1D).
Discussion
Blockade of the RAS is a well‐accepted therapeutic strategy for the management of hypertension and kidney disease in patients with CKD. In fact, ACEI and ARB are the mainstay of the current clinical therapy for CKD and have been proven to reduce proteinuria, retard the rate of the decline in renal function and progression to end‐stage renal disease (ESRD). However, the following factors limit the efficacy of these agents: (i) administration of ACEI and ARB provokes a compensatory up‐regulation of renin expression via a homeostatic mechanism; (ii) AngII and aldosterone levels rise after ACEI or ARB administration and AngII‐escape and aldosterone escape occurs, when single components of the RAS are blocked; and (iii) the intrarenal RAS may remain active when the systemic RAS is fully blocked (Zhou and Liu, 2016). In addition, renal tubular AngII concentrations significantly exceed their systemic concentrations (Zhou and Liu, 2016). Therefore, therapeutic strategies that simultaneously target multiple RAS components will be a more effective treatment for CKD.
There is an accumulation of evidence demonstrating that numerous traditional Chinese medicines (TCM), which are widely used for treatment of various kidney diseases, confer beneficial and reliable therapeutic efficacy (Zhao et al., 2012a,c; 2013d; 2014; Zhang et al., 2016; Chen et al., 2018a). P. cocos is commonly prescribed as one of the main ingredients in composite prescriptions in TCM. Nearly 10% of the TCM preparations, recorded in the Chinese Pharmacopoeia (2015 edition), contain P. cocos. One of these preparation, Wulinsan, was shown to confer a significant renoprotective effect (Yang et al., 2015; Yoon et al., 2015) and is widely used in the treatment of patients with CKD. A number of studies have demonstrated the renoprotective effect of P. cocos (Lee et al., 2012; Yoon et al., 2013; Lee et al., 2014). In addition, several studies have shown the protective effect of lanostane triterpenoids derived from the sclerotia of P. cocos on renal tubular epithelial cells including LLC‐PK1 and HK‐2 cells (Lee et al., 2017; Wang et al., 2017a). In this study, we demonstrated for the first time that three novel RAS inhibitors including PZC, PZD and PZE, as tetracyclic triterpenoid compounds extracted and isolated from SLPC, significantly attenuated TIF by blocking the Wnt/β‐catenin and TGF‐β/Smad signalling cascades in vitro and in vivo (Figure 10). PZC, PZD and PZE selectively inhibited TGFβ1‐ and AngII‐induced Smad3 phosphorylation by blocking the interaction of TGFBR1 with Smad3, suggesting that these three compounds might be specific inhibitors of TGFβ1/Smad3 signalling. Our results further demonstrated that the bioactivity of secolanostane tetracyclic triterpenoid compounds PZC and PZD is stronger than that of PZE indicating that the bioactivity of compounds with a secolanostance skeleton is stronger than that of compounds with a lanostance skeleton. An increased number of carboxyl groups may be associated with their bioactivities. The OH group in the side chain was important for the anti‐fibrotic effect, and the presence of more than one OH group in the side chain increased anti‐fibrotic activity. These findings elucidate the mechanism of the anti‐fibrotic activity of many fungi containing triterpenoids.
Figure 10.
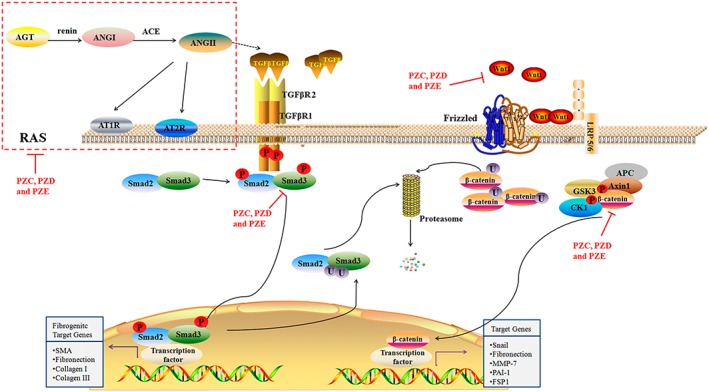
The signalling pathways involved in the intervention effects of PZC, PZD and PZE on TIF. The activated RAS, Wnt/β‐catenin and TGFβ1/Smad pathways in the HK‐2 cells and podocytes and the renoprotective effect of PZC, PZD and PZE.
Activation of the RAS is a cascade event in which two major enzymes, renin and ACE, convert AGT to biologically active AngII. The secolanostane tetracyclic triterpenoids PZC and PZD showed a strong inhibitory effect on all RAS components in vitro and in vivo. Therefore, secolanostane tetracyclic triterpenoids could be considered as RAS inhibitors. The interactions between AngII and the TGFβ axis are complex and multiple, while both AngII and TGFβ accelerate renal fibrosis. Many reports have shown that TGFβ1 is a downstream mediator of AngII‐induced renal fibrosis and both factors share several intracellular signalling pathways involved in the regulation of ECM synthesis and accumulation (Urushihara and Kagami, 2017; Yang and Xu, 2017). AngII can cause pro‐fibrotic effects in proximal tubular epithelial cells, podocytes and mesangial cells of the kidney. AngII stimulates the transcription and synthesis of TGFβ1 in murine proximal tubular epithelial cells and up‐regulates TGFβ receptors, further amplifying the fibrotic process (Yang and Xu, 2017). AngII activates kidney cells to produce numerous pro‐fibrotic factors and increase ECM production (Urushihara and Kagami, 2017; Yang and Xu, 2017). In particular, both AngII and TGFβ1 induce EMT production in tubular epithelial cells (Chen et al., 2017c). Several studies have demonstrated that AngII causes cell proliferation and ECM expansion via a TGFβ1‐dependent signalling pathway in murine proximal tubular epithelial cells (Yang et al., 2010). AngII directly stimulates complex interactions in the TGFβ axis and gene encoding TGFβ transcription. The AngII‐mediated stimulation of collagen expression and cellular hypertrophy depend on TGFβ expression (Urushihara and Kagami, 2017). AngII stimulates the proliferation of renal fibroblasts and increases mRNA expressions of TGFβ, fibronectin and collagen I. TGFβ subsequently stimulates the accumulation of ECM and inflammation. Recent studies have demonstrated that TGFβ1 induces an up‐regulation of RAS genes including AGT, renin and AT1 receptors in proximal tubular epithelial cells and fibroblasts (Zhou and Liu, 2016). Hence, the suppression on TGFβ1 and Ang II plays a decisive role in attenuating TIF. Our study demonstrated that PZC and PZD strongly inhibit the effects of both TGFβ1 and AngII on TIF. Thus, secolanostane tetracyclic triterpenoid compounds may simultaneously inhibit multiple RAS components.
PZE only had a strong inhibitory effect on AGT and renin in HK‐2 cells and UUO mice. This lanostane tetracyclic triterpenoid compound could be considered as a renin‐specific inhibitor. An overexpression of renin and AGT leads to an increased production of ECM without inducing systemic hypertension. Renin increases ECM production by up‐regulating TGFβ1 (Urushihara and Kagami, 2017), which is independent of its enzymatic action, that is, AngII generation. Renin can trigger multiple intracellular signalling pathways and promote the protein expression of TGFβ1 and EMT via an AngII‐independent mechanism (Zhang et al., 2012). Renin is the rate‐limiting step in RAS activation, and direct renin inhibition has been tested in a clinical setting (Jensen et al., 2008). Hence, the inhibition of RAS plays a beneficial role in the treatment of renal fibrosis. Another large clinical trial comparing the efficacy of the AT1 receptor blocker, telmisartan, with that of the ACE inhibitor, ramipril, or their combination, in a large group of patients with established atherosclerotic vascular disease and patients with diabetes and end‐organ damage demonstrated significant adverse renal outcomes in patients treated with a combination of ARB and ACE inhibitor (Mann et al., 2008).
Numerous genes that encode RAS are direct downstream targets of Wnt/β‐catenin, which is an evolutionarily developmental signalling pathway that plays a critical role in organ development and tissue homeostasis. In the adult kidney, Wnt/β‐catenin signalling is silenced. However, a wide variety of kidney diseases such as obstructive nephropathy, remnant kidney and diabetic nephropathy result in reactivation of the Wnt/β‐catenin system (Tan et al., 2014; Zhou and Liu, 2015; Zhou and Liu, 2016). Activated β‐catenin, in turn, promotes the binding of LEF‐1/TCF‐4 to these sites and induces multiple RAS gene expressions in proximal tubular epithelial cells (Zhou et al., 2015). These findings have identified Wnt/β‐catenin as an important upstream regulator that controls the expression of multiple RAS genes. Our results showed that TGFβ1 and AngII up‐regulate the expression of β‐catenin and its target genes including Snail1, Twist, MMP7, PAI‐1 and FSP1 in HK‐2 cells. PZC, PZD and PZE treatment significantly decreased the expression of these proteins in HK‐2 cells. Interestingly, PZD showed a stronger inhibitory effect than the other two compounds. Our findings demonstrate that these three new compounds inhibit activation of the Wnt/β‐catenin pathway.
Several extensive reviews have described the critical role of TGFβ/Smad signalling in the pathogenesis of CKD in humans and experimental animals (Meng et al., 2015; Meng et al., 2016; Walton et al., 2017; Chen et al., 2018b). It was reported that AngII comes into play through AT1 receptors to activate Smad3 and induce EMT production in AngII‐stimulated rat tubular epithelial cells (Yang et al., 2010). The present study showed that treatment with PZC, PZD and PZE dramatically suppressed the rise in p‐Smad3 both in vitro and in vivo. PZC, PZD and PZE treatment inhibited the nuclear translocation of the Smads complex and expression of Smad‐driven genes including collagen I, α‐SMA and fibronectin. The inhibitory effects of PZC, PZD and PZE were specific for Smad3 phosphorylation, they did not affect Smad2 phosphorylation or the protein expressions of Smad4 and Smad7.
To investigate whether PZC, PZD and PZE specifically inhibit p‐Smad3, we knocked down the expression of Smad3 by RNA interference in TGFβ1‐stimulated HK‐2 cells. After depletion of Smad3 by siRNA, the inhibitory effects of PZC, PZD and PZE on collagen I, fibronectin, α‐SMA and E‐cadherin were partially inhibited. These results further demonstrate that PZC, PZD and PZE attenuated renal injury via specific blockade of Smad3 phosphorylation. These findings identified PZC, PZD and PZE as potential inhibitors of TGFβ1/Smad3 signalling.
The generation of p‐Smad3 induced by the interaction of TGFBR1 and Smad3 is a pivotal step in TGFβ1/Smad pathway. To further illuminate the mechanisms by which PZC, PZD and PZE specifically inhibited Smad3 phosphorylation, we investigated the effects of PZC, PZD and PZE on the interaction of Smad2/Smad3 with TGFβ receptors. Co‐immunoprecipitation analysis indicated that PZC, PZD and PZE selectively blocked the interaction of TGFBR1 and Smad3 without interfering with the interaction of TGFBR1 with Smad2.
In conclusion, the present study identified three novel natural RAS inhibitors, PZC, PZD and PZE, which were derived from SLPC. PZC and PZD showed strong inhibitory effects on all RAS components while PZE showed a strong inhibitory effect on renin. They significantly attenuated TIF by inhibiting activation of the Wnt/β‐catenin pathway and blocking Smad3 phosphorylation in vitro and in vivo. These compounds selectively inhibited TGFβ1‐ and AngII‐induced Smad3 phosphorylation by blocking the interaction of TGFBR1 and Smad3. Our results further indicated that the secolanostane tetracyclic triterpenoid compounds PZC and PZD conferred stronger renoprotective effects than the lanostane tetracyclic triterpenoid compound PZE. Thus, secolanostane tetracyclic triterpenoids can be developed as novel inhibitors that simultaneously target multiple RAS components, and lanostane tetracyclic triterpenoid compounds can be developed as novel renin inhibitors. Our results provide several potential leads for the development of novel compounds for effective treatment of CKD.
Author contributions
M.W., D.Q.C., H.Z., L.C. and D.L. performed the experiments and analysed the data. M.W., D.Q.C. and Y.Y.Z. analysed the data and contributed to the manuscript writing. N.D.V. and Y.G. contributed to the manuscript writing.
Conflict of interest
The authors declare no conflict of interest.
Declaration of transparency and scientific rigour
This http://onlinelibrary.wiley.com/doi/10.1111/bph.13405/abstract acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Nos 81673578 and 81603271).
Wang, M. , Chen, D.‐Q. , Chen, L. , Cao, G. , Zhao, H. , Liu, D. , Vaziri, N. D. , Guo, Y. , and Zhao, Y.‐Y. (2018) Novel inhibitors of the cellular renin‐angiotensin system components, poricoic acids, target Smad3 phosphorylation and Wnt/β‐catenin pathway against renal fibrosis. British Journal of Pharmacology, 175: 2689–2708. doi: 10.1111/bph.14333.
References
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174 (Suppl 1): S17–s129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: enzymes. Br J Pharmacol 174 (Suppl 1): S272–s359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD et al (2017c). The Concise Guide to PHARMACOLOGY 2017/18: overview. Br J Pharmacol 174 (Suppl 1): S1–s16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DQ, Cao G, Chen H, Liu D, Su W, Yu XY et al (2017a). Gene and protein expressions and metabolomics exhibit activated redox signaling and wnt/β‐catenin pathway are associated with metabolite dysfunction in patients with chronic kidney disease. Redox Biol 12: 505–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DQ, Chen H, Chen L, Vaziri ND, Wang M, Li XR et al (2017b). The link between phenotype and fatty acid metabolism in advanced chronic kidney disease. Nephrol Dial Transplant 32: 1154–1166. [DOI] [PubMed] [Google Scholar]
- Chen H, Cao G, Chen DQ, Wang M, Vaziri ND, Zhang ZH et al (2016). Metabolomics insights into activated redox signaling and lipid metabolism dysfunction in chronic kidney disease progression. Redox Biol 10: 168–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Yang T, Wang MC, Chen DQ, Yang Y, Zhao YY (2018a). Novel RAS inhibitor 25‐O‐methylalisol F attenuates epithelial‐to‐mesenchymal transition and tubulo‐interstitial fibrosis by selectively inhibiting TGF‐β‐mediated Smad3 phosphorylation. Phytomedicine 41: 207–218. [DOI] [PubMed] [Google Scholar]
- Chen L, Chen DQ, Wang M, Liu D, Chen H, Dou F et al (2017c). Role of RAS/Wnt/β‐catenin axis activation in the pathogenesis of podocyte injury and tubulo‐interstitial nephropathy. Chem Biol Interact 273: 56–72. [DOI] [PubMed] [Google Scholar]
- Chen L, Yang T, Lu DW, Zhao H, Feng YL, Chen H et al (2018b). Central role of dysregulation of TGF‐β/Smad in CKD progression and potential targets of its treatment. Biomed Pharmacother 101: 670–681. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agati VD, Chagnac A, de Vries AP, Levi M, Porrini E, Herman‐Edelstein M et al (2016). Obesity‐related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat Rev Nephrol 12: 453–471. [DOI] [PubMed] [Google Scholar]
- Edeling M, Ragi G, Huang S, Pavenstadt H, Susztak K (2016). Developmental signalling pathways in renal fibrosis: the roles of Notch, Wnt and Hedgehog. Nat Rev Nephrol 12: 426–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng YL, Lei P, Tian T, Yin L, Chen DQ, Chen H et al (2013). Diuretic activity of some fractions of the epidermis of Poria cocos . J Ethnopharmacol 150: 1114–1118. [DOI] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res 46: D1091–d1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen C, Herold P, Brunner HR (2008). Aliskiren: the first renin inhibitor for clinical treatment. Nat Rev Drug Discov 7: 399–410. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klahr S, Morrissey J (2002). Obstructive nephropathy and renal fibrosis. Am J Physiol Renal Physiol 283: F861–F875. [DOI] [PubMed] [Google Scholar]
- Kobori H, Nangaku M, Navar LG, Nishiyama A (2007). The intrarenal renin‐angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251–287. [DOI] [PubMed] [Google Scholar]
- Lee D, Lee S, Shim SH, Lee HJ, Choi Y, Jang TS et al (2017). Protective effect of lanostane triterpenoids from the sclerotia of Poria cocos Wolf against cisplatin‐induced apoptosis in LLC‐PK1 cells. Bioorg Med Chem Lett 27: 2881–2885. [DOI] [PubMed] [Google Scholar]
- Lee SM, Lee YJ, Yoon JJ, Kang DG, Lee HS (2012). Effect of Poria cocos on hypertonic stress‐induced water channel expression and apoptosis in renal collecting duct cells. J Ethnopharmacol 141: 368–376. [DOI] [PubMed] [Google Scholar]
- Lee SM, Lee YJ, Yoon JJ, Kang DG, Lee HS (2014). Effect of Poria cocos on puromycin aminonucleoside‐induced nephrotic syndrome in rats. Evid Based Complement Alternat Med 2014: 570420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J et al (2008). Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double‐blind, controlled trial. Lancet 372: 547–553. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng XM, Nikolic‐Paterson DJ, Lan HY (2016). TGF‐β: the master regulator of fibrosis. Nat Rev Nephrol 12: 325–338. [DOI] [PubMed] [Google Scholar]
- Meng XM, Tang PMK, Li J, Lan HY (2015). TGF‐β/Smad signaling in renal fibrosis. Front Physiol 6: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H, Li MH, Zhang X, Yuan SJ, Ho CC, Zhao YY (2015). The antihyperlipidemic effect of Fu‐Ling‐Pi is associated with abnormal fatty acid metabolism as assessed by UPLC‐HDMS‐based lipidomics. RSC Adv 5: 64208–64219. [Google Scholar]
- Miao H, Zhao YH, Vaziri ND, Tang DD, Chen H, Chen H et al (2016). Lipidomics biomarkers of diet‐induced hyperlipidemia and Its treatment with Poria cocos . J Agric Food Chem 64: 969–979. [DOI] [PubMed] [Google Scholar]
- Siragy HM, Carey RM (2010). Role of the intrarenal renin‐angiotensin‐aldosterone system in chronic kidney disease. Am J Nephrol 31: 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spranger S, Bao R, Gajewski TF (2015). Melanoma‐intrinsic β‐catenin signalling prevents anti‐tumour immunity. Nature 523: 231–235. [DOI] [PubMed] [Google Scholar]
- Tan RJ, Zhou D, Zhou L, Liu Y (2014). Wnt/β‐catenin signaling and kidney fibrosis. Kidney Int Suppl (2011) 4: 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian T, Chen H, Zhao YY (2014). Traditional uses, phytochemistry, pharmacology, toxicology and quality control of Alisma orientale (Sam.) Juzep: a review. J Ethnopharmacol 158: 373–387. [DOI] [PubMed] [Google Scholar]
- Urushihara M, Kagami S (2017). Role of the intrarenal renin‐angiotensin system in the progression of renal disease. Pediatr Nephrol 32: 1471–1479. [DOI] [PubMed] [Google Scholar]
- Walton KL, Johnson KE, Harrison CA (2017). Targeting TGF‐β mediated SMAD signaling for the prevention of fibrosis. Front Pharmacol 8: 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Chen DQ, Chen L, Zhao H, Liu D, Zhang ZH et al (2018). Novel RAS inhibitors poricoic acid ZG and poricoic acid ZH attenuate renal fibrosis via Wnt/β‐catenin pathway and targeted phosphorylation of smad3 signaling. J Agric Food Chem 66: 1828–1842. [DOI] [PubMed] [Google Scholar]
- Wang M, Chen DQ, Wang MC, Chen H, Chen L, Liu D et al (2017a). Poricoic acid ZA, a novel RAS inhibitor, attenuates tubulo‐interstitial fibrosis and podocyte injury by inhibiting TGF‐β/Smad signaling pathway. Phytomedicine 36: 243–253. [DOI] [PubMed] [Google Scholar]
- Wang M, Chen L, Liu D, Chen H, Tang DD, Zhao YY (2017b). Metabolomics highlights pharmacological bioactivity and biochemical mechanism of traditional Chinese medicine. Chem Biol Interact 273: 133–141. [DOI] [PubMed] [Google Scholar]
- Wang YZ, Zhang J, Zhao YL, Li T, Shen T, Li JQ et al (2013). Mycology, cultivation, traditional uses, phytochemistry and pharmacology of Wolfiporia cocos (Schwein.) Ryvarden et Gilb.: a review. J Ethnopharmacol 147: 265–276. [DOI] [PubMed] [Google Scholar]
- Yang F, Huang XR, Chung AC, Hou CC, Lai KN, Lan HY (2010). Essential role for Smad3 in angiotensin II‐induced tubular epithelial‐mesenchymal transition. J Pathol 221: 390–401. [DOI] [PubMed] [Google Scholar]
- Yang T, Xu C (2017). Physiology and pathophysiology of the intrarenal renin‐angiotensin system: an update. J Am Soc Nephrol 28: 1040–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Zhang DM, Liu JH, Hu LS, Xue QC, Ding XQ et al (2015). Wuling San protects kidney dysfunction by inhibiting renal TLR4/MyD88 signaling and NLRP3 inflammasome activation in high fructose‐induced hyperuricemic mice. J Ethnopharmacol 169: 49–59. [DOI] [PubMed] [Google Scholar]
- Yoon JJ, Lee YJ, Lee SM, Jin SN, Kang DG, Lee HS (2013). Poria cocos inhibits high glucose‐induced proliferation of rat mesangial cells. Am J Chin Med 41: 71–83. [DOI] [PubMed] [Google Scholar]
- Yoon JJ, Lee YJ, Lee SM, Kang DG, Lee HS (2015). Oryeongsan suppressed high glucose‐induced mesangial fibrosis. BMC Complement Altern Med 15: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wu J, Gu C, Noble NA, Border WA, Huang Y (2012). Receptor‐mediated nonproteolytic activation of prorenin and induction of TGF‐β1 and PAI‐1 expression in renal mesangial cells. Am J Physiol Renal Physiol 303: F11–F20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZH, Vaziri ND, Wei F, Cheng XL, Bai X, Zhao YY (2016). An integrated lipidomics and metabolomics reveal nephroprotective effect and biochemical mechanism of Rheum officinale in chronic renal failure. Sci Rep 6: 22151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZH, Wei F, Vaziri ND, Cheng XL, Bai X, Lin RC et al (2015). Metabolomics insights into chronic kidney disease and modulatory effect of rhubarb against tubulointerstitial fibrosis. Sci Rep 5: 14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YY (2013). Traditional uses, phytochemistry, pharmacology, pharmacokinetics and quality control of Polyporus umbellatus (Pers.) Fries: a review. J Ethnopharmacol 149: 35–48. [DOI] [PubMed] [Google Scholar]
- Zhao YY, Chen H, Tian T, Chen DQ, Bai X, Wei F (2014). A pharmaco‐metabonomic study on chronic kidney disease and therapeutic effect of ergone by UPLC‐QTOF/HDMS. PLoS One 23: e115467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YY, Cheng XL, Cui JH, Yan XR, Wei F, Bai X et al (2012a). Effect of ergosta‐4,6,8(14),22‐tetraen‐3‐one (ergone) on adenine‐induced chronic renal failure rat: a serum metabonomic study based on ultra performance liquid chromatography/high‐sensitivity mass spectrometry coupled with MassLynx i‐FIT algorithm. Clin Chim Acta 413: 1438–1445. [DOI] [PubMed] [Google Scholar]
- Zhao YY, Feng YL, Bai X, Tan XJ, Lin RC, Mei Q (2013a). Ultra performance liquid chromatography‐based metabonomic study of therapeutic effect of the surface layer of Poria cocos on adenine‐induced chronic kidney disease provides new insight into anti‐fibrosis mechanism. PLoS One 8: e59617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YY, Feng YL, Du X, Xi ZH, Cheng XL, Wei F (2012b). Diuretic activity of the ethanol and aqueous extracts of the surface layer of Poria cocos in rat. J Ethnopharmacol 144: 775–778. [DOI] [PubMed] [Google Scholar]
- Zhao YY, Lei P, Chen DQ, Feng YL, Bai X (2013b). Renal metabolic profiling of early renal injury and renoprotective effects of Poria cocos epidermis using UPLC Q‐TOF/HSMS/MSE . J Pharm Biomed Anal 81‐82: 202–209. [DOI] [PubMed] [Google Scholar]
- Zhao YY, Li HT, Feng YI, Bai X, Lin RC (2013c). Urinary metabonomic study of the surface layer of Poria cocos as an effective treatment for chronic renal injury in rats. J Ethnopharmacol 148: 403–410. [DOI] [PubMed] [Google Scholar]
- Zhao YY, Shen X, Cheng XL, Wei F, Bai X, Lin RC (2012c). Urinary metabonomics study on the protective effects of ergosta‐4,6,8(14),22‐tetraen‐3‐one on chronic renal failure in rats using UPLC Q‐TOF/MS and a novel MSE data collection technique. Process Biochem 47: 1980–1987. [Google Scholar]
- Zhao YY, Zhang L, Long FY, Cheng XL, Bai X, Wei F et al (2013d). UPLC‐Q‐TOF/HSMS/MSE‐based metabonomics for adenine‐induced changes in metabolic profiles of rat faeces and intervention effects of ergosta‐4,6,8(14),22‐tetraen‐3‐one. Chem Biol Interact 201: 31–38. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Deng Y, Chen Y, Chuang PY, Cijiang He J (2013). Therapeutic use of traditional Chinese herbal medications for chronic kidney diseases. Kidney Int 84: 1108–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Menon MC, Deng Y, Chen Y, He JC (2015). Recent advances in traditional Chinese medicine for kidney disease. Am J Kidney Dis 66: 513–522. [DOI] [PubMed] [Google Scholar]
- Zhou L, Li Y, Hao S, Zhou D, Tan RJ, Nie J et al (2015). Multiple genes of the renin‐angiotensin system are novel targets of Wnt/β‐catenin signaling. J Am Soc Nephrol 26: 107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Liu Y (2015). Wnt/β‐catenin signalling and podocyte dysfunction in proteinuric kidney disease. Nat Rev Nephrol 11: 535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Liu Y (2016). Wnt/β‐catenin signaling and renin‐angiotensin system in chronic kidney disease. Curr Opin Nephrol Hypertens 25: 100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]


