Abstract
Cumulative risk assessment (CRA) methods promote the use of a conceptual site model (CSM) to apportion exposures and integrate risk from multiple stressors. While CSMs may encompass multiple species, evaluating endpoints across taxa can be challenging due to data availability and physiological differences among organisms. Adverse Outcome Pathways (AOPs) describe biological mechanisms leading to adverse outcomes (AOs) by assembling causal pathways with measurable intermediate steps termed key events (KEs), thereby providing a framework for integrating data across species. We used a case study focused on the perchlorate anion (ClO4−) to highlight the value of the AOP framework for cross-species data integration. Computational models and dose-response data were used to evaluate the effects of ClO4− in twelve species and revealed a dose-response concordance across KEs and taxa. The Aggregate Exposure Pathway (AEP) tracks stressors from sources to the exposures and serves as a complement to the AOP. We discuss how the combined AEP-AOP construct helps to maximize the use of existing data and advances CRA by 1) organizing toxicity and exposure data, 2) providing a mechanistic framework of KEs for integrating data across human health and ecological endpoints, 3) facilitating cross-species dose-response evaluation, and 4) highlighting data gaps and technical limitations.
Keywords: Cumulative risk assessment, Adverse Outcome Pathway (AOP), Ecotoxicology, Human Health, Aggregate exposure
Graphical abstract
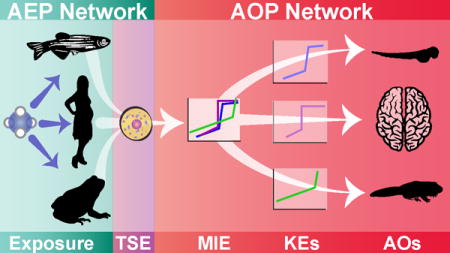
1. Introduction
Researchers commonly evaluate risk from chemical stressors in the context of a single substance and target organism1. As a regulatory agency, the United States Environmental Protection Agency (EPA) is often required to meet its statutory mandate on a chemical-by-chemical basis. However, understanding how simultaneous exposure to multiple environmental contaminants and non-chemical stressors affects the health of individuals and populations is often relevant2,3. Growing concerns about this issue led the EPA to develop a framework for cumulative risk assessment (CRA)4. CRA methods, which can be qualitative or quantitative5, evaluate the effects of aggregated exposure from multiple sources to characterize risk for adverse outcomes (AOs)6,7. These techniques promote the use of a conceptual site model (CSM) to account for the stressors in a given environment, and thus can facilitate place-based assessments by incorporating relevant exposure data for that location8. While the emphasis of a CRA framework is on the effects of multiple chemicals and stressors4, the 2009 NRC report “Science and Decisions”6 highlighted the importance of considering “human health or ecological effects, taking account of such factors as vulnerability and background exposures”, and the recent National Academies report on the use of new technologies for decision support9 emphasizes the same for both data integration and to ensure comprehensive risk characterization corresponding to real-world scenarios. Therefore, in this work CRA is considered to evaluate the effects of multiple stressors on multiple endpoints.
Integration of human health and ecological endpoints can be difficult because confounding factors such as physiological differences may lead to a diversity of species-specific endpoints, relevant exposure concentrations, experimental designs, and assessment approaches. For example, human health risk assessments have traditionally focused on the occurrence of an adverse health effect at the level of the individual by identifying hazards, collecting and evaluating dose-response and exposure data, and combining this information to characterize risk10. However, guidance for ecological endpoints, provided in a report on Generic Ecological Assessment Endpoints (GEAEs) for ecological risk assessment11,12,13, includes organism-level (e.g. survival or growth), population-level (e.g., abundance), ecosystem-level (e.g., production), and ecosystem service-level (e.g. waste treatment) responses. The assessment endpoints at these levels of organization can vary, and identifying assessment endpoints that are relevant for receptors in potentially affected organisms is a well-recognized step in the problem formulation process within ecological risk assessment14. Additional challenges such as differences between field and laboratory studies or differences in experimental design that include exposure media, magnitude, and duration can further complicate the integration of human health and ecological data. Despite these challenges, however, integration of mechanistic data across species remains essential for site-specific, place-based community risk assessments and the maturation of CRA15.
Advances in modeling frameworks and computational tools have enabled researchers to begin to address the challenges associate with this process. In recent years, CRA frameworks have been developed to address the challenge of cross-species data integration. For example, multi-criteria decision analysis (MCDA)16 and combined exposures decision trees17 are frameworks specifically designed to facilitate the evaluation of risk in both human health and ecological endpoints by summing hazard indices for endpoints in human and non-human species to estimate overall risk. However, a mechanism-based framework for organizing and integrating data to evaluate relative risk across multiple species remains to be developed. This work begins to address this challenge by combining the Adverse Outcome Pathway (AOP)18,19 and Aggregate Exposure Pathway (AEP)20 frameworks to integrate dose-response data across multiple endpoints in multiple taxa.
AOPs describe biological pathways that link perturbations at the molecular level (molecular initiating events; MIEs) to AOs through a series of causal key events (KEs)18. These KEs can be measured and used to confirm the activation of an AOP. AOs can encompass both the organismal and population levels21,22, making AOPs useful for integrating mechanistic data across multiple species. Furthermore, AOPs are chemical-agnostic and pertain to any stressor(s) that activate a KE in the pathway19,23; therefore, these constructs can form networks that encompass multiple chemicals and endpoints. AOP networks provide an organizing framework for toxicity data that describe responses after exposure to contaminants, but do not address the mechanisms of environmental fate and transport leading to these exposures.
The AEP framework parallels the AOP framework, but tracks contaminants from sources, through the environment, to target site exposures (TSEs) in affected organisms20. Ideally, the TSE will correspond to the same level of biological organization as the MIE, thereby allowing prediction of the expected perturbation of the AOP based on in silico or in vitro dose-response information. This framework builds off previous ideas about the importance of incorporating multiple routes of exposure into CRA6,24,25,26 by creating a generic CSM that is not constrained to a single site. The AEP consists of two entities: a key exposure state (KES) that describes the amount of a stressor at a given location and time, and a key transitional relationship (KTR) that describes either the transport of that stressor to a different location or the transformation of that stressor into a different molecule. Individual AEPs can be assembled into AEP networks to characterize the movement of multiple stressors and transformations from one stressor to another20. As with the AOP, these AEP networks are useful for CRA because they can organize exposure data from multiple sources, make that information readily available for the development of site-specific CSMs, and highlight data gaps and uncertainties at study locations.
These organizing frameworks can be linked to form a joint AEP-AOP construct that provides a mechanistic scaffold for organizing and integrating exposure and toxicity data from multiple species20,27,28. This construct has the potential to be applied to CRA across classes of environmental contaminants affecting the same MIE29,30, as well as across species affected by the same MIE. Cross-species AOP networks provide a common ground for evaluating species-specific responses to environmental stressors across taxa by highlighting the effects of stressors at the same KEs in different species. In this work, we develop techniques to apply the joint AEP-AOP construct to integrate human health and ecological data. We conducted a case study using a single contaminant, the perchlorate anion (ClO4−), to provide a simple example of this approach. The results highlight the potential of the AEP-AOP construct to advance CRA by enabling evaluation of risk from aggregate exposures across multiple species and endpoints.
2. Methods
2.1 Case study
ClO4− was selected for the case study presented in this work because it is a wide-spread environmental contaminant that has been heavily studied in several taxa31,32. This contaminant, which is the subject of a National Primary Drinking Water Standard under development at EPA33, is found in parts per billion (ppb) concentrations in sources ranging from drinking water, to soil, to plant material, to cow’s milk34, and can reach concentrations over 100 parts per million (ppm) in surface water at point locations32. Relevant exposure concentrations can vary greatly between locations31. Crucially, this anion affects organisms primarily through a well-defined mechanism involving the competitive inhibition of iodide uptake into the thyroid at the sodium-iodide symporter (NIS)6,31,35,36,37. NIS inhibition affects the hypothalamic-pituitary-thyroid (HPT) axis by decreasing iodide availability in the thyroid gland, resulting in decreased thyroxin (T4) and triiodothyronine (T3) synthesis and may result in a feedback response of increases in thyroid stimulating hormone (TSH) production in the pituitary gland. Reductions in these TH levels can have important effects on the growth and development of organisms (Figure 1)38 and circulating T4 and T3 often serve as surrogates for thyroid hormone (TH) influences in the brain. Conservation of HPT axis biology, and therefore pieces of the NIS inhibition AOP, across diverse taxa make ClO4− a straightforward compound for demonstrating proof of concept and developing techniques for cross-species data integration using a joint AEP-AOP construct.
Figure 1.

The joint AEP-AOP construct applied to the ClO4− case study. White clouds represent different exposure media, while white boxes rectangles represent KESs in the AEP and the purple circle represents the sum of human consumption from KESs. Different colored pathways in the AEP depict different KTRs. Yellow boxes and black arrows depict KEs and KE relationships in the AOP, and the black box represents the unknown effects of thyroid histology changes. Silhouettes of different species show how species-specific AOPs were integrated into the AOP network.
Dose-response data for ClO4− were assembled across multiple endpoints from published literature covering twelve species, eight vertebrate and four invertebrate; all doses were adjusted by the molecular weight of the perchlorate salt used and anion concentrations were converted to units of μg/kg/d. In addition to humans, vertebrate species included three representative small mammals: rats (Rattus sp.), meadow voles (Microtus sp.), and rabbits (Oryctolagus cuniculus), one representative amphibian: the African clawed frog (Xenopus laevis), two representative fishes: zebrafish (Danio rerio) and mosquito fish (Gambusia holbrooki), and one representative bird: the bobwhite quail (Colinus virginianus). Invertebrate species examined in this study included earthworms (Eisenia foetida), mosquito larvae (Culex quinquefasciatus), the water flea (Daphnia magna), and the sand dollar (Peronella japonica). Although invertebrates lack an HPT axis, these organisms were included in this study because NIS homologs, as well as adverse effects of ClO4− exposure, have been reported in invertebrates39,40. Furthermore, TH production has been suggested to occur in invertebrates41; thus, a plausible basis exists for AOPs linking NIS inhibition to AOs in these organisms.
The dataset of species included in this work possessed a variety of relevant exposure pathways, experimental design, physiology, life-history traits, and AOs, allowing for the demonstration of data integration in the presence of these complicating factors. The organisms included in this type of analysis can serve as surrogates for affected wildlife species, and the set of species included in other applications of the AEP-AOP approach will vary based on the objective of the risk assessment. For example, predictive assessments may use a broad range of species, while place-based evaluations may consider a much more focused assemblage. This study focused on representative members from the different taxa of animals examined in prior ecological risk assessments for ClO4−, and species were included based on the availability of dose-response data and evidence for the presence of the NIS inhibition AOP31,42,43.
The ClO4− case study focused on twenty-nine endpoints contributing to four AOs among these species (Supporting Information; Table SI1). Developmental neurotoxicity was selected as the AO to inform human health risk because NIS inhibition can reduce maternal serum TH levels available to the fetus and passive transport of ClO4− across the placental barrier can additionally affect TH production in the fetus, making this the most susceptible human life stage and endpoint to exposure32,44,45. We used findings from established assays such as the FETAX (Frog Embryo Teratogenesis Assay – Xenopus) assay46, which relies on the link between THs and metamorphosis in amphibians, to inform selection of AOs for non-human species. Data supporting developmental neurotoxicity as a result of ClO4− exposure were also available for rats, the African clawed frog, and zebrafish31,47,48; therefore, we considered this AO in these species as well. This study did not consider thyroid cancer since there are no data suggesting that NIS inhibition leads to cancer in humans and developmental neurotoxicity is established as an outcome of greater concern at environmental exposure levels; further, rats are considered more susceptible to thyroid malignancy due to a number of physiological factors49. Other AOs considered in this work were based on the reported effects of ClO4− in the examined species and conformed to the guidelines for GEAEs13. These GEAEs included altered growth in zebrafish, mosquito larvae, and water fleas, decreased survival in mosquito fish, earthworms, and mosquito larvae, and decreased fecundity in zebrafish, meadow voles, earthworms, and water fleas. Impaired metamorphosis was used as a measurement endpoint in frogs and sand dollars; therefore, we included these organisms in the altered growth GEAE (Figure 1). Data for specific AOs were not available for rabbits and quails, but these species were included in this study because dose-response data for intermediate KEs in the NIS inhibition AOP were available.
2.2 Adverse Outcome Pathway (AOP) network
The AOP network portion of the AEP-AOP construct assembled the NIS inhibition AOPs of the different species examined to provide a common ground for comparison. We based this network on the preliminary AOP for NIS inhibition in mammals authored by Gilbert and colleagues in the AOP-Wiki (https://aopwiki.org/aops/134), the 2002 EPA perchlorate report31, and the 2005 NRC assessment for ingested perchlorate32. NIS inhibition was the only MIE in this network, and led to decreased synthesis and depletion of TH in the thyroid as an early biological effect for all of the vertebrate species examined. We included a separate KE for depletion of TH in the thyroid because measurement of depleted stores is an early histopathological indicator of perturbation in the HPT axis. Data on decreases in circulating TH and the subsequent upregulation of TSH in a feedback response were also available across the taxa examined. Decreased serum TH levels result from these early biological effects in all vertebrates, and the branching of subsequent KEs reflected the diversity of the AOPs across all of the species examined (Figure 1). For example, altered hormone economy can result in hypertrophy and hyperplasia of the thyroid gland to compensate. Deficits in TH ultimately lead to developmental neurotoxicity in rats as assessed by brain morphometry and behavior31,32, and to different adverse effects in other species such as impaired metamorphosis (growth) in the African clawed frog47 (Figure 1). Altered structure and functions of thyroid tissues aside from colloid depletion were classified as “other thyroid histology changes” (Figure 1), and included angiogenesis50, follicle cell hypertrophy51, and hyperplasia52. AOs and GEAEs stemming from altered development were used to categorize the different effects of NIS inhibition across species. The resulting AOP network was used to organize dose-response data for endpoints pertaining to KEs and facilitate the integration of this information to inform CRAs.
2.3 Aggregate Exposure Pathway network
The AEP network portion of the AEP-AOP construct represents the movement of the ClO4− in the environment from its sources to different TSEs in the species examined. This places the measured exposures used as input for the AOP network in a relevant context and could be used to relate the current results to a place-based risk assessment by populating the exposure pathways for different species with site-specific data. Thus, the AEP network can serve as a general representation of a conceptual site model (CSM)20, as is highlighted by the generic AEP network for ClO4− presented in this work.
The generic AEP network includes multiple sources (atmospheric deposition, water discharge, and disposal in the form of ClO4− salts) and transport to several different media including groundwater, surface water, and soil (Figure 1). The resulting pathways include both direct exposure to organisms through contaminated media and exposure through trophic interactions with organisms in intermediate KESs. Previous ecological assessments for ClO4− have considered possible exposure to animals through accumulation of the ClO4− in the plant matter that they graze on42,53. Therefore, we included consumption of plant matter for both human and non-human species into the AEP. TSEs in the AEP are driven by absorption, distribution, metabolism, and elimination (ADME) processes, which describe the amount of ClO4− affecting the NIS inhibition MIE at the thyroid after external exposure in each organism (Figure 1). In mammals, these processes are complicated by the fact that both ClO4− and maternal TH cross the placenta into the fetus, thus adding additional ADME considerations. Species-specific TSEs resulting from ADME processes link the AEP network and the AOP network. Flows between KESs in the AEP were not quantified for the purpose of this case study, as environmental exposure data were not necessary to demonstrate cross-species integration of toxicity data and the generic AEP network does not represent a specific, measurable location. However, the incorporation of the AEP network into the AEP-AOP construct is crucial to illustrate how the techniques presented in this work can inform CRAs by creating a context for considering relevant environmental exposures and by highlighting knowledge gaps in relevant exposure mechanisms.
2.4 Data acquisition
Dose-response data for KEs in the NIS inhibition AOP network were obtained from literature sources and included a combination of laboratory experiments and model outputs. For humans, the point of departure (POD) of 7.0 μg/kg/d was obtained from Greer et al. (2002)54, which provided in-vivo data for NIS inhibition from ClO4−; however, no further human in vivo empirical data for NIS inhibition were available. We used the biologically based dose-response (BBDR) model for a pregnant mother and fetus presented in Lumen et al. (2013)55 to simulate ADME processes in humans and to estimate hypothyroxinemia (serum T4 below 10 pmol/l) as an early biological effect of NIS inhibition55. This model allowed for consideration of susceptible populations of interest by predicting serum-free T4 levels in both the mother and fetus at different levels of ClO4− and iodide intake. We considered the doses of ClO4− necessary to induce predicted hypothyroxinemia in women receiving the World Health Organization recommended 200 μg iodide per day56, as well as in iodide-deficient women receiving just 75 μg iodide per day, to provide estimations across a range of relevant iodide intake conditions55. For KEs downstream of serum T4 reduction, which were not included in the BBDR model, we hypothesized that a ClO4− dose at least as high as the dose necessary to induce hypothyroxinemia would be required for activation. This hypothesis assumes a 1:1 relationship among upstream and downstream events, and therefore does not take into account possible biological feedbacks explicitly except those that were empirically inherent by time point in the experimental design. The reference dose (RfD) for oral consumption of the ClO4− presented in the EPA Integrated Risk Information System (0.7 μg/kg/d)32,57 was also included in this case study to provide a context for dose-response data.
Data sources for non-human species were obtained through prescribed searches of the ECOTOXicology knowledgebase58, as well as from the 2002 EPA ClO4− toxicological review and risk characterization31. ECOTOX search parameters for each species included an exact match for genus and species name, “Perchlorates” as a predefined chemical group, and Lowest-Observed-Effect-Concentration (LOEC), No-Observed-Effect-Concentration (NOEC), Lowest-Observed-Effect-Level (LOEL), and No-Observed-Effect-Level (NOEL) as endpoints. No further search parameters were provided and results were sorted by response site and organism life stage. The relevant data identified through the ECOTOX search was added to the data included in the 2002 EPA review31, which contained Lowest-Observed-Adverse-Effect-Level (LOAEL), and No-Observed-Adverse-Effect-Level (NOAEL) findings in addition to these other metrics. Therefore, the final data set included NOEC, LOEC, NOEL, LOEL, NOAEL, and LOAEL observations. Statistical approaches, including benchmark dose (BMD) modelling values and the 95% confidence limit of the BMD (BMDL) values according to the methods described in the 2002 EPA assessment31, were used to characterize hormone, histopathological, and brain morphometry responses in rats and rabbits because data were available.
2.5 Data integration
The AOP network allowed for the organization of data from different studies into aggregated KEs, each of which spanned multiple measured endpoints, to create a context for comparison. We used the conceptual AOP network (Figure 1) to assemble a total of nine KEs from twenty-nine different measurement endpoints (Supporting Information). Although data were not available for every KE in all species, each AOP in the AOP network shared the NIS inhibition MIE and the early KEs of T4/T3 decrease, and TSH increase. When assembling data, we separated colloid depletion from other compensatory thyroid histological changes to reflect the fact that colloid depletion must occur before downstream KEs and AOs. The four AOs considered in this study had equal proximity to the MIE and were not assumed to occur sequentially.
The measurement units of the data generated by different experimental designs were made to be consistent within and across aggregated KEs to allow for comparison across species. The set of studies used in this work dosed organisms using ammonium, potassium, or sodium perchlorate salts, and reported data were adjusted by the ratio of the molecular weight of ClO4− to the appropriate source molecule (0.85, 0.72, 0.81, respectively) to ensure that appropriate anion concentrations were considered. While doses for terrestrial organisms were often reported in units of μg/kg/d, aquatic organisms are dosed by media concentration (μg/L or ppb). The dose metrics from all studies were converted to μg/kg/d, and the blood concentration of ClO4− for aquatic organisms was assumed to be equal to the medium (water) concentration. This assumption is plausible in this case study due to the high mobility, stability and poor complexing properties of ClO4−, and equilibration of these organisms with their aqueous environment59. However, tools such as physiologically based pharmacokinetic (PBPK) models may be necessary to simulate ADME properties and estimate the internal dosimetry of other compounds.
We visually compared and evaluated data for the NOECs, LOECs, NOELs, LOELs, NOAELs, LOAELs, BMDs, and BMDLs from different studies for each species and endpoint at each KE in the AOP network (Figure 2). Data for lethal concentration (LC50) were also considered, but were restricted to invertebrates to highlight the existing literature data for these organisms. LC50 data points were not considered when comparing dose-response data across species. We arrange the KEs in the NIS inhibition AOP network from the MIE to the AOs, then plotted the ClO4− dose-response data (μg/kg/d) versus the KEs. This linearized arrangement of KEs (Figure 2) does not imply causal relationships, but rather these relationships are described by AOP network layout presented in Figure 1. To provide a conservative estimate of sensitivity to ClO4− across KEs and species, we determined the lowest reported dose observed to activate each KE for each species, regardless of experimental design or duration. While it is possible that this approach could bias results in AOPs where the timing of activation differs greatly among early and late KEs, no patterns were observed between experimental duration and KE activation in the NIS inhibition AOP, suggesting that ClO4− concentration at activation of a KE is an appropriate conservative indicator of sensitivity. We examined the variability both within and among each species and KE by calculating the coefficient of variation (standard deviation divided by mean) for each comparison (Table 1).
Figure 2.
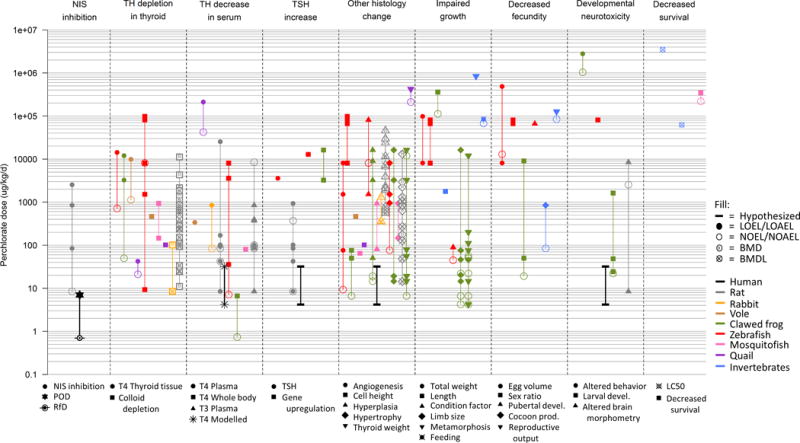
Dose-response data for the nine aggregated KEs in the ClO4− AOP network. Each data point represents a different study result, and the point fill represents the type of data. The shape of points corresponds to the legend beneath each KE column, which specifies the measurement endpoint. Colors depict different species, and vertical lines link studies with the same endpoints in the same species. Black asterisks and vertical line in the “TH decrease in serum” KE show the range of predictions from the Lumen et al. (2013) model for hypothyroxinemia based on daily iodide intakes of 200 μg (top) and 75 μg (bottom) to represent iodide sufficient and deficient populations, respectively. Black lines in KEs downstream of “TH decrease in serum” show the hypothesized activation of KEs in humans based on upstream events. Data points were scaled by the ratio of the molecular weight of ClO4− to the source salts of ammonium (0.85), potassium (0.72), or sodium (0.81) perchlorate used in each experiment.
Table 1.
Coefficients of variation (CV) for the lowest reported activation dose across early and intermediate (E/I) KEs and all KEs the species examined in this study.
| Organism | CV (E/I KEs) | CV (all KEs) |
|---|---|---|
| Humans (Homo sapiens) | 0.9* | NA |
| rats (Rattus sp.) | 1.3 | 1.4 |
| meadow voles (Microtus sp.) | 0.17 | 0.17 |
| rabbits (Oryctolagus cuniculus) | 1.0 | 1.0 |
| African clawed frog (Xenopus laevis) | 1.1 | 1.7 |
| zebrafish (Danio rerio) | 1.9 | 2.3 |
| mosquito fish (Gambusia holbrooki) | 0.44 | 2.0 |
| bobwhite quail (Colinus virginianus) | 1.7 | 1.7 |
NA indicates not applicable;
indicates based on modeled results.
3. Results and Discussion
3.1 ClO4− case study
The data assembled for the ClO4− case study focused on toxicity and included empirical dose-response results for 29 endpoints linked to 9 KEs across the AOP network (Supporting Information; Table SI1). Data points indicating KE activation spanned 7 orders of magnitude (Figure 2) and ranged from 4.2 μg/kg/d for impaired growth in African clawed frogs47 to 3.5 g/kg/d for LC50 in earthworms60 after adjusting for the type of perchlorate salts used in each experiment. The mechanistic nature of the AOP network and conserved KEs facilitated data evaluation across endpoints in human and non-human targets, which were measured using different experimental designs, and provided an organizing framework for the display of dose-response data across the species-specific mechanisms of action (Figures 2 and 3).
Figure 3.

Trajectories of the lowest endpoint observed to activate each KE in the ClO4− AOP network for vertebrate each species. Invertebrates were excluded from this analysis because the data points included four different species. Dotted black line for human indicates that the points shown for these KEs are hypothesized from the Lumen et al. (2013) model results for pregnant women receiving 200 μg iodide per day. A heatmap highlighting the relative activation doses for each species at each KE, as well as data gaps, is available in the Supporting Information (Figure SI1).
The dose-response data showed relatively flat trends in the amount of ClO4− necessary to activate KEs across NIS inhibition AOPs for the species examined, particularly in early and intermediate KEs (Figure 3). The coefficient of variation for the ClO4− dose required to activate the early and intermediate KEs (Table 1) was less than two for all species and less than or equal to one for four of the species examined (humans, meadow voles, rabbits, and mosquito fish), indicating relatively little within-species variation. Rats consistently displayed among the lowest reported ClO4− doses for KE activation of the species examined, and closely mimicked the human responses hypothesized from the Lumen et al. (2013) model predictions55. In this way, cross-species comparisons can be used to support the plausibility of hypothesized responses in data-limited species. The fact that KEs within a species were activated at similar concentrations in multiple studies suggests that the AOs in the NIS inhibition AOP are similarly sensitive to the activation of the MIE.
KE activation doses for invertebrate organisms were generally higher than those for vertebrates, but some endpoints were lower than those for fish at the impaired reproduction, developmental effects, and mortality AOs (Figures 2). This finding may be the result of limited testing efforts at lower concentrations in fish. Alternatively, different biology among the diverse taxa that make up invertebrates may cause some species to be more sensitive to ClO4− exposure than others. For example, the two LC50 data points for the mortality AO correspond to earthworms (3.5 g/kg/d)60 and mosquito larvae (63 mg/kg/d)40, and differences between these organisms such as ADME properties or life history traits may account for this discrepancy (Figure 2). However, the fact that invertebrates showed responses within the same order of magnitude as vertebrates for AOs, despite lacking an HPT axis, gives support to the hypothesis that control pathways similar to the NIS inhibition AOP are present in these organisms. This finding highlights the utility of the approach presented in this work to leverage the conserved mechanism of action for ClO4− to integrate and interpret data across species.
The AOP network (Figure 1) facilitates the interpretation of dose-response data both by describing the sequential relationship among KEs and highlighting uncertainties. For example, the lowest doses reported to induce TSH increases in zebrafish and African clawed frogs were approximately 100-fold greater at this KE than at other early and intermediate KEs in these species such as TH decrease in the serum and thyroid histology changes. However, direct measurement of TSH in these organisms during their development is challenging and sampling efforts were only made at higher doses61,62, resulting in an apparent increase in the amount of perchlorate necessary to active this KE. It is likely that this KE is activated at lower doses than those reported for each species, and the fact that KEs both upstream and downstream of TSH increase are activated at lower doses in these species supports this claim (Figures 2 and 3). Similarly, measurements of T4 decrease in the plasma of quails were observed at doses over 100 times the upper limit of the dose range reported for other species, but effects were observed at lower activation doses for both upstream and downstream KEs. This finding suggests that more work is required to verify the dose-response relationship for ClO4− on TH in the serum of these organisms, despite an observed NOEL (Figure 2). In a separate example, the ClO4− dose (81.2 mg/kg/d) required to cause altered jaw development in zebrafish larvae63 is relatively high compared to other KEs and other species at the development KE. The AOP construct highlights that this finding may be an overestimation because few NOELs for this endpoint have been reported (Figure 2), and therefore indicates that the lack of response observed at low doses may be a result of sampling bias due to experimental design rather than an absence of effect. By highlighting considerations such as these in the context of sequential KEs, the AOP network facilitates the evaluation of experimental results from disparate studies across multiple taxa.
The framework provided by the AOP network also facilitated comparison of the dose-response data across species at each KE. Although the activation doses of ClO4− for early and intermediate KEs spanned three orders of magnitude across the organisms examined, four of the species (rats, rabbits, African clawed frogs, and zebrafish) showed activation of at least one KE within the same order of magnitude as the human POD (7.0 μg/kg/d; Figure 3). African clawed frogs and rats had the lowest activation doses at seven of the nine KEs (see Supporting Information; Figure SI1 for additional information), suggesting that these species may be more vulnerable than others to ClO4− exposure; however, this apparent sensitivity is likely biased by data availability and sampling efforts. For example, data were not available for meadow voles at doses lower that 338 μg/kg/d for any KEs in the NIS inhibition AOP. Therefore, we were unable to confirm NOELs at concentrations similar to the human POD and the higher ClO4− dose needed for KE activation in meadow voles observed in Figure 3 is likely an underestimation driven by experimental design. Taken in total, similar trends were observed in data reported in studies across species and KEs (Figure 3), a finding that reflects the highly conserved nature of HPT axis biology among vertebrate organisms64. Thus, this case study demonstrates how disparate human health and ecological data sources can be integrated to inform a CRA. However, this case study also highlights that additional efforts directed at filling in data gaps and testing the same concentrations across species are necessary to strengthen cross-species comparisons.
3.2 The role of uncertainties
Confidence in the interpretation of results from cross-species comparisons within an AEP-AOP construct is dependent on the availability and quality of data used to inform dose-response relationships, the completeness of data sets for each organism, and the strength of the evidence for parallel mechanisms of action across organisms that includes the utility of the given experimental design and relevance to the particular KE. In the ClO4− case study, for example, the absence of dose-response data for fishes at relatively low concentrations that result in AOs for other species (Figure 2) implies an increased uncertainty in the dose that will result in AOs for fishes. Identification of data gaps such as these can guide the exposure dose-range for future studies to refine results. Additionally, uncertainties such as assumptions about the ADME properties of different species or biological mechanisms of action can decrease confidence in results. While challenges such as these can make cross-species data integration difficult, one strength of the combined AEP-AOP approach used in this work is that it provides a mechanistic structure to assist with identifying and documenting data gaps and assumptions that are made in the absence of data. These assumptions can then be tested and relaxed when data become available. Thus, AEP-AOP constructs can assist with transparent communication of risk assessment results.
3.3 AEP-AOP construct applications in CRA
Hazard identification, exposure, and toxicity have long been recognized as integral components of human health and ecological risk assessment6,65,66. The combined AEP-AOP construct provides a framework for integrating data that facilitates place-based, community-scale applications of the phased approach to CRAs described in Menzie et al. (2007)67. In this approach67, a conceptual model is first constructed, environmental hazards are then identified, and the individual and combined effects of hazards are assessed through toxicity screening. The CSM for relevant exposure mechanisms is extracted from the AEP and the conceptual model for toxicity effects is captured in the AOP network. This approach has the potential to accommodate multiple stressors by assembling the CSM from AEP networks covering the stressors of interest. Additionally, these AEP networks can include transformational relationships whereby one stressor is converted into another. The chemical-agnostic nature of AOP networks19,23 implies that multiple stressors affecting an MIE individually or in concert could be evaluated by converting TSEs to equivalent doses informed by toxicity testing. Thus, the AEP-AOP construct enables the combination of exposure from multiple chemicals and stressors with relevant toxicity data, as advocated by World Health Organization’s International Programme on Chemical Safety15 and the National Academies of Sciences, Engineering, and Medicine9, to inform cross-species CRA.
While the ClO4− case study presented in this work focused on a single substance to provide an example of how the AEP-AOP construct can organize and integrate data across species, the NIS inhibition AOP network used in this work is relevant to all chemicals that perturb the NIS inhibition MIE or other KEs in the network. Therefore, if data are sufficiently robust, exposure and toxicity data for other NIS inhibitors such as nitrate and thiocyanate44,68,69,70 could be combined with ClO4− data, and, assuming sufficient data exist for the additional chemicals, cumulative risk could be assessed based on the exposures of each substance described by the AEP network9,15.
For chemicals with complex relationships between environmental exposure and TSEs, species-specific information on the ADME properties of a contaminant can be incorporated into PBPK models to quantitatively describe the mechanistic links between the AEP and AOP networks. The resulting AEP-AOP continuum can qualitatively assess risk across human health and ecological endpoints to identify species of concern, or can be used quantitatively to calculate species-specific hazard indices such as those as in Price et al. (2012)17. However, caution should be taken when conducting quantitative analyses to ensure that a consistent set of doses with relevant experimental conditions has been tested across species as well as across KEs in the AOP network to avoid observational bias.
The AEP-AOP approach presented in this work employs the AOP framework differently from toxicity screening applications such as those presented in Angrish et al. (2015)71 and Knapen et al. (2015)72. Instead of identifying potentially hazardous chemicals, the AOP network highlights similarities and differences among species responses to contaminants and can be used in support of site-specific community decision making. The mechanistic emphasis of the AEP-AOP approach provides a systematic construct for communities to both understand components of risk characterization and voice valuation preferences regarding either exposures or species and endpoint of interest, and affords decision makers a more comprehensive characterization of alternatives and impacts. Thus, the AEP-AOP approach readily informs decision support tools such as MCDA16. Additionally, the organization of data provided by the AEP-AOP construct identifies data gaps that can help to prioritize future research efforts.
3.4 Benefits of a holistic perspective
The joining of the AEP framework to the AOP framework is essential for the application of the data integration techniques described in this case study to CRA. This construct provides a holistic perspective that allows for the incorporation of relevant TSEs derived from site-based AEPs to be used in conjunction with toxicity data describing KEs in the multi-species AOP network for a community risk assessment. These AEPS can consider exposure differences among organisms based on life-history characteristics, exposure media, or other factors such as degradation of contaminants by bacteria. Exposures experienced by different organisms can be projected onto the dose-response data for KEs in an AOP network of representative species to identify species or groups at risk. For example, the ClO4− case study demonstrates that vertebrate organisms exposed to environmental conditions leading to TSEs of 100 μg/kg/d or greater, including mammals, fish, amphibians, and birds, may also be at risk for NIS inhibition and the downstream KEs and AOs in the NIS inhibition AOP network (Figure 3). Using the same logic, the dose-response data assembled in this case study suggest that ClO4− exposures leading to the EPA RfD of 0.7 μg/kg/d57 (Figure 2) would not result in high risk of AOs in either human health or ecological endpoints from NIS inhibition.
The holistic approach provided by the combined AEP-AOP construct is also useful for informing the problem formulation stage of risk assessments. For example, the inclusion of invertebrates in the ClO4− case study, despite the fact that these organisms do not have a well-characterized AOP that is analogous to the AOP for NIS inhibition in vertebrates, highlights how this approach can help to identify potentially affected species in a community. Additionally, the inclusion of invertebrate species demonstrates how the limited data available for these organisms can be placed in the context of the data for other species in a community. The ability of the AEP-AOP construct to organize data based on mechanistic processes enables a systematic evaluation of knowledge gaps.
3.5 Towards a mechanistic approach for place-based community CRAs
Developing a site-specific CSM based on AEPs for an area of concern should be an early goal for conducting community based CRAs. These AEPs could be either qualitative or quantitative in nature, depending on the substance(s) and availability of data, and describe the drivers of risk for the organisms inhabiting the study site. Exposure pathways that use different media such as water, air, or food may result in different internal doses for different organisms, and can be captured in an AEP. Additionally, the species included in an AEP network, along with risk assessment goals, can inform which AOs are relevant for inclusion in an AOP network. Recent work has encouraged a paradigm shift for risk assessments that focuses first on exposure, then on toxicity73, and consideration of exposure scenarios to inform problem formulation is emphasized in the new TSCA law. The AEP-AOP construct facilitates a mechanistic application of this paradigm by linking exposure information in the form of an AEP network to toxicity for individual KEs in an AOP network and highlights the importance of ADME considerations to describe the appropriate TSE as the critical link to an MIE. Future efforts are needed to address this linkage by developing quantitative AEPs that link the dose-response data for an AOP network to environmental exposures, and efforts are currently underway to provide an example application of this mechanistic continuum for the ClO4− case study presented in this work. Implementation of this approach could facilitate the comparison of risk across species and identify species at greater risk due to either higher internal doses from exposure pathways or more severe toxicological effects.
Connecting toxicity data across species with KEs can be challenging and illustrates the need for careful attention to experimental design and a common ontology across disciplines, especially when performing systematic reviews. A KE for an AOP can be described in term of specific processes and objects based on existing biological ontologies74. For example, the impaired growth KE in the ClO4− case study could be assigned the “growth” process term from the Gene Ontology75. These biological process and objects can be further related to specific ontological terms from ontologies focused on experimental measurements or toxicological outcomes. For example, in the ClO4− case study the growth process can be related to endpoints such as “body weight”, “height growth measurement”, and “growth condition” from the Experimental Factor Ontology76 to allow endpoints to be matched to KEs in searchable databases. This functionality could contribute to automated population of dose-response data into cross-species AOP networks. Furthermore, adherence to reporting criteria for toxicological data such as those presented in Hanson et al. (2017)77 could assist in the development of these ontologies and help to ensure that high quality data are available for risk assessments. Combining well-described AOPs with an ontology linking KEs to measurement endpoints will increase the utility of the AEP-AOP construct for integrating human health and ecological endpoints into CRA.
The techniques presented in this work demonstrate how a joint AEP-AOP construct can be used to integrate data on human health and ecological endpoints. This approach can advance CRA applications in at least four ways. First, the AEP-AOP construct facilitates the organization of data into mechanistic pathways linking sources of contamination to outcomes in multiple species (Figure 1). This organization provides a common basis for comparing the results of exposure and toxicity experiments and can be used to link a CSM for place-based community CRA to toxicity data. Second, the AEP-AOP construct can inform the evaluation of risk across communities by integrating data from studies spanning multiple taxa (Figure 2). For example, in the ClO4− case study, we observed a general concordance across species with regard to dose necessary to activate the MIE and early KEs, suggesting similar susceptibility to ClO4− exposure across the taxa examined. This approach could provide insight into vulnerable populations and lifestages, elucidate differences and similarities in sensitivity among species or experimental design, and inform decision support tools such as MCDA. Third, the AEP-AOP construct highlights data gaps and can identify areas where more data would be most useful. Identifying and understanding the effects of data gaps is an essential task for guiding the interpretation of existing data and identifying research needs. Furthermore, organizing the information in a systematic way highlights the caveats associated with those data (Figure 2–3, Supplemental Information Figure SI1), and can be used to identify areas where additional caution is needed when interpreting data. Finally, the AEP-AOP construct emphasizes the need for a common ontology to guide the integration of endpoints across species. The mechanistic approach for cross-species data integration described in this work represents an important step for informing CRA.
Supplementary Material
Acknowledgments
Development of this document was funded wholly by the U. S. Environmental Protection Agency. This document has been subjected to review by the National Health and Environmental Effects Research Laboratory and approved for publication. Approval does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use. The authors would like to thank Allyson P. Scatterday for her help in assembling dose-response data, as well as Dr. Glenn Suter and Dr. Mary Gilbert for providing technical reviews of this manuscript.
Acronyms
- AEP
Aggregate Exposure Pathway
- AO
Adverse Outcome
- AOP
Adverse Outcome Pathway
- BMD
benchmark dose
- BMDL
benchmark dose 95% confidence limit
- ClO4−
perchlorate anion
- CRA
Cumulative Risk Assessment
- CSM
Conceptual Site Model
- GEAE
Generic Ecological Assessment Endpoint
- HPT
Hypothalamic-Pituitary-Thyroid
- KE
Key Event
- KER
Key Event Relationship
- KES
Key Exposure State
- KTR
Key Transitional Relationship
- LOAEL
Lowest-Observed-Adverse-Effect-Level
- LOEC
Lowest-Observed-Effect-Concentration
- LOEL
Lowest-Observed-Effect-Level
- MCDA
Multi-Criteria Decision Analysis
- MIE
Molecular Initiating Event
- NIS
sodium-Iodide Symporter
- NOAEL
No-Observed-Adverse-Effect-Level
- NOEC
No-Observed-Effect-Concentration
- NOEL
No-Observed-Effect-Level
- PBPK
Physiologically-Based Pharmacokinetic
- T3
triiodothyronine
- T4
thyroxin
- TH
Thyroid Hormone
- TSE
Target Site Exposure
- TSH
Thyroid Stimulating Hormone
Footnotes
Supporting Information. Table detailing the endpoints used to measure each KE (Table SI1); Heatmap highlighting the relative activation doses for each species at each KE (Figure SI1)
References
- 1.Moretto A, Bachman A, Boobis A, Solomon KR, Pastoor TP, Wilks MF, Embry MR. A framework for cumulative risk assessment in the 21st century. Crit Rev Toxicol. 2017;47(2):85–97. doi: 10.1080/10408444.2016.1211618. [DOI] [PubMed] [Google Scholar]
- 2.Lentz TJ, Dotson GS, Williams PRD, Maier A, Gadagbui B, Pandalai SP, Lamba A, Hearl F, Mumtaz M. Aggregate exposure and cumulative risk assessment—Integrating occupational and non-occupational risk factors. J Occup Environ Hyg. 2015;12(sup1):S112–S126. doi: 10.1080/15459624.2015.1060326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ankley G, Escher B, Hartung T, Shah I. Pathway-Based Approaches for Environmental Monitoring and Risk Assessment. Chem Res Toxicol. 2016;29:1789–1790. doi: 10.1021/acs.chemrestox.6b00321. [DOI] [PubMed] [Google Scholar]
- 4.EPA (U.S. Environmental Protection Agency) Framework for Cumulative Risk Assessment. U.S. Environmental Protection Agency, Office of Research and Development, National Center for Environmental Assessment, Washington Office; Washington, D.: 2003. EPA/600/P-02/001F. https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?substance_nmbr=1007(accessed July 21, 2017) [Google Scholar]
- 5.Callahan MA, Sexton K. If cumulative risk assessment is the answer, what is the question? . Environ Health Persp. 2007;115(5):799–806. doi: 10.1289/ehp.9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.NRC (National Research Council) Science and decisions: advancing risk assessment. National Academies Press; 2009. [PubMed] [Google Scholar]
- 7.Sexton K. Cumulative risk assessment: an overview of methodological approaches for evaluating combined health effects from exposure to multiple environmental stressors. Int J Environ Res Pub He. 2012;9(2):370–390. doi: 10.3390/ijerph9020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levy JI. Is epidemiology the key to cumulative risk assessment? . Risk Anal. 2008;28(6):1507–1513. doi: 10.1111/j.1539-6924.2008.01121.x. [DOI] [PubMed] [Google Scholar]
- 9.NASEM (National Academies of Sciences, Engineering, and Medicine) Using 21st century science to improve risk-related evaluations. National Academies Press; 2017. [PubMed] [Google Scholar]
- 10.NRC (National Research Council) Risk Assessment in the Federal Government: Managing the Process. National Academies Press; 1983. [PubMed] [Google Scholar]
- 11.Suter GW. Ecological Risk Assessment Second edition. CRC Press; Boca Raton, FL: 2007. [Google Scholar]
- 12.Paustenbach DJ. Human and Ecological Risk Assessment: Theory and Pactice. John Wiley & Sons; 2015. [Google Scholar]
- 13.Barron M, Suter GW. Generic Ecological Assessment Endpoints (GEAEs) for ecological risk assessment: Second edition with generic ecosystem services endpoints added. 2016 EPA/100/F15/005. [Google Scholar]
- 14.Reinert KH, Bartell SM, Biddinger GR. Ecological risk assessment decision support system: a conceptual design; Proceedings of the Pellston Workshop on Ecological Risk Assessment Modeling; 23- Pellston, Michigan, SETAC Foundation. 1998. [Google Scholar]
- 15.Meek MB, Boobis AR, Crofton KM, Heinemeyer G, Kleiner J, Lund BO, Olin S, Pavittranon S, Rodriguez C, Van Raaij M, Vickers C. Assessment of Combined Exposures to Multiple Chemicals: Report of a WHO/IPCS International Workshop on Aggregate/Cumulative Risk Assessment. World Health Organization; 2009. [Google Scholar]
- 16.Kiker GA, Bridges TS, Varghese A, Seager TP, Linkov I. Application of multicriteria decision analysis in environmental decision making. Integ Environ Asses. 2005;1(2):95–108. doi: 10.1897/IEAM_2004a-015.1. [DOI] [PubMed] [Google Scholar]
- 17.Price P, Han X, Junghans M, Kunz P, Watts C, Leverett D. An application of a decision tree for assessing effects from exposures to multiple substances to the assessment of human and ecological effects from combined exposures to chemicals observed in surface waters and waste water effluents. Environ Sci Eur. 2012;24(1):24–34. [Google Scholar]
- 18.Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, Serrrano JA. Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem. 2010;29(3):730–741. doi: 10.1002/etc.34. [DOI] [PubMed] [Google Scholar]
- 19.Villeneuve DL, Crump D, Garcia-Reyero N, Hecker M, Hutchinson TH, LaLone CA, Landesmann B, Lettieri T, Munn S, Nepelska M, Ottinger MA, Vergauwen L, Whelan M. Adverse outcome pathway (AOP) development I: Strategies principles. Toxicol Sci. 2014;142(2):312–320. doi: 10.1093/toxsci/kfu199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teeguarden JG, Tan Y, Edwards SW, Leonard JA, Anderson KA, Corley RA, Kile ML, Simonich SM, Stone D, Tanquay RL, Waters KM, Harper SL, Williams DE. Completing the link between exposure science and toxicology for improved environmental health decision making: The Aggregate Exposure Pathway framework. Environ Sci Technol. 2016;50:4579–4586. doi: 10.1021/acs.est.5b05311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kramer VJ, Etterson MA, Hecker M, Murphy CA, Roesijadi G, Spade DJ, Spromberg JA, Wang M, Ankley GT. Adverse Outcome Pathways and ecological risk assessment: Bridging to population-level effects. Environ Toxicol Chem. 2011;30(1):64–76. doi: 10.1002/etc.375. [DOI] [PubMed] [Google Scholar]
- 22.Villeneuve DL, Crump D, Garcia-Reyero N, Hecker M, Hutchinson TH, LaLone CA, Landesmann B, Lettieri T, Munn S, Nepelska M, Ottinger MA. Adverse Outcome Pathway development II: best practices. Toxicol Sci. 2014;142(2):321–330. doi: 10.1093/toxsci/kfu200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards SW, Tan YM, Villeneuve DL, Meek ME, McQueen CA. Adverse Outcome Pathways—organizing toxicological information to improve decision making. JPharmacol Exp Ther. 2016;356(1):170–181. doi: 10.1124/jpet.115.228239. [DOI] [PubMed] [Google Scholar]
- 24.Wilkinson CF, Christoph GR, Julien E, Kelley JM, Kronenberg J, McCarthy J, Reiss R. Assessing the risks of exposures to multiple chemicals with a common mechanism of toxicity: how to cumulate? . Regul Toxicol Pharm. 2000;31(1):30–43. doi: 10.1006/rtph.1999.1361. [DOI] [PubMed] [Google Scholar]
- 25.Robson MG, Toscano WA, editors. Risk assessment for environmental health. Vol. 2. John Wiley & Sons; 2007. [Google Scholar]
- 26.Sexton K, Linder SH. Cumulative risk assessment for combined health effects from chemical and nonchemical stressors. Am J Public Health. 2011;101(S1):S81–S88. doi: 10.2105/AJPH.2011.300118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Escher BI, Hackermüller J, Polte T, Scholz S, Aigner A, Altenburger R, Böhme A, Bopp SK, Brack W, Busch W, Chadeau-Hyam M. From the exposome to mechanistic understanding of chemical-induced adverse effects. Environ Int. 2017;99:97–106. doi: 10.1016/j.envint.2016.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Norton SB, Schofield KA. Conceptual model diagrams as evidence scaffolds for environmental assessment and management. Freshw Sci. 2017;36(1):231–239. [Google Scholar]
- 29.Lumen A, George NI. Estimation of iodine nutrition and thyroid function status in late-gestation pregnant women in the United States: Development and application of a population-based pregnancy model. Toxicol Appl Pharm. 2017;314:24–38. doi: 10.1016/j.taap.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 30.Willemin ME, Lumen A. Thiocyanate: a review and evaluation of the kinetics and the modes of action for thyroid hormone perturbations. Crit Rev Toxicol. 2017:1–27. doi: 10.1080/10408444.2017.1281590. [DOI] [PubMed] [Google Scholar]
- 31.EPA (U.S. Environmental Protection Agency) Perchlorate environmental contamination: Toxicological review and risk characterization (external review draft) U.S. Environmental Protection Agency, Office of Research and Development, National Center for Environmental Assessment, Washington Office; Washington, DC: 2002. NCEA-1-0503. [Google Scholar]
- 32.NRC (National Research Council) Health implications of perchlorate ingestion. National Academies Press; 2005. [Google Scholar]
- 33.EPA (U.S. Environmental Protection Agency) Perchlorate in drinking water. Website; https://www.epa.gov/dwstandardsregulations/perchlorate-drinking-water.
- 34.Leung AM, Pearce EN, Braverman LE. Perchlorate, iodine and the thyroid. Best Pract Res Cl En. 2010;24(1):133–141. doi: 10.1016/j.beem.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carrasco N. Iodide transport in the thyroid gland. BBA-Rev Biomembranes. 1993;1154(1):65–82. doi: 10.1016/0304-4157(93)90017-i. [DOI] [PubMed] [Google Scholar]
- 36.Eskandari S, Loo DD, Dai G, Levy O, Wright EM, Carrasco N. Thyroid Na+/I− symporter mechanism, stoichiometry, and specificity. J Biol Chem. 1997;272(43):27230–27238. doi: 10.1074/jbc.272.43.27230. [DOI] [PubMed] [Google Scholar]
- 37.Sijimol MR, Jyothy S, Pradeepkumar AP, Chandran MS, Ghouse SS, Mohan M. Review on fate, toxicity, and remediation of perchlorate. Environ Forensics. 2015;16(2):125–134. [Google Scholar]
- 38.de Groef B, Decallonne BR, van der Geyten S, Darras VM, Bouillon R. Perchlorate versus other environmental sodium/iodide symporter inhibitors: potential thyroid-related health effects. Eur J Endocrinol. 2006;155(1):17–25. doi: 10.1530/eje.1.02190. [DOI] [PubMed] [Google Scholar]
- 39.Benvenga S, Alesci S, Trimarchi F, Facchiano A. Homologies of the thyroid sodium-iodide symporter with bacterial and viral proteins. J Endocrinol Invest. 1999;22(7):535–540. doi: 10.1007/BF03343605. [DOI] [PubMed] [Google Scholar]
- 40.Sorensen MA, Jensen PD, Walton WE, Trumble JT. Acute and chronic activity of perchlorate and hexavalent chromium contamination on the survival and development of Culex quinquefasciatus Say (Diptera: Culicidae) Environ Pollut. 2006;144(3):759–764. doi: 10.1016/j.envpol.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 41.Saito M, Seki M, Amemiya S, Yamasu K, Suyemitsu T, Ishihara K. Induction of metamorphosis in the sand dollar Peronella japonica by thyroid hormones. Dev Growth Differ. 1998;40(3):307–312. doi: 10.1046/j.1440-169x.1998.t01-1-00006.x. [DOI] [PubMed] [Google Scholar]
- 42.Smith PN, Theodorakis CW, Anderson TA, Kendall RJ. Preliminary assessment of perchlorate in ecological receptors at the Longhorn Army Ammunition Plant (LHAAP), Karnack, Texas. Ecotoxicology. 2001;10(5):305–313. doi: 10.1023/a:1016715502717. [DOI] [PubMed] [Google Scholar]
- 43.Theodorakis C, Rinchard J, Anderson T, Liu F, Park JW, Costa F, McDaniel L, Kendall R, Waters A. Perchlorate in fish from a contaminated site in east-central Texas. Environ Pollut. 2006;139(1):59–69. doi: 10.1016/j.envpol.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 44.Yarrington C, Pearce EN. Iodine and pregnancy. J Thyroid Res. 2011:1–8. doi: 10.4061/2011/934104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suh M, Abraham L, Hixon JG, Proctor DM. The effects of perchlorate, nitrate, and thiocyanate on free thyroxine for potentially sensitive subpopulations of the 2001–2002 and 2007–2008 National Health and Nutrition Examination Surveys. J Expo Sci Env Epid. 2014;24(6):579–587. doi: 10.1038/jes.2013.67. [DOI] [PubMed] [Google Scholar]
- 46.Dawson DA, Bantle JA. Development of a reconstituted water medium and preliminary validation of the frog embryo teratogenesis assay—Xenopus (FETAX) J Appl Toxicol. 1987;7(4):237–244. doi: 10.1002/jat.2550070403. [DOI] [PubMed] [Google Scholar]
- 47.Goleman WL, Urquidi LJ, Anderson TA, Smith EE, Kendall RJ, Carr JA. Environmentally relevant concentrations of ammonium perchlorate inhibit development and metamorphosis in Xenopus laevis. Environ Toxicol Chem. 2002;21(2):424–430. [PubMed] [Google Scholar]
- 48.Mukhi S, Torres L, Patino R. Effects of larval–juvenile treatment with perchlorate and co-treatment with thyroxine on zebrafish sex ratios. Gen Comp Endocr. 2007;150(3):486–494. doi: 10.1016/j.ygcen.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 49.EPA (U.S. Environmental Protection Agency) Assessment of thyroid follicular cell tumors. Washington DC: Office of Research and Development; 1998. (report no. EPA/630/R-96/009). [Google Scholar]
- 50.Patiño R, Wainscott MR, Cruz-Li EI, Balakrishnan S, McMurry C, Blazer VS, Anderson TA. Effects of ammonium perchlorate on the reproductive performance and thyroid follicle histology of zebrafish. Environ Toxicol Chem. 2003;22(5):1115–1121. [PubMed] [Google Scholar]
- 51.Tietge JE, Holcombe GW, Flynn KM, Kosian PA, Korte JJ, Anderson LE, Wolf DC, Degitz SJ. Metamorphic inhibition of Xenopus laevis by sodium perchlorate: effects on development and thyroid histology. Environ Toxicol Chem. 2005;24(4):926–933. doi: 10.1897/04-105r.1. [DOI] [PubMed] [Google Scholar]
- 52.Bradford CM, Rinchard J, Carr JA, Theodorakis C. Perchlorate affects thyroid function in eastern mosquitofish (Gambusia holbrooki) at environmentally relevant concentrations. Environ Sci Technol. 2005;39(14):5190–5195. doi: 10.1021/es0484505. [DOI] [PubMed] [Google Scholar]
- 53.Smith PN, Yu L, McMurry ST, Anderson TA. Perchlorate in water, soil, vegetation, and rodents collected from the Las Vegas Wash, Nevada, USA. Environ Pollut. 2004;132(1):121–127. doi: 10.1016/j.envpol.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 54.Greer MA, Goodman G, Pleus RC, Greer SE. Health effects assessment for environmental perchlorate contamination: the dose response for inhibition of thyroidal radioiodine uptake in humans. Environ Health Persp. 2002;110(9):927–937. doi: 10.1289/ehp.02110927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lumen A, Mattie DR, Fisher JW. Evaluation of perturbations in serum thyroid hormones during human pregnancy due to dietary iodide and perchlorate exposure using a biologically based dose-response model. Toxicol Sci. 2013;133(2):320–341. doi: 10.1093/toxsci/kft078. [DOI] [PubMed] [Google Scholar]
- 56.Vitamin and mineral requirements in human nutrition. 2nd. World Health Organization; Geneva: 2005. (FAO (Food and Agriculture Organization) and WHO (World Health Organization)). [Google Scholar]
- 57.EPA (U.S. Environmental Protection Agency) Integrated Risk Information System chemical assessment summary. Office of Research and Development, National Center for Environmental Assessment, Washington Office; Washington, DC: 2005. Perchlorate (ClO4−) and perchlorate salts. [Google Scholar]
- 58.EPA (U.S. Environmental Protection Agency) ECOTOX user guide: ECOTOXicology knowledgebase system, Version 40. Washington, DC: 2016. http:/www.epa.gov/ecotox/, accessed June 21, 2017. [Google Scholar]
- 59.Liu FJ, Cobb GP, Anderson TA, Cheng QQ, Theodorakis CW. Uptake, accumulation and depuration of sodium perchlorate and sodium arsenate in zebrafish (Danio rerio) Chemosphere. 2006;65(10):1679–1689. doi: 10.1016/j.chemosphere.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 60.Landrum M, Canas JE, Coimbatore G, Cobb GP, Jackson WA, Zhang B, Anderson TA. Effects of perchlorate on earthworm (Eisenia fetida) survival and reproductive success. Sci Total Environ. 2006;363(1):237–244. doi: 10.1016/j.scitotenv.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 61.Opitz R, Trubiroha A, Lorenz C, Lutz I, Hartmann S, Blank T, Braunbeck T, Kloas W. Expression of sodium-iodide symporter mRNA in the thyroid gland of Xenopus laevis tadpoles: developmental expression, effects of antithyroidal compounds, and regulation by TSH. Journal of endocrinology. 2006;190(1):157–170. doi: 10.1677/joe.1.06606. [DOI] [PubMed] [Google Scholar]
- 62.Schmidt F, Schnurr S, Wolf R, Braunbeck T. Effects of the anti-thyroidal compound potassium-perchlorate on the thyroid system of the zebrafish. Aquat Toxicol. 2012;109:47–58. doi: 10.1016/j.aquatox.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 63.Mukhi S, Patiño R. Effects of prolonged exposure to perchlorate on thyroid and reproductive function in zebrafish. Toxicol Sci. 2007;96(2):246–254. doi: 10.1093/toxsci/kfm001. [DOI] [PubMed] [Google Scholar]
- 64.Zoeller RT, Tan SW, Tyl RW. General background on the hypothalamic-pituitary-thyroid (HPT) axis. Crit Rev Toxicol. 2007;37(1–2):11–53. doi: 10.1080/10408440601123446. [DOI] [PubMed] [Google Scholar]
- 65.Barnes DG, Dourson M, Preuss P, Bellin J, Derosa C, Engler R, Erdreich L, Farber T, Fenner-Crisp P, Francis E, Ghali G. Reference dose (RfD): description and use in health risk assessments. Regul Toxicol Pharm. 1988;8(4):471–486. doi: 10.1016/0273-2300(88)90047-5. [DOI] [PubMed] [Google Scholar]
- 66.de Lange HJ, Sala S, Vighi M, Faber JH. Ecological vulnerability in risk assessment—a review and perspectives. Sci Total Environ. 2010;408(18):3871–3879. doi: 10.1016/j.scitotenv.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 67.Menzie CA, MacDonell MM, Mumtaz M. A phased approach for assessing combined effects from multiple stressors. Environ Health Persp. 2007;115(5):807–816. doi: 10.1289/ehp.9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pickford DB. Screening chemicals for thyroid-disrupting activity: A critical comparison of mammalian and amphibian models. Crit Rev Toxicol. 2010;40(10):845–892. doi: 10.3109/10408444.2010.494250. [DOI] [PubMed] [Google Scholar]
- 69.Pearce EN, Braverman LE. Environmental Iodine Uptake Inhibitors. In: Pearce EN, editor. Iodine Deficiency Disorders and Their Elimination. Springer International Publishing; 2017. pp. 141–153. [Google Scholar]
- 70.Willemin ME, Lumen A. Development of a PBPK model of thiocyanate in rats with an extrapolation to humans: A computational study to quantify the mechanism of action of thiocyanate kinetics in thyroid. Toxicol Appl Pharm. 2016;307:19–34. doi: 10.1016/j.taap.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 71.Angrish MM, Madden MC, Pleil JD. Probe molecule (PrM) approach in Adverse Outcome Pathway (AOP) based High-Throughput Screening (HTS): In vivo discovery for developing in vitro target methods. Chem Res Toxicol. 2015;28(4):551–559. doi: 10.1021/acs.chemrestox.5b00024. [DOI] [PubMed] [Google Scholar]
- 72.Knapen D, Vergauwen L, Villeneuve DL, Ankley GT. The potential of AOP networks for reproductive and developmental toxicity assay development. Reprod Toxicol. 2015;56:52–55. doi: 10.1016/j.reprotox.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 73.Pastoor TP, Bachman AN, Bell DR, Cohen SM, Dellarco M, Dewhurst IC, Doe JE, Doerrer NG, Embry MR, Hines RN, Moretto A. A 21st century roadmap for human health risk assessment. Crit Rev Toxicol. 2014;44(sup3):1–5. doi: 10.3109/10408444.2014.931923. [DOI] [PubMed] [Google Scholar]
- 74.Ives C, Campia I, Wang RL, Wittwehr C, Edwards SW. Appl In Vitro Toxicol. Mary Ann Liebert, Inc.; Creating a Structured AOP knowledgebase via ontology-based annotations. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA. Gene Ontology: tool for the unification of biology. Nat Genet. 2000;25(1):25. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Malone J, Holloway E, Adamusiak T, Kapushesky M, Zheng J, Kolesnikov N, Zhukova A, Brazma A, Parkinson H. Modeling sample variables with an Experimental Factor Ontology. Bioinformatics. 2010;26(8):1112–1118. doi: 10.1093/bioinformatics/btq099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hanson ML, Wolff BA, Green JW, Kivi M, Panter GH, Warne MSJ, Ågerstrand M, Sumpter JP. How we can make ecotoxicology more valuable to environmental protection. Sci Total Environ. 2017;578:228–235. doi: 10.1016/j.scitotenv.2016.07.160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


