Abstract
In the last few decades, the endocannabinoid system has attracted a great deal of interest in terms of its applications to clinical medicine. In particular, its applications in cancer probably represent one of the therapeutic areas with most promise. On the one hand, expression of the endocannabinoid system is altered in numerous types of tumours, compared to healthy tissue, and this aberrant expression has been related to cancer prognosis and disease outcome, suggesting a role of this system in tumour growth and progression that depends on cancer type. On the other hand, cannabinoids exert an anticancer activity by inhibiting the proliferation, migration and/or invasion of cancer cells and also tumour angiogenesis. However, some cannabinoids, at lower concentrations, may increase tumour proliferation, inducing cancer growth. Enough data has been provided to consider the endocannabinoid system as a new therapeutic target in cancer, although further studies to fully establish the effect of cannabinoids on tumour progression are still needed.
Abbreviations
- 2‐AG
2‐arachidonoylglycerol
- ABCP
breast cancer resistance protein
- AM‐356
methanandamide
- CBD
cannabidiol
- ECS
endocannabinoid system
- HER‐2
human epidermal growth factor receptor
- MDR1
multidrug resistance protein 1
- THC
Δ9‐tetrahydrocannabinol
Introduction
Nowadays, the term “endocannabinoid system” (ECS) comprises cannabinoid (CB) receptors, endogenous cannabinoids, also called endocannabinoids, and the enzymes involved in their biosynthesis, transport and degradation. The most important endocannabinoids are http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2364, also called anandamide, and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=729, which are derived from arachidonic acid and synthesized on demand. The main synthesis and degradation pathways are shown in Figure 1 (Fraguas‐Sanchez et al., 2016; Schurman and Lichtman, 2017). Other minor endocannabinoids have also been identified, such as http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=284, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5554 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5545, also known as noladin‐ether.
Figure 1.
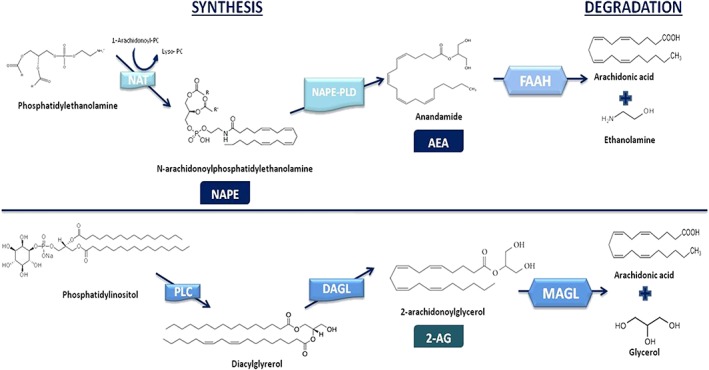
Diagram showing the main biosynthetic and degradation pathways of both AEA and 2‐AG. Anandamide is synthesized via a phospholipase‐D (NAPE‐PLD), which converts N‐arachidonoylphosphatidylethanolamine (NAPE), formed by the transfer of an arachidonoyl group to phosphatidylethanolamine by the action of N‐acetyltransferase (NAT), to AEA. 2‐AG is formed from diacylglycerol, via diacylglycerol lipase (DGL). Diacylglycerol is synthesized from phosphatidylinositol by the action of a PLC. In terms of degradation pathways, FAAH and MAGL are the most important enzymes responsible for inactivation of AEA and 2‐AG respectively. However, other pathways also participate in their degradation including lipoxygenases, cytochrome P450, COX‐2 and the domains 6 and 12 of serine lipases a/ß hydrolases.
The http://www.guidetopharmacology.org/GRAC/FamilyIntroductionForward?familyId=13 receptors belong to the superfamily of GPCRs and two distinct receptors have been identified and characterized to date, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=56 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=57, exhibiting around 44% homology. The CB1 receptor (first cloned from a rat brain in 1990) has an extended distribution, and although it is mostly expressed in the CNS, it is also found in peripheral nerve terminals and extra‐neuronal tissues, including the vascular endothelium, adipose tissue, lungs, liver, spleen, kidneys, uterus, prostate, testis and stomach. The CB2 receptor (isolated for the first time in 1993 from human promyelocytic HL‐60 cells) has a more localized distribution, being found predominantly in the immune system (tissues and cells). Nonetheless, it is also detected in the CNS, primarily after certain circumstances such as inflammation (Console‐Bram et al., 2012; Kendall and Yudowski, 2016). Finally, some endocannabinoid effects are mediated by non‐CB receptors, particularly the orphan receptors http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=114 and http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=114, which have also been postulated to be members of the ECS (Okuno and Yokomizo, 2011; Pyszniak et al., 2016; Morales and Reggio, 2017).
Since the discovery of the ECS, it has attracted a great deal of interest in therapeutics due to its involvement in several physiopathological processes including energy balance, appetite stimulation, nociception, embryogenesis, immune response and control of nausea and vomiting (Cunha et al., 2011; Katchan et al., 2016; Laprairie et al., 2017). Alterations of ECS expression have been found in many different disease conditions including cancer and neurological disorders such as Parkinson's disease, Huntington's disease and multiple sclerosis (Hasenoehrl et al., 2016; Ligresti et al., 2016; Bridgeman and Abazia, 2017). This review focuses on the role of the ECS in cancer disease progression and as a novel therapeutic target for anticancer treatments.
Endocannabinoid system expression in cancer
Cannabinoid receptors
The expression of both CB1 and CB2 receptors is altered in numerous types of tumours (summarized in Figure 2) and has been related to cancer prognosis. The altered expression is correlated with positive and negative survival indicators, depending on the origin of the cancer.
Figure 2.
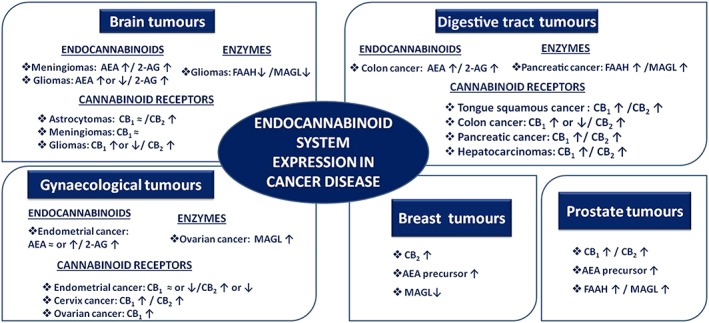
Altered expression of endocannabinoid system in brain, breast, digestive tract, gynaecological and prostate carcinomas.
In brain carcinomas, both CB1 and CB2 receptor expression is different from that in healthy tissue. Although, in astrocytomas and meningiomas, no differences in the expression of CB1 receptors have been detected, some studies have reported that in glioblastoma multiforme, their expression is lower (De Jesus et al., 2010). However, recent studies have demonstrated opposite results, showing CB1 receptors to be highly expressed in high‐grade gliomas compared to low‐grade tumours and healthy brain samples (Wu et al., 2012). Interestingly, these results have also been found in samples of low‐grade paediatric glioma, where overexpression has been associated with tumour regression, due to the apoptosis and cell cycle arrest induced by activation of CB1 receptors by endocannabinoids (Sredni et al., 2016). Concerning CB2 receptors, Schley et al. (2009) revealed an up‐regulation of these receptors in the endothelial cells of blood vessels of glioblastoma tissues. Finally, Wu et al. reported an overexpression of both CB1 and CB2 receptors in gliomas compared to the healthy brain. Whereas up‐regulation of CB1 receptors was associated with low‐grade tumours, over‐expression of CB2 receptors was related to high‐grade gliomas (Wu et al., 2012).
In breast tumours, which are mainly grouped into three categories depending on the molecular profile, hormone‐receptor positive, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2019 and triple negative, an increased expression of CB2 receptors has been detected. More than 90% of HER‐2‐positive tumours overexpressed this receptor (Caffarel et al., 2010) and this finding was related to a poor prognosis, probably due to the activation of HER‐2 pro‐oncogenic pathways (Perez‐Gomez et al., 2015). However, Elbaz et al. (2016) reported that the expression of these receptors in oestrogen‐receptor‐positive and oestrogen‐receptor‐negative mammary tumours was related to a better prognosis. In fact, they suggested CB2 receptors as a potential therapeutic target for treating breast cancer metastases. They demonstrated in vitro and in vivo (using mice orthotopic models) that the activation of CB2 receptors decreased the migration and invasion of oestrogen‐positive and ‐negative breast cancer cells, suppressing epidermal growth factor and insulin‐like growth factor tumourigenic pathways. Similar results were also found by Murase et al. (2014) who reported in mice models of breast cancer that the activation of CB2 receptors by O‐1665, a resorcinol compound analogue of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4150, reduced gene expression of the Id‐1 protein, associated with breast cancer metastases, and also increased the survival rate in advanced stages of carcinoma.
In prostate carcinomas, expressions of both CB1 and CB2 receptors were increased compared to the normal prostatic tissue (Orellana‐Serradell et al., 2015), and the overexpression of CB1 receptors has been associated with a higher Gleason score and metastasis incidence, being a negative marker of disease outcome. (Chung et al., 2009; Cipriano et al., 2013).
With respect to cancers of the digestive tract, the overexpression of CB receptors has also been related to cancer prognosis. In this sense, in tongue squamous tumour cells, both CB1 and CB2 receptors were overexpressed and this up‐regulation has been postulated to be an indicator of cancer outcome, with high levels of CB1 receptors being a better marker of disease survival than the overexpression of CB2, or of both CB receptors (Theocharis et al., 2016). Similarly, in colon tumours, both CB receptors were detected. While some studies reveal that CB1 receptors were down‐regulated in colon cancer compared to normal mucosa (Cianchi et al., 2008), others indicate that some tumours had high levels of this CB receptor, and it was an indicator of a poor disease outcome in patients with stage II microsatellite‐stable (Gustafsson et al., 2011) or stage IV (Jung et al., 2013) tumours. Targeting the CB1 receptors could be a good strategy to increase the efficacy of anticancer treatments. Thus their inactivation with http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=743, an inverse agonist at CB1 receptors. decreased the growth of colon cancer tumours in vivo by inhibiting the canonical Wnt/http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5371‐pathway through the inhibition of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2735 activity (Proto et al., 2017). In fact, a recent paper has reported, using 3D cultures of colon cancer cells that this compound acted specifically in tumour cells. Whereas in HTC116 cells, it demonstrated a strong synergism with http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4789 in overcoming tumour resistances, in GTG7 cells, it slightly increased 5‐fluorouracil efficacy and showed an antagonism with http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7433, with further studies being necessary (Fiore et al., 2017). As for CB2 receptors, an up‐regulated expression has also been associated with lower survival (Martinez‐Martinez et al., 2015). In pancreatic cancer, expression of both CB1 and CB2 receptors was increased compared to the normal pancreas (Carracedo et al., 2006), and CB1 up‐regulation has been related to a worse cancer prognosis (Michalski et al., 2008). In contrast, in hepatocarcinoma, where CB1 and CB2 receptors were also over‐expressed comparing to normal liver (307‐ and 5.44‐fold respectively) (Suk et al., 2016), high levels of both receptors have been associated with better disease‐free survival rates (Xu et al., 2006).
In several gynaecological tumours, the expression of CB receptors was also altered. In this context, studies performed in biopsies of patients with endometrial cancer showed that CB2 receptors, which are barely expressed in the healthy endometrial tissue, were up‐regulated, but no significant differences in CB1 receptor expression were detected (Guida et al., 2010). However, other researchers found low levels of both CB1 and CB2 receptors in endometrial carcinoma (Ayakannu et al., 2014). On the other hand, in cervix cancer, high levels of both CB were detected (Contassot et al., 2004a). In invasive ovarian tumours, only higher levels of CB1 were found and this up‐regulation was related to the invasiveness of ovarian cancer (Messalli et al., 2014).
Finally, in melanoma cells (Blazquez et al., 2006) and lung carcinomas, CB1 and CB2 receptors are present in 24% and 55% of patients with non‐small cell lung cancer respectively (Preet et al., 2011). In leukaemia and lymphomas, the opposite results have been reported. While some murine leukaemia cell lines expressed both CB1 and CB2 receptors, human leukaemia cells only expressed CB2 receptors (high levels) (McKallip et al., 2002). A high CB1 receptor expression has also been found in Hodgkin lymphoma cells (Benz et al., 2013).
Table 1 summarizes the alterations in expression of CB1 and CB2 receptors in several types of tumours.
Table 1.
Expression of CB1 and CB2 receptors in several carcinomas, compared with that in normal tissue.
| Cancer type | CB1 | CB2 | Relation to disease outcome | Reference | |
|---|---|---|---|---|---|
| Brain tumours | Astrocytomas | ≈ | ↑ | – | (De Jesus et al., 2010) |
| Meningiomas | ≈ | – | – | ||
| Gliomas | ↑ | ↑ |
• CB1 receptor overexpression is associated with tumour regression in glioblastoma and paediatric‐low‐gliomas • CB2 receptors; higher levels are related to tumour grade |
(Schley et al., 2009, Wu et al., 2012, Sredni et al., 2016) | |
| – | Breast | – | ↑ |
• CB2 receptors overexpression in more than 90% of HER‐2 positive tumours; is a negative prognosis marker. • CB2 receptor overexpression is a good prognosis marker in oestrogen negative and positive tumours. |
(Caffarel et al., 2010, Perez‐Gomez et al., 2015) |
| Prostate | ↑ | ↑ | • CB1 receptor overexpression is a good prognosis marker | (Chung et al., 2009, Cipriano et al., 2013, Orellana‐Serradell et al., 2015) | |
| Digestive tract tumours | Tongue squamous | ↑ | ↑ | • CB1 receptor overexpression is a positive marker of disease outcome | (Theocharis et al., 2016) |
| Colon | ↑ or ↓ | ↑ | • CB1and CB2 receptor overexpression is related to a poor disease outcome. | (Cianchi et al., 2008, Gustafsson et al., 2011, Jung et al., 2013, Martinez‐Martinez et al., 2015) | |
| Pancreas | ↑ | ↑ | • CB1 receptor overexpression is a negative marker of disease outcome | (Carracedo et al., 2006, Michalski et al., 2008) | |
| Hepatocarcinoma | ↑ | ↑ | • CB1 and CB2 receptor overexpression is a good indicator of survival | (Xu et al., 2006, Suk et al., 2016) | |
| Gynaecological tumours | Endometrial | ≈ or ↓ | ↑ or ↓ | – | (Guida et al., 2010, Ayakannu et al.) |
| Cervix | ↑ | ↑ | – | (Contassot et al., 2004a) | |
| Ovary | ↑ | – | • CB1 receptor up‐regulation is associated with a higher tumour aggressiveness | (Messalli et al., 2014) | |
| – | Hodgkin lymphoma | ↑ | – | – | (Benz et al., 2013) |
In the Table, ≈ denotes a similar expression, ↑ higher levels and ↓ lower levels of expression compared with normal tissues
Endocannabinoid levels
The levels of endocannabinoids, especially AEA and 2‐AG, are also abnormal in some tumours, compared with normal tissues. Regarding brain tumours, conflicting information has been documented. While some authors have reported that AEA levels were lower in gliomas compared to non‐tumour tissues (Maccarrone et al., 2001; Wu et al., 2012), others have detected higher levels of this endocannabinoid in gliomas and also in meningiomas (Petersen et al., 2005). With respect to 2‐AG levels, they were up‐regulated in both kinds of brain tumours (Petersen et al., 2005; Wu et al., 2012). In prostate tumours, increased levels of AEA have also been reported (Schmid et al., 2002). However, in breast carcinoma, AEA levels were not increased. Nevertheless, high levels of the AEA precursor, N‐acylphosphatidylethanolamine, have been detected. In colon cancer, several authors have also reported that levels of both AEA and 2‐AG were increased, threefold and twofold respectively (Ligresti et al., 2003). Interestingly, especially AEA levels were increased in lymphatic metastasis (Chen et al., 2015). In patients with endometrial carcinoma, elevated levels of 2‐AG have also been shown compared to healthy tissues, but with respect to AEA levels, the opposite results were detected. While some authors showed no significant differences in AEA levels (Guida et al., 2010), in other studies, high levels have been reported (Schmid et al., 2002). Finally, elevated levels of both AEA and 2‐AG have been found in pituitary adenomas, correlated with the presence of CB1 receptors. While in CB1 receptor‐positive samples, higher endocannabinoid levels have been detected, in samples with a low expression of CB1, endocannabinoid levels were lower (Pagotto et al., 2001).
Endocannabinoid degrading enzymes
Taking into account that, in some tumours, endocannabinoid levels were increased compared to normal tissues, an inhibition of the main enzymes responsible for their degradation could be expected. This happens in gliomas, where the expression and the activity of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1400 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1399 enzymes were reduced, compared with normal brain tissue (Wu et al., 2012). However, in some studies, an increase of these enzymes has been reported. For example, in breast ductal carcinomas, MAGL expression was increased (Gjerstorff et al., 2006), and in prostate tumours, high levels of AEA and its major degradative enzyme were detected compared to normal prostate tissue (Endsley et al., 2008). FAAH overexpression has been associated with cancer invasion and disease outcome (Thors et al., 2010). In androgen‐independent prostate tumours, elevated levels of MAGL have also been found (Nithipatikom et al., 2005). The expression and the activity of the MAGL enzyme were also increased in invasive ovarian and melanoma tumours, and interestingly, the up‐regulation of MAGL in ovarian, melanoma and non‐aggressive prostate cancer was associated with higher tumour cell migration and invasion (Van Dross et al., 2013; Qin and Ruan, 2014). Nevertheless, the opposite results were found in pancreatic ductal adenocarcinomas, where both FAAH and MAGL enzymes were overexpressed and related to a good prognosis (Michalski et al., 2008).
Table 2 summarizes the levels of endocannabinoids and expression of endocannabinoid degradative enzymes in several cancer types.
Table 2.
Expression of the major endocannabinoids and their major degradative enzymes in several carcinomas, compared with normal tissue.
| Cancer type | AEA | 2‐AG | FAAH | MAGL | Observations | References | |
|---|---|---|---|---|---|---|---|
| Brain cancer | Meningioma | ↑ | ↑ | – | – | – | (Maccarrone et al., 2001, Petersen et al., 2005, Wu et al., 2012) |
| Glioma | ↑ or ↓ | ↑ | ↓ | ↓ | – | ||
| – | Pituitary adenomas | ↑ | ↑ | – | – | Correlation with CB1 receptor expression | (Pagotto et al., 2001) |
| Breast | Precursor ↑ | – | – | ↑ | Higher levels of AEA precursor have been detected | (Gjerstorff et al., 2006) | |
| Prostate | ↑ | – | ↑ | ↑ | FAAH and MAGL overexpression related to cancer invasion and disease outcome | (Schmid et al., 2002, Nithipatikom et al., 2005, Endsley et al., 2008, Thors et al., 2010) | |
| Digestive tract tumours | Colon | ↑ | ↑ | – | – | High levels of AEA in lymphatic metastases | (Ligresti et al., 2003, Chen et al., 2015) |
| Pancreas | – | – | ↑ | ↑ | FAAH and MAGL overexpression is a good cancer prognosis | (Michalski et al., 2008) | |
| Gynaecological tumours | Endometrial | ≈ or ↑ | ↑ | – | – | – | (Schmid et al., 2002, Guida et al., 2010) |
| Ovarian | – | – | – | ↑ | MAGL up‐regulation associate with cancer invasiveness. | (Van Dross et al., 2013, Qin and Ruan, 2014) | |
| – | Melanoma | – | – | – | ↑ |
In the Table, ≈ denotes a similar expression, ↑ higher levels and ↓ lower levels of expression compared with normal tissues
Non‐cannabinoid receptors: GPR55
Levels of the orphan GPR55 were associated with high proliferation rates of tumour cells. In this context, in vitro and in vivo studies undertaken in several tumour models, including pancreas, breast, brain (Andradas et al., 2011; Andradas et al., 2016), prostate, ovary (Pineiro et al., 2011) and skin carcinomas (Perez‐Gomez et al., 2013) reported that GPR55 receptors were implicated in the proliferation and progression of cancer. This may be attributed to GPR55 activation by the agonist http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4028 (Ford et al., 2010; Hofmann et al., 2015) and, in patients with ovarian cancer, elevated levels of LPI were detected in plasma (Xiao et al., 2001; Sutphen et al., 2004).
The overexpression of these receptors has also been related to cancer disease aggressiveness and a poor prognosis. For example, in glioblastoma, increased levels of GPR55 were correlated with higher tumour grades and a lower survival rate, and provided a marker of a negative cancer prognosis. This relationship has also been shown in breast and pancreatic tumours (Andradas et al., 2011). Hasenoehrl et al. (2018) reported a pro‐tumour activity of these receptors in a recent study undertaken in mouse models of colon carcinoma. Although no effect on cell proliferation was detected with an agonist or antagonist of GPR55 receptors, they interfered with the composition of the leukocyte population during carcinogenesis, triggering a tumour‐promoting micro‐environment with the increase of tumourigenic factors (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1376, STAT3 and NF‐κB). Knockout mice exhibited reduced levels. In samples obtained from colon cancer patients, a correlation between high levels of GPR55 and a decrease in relapse‐free survival has been also reported, supporting the implication of GPR55 in carcinogenesis (Hasenoehrl et al., 2018).
Interestingly, some studies have reported that the heterodimerization between GPR55 and CB2 receptors (Balenga et al., 2014) may modulate, in breast and brain tumours, the antitumour activity of some cannabinoids.
Endocannabinoid antitumour activity
A large number of studies have been undertaken to evaluate the anticancer activity of plant and synthetic cannabinoids (Guzman, 2003; Fraguas‐Sanchez et al., 2016; Velasco et al., 2016; Bogdanovic et al., 2017). Regarding endocannabinoids, their exogenous administration has also been reported to reduce the proliferation, migration and invasion of tumours. Finally, inhibition of the MAGL and FAAH enzymes has also shown anticancer activity.
Several authors have demonstrated that endocannabinoids exert an anticancer activity in brain tumours. Thus, AEA inhibited the proliferation of C6 glioma cells in a mechanism that involves both http://www.guidetopharmacology.org/GRAC/FamilyIntroductionForward?familyId=78 and CB receptors (Fowler et al., 2003). Contassot et al. (2004b) reported that this compound also inhibited the proliferation of U87, U251, C6 and H4 glioma cells. However, these authors found that the blockade of both CB1 and CB2 receptors did not protect from this activity; on the contrary, blocking these receptors seemed to exacerbate proliferation of these cells. The use of TRPV‐1 channel antagonists significantly decreased the antiproliferative effect, suggesting that this was responsible for AEA action. The involvement of these receptors was also demonstrated by Bari et al. (2005), who found that the pretreatment of C6 glioma cells with the lipid raft disruptor, methyl‐β‐cyclodextrin, decreased the apoptosis induced by AEA. While co‐incubation with the TRPV‐1 channel antagonist significantly reduced the apoptotic effect, CB1 antagonists almost doubled it, also preventing methyl‐β‐cyclodextrin activity. All these data support the participation of TRPV‐1 channels in AEA apoptotic activity. Finally, the involvement of the COX‐2 enzyme in cell death induced by this endocannabinoid has also been suggested in H4 cells (Hinz et al., 2004). Ma et al. (2016) also found in vitro that AEA decreased not only the proliferation but also the migration and invasion of U251 glioma cancer cells, showing, in agreement with earlier results, that the inhibition of cell growth was due to induction of apoptosis and even a cell cycle arrest at the G0/G1 phase. They also demonstrated the antiproliferative effect of this cannabinoid in mice, reporting a significant tumour growth inhibition compared to the control.
In addition to AEA, other endocannabinoids have been investigated. 2‐AG also decreased the proliferation of C6 glioma cells, showing a similar IC50 value to AEA (1.8 and 1.6 μM respectively) (Fowler et al., 2003). Jacobsson et al. (2001) also reported that 1‐AG and 2‐AG inhibited the proliferation of C6 glioma cells. Interestingly, both endocannabinoids showed practically the same inhibitory profile and sensitivity as TRPV‐1 and CB receptor antagonists; blocking completely their activity. All these data suggest that the action of 2‐AG may be secondary to its conversion to 1‐AG (Jacobsson et al., 2001).
With respect to breast cancer, both the major endogenous cannabinoids, AEA and 2‐AG, and also minor compounds such as oleamide, inhibited the proliferation of breast cancer cells (EFM‐19, MCF‐7 T‐47D and BT4744–6) in vitro by cell cycle arrest and/or the induction of apoptosis. The involvement of CB1 receptors has also been reported (Bisogno et al., 1998; Melck et al., 2000). The nerve growth factor (NGF) induced proliferation was also inhibited by both AEA and 2‐AG suppressing http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1817 levels, and the co‐administration of several endocannabinoids, such as the combination of AEA with oleamide, potentiated this antiproliferative effect (De Petrocellis et al., 1998).
Concerning prostate carcinomas, various studies have reported the anti‐proliferative activity of endocannabinoids. In a CB1 receptor‐dependent manner, AEA has been shown to inhibit the proliferation of PC‐3, DU‐145 and LNCaP cells, including the proliferation induced by http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4916, by decreasing the expression of its http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=320 and blocking the cell cycle at the G1 phase (Mimeault et al., 2003; Nithipatikom et al., 2011). The growth of primary cultures of prostate tumours was also inhibited by AEA, triggering apoptosis (Orellana‐Serradell et al., 2015). It has been reported that AEA and also 2‐AG decreased the http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5049‐induced growth of DU‐145 prostate cancer cells. All these effects seem to involve CB1 receptors. On the other hand, noladin ether, a minor endogenous cannabinoid, also reduced the proliferation of prostate tumour cells, but this action was not mediated by CB receptors (Nithipatikom et al., 2011).
The effect of endocannabinoids on prostate tumour invasion has also been investigated. The increase in endogenous 2‐AG levels via http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=943 inhibition enhanced the invasion of PC‐3 and DU‐145 cells (androgen‐independent) (4.2‐ and 2.0‐fold increase respectively). However, in LNCaP (androgen‐dependent), the opposite results were found, suggesting that this endocannabinoid may be a potential inhibitor of androgen‐dependent prostate tumours. This anti‐invasive effect was related to inhibition of http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=257 and a reduction of http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=284 activity in a mechanism that seems to involve CB1 receptors (Nithipatikom et al., 2004). Interestingly, Endsley et al. (2007) showed endogenous 2‐AG to be an anti‐invasive compound in PC‐3 cells, while its exogenous administration had the opposite effect, stimulating the invasion capacity of these cells. The administration of exogenous http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2391 also showed this effect, suggesting that the rapid hydrolysis of 2‐AG may be responsible for the increase in the invasion rate. In fact, Nomura et al. (2011) reported that MAGL inhibitors decreased the invasion capacity of prostate carcinomas and that this effect was partially reversed by blocking CB1 receptors. The disruption of MAGL activity also interfered with the expression of the epidermal growth factor receptor, decreasing the proliferation induced by epidermal growth factor (Cipriano et al., 2014). Finally, noladin ether has also been reported to inhibit the invasion of androgen‐independent prostate tumours, inhibiting PKA activity via the CB1 receptors (Nithipatikom et al., 2004).
Regarding tumours of the digestive tract, AEA and 2‐AG have been demonstrated to reduce the proliferation of several lines of colon cancer cells (DLD‐1, HT‐29, SW620 and CaCo‐2). The involvement of CB receptors in this antiproliferative effect is disputed and may depend on cell type. While some researchers reported that these effects were mediated by CB1 receptors and CB2 receptors in the case of DLD‐1 cells (Ligresti et al., 2003; Linsalata et al., 2010), others showed that CB were not involved (Gustafsson et al., 2009b; Patsos et al., 2010). Interestingly, Gustafsson et al. (2009b) demonstrated the involvement of oxidative stress due to the use of α‐tocopherol, and a http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=253 inhibitor attenuated the AEA antiproliferative activity. Linsalata et al. (2010) postulated that the antiproliferative activity of AEA may be due to the reduction of polyamine levels that play a critical role in cell proliferation. Finally, the inhibition of FAAH also reduced the viability, migration and invasion of Colo‐205 cells in vitro (Wasilewski et al., 2017).
In gastric carcinomas, some researchers have reported that AEA diminished the proliferation of cancer cells, inducing cell cycle arrest at the G0/G1 phase (Park et al., 2011; Ortega et al., 2016). It also enhanced the pro‐apoptotic effect of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2770, being a promising combination drug in chemotherapy (Miyato et al., 2009). In hepatocarcinomas, the opposite results were found. While anandamide showed an in vitro antiproliferative activity in cholangiocarcinoma cells, 2‐AG stimulated cell growth. Both effects were not mediated by CB receptors and involved lipid rafts. AEA seemed to exert its action by stabilizing lipid rafts and recruiting Fas and FasL. 2‐AG disrupted the lipid raft structure (DeMorrow et al., 2007). In vivo studies undertaken in mice supported the antiproliferative activity of AEA and reported the activation of non‐canonical Wnt signalling pathway as one of the mechanisms responsible for its action, increasing the expression of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3548. The increase of this protein induced the triggering of calcium‐independent pathways that involved the orphan receptor http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1846 (DeMorrow et al., 2008).
With regard to gynaecological cancers, AEA has been reported to inhibit the proliferation of several cervical cell lines (CC299, CasKi and HeLa) by the induction of apoptosis (activating the cleavage of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1623). Interestingly, CB receptors were not involved in this induction of apoptosis, although they were expressed by these cells. By contrast, the blockade of both CB1 and CB2 receptors with selective agonists did not prevent the induction of apoptosis but potentiated it, suggesting that these receptors may have a protective role in death of cervical cancer cells and the apoptotic effect was attributed, at least in part, to TRPV‐1 receptors (Contassot et al., 2004a). Nevertheless, CB receptors were involved in the reduction of the migration and invasion of cervical cancer cells triggered by some cannabinoid compounds. For example, Rudolph et al. (2008) reported that CB1 receptors mediated the anti‐migratory effect of 2‐AG in SW 756 cancer cells.
The synthetic analogue of anandamide, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2506, has been reported to inhibit the growth of established thyroid cancer in vivo and also to decrease the expression of http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=963, producing anti‐angiogenic effects. All these actions were attenuated by CB1 receptor antagonists, so CB1 receptors are involved in the anti‐tumour activity of AM‐356. The same authors also demonstrated that AM‐356 inhibited the in vitro proliferation of metastasis‐derived thyroid cancer cells, especially lung metastasis cells (Portella et al., 2003). Interestingly, in vivo studies in thyroid tumour xenografts induced in mice reported that arachidonyl‐5‐HT, an FAAH inhibitor, and VDM‐11, an inhibitor of endocannabinoid re‐uptake, decreased tumour growth, involving both non‐cannabinoid and CB1 receptors (Bifulco et al., 2004).
In lymphomas, some murine cell lines that express both CB1 and CB2 receptors were sensitive to the action of AEA and also to other CB receptor agonists which induce apoptosis (McKallip et al., 2002). AM‐356 also decreased the proliferation of mantle cell lymphoma, normally a very aggressive carcinoma, via the activation of the novo ceramide synthesis pathway in a CB1 receptor‐dependent manner (Gustafsson et al., 2009a).
Finally, AEA has been shown to reduce the proliferation of human leukaemia cells, such as Jurkat, Mol‐4 and Sup‐1 cells, inducing apoptosis with the involvement of CB2 receptors (McKallip et al., 2002).
The anti‐tumour activities of endocannabinoids are summarized in Table 3.
Table 3.
Anti‐tumour actions of endocannabinoids
| Cancer type | Cannabinoid | Cancer cells | Anti‐tumour effect | Mechanism | References |
|---|---|---|---|---|---|
| Glioma | AEA | U87, U251, C6, H4 | Inhibition of cell proliferation |
• Apoptosis induction • Involvement of TRPV‐1 channels |
(Hinz et al., 2004, Contassot et al., 2004b, Bari et al., 2005) |
| U251 | Inhibition of cell proliferation, migration and invasion | • Induction of apoptosis and cell cycle arrest at G0/G1 phase | (Ma et al., 2016) | ||
|
2‐AG 1‐AG |
C6 | Inhibition of cell proliferation |
• Involvement of CB receptors and TRPV‐1 channels. • The action of 2‐AG seems to be secondary to its conversion to 1‐AG |
(Jacobsson et al., 2001, Fowler et al., 2003) | |
| Breast cancer | AEA, 2‐AG | EFM‐19, MCF‐7, T47‐D, BT‐47446 | Inhibition of cell proliferation |
• Induction of apoptosis and cell cycle arrest at G1/S phase via CB1 receptors • Suppression of NGF induced proliferation probably due to a reduction of NGF/TrK receptor levels • Oleamide potentiates the antiproliferative activity of AEA. |
(Bisogno et al., 1998, De Petrocellis et al., 1998, Melck et al., 2000) |
| Prostate cancer | AEA | PC‐3, DU‐145 and LNCaP | Inhibition of cell proliferation. | • Apoptosis induction | (Mimeault et al., 2003, Nithipatikom et al., 2011, Orellana‐Serradell et al., 2015) |
| AEA, 2‐AG | DU‐145 | Reduction of prolactin induced proliferation | – | (Nithipatikom et al., 2011) | |
| Noladin‐Ether | DU‐145 | Inhibition of cell proliferation | • In a CB receptor independent manner | ||
| 2‐AG | Androgen‐independent tumour cells | Inhibition of cell invasion | • Involvement of CB1 receptors | (Endsley et al., 2007) | |
| Colon cancer | AEA, 2‐AG | – | Inhibition of cell proliferation | • Opposite results have been reported in the participation of CB receptors. | (Ligresti et al., 2003, Gustafsson et al., 2009b, Linsalata et al., 2010, Patsos et al., 2010) |
| Gastric cancer | AEA | AGS | Inhibition of cell proliferation | • Cell cycle arrest at G0/G1and apoptosis induction. | (Ortega et al., 2016) |
| Hepatocarcinoma | AEA | – |
Inhibition of cell proliferation. Reduction of tumour growth in vivo |
• CB receptor independent mechanism | (DeMorrow et al., 2007, DeMorrow et al., 2008) |
| Cervical cancer | AEA | CC299, CasKi, HELA | Inhibition of cell proliferation |
• Cell cycle arrest at G0/G1 phase and apoptosis induction. • Involvement of non‐CB receptors in the generation of apoptosis |
(Contassot et al., 2004a) |
| 2‐AG | SW‐756 | Inhibition of cell migration and invasion | • Involvement of CB1 receptors | (Rudolph et al., 2008) | |
| Thyroid cancer | AM‐356 | – | In vivo inhibition of tumour growth | • Inhibition of tumour angiogenesis due to the reduction of VEGF expression via CB1 receptors | (Portella et al., 2003) |
| Lymphomas | AEA | Murine cells | Inhibition of cell proliferation | – | (McKallip et al., 2002) |
| Leukaemia | AEA | Jurkat, Mol‐4 and Sup‐1 | Inhibition of cell proliferation | • Induction of apoptosis via CB2 receptors |
Involvement of the endocannabinoid system in tumour progression
Effect on tumour neovascularization
The ECS has also been postulated as a modulator of tumour angiogenesis, being implicated in anti‐angiogenic action by decreasing the survival and migration of endothelial cells and/or reducing the expression of pro‐angiogenic factors. In fact, several cannabinoids, including AEA and CBD, have been shown to have anti‐angiogenic properties (Rajesh et al., 2010; Thapa et al., 2011; Solinas et al., 2012). This effect is involved in the inhibition of the growth of numerous kinds of tumours such as lung, breast, skin and brain carcinomas. For example, in vitro and in ovo (in the chick chorioallantoic neovascularization model), AM‐356 exerted an anti‐angiogenic effect, decreasing the proliferation and inducing apoptosis of endothelial cells, with the diminution of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1629 activity. The mechanisms responsible for these effects involved CB1 receptors and were also involved in the anti‐tumour activity of cannabinoids in thyroid cancer (Pisanti et al., 2007). Similar results were previously reported by Portella et al. (2003), showing that the inhibition of angiogenesis in thyroid tumours by AM‐356 involved a reduction of the expression of VEGF, a pro‐angiogenic factor, also showing the participation of CB1 receptors. This effect was attributed to inhibition of p21ras activity (necessary for VEGF action). AEA was also reported to reduce pro‐angiogenic pathways in breast cancer cells. In an in vitro model of angiogenesis, AEA decreased the MDA‐MB‐231‐induced proliferation of endothelial cells and reduced the levels of a significant number of pro‐angiogenic factors, especially those of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4968, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5015, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5060, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5309, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5310, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5063 and VEGF (Picardi et al., 2014).
CB receptors are also implicated in the anti‐angiogenic activity of other synthetic cannabinoids such as WIN‐55212–2 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=747, in brain tumours (gliomas and astrocytomas). Blazquez et al. (2003) reported that these compounds impaired tumour neovascularization by inhibiting cell survival with the induction of apoptosis and the migration of vascular endothelial cells. They also reduced the expression of several angiogenesis stimulation factors, specifically VEGF and angiotensin II.
The inhibition of the MAGL enzyme with the consequent increase of 2‐AG levels was also reported to decrease tumour growth, exerting an anti‐angiogenic activity. In vivo studies reported that MAGL inhibitors down‐regulated pro‐angiogenic factors, particularly VEGF and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4924, and also reduced the number of vessels (Pagano et al., 2017).
In spite of this anti‐angiogenic effect of cannabinoids, minor endocannabinoid compounds have been postulated as pro‐angiogenic factors, stimulating angiogenesis in a GPR55 receptor‐dependent manner (Zhang et al., 2010). Indeed, this effect has also been reported for AEA. In the nanomolar range, this cannabinoid induced the angiogenesis stimulated by FGF‐2, in a pathway that involved CB1 receptors, which were found to be overexpressed during the angiogenesis process. Their involvement in the angiogenesis process has been corroborated in vivo. On the one hand, studies in CB1 receptor knockdown mice reported an inactivation of the proliferation, migration and capillary‐like tube formation induced by pro‐angiogenic factors (particularly FGF‐2). On the other hand, CB1 blockage also reduced the FGF‐2‐induced neovascular proliferation in the rabbit cornea assay (Pisanti et al., 2011). The implication of these receptors in carcinogenesis has also been reported by other authors. Malfitano et al. (2012) demonstrated using an ascitic tumour model (Meth‐A cells specifically injected into mice) that CB1 receptor blockade reduced tumour growth. Ciaglia et al. (2015) found similar results in an in vivo model of glioma, also involving the participation of STAT3 pathway. Additionally, in glioma samples from patients, they related low levels of active STAT3 to a lower expression of CB1 receptors. Nevertheless, Wang et al. (2008) found in mice models of colon cancer that the loss or inhibition of CB1 receptors induced tumour growth. So the involvement of these receptors in cancer probably depends on the origin of the tumour.
Stimulation of tumour growth by cannabinoids
In spite of the establishment of anticancer activity of cannabinoids and the involvement of the ECS, the implication of the ECS in cancer progression has also been reported in some tumours. This biphasic effect seems to depend on the cannabinoid concentration and involves CB receptors, specifically CB2.
Thus, AEA at concentrations of 1 μM stimulated the proliferation of gastric cancer cells (Miyato et al., 2009), and its analogue, AM‐356, at lower concentrations also exerted a mitogenic activity in prostate carcinomas. While, in the micromolar range, AM‐356 inhibited the proliferation of LNCap prostate cancer cells, at nanomolar concentrations (100–200 nM), it stimulated cell growth. Similarly, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2424 and JWH‐133 exerted pro‐proliferative effects in prostate cancer (Sanchez et al., 2003).
The proliferation induced by THC has also been reported in lung, breast and even in brain tumours. This phytocannabinoid, in concentrations of 100–300 nM, stimulated the proliferation of lung tumours and gliomas in vitro (Hart et al., 2004). In vivo, in mice models, Δ9‐THC also improved the growth and metastases formation of breast tumours (McKallip et al., 2005). Interestingly, this pro‐cancer activity has been related to the immune response against the tumour and involves CB2 receptors. By the activation of these receptors, THC increased the production of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4996 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4975, stimulating a Th‐2‐type immune response and inhibiting the Th‐1 response (Zhu et al., 2000; McKallip et al., 2005).
ECS and drug resistance
Multidrug resistance is probably one of the major problems in chemotherapy of cancer. Tumours become resistant to a great number of different drugs without structural or functional similarities, including anthracyclines, taxanes and Vinca alkaloids, and this hampers cancer treatments (An et al., 2017). Much of such resistance to cancer chemotherapy involves the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=768), an efflux pump that belongs to the family of ATP‐binding cassette transporters (ABC) (Lopes‐Rodrigues et al., 2016) and other proteins of the ABC superfamily such as the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=792 (Chen et al., 2010). Several studies have reported the involvement of the ECS in the mediation of resistance to anticancer drugs.
In T‐lymphoblastic leukaemia cells (CEM/VLB100), the two major plant cannabinoids, THC and CBD, modulate the expression of MDR1. While, at shorter incubation times (4 h), they increased its expression and consequently the activity of MDR1, at longer times (72 h), they decreased MDR1 action. The ECS is involved in these actions, although differences in the mechanisms of both cannabinoids have been reported. Although the THC effect was only mediated by CB2 receptors, the activity of CBD was mediated by both CB2 and non‐CB receptors, specifically TRPV‐1 channels (Arnold et al., 2012). Holland et al. also reported that both cannabinoids and also http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=740 reduced the expression of MDR1 protein in these leukaemia cells, without inhibiting its efflux activity. In breast cancer (MCF‐7 cells), CBD also inhibited the expression of MDR1, but it increased expression of ABCP (Feinshtein et al., 2013).
By contrast, other authors have shown that several plant cannabinoids, including CBD, THC and cannabinol, inhibited ABCP (Holland et al., 2007; Tournier et al., 2010) and also MDR1, with CBD being the most potent inhibitor (Holland et al., 2008).
Concluding remarks
In tumours, the abnormal expression of the different components of the ECS, especially CB1 and CB2 receptors, compared with that healthy tissues reveals its involvement in cancer. However, this aberrant expression is not consistent and varies with cancer type. Although in some tumours these receptors are up‐regulated, in others, their expression is lower. Therefore, it is important to consider the participation of CB2 receptors, whose levels seem to be up‐regulated after certain pathological conditions. The correlation of the aberrant expression in the ECS with cancer outcome reinforces its involvement in tumourigenesis. The ECS either participates in disease progression or exerts a protective role and becomes a potential therapeutic target. In general, for example, in prostate, colon and pancreatic carcinomas, an altered ECS is a negative marker for cancer, being related to a more invasive tumour and a lower survival rate, except in hepatocarcinoma where it is an indicator of good prognosis.
CB receptors also mediate anticancer activity, at least in part, of other cannabinoid compounds, including phytocannabinoids such as THC, CBD and cannabinol, and even synthetic cannabinoids, including AM‐356. The efficacy of CBD alone and in combination with THC is being extensively evaluated for the treatment of different solid tumours (Ramer and Hinz, 2016).
Besides CB receptors, endocannabinoid levels are also altered in some carcinomas. In this context, it is important to emphasize that the two major endogenous cannabinoids (AEA and 2‐AG) administered exogenously inhibit the growth of several kinds of tumours, demonstrating an anti‐proliferative, anti‐invasive and anti‐angiogenic activity. The increase in endocannabinoid levels as a result of inhibiting their degradation pathways, especially the FAAH and MAGL enzymes, has also been useful in reducing cancer proliferation.
In spite of the promising activity of cannabinoids as anticancer treatments, it has to be taken into account that some cannabinoids have also been shown to increase tumour growth, exerting pro‐angiogenic and pro‐proliferative effects. This biphasic activity has been related to cannabinoid concentration. It seems that while cannabinoids stimulated cancer growth at low concentrations (in the nanomolar range), they inhibited it at higher concentrations (micromolar range). While both CB1 and CB2 receptors are involved in cannabinoid anticancer activity, the stimulation of cancer proliferation appears to be mainly attributable to CB2 receptors.
Finally, cannabinoids have also been associated with resistance to anticancer drugs, interfering in the expression of several ABC drug transporter proteins, especially MDR1, implying that these proteins could provide a good target for cannabinoids to overcome cancer resistance. In fact, some cannabinoids at non‐toxic concentrations increase the sensitivity of cancer cells to chemotherapy. For example, THC and CBD potentiate the cytotoxicity of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6851 (Holland et al., 2006) and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7301 in leukaemia and glioma respectively. Consequently, cannabinoids could be a good strategy as co‐adjuvant treatments. In fact, a clinical study is currently being performed to evaluate the efficacy and safety of a cannabinoid‐based spray containing the two major natural cannabinoids present in Cannabis sativa, THC and CBD, in combination with temozolomide for the treatment of glioma (NCT01812603) (Holland et al., 2006; Zogopoulos et al., 2015).
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a, 2017b, 2017c, 2017d, 2017e, 2017f).
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
A.I. Fraguas‐Sánchez was granted a research fellowship (ref: FPU14/06441) from the Spanish Ministry of Education. This work was supported by the Complutense University of Madrid (ref: FEI16/83) and the drug parenteral administration group (ref: 910939).
Fraguas‐Sánchez, A. I. , Martín‐Sabroso, C. , and Torres‐Suárez, A. I. (2018) Insights into the effects of the endocannabinoid system in cancer: a review. British Journal of Pharmacology, 175: 2566–2580. doi: 10.1111/bph.14331.
References
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD et al (2017c). The Concise Guide to PHARMACOLOGY 2017/18: Other proteins. Br J Pharmacol 174: S1–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Striessnig J, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017d). The Concise Guide to PHARMACOLOGY 2017/18: Voltage‐gated ion channels. Br J Pharmacol 174: S160–S194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017e). The Concise Guide to PHARMACOLOGY 2017/18: Catalytic receptors. Br J Pharmacol 174: S225–S271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD et al (2017f). The Concise Guide to PHARMACOLOGY 2017/18: Transporters. Br J Pharmacol 174: S360–S446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An X, Sarmiento C, Tan T, Zhu H (2017). Regulation of multidrug resistance by microRNAs in anti‐cancer therapy. Acta Pharm Sin B 7: 38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andradas C, Blasco‐Benito S, Castillo‐Lluva S, Dillenburg‐Pilla P, Diez‐Alarcia R, Juanes‐Garcia A et al (2016). Activation of the orphan receptor GPR55 by lysophosphatidylinositol promotes metastasis in triple‐negative breast cancer. Oncotarget 7: 47565–47575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andradas C, Caffarel MM, Perez‐Gomez E, Salazar M, Lorente M, Velasco G et al (2011). The orphan G protein‐coupled receptor GPR55 promotes cancer cell proliferation via ERK. Oncogene 30: 245–252. [DOI] [PubMed] [Google Scholar]
- Arnold JC, Hone P, Holland ML, Allen JD (2012). CB2 and TRPV1 receptors mediate cannabinoid actions on MDR1 expression in multidrug resistant cells. Pharmacol Rep 64: 751–757. [DOI] [PubMed] [Google Scholar]
- Ayakannu T, Taylor AH, Marczylo TH, Willets JM, Brown L, Davies Q et al (2014). Association of cannabinoid receptor expression with anandamide concentrations in endometrial cancer. The Lancet 383: S23. [Google Scholar]
- Balenga NA, Martinez‐Pinilla E, Kargl J, Schroder R, Peinhaupt M, Platzer W et al (2014). Heteromerization of GPR55 and cannabinoid CB2 receptors modulates signalling. Br J Pharmacol 171: 5387–5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari M, Battista N, Fezza F, Finazzi‐Agro A, Maccarrone M (2005). Lipid rafts control signaling of type‐1 cannabinoid receptors in neuronal cells. Implications for anandamide‐induced apoptosis. J Biol Chem 280: 12212–12220. [DOI] [PubMed] [Google Scholar]
- Benz AH, Renne C, Maronde E, Koch M, Grabiec U, Kallendrusch S et al (2013). Expression and functional relevance of cannabinoid receptor 1 in Hodgkin lymphoma. PLoS One 8: e81675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bifulco M, Laezza C, Valenti M, Ligresti A, Portella G, DI Marzo V (2004). A new strategy to block tumor growth by inhibiting endocannabinoid inactivation. FASEB J 18: 1606–1608. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Katayama K, Melck D, Ueda N, De Petrocellis L, Yamamoto S et al (1998). Biosynthesis and degradation of bioactive fatty acid amides in human breast cancer and rat pheochromocytoma cells – implications for cell proliferation and differentiation. Eur J Biochem 254: 634–642. [DOI] [PubMed] [Google Scholar]
- Blazquez C, Carracedo A, Barrado L, Real PJ, Fernandez‐Luna JL, Velasco G et al (2006). Cannabinoid receptors as novel targets for the treatment of melanoma. FASEB J 20: 2633–2635. [DOI] [PubMed] [Google Scholar]
- Blazquez C, Casanova ML, Planas A, Gomez Del Pulgar T, Villanueva C, Fernandez‐Acenero MJ et al (2003). Inhibition of tumor angiogenesis by cannabinoids. FASEB J 17: 529–531. [DOI] [PubMed] [Google Scholar]
- Bogdanovic V, Mrdjanovic J, Borisev I (2017). A review of the therapeutic antitumor potential of cannabinoids. J Altern Complement Med (New York, NY) 23: 831–836. [DOI] [PubMed] [Google Scholar]
- Bridgeman MB, Abazia DT (2017). Medicinal cannabis: history, pharmacology, and implications for the acute care setting. P T 42: 180–188. [PMC free article] [PubMed] [Google Scholar]
- Caffarel MM, Andradas C, Mira E, Perez‐Gomez E, Cerutti C, Moreno‐Bueno G et al (2010). Cannabinoids reduce ErbB2‐driven breast cancer progression through Akt inhibition. Mol Cancer 9: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carracedo A, Gironella M, Lorente M, Garcia S, Guzman M, Velasco G et al (2006). Cannabinoids induce apoptosis of pancreatic tumor cells via endoplasmic reticulum stress‐related genes. Cancer Res 66: 6748–6755. [DOI] [PubMed] [Google Scholar]
- Chen L, Chen H, Li Y, Li L, Qiu Y, Ren J (2015). Endocannabinoid and ceramide levels are altered in patients with colorectal cancer. Oncol Rep 34: 447–454. [DOI] [PubMed] [Google Scholar]
- Chen WJ, Wang H, Tang Y, Liu CL, Li HL, Li WT (2010). Multidrug resistance in breast cancer cells during epithelial‐mesenchymal transition is modulated by breast cancer resistant protein. Chin J Cancer 29: 151–157. [DOI] [PubMed] [Google Scholar]
- Chung SC, Hammarsten P, Josefsson A, Stattin P, Granfors T, Egevad L et al (2009). A high cannabinoid CB(1) receptor immunoreactivity is associated with disease severity and outcome in prostate cancer. Eur J Cancer 45: 174–182. [DOI] [PubMed] [Google Scholar]
- Ciaglia E, Torelli G, Pisanti S, Picardi P, D'Alessandro A, Laezza C et al (2015). Cannabinoid receptor CB1 regulates STAT3 activity and its expression dictates the responsiveness to SR141716 treatment in human glioma patients' cells. Oncotarget 6: 15464–15481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianchi F, Papucci L, Schiavone N, Lulli M, Magnelli L, Vinci MC et al (2008). Cannabinoid receptor activation induces apoptosis through tumor necrosis factor alpha‐mediated ceramide de novo synthesis in colon cancer cells. Clin Cancer Res 14: 7691–7700. [DOI] [PubMed] [Google Scholar]
- Cipriano M, Gouveia‐Figueira S, Persson E, Nording M, Fowler CJ (2014). The influence of monoacylglycerol lipase inhibition upon the expression of epidermal growth factor receptor in human PC‐3 prostate cancer cells. BMC Res Notes 7: 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriano M, Haggstrom J, Hammarsten P, Fowler CJ (2013). Association between cannabinoid CB(1) receptor expression and Akt signalling in prostate cancer. PLoS One 8: e65798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Console‐Bram L, Marcu J, Abood ME (2012). Cannabinoid receptors: nomenclature and pharmacological principles. Prog Neuropsychopharmacol Biol Psychiatry 38: 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contassot E, Tenan M, Schnuriger V, Pelte MF, Dietrich PY (2004a). Arachidonyl ethanolamide induces apoptosis of uterine cervix cancer cells via aberrantly expressed vanilloid receptor‐1. Gynecol Oncol 93: 182–188. [DOI] [PubMed] [Google Scholar]
- Contassot E, Wilmotte R, Tenan M, Belkouch MC, Schnuriger V, de Tribolet N et al (2004b). Arachidonylethanolamide induces apoptosis of human glioma cells through vanilloid receptor‐1. J Neuropathol Exp Neurol 63: 956–963. [DOI] [PubMed] [Google Scholar]
- Cunha P, Romão AM, Mascarenhas‐Melo F, Teixeira HM, Reis F (2011). Endocannabinoid system in cardiovascular disorders – new pharmacotherapeutic opportunities. J Pharm Bioallied Sci 3: 350–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jesus ML, Hostalot C, Garibi JM, Salles J, Meana JJ, Callado LF (2010). Opposite changes in cannabinoid CB1 and CB2 receptor expression in human gliomas. Neurochem Int 56: 829–833. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Melck D, Palmisano A, Bisogno T, Laezza C, Bifulco M et al (1998). The endogenous cannabinoid anandamide inhibits human breast cancer cell proliferation. Proc Natl Acad Sci U S A 95: 8375–8380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMorrow S, Francis H, Gaudio E, Venter J, Franchitto A, Kopriva S et al (2008). The endocannabinoid anandamide inhibits cholangiocarcinoma growth via activation of the noncanonical Wnt signaling pathway. Am J Physiol Gastrointest Liver Physiol 295: G1150–G1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMorrow S, Glaser S, Francis H, Venter J, Vaculin B, Vaculin S et al (2007). Opposing actions of endocannabinoids on cholangiocarcinoma growth: recruitment of Fas and Fas ligand to lipid rafts. J Biol Chem 282: 13098–13113. [DOI] [PubMed] [Google Scholar]
- Elbaz M, Ahirwar D, Ravi J, Nasser MW, Ganju RK (2016). Novel role of cannabinoid receptor 2 in inhibiting EGF/EGFR and IGF‐I/IGF‐IR pathways in breast cancer. Oncotarget 8: 29668–29678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endsley MP, Aggarwal N, Isbell MA, Wheelock CE, Hammock BD, Falck JR et al (2007). Diverse roles of 2‐arachidonoylglycerol in invasion of prostate carcinoma cells: Location, hydrolysis and 12‐lipoxygenase metabolism. Int J Cancer 121: 984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endsley MP, Thill R, Choudhry I, Williams CL, Kajdacsy‐Balla A, Campbell WB et al (2008). Expression and function of fatty acid amide hydrolase in prostate cancer. Int J Cancer 123: 1318–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinshtein V, Erez O, Ben‐Zvi Z, Erez N, Eshkoli T, Sheizaf B et al (2013). Cannabidiol changes P‐gp and BCRP expression in trophoblast cell lines. PeerJ 1: e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore D, Ramesh P, Proto MC, Piscopo C, Franceschelli S, Anzelmo S et al (2017). Rimonabant kills colon cancer stem cells without inducing toxicity in normal colon organoids. Front Pharmacol 8: 949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford LA, Roelofs AJ, Anavi‐Goffer S, Mowat L, Simpson DG, Irving AJ et al (2010). A role for L‐alpha‐lysophosphatidylinositol and GPR55 in the modulation of migration, orientation and polarization of human breast cancer cells. Br J Pharmacol 160: 762–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CJ, Jonsson KO, Andersson A, Juntunen J, Jarvinen T, Vandevoorde S et al (2003). Inhibition of C6 glioma cell proliferation by anandamide, 1‐arachidonoylglycerol, and by a water soluble phosphate ester of anandamide: variability in response and involvement of arachidonic acid. Biochem Pharmacol 66: 757–767. [DOI] [PubMed] [Google Scholar]
- Fraguas‐Sanchez AI, Fernandez‐Carballido A, Torres‐Suarez AI (2016). Phyto‐, endo‐ and synthetic cannabinoids: promising chemotherapeutic agents in the treatment of breast and prostate carcinomas. Expert Opin Investig Drugs 25: 1311–1323. [DOI] [PubMed] [Google Scholar]
- Gjerstorff MF, Benoit VM, Laenkholm AV, Nielsen O, Johansen LE, Ditzel HJ (2006). Identification of genes with altered expression in medullary breast cancer vs. ductal breast cancer and normal breast epithelia. Int J Oncol 28: 1327–1335. [PubMed] [Google Scholar]
- Guida M, Ligresti A, De Filippis D, D'Amico A, Petrosino S, Cipriano M et al (2010). The levels of the endocannabinoid receptor CB2 and its ligand 2‐arachidonoylglycerol are elevated in endometrial carcinoma. Endocrinology 151: 921–928. [DOI] [PubMed] [Google Scholar]
- Gustafsson K, Sander B, Bielawski J, Hannun YA, Flygare J (2009a). Potentiation of cannabinoid‐induced cytotoxicity in mantle cell lymphoma through modulation of ceramide metabolism. Mol Cancer Res 7: 1086–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson SB, Lindgren T, Jonsson M, Jacobsson SO (2009b). Cannabinoid receptor‐independent cytotoxic effects of cannabinoids in human colorectal carcinoma cells: synergism with 5‐fluorouracil. Cancer Chemother Pharmacol 63: 691–701. [DOI] [PubMed] [Google Scholar]
- Gustafsson SB, Palmqvist R, Henriksson ML, Dahlin AM, Edin S, Jacobsson SO et al (2011). High tumour cannabinoid CB1 receptor immunoreactivity negatively impacts disease‐specific survival in stage II microsatellite stable colorectal cancer. PLoS One 6: e23003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman M (2003). Cannabinoids: potential anticancer agents. Nat Rev Cancer 3: 745–755. [DOI] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 46: D1091–d1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart S, Fischer OM, Ullrich A (2004). Cannabinoids induce cancer cell proliferation via tumor necrosis factor alpha‐converting enzyme (TACE/ADAM17)‐mediated transactivation of the epidermal growth factor receptor. Cancer Res 64: 1943–1950. [DOI] [PubMed] [Google Scholar]
- Hasenoehrl C, Feuersinger D, Sturm EM, Barnthaler T, Heitzer E, Graf R et al (2018). G protein‐coupled receptor GPR55 promotes colorectal cancer and has opposing effects to cannabinoid receptor 1. Int J Cancer 142: 121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenoehrl C, Taschler U, Storr M, Schicho R (2016). The gastrointestinal tract – a central organ of cannabinoid signaling in health and disease. Neurogastroenterol Motil 28: 1765–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Ramer R, Eichele K, Weinzierl U, Brune K (2004). Up‐regulation of cyclooxygenase‐2 expression is involved in R(+)‐methanandamide‐induced apoptotic death of human neuroglioma cells. Mol Pharmacol 66: 1643–1651. [DOI] [PubMed] [Google Scholar]
- Hofmann NA, Yang J, Trauger SA, Nakayama H, Huang L, Strunk D et al (2015). The GPR 55 agonist, L‐alpha‐lysophosphatidylinositol, mediates ovarian carcinoma cell‐induced angiogenesis. Br J Pharmacol 172: 4107–4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland ML, Allen JD, Arnold JC (2008). Interaction of plant cannabinoids with the multidrug transporter ABCC1 (MRP1). Eur J Pharmacol 591: 128–131. [DOI] [PubMed] [Google Scholar]
- Holland ML, Lau DT, Allen JD, Arnold JC (2007). The multidrug transporter ABCG2 (BCRP) is inhibited by plant‐derived cannabinoids. Br J Pharmacol 152: 815–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland ML, Panetta JA, Hoskins JM, Bebawy M, Roufogalis BD, Allen JD et al (2006). The effects of cannabinoids on P‐glycoprotein transport and expression in multidrug resistant cells. Biochem Pharmacol 71: 1146–1154. [DOI] [PubMed] [Google Scholar]
- Jacobsson SO, Wallin T, Fowler CJ (2001). Inhibition of rat C6 glioma cell proliferation by endogenous and synthetic cannabinoids. Relative involvement of cannabinoid and vanilloid receptors. J Pharmacol Exp Ther 299: 951–959. [PubMed] [Google Scholar]
- Jung CK, Kang WK, Park JM, Ahn HJ, Kim SW, Taek OS et al (2013). Expression of the cannabinoid type I receptor and prognosis following surgery in colorectal cancer. Oncol Lett 5: 870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katchan V, David P, Shoenfeld Y (2016). Cannabinoids and autoimmune diseases: a systematic review. Autoimmun Rev 15: 513–528. [DOI] [PubMed] [Google Scholar]
- Kendall DA, Yudowski GA (2016). Cannabinoid receptors in the central nervous system: their signaling and roles in disease. Front Cell Neurosci 10: 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprairie RB, Bagher AM, Denovan‐Wright EM (2017). Cannabinoid receptor ligand bias: implications in the central nervous system. Curr Opin Pharmacol 32: 32–43. [DOI] [PubMed] [Google Scholar]
- Ligresti A, Bisogno T, Matias I, De Petrocellis L, Cascio MG, Cosenza V et al (2003). Possible endocannabinoid control of colorectal cancer growth. Gastroenterology 125: 677–687. [DOI] [PubMed] [Google Scholar]
- Ligresti A, De Petrocellis L, Di Marzo V (2016). From phytocannabinoids to cannabinoid receptors and endocannabinoids: pleiotropic physiological and pathological roles through complex pharmacology. Physiol Rev 96: 1593–1659. [DOI] [PubMed] [Google Scholar]
- Linsalata M, Notarnicola M, Tutino V, Bifulco M, Santoro A, Laezza C et al (2010). Effects of anandamide on polyamine levels and cell growth in human colon cancer cells. Anticancer Res 30: 2583–2589. [PubMed] [Google Scholar]
- Lopes‐Rodrigues V, Sousa E, Vasconcelos MH (2016). Curcumin as a modulator of P‐glycoprotein in cancer: challenges and perspectives. Pharmaceuticals (Basel) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Wu TT, Jiang PC, Li ZQ, Chen XJ, Fu K et al (2016). Anti‐carcinogenic activity of anandamide on human glioma in vitro and in vivo. Mol Med Rep 13: 1558–1562. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Maccarrone M, Attina M, Cartoni A, Bari M, Finazzi‐Agro A (2001). Gas chromatography‐mass spectrometry analysis of endogenous cannabinoids in healthy and tumoral human brain and human cells in culture. J Neurochem 76: 594–601. [DOI] [PubMed] [Google Scholar]
- Malfitano AM, Laezza C, Galgani M, Matarese G, D'Alessandro A, Gazzerro P et al (2012). The CB1 receptor antagonist rimonabant controls cell viability and ascitic tumour growth in mice. Pharmacol Res 65: 365–371. [DOI] [PubMed] [Google Scholar]
- Martinez‐Martinez E, Gomez I, Martin P, Sanchez A, Roman L, Tejerina E et al (2015). Cannabinoids receptor type 2, CB2, expression correlates with human colon cancer progression and predicts patient survival. Oncoscience 2: 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKallip RJ, Lombard C, Fisher M, Martin BR, Ryu S, Grant S et al (2002). Targeting CB2 cannabinoid receptors as a novel therapy to treat malignant lymphoblastic disease. Blood 100: 627–634. [DOI] [PubMed] [Google Scholar]
- McKallip RJ, Nagarkatti M, Nagarkatti PS (2005). Delta‐9‐tetrahydrocannabinol enhances breast cancer growth and metastasis by suppression of the antitumor immune response. J Immunol 174: 3281–3289. [DOI] [PubMed] [Google Scholar]
- Melck D, De Petrocellis L, Orlando P, Bisogno T, Laezza C, Bifulco M et al (2000). Suppression of nerve growth factor Trk receptors and prolactin receptors by endocannabinoids leads to inhibition of human breast and prostate cancer cell proliferation. Endocrinology 141: 118–126. [DOI] [PubMed] [Google Scholar]
- Messalli EM, Grauso F, Luise R, Angelini A, Rossiello R (2014). Cannabinoid receptor type 1 immunoreactivity and disease severity in human epithelial ovarian tumors. Am J Obstet Gynecol 211: 234.e231–234.e236. [DOI] [PubMed] [Google Scholar]
- Michalski CW, Oti FE, Erkan M, Sauliunaite D, Bergmann F, Pacher P et al (2008). Cannabinoids in pancreatic cancer: correlation with survival and pain. Int J Cancer 122: 742–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimeault M, Pommery N, Wattez N, Bailly C, Henichart JP (2003). Anti‐proliferative and apoptotic effects of anandamide in human prostatic cancer cell lines: implication of epidermal growth factor receptor down‐regulation and ceramide production. Prostate 56: 1–12. [DOI] [PubMed] [Google Scholar]
- Miyato H, Kitayama J, Yamashita H, Souma D, Asakage M, Yamada J et al (2009). Pharmacological synergism between cannabinoids and paclitaxel in gastric cancer cell lines. J Surg Res 155: 40–47. [DOI] [PubMed] [Google Scholar]
- Morales P, Reggio PH (2017). An update on non‐CB1, non‐CB2 cannabinoid related G‐protein‐coupled receptors. Cannabis Cannabinoid Res 2: 265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase R, Kawamura R, Singer E, Pakdel A, Sarma P, Judkins J et al (2014). Targeting multiple cannabinoid anti‐tumour pathways with a resorcinol derivative leads to inhibition of advanced stages of breast cancer. Br J Pharmacol 171: 4464–4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nithipatikom K, Endsley MP, Isbell MA, Falck JR, Iwamoto Y, Hillard CJ et al (2004). 2‐arachidonoylglycerol: a novel inhibitor of androgen‐independent prostate cancer cell invasion. Cancer Res 64: 8826–8830. [DOI] [PubMed] [Google Scholar]
- Nithipatikom K, Endsley MP, Isbell MA, Wheelock CE, Hammock BD, Campbell WB (2005). A new class of inhibitors of 2‐arachidonoylglycerol hydrolysis and invasion of prostate cancer cells. Biochem Biophys Res Commun 332: 1028–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nithipatikom K, Isbell MA, Endsley MP, Woodliff JE, Campbell WB (2011). Anti‐proliferative effect of a putative endocannabinoid, 2‐arachidonylglyceryl ether in prostate carcinoma cells. Prostaglandins Other Lipid Mediat 94: 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura DK, Lombardi DP, Chang JW, Niessen S, Ward AM, Long JZ et al (2011). Monoacylglycerol lipase exerts dual control over endocannabinoid and fatty acid pathways to support prostate cancer. Chem Biol 18: 846–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno T, Yokomizo T (2011). What is the natural ligand of GPR55? J Biochem 149: 495–497. [DOI] [PubMed] [Google Scholar]
- Orellana‐Serradell O, Poblete CE, Sanchez C, Castellon EA, Gallegos I, Huidobro C et al (2015). Proapoptotic effect of endocannabinoids in prostate cancer cells. Oncol Rep 33: 1599–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega A, Garcia‐Hernandez VM, Ruiz‐Garcia E, Meneses‐Garcia A, Herrera‐Gomez A, Aguilar‐Ponce JL et al (2016). Comparing the effects of endogenous and synthetic cannabinoid receptor agonists on survival of gastric cancer cells. Life Sci 165: 56–62. [DOI] [PubMed] [Google Scholar]
- Pagano E, Borrelli F, Orlando P, Romano B, Monti M, Morbidelli L et al (2017). Pharmacological inhibition of MAGL attenuates experimental colon carcinogenesis. Pharmacol Res 119: 227–236. [DOI] [PubMed] [Google Scholar]
- Pagotto U, Marsicano G, Fezza F, Theodoropoulou M, Grubler Y, Stalla J et al (2001). Normal human pituitary gland and pituitary adenomas express cannabinoid receptor type 1 and synthesize endogenous cannabinoids: first evidence for a direct role of cannabinoids on hormone modulation at the human pituitary level. J Clin Endocrinol Metab 86: 2687–2696. [DOI] [PubMed] [Google Scholar]
- Park JM, Xian XS, Choi MG, Park H, Cho YK, Lee IS et al (2011). Antiproliferative mechanism of a cannabinoid agonist by cell cycle arrest in human gastric cancer cells. J Cell Biochem 112: 1192–1205. [DOI] [PubMed] [Google Scholar]
- Patsos HA, Greenhough A, Hicks DJ, Al Kharusi M, Collard TJ, Lane JD et al (2010). The endogenous cannabinoid, anandamide, induces COX‐2‐dependent cell death in apoptosis‐resistant colon cancer cells. Int J Oncol 37: 187–193. [DOI] [PubMed] [Google Scholar]
- Perez‐Gomez E, Andradas C, Blasco‐Benito S, Caffarel MM, Garcia‐Taboada E, Villa‐Morales M et al (2015). Role of cannabinoid receptor CB2 in HER2 pro‐oncogenic signaling in breast cancer. J Natl Cancer Inst 107: djv077. [DOI] [PubMed] [Google Scholar]
- Perez‐Gomez E, Andradas C, Flores JM, Quintanilla M, Paramio JM, Guzman M et al (2013). The orphan receptor GPR55 drives skin carcinogenesis and is upregulated in human squamous cell carcinomas. Oncogene 32: 2534–2542. [DOI] [PubMed] [Google Scholar]
- Petersen G, Moesgaard B, Schmid PC, Schmid HH, Broholm H, Kosteljanetz M et al (2005). Endocannabinoid metabolism in human glioblastomas and meningiomas compared to human non‐tumour brain tissue. J Neurochem 93: 299–309. [DOI] [PubMed] [Google Scholar]
- Picardi P, Ciaglia E, Proto M, Pisanti S (2014). Anandamide inhibits breast tumor‐induced angiogenesis. Transl Med UniSa 10: 8–12. [PMC free article] [PubMed] [Google Scholar]
- Pineiro R, Maffucci T, Falasca M (2011). The putative cannabinoid receptor GPR55 defines a novel autocrine loop in cancer cell proliferation. Oncogene 30: 142–152. [DOI] [PubMed] [Google Scholar]
- Pisanti S, Borselli C, Oliviero O, Laezza C, Gazzerro P, Bifulco M (2007). Antiangiogenic activity of the endocannabinoid anandamide: correlation to its tumor‐suppressor efficacy. J Cell Physiol 211: 495–503. [DOI] [PubMed] [Google Scholar]
- Pisanti S, Picardi P, Prota L, Proto MC, Laezza C, McGuire PG et al (2011). Genetic and pharmacologic inactivation of cannabinoid CB1 receptor inhibits angiogenesis. Blood 117: 5541–5550. [DOI] [PubMed] [Google Scholar]
- Portella G, Laezza C, Laccetti P, De Petrocellis L, Di Marzo V, Bifulco M (2003). Inhibitory effects of cannabinoid CB1 receptor stimulation on tumor growth and metastatic spreading: actions on signals involved in angiogenesis and metastasis. FASEB J 17: 1771–1773. [DOI] [PubMed] [Google Scholar]
- Preet A, Qamri Z, Nasser MW, Prasad A, Shilo K, Zou X et al (2011). Cannabinoid receptors, CB1 and CB2, as novel targets for inhibition of non‐small cell lung cancer growth and metastasis. Cancer Prev Res (Phila) 4: 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proto MC, Fiore D, Piscopo C, Franceschelli S, Bizzarro V, Laezza C et al (2017). Inhibition of Wnt/beta‐catenin pathway and Histone acetyltransferase activity by Rimonabant: a therapeutic target for colon cancer. Sci Rep 7: 11678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyszniak M, Tabarkiewicz J, Luszczki JJ (2016). Endocannabinoid system as a regulator of tumor cell malignancy – biological pathways and clinical significance. Onco Targets Ther 9: 4323–4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Ruan ZH (2014). The role of monoacylglycerol lipase (MAGL) in the cancer progress. Cell Biochem Biophys 70: 33–36. [DOI] [PubMed] [Google Scholar]
- Rajesh M, Mukhopadhyay P, Hasko G, Liaudet L, Mackie K, Pacher P (2010). Cannabinoid‐1 receptor activation induces reactive oxygen species‐dependent and ‐independent mitogen‐activated protein kinase activation and cell death in human coronary artery endothelial cells. Br J Pharmacol 160: 688–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramer R, Hinz B (2016). Antitumorigenic targets of cannabinoids – current status and implications. Expert Opin Ther Targets 20: 1219–1235. [DOI] [PubMed] [Google Scholar]
- Rudolph MI, Boza Y, Yefi R, Luza S, Andrews E, Penissi A et al (2008). The influence of mast cell mediators on migration of SW756 cervical carcinoma cells. J Pharmacol Sci 106: 208–218. [DOI] [PubMed] [Google Scholar]
- Sanchez MG, Sanchez AM, Ruiz‐Llorente L, Diaz‐Laviada I (2003). Enhancement of androgen receptor expression induced by (R)‐methanandamide in prostate LNCaP cells. FEBS Lett 555: 561–566. [DOI] [PubMed] [Google Scholar]
- Schley M, Stander S, Kerner J, Vajkoczy P, Schupfer G, Dusch M et al (2009). Predominant CB2 receptor expression in endothelial cells of glioblastoma in humans. Brain Res Bull 79: 333–337. [DOI] [PubMed] [Google Scholar]
- Schmid PC, Wold LE, Krebsbach RJ, Berdyshev EV, Schmid HH (2002). Anandamide and other N‐acylethanolamines in human tumors. Lipids 37: 907–912. [DOI] [PubMed] [Google Scholar]
- Schurman LD, Lichtman AH (2017). Endocannabinoids: a promising impact for traumatic brain injury. Front Pharmacol 8: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Massi P, Cantelmo AR, Cattaneo MG, Cammarota R, Bartolini D et al (2012). Cannabidiol inhibits angiogenesis by multiple mechanisms. Br J Pharmacol 167: 1218–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sredni ST, Huang CC, Suzuki M, Pundy T, Chou P, Tomita T (2016). Spontaneous involution of pediatric low‐grade gliomas: high expression of cannabinoid receptor 1 (CNR1) at the time of diagnosis may indicate involvement of the endocannabinoid system. Childs Nerv Syst 32: 2061–2067. [DOI] [PubMed] [Google Scholar]
- Suk KT, Mederacke I, Gwak GY, Cho SW, Adeyemi A, Friedman R et al (2016). Opposite roles of cannabinoid receptors 1 and 2 in hepatocarcinogenesis. Gut 65: 1721–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutphen R, Xu Y, Wilbanks GD, Fiorica J, Grendys EC Jr, LaPolla JP et al (2004). Lysophospholipids are potential biomarkers of ovarian cancer. Cancer Epidemiol Biomarkers Prev 13: 1185–1191. [PubMed] [Google Scholar]
- Thapa D, Lee JS, Heo SW, Lee YR, Kang KW, Kwak MK et al (2011). Novel hexahydrocannabinol analogs as potential anti‐cancer agents inhibit cell proliferation and tumor angiogenesis. Eur J Pharmacol 650: 64–71. [DOI] [PubMed] [Google Scholar]
- Theocharis S, Giaginis C, Alexandrou P, Rodriguez J, Tasoulas J, Danas E et al (2016). Evaluation of cannabinoid CB1 and CB2 receptors expression in mobile tongue squamous cell carcinoma: associations with clinicopathological parameters and patients' survival. Tumour Biol 37: 3647–3656. [DOI] [PubMed] [Google Scholar]
- Thors L, Bergh A, Persson E, Hammarsten P, Stattin P, Egevad L et al (2010). Fatty acid amide hydrolase in prostate cancer: association with disease severity and outcome, CB1 receptor expression and regulation by IL‐4. PLoS One 5: e12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier N, Chevillard L, Megarbane B, Pirnay S, Scherrmann JM, Decleves X (2010). Interaction of drugs of abuse and maintenance treatments with human P‐glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2). Int J Neuropsychopharmacol 13: 905–915. [DOI] [PubMed] [Google Scholar]
- Van Dross R, Soliman E, Jha S, Johnson T, Mukhopadhyay S (2013). Receptor‐dependent and receptor‐independent endocannabinoid signaling: a therapeutic target for regulation of cancer growth. Life Sci 92: 463–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco G, Sanchez C, Guzman M (2016). Anticancer mechanisms of cannabinoids. Current oncology (Toronto, Ont) 23: S23–S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Wang H, Ning W, Backlund MG, Dey SK, DuBois RN (2008). Loss of cannabinoid receptor 1 accelerates intestinal tumor growth. Cancer Res 68: 6468–6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasilewski A, Krajewska U, Owczarek K, Lewandowska U, Fichna J (2017). Fatty acid amide hydrolase (FAAH) inhibitor PF‐3845 reduces viability, migration and invasiveness of human colon adenocarcinoma Colo‐205 cell line: an in vitro study. Acta Biochim Pol 64: 519–525. [DOI] [PubMed] [Google Scholar]
- Wu X, Han L, Zhang X, Li L, Jiang C, Qiu Y et al (2012). Alteration of endocannabinoid system in human gliomas. J Neurochem 120: 842–849. [DOI] [PubMed] [Google Scholar]
- Xiao YJ, Schwartz B, Washington M, Kennedy A, Webster K, Belinson J et al (2001). Electrospray ionization mass spectrometry analysis of lysophospholipids in human ascitic fluids: comparison of the lysophospholipid contents in malignant vs nonmalignant ascitic fluids. Anal Biochem 290: 302–313. [DOI] [PubMed] [Google Scholar]
- Xu X, Liu Y, Huang S, Liu G, Xie C, Zhou J et al (2006). Overexpression of cannabinoid receptors CB1 and CB2 correlates with improved prognosis of patients with hepatocellular carcinoma. Cancer Genet Cytogenet 171: 31–38. [DOI] [PubMed] [Google Scholar]
- Zhang X, Maor Y, Wang JF, Kunos G, Groopman JE (2010). Endocannabinoid‐like N‐arachidonoyl serine is a novel pro‐angiogenic mediator. Br J Pharmacol 160: 1583–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu LX, Sharma S, Stolina M, Gardner B, Roth MD, Tashkin DP et al (2000). Delta‐9‐tetrahydrocannabinol inhibits antitumor immunity by a CB2 receptor‐mediated, cytokine‐dependent pathway. J Immunol 165: 373–380. [DOI] [PubMed] [Google Scholar]
- Zogopoulos P, Korkolopoulou P, Patsouris E, Theocharis S (2015). The antitumor action of cannabinoids on glioma tumorigenesis. Histol Histopathol 30: 629–645. [DOI] [PubMed] [Google Scholar]


