Abstract
Alterations in peripheral immune markers are observed in individuals with post-traumatic stress disorder (PTSD). PTSD is characterized in part by impaired extinction of fear memory for a traumatic experience. We hypothesized that fear memory extinction is regulated by immune signaling stimulated when fear memory is retrieved. The relationship between fear memory and the peripheral immune response was tested using auditory Pavlovian fear conditioning in mice. Memory for the association was quantified by the amount of conditioned freezing exhibited in response to the conditioned stimulus (CS), extinction and time-dependent changes in circulating inflammatory cytokines. Brief extinction training with 12 CS rapidly and acutely increased circulating levels of the cytokine interleukin-6 (IL-6), downstream IL-6 signaling, other IL-6 related pro-inflammatory cytokines. Transgenic manipulations or neutralizing antibodies that inhibit IL-6 activity did not affect conditioned freezing during the acquisition of fear conditioning or extinction but significantly reduced conditioned freezing 24 h after extinction training with 12 CS. Conversely, conditioned freezing after extinction training was unchanged by IL-6 inhibition when 40 CS were used during the extinction training session. In addition to effectively diminishing conditioned freezing, extinction training with 40 CS also diminished the subsequent IL-6 response to the CS. These data demonstrate that IL-6 released following fear memory retrieval contributes to the maintenance of that fear memory and that this effect is extinction dependent. These findings extend the current understanding for the role of the immune system in PTSD and suggest that IL-6 and other IL-6 related pro-inflammatory cytokines may contribute to the persistence of fear memory in PTSD where fear memory extinction is impaired.
Keywords: Inflammation, fear, reconsolidation, extinction, immune system, interleukin-6, PTSD
INTRODUCTION
The fear response to a memory is diminished or ‘extinguished’ by neurobiological processes that are initiated when the fear response repeatedly fails to predict an actual threat (Milad and Quirk, 2012). Persistent and powerful fear responses to remembered trauma in post-traumatic stress disorder (PTSD) are hypothesized to result from impairments in extinction processes that regulate fear memory maintenance (Rothbaum and Davis, 2003). Although the maintenance of a retrieved fear memory can be disrupted in animal models by targeting neurobiological processes known to mediate extinction, translation of such approaches in humans does not reliably disrupt the maintenance of powerful fear responses in PTSD (Singewald et al., 2015). Characterizing alternative biological substrates that influence fear memory extinction may provide better targets for improving fear extinction in PTSD.
Individuals with PTSD exhibit a range of altered peripheral immune markers at baseline, in response to psychological stressors, or in response to immune challenges (Breen et al., 2015; Michopoulos et al., 2016). Baseline immune alterations include elevated peripheral serum levels of pro-inflammatory cytokines (Gill et al., 2009; Tursich et al., 2014), increased in vitro cytokine release from peripheral blood mononuclear cells (Gola et al., 2013), and altered proportion or activity of T lymphocytes (Jergović et al., 2014; Lemieux et al., 2008). In addition to baseline alterations, PTSD has been associated with heightened immune responses to psychological stressors (Newton et al., 2014) and a greater likelihood of developing an autoimmune disorder (O’Donovan et al., 2015). Despite known physiological consequences, the relevance of altered immune signaling to the lasting psychological symptoms of PTSD has not been determined.
Studies in rodents using Pavlovian fear conditioning support a role for the immune system in the formation and maintenance of fear memory. Impaired fear memory maintenance is observed in mice lacking peripheral T lymphocytes (Clark et al., 2016). Broad anti-inflammatory treatments prior to extinction training enhance contextual fear extinction and reverse fear conditioning-induced hippocampal levels of TNF-alpha (Yu et al., 2016). Conversely, extinction acquisition is impaired by direct administration of the cytokines interferon-α or interleukin-6 (IL-6) to the amygdala prior to extinction training (Bi et al., 2016; Hao et al., 2014). Despite previous observations using exogenous immune manipulations the role of endogenous peripheral immune responses in fear memory processes has not been well studied.
We hypothesized that alterations in immune signaling triggered by fear memory retrieval serves the maintenance of that memory. Using a mouse model of Pavlovian fear conditioning, we report that peripheral IL-6 is a key signaling mechanisms involved in a labile immune response to fear memory that also contributes to fear memory extinction. These observations provide new insights regarding the role of the immune system in modulating the maintenance of fear memory.
MATERIALS and METHODS
Animals
C57BL/6J and IL-6-deficient mice (IL-6−/−) were from Jackson Laboratory (Bar Harbor, ME, USA) and subsequently bred in-house at the Yerkes National Primate Research Center at Emory University or George Washington University. Mice were group housed in ventilated cages and maintained on ad libitum food and water. Lights turned on at 7:00 A.M. and turned off at 9:00 P.M. All experiments were performed between 9:00 A.M. and 5:00 P.M. in 10-16 week-old male mice. Studies were in accordance with NIH guidelines and all procedures were approved by the IACUC at Emory and George Washington University.
Experimental Compounds
Mouse IL-6 monoclonal antibody (IL6Ab; clone MP5-20F3) and Normal Rat IgG (ControlAb) was obtained from R&D Systems (Minneapolis, MN, USA). All antibodies were diluted in sterile 0.9% saline and injected intraperitoneally once per day (4ug, 0.2 mL). This concentration was used based on literature demonstrating tolerability and minimal effects on motor behavior following repeated dosing (Hodes et al., 2014).
Behavioral Approaches
Fear conditioning, extinction training and memory maintenance testing took place over five days. Two days prior to conditioning animals were habituated to the conditioning apparatus (Med-Associates; Georgia, VT, USA) in a brightly-lit room for 20 min. On the conditioning day animals were trained to 20 pairings of an auditory CS (6 kHz, 75-80dB) and an US-shock (0.7 mA, 2-4 s). The CS varied 15-30 s before co-terminating with the US and the inter-trial interval varied 45-90s. This unpredictable conditioning protocol was adapted from other unpredictable conditioning paradigms (Moberg and Curtin, 2009; Walker et al., 2003) was used to model significant psychological and physical trauma that precedes the development of PTSD. In total, the conditioning phase lasted 27 min. “Naïve” animals were placed in the conditioning apparatus and exposed to an identical regimen of CS, but without the US. During both habituation and conditioning the behavioral apparatus was cleaned with 70% ethanol. In antibody experiments mice were treated with 4 μg of either IL6Ab or ControlAb in 200 uL of saline beginning the day after conditioning and ending on the day of re-exposure. Four days after conditioning all animals were placed in a novel testing chamber with a black Plexiglas floor, cleaned with 5% Roccal-D (Pfizer; New York, NY, USA) and in a different room lit only by red light. “Naïve” animals and half of the fear-conditioned animals (‘CS’ group) were exposed to the CS-tone. The remaining half of fear-conditioned animals (‘No CS’) were placed in the testing apparatus without re-exposure to the CS (Figure 1A). For experiments investigating immune responses to fear memory animals were exposed to 12 or 1 CS-tones (15-30 s; 45 s intertrial interval). For behavioral experiments investigating extinction animals were exposed to either an abbreviated extinction session involving 12 CS-tones or an extended extinction session involving 40 CS-tones. Memory maintenance testing was conducted the following day in the same apparatus used the previous day.
Figure 1.
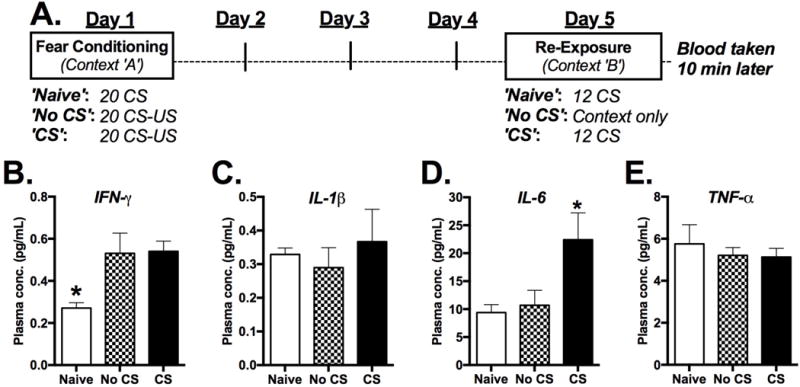
Rapid effect of fear memory retrieval on plasma cytokine levels. a Study design. b-e Plasma levels of IFN-γ, IL-1β IL-6 and TNF-α 10 minutes after mice were removed from the re-exposure apparatus. “Naïve” mice were not fear-conditioned, but were re-exposed to the CS. “No CS” mice were fear conditioned, but were not re-exposed to the CS while in Context B. “CS” mice were both fear conditioned and re-exposed to the CS. (n=7-8/group) *P<0.05.
Blood Collection and Multiplex Cytokine ELISA
10 min after the final CS re-exposure (or an equivalent amount of time for animals not re-exposed to the CS), animals were anesthetized with 4% isoflurane and quickly decapitated. Trunk blood was collected in EDTA-coated centrifuge tubes before being centrifuged at 1500 g (4° C) for 15 min. Isolated plasma was stored at −80° C. Plasma samples cytokine analysis was conducted by the Emory Immunoassay Core in the Department of Physiology (Atlanta, GA) for interleukin-1β (IL-1β), IL-6, interferon-γ (IFN- γ) and tumor necrosis factor-α (TNF- α) using multiplex cytokine ELISA assays (Meso Scale; Rockville, MD, USA). Additional cytokine multiplex assays were conducted using the Biolegend LEGENDplex kit (Cat# 740134) and Quantikine ELISA kit (Cat#M6000B R&D systems).
Western Blot
Mouse spleens were homogenized in RIPA buffer containing protease and phosphatase inhibitors (Thermo Fisher Scientific) at a ratio of 75 ul/gram tissue. After lysed on ice for 30 min, the lysates were centrifuged at 13,000 × g for 15 min at 4°C, and the supernatants were collected for further examination. For western blot, a total of 50 ug protein were separated by SDS-PAGE and transferred to nitrocellulose (NC) membranes (Bio-Rad Laboratories). After blocking with 5% non-fat milk (Bio-Rad Laboratories), blots were incubated overnight at 4 °C with anti-phospho-Stat3 (Tyr 705) antibody (1:2000, #9145, Cell Signaling) or anti-Stat3 antibody (1:2000, #12640, Cell Signaling). Secondary antibodies conjugated to horseradish peroxidase (HRP) were added to the blots and then visualized in Clarity™ Western ECL Blotting Substrates (Bio-Rad Laboratories). The blots were then quantified with the Image J software and the optic density of p-Stat3 was normalized to Stat3.
Data Presentation and Statistical Analysis
Behavioral data were analyzed with SPSS 22.0 (IBM) and GraphPad Prism 5.0 using a t-test, one- or two-way ANOVA, or a repeated-measures ANOVA. Post hoc ANOVA comparisons were made using Bonferroni test. Data in figures are presented as mean ± standard error from the mean and p values <.05 were considered statistically significant. All animals were randomly allocated into experimental and control groups and counterbalanced across groups without bias. Sample sizes were determined and consistent based on previous publications using statistical power analysis for similar experimental models and behavioral experiments. Based on pre-defined criterion (values that are more than 2 standard deviations above or below the mean) samples were excluded from some multi-plex assay analysis. All attempts at replication in data sets were successful.
RESULTS
Fear conditioning and retrieval of fear memory stimulate a peripheral cytokine response
Three groups of mice were used to determine the effect of fear memory retrieval during extinction training on cytokine release in plasma (Figure 1A). One-way ANOVA revealed significant between-group differences in levels of IFN-γ and IL-6, but in not levels of IL-1β and TNF-β (Figure 1B–1E) [IFN-γ: F(2,19)=5.81, p=0.011; IL-1β: F(2,21)=0.34; p=0.715; IL-6: F(2,21)=4.82, p=0.019; TNF-α F(2,21)=0.307, p=0.739; n=7 or 8/group]. Both groups of fear-conditioned mice exhibited significantly greater levels of IFN-γ compared to non-fear-conditioned controls regardless of whether they were re-exposed to the CS (Figure 1B) [Dunnett’s: ‘No CS’ p=0.027; ‘CS’ p=0.019]. However no difference in IFN-γ was observed between fear-conditioned groups. Plasma levels of IL-6 in the ‘Naïve’ group differed only from those in fear-conditioned mice that were re-exposed to the CS (Figure 1D) [Dunnett’s: ‘No CS’ p=0.942; ‘CS’ p=0.019]. To determine whether other IL-6 related pro and anti-inflammatory cytokines were changed following CS exposure additional peripheral cytokines were analyzed at multiple time-points (0 and 60 min) following CS exposure (Supplemental Figure 1). These data show that following CS exposure the elevated cytokine response is transient showing increased levels at 0 and 10 min post CS but not 60 minutes following CS exposure. Moreover, phosphorylation of STAT3, a primary transcriptional effector of IL-6 receptor activation, was significantly increased in the spleen, a principal peripheral immune organ, immediately following 12 CS exposure but not 60 minutes post-CS (Figure S1) [t(14)=2.23, p=0.04; n=8/group].
Genetic and pharmacological inhibition of IL-6 improves the maintenance of fear memory extinction following brief, but not prolonged, extinction training
Conditioned freezing behavior was increased across the conditioning session in both wild-type (WT) mice and mice congenitally lacking IL-6 (IL-6−/−) but there was no main effect of genotype on freezing behavior (Figure 2b) [F(5,10)=79.48, p<0.0001 for main effect of CS trial; F(1,14)=0.02, p=0.892 for main effect of genotype; F(5,10)=2.39, p=0.114 for interaction; n=8/group]. Four days after conditioning, WT and IL-6−/− mice exhibited similar conditioned freezing prior to CS re-exposure [t(14)=0.24, p=0.818; n=8/group] and also across all re-exposures to 12 CS (Figure 2c) [F(1,14)=4.45, p=0.739 for main effect of genotype; F(5,10)=7.80, p=0.003 for main effect of CS trial; F(5,10)=1.31 for interaction, p=0.333; n=8/group]. Both groups exhibited significant reductions of conditioned freezing from the first to the last CS re-exposures demonstrating within-session extinction in both genotypes [Bonferroni’s: p<0.001]. However, during memory maintenance testing (4 CS re-exposures) 24 h later IL-6−/− mice exhibited significantly less within-session conditioned freezing than WT mice (Figure 2d) [F(1,14)=8.94, P=0.005 for main effect of genotype; F(1,14)=27.70, P<0.001 for main effect of test day; F(1,14)=4.37, P=0.042 for interaction; n=8/group]. Although WT mice appeared to exhibit extinction during extinction training, maintenance of extinction between-sessions was not observed. Comparison of WT conditioned freezing during the first 4 CS of extinction training to 4 CS re-exposures during extinction testing did not reveal a significant difference. Conversely, the same comparison in IL-6−/− mice revealed significantly less conditioned freezing during extinction testing than during extinction training the previous day, demonstrating a significant effect of genotype on between-session retention of extinction (Figure 2e) [F(1,14)=8.24, p=0.008 for main effect of genotype; F(1,14)=36.52, p<0.001 for main effect of test day; F(1,14)=4.76, p=0.038 for interaction, n=8/group].
Figure 2.
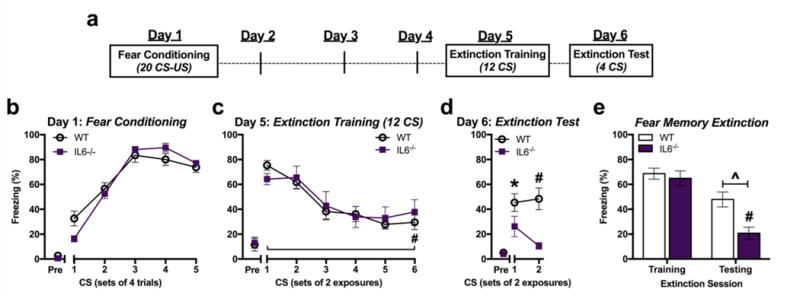
Genetic knockout of IL-6 selectively improves retention of fear memory extinction. a Study design. b Freezing during CS exposures terminating with a footshock during fear conditioning in wild-type (WT) and IL-6−/− mice. c Conditioned freezing in response to extinction training with 12 CS four days after conditioning. d Testing for retention of fear memory extinction with 4 CS one day after extinction training. e Total average conditioned freezing in response to four CS during extinction training (Day 5) and testing (Day 6). (n=8/group) *P<0.05, ^P<0.01, #P<0.001. “Pre” = 2-min period before CS presentations begin.
An IL-6-disrupting antibody was administered beginning the day after fear conditioning and continuing up to CS re-exposure on Day 5 in order to allow intact IL-6 signaling during learning and memory processes that take place during and soon after fear conditioning (Figure 3a). No differences in conditioned freezing were observed between the two treatment during the 12 CS extinction training session (Figure 3c) [F(5,18)=31.47, P< 0.001 for main effect of CS trial; F(1,22)=2.83, P=0.096 for main effect of treatment; F(5,18)=0.108, P=0.995 for interaction; n=12/group]. Similar to the previous experiment (Figure 2), both groups exhibited significant within-session extinction of conditioned freezing at the end of the of the re-exposure session [CS1 vs. CS6: p<0.001] (Figure 2c and 3c). However, during extinction testing 24 h after extinction training, IL6Ab-treated mice exhibited less conditioned freezing than ControlAb-treated mice (Figure 3d) [F(1,22)=5.27, p=0.032 for main effect of genotype; F(1,22)=1.95, p=0.176 for main effect of CS; F(1,22)=0.963, p=0.337 for interaction; n=12/group]. Moreover, mice treated with IL6Ab but not those treated with ControlAb exhibited significant reductions in conditioned freezing during extinction testing compared to extinction training (comparison of first 4 CS on Day 5 and 6) (Figure 3e) [F(1,44)=8.94, P=0.005 for main effect of treatment; F(1,44)=27.70, P<0.001 for main effect of test day; F(1,44)=4.37, P=0.042 for interaction; n=12/group].
Figure 3.
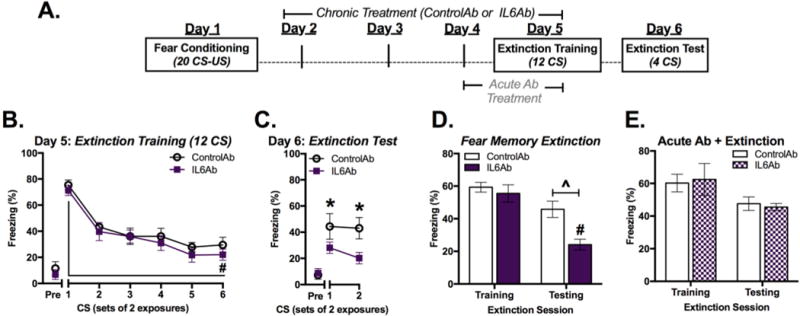
Pharmacological inhibition of IL-6 prior to extinction training improves retention of fear memory extinction. a Study design. b Conditioned freezing in response to 12 CS following four days of Ab treatment. c Testing for retention of fear memory extinction one day after extinction training. d Total average conditioned freezing in response to four CS during extinction training (Day 5) and testing (Day 6). e Total average conditioned freezing in response to four CS during extinction training and testing in mice treated with only two doses of ControlAb or IL6Ab prior to extinction training. (n=10/group) *P<0.05, ^P<0.01, #P<0.001.
To determine whether timing and duration of IL6Ab administration affects fear memory extinction, a separate group of mice were treated with ControlAb or IL6Ab for only two days prior to extinction training (Figure 3a; ‘Acute Ab Treatment’). Both groups exhibited similar levels of conditioned freezing to one another during extinction training and during the extinction testing sessions (Figure 3e) [F(1,36)=0.001, p=0.99 for main effect of treatment; F(1,36)=5.95, p=0.02 for main effect of test day; F(1,36)=0.123, p=0.73 for interaction; n=10/group] indicating that a 2 day regimen of IL-6 inhibition is not sufficient to alter the maintenance of extinction.
ControlAb or IL6Ab was administered to a separate group of fear-conditioned mice for four days leading up to extinction training with 40 CS tones (Figure 4a). Although some differences in conditioned freezing were observed between treatment groups during the latter half of extinction training, overall, IL6Ab treatment did not significantly alter conditioned freezing across the prolonged 40 re-exposure trials relative to ControlAb group (Figure 4b) [F(5,15)=8.03, p=0.007 for main effect of trial; F(1,19)=2.54, p=0.135 for main effect of treatment; F(5,15)=1.33, p=0.357 for interaction; n=10 or 11/group]. In contrast to extinction training with 12 CS, IL6Ab treatment did not significantly alter conditioned freezing during the fear extinction test compared to ControlAb the following day (Figure 4c). Although a significant main effect of antibody treatment on conditioned freezing was observed, post-hoc analysis did not reveal significant differences at any time point [F(1,19)=1.37, p=0.253 for main effect of CS trial; F(1,19)=4.65, p=0.041 for main effect of treatment; F(1,19)=0.004, p=0.947 for interaction; n=10 or 11/group]. Conditioned freezing to the first 4 CS exposures was significantly decreased from the extinction training day to the subsequent extinction testing day in both antibody treatment groups, indicating significant extinction between test days, but no difference between treatment groups was observed (Figure 4d) [F(1,28)=45.58, p<0.0001 for main effect of test day; F(1,28)=0.586, p=0.451 for main effect of treatment; F(1,28)=0.291, p=0.594 for interaction; n=10 or 11/group].
Figure 4.
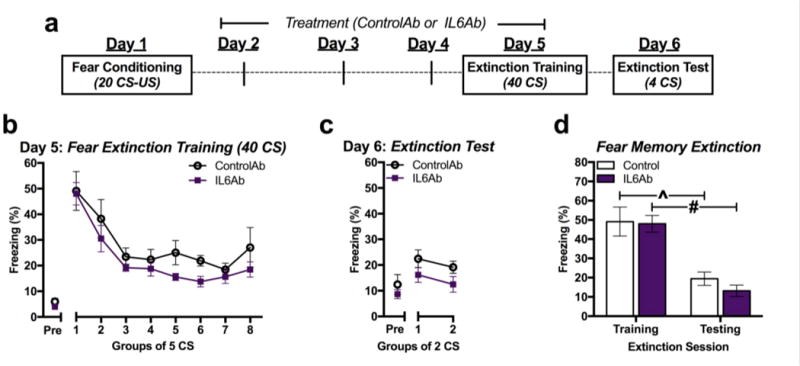
Extinction by prolonged training is unaffected by IL-6 inhibition. a Study design. b Conditioned freezing measured during extinction training with 40 CS in ControlAb- and IL6Ab-treated mice. (n=10-11/group). c Conditioned freezing measured during extinction testing with four CS. d Total average conditioned freezing in response to four CS during extinction training and testing. *P<0.05, ^P<0.01, #P<0.001.
Prolonged fear extinction training diminishes both the fear response and the IL-6 response to aversive conditioned stimuli
A separate group of fear-conditioned mice either underwent lasting fear memory extinction training by re-exposure to 40 CS tones (‘Ext’) or were placed in the extinction apparatus without re-exposure to the CS tones (‘No Ext’) (Figure 5a). Mice that underwent prolonged extinction training exhibited significantly lower levels of 12 CS-induced plasma IL-6 than No Ext controls during extinction testing the following day (Figure 5b) [t(14)=2.86, p=0.029; n=8/group]. Moreover, when levels of freezing during the 12 CS re-exposures and levels of plasma IL-6 taken afterward were compiled, a significant correlation between conditioned freezing during re-exposure and the amount of IL-6 released was revealed [r2=0.328, p=0.02; N=16] (Figure 5c).
Figure 5.
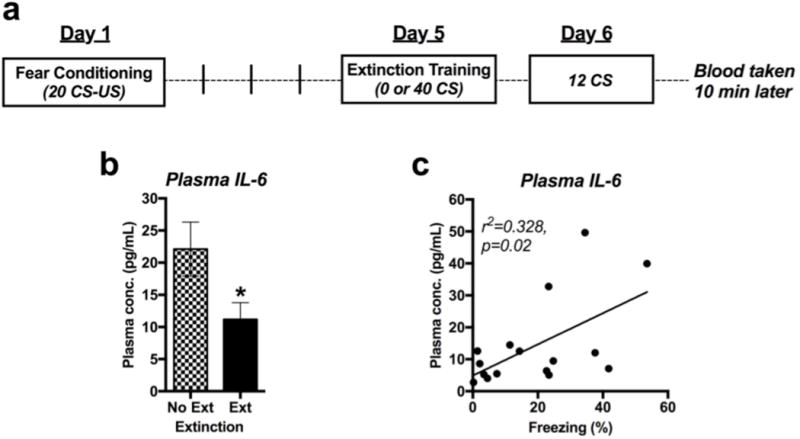
Prolonged extinction training has lasting effect on peripheral and central IL-6 signaling. a Study design. b Correlation between plasma levels of IL-6 after 12 CS re-exposures and total average freezing in response to those 12 CS (N=16). c Plasma IL-6 response to 12 CS in fear-conditioned mice exposed to either extinction training with 40 CS (Ext) or to 0 CS (No Ext) (n=8/group).
A single CS re-exposure increases circulating IL-6 levels that are not required for reconsolidation of fear memory
Fear memory maintenance after retrieval can be affected by modulating extinction learning or memory reconsolidation. We therefore examined the relationship between IL-6 and a brief CS re-exposure in regulating fear memory maintenance. Similar to the effect of 12 CS, re-exposing fear-conditioned mice to a single CS resulted in a rapid elevation of plasma IL-6 (Figure 6b) [t(14) =3.11, p=0.008; n=8/group]. WT and IL-6−/− mice exhibited similar amounts of conditioned freezing in response to a single CS re-exposure (Figure 6c) and also similar maintenance of fear memory the following day (Figure 6d-e). These results were replicated when mice were treated with either ControlAb or IL6Ab in the days between fear conditioning and re-exposure (Figure 6f-h), suggesting that IL-6, despite being elevated following a single CS exposure, does not contribute to the conditioned freezing response or memory maintenance in the absence of extinction training.
Figure 6.
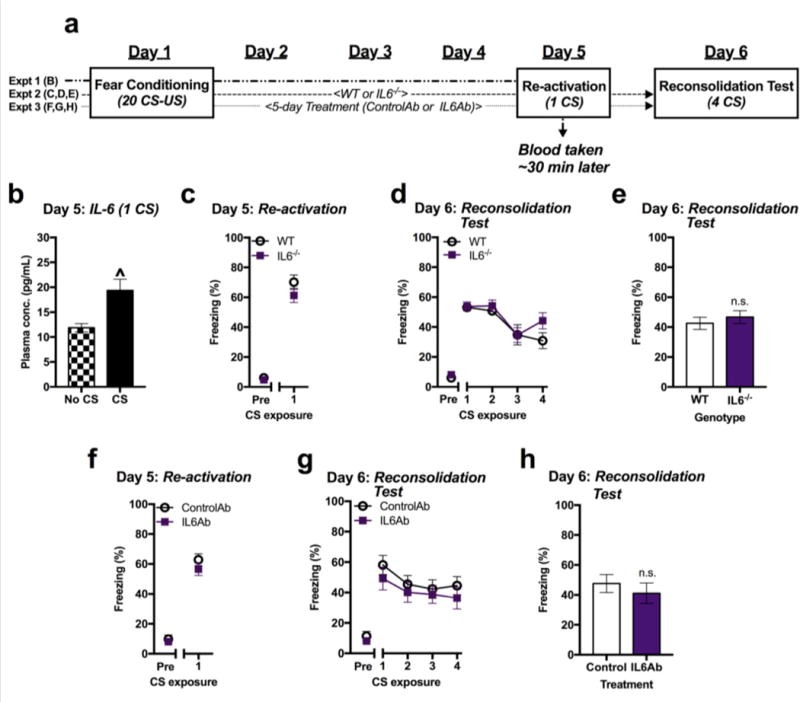
IL-6 is not required for the reconsolidation of fear memory. a Study design. b Plasma levels of IL-6 in fear-conditioned mice re-exposed to 0 (No CS) or 1 CS. c Conditioned freezing in response to 1 CS in WT and IL-6−/− mice. d Conditioned freezing of WT and IL-6−/− mice in response to each of 4 CS presented 24 hours after initial re-exposure. e Conditioned freezing averaged across 4 CS re-exposures during test. f Conditioned freezing in response to 1 CS re-exposure in ControlAb- and IL6Ab-treated mice. g Maintenance of fear memory in ControlAb-and IL6Ab-treated mice during each of 4 CS in a test of reconsolidation h and averaged across 4 CS. (n=8/group). ^P<0.01.
DISCUSSION
Despite the current understanding of IL-6 in the generalized stress response far less is known in respect to its role fear learning behavior. Limited research to date has either identified an immune response to fear-eliciting stimuli or demonstrated that immune signals can affect fear memory. Here we report that peripheral levels of IL-6 increase soon after fear memory retrieval and this is dependent on timing following CS exposure and extent of extinction training. We also demonstrate that inhibiting IL-6 by pharmacological or transgenic approaches during extinction training improves the lasting strength of extinction learning. These observations are relevant to individuals with PTSD who exhibit altered immune signaling and also suffer from persistent and powerful memories of trauma even after threat cues no longer predict harm.
Several observations here support peripheral IL-6 as a distinct immunological component of the biological fear response. A powerful fear conditioning procedure did not persistently affect plasma levels of IL-6 despite the repeated physical and psychological stress. The initial pro-inflammatory cytokines measured in the present study included IFN-γ, IL-1β, IL-6, and TNF-α. Only levels of IFN-γ were elevated in plasma days after fear conditioning. Similar increases in baseline IFN-γ have been observed in individuals with PTSD, supporting the hypothesis that IFN-γ represents a lasting immunological marker of acute trauma (Breen et al., 2015; J. Zhou et al., 2014). Changes in brain levels of IL-1β and TNF-α have been observed days after a single fear-conditioning session, but similar baseline changes in the periphery are not observed in the present study or elsewhere (M. E. Jones et al., 2015; Yu et al., 2016). Although baseline plasma levels of IL-6 were unchanged days after conditioning, we and others observed that plasma IL-6 quickly increases following re-exposure to an aversive non-physiological CS (D. Zhou et al., 1993). Rapid fear-induced increases in plasma IL-6 have also been observed in humans exposed to the threat of an impending electrical shock (Breznitz et al., 1998), suggesting that the anticipation of harm mobilizes the IL-6 response. Further supporting this hypothesis, extinguishing the fear response in the current study significantly diminished the IL-6 response to the aversive CS. Also, CS-induced elevations in plasma IL-6 correlated with the amount of fear behavior elicited by re-exposure to the CS. Combined with the observation that IL-6-deficient mice exhibited similar fear memory expression to WT mice, these data support the hypothesis that re-living highly traumatic fear memory results in an exacerbated immune response (Baker et al., 2001; Gola et al., 2013; Jergović et al., 2014; Lindqvist et al., 2014).
Given that IL-6 is pleiotropic cytokine, having both pro- and anti-inflammatory properties, we examined other pro-inflammatory cytokines at different time-points following fear exposure (Hunter and S. A. Jones, 2015). In these studies, we observed increases in both IL-6 and IL-6-related pro-inflammatory plasma cytokines immediately following CS exposure up to 10 minutes, but not beyond 60 minutes, suggesting a transient time dependent affect. In addition, phosphorylation of STAT3, a primary transcriptional effector of IL-6 receptor activation, was significantly increased in the spleen, a principal peripheral immune organ, immediately following CS re-exposure, but not 60 minutes post CS. These data extend our IL-6 plasma cytokine data and provide further evidence for an important role of peripheral immunity and IL-6 signaling in fear recall. Based on these data we speculate that peripheral IL-6 signaling, through an undetermined pathway and mechanism, maybe altering a critical period of consolidation of extinction during this short-term period of fear recall.
Inhibition of IL-6 signaling in the current study appeared to facilitate the maintenance of within-session fear memory extinction the following day. We utilized an abbreviated 12-CS extinction training procedure because it resulted in significant within-session extinction that did not robustly persist the following day. 12 CS re-exposures also resulted in rapid elevation of plasma IL-6. The limited effect of IL-6 inhibition on the retention of extinction memory following 40 CS re-exposures was likely due to a floor effect on the extinction of conditioned freezing maintained the following day. Interestingly, congenital IL-6 deletion affected maintenance of within-session extinction with 12 CS, but did not affect fear conditioning or the expression of conditioned freezing during the extinction session. Pharmacological inhibition of IL-6 initiated after the memory consolidation window for fear conditioning also improved extinction retention without affecting extinction acquisition, suggesting that the effect of IL-6 on fear memory occurs during the consolidation and not the encoding of extinction memory. Improvement of extinction retention by both genetic and pharmacological inhibition of IL-6 are likely due to their effects on peripheral IL-6 only, as systemically-administered IL6Ab is unlikely to cross the blood-brain barrier. The four-day regimen of IL6Ab treatment was based upon previous literature demonstrating its effectiveness (Hodes et al., 2014). Indeed, IL6Ab’s effect on extinction retention was abolished when we began administration only the day before extinction training. In contrast to the effects of IL-6 inhibition, central administration of IL-6 prior to extinction training has been observed to impair within-session extinction acquisition (Hao et al., 2014). This indicates that, although normal extinction acquisition does not require IL-6, it can be impaired by abnormally high IL-6 levels in the brain.
Although re-exposure to either 1 or 12 CS rapidly increased plasma levels of IL-6, we are confident that the effect on IL-6 inhibition on fear memory is due to regulation of extinction and not reconsolidation. As re-exposures to a CS accumulate, the brain transitions from reconsolidation processes to extinction processes (Monfils et al., 2009). Both 1 and 12 CS re-exposures increased plasma IL-6 levels, but IL-6 inhibition affected fear memory the following day only when within-session extinction to 12 CS was observed. Therefore, the effect of IL-6 inhibition of fear memory maintenance is likely due to an effect on extinction and not on reconsolidation of fear memory. A relationship between IL-6 and the maintenance of fear memory extinction has previously been alluded to in studies of vagus nerve stimulation. Vagal nerve stimulation during shortened fear memory extinction training improves lasting extinction retention in rodents (Peña et al., 2013) and has effects on neuronal excitability in the medial prefrontal cortex (Garcia-Oscos et al., 2015) and brainstem regions (Johnson et al., 2016). The medial prefrontal cortex is a key site for the extinction of fear memory, and may therefore represent a key site the effect of fear-induced IL-6 release on fear memory extinction (Milad and Quirk, 2012).
How peripheral increases in IL-6 affect the maintenance of fear memory extinction processes is beyond the scope of the findings of the current study. However, because systemically-administered IL6Ab is unlikely to cross the blood-brain barrier in its bound or unbound state, and whether peripherally-released IL-6 directly affects extinction processes in the brain is unclear. Local cytokine release in the brain by microglia could potentially play a role in fear memory maintenance, as stimuli that induce peripheral cytokine release typically also have the same effect in the brain. For example, upregulation of TNF-α, IL-1β and IL-6 is observed in the hippocampus days after fear conditioning, and can be reversed by complete extinction training (Scholz et al., 2016; Yu et al., 2016). Administration of either IL-6 or interferon-α in the amygdala prior to extinction training impairs fear extinction memory (Bi et al., 2016; Hao et al., 2014), and inflammatory stimuli alter synaptic activity in the prefrontal cortex via increased local IL-6 signaling (Garcia-Oscos et al., 2015). While the blood-brain barrier would have prevented IL6Ab from inhibiting IL-6 locally-derived in the brain, peripherally-released IL-6 may still regulate central cytokine signaling in the brain through a variety of indirect mechanisms.
One potential indirect pathway through which IL-6 may regulate the maintenance of extinction processes in the brain is through T lymphocytes (Hundhausen et al., 2016; G. W. Jones et al., 2010; Nish et al., 2014). A growing body of literature indicates that peripheral T lymphocytes contribute to normal memory function (Kipnis et al., 2012). In particular, mice that lack peripheral T lymphocytes exhibit impaired fear memory maintenance (Clark et al., 2016).
Regulation of learning by T lymphocytes is hypothesized to exert its influence through lymphocyte infiltration and/or their release of cytokines across blood-meningeal and blood-brain barriers (Kipnis, 2016). However, it is unclear how T lymphocytes signal across these largely impermeable barriers during learning. Notably, blood-brain barrier permeability is increased within minutes of peripheral or central IL-6 administration (Paul et al., 2003; Saija et al., 1995), and systemic IL-6 administration increases markers of brain permeability in the frontal cortex, hippocampus and hypothalamus that were not observed in response to IL-1β (Saija et al., 1995). Future studies should explore central IL-6 signaling and potential role of T lymphocytes in the extinction of fear memory. Whether IL-6-deficient mice exhibit different peripheral and central T lymphocyte responses to fear memory retrieval is unknown but may shed light on the significance of altered T lymphocytes signaling in individuals with PTSD (Jergović et al., 2014; Lemieux et al., 2008).
Individuals with PTSD exhibit alterations in IL-6 markers that correlate with the response to psychological stress (Baker et al., 2001; Newton et al., 2014). However, the biological relevance of these markers to the symptoms of PTSD is unclear. We address this question by demonstrating that peripheral levels of IL-6 respond to the retrieval of fear memory and that IL-6 signaling is essential to the future maintenance of within-session fear memory extinction. The latter is of particular importance, as the persistence of powerful fear memory in PTSD has been theorized to result from an inability to completely extinguish the fear response (Rothbaum et al., 2014). Potentiated IL-6 signaling consistently observed in PTSD (Baker et al., 2001; Gola et al., 2013; Jergović et al., 2014; Lindqvist et al., 2014) may maladaptively inhibit the maintenance of fear memory extinction in PTSD each time a traumatic experience is remembered.
Additional future studies aimed at understanding the mechanism for IL-6 in fear learning and extinction, such as the cell source and trigger for IL-6 synthesis, secretion and associated downstream signaling pathways, will be important for evaluating this pathway as a potential therapeutic target for PTSD. Our findings directly suggest that fear memory extinction could be enhanced in PTSD patients by disrupting the IL-6 signaling axis. Moreover, these data support recent literature suggesting an important role for targeting peripheral IL-6 in the pathogenesis of depression, a common mood disorder that often occurs following trauma (Zhang et al., 2017). Indeed, FDA-approved biologics that antagonize IL-6 (siltuximab) or the IL-6 receptor (tocilizumab) may offer an attractive, novel avenue for rapidly translating our findings into a viable treatment paradigm for PTSD and related co-morbidities.
Acknowledgments
We thank the animal care and veterinary staff at the Yerkes National Primate Research Center (YNPRC) and George Washington University for maintaining the health and well-being of our research subjects, whom we thank greatly for their contribution. M.B.Y. thanks Dr. Brian Dias for use of valuable behavioral equipment. M.B.Y. also thanks Daniel Curry and Karly Hampshire for assistance with behavioral experiments. The YNPRC is fully accredited by the American Association for Accreditation for Laboratory Animal Care. This research complied with all laws of the United States of America.
FUNDING AND DISCLOSURES
M.B.Y. became employed by Shire Pharmaceuticals (Lexington, MA, USA) after completion of the studies described herein. M.B.Y. was supported by a NIH/NIGMS IRACDA grant K21 GM000680 awarded to Emory University. L.L.H. was supported by NIH/NIDA K05 DA031246. P.J.M. was supported by NIH R00 HL 107675-03 and the American Heart Association 15CSA24340001. L.L.H. was supported by NIH/NIDA K05 DA031246.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All other authors declare no conflicts of interest pertinent to this manuscript.
References
- Baker DG, Ekhator NN, Kasckow JW, Hill KK, Zoumakis E, Dashevsky BA, Chrousos GP, Geracioti TD. Plasma and cerebrospinal fluid interleukin-6 concentrations in posttraumatic stress disorder. Neuroimmunomodulation. 2001;9:209–217. doi: 10.1159/000049028. [DOI] [PubMed] [Google Scholar]
- Bi Q, Shi L, Yang P, Wang J, Qin L. Minocycline attenuates interferon-α-induced impairments in rat fear extinction. Journal of Neuroinflammation. 2016;13:172. doi: 10.1186/s12974-016-0638-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen MS, Maihofer AX, Glatt SJ, Tylee DS, Chandler SD, Tsuang MT, Risbrough VB, Baker DG, O’Connor DT, Nievergelt CM, Woelk CH. Gene networks specific for innate immunity define post-traumatic stress disorder. Mol Psychiatry. 2015;20:1538–1545. doi: 10.1038/mp.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breznitz S, Ben-Zur H, Berzon Y, Weiss DW, Levitan G, Tarcic N, Lischinsky S, Greenberg A, Levi N, Zinder O. Experimental induction and termination of acute psychological stress in human volunteers: effects on immunological, neuroendocrine, cardiovascular, and psychological parameters. Brain Behavior and Immunity. 1998;12:34–52. doi: 10.1006/brbi.1997.0511. [DOI] [PubMed] [Google Scholar]
- Clark SM, Soroka JA, Song C, Li X, Tonelli LH. CD4(+) T cells confer anxiolytic and antidepressant-like effects, but enhance fear memory processes in Rag2(−/−) mice. Stress. 2016;19:303–311. doi: 10.1080/10253890.2016.1191466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Oscos F, Peña D, Housini M, Cheng D, Lopez D, Borland MS, Salgado-Delgado R, Salgado H, D’Mello S, Kilgard MP, Rose-John S, Atzori M. Vagal nerve stimulation blocks interleukin 6-dependent synaptic hyperexcitability induced by lipopolysaccharide-induced acute stress in the rodent prefrontal cortex. Brain Behavior and Immunity. 2015;43:149–158. doi: 10.1016/j.bbi.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill JM, Saligan L, Woods S, Page G. PTSD is associated with an excess of inflammatory immune activities. Perspect Psychiatr Care. 2009;45:262–277. doi: 10.1111/j.1744-6163.2009.00229.x. [DOI] [PubMed] [Google Scholar]
- Gola H, Engler H, Sommershof A, Adenauer H, Kolassa S, Schedlowski M, Groettrup M, Elbert T, Kolassa IT. Posttraumatic stress disorder is associated with an enhanced spontaneous production of pro-inflammatory cytokines by peripheral blood mononuclear cells. BMC Psychiatry. 2013;13:40. doi: 10.1186/1471-244X-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Jing H, Bi Q, Zhang J, Qin L, Yang P. Intra-amygdala microinfusion of IL-6 impairs the auditory fear conditioning of rats via JAK/STAT activation. Behavioural Brain Research. 2014;275:88–95. doi: 10.1016/j.bbr.2014.08.052. [DOI] [PubMed] [Google Scholar]
- Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, Rebusi N, Heshmati M, Aleyasin H, Warren BL, Lebonté B, Horn S, Lapidus KA, Stelzhammer V, Wong EHF, Bahn S, Krishnan V, Bolaños-Guzman CA, Murrough JW, Merad M, Russo SJ. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proceedings of the National Academy of Sciences. 2014;111:16136–16141. doi: 10.1073/pnas.1415191111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundhausen C, Roth A, Whalen E, Chen J, Schneider A, Long SA, Wei S, Rawlings R, Kinsman M, Evanko SP, Wight TN, Greenbaum CJ, Cerosaletti K, Buckner JH. Enhanced T cell responses to IL-6 in type 1 diabetes are associated with early clinical disease and increased IL-6 receptor expression. Science Translational Medicine. 2016;8:356ra119. doi: 10.1126/scitranslmed.aad9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter CA, Jones SA. IL-6 as a keystone cytokine in health anddisease. Nat Immunol. 2015;16:448–457. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- Jergović M, Bendelja K, Vidović A, Savić A, Vojvoda V, Aberle N, Rabatić S, Jovanovic T, Sabioncello A. Patients with posttraumatic stress disorder exhibit an altered phenotype of regulatory T cells. Allergy Asthma Clin Immunol. 2014;10:43. doi: 10.1186/1710-1492-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RL, Murray ST, Camacho DK, Wilson CG. Vagal nerve stimulation attenuates IL-6 and TNFα expression in respiratory regions of the developing rat brainstem. Respiratory Physiology & Neurobiology. 2016;229:1–4. doi: 10.1016/j.resp.2016.03.014. [DOI] [PubMed] [Google Scholar]
- Jones GW, McLoughlin RM, Hammond VJ, Parker CR, Williams JD, Malhotra R, Scheller J, Williams AS, Rose-John S, Topley N, Jones SA. Loss of CD4+ T cell IL-6R expression during inflammation underlines a role for IL-6 trans signaling in the local maintenance of Th17 cells. The Journal of Immunology. 2010;184:2130–2139. doi: 10.4049/jimmunol.0901528. [DOI] [PubMed] [Google Scholar]
- Jones ME, Lebonville CL, Barrus D, Lysle DT. The role of brain interleukin-1 in stress-enhanced fear learning. Neuropsychopharmacology. 2015;40:1289–1296. doi: 10.1038/npp.2014.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J. Multifaceted interactions between adaptive immunity and the central nervous system. Science. 2016;353:766–771. doi: 10.1126/science.aag2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Gadani S, Derecki NC. Pro-cognitive properties of T cells. Nat Rev Immunol. 2012;12:663–669. doi: 10.1038/nri3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, Schuurman PR, Mehta AD, Levine YA, Faltys M, Zitnik R, Tracey KJ, Tak PP. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proceedings of the National Academy of Sciences. 2016;113:8284–8289. doi: 10.1073/pnas.1605635113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux A, Coe CL, Carnes M. Symptom severity predicts degree of T cell activation in adult women following childhood maltreatment. Brain Behavior and Immunity. 2008;22:994–1003. doi: 10.1016/j.bbi.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D, Wolkowitz OM, Mellon S, Yehuda R, Flory JD, Henn-Haase C, Bierer LM, Abu-Amara D, Coy M, Neylan TC, Makotkine I, Reus VI, Yan X, Taylor NM, Marmar CR, Dhabhar FS. Proinflammatory milieu in combat-related PTSD is independent of depression and early life stress. Brain Behavior and Immunity. 2014;42:81–88. doi: 10.1016/j.bbi.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Michopoulos V, Powers A, Gillespie CF, Ressler KJ, Jovanovic T. Inflammation in Fear- and Anxiety-Based Disorders: PTSD, GAD and Beyond. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberg CA, Curtin JJ. Alcohol selectively reduces anxiety but not fear: startle response during unpredictable versus predictable threat. J Abnorm Psychol. 2009;118:335–347. doi: 10.1037/a0015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324:951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton TL, Fernandez-Botran R, Miller JJ, Burns VE. Interleukin-6 and soluble interleukin-6 receptor levels in posttraumatic stress disorder: associations with lifetime diagnostic status and psychological context. Biological Psychology. 2014;99:150–159. doi: 10.1016/j.biopsycho.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nish SA, Schenten D, Wunderlich FT, Pope SD, Gao Y, Hoshi N, Yu S, Yan X, Lee HK, Pasman L, Brodsky I, Yordy B, Zhao H, Brüning J, Medzhitov R. T cell-intrinsic role of IL-6 signaling in primary and memory responses. Elife. 2014;3:e01949. doi: 10.7554/eLife.01949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Cohen BE, Seal KH, Bertenthal D, Margaretten M, Nishimi K, Neylan TC. Elevated risk for autoimmune disorders in iraq and afghanistan veterans with posttraumatic stress disorder. Biol Psychiatry. 2015;77:365–374. doi: 10.1016/j.biopsych.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Koedel U, Winkler F, Kieseier BC, Fontana A, Kopf M, Hartung HP, Pfister HW. Lack of IL-6 augments inflammatory response but decreases vascular permeability in bacterial meningitis. Brain. 2003;126:1873–1882. doi: 10.1093/brain/awg171. [DOI] [PubMed] [Google Scholar]
- Peña DF, Engineer ND, McIntyre CK. Rapid remission of conditioned fear expression with extinction training paired with vagus nerve stimulation. Biol Psychiatry. 2013;73:1071–1077. doi: 10.1016/j.biopsych.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. Annals of the New York Academy of Sciences. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Price M, Jovanovic T, Norrholm SD, Gerardi M, Dunlop B, Davis M, Bradley B, Ressler KJ. A randomized, double-blind evaluation of D-cycloserine or alprazolam combined with virtual reality exposure therapy for posttraumatic stress disorder (PTSD) in Iraq and Afghanistan war veterans. American Journal of Psychiatry. 2014 doi: 10.1176/appi.ajp.2014.13121625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saija A, Princi P, Lanza M, Scalese M, Aramnejad E, De Sarro A. Systemic cytokine administration can affect blood-brain barrier permeability in the rat. Life Sciences. 1995;56:775–784. doi: 10.1016/0024-3205(95)00008-t. [DOI] [PubMed] [Google Scholar]
- Scholz B, Doidge AN, Barnes P, Hall J, Wilkinson LS, Thomas KL. The Regulation of Cytokine Networks in Hippocampal CA1 Differentiates Extinction from Those Required for the Maintenance of Contextual Fear Memory after Recall. PLoS ONE. 2016;11:e0153102. doi: 10.1371/journal.pone.0153102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singewald N, Schmuckermair C, Whittle N, Holmes A, Ressler KJ. Pharmacology of cognitive enhancers for exposure-based therapy of fear, anxiety and trauma-related disorders. Pharmacol Ther. 2015;149:150–190. doi: 10.1016/j.pharmthera.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tursich M, Neufeld RWJ, Frewen PA, Harricharan S, Kibler JL, Rhind SG, Lanius RA. Association of trauma exposure with proinflammatory activity: a transdiagnostic meta-analysis. Transl Psychiatry. 2014;4:e413. doi: 10.1038/tp.2014.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. European Journal of Pharmacology. 2003;463:199–216. doi: 10.1016/S0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Yu Z, Fukushima H, Ono C, Sakai M, Kasahara Y, Kikuchi Y, Gunawansa N, Takahashi Y, Matsuoka H, Kida S, Tomita H. Microglial production of TNF- alpha is a key element of sustained fear memory. Brain Behavior and Immunity. 2016 doi: 10.1016/j.bbi.2016.08.011. [DOI] [PubMed] [Google Scholar]
- Zhang JC, Yao W, Dong C, Yang C, Ren Q, Ma M, Hashimoto K. Blockade of interleukin-6 receptor in the periphery promotes rapid and sustained antidepressant actions: a possible role of gut-microbiota-brain axis. Transl Psychiatry. 2017;7:e1138. doi: 10.1038/tp.2017.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Kusnecov AW, Shurin MR, DePaoli M, Rabin BS. Exposure to physical and psychological stressors elevates plasma interleukin 6: relationship to the activation of hypothalamic-pituitary-adrenal axis. Endocrinology. 1993;133:2523–2530. doi: 10.1210/endo.133.6.8243274. [DOI] [PubMed] [Google Scholar]
- Zhou J, Nagarkatti P, Zhong Y, Ginsberg JP, Singh NP, Zhang J, Nagarkatti M. Dysregulation in microRNA expression is associated with alterations in immune functions in combat veterans with post-traumatic stress disorder. PLoS ONE. 2014;9:e94075. doi: 10.1371/journal.pone.0094075. [DOI] [PMC free article] [PubMed] [Google Scholar]


