Abstract
Introduction
We evaluated the diagnostic and prognostic value of quantification of myocardial flow reserve (MFR) with positron emission tomography (PET) in orthotopic heart transplant (OHT) patients.
Methods and Results
We retrospectively identified OHT patients who underwent rubidium-82 cardiac PET imaging. The primary outcome was the composite of cardiovascular death, acute coronary syndrome, coronary revascularization, and heart failure hospitalization. Cox regression was used to evaluate the association of MFR with the primary outcome. The relationship of MFR and cardiac allograft vasculopathy (CAV) severity in patients with angiography within one year of PET imaging was assessed using Spearman rank correlation and logistic regression. A total of 117 patients (median age 60 years, 71% male) were identified. Twenty-one of 62 patients (34%) who underwent angiography before PET had CAV. The median time from OHT to PET imaging was 6.4 years (median global MFR = 2.31). After a median of 1.4 years, 22 patients (19%) experienced the primary outcome. On an unadjusted basis, global MFR (HR 0.22 per unit increase, 95% CI 0.09-0.50, p < 0.001) and stress myocardial blood flow (MBF, HR 0.48 per unit increase, 95% CI 0.29-0.79, p=0.004) were associated with the primary outcome. Decreased MFR independently predicted the primary outcome after adjustment for other variables. In 42 patients who underwent angiography within 12 months of PET, MFR and stress MBF were associated with moderate-severe CAV (ISHLT grade 2-3).
Conclusions
MFR assessed by cardiac rubidium-82 PET imaging is a predictor of cardiovascular events following OHT and is associated with CAV severity.
Keywords: cardiac transplant, vasculopathy, positron emission tomography, myocardial perfusion imaging
Subject Terms: heart failure, transplantation, nuclear cardiology and PET, coronary circulation
Introduction
Cardiac allograft vasculopathy (CAV) is a leading cause of morbidity and mortality following cardiac transplantation (1). CAV is a process of circumferential intimal thickening due to smooth muscle proliferation, inflammation, lipid deposition, and perivascular fibrosis. Unlike the focal lesions of coronary artery disease, it is a diffuse process affecting both the epicardial vessels as well as the microvasculature(1, 2). Both immune-mediated mechanisms and traditional cardiovascular risk factors have been implicated in the disease process (1, 2). Unfortunately, CAV is quite common following transplantation with registry data showing 30% of patients developing CAV within five years of transplantation (3). Progressive CAV can lead to acute coronary syndrome, heart failure, arrhythmias, and sudden cardiac death.
Because of allograft denervation, CAV may remain asymptomatic until the onset of severe complications; therefore, routine surveillance is generally considered mandatory (1). ISHLT guidelines currently recommend annual or biannual coronary angiography for at least the first several years following transplantation (4). Unfortunately, even when angiography is performed regularly, it may not fully characterize CAV severity or the impact of CAV on allograft function. Because of the diffuse nature of intimal thickening, it can be missed on standard angiography, especially in the presence of vascular remodeling which may counterbalance the impact of CAV on the epicardial coronary lumen (1, 2, 5–9). The addition of intravascular ultrasound (IVUS) to coronary angiography has been shown to increase sensitivity for the detection of CAV (9). However, both are invasive procedures that expose transplant patients to intravenous contrast and associated complications, including exacerbation of widely prevalent renal dysfunction.
For these reasons, non-invasive imaging that can reliably detect and quantify CAV, especially in its early stages, is needed. Although several non-invasive modalities have utility for identification of obstructive coronary disease, data is lacking to support their ability to detect early and non-obstructive CAV. Earlier CAV detection would allow for changes in medical therapy and immunosuppression that could possibly prevent progression of CAV, graft failure, and other cardiovascular outcomes (2).
Myocardial perfusion imaging with rubidium-82 cardiac positron emission tomography (PET) has the ability to noninvasively assess both epicardial and microvascular coronary flow. Myocardial flow reserve (MFR), the ratio of stress to rest myocardial blood flow, is a well validated evaluation of abnormal coronary vasodilatory capacity that is now routinely measured during PET imaging (10). Among patients without OHT, impaired MFR has been associated with cardiovascular outcomes such as cardiac death, nonfatal myocardial infarction, revascularization, and heart failure hospitalization (10). While PET MFR has been shown to correlate with IVUS assessments of plaque burden (11), only one study has evaluated the relationship between MFR assessed with PET imaging and cardiovascular outcomes among cardiac transplant patients (5).
We sought to extend these findings to an independent population of heart transplant patients. We hypothesized that decreased MFR predicts cardiovascular events following heart transplantation. We also hypothesized that patients with CAV would have lower MFR values than those without CAV.
Methods
Study Design and Patient Selection
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure due to patient privacy regulations. We retrospectively identified patients with prior OHT who underwent rest/stress rubidium-82 cardiac PET imaging at any point following OHT. For patients that underwent multiple rest/stress rubidium-82 cardiac PET studies, the first scan was selected for analysis. For patients with serial scans, change in MFR between studies was recorded. Patient data including demographics, comorbidities, medications, prior coronary angiography, and rejection history were gathered through chart review. The study was approved by the University of Michigan Institutional Review Board. Informed consent was not required from participants for this retrospective study.
PET Imaging Protocol
Patients were studied with a whole-body PET-computed tomography scanner (Siemens mCT, Knoxville, TN) after an overnight fast. Patients refrained from caffeine- and methylxanthine-containing substances and drugs for 24 hours before their scans. Myocardial blood flow was measured during rest and peak stress with rubidium-82 as a perfusion tracer, as described previously (12, 13). Briefly, after transmission imaging and beginning with the intravenous bolus administration of rubidium-82, list mode images were acquired for 7 minutes. Then, a standard intravenous infusion of regadenoson was given. A second dose of rubidium-82 was injected 30-45 seconds after regadenoson administration, and images were recorded in the same manner. Heart rate, blood pressure, and 12-lead ECG were recorded at baseline and every minute during or after pharmacologic stress.
Image Interpretation
Semiquantitative 17-segment visual interpretation of the gated myocardial perfusion images was performed by experienced observers using a standard 5-point scoring system (14). Summed rest and stress scores were calculated as the sum of individual segmental scores on the respective images, and their difference was recorded as a summed difference score.
Absolute myocardial blood flow (MBF, mL/g/min) was computed from the dynamic rest and stress imaging series with commercially available software (Corridor4DM; Ann Arbor, MI) and previously validated methods (12, 13, 15). Factor analysis was used to generate blood pool (arterial input function) and tissue time-activity curves to a 2-compartment tracer kinetic model, as described previously (15). Per-patient global MFR was calculated as the ratio of absolute MBF at stress over rest for the entire left ventricle. For survival analyses, an abnormal MFR was defined as a ratio < 2.0 as this has been used to define coronary microvascular dysfunction in prior studies. Quantification of MBF was performed by 2 operators.
Primary Outcome Measure
The primary outcome was the composite of cardiovascular death, acute coronary syndrome, coronary revascularization, and heart failure hospitalization. The events were identified by reviewing each patient’s electronic medical record. One patient in this study underwent retransplantation following PET imaging. Because this patient also experienced a heart failure hospitalization, we did not include retransplantation in the primary outcome.
Evaluation of MFR in Patients with CAV
For our comparison of MFR values in patients with and without CAV, we selected only patients who had undergone coronary angiography within one year of PET imaging. We excluded patients with intervening revascularization, myocardial infarction, or acute coronary syndrome between angiography and PET imaging. Catheterization and echocardiography reports were reviewed, and CAV presence and severity was characterized using ISHLT standardized nomenclature (16). For the purpose of our analysis, ISHLT grade 2-3 was considered moderate-severe CAV as this represents the presence of at least one obstructive lesion in the proximal-mid segment of the left anterior descending, left circumflex, or right coronary arteries.
Statistical Analysis
Data were evaluated for normality and summarized with means and standard deviations or median and interquartile range, as appropriate. Kaplan-Meier analyses, and unadjusted and adjusted Cox regression were used to evaluate the relationship between clinical variables and PET measurements with the primary outcome. Due to the small number of patients experiencing the primary outcome, adjusted analyses were limited to a single additional covariate per regression model. Spearman rank correlation, logistic regression, and ROC curves were used to assess the relationships between MFR, stress myocardial blood flow (MBF), summed stress score (SSS), and angiographic CAV. The Kruskal-Wallis rank sum test was used to compare differences in characteristics between patients with and without CAV. All statistical analyses were performed in R version 3.4 (R Foundation for Statistical Computing).
Results
A total of 117 patients (median age 60, 71% male) underwent rest/stress rubidium-82 cardiac PET imaging between May 2011 and February 2016. Baseline characteristics are reported in Table 1. Comorbidities were common [40% diabetes, 86% hypertension, 64% chronic kidney disease (GFR < 60 mL/min per 1.73 m2), median BMI 30.0 kg/m2]. Immunosuppression regimens varied but most commonly utilized tacrolimus. Twenty-one of 62 patients (34%) who received angiography before PET had CAV by ISHLT criteria. The median time from OHT to PET imaging was 6.4 years. The median global MFR was 2.31 (IQR 1.84 – 2.72); 33 patients (28%) had a MFR less than 2.0. Forty-eight patients underwent repeat PET studies during the study period. The median change in MFR observed was as follows: 0.01 (IQR −0.21, +0.22) in 7 patients with a repeat study within 12 months, 0.10 (IQR −0.12, +0.36) in 25 patients with a repeat study 12-24 months later, and −0.10 (IQR −0.28, +0.02) in 16 patients with a repeat study over 24 months later.
Table 1.
Baseline characteristics.
| Baseline Characteristics | Total (n=117), n (%) |
|---|---|
| Demographics | |
| Age, years | 60 (52-66) |
| Men | 83 (71%) |
| Time from transplant to PET imaging, years | 6.4 (3.5-10.0) |
| Follow-up, years | 1.4 (0.9-2.4) |
| Transplant Indication | |
| Ischemic cardiomyopathy | 77 (66%) |
| Nonischemic cardiomyopathy | 40 (34%) |
| Immunosuppression | |
| Tacrolimus | 102 (87%) |
| Sirolimus or Everolimus | 24 (21%) |
| Cyclosporine | 12 (10%) |
| Mycophenolate mofetil | 63 (54%) |
| Azathioprine | 8 (7%) |
| Prednisone | 77 (66%) |
| Other Medications | |
| Aspirin | 103 (88%) |
| Statin | 102 (87%) |
| Vitamin C | 88 (75%) |
| Vitamin E | 82 (70%) |
| Comorbidities | |
| Diabetes mellitus | 47 (40%) |
| Hypertension | 101 (86%) |
| Chronic kidney disease (GFR < 60 mL/min per 1.73 m2) | 75 (64%) |
| Body mass index, kg/m2 | 30.0 (26.1-34.6) |
| Previously documented cardiac allograft vasculopathy, n | 21 (18%) |
| Previous revascularization | 4 (3%) |
| Prior ≥ 2R cellular rejection or antibody-mediated rejection | 42 (36%) |
| PET Imaging Data | |
| Summed stress score | 1 (0-2) |
| Summed rest score | 1 (0-2) |
| Summed difference score | 0 (0-1) |
| Rest ejection fraction, % | 61 (56-65) |
| LV volume ratio at stress/rest | 1.02 (0.96-1.06) |
| Rest myocardial blood flow, mL/g per minute | 1.59 (1.35-1.93) |
| Stress myocardial blood flow, mL/g per minute | 3.60 (3.11-4.16) |
| Myocardial flow reserve | 2.31 (1.84-2.72) |
| Myocardial flow reserve < 2.0 | 33 (28%) |
Continuous variables reported as median (25% and 75% percentiles). GFR = glomerular filtration rate.
Primary Outcome Analyses
After a median of 1.4 years (IQR 0.9-2.4 years), 22 patients experienced the primary outcome. There were 2 cardiovascular deaths. Five patients experienced an acute coronary syndrome, 8 patients underwent revascularization, and 15 patients were hospitalized for heart failure.
In unadjusted analyses, when evaluated as continuous variables, MFR (HR 0.22 per unit increase in MFR, 95% CI 0.09-0.50, p = <0.001) and stress MBF (HR 0.48 per unit increase in stress MBF, 95% CI 0.29-0.79, p = 0.004) were associated with the primary outcome. Other PET measures including summed stress score (SSS), summed rest score (SRS), summed difference score (SDS), and rest ejection fraction (EF) were also significantly associated with the primary outcome (Table 2). Time since orthotopic heart transplantation was significantly associated with the primary outcome; however, no other clinical variables including patient comorbidities and history of rejection predicted the primary outcome.
Table 2.
Unadjusted predictors of cardiovascular events.
| Variable | Outcome (n = 22) |
No Outcome (n = 95) |
Hazard Ratio (95% CI) |
χ2 | P Value |
|---|---|---|---|---|---|
| Age, yrs | 57 (44-65) | 61 (55-66) | 0.98 (0.95-1.02) | 0.69 | 0.405 |
| Men | 16 (73%) | 68 (72%) | 0.88 (0.34-2.25) | 0.07 | 0.788 |
| Time since OHT, yrs | 9.9 (6.4-13.0) | 6.0 (3.2-9.2) | 1.08 (1.00-1.16) | 4.16 | 0.041 |
| Hypertension | 19 (86%) | 82 (86%) | 0.86 (0.25-2.95) | 0.06 | 0.814 |
| Diabetes mellitus | 8 (36%) | 39 (41%) | 0.91 (0.38-2.17) | 0.05 | 0.825 |
| GFR < 60 mL/min/1.73 m2 | 17 (77%) | 58 (61%) | 1.90 (0.70-5.16) | 1.59 | 0.207 |
| BMI, kg/m2 | 28 (27-36) | 30 (26-34) | 1.03 (0.95-1.10) | 0.47 | 0.492 |
| Previous revascularization | 2 (9%) | 2 (2%) | 4.22 (0.97-18.32) | 3.69 | 0.054 |
| Previous CAV* | 8 (8/14; 57%) | 13 (13/48; 27%) | 4.70 (0.61-36.08) | 2.21 | 0.137 |
| Prior rejection ≥ 2R or AMR | 10 (45%) | 32 (34%) | 1.60 (0.69 – 3.72) | 1.20 | 0.272 |
| SSS | 3 (1-6) | 0 (0-2) | 1.23 (1.13-1.34) | 22.6 | <0.001 |
| SRS | 1.5 (0-5) | 0 (0-2) | 1.16 (1.06-1.27) | 10.46 | 0.001 |
| SDS | 1 (0-2) | 0 (0-0) | 1.25 (1.09-1.43) | 10.35 | 0.001 |
| Rest EF, % | 56 (42-62) | 62 (58-66) | 0.92 (0.88-0.96) | 17.88 | <0.001 |
| EF reserve with stress, % | 4 (1-8) | 5 (2-8) | 0.97 (0.88-1.07) | 0.32 | 0.570 |
| LV volume ratio (stress/rest) | 1.04 (1.01-1.06) | 1.02 (0.98-1.06) | 9.62 (0.02-5856) | 0.48 | 0.489 |
| Rest MBF, mL/g/min | 1.62 (1.46-1.94) | 1.57 (1.32-1.93) | 1.79 (0.68-4.68) | 1.41 | 0.234 |
| Stress MBF, mL/g/min | 2.90 (2.34-4.03) | 3.67 (3.22-4.24) | 0.48 (0.29-0.79) | 8.15 | 0.004 |
| MFR | 1.86 (1.38-2.37) | 2.38 (2.05-2.79) | 0.22 (0.09-0.50) | 12.62 | <0.001 |
| MFR < 2.0 | 12 (54%) | 21 (22%) | 0.21 (0.09-0.50) | 12.59 | <0.001 |
Only 63 patients had angiography prior to PET imaging (14 patients experienced primary outcome). Continuous variables reported as median (25% and 75% percentiles) unless otherwise stated. Categorical variables reported as n (%). CI indicates confidence interval; OHT, orthotopic heart transplantation; CAV, cardiac allograft vasculopathy; GFR, glomerular filtration rate; BMI, body mass index; SSS, summed stress score; SRS, summed rest score; SDS, summed difference score; EF, left ventricular ejection fraction; MBF, myocardial blood flow; MFR, myocardial flow reserve.
On several adjusted analyses each with the addition of a single covariate, MFR remained a significant predictor of the primary outcome (Table 3). In contrast, stress myocardial blood flow remained a significant predictor of the primary outcome on some but not all of the adjusted analyses (Table 3).
Table 3.
Adjusted analyses for PET measures of myocardial flow and the primary outcome.
| Myocardial Flow Reserve (MFR) | |||
|---|---|---|---|
| Covariates | Hazard Ratio (95% CI) |
χ2 | P Value |
| MFR + rest EF | 0.31 (0.13-0.76) | 23.58 | 0.010 |
| MFR + SSS | 0.36 (0.15-0.86) | 27.35 | 0.021 |
| MFR + SDS | 0.27 (0.11-0.64) | 18.51 | 0.003 |
| MFR + SRS | 0.26 (0.11-0.60) | 20.75 | 0.002 |
| MFR + Time since OHT | 0.23 (0.10-0.53) | 16.76 | <0.001 |
| MFR + Prior Diagnosis of CAV* | 0.20 (0.07-0.58) | 9.97 | 0.003 |
| Stress Myocardial Blood Flow (Stress MBF) | |||
| Covariates |
Hazard Ratio (95% CI) |
χ2 | P Value |
| Stress MBF + rest EF | 0.67 (0.38-1.18) | 19.59 | 0.165 |
| Stress MBF + SSS | 0.68 (0.40-1.16) | 24.37 | 0.155 |
| Stress MBF + SDS | 0.54 (0.32-0.92) | 14.55 | 0.023 |
| Stress MBF + SRS | 0.56 (0.34-0.91) | 16.77 | 0.020 |
| Stress MBF + Time since OHT | 0.52 (0.30-0.88) | 9.50 | 0.015 |
| Stress MBF + Prior Diagnosis of CAV* | 0.51 (0.27-0.96) | 5.84 | 0.037 |
EF indicates left ventricular ejection fraction; SSS, summed stress score; SDS, summed difference score; SRS, summed rest score; OHT, orthotopic heart transplantation; CAV, cardiac allograft vasculopathy.
Analyses included 62 patients as 55 patients did not have angiography available. Of the 62 patients, 21 patients had a prior diagnosis of CAV. The primary outcome was observed in 17 of 62 patients.
Kaplan-Meier analysis demonstrated that patients with an MFR < 2.0 were at increased risk for experiencing the primary outcome (cox proportional HR 0.21, 95% CI 0.09-0.50, p < 0.0001, Figure 1). In contrast, a stress MBF below the mean for this sample (< 3.7 mL/g/min) was not significantly associated with the primary outcome (cox proportional HR 0.55, 95% CI 0.22-1.34, p=0.18, Figure 1).
Figure 1.
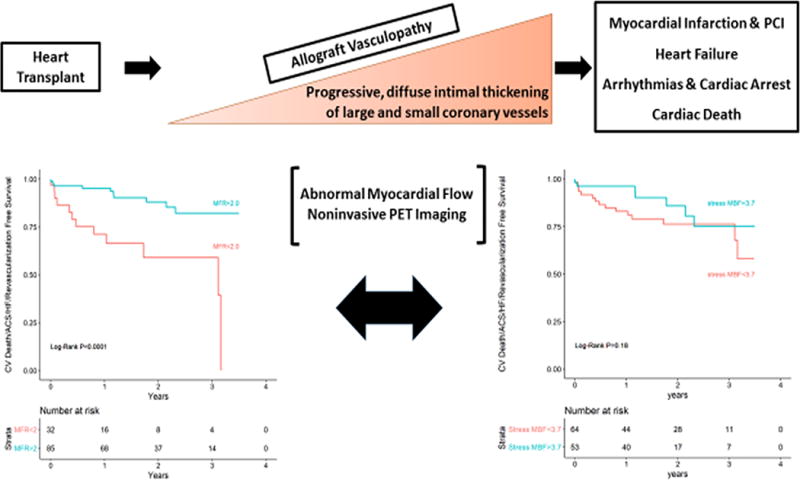
Kaplan-Meier analysis for the primary outcome using myocardial flow reserve (MFR) and stress myocardial blood flow (MBF) as a dichotomous variables.
Myocardial Flow Reserve, Stress Myocardial Blood Flow, and Cardiac Allograft Vasculopathy
A total of 42 patients (median age 59 years, IQR 55-65 years, 64% male, Table 4) underwent cardiac rubidium-82 PET imaging within 12 months of coronary angiography (median 110 days between PET and angiogram, IQR 26-270 days). The median time from OHT to first PET imaging was 5.6 years (IQR 3.0-9.7 years). Comorbidities were common [48% diabetes, 81% hypertension, 67% chronic kidney disease (GFR < 60 mL/min), 50% obesity]. The median global MFR was 2.18 (IQR1.62-2.60). Twenty-two patients (52%) had CAV by ISHLT criteria with 11 patients having moderate-severe CAV (ISHLT grade 2-3).
Table 4.
Baseline characteristics for patients with and without CAV by ISHLT criteria.
| Variable | CAV (n=22) | No CAV (n = 20) | P Value |
|---|---|---|---|
| Age, years | 58.5 (53.5-64.5) | 61.0 (56.0-66.3) | 0.503 |
| Men | 12 (54%) | 15 (75%) | 0.289 |
| Time OHT to PET, yrs | 8.69 (4.34-14.4) | 3.84 (2.22-5.44) | 0.001 |
| Immunosuppression | |||
| Tacrolimus | 17 (77%) | 20 (100%) | 0.073 |
| Sirolimus or Everolimus | 11 (50%) | 2 (10%) | 0.014 |
| Cyclosporine | 3 (14%) | 0 | 0.265 |
| Mycophenolate mofetil | 8 (36%) | 12 (60%) | 0.222 |
| Azathioprine | 2 (9%) | 0 | 0.512 |
| Prednisone | 14 (64%) | 12 (60%) | 0.999 |
| Comorbidities | |||
| Diabetes mellitus | 9 (41%) | 11 (55%) | 0.546 |
| Hypertension | 18 (82%) | 16 (80%) | 0.999 |
| CKD (GFR < 60 mL/min) | 18 (82%) | 10 (50%) | 0.512 |
| Body mass index, kg/m2 | 31.2 (26.5-35.4) | 28.1 (24.5-32.0) | 0.064 |
| Hx ≥ 2R rejection or AMR | 8 (36%) | 7 (35%) | 0.999 |
| PET Imaging Data | |||
| Summed stress score | 4.5 (2.0-7.5) | 1.0 (0-3.25) | 0.220 |
| Summed rest score | 2.0 (1.0-3.0) | 1.0 (0-2.0) | 0.272 |
| Summed difference score | 1.5 (0-4.8) | 0 (0-0.2) | 0.034 |
| Rest ejection fraction, % | 58 (55-64) | 63 (59-68) | 0.398 |
| Rest MBF, mL/g/min | 1.55 (1.37-1.93) | 1.66 (1.31-2.08) | 0.537 |
| Stress MBF mL/g/min | 2.96 (2.42-3.48) | 3.90 (3.31-4.90) | < 0.001 |
| Myocardial flow reserve | 1.75 (1.46-2.52) | 2.46 (2.14-2.84) | 0.017 |
| MFR < 2.0 | 12 (54%) | 3 (15%) | 0.019 |
Continuous variables reported as median (25% and 75% percentiles).
MFR was significantly correlated with the ISHLT CAV grade (Spearman ρ = −0.47, p=0.002, Figure 2A). MFR was associated with the presence of moderate-severe CAV on unadjusted analysis (OR 0.15 per unit increase in MFR, 95% CI 0.03-0.58, p=0.01, Table 5) and when adjusted for SSS (OR=0.19 per unit increase in MFR, 95% CI 0.03-0.80, p=0.04, Table 5). Stress MBF was also significantly correlated with ISHLT CAV grade (Spearman ρ = −0.60, p<0.0001, Figure 2B). Stress MBF was associated with the presence of moderate-severe CAV on unadjusted analysis (OR 0.21 per unit increase in stress MBF, 95% CI 0.05-0.58, p=0.009, Table 5) and when adjusted for SSS (OR=0.24 per unit increase in stress MBF, 95% CI 0.06-0.70, p=0.03, Table 5). We did not identify a statistically significant association between SSS (p= 0.07, Table 5), SRS, LVEF or rest MBF and moderate-severe CAV.
Figure 2.
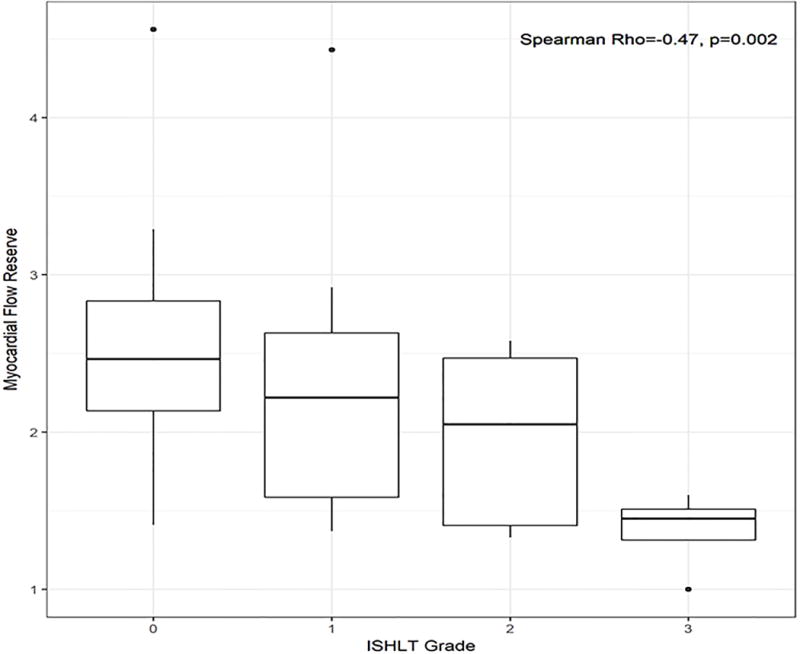
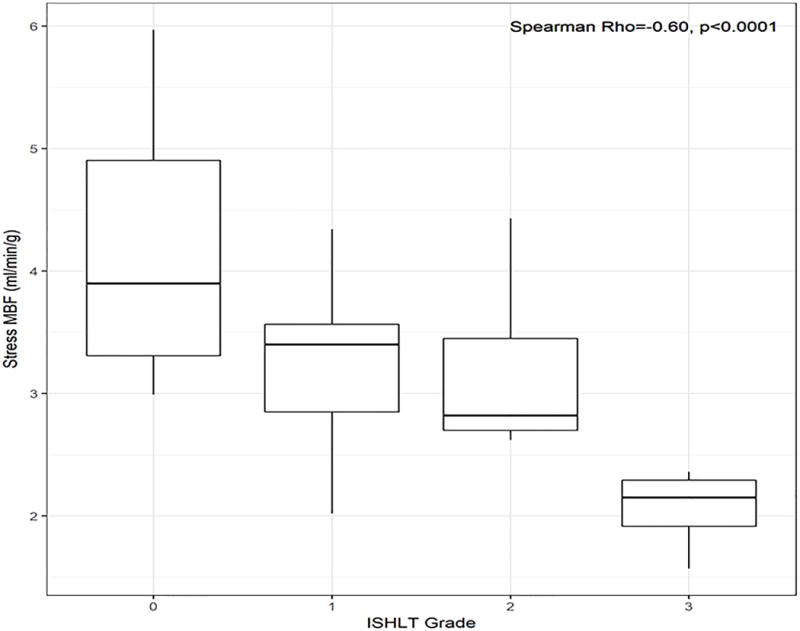
Correlations between (A) myocardial flow reserve and (B) stress myocardial blood flow (MBF) with severity of CAV.
Table 5.
Logistic regression of PET variables and ISHLT grade 2-3 CAV (n=11)
| Odds Ratio (95% CI) | P Value | |
|---|---|---|
| Summed Stress Score (SSS) | ||
| SSS (unajdusted) | 1.18 (0.99-1.44) | 0.070 |
| Myocardial Flow Reserve (MFR) | ||
| MFR (unadjusted) | 0.15 (0.03-0.58) | 0.010 |
| MFR adjusted for SSS | 0.19 (0.03-0.80) | 0.040 |
| Stress Myocardial Blood Flow (Stress MBF) | ||
| Stress MBF (unadjusted) | 0.21 (0.05-0.58) | 0.009 |
| Stress MBF adjusted for SSS | 0.24 (0.06-0.70) | 0.030 |
Both MFR (AUC=0.76, p=0.36 vs. SSS, Figure 3A) and stress MBF (AUC=0.79, p=0.22 vs. SSS, Figure 3B) had non-significantly higher AUC values for ISHLT moderate to severe CAV than SSS (AUC=0.66). The addition of MFR to SSS (AUC=0.76, p=0.30 vs. SSS, Figure 3A) and stress MBF to SSS (AUC=0.80, p=0.15 vs. SSS, Figure 3B) showed non-significantly higher AUC values for ISHLT moderate to severe CAV.
Figure 3.
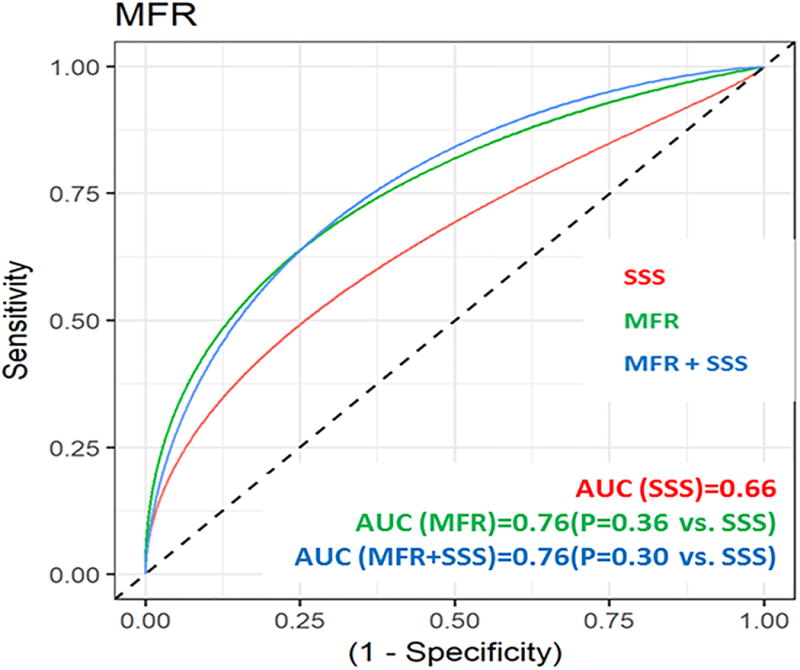
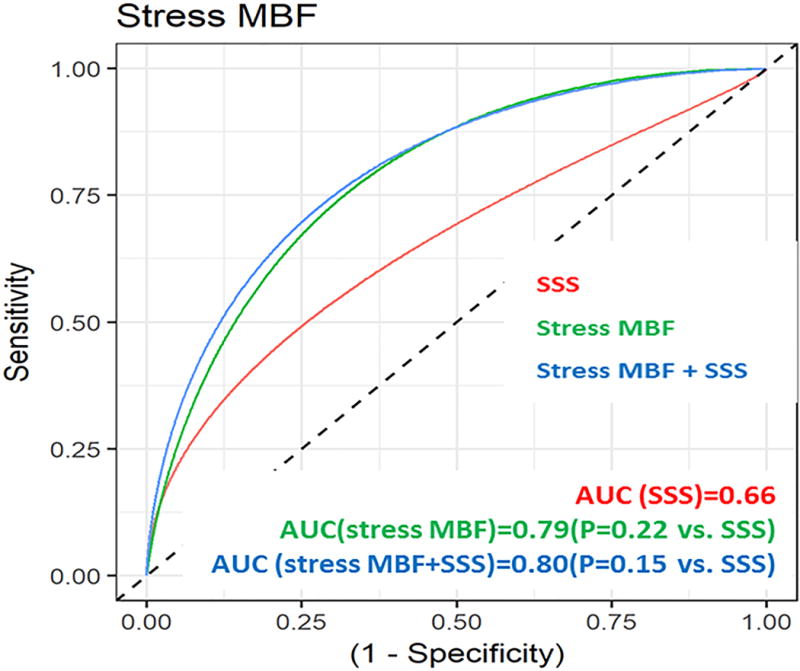
Receiver operator curves for (A) myocardial flow reserve (MFR) and (B) stress myocardial blood flow (MBF) for the diagnosis of CAV.
Discussion
In this study of cardiac transplant patients, decreased global MFR was the most significant predictor of the composite primary outcome of cardiovascular death, acute coronary syndrome, coronary revascularization, and heart failure hospitalization. In addition, MFR and stress MBF were both associated with the severity of CAV. These findings demonstrate the added value of quantitative measures of myocardial blood flow and vasomotor function provided by PET imaging.
Stress echocardiography and other forms of perfusion imaging rely on heterogeneity in perfusion or contractility to identify disease (2). These changes occur with obstructive epicardial disease which would be a late manifestation of CAV. This results in these modalities having a decreased sensitivity for identifying CAV, particularly in its earliest stages. This may explain why EF reserve, a measurement used to assess for CAV risk on stress echocardiography, was not a significant predictor of the primary outcome in this study. The limitations of stress echocardiography and other forms of perfusion imaging explain why guidelines continue to recommend angiography as the preferred assessment for CAV (4). Unfortunately, angiography is an invasive procedure which exposes patients to risk of vascular complications and worsening renal function. Standard angiography can also miss a diagnosis of CAV as the diffuse nature of intimal thickening as well as vascular remodeling may lead to no appreciable disease on longitudinal assessment of the epicardial coronary lumen (1, 2, 5–9). As a result, IVUS is frequently added, with additional limitations and risk. Most notably, although IVUS provides excellent assessment of the coronary arterial wall structure, vasomotor function and the coronary microvasculature cannot be evaluated. Importantly, multiple coronary arteries must be imaged to maximize the sensitivity of IVUS for CAV, further increasing the risk of complications (17).
Rather than relying solely on an anatomic characterization of CAV, MFR provides an integrated assessment of both macro- and microvascular function. Factors other than luminal narrowing, such as inflammation from rejection, infection, or other risk factors, can contribute to impaired vasomotor function and decreased MFR (18). Abnormal coronary vasodilation, assessed by Doppler echocardiography MFR, has been associated with increased prevalence of CAV, reduced LV longitudinal myocardial deformation, and decreased exercise capacity following cardiac transplantation (19). Similarly, angiographic markers of endothelial dysfunction precede the development of CAV and other cardiovascular events in cardiac transplant patients (20–22). These data argue that CAV is more than simply a disease of luminal narrowing; it has effects on coronary vasomotor function that may signify CAV progression and impact allograft function.
Global MFR, as assessed with PET imaging, has the potential to detect homogenous reductions in blood flow seen in CAV, a diffuse process affecting both the epicardial coronary vessels as well as the microvasculature. MFR, as assessed with cardiac magnetic resonance imaging (MRI), has been shown to have a high sensitivity for CAV and reliably exclude severe CAV at a value above 2.3 (23, 24). Similar to CMR measurements of MFR, PET MFR may be superior to other forms of stress imaging by identifying earlier stages of CAV. Indeed, in studies of patients within the first few years of transplantation, MFR by 13N-ammonia PET imaging has been found to correlate with plaque volume index and maximal intimal thickness as assessed by intravascular ultrasound (IVUS) (11, 25). In another study of 19 patients 18 ± 6 months following transplantation, MFR by 13N-ammonia PET imaging was associated with IVUS measurements of total vessel area and lumen diameter (26). Similar to IVUS measurements of CAV (27–31), MFR assessed with PET previously has been shown to predict adverse outcomes following cardiac transplantation (5).
The assessment of MFR by PET imaging has clinical implications for the diagnosis and management of CAV. Future studies should continue to evaluate the relationship between abnormal PET MFR and the characterization of CAV by coronary angiography. In patients with contraindications to or a preference to avoid coronary angiography at regular intervals, measurement of MFR may serve as a screening tool to determine which patients should proceed with coronary angiography. Through its assessment of blood flow and vasomotor function of the entire coronary arterial bed, MFR has the potential to identify CAV in its very early stages, even before it can be detected by angiography or IVUS. Treatment of CAV appears to be more effective when initiated early in its course (32–35); this suggests that earlier detection by PET imaging could impact cardiovascular outcomes though this requires further study (21). The mTOR inhibitors sirolimus and everolimus have been shown to reduce CAV incidence and progression (36–41) as well as improve coronary artery function (42). However, the adverse effects associated with these agents lead many providers to avoid routine use of these therapies in the absence of CAV. More data is needed to determine if PET imaging could identify patients who would benefit from an earlier transition to an mTOR inhibitor and whether serial imaging could also non-invasively monitor treatment response and disease progression.
There are several limitations to this study. First, while one of the larger studies of PET imaging in cardiac transplant patients, this was a single-center study with a limited number of adverse outcomes. Our findings do support those from a previous study that demonstrated prognostic value of PET imaging following cardiac transplantation (5). In addition, specialists at our center vary in their preferred means for CAV screening. Patients varied in regards to the timing and number of PET studies and coronary angiograms performed. Most patients underwent PET imaging several years after transplant, limiting our evaluation of imaging early after transplant when CAV presumably is less severe. Thus, recommendations regarding the appropriate clinical use of PET imaging cannot be made based on these data alone. For example, because of selection bias, we cannot conclude that abnormal MFR predicts the presence of CAV. Future prospective studies are needed to determine if protocols utilizing PET imaging could replace routine coronary angiography or have implications for the therapy of CAV. Lastly, PET imaging may not be widely available though it is the authors’ impression that most transplant centers have access to this technology.
Conclusions
MFR non-invasively assessed by cardiac rubidium-82 PET imaging is a powerful predictor of cardiovascular events following OHT. We also observed that MFR and stress MBF are decreased in patients with CAV. Prospective studies are needed to determine if PET imaging can serve as an adjunct to coronary angiography as a preferred method for CAV screening and treatment monitoring.
Commentary.
WHAT IS NEW?
In this retrospective study of heart transplant patients, noninvasive assessments of coronary flow and vasomotor function obtained with PET imaging were associated with the primary composite outcome of acute coronary syndrome, coronary revascularization, heart failure hospitalization, and cardiovascular death.
These measures were also associated with the severity of cardiac allograft vasculopathy as assessed with coronary angiography.
WHAT ARE THE CLINICAL IMPLICATIONS?
Coronary angiography is recommended for cardiac allograft vasculopathy screening as conventional stress imaging has decreased sensitivity, especially for earlier stages of allograft vasculopathy.
Coronary angiography has limitations for the diagnosis of allograft vasculopathy and exposes patients to the risk of vascular complications and renal injury.
PET imaging may be able to identify early stages of allograft vasculopathy through its assessment of epicardial and microvascular blood flow and vasomotor function.
Prospective studies should investigate whether PET imaging can serve as an adjunct to coronary angiography as a preferred method for CAV screening and treatment monitoring.
Acknowledgments
Dr. Murthy is principal investigator for research supported by the National Heart, Lung, and Blood Institute (1R01HL136685), Singulex, and Siemens Medical Imaging. He receives salary support on grant P01CA059827 from the National Cancer Institute.
Footnotes
Disclosures
He serves on an advisory board for Ionetix. He owns stock in General Electric and Cardinal Health and stock options in Ionetix. Analytic methods utilized were from INVIA in commercially available software (Corbett: Significant).
References
- 1.Pollack A, Nazif T, Mancini D, Weisz G. Detection and imaging of cardiac allograft vasculopathy. J ACC Cardiovascular imaging. 2013;6:613–23. doi: 10.1016/j.jcmg.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Chih S, Chong AY, Mielniczuk LM, Bhatt DL, Beanlands RS. Allograft Vasculopathy: The Achilles’ Heel of Heart Transplantation. Journal of the American College of Cardiology. 2016;68:80–91. doi: 10.1016/j.jacc.2016.04.033. [DOI] [PubMed] [Google Scholar]
- 3.Lund LH, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Goldfarb S, Levvey BJ, Meiser B, Rossano JW, Yusen RD, Stehlik J. The Registry of the International Society for Heart and Lung Transplantation: Thirty-second Official Adult Heart Transplantation Report—2015; Focus Theme: Early Graft Failure. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2015;34:1244–54. doi: 10.1016/j.healun.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Costanzo MR, Dipchand A, Starling R, Anderson A, Chan M, Desai S, Fedson S, Fisher P, Gonzales-Stawinski G, Martinelli L, McGiffin D, Smith J, Taylor D, Meiser B, Webber S, Baran D, Carboni M, Dengler T, Feldman D, Frigerio M, Kfoury A, Kim D, Kobashigawa J, Shullo M, Stehlik J, Teuteberg J, Uber P, Zuckermann A, Hunt S, Burch M, Bhat G, Canter C, Chinnock R, Crespo-Leiro M, Delgado R, Dobbels F, Grady K, Kao W, Lamour J, Parry G, Patel J, Pini D, Towbin J, Wolfel G, Delgado D, Eisen H, Goldberg L, Hosenpud J, Johnson M, Keogh A, Lewis C, O’Connell J, Rogers J, Ross H, Russell S, Vanhaecke J, International Society of H, Lung Transplantation G The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2010;29:914–56. doi: 10.1016/j.healun.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 5.Mc Ardle BA, Davies RA, Chen L, Small GR, Ruddy TD, Dwivedi G, Yam Y, Haddad H, Mielniczuk LM, Stadnick E, Hessian R, Guo A, Beanlands RS, deKemp RA, Chow BJ. Prognostic value of rubidium-82 positron emission tomography in patients after heart transplant. Circulation Cardiovascular imaging. 2014;7:930–7. doi: 10.1161/CIRCIMAGING.114.002184. [DOI] [PubMed] [Google Scholar]
- 6.Sharples LD, Jackson CH, Parameshwar J, Wallwork J, Large SR. Diagnostic accuracy of coronary angiography and risk factors for post-heart-transplant cardiac allograft vasculopathy. Transplantation. 2003;76:679–82. doi: 10.1097/01.TP.0000071200.37399.1D. [DOI] [PubMed] [Google Scholar]
- 7.Gao SZ, Alderman EL, Schroeder JS, Hunt SA, Wiederhold V, Stinson EB. Progressive coronary luminal narrowing after cardiac transplantation. Circulation. 1990;82:IV269–75. [PubMed] [Google Scholar]
- 8.St Goar FG, Pinto FJ, Alderman EL, Fitzgerald PJ, Stinson EB, Billingham ME, Popp RL. Detection of coronary atherosclerosis in young adult hearts using intravascular ultrasound. Circulation. 1992;86:756–63. doi: 10.1161/01.cir.86.3.756. [DOI] [PubMed] [Google Scholar]
- 9.St Goar FG, Pinto FJ, Alderman EL, Valantine HA, Schroeder JS, Gao SZ, Stinson EB, Popp RL. Intracoronary ultrasound in cardiac transplant recipients. In vivo evidence of “angiographically silent” intimal thickening. Circulation. 1992;85:979–87. doi: 10.1161/01.cir.85.3.979. [DOI] [PubMed] [Google Scholar]
- 10.Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, Dorbala S, Blankstein R, Rimoldi O, Camici PG, Di Carli MF. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129:2518–27. doi: 10.1161/CIRCULATIONAHA.113.008507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu YW, Chen YH, Wang SS, Jui HY, Yen RF, Tzen KY, Chen MF, Lee CM. PET assessment of myocardial perfusion reserve inversely correlates with intravascular ultrasound findings in angiographically normal cardiac transplant recipients. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2010;51:906–12. doi: 10.2967/jnumed.109.073833. [DOI] [PubMed] [Google Scholar]
- 12.El Fakhri G, Sitek A, Guerin B, Kijewski MF, Di Carli MF, Moore SC. Quantitative dynamic cardiac 82Rb PET using generalized factor and compartment analyses. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2005;46:1264–71. [PubMed] [Google Scholar]
- 13.Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, Blankstein R, Dorbala S, Sitek A, Pencina MJ, Di Carli MF. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011;124:2215–24. doi: 10.1161/CIRCULATIONAHA.111.050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS, American Heart Association Writing Group on Myocardial S, Registration for Cardiac I Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–42. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 15.El Fakhri G, Kardan A, Sitek A, Dorbala S, Abi-Hatem N, Lahoud Y, Fischman A, Coughlan M, Yasuda T, Di Carli MF. Reproducibility and accuracy of quantitative myocardial blood flow assessment with (82)Rb PET: comparison with (13)N-ammonia PET. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2009;50:1062–71. doi: 10.2967/jnumed.104.007831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehra MR, Crespo-Leiro MG, Dipchand A, Ensminger SM, Hiemann NE, Kobashigawa JA, Madsen J, Parameshwar J, Starling RC, Uber PA. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy-2010. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2010;29:717–27. doi: 10.1016/j.healun.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 17.Kapadia SR, Ziada KM, L’Allier PL, Crowe TD, Rincon G, Hobbs RE, Bott-Silverman C, Young JB, Nissen SE, Tuzcu EM. Intravascular ultrasound imaging after cardiac transplantation: advantage of multi-vessel imaging. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2000;19:167–72. doi: 10.1016/s1053-2498(99)00128-x. [DOI] [PubMed] [Google Scholar]
- 18.Vassalli G, Gallino A, Weis M, von Scheidt W, Kappenberger L, von Segesser LK, Goy JJ, Working Group Microcirculation of the Eurpean Society of C Alloimmunity and nonimmunologic risk factors in cardiac allograft vasculopathy. European heart journal. 2003;24:1180–8. doi: 10.1016/s0195-668x(03)00237-9. [DOI] [PubMed] [Google Scholar]
- 19.Clemmensen TS, Logstrup BB, Eiskjaer H, Poulsen SH. Coronary Flow Reserve Predicts Longitudinal Myocardial Deformation Capacity in Heart-Transplanted Patients. Echocardiography. 2016;33:562–71. doi: 10.1111/echo.13123. [DOI] [PubMed] [Google Scholar]
- 20.Hollenberg SM, Klein LW, Parrillo JE, Scherer M, Burns D, Tamburro P, Oberoi M, Johnson MR, Costanzo MR. Coronary endothelial dysfunction after heart transplantation predicts allograft vasculopathy and cardiac death. Circulation. 2001;104:3091–6. doi: 10.1161/hc5001.100796. [DOI] [PubMed] [Google Scholar]
- 21.Haddad F, Khazanie P, Deuse T, Weisshaar D, Zhou J, Nam CW, Vu TA, Gomari FA, Skhiri M, Simos A, Schnittger I, Vrotvec B, Hunt SA, Fearon WF. Clinical and functional correlates of early microvascular dysfunction after heart transplantation. Circulation Heart failure. 2012;5:759–68. doi: 10.1161/CIRCHEARTFAILURE.111.962787. [DOI] [PubMed] [Google Scholar]
- 22.Tona F, Osto E, Famoso G, Previato M, Fedrigo M, Vecchiati A, Perazzolo Marra M, Tellatin S, Bellu R, Tarantini G, Feltrin G, Angelini A, Thiene G, Gerosa G, Iliceto S. Coronary microvascular dysfunction correlates with the new onset of cardiac allograft vasculopathy in heart transplant patients with normal coronary angiography. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015;15:1400–6. doi: 10.1111/ajt.13108. [DOI] [PubMed] [Google Scholar]
- 23.Korosoglou G, Osman NF, Dengler TJ, Riedle N, Steen H, Lehrke S, Giannitsis E, Katus HA. Strain-encoded cardiac magnetic resonance for the evaluation of chronic allograft vasculopathy in transplant recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9:2587–96. doi: 10.1111/j.1600-6143.2009.02769.x. [DOI] [PubMed] [Google Scholar]
- 24.Muehling OM, Wilke NM, Panse P, Jerosch-Herold M, Wilson BV, Wilson RF, Miller LW. Reduced myocardial perfusion reserve and transmural perfusion gradient in heart transplant arteriopathy assessed by magnetic resonance imaging. Journal of the American College of Cardiology. 2003;42:1054–60. doi: 10.1016/s0735-1097(03)00924-0. [DOI] [PubMed] [Google Scholar]
- 25.Kofoed KF, Czernin J, Johnson J, Kobashigawa J, Phelps ME, Laks H, Schelbert HR. Effects of cardiac allograft vasculopathy on myocardial blood flow, vasodilatory capacity, and coronary vasomotion. Circulation. 1997;95:600–6. doi: 10.1161/01.cir.95.3.600. [DOI] [PubMed] [Google Scholar]
- 26.Allen-Auerbach M, Schoder H, Johnson J, Kofoed K, Einhorn K, Phelps ME, Kobashigawa J, Czernin J. Relationship between coronary function by positron emission tomography and temporal changes in morphology by intravascular ultrasound (IVUS) in transplant recipients. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 1999;18:211–9. doi: 10.1016/s1053-2498(98)00037-0. [DOI] [PubMed] [Google Scholar]
- 27.Mehra MR, Ventura HO, Stapleton DD, Smart FW, Collins TC, Ramee SR. Presence of severe intimal thickening by intravascular ultrasonography predicts cardiac events in cardiac allograft vasculopathy. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 1995;14:632–9. [PubMed] [Google Scholar]
- 28.Kobashigawa JA, Tobis JM, Starling RC, Tuzcu EM, Smith AL, Valantine HA, Yeung AC, Mehra MR, Anzai H, Oeser BT, Abeywickrama KH, Murphy J, Cretin N. Multicenter intravascular ultrasound validation study among heart transplant recipients: outcomes after five years. Journal of the American College of Cardiology. 2005;45:1532–7. doi: 10.1016/j.jacc.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 29.Rickenbacher PR, Pinto FJ, Lewis NP, Hunt SA, Alderman EL, Schroeder JS, Stinson EB, Brown BW, Valantine HA. Prognostic importance of intimal thickness as measured by intracoronary ultrasound after cardiac transplantation. Circulation. 1995;92:3445–52. doi: 10.1161/01.cir.92.12.3445. [DOI] [PubMed] [Google Scholar]
- 30.Potena L, Masetti M, Sabatino M, Bacchi-Reggiani ML, Pece V, Prestinenzi P, Dall’Ara G, Taglieri N, Saia F, Fallani F, Magnani G, Rapezzi C, Grigioni F. Interplay of coronary angiography and intravascular ultrasound in predicting long-term outcomes after heart transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2015;34:1146–53. doi: 10.1016/j.healun.2015.01.990. [DOI] [PubMed] [Google Scholar]
- 31.Tuzcu EM, Kapadia SR, Sachar R, Ziada KM, Crowe TD, Feng J, Magyar WA, Hobbs RE, Starling RC, Young JB, McCarthy P, Nissen SE. Intravascular ultrasound evidence of angiographically silent progression in coronary atherosclerosis predicts long-term morbidity and mortality after cardiac transplantation. Journal of the American College of Cardiology. 2005;45:1538–42. doi: 10.1016/j.jacc.2004.12.076. [DOI] [PubMed] [Google Scholar]
- 32.Raichlin E, Bae JH, Khalpey Z, Edwards BS, Kremers WK, Clavell AL, Rodeheffer RJ, Frantz RP, Rihal C, Lerman A, Kushwaha SS. Conversion to sirolimus as primary immunosuppression attenuates the progression of allograft vasculopathy after cardiac transplantation. Circulation. 2007;116:2726–33. doi: 10.1161/CIRCULATIONAHA.107.692996. [DOI] [PubMed] [Google Scholar]
- 33.Arora S, Ueland T, Wennerblom B, Sigurdadottir V, Eiskjaer H, Botker HE, Ekmehag B, Jansson K, Mortensen SA, Saunamaki K, Simonsen S, Gude E, Bendz B, Solbu D, Aukrust P, Gullestad L. Effect of everolimus introduction on cardiac allograft vasculopathy–results of a randomized, multicenter trial. Transplantation. 2011;92:235–43. doi: 10.1097/TP.0b013e31822057f1. [DOI] [PubMed] [Google Scholar]
- 34.Matsuo Y, Cassar A, Yoshino S, Flammer AJ, Li J, Gulati R, Topilsky Y, Raichlin E, Lennon RJ, Lerman LO, Rihal CS, Kushwaha SS, Lerman A. Attenuation of cardiac allograft vasculopathy by sirolimus: Relationship to time interval after heart transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2013;32:784–91. doi: 10.1016/j.healun.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masetti M, Potena L, Nardozza M, Prestinenzi P, Taglieri N, Saia F, Pece V, Magnani G, Fallani F, Coccolo F, Russo A, Rapezzi C, Grigioni F, Branzi A. Differential effect of everolimus on progression of early and late cardiac allograft vasculopathy in current clinical practice. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13:1217–26. doi: 10.1111/ajt.12208. [DOI] [PubMed] [Google Scholar]
- 36.Kobashigawa JA, Pauly DF, Starling RC, Eisen H, Ross H, Wang SS, Cantin B, Hill JA, Lopez P, Dong G, Nicholls SJ, Investigators AIS Cardiac allograft vasculopathy by intravascular ultrasound in heart transplant patients: substudy from the Everolimus versus mycophenolate mofetil randomized, multicenter trial. JACC Heart failure. 2013;1:389–99. doi: 10.1016/j.jchf.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 37.Keogh A, Richardson M, Ruygrok P, Spratt P, Galbraith A, O’Driscoll G, Macdonald P, Esmore D, Muller D, Faddy S. Sirolimus in de novo heart transplant recipients reduces acute rejection and prevents coronary artery disease at 2 years: a randomized clinical trial. Circulation. 2004;110:2694–700. doi: 10.1161/01.CIR.0000136812.90177.94. [DOI] [PubMed] [Google Scholar]
- 38.Arora S, Andreassen AK, Andersson B, Gustafsson F, Eiskjaer H, Botker HE, Radegran G, Gude E, Ioanes D, Solbu D, Sigurdardottir V, Dellgren G, Erikstad I, Solberg OG, Ueland T, Aukrust P, Gullestad L, Investigators S The Effect of Everolimus Initiation and Calcineurin Inhibitor Elimination on Cardiac Allograft Vasculopathy in De Novo Recipients: One-Year Results of a Scandinavian Randomized Trial. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015;15:1967–75. doi: 10.1111/ajt.13214. [DOI] [PubMed] [Google Scholar]
- 39.Eisen HJ, Tuzcu EM, Dorent R, Kobashigawa J, Mancini D, Valantine-von Kaeppler HA, Starling RC, Sorensen K, Hummel M, Lind JM, Abeywickrama KH, Bernhardt P, Group RBS Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. The New England journal of medicine. 2003;349:847–58. doi: 10.1056/NEJMoa022171. [DOI] [PubMed] [Google Scholar]
- 40.Mancini D, Pinney S, Burkhoff D, LaManca J, Itescu S, Burke E, Edwards N, Oz M, Marks AR. Use of rapamycin slows progression of cardiac transplantation vasculopathy. Circulation. 2003;108:48–53. doi: 10.1161/01.CIR.0000070421.38604.2B. [DOI] [PubMed] [Google Scholar]
- 41.Topilsky Y, Hasin T, Raichlin E, Boilson BA, Schirger JA, Pereira NL, Edwards BS, Clavell AL, Rodeheffer RJ, Frantz RP, Maltais S, Park SJ, Daly RC, Lerman A, Kushwaha SS. Sirolimus as primary immunosuppression attenuates allograft vasculopathy with improved late survival and decreased cardiac events after cardiac transplantation. Circulation. 2012;125:708–20. doi: 10.1161/CIRCULATIONAHA.111.040360. [DOI] [PubMed] [Google Scholar]
- 42.Sinha SS, Pham MX, Vagelos RH, Perlroth MG, Hunt SA, Lee DP, Valantine HA, Yeung AC, Fearon WF. Effect of rapamycin therapy on coronary artery physiology early after cardiac transplantation. American heart journal. 2008;155:889 e1–6. doi: 10.1016/j.ahj.2008.02.004. [DOI] [PubMed] [Google Scholar]


