Abstract
Background
Toxoplasmosis is a cosmopolitan infection caused by an intracellular obligatory protozoan, Toxoplasma gondii. Infection to this parasite in immunocompetent patients is usually asymptomatic, but today it is believed that the infection can be a risk factor for a variety of diseases, including rheumatoid arthritis (RA). RA is an autoimmune disease and the most common type of inflammatory arthritis that is a major cause of disability. The aim of this systematic review and meta-analysis was to address the association between RA and toxoplasmosis in light of the available research.
Methods
Based on the keywords, a systematic search of eight databases was conducted to retrieve the relevant English-language articles. Then, the studies were screened based on the inclusion and exclusion criteria. The random effect model was used to calculate the odds ratio (OR) using forest plot with 95% confidence interval (CI).
Results
Overall, 4168 Individual, extracted from 9 articles were included for systematic review evaluation, with 1369 RA patients (46% positive toxoplasmosis) and 2799 individuals as controls (21% positive toxoplasmosis). Then, eight articles (10 datasets) were used for meta-analysis (1244 rheumatoid arthritis patients and 2799 controls). By random effect model, the combined OR was 3.30 (95% CI: 2.05 to 5.30) with P < 0.0001.
Conclusion
Although toxoplasmosis could be considered as a potential risk factor for rheumatoid arthritis, more and better quality studies are needed to determine the effect of T. gondii infection on induction or exacerbation of RA. Our study was registered at the International Prospective Register of Systematic Reviews (PROSPERO; code: CRD42017069384).
Author summary
Toxoplasma gondii is an intracellular obligatory protozoan, which causes toxoplasmosis. T. gondii infection in immunocompetent individuals is mostly asymptomatic, but it may be reactivated as a result of immune disorders inducing serious complications. Rheumatoid arthritis (RA), as a complex autoimmune disease, is a major cause of significant and progressive disability, articular complications, and premature death. Studies confirmed an interaction between infections and environmental factors as the potential risk or protective factors determining the development of autoimmune diseases. In this study, we investigated the association between toxoplasmosis and RA.
Introduction
Toxoplasmosis is a parasitic disease with worldwide distribution caused by obligate intracellular coccidian protozoan Toxoplasma gondii (T. gondii) [1]. It is estimated that one-third of the world’s population are infected with this parasite in both developed and developing countries [2, 3]. Humans can be infected with the parasite through different routes, including consumption of raw or undercooked meat containing tissue cysts of the parasite, ingestion of sporulated oocysts from contaminated water and food, and vertical transmission during pregnancy through the placenta to the fetus [4].
T. gondii remains in the infected host tissues perpetually [5]. Most immunocompetent individuals, if infected with this parasite, are asymptomatic or show minor symptoms [6]. The most common symptom of toxoplasmosis in humans is lymphadenopathy that may be associated with fever, sore throat, muscle pain, fatigue, and headache [4]. In congenitally infected and immunocompromised patients, this disease is more likely to bring about severe complications [7]. Myocarditis and polymyositis have been reported in immunocompetent individuals with acute toxoplasmosis [8]. Furthermore, toxoplasmosis may cause polyarthritis in the hand and knee joints [9]. Polytenosynovitis (inflammation of a tendon sheath) caused by T. gondii has also been reported [10].
Rheumatoid arthritis (RA) is a common autoimmune disease, which is a major cause of inflammation of the joints and the principal cause of disability that affects 0.5–1% of the population [11, 12]. The disease presents with swollen joints, production of autoantibodies (rheumatoid factor), and systemic effects [13].
In recent years, the role of infectious agents, especially bacteria and viruses, has been identified in the pathogenesis of autoimmune diseases, while the role of parasitic infections due to their vague effects on host immunity has not been well-investigated. Experimental evidence may support the protective effect of specific parasitic infections in the susceptibility to autoimmunity [14]. Some geoepidemiological studies showed that host genetic susceptibility interacts with lifestyle and environmental factors, such as socioeconomic status, dietary habits, environmental pollutants, and ultraviolet radiation exposure; further, infections increase the risk of developing autoimmunity [15]. On the other hand, infectious diseases may contribute to the development of autoimmune diseases through molecular mimicry and epitope spreading [16]. Therefore, the aim of this systematic review and meta-analysis was to provide an updated review of data about the relationship between toxoplasmosis and RA.
Methods
Search strategy
This study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) and its checklist [17]. Individuals with RA, along with a control group, were surveyed. To begin, we searched scientific databases for all the articles on the association between toxoplasmosis and RA published up to the first of January 2018. These keywords were used alone or in combination: “Toxoplasma gondii”, “toxoplasmosis”, “seroprevalence”, “prevalence”, “rheumatoid arthritis”, “rheumatoid factor”, “meta-analysis”, and “systematic review”.
A literature review was carried out using English databases including “PubMed”, “Google Scholar”, “Science Direct”, “Scopus”, “Web of Science”, “EMBASE”, “CINAHL”, and “ProQuest”. The systematic search of articles was conducted from March 4 to December 31, 2017 by two researchers independently. Also, for completing the checklist, we investigated all the references lists of the selected articles manually. In this study, only English-language articles were analyzed; furthermore, unpublished studies were not evaluated.
Study selection
After completing the search, the selected articles were reviewed by the two researchers independently. All the duplicate and irrelevant studies were excluded after reviewing the title, abstract, and full text of the articles. Moreover, to prevent reprint bias, the results of the articles were carefully investigated and duplicates were omitted.
Quality assessment
In order to assess the quality of reporting of the studies, standard Strengthening the Reporting of Observational Studies in Epidemiology checklist (STROBE) was used [18]. S1 Checklist represents the quality score of different eligible studies. This checklist included items assessing the study methodology, study type, study population, sample size, sample collection methods, statistical tests, and presentations. In our study, articles were evaluated based on STROBE assessment (low quality: less than 16.5, moderate quality: 16.6–25.5, and high quality: 25.6–34). The articles we entered in our meta-analysis had acceptable quality.
Inclusion and exclusion criteria
Abstracts and full texts were assessed independently by the two researchers using a piloted form. The final decisions about the eligibility or exclusion of studies were made separately. Disagreements were resolved with provision for arbitration from a third reviewer. Following the removal of duplicate entries, articles were evaluated according to the following criteria: (1) cohort or case-control studies about the relationship between toxoplasmosis as an exposure and rheumatoid arthritis as a disease, (2) the studies conducted only on humans, (3) the presence of case and control groups, (4) the studies where toxoplasmosis was diagnosed by detecting IgG and/or IgM antibodies against T. gondii in individuals with definitive diagnosis of RA, and (5) the studies providing details on the seroprevalence rate of toxoplasmosis and RA.
The exclusion criteria comprised: (1) studies that were only descriptive, (2) studies that only presented the final result and did not provide the raw data, (3) articles that were not available in English language, and (4) the studies conducted on animals.
Data extraction
Articles were carefully studied and the following data were extracted: first author, year of publication, the number of patients and controls, the number and percentage of the positive and negative cases of serum IgG and IgM in patients and controls, as well as information about age and gender and laboratory results. In studies where two different populations were studied, data were extracted separately.
Statistical analysis
The meta-analysis was executed with the Stats Direct statistical software (http://statsdirect.com). For displaying the heterogeneity between studies, χ2-based Cochrane test (Q) and I2 index were applied [19]. Due to significant heterogeneity between the studies, a random effect model was used to combine the results of the studies. Forest plot was used to indicate the prevalence of toxoplasmosis in each study and to determine pooled estimate prevalence in the studies. Odds ratios (ORs) and 95% confidence intervals (CI) were used for estimating the risk of T. gondii infection (the significance of P<0.05). OR > 1 indicates the positive effect of Toxoplasma on RA and an OR < 1 shows that toxoplasmosis has a protective effect against RA. Publication bias was examined by funnel plots and the statistical significance was assessed by the Egger test [20]. Also, it was performed a sensitivity analysis to identify probably effect of each article on the overall results by excluding them using Stata version 14 (Stata Corp, College Station, TX, USA).
The study protocol (CRD42017069384) was registered on the website of the International Prospective Register of Systematic Reviews (PROSPERO) [21].
Results
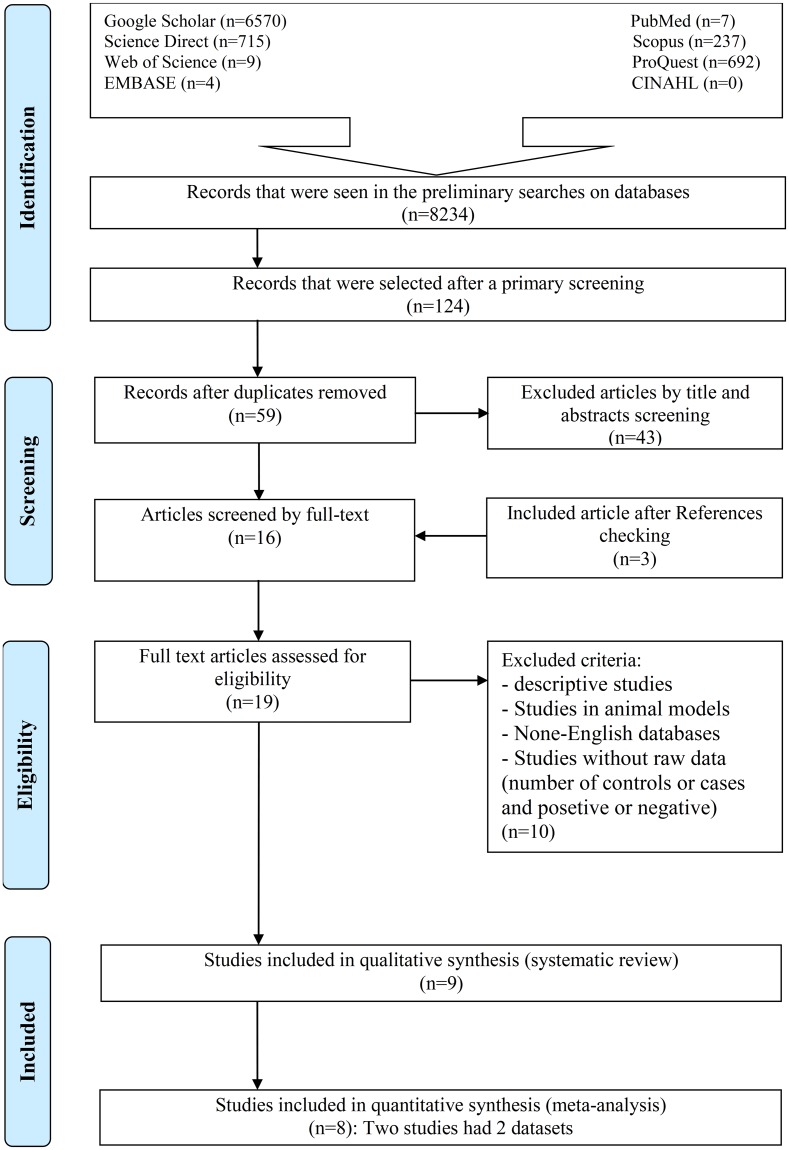
Our preliminary search of eight databases yielded 8234 papers. After a primary screening of the titles of the articles based on keywords, 124 studies were extracted. Sixty-five articles were also excluded from the study due to duplication. In the next step, by screening the abstracts of the articles and based on the inclusion/exclusion criteria, 43 other articles were excluded. After reading the full text of the articles, 10 other papers were omitted, and three studies were added to the collection after reviewing the references. After the final review of the articles, nine eligible studies [14, 16, 22–28] were identified for systematic review. Another study was excluded due to the absence of a healthy control group [16]. Finally, eight of these nine articles [14, 22–28] were entered into the meta-analysis with respect to the inclusion/exclusion criteria (Fig 1). The studied articles were published between 2007 and 2017. We identified 11 datasets from the nine articles that met the inclusion criteria, eight of which were case-control, two cross-sectional, and one were cohort studies (Table 1). The surveys were conducted in Latin America [14], Europe [14, 16], Egypt [24, 25], Iraq [22, 23, 27], Czech and Slovak [26], and China [28].
Fig 1. Flow diagram of the study design process.
Table 1. Baseline characteristics of the included studies in the systematic review and meta-analysis of the relationship between T. gondii infection and RA patients.
| No | First author | Publication year | Place of study | Type of study | Method | Test | Results | Age | Sex |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Shapira Y [14] | 2012 | Europe | Case control | BioPlex 2200 system | IgG IgM | Significant | ---- | ---- |
| 2 | Shapira Y [14] | 2012 | Latin America | Case control | BioPlex 2200 system | IgG IgM | Not significant | ---- | ---- |
| 3 | Fischer S [16] | 2013 | Europe | Cross sectional | Chemi luminescence | IgG | Significant | P: 40–74 C:-- | ---- |
| 4 | Kuba RH [27] | 2014 | Iraq (Treated patients) | Case control | ELISA | IgG IgM | Significant | P: 20–80 C:-- | ---- |
| 5 | Kuba RH [27] | 2014 | Iraq (Untreated patients) | Case control | ELISA | IgG IgM | Significant | 20–80 C:-- | ---- |
| 6 | Al kalaby RF [22] | 2016 | Iraq | Case control | ELISA | IgG IgM | Significant | P: 16–50 C: 16–50 | F |
| 7 | El-Sayed NM [25] | 2016 | Egypt | Case control | EIA | IgG IgM | Significant | P: 30–58 C: 29–57 | P: (F:70, M:30) C: (F:34, M:16) |
| 8 | Flegr J [26] | 2016 | Czech and Slovak | cohort | ELISA CFT | IgG IgM | Significant | ---- | P: (F:10, M:3) C: (F:935, M:372) |
| 9 | El- Henawy AA [24] | 2017 | Egypt | Cross sectional | ELISA | IgG IgM | Significant | P: <60 C: <60 | P: (F:29, M:31) C: (F:28, M:32) |
| 10 | Tian A-L [28] | 2017 | China | Case control | ELISA | IgG IgM | Significant | ---- | P: (—) C: (F:454, M:366) |
| 11 | Al- Oqaily MA [23] | 2017 | Iraq | Case control | ELISA | IgG IgM | Significant | P: 13–68 C: 13–68 | ---- |
Age is in years, ELISA: enzyme-linked immunosorbent assay, EIA: enzyme immunoassay, CFT: complement fixation test, IgG: Immunoglobulin G, IgM: Immunoglobulin M, P: Patient, C: Control, F: Female, M: Male
Our meta-analysis was performed among 4168 people including 1369 RA patients and 2799 controls. In all the studies, blood samples were collected from patients and controls. To identify anti-Toxoplasma antibodies (IgG and IgM) in those studies, ELISA [22–24, 26–28], CFT [26], EIA [25], chemiluminescence [16], and BioPlex 2200 system [14] were used (Table 1).
Except for Fischer et al. [16], who only evaluated IgG, other authors surveyed both IgG and IgM antibodies. However, only three studies had reported a titer of antibodies [23–25], and others had described the percentage of positive antibodies in patients and controls. Finally, all the studies analyzed the relationship between toxoplasmosis and RA with respect to the percentage of seropositive and seronegative individuals (patients and controls).
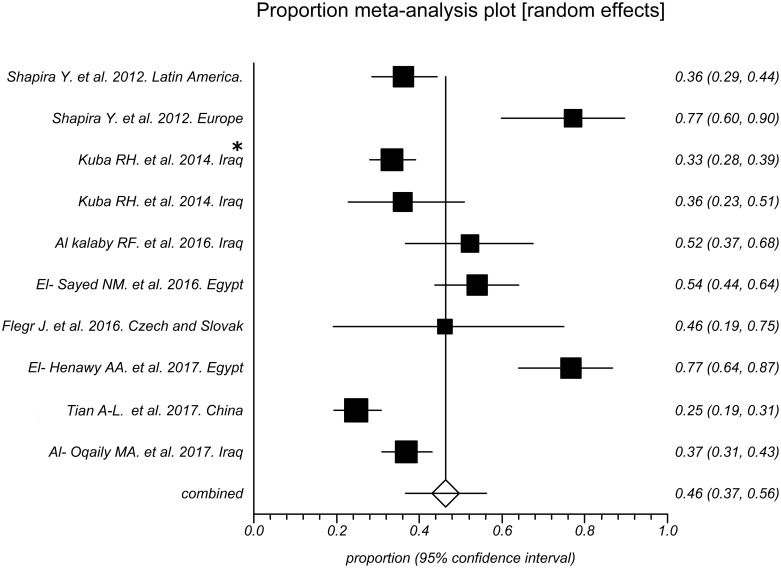
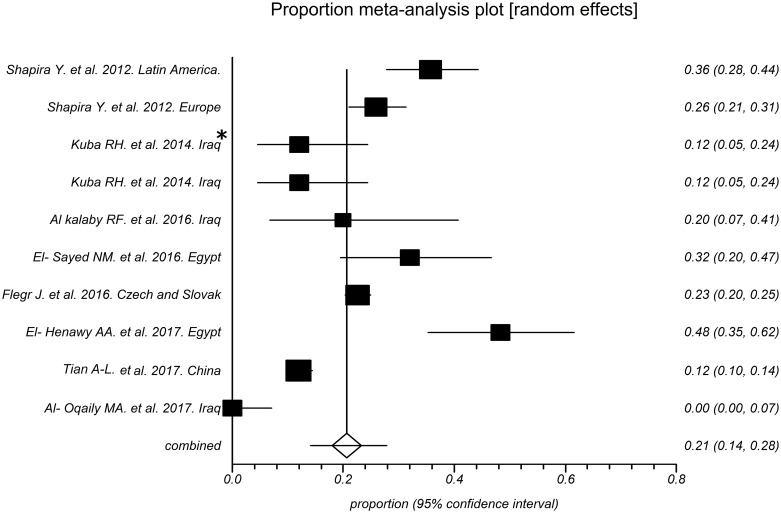
As shown in Fig 2, the prevalence of toxoplasmosis in RA patients in these studies varied from 25% to 77% with an overall seroprevalence of 46% (95% CI [37; 56]). However, the total prevalence of this disease in the control subjects entered in these studies was 21% (95% CI [14; 28]), which varied from 0% to 48% in various studies (Fig 3).
Fig 2. Forest plot of seroprevalence rates of toxoplasmosis in rheumatoid arthritis patients.
* Patients under treatment.
Fig 3. Forest plot of seroprevalence rates of toxoplasmosis in controls groups.
* Patients under treatment.
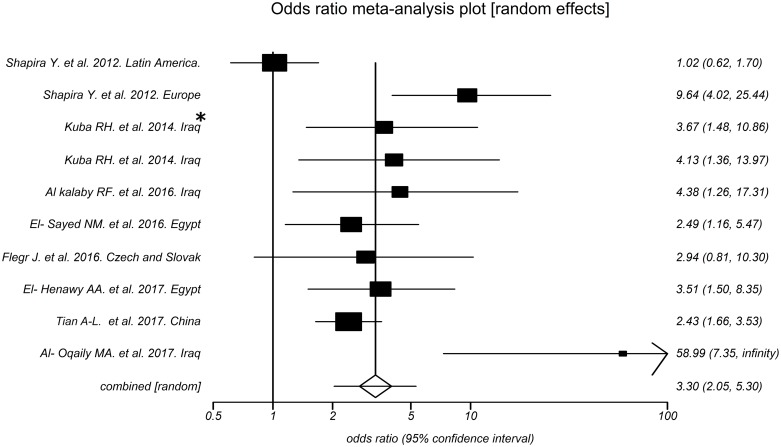
According to Fig 4, the odds of toxoplasmosis in RA patients are 3.30 times compared to that of controls with 95% CI: 2.05 to 5.30 and P < 0.0001. Nonetheless, the heterogeneity analysis of the effect size of arthritis (Q = 32.77, P = 0.0001, I2 = 72.5%) showed a relatively high heterogeneity in our meta-analysis.
Fig 4. Forest plot of odds ratios for correlation between toxoplasmosis and rheumatoid arthritis.
* Patients under treatment.
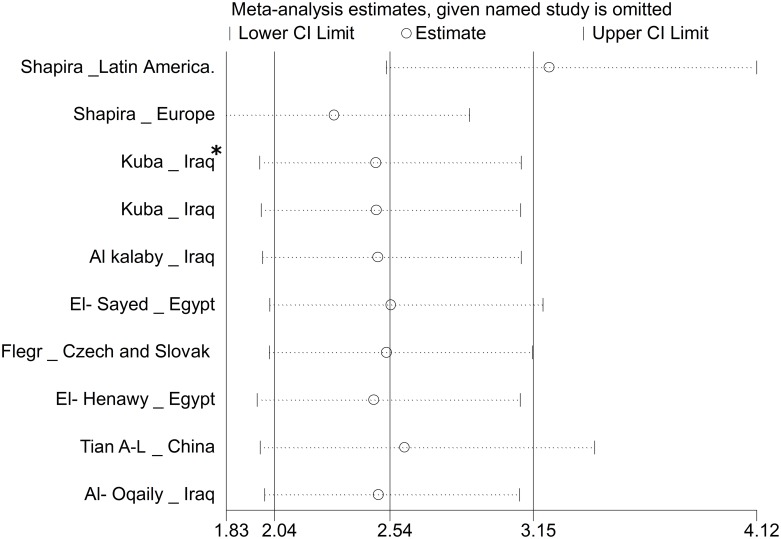
Begg and Egger tests were used to evaluate publication bias. Negligible publication bias was observed using both Begg test (P = 0.0286) and Egger test (P = 0.0446) in the included studies. The results of sensitivity analysis showed that the impact of each study on meta-analysis was not significant on overall estimates (Fig 5).
Fig 5. Sensitivity analysis for assessing the effect of each primary study on the total estimates.
* Patients under treatment.
Discussion
Toxoplasma gondii is an important opportunistic parasite infecting one-third of the world’s human population and it is considered a silent threat [29]. Though a clear relationship between toxoplasmosis and autoimmune diseases, including RA, has not yet been well documented, a higher prevalence of anti-T. gondii antibodies was reported in patients with rheumatoid arthritis [16]. Thus, we designed this systematic review and meta-analysis to explore the possible association between Toxoplasma infection and RA, an autoimmune disease causing pain and disability [30].
Although few studies were included in our meta-analysis, our findings showed that the prevalence of toxoplasmosis in the control group was 21%, which is almost in agreement with the results obtained by Dubey and Beattie [31]. This seroprevalence was significantly different from the prevalence in RA patients (46%).
According to Table 2, the lowest OR was reported in Latin America and the highest OR in Europe. The difference in ORs can be attributed to the significant difference in host response and virulence of parasitic strains [32]. In addition, we found high heterogeneity in the relationship between RA and T. gondii infection in this systematic review. The high heterogeneity index is suggestive of potential variation, which could be due to difference in genetic potential of humans, which is affected by lifestyle and environmental factors such as dietary habits, environmental pollution, exposure to ultraviolet radiation, various types of infections, and socioeconomic status [33].
Table 2. Data extracted from the included studies in the meta-analysis for an association between toxoplasmosis and RA.
| No | Reference | N | Case: RA+ (n) | Control: RA- (n) | RA+ & T+ (n, %) | RA- & T+ (n, %) | OR (95% CI) | P-value |
|---|---|---|---|---|---|---|---|---|
| 1 | Shapira Y [14] (Latin America) | 292 | 152 | 140 | 55 (36.18%) | 50 (35.71%) | 1.02 (0.62–1.70) | NS |
| 2 | Shapira Y [14] (Europe) | 332 | 35 | 297 | 27 (77.14%) | 77 (25.93%) | 9.64 (4.02–25.44) | < 0.0001 |
| 3 | Kuba RH [27] (Treated) | 344 | 294 | 50 | 98 (33.33%) | 6 (12%) | 3.67 (1.48–10.86) | < 0.05 |
| 4 | Kuba RH [27] (Untreated) | 100 | 50 | 50 | 18 (36%) | 6 (12%) | 4.13 (1.36–13.97) | < 0.05 |
| 5 | Al kalaby RF [22] | 69 | 44 | 25 | 23 (52.27%) | 5 (20%) | 4.38 (1.26–17.31) | 0.01 |
| 6 | El-Sayed NM [25] | 150 | 100 | 50 | 54 (54%) | 16 (32%) | 2.49 (1.16–5.47) | S |
| 7 | Flegr J [26] | 1320 | 301 | 1019 | 6 (46.15%) | 295 (22.57%) | 2.94 (0.81–10.30) | 0.012 |
| 8 | El- Henawy AA [24] | 120 | 60 | 60 | 46 (76.67%) | 29 (48.3%) | 3.51 (1.50–8.35) | < 0.001 |
| 9 | Tian A-L [28] | 1058 | 157 | 901 | 59 (24.79%) | 98 (11.59%) | 2.43 (1.66–3.53) | < 0.001 |
| 10 | Al- Oqaily MA [23] | 308 | 258 | 50 | 95 (36.82) | 0 (0%) | 58.99 (7.35-infinity) | < 0.0001 |
N and n: Number, CI: Confidence interval; RA+: People with rheumatoid arthritis; RA-: People without rheumatoid arthritis; RA+ & T+: People with rheumatoid arthritis and Toxoplasma positive; RA- & T+: People without rheumatoid arthritis and Toxoplasma positive; OR: Odds ratio; NS: Not significant; S: Significant
Our findings suggest that T. gondii may trigger a pathologic process in individuals, which can ultimately lead to RA. This finding has been reported in other autoimmune diseases such as diabetes mellitus [34, 35], lupus erythematosus [36], and autoimmune thyroid diseases [37]. The higher prevalence of T. gondii in people with chronic diseases can be explained by the following reasons: 1) toxoplasmosis can contribute to the progression of chronic diseases and 2) treatment of these diseases with immunosuppressive drugs increases the susceptibility of patients to infections, including toxoplasmosis [6]. Recent treatments for RA patients with anti-tumor necrosis factor-α (TNF-α), which leads to brain toxoplasmosis, are indicative of this issue [27, 38]. On the other hand, some toll-like receptors (TLRs) have been identified in mammals, for which some pathogens act as ligands, and as a result of binding between the TLRs and pathogens different types of immune responses can be induced. Based on reference, T. gondii may be used as ligands for TLRs, which can induce inflammatory response [39].
Also, studies show that T. gondii increases the expression of interleukin 17 (IL-17) in patients [40], and since this cytokine is involved in the pathogenesis of many autoimmune diseases, including RA [41], a significant relationship between toxoplasmosis and RA can be explained.
RA patients have autoantibodies and rheumatoid factors in their blood [42]. In two studies, these disease activity markers were found to have a significant relationship with toxoplasmosis, especially in high titers [24, 25]. This indicates that T. gondii can induce or exacerbate arthritis symptoms [43–45].
Because in the studied articles the relationship of age and sex with the prevalence of toxoplasmosis was not evaluated, we avoided the meta-analysis of these risk factors. In addition, diversity in the quality of studies and methods of measuring antibodies limited the interpretation and analysis of these items. These two issues were the important limitations of our meta-analysis.
Despite the significant relationship found between T. gondii infection and RA in this systematic review and meta-analysis study, further studies are needed on the following grounds: 1) the limited sample sizes in the articles, 2) difference in the quality of the reports, 3) diverse methods of measuring anti-parasitic antibodies, and 4) lack of evaluation of various risk factors such as age and gender.
Conclusions
One of the most important achievements of our study is that although T. gondii infection affects about one-third of the world’s population and possibly causes and exacerbates the symptoms of RA, only few studies have addressed this subject. These studies were conducted only in Latin America, Europe, and few regions of Asia and Africa. Accordingly, further studies are needed to achieve accurate results from other parts of the world. Also, further studies will be necessary to clarify the pathogenesis of T. gondii in humans to understand whether T. gondii is a cofactor in the development of autoimmune diseases.
Supporting information
(DOCX)
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Robert-Gangneux F, Dardé M-L. Epidemiology of and diagnostic strategies for toxoplasmosis. Clinical microbiology reviews. 2012;25(2):264–96. doi: 10.1128/CMR.05013-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mizani A, Alipour A, Sharif M, Sarvi S, Amouei A, Shokri A, et al. Toxoplasmosis seroprevalence in Iranian women and risk factors of the disease: a systematic review and meta-analysis. Tropical medicine and health. 2017;45(1):7 doi: 10.1186/s41182-017-0048-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montoya JG, Liesenfeld O. Toxoplasmosis. The Lancet. 363(9425):1965–76. doi: 10.1016/S0140-6736(04)16412-x [DOI] [PubMed] [Google Scholar]
- 4.Hill D, Dubey J. Toxoplasma gondii: transmission, diagnosis and prevention. Clinical microbiology and infection. 2002;8(10):634–40. [DOI] [PubMed] [Google Scholar]
- 5.Wilking H, Thamm M, Stark K, Aebischer T, Seeber F. Prevalence, incidence estimations, and risk factors of Toxoplasma gondii infection in Germany: a representative, cross-sectional, serological study. Scientific reports. 2016;6:22551 doi: 10.1038/srep22551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flegr J, Prandota J, Sovičková M, Israili ZH. Toxoplasmosis–a global threat. Correlation of latent toxoplasmosis with specific disease burden in a set of 88 countries. PloS one. 2014;9(3):e90203 doi: 10.1371/journal.pone.0090203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daryani A, Sharif M, Dadimoghaddam Y, Souteh MBH, Ahmadpour E, Khalilian A, et al. Determination of parasitic load in different tissues of murine toxoplasmosis after immunization by excretory–secretory antigens using Real time QPCR. Experimental parasitology. 2014;143:55–9. doi: 10.1016/j.exppara.2014.05.008 [DOI] [PubMed] [Google Scholar]
- 8.Montoya JG, Jordan R, Lingamneni S, Berry GJ, Remington JS. Toxoplasmic myocarditis and polymyositis in patients with acute acquired toxoplasmosis diagnosed during life. Clinical infectious diseases. 1997;24(4):676–83. [DOI] [PubMed] [Google Scholar]
- 9.Balleari E, Cutolo M, Accardo S. Adult-onset Still’s disease associated to toxoplasma gondii infection. Clinical rheumatology. 1991;10(3):326–7. [DOI] [PubMed] [Google Scholar]
- 10.Vass M, Kullmann L, Csoka R, Magyar E. Polytenosynovitis caused by Toxoplasma gondii. Bone & Joint Journal. 1977;59(2):229–32. [DOI] [PubMed] [Google Scholar]
- 11.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356–61. doi: 10.1038/nature01661 [DOI] [PubMed] [Google Scholar]
- 12.Prasad SK, Vassiliou VS. Rheumatoid Arthritis: Mapping the Future. JACC: Cardiovascular Imaging. 2015;8(5):537–9. doi: 10.1016/j.jcmg.2014.12.024 [DOI] [PubMed] [Google Scholar]
- 13.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. New England Journal of Medicine. 2011;365(23):2205–19. doi: 10.1056/NEJMra1004965 [DOI] [PubMed] [Google Scholar]
- 14.Shapira Y, Agmon-Levin N, Selmi C, Petríková J, Barzilai O, Ram M, et al. Prevalence of anti-toxoplasma antibodies in patients with autoimmune diseases. Journal of autoimmunity. 2012;39(1–2):112–6. doi: 10.1016/j.jaut.2012.01.001 [DOI] [PubMed] [Google Scholar]
- 15.Shapira Y, Agmon-Levin N, Shoenfeld Y. Defining and analyzing geoepidemiology andhuman autoimmunity. Journal of autoimmunity. 2010;34(3):J168–J77. doi: 10.1016/j.jaut.2009.11.018 [DOI] [PubMed] [Google Scholar]
- 16.Fischer S, Agmon-Levin N, Shapira Y, Katz B-SP, Graell E, Cervera R, et al. Toxoplasma gondii: bystander or cofactor in rheumatoid arthritis. Immunologic research. 2013;56(2–3):287–92. doi: 10.1007/s12026-013-8402-2 [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine. 2009;6(7):e1000097 doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS medicine. 2007;4(10):e296 doi: 10.1371/journal.pmed.0040296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins J, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in medicine. 2002;21(11):1539–58. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 20.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daryani A, Hosseininejad Z, Amouei A. Relationship between toxoplasmosis and rheumatoid arthritis: A systematic review and meta-analysis: PROSPERO; 2017 [cited 2017].
- 22.Al kalaby RF, Sultan B, AL-Fatlawi S, AbdulKadhim H, RF O. Relationship between Toxoplasma gondii and Autoimmune Disease in Aborted Women in Najaf Province. Karbala J Med. 2016;9(1):6. [Google Scholar]
- 23.Al-Oqaily MA, kasim Al-Ubaidi I. Prevavalence of toxoplasmosis of in Iraqi rhumatoid arthritis patients and detection levels of and TGF-β chemokines during infection. I.J.S.N. 2017;8 (4):824–829. [Google Scholar]
- 24.El-Henawy AA, Hafez EAR, Nabih N, Shalaby NM, Mashaly M. Anti-Toxoplasma antibodies in Egyptian rheumatoid arthritis patients. Rheumatology international. 2017;37(5):785–90. doi: 10.1007/s00296-017-3703-8 [DOI] [PubMed] [Google Scholar]
- 25.El-Sayed NM, Kishik SM, Fawzy RM. The current status of Toxoplasma gondii infection among Egyptian rheumatoid arthritis patients. Asian Pacific Journal of Tropical Disease. 2016;6(10):797–801. doi: 10.1016/S2222-1808(16)61133-7 [Google Scholar]
- 26.Flegr J, Escudero DQ. Impaired health status and increased incidence of diseases in Toxoplasma-seropositive subjects—an explorative cross-sectional study. Parasitology. 2016;143(14):1974–89. doi: 10.1017/S0031182016001785 [DOI] [PubMed] [Google Scholar]
- 27.Kuba RH, Zghair KH, Alosami MH. Detection of Toxoplasma antibodies and TNF-α in rheumatoid arthritis patients treated with methotrexate. Iraqi J Sci. 2014;55:1535–40. [Google Scholar]
- 28.Tian A-L, Gu Y-L, Zhou N, Cong W, Li G-X, Elsheikha HM, et al. Seroprevalence of Toxoplasma gondii infection in arthritis patients in eastern China. Infectious diseases of poverty. 2017;6(1):153 doi: 10.1186/s40249-017-0367-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nissapatorn V. Toxoplasmosis: A silent threat in Southeast Asia. Res J Parasitol. 2007;2(1):1–12. doi: 10.3923/jp.2007.1.12 [Google Scholar]
- 30.Gibofsky A. Overview of epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis. The American journal of managed care. 2012;18(13 Suppl):S295–302. [PubMed] [Google Scholar]
- 31.Dubey JP, Beattie C. Toxoplasmosis of animals and man: CRC Press, Inc.; 1988. [Google Scholar]
- 32.Dardé M-L. Toxoplasma gondii, “new” genotypes and virulence. Parasite. 2008;15(3): 366–71. doi: 10.1051/parasite/2008153366 [DOI] [PubMed] [Google Scholar]
- 33.Shapira Y, Agmon-Levin N, Shoenfeld Y. Geoepidemiology of autoimmune rheumatic diseases. Nature Reviews Rheumatology. 2010;6(8): 468–76. doi: 10.1038/nrrheum.2010.86 [DOI] [PubMed] [Google Scholar]
- 34.Goekce C, Yazar S, Bayram F, GÜNDOĞAN K. Toxoplasma gondii antibodies in type 1 diabetes mellitus. Turkiye Klinikleri Journal of Medical Sciences. 2008;28(5):619–22. [Google Scholar]
- 35.Majidiani H, Dalvand S, Daryani A, Galvan-Ramirez MdlL, Foroutan-Rad M. Is chronic toxoplasmosis a risk factor for diabetes mellitus? A systematic review and meta-analysis of case-control studies. Brazilian Journal of Infectious Diseases. 2016;20(6):605–9. doi: 10.1016/j.bjid.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilcox M, Powell R, Pugh S, Balfour A. Toxoplasmosis and systemic lupus erythematosus. Annals of the rheumatic diseases. 1990;49(4):254–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaňková Š, Prochazkova L, Flegr J, Calda P, Springer D, Potlukova E. Effects of latent toxoplasmosis on autoimmune thyroid diseases in pregnancy. PloS one. 2014;9(10):e110878 doi: 10.1371/journal.pone.0110878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cren J, Bouvard B, Crochette N. Cerebral toxoplasmosis and anti-TNFα: a case report. IDCases. 2016;5:40–2. doi: 10.1016/j.idcr.2016.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ali T, Kaitha S, Mahmood S, Ftesi A, Stone J, Bronze MS. Clinical use of anti-TNF therapy and increased risk of infections. Drug, healthcare and patient safety. 2013;5:79 doi: 10.2147/DHPS.S28801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guiton R, Vasseur V, Charron S, Torres Arias M, Van Langendonck N, Buzoni-Gatel D, et al. Interleukin 17 receptor signaling is deleterious during Toxoplasma gondii infection in susceptible BL6 mice. The Journal of infectious diseases. 2010;202(3):427–35. doi: 10.1086/653738 [DOI] [PubMed] [Google Scholar]
- 41.Gaffen SL. The role of interleukin-17 in the pathogenesis of rheumatoid arthritis. Current rheumatology reports. 2009;11(5):365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nielen MM, van Schaardenburg D, Reesink HW, Van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MH, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis & Rheumatology. 2004;50(2):380–6. doi: 10.1002/art.20018 [DOI] [PubMed] [Google Scholar]
- 43.Abbasi J. To prevent rheumatoid arthritis, look past the joints to the gums. Jama. 2017;317(12):1201–2. doi: 10.1001/jama.2017.0764 [DOI] [PubMed] [Google Scholar]
- 44.Li S, Yu Y, Yue Y, Zhang Z, Su K. Microbial infection and rheumatoid arthritis. Journal of clinical & cellular immunology. 2013;4(6):174 doi: 10.4172/2155-9899.1000174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bennett JC. The infectious etiology of rheumatoid arthritis. Arthritis & Rheumatology. 1978;21(5):531–8. doi: 10.1002/art.1780210507 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.