Abstract
OBJECTIVES
This study sought to describe the health status of outpatients with heart failure and reduced ejection fraction (HFrEF) by sex, race/ethnicity, and socioeconomic status (SES).
BACKGROUND
Although a primary goal in treating patients with HFrEF is to optimize health status, whether disparities by sex, race/ethnicity, and SES exist is unknown.
METHODS
In the CHAMP-HF (Change the Management of Patients with Heart Failure) registry, the associations among sex, race, and SES and health status, as measured by the Kansas City Cardiomyopathy Questionnaire-overall summary (KCCQ-os) score (range 0 to 100; higher scores indicate better health status) was compared among 3,494 patients from 140 U.S. clinics. SES was categorized by total household income. Hierarchical multivariate linear regression estimated differences in KCCQ-os score after adjusting for 31 patient characteristics and 10 medications.
RESULTS
Overall mean KCCQ-os scores were 64.2 ± 24.0 but lower for women (29% of sample; 60.3 ± 24.0 vs. 65.9 ± 24.0, respectively; p < 0.001), for blacks (60.5 ± 25.0 vs. 64.9 ± 23.0, respectively; p < 0.001), for Hispanics (59.1 ± 21.0 vs. 64.9 ± 23.0, respectively; p < 0.001), and for those with the lowest income (<$25,000; mean: 57.1 vs. 63.1 to 74.7 for other income categories; p < 0.001). Fully adjusted KCCQ-os scores were 2.2 points lower for women (95% confidence interval [CI]: −3.8 to −0.6; p = 0.007), no different for blacks (p = 0.74), 4.0 points lower for Hispanics (95% CI: −6.6 to −1.3; p = 0.003), and lowest in the poorest patients (4.7 points lower than those with the highest income (95% CI: 0.1 to 9.2; p = 0.045; p for trend = 0.003).
CONCLUSIONS
Among outpatients with HFrEF, women, blacks, Hispanics, and poorer patients had worse health status, which remained significant for women, Hispanics, and poorer patients in fully adjusted analyses. This suggests an opportunity to further optimize treatment to reduce these observed disparities.
Keywords: health disparities, heart failure, quality of life
Aprimary goal of U.S. health care, as articulated by the Department of Health and Human Services’ Healthy People 2020 initiative, is to eradicate disparities in health status by sex, race/ethnicity, and socioeconomic status (SES) (1). Prior studies have demonstrated worse outcomes, principally mortality and hospitalization rates, in women, blacks, Hispanics, and patients with lower SES in the setting of heart failure (2,3). However, a primary treatment goal in heart failure is to optimize patients’ health status, including their symptoms, function, and quality of life. To date, no studies have described the health status of patients with heart failure and reduced ejection fraction (HFrEF) in routine clinical care. Given the many potential interventions available to improve the health status of patients with HFrEF, identifying differences by sex, race/ethnicity, or SES can highlight new opportunities to further reduce these disparities in care.
To address this gap in knowledge, we compared the health status of patients with HFrEF by sex, race/ethnicity, and SES in the CHAMP-HF (Change the Management of Patients with Heart Failure) registry. CHAMP-HF is a large, prospective, multicenter, observational study of outpatients with HFrEF that captured patients’ health status by using the short form of the Kansas City Cardiomyopathy Questionnaire-12 (KCCQ-12), a well-validated measurement of patients’ symptoms, function, and quality of life (4). Moreover, as payers increasingly turn to patient-reported outcome measures, such as the KCCQ-12 instrument, to quantify health care quality, identifying populations of patients with worse health status can form the foundation with which to evaluate whether the use of such performance measures can successfully reduce health status disparities.
METHODS
STUDY DESIGN
CHAMP-HF, as previously described, is a multicenter, observational registry developed with the primary objective of capturing the outcomes and real-world treatment patterns of patients with HFrEF in the United States (5). Briefly, patients with chronic HFrEF (left ventricular ejection fraction [LVEF] ≤40%) being treated with at least 1 guideline-recommended pharmacotherapy were consecutively recruited from outpatient heart failure clinics. Subjects were excluded if they were enrolled in a hospice program or estimated to have a life expectancy of <1 year or had a history of heart transplantation, left ventricular assist device implantation, or end-stage kidney disease requiring hemodialysis. Eligible sites were identified based upon the completion of a feasibility survey, which provided investigators with the opportunity to ensure broad geographic and provider specialty representation. Study coordinators at each site were responsible for identification and enrollment of subjects during the course of a scheduled outpatient visit. CHAMP-HF was sponsored by Novartis Pharmaceuticals Corp., and all participating sites obtained local or central institutional review board approval before subject enrollment as well as informed consent from each participant. This study leveraged baseline data from all patients enrolled before March 6, 2017.
DATA COLLECTION
At the time of study subject enrollment, site coordinators interviewed patients to collect their self-identified race/ethnicity as well as household income and health status and abstracted their clinical history and medications. The primary outcome for this analysis was disease-specific health status, as assessed by the 12-item KCCQ-12. The KCCQ-12 is a valid, reliable, and sensitive 12-item HF-specific patient-reported outcome form that quantifies patients’ HF symptoms, physical and social limitations, and quality of life (4,6). KCCQ-12 domains can be summarized as an overall summary score that ranges from 0 to 100, where higher scores reflect better health status (fewer symptoms, less social or physical limitations, and better quality of life). A 5-point change in KCCQ-os is considered a clinically meaningful difference in scores from both patients’ and providers’ perspectives (7,8). For descriptive purposes, the KCCQ-os was divided (9) into poor health status (score: <25), fair health status (score: 25 to 49), good health status (score: 50 to 74), and excellent health status (score: 75 to 100). SES was characterized as total annual household income and assessed by asking patients to use ordinal categories of annual household income ranging from <$25,000 to >$150,000 per year.
STATISTICAL ANALYSIS
Distribution of continuous KCCQ-os scores was described by mean ± SD, median, and 25th and 75th percentiles according to patient characteristics that included sex, race/ethnicity, and SES. We then used hierarchical linear regression models, with site as a random effect to account for clustering within sites, to identify patient characteristics associated with patients’ health status. Our first model incorporated patient sociodemographic and clinical characteristics (model 1) with subsequent adjustment for medical therapies (model 2) present on enrollment. Backward selection was performed to obtain the final models. Full models included all variables shown in Table 1, except for laboratory results. Age, sex, and race/ethnicity were permanently retained in the model. The maximum p value for covariates to be retained in the model was set at 0.05. The relationship between mean KCCQ-os score and continuous variables are reported in units of 1 SD, except for age, which was reported per 10 year intervals. We tested the nonlinearity by using restricted cubic splines. There was no evidence of nonlinearity except for age, and therefore we used a linear spline with a knot of 70 for age.
TABLE 1.
Distribution of Patient Characteristics (N = 3,494)
| Age, yrs | 68.0 (59.0, 75.0) |
| <40 | 111 (3.2) |
| 40–64 | 1,307 (37.4) |
| 65–80 | 1,638 (46.9) |
| >80 | 438 (12.5) |
|
| |
| Male | 2,473 (70.8) |
|
| |
| White | 2,616 (74.9) |
|
| |
| Black | 572 (16.4) |
|
| |
| Hispanic | 589 (16.9) |
|
| |
| BMI, kg/m2 | 29.2 (25.5, 33 |
|
| |
| Insurance status | |
| Managed care | 574 (16.4) |
| Private insurance | 330 (9.4) |
| Medicare | 2,038 (58.3) |
| Medicaid | 317 (9.1) |
|
| |
| Highest level of education | |
| Less than high school | 425 (12.2) |
| High school | 1,187 (34.0) |
| Some college | 1,094 (31.3) |
| 4–yr college | 440 (12.6) |
| Graduate or other professional degree | 348 (10.0) |
|
| |
| Total household income | |
| <$25,000 | 1,076 (30.8) |
| $25,000–$49,999 | 685 (19.6) |
| $50,000–$74,999 | 417 (11.9) |
| $75,000–$99,999 | 212 (6.1) |
| $100,000–$149,999 | 184 (5.3) |
| $150,000 or more | 95 (2.7) |
|
| |
| Employee status | |
| Full-time | 496 (14.2) |
| Part–time | 252 (7.2) |
| Disability for medical reasons | 877 (25.1) |
| Not employed for other reasons | 1,869 (53.5) |
|
| |
| Medical history | |
| COPD | 1,054 (30.2) |
| CKD | 693 (19.8) |
| Depression | 874 (25.0) |
| Diabetes mellitus | 1,426 (40.8) |
| Tobacco use/smoking | 689 (19.7) |
| Atrial fibrillation | 1,258 (36.0) |
| Coronary artery disease | 2,177 (62.3) |
| Hyperlipidemia | 2,643 (75.6) |
| Hypertension | 2,872 (82.2) |
| VT/VF | 661 (18.9) |
| CRT therapy | 234 (6.7) |
|
| |
| NYHA functional classification | |
| I | 344 (9.8) |
| II | 1,914 (54.8) |
| III | 1,004 (28.7) |
| IV | 87 (2.5) |
| Unknown | 145 (4.1) |
|
| |
| Number of prior hospitalizations within 12 months of screening | |
| 0 | 2,173 (62.2) |
| 1 | 886 (25.4) |
| ≥2 | 435 (12.4) |
|
| |
| Vital signs on enrollment | |
| Systolic pressure, mm Hg | 120 (110, 131) |
| Diastolic pressure, mm Hg | 72 (64, 80) |
| Heart rate, beats/min | 72 (66, 81) |
|
| |
| Clinical measurements and laboratory results | |
| LVEF, % | 30 (23, 35) |
| NT-proBNP, pg/ml | 2,013 (794, 5,490) |
| HbA1c, % | 6.4 (5.8, 7.6) |
| Hemoglobin, g/dl | 13.2 (11.8, 14.4) |
| Serum creatinine, mg/dl | 1.1 (0.9, 1.4) |
| BUN, mg/dl | 20.0 (16.0, 28.0) |
| Sodium, mmol | 139 (137, 141) |
| eGFR, ml/min/m2 | |
| <30 | 122 (3.5) |
| 30–45 | 304 (8.7) |
| 45–60 | 491 (14.1) |
| >60 | 1,200 (34.3) |
| Missing | 1,377 (39.4) |
|
| |
| Medication on enrollment | |
| ACE inhibitor/ARB | 2,102 (60.2) |
| Beta-blocker | 2,894 (82.8) |
| MRA | 1,161 (33.2) |
| ARNI | 451 (12.9) |
| Loop diuretic agent | 2,139 (61.2) |
| Hydralazine | 193 (5.5) |
| Digoxin | 475 (13.6) |
| Ivabradine | 42 (1.2) |
| Inotrope | 14 (0.4) |
| Number of medications | 3.0 (2.0, 4.0) |
|
| |
| Site characteristics | |
| Physician specialty | |
| Family practice | 219 (6.3) |
| Internal medicine | 266 (7.6) |
| HF specialist | 718 (20.5) |
| Other cardiologist | 2,086 (59.7) |
| Number of HF patients managed annually | 1,200 (480, 3,000) |
Values are median (Q1, Q3) or n (%).
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; ARNI = angiotensin-receptor neprilysin inhibitor; BMI = body mass index; BUN = blood urea nitrogen; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; CRT = cardiac resynchronization therapy; eGFR = estimated glomerular filtration rate; HbA1c = Hemoglobin A1c; HF = heart failure; LVEF = left ventricular ejection fraction; MRA = mineralocorticoid antagonist; VT/VF = ventricular tachycardia/ventricular fibrillation.
Rates of missing data for patient-level variables, overall, were small (<8%), except for household income, which was not reported by ~24% of patients. Missing values for continuous variables were imputed using the sex/age/KCCQ group-specific median for patient-level covariates. For categorical variables, missing medical history variables were imputed to the most common value. Missing procedures were imputed as “no.” All estimates were reported using 95% confidence intervals (CIs) and an α = 0.05 was used to determine statistical significance. All analyses were performed using SAS software version 9.4 (SAS Institute, Cary, North Carolina). Analyses were performed independently by Duke Clinical Research Institute, and the lead author takes responsibility for guiding data analysis and interpretation of the results.
RESULTS
A total of 3,552 patients were enrolled in the CHAMPHF registry before March 6, 2017. Of that sample, our final analytic cohort consisted of 3,494 patients across 140 sites after excluding patients with missing KCCQ-os data (n = 14), demographic data (n = 10), and those ineligible according to the study protocol (n = 34) (Figure 1). There was a broad range of patient-reported KCCQ-os scores, encompassing poor (n = 228), fair (n = 785), good (n = 1,101), and excellent (n = 1,380) health status.
FIGURE 1.
Patient Exclusion Flowsheet
PATIENT CHARACTERISTICS AND HEALTH STATUS ACROSS SUBGROUPS
Characteristics of the analytic cohort are described in Tables 1 and 2, with information on medication prescription by sex, race/ethnicity, and SES provided in Online Tables 3a to 3d and New York Heart Association (NYHA) functional classification by sex, in Online Table 3e. Of the total sample, the median age was 68.0 (interquartile range [IQR]: 59.0 to 75.0) years, with more men than women (70.8% vs. 29.2%) and white (74.9%) than black (16.4%) or Hispanic (16.9%) patients. The total annual household income was <$25,000 in 30.8% of participants, whereas 2.7% reported incomes >$150,000. A significant proportion had concomitant diagnoses of atrial fibrillation, coronary artery disease, chronic obstructive lung disease, diabetes mellitus, and hypertension. Finally, median documented LVEF was 30% (IQR: 23%, 35%). Supporting the stability of this outpatient population, 87.6% of the cohort had no or only one hospitalization within 12 months of enrollment in the registry.
TABLE 2.
Distribution of KCCQ-os Score by Patient Subgroup (N = 3,494)
| Age, yrs | ||
| <40 | 61.2 ± 26.1 | 62.5 (40.1, 85.9) |
| 40–64 | 61.9 ± 25.0 | 63.9 (43.8, 83.3) |
| 65–80 | 66.5 ± 22.6 | 68.8 (50.0, 84.9) |
| >80 | 63.4 ± 23.7 | 64.6 (44.8, 83.9) |
|
| ||
| Sex | ||
| Male | 65.9 ± 23.7 | 68.8 (47.9, 85.4) |
| Female | 60.3 ± 23.8 | 61.5 (43.8, 80.2) |
|
| ||
| Race | ||
| White | 64.9 ± 23.4 | 67.7 (47.9, 84.4) |
| Black | 60.5 ± 25.2 | 61.2 (41.7, 82.3) |
| Hispanic | 59.1 ± 21.0 | 58.3 (43.8, 75.0) |
|
| ||
| Insurance status | ||
| Managed care | 68.2 ± 24.1 | 71.9 (51.6, 88.5) |
| Private insurance | 70.2 ± 22.1 | 73.4 (55.7, 88.5) |
| Medicare | 63.7 ± 23.3 | 65.1 (46.4, 82.3) |
| Medicaid | 56.1 ± 24.6 | 54.2 (38.5, 76.6) |
|
| ||
| Highest level of education | ||
| Less than high school | 58.3 ± 23.2 | 57.3 (41.7, 76.6) |
| High school | 62.5 ± 24.0 | 64.6 (44.8, 81.8) |
| Some college | 65.3 ± 24.2 | 67.7 (46.9, 85.9) |
| 4-yr college | 67.8 ± 22.9 | 71.1 (51.3, 87.5) |
| Graduate or other professional degree | 69.8 ± 22.2 | 75.0 (53.4, 87.5) |
|
| ||
| Total household income | ||
| <$25,000 | 57.1 ± 23.2 | 56.3 (40.6, 75.0) |
| $25,000–$49,999 | 63.1 ± 24.2 | 66.1 (44.3, 83.3) |
| $50,000–$74,999 | 68.8 ± 22.6 | 71.9 (53.1, 87.5) |
| $75,000–$99,999 | 69.9 ± 22.6 | 75.0 (56.3, 87.5) |
| $100,000–$149,999 | 73.5 ± 20.9 | 77.1 (58.9, 92.2) |
| $150,00 or More | 74.6 ± 21.0 | 83.3 (62.5, 89.6) |
|
| ||
| Employment status | ||
| Working full-time | 74.6 ± 21.9 | 80.2 (62.5, 91.7) |
| Working part-time | 70.7 ± 22.6 | 77.1 (57.3, 88.5) |
| Disability for medical reasons | 52.9 ± 23.7 | 52.1 (34.9, 70.8) |
| Not employed for other reasons | 65.9 ± 22.6 | 68.8 (49.0, 84.4) |
Values are mean ± SD and median (Q1, Q3).
KCCQ-os = Kansas City Cardiomyopathy Questionnaire-overall summary score.
In regard to HF-related quality of life, the mean KCCQ-os score was 64.2 ± 23.9 in the overall sample. Participants with good to excellent health status were more often older (≥65), male, and white. Online Table S1 provides a detailed overview of patient subgroup characteristics by ranges of KCCQ-os scores.
DIFFERENCES IN HEALTH STATUS BY SEX, RACE/ETHNICITY, AND SES STATUS
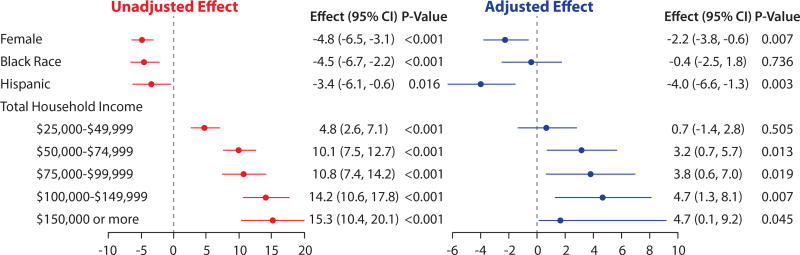
Significant differences in KCCQ-os scores by sex were observed in both unadjusted and adjusted analyses. In the unadjusted model, women had worse KCCQ-os scores than men (−4.8 points; 95% CI: −6.5 to −3.1; p < 0.001). This variability was modestly attenuated after adjusting for other patient-level characteristics in model 1 (−2.2 points; 95% CI: −3.7 to −0.6; p = 0.007) (Online Table S2), and remained statistically significant even after adjusting for HF medications (−2.2 points; 95% CI: −3.8 to −0.6; p = 0.007) (Table 3). Differences by race/ethnicity were observed in unadjusted analyses, with blacks (−4.5 points; 95% CI: −6.7 to −2.2; p < 0.001) and Hispanics (−3.4 points; 95% CI: −6.1 to −0.6; p = 0.016) having worse health status scores than those of whites. For blacks, this difference was fully explained after the addition of other patient characteristics to model 1 (−0.7 points; 95% CI: −2.9 to 1.4; p = 0.52) (Online Table 2) and remained insignificant after adjusting for medical therapies (−0.4 points; 95% CI: −2.5 to 1.8; p = 0.736) (Table 3). For Hispanics, clinically significant differences remained after adjusting only for patient characteristics in model 1 (−3.4 points; 95% CI: −6.0 to −0.8; p = 0.011) (Online Table S2) and then medications (−4.0 points; 95% CI: −6.6 to −1.3; p = 0.003) (Table 3) in model 2.
TABLE 3.
Model 2: Unadjusted and Adjusted Association Between Patient Characteristics and Medications at Enrollment with KCCQ-os (N = 3,494)
| Unadjusted Effect (95% CI) |
p Value | Adjusted Effect (95% CI) |
p Value | |
|---|---|---|---|---|
| Age, 10-yr increments | ||||
| ≤70 yrs | 1.2 (0.5 to 1.8) | <0.001 | 1.6 (0.6 to 2.6) | 0.002 |
| ≥70 yrs | −0.2 (−1.7 to 1.3) | 0.765 | −5.5 (−7.5 to −3.4) | <0.001 |
|
| ||||
| Female (ref: male) | −4.8 (−6.5 to −3.1) | <0.001 | −2.2 (−3.8 to −0.6) | 0.007 |
|
| ||||
| Race/ethnicity (ref: white) | ||||
| Black | −4.5 (−6.7 to −2.2) | <0.001 | −0.4 (−2.5 to 1.8) | 0.736 |
| Other | 0.1 (−2.8 to 3.1) | 0.930 | 3.1 (0.3 to 5.9) | 0.031 |
| Hispanic (ref: non-Hispanic) | −3.4 (−6.1 to −0.6) | 0.016 | −4.0 (−6.6 to −1.3) | 0.003 |
|
| ||||
| BMI to per 7.2 U | −3.3 (−4.0 to −2.5) | <0.001 | −2.5 (−3.3 to −1.8) | <0.001 |
|
| ||||
| Total household income (ref: <$25,000) | ||||
| $25,000–$49,999 | 4.8 (2.6 to 7.1) | <0.001 | 0.7 (−1.4 to 2.8) | 0.505 |
| $50,000–$74,999 | 10.1 (7.5 to 12.7) | <0.001 | 3.2 (0.7 to 5.7) | 0.013 |
| $75,000–$99,999 | 10.8 (7.4 to 14.2) | <0.001 | 3.8 (0.6 to 7.0) | 0.019 |
| $100,000–$149,999 | 14.2 (10.6 to 17.8) | <0.001 | 4.7 (1.3 to 8.1) | 0.007 |
| $150,000 or more | 15.3 (10.4 to 20.1) | <0.001 | 4.7 (0.1 to 9.2) | 0.045 |
| Prefer not to answer | −0.9 (−2.6 to 0.7) | 0.267 | −1.8 (−3.3 to −0.2) | 0.029 |
|
| ||||
| Employment status (ref: working full-time) | ||||
| Working part-time | −2.8 (−6.1 to 0.6) | 0.107 | −1.3 (−4.5 to 1.9) | 0.417 |
| Disability for medical reasons | −20.4 (−22.8 to −17.9) | <0.001 | −14.3 (−16.8 to −11.8) | <0.001 |
| Not employed for other reasons | −7.4 (−9.6 to −5.2) | <0.001 | −5.0 (−7.4 to −2.5) | <0.001 |
|
| ||||
| COPD | −10.4 (−12.1 to −8.7) | <0.001 | −6.2 (−7.7 to −4.6) | <0.001 |
|
| ||||
| Chronic kidney disease | −6.4 (−8.3 to −4.4) | <0.001 | −2.6 (−4.4 to −0.8) | 0.005 |
|
| ||||
| Depression | −10.5 (−12.3 to −8.7) | <0.001 | −7.3 (−9.0 to −5.7) | <0.001 |
|
| ||||
| Atrial fibrillation | −2.0 (−3.6 to −0.4) | 0.015 | −2.0 (−3.5 to −0.5) | 0.011 |
|
| ||||
| Coronary artery disease | −0.9 (−2.6 to 0.7) | 0.267 | −1.8 (−3.3 to −0.2) | 0.029 |
|
| ||||
| Prior HF hospitalization in past year (ref: 0) | ||||
| 1 | −5.2 (−7.1 to −3.4) | <0.001 | −2.8 (−4.5 to −1.2) | 0.001 |
| ≥2 | −13.2 (−15.6 to −10.7) | <0.001 | −6.6 (−8.9 to −4.3) | <0.001 |
|
| ||||
| Pulse, per 12.5 beats/min | −4.0 (−4.8 to −3.2) | <0.001 | −2.4 (−3.1 to −1.7) | <0.001 |
|
| ||||
| LVEF, per 8% | 2.2 (1.5 to 3.0) | <0.001 | 1.1 (0.4 to 1.9) | 0.003 |
|
| ||||
| ARNI | 1.2 (−1.3 to 3.6) | 0.3474 | 3.9 (1.5 to 6.4) | 0.002 |
|
| ||||
| ACEi/ARB | 3.6 (2.0 to 5.2) | <0.001 | 3.6 (2.0 to 5.3) | <0.001 |
|
| ||||
| Loop diuretic agent | −8.2 (−9.8 to −6.6) | <0.001 | −4.4 (−6.0 to −2.9) | <0.001 |
|
| ||||
| Ivabradine | −10.2 (−17.2 to −3.2) | 0.004 | −6.9 (−13.2 to −0.6) | 0.033 |
|
| ||||
| Inotrope | −25.8 (−37.9 to −13.6) | <0.001 | −17.0 (−27.9 to −6.1) | 0.002 |
Abbreviations as in Table 1.
Finally, large differences by household income were observed between the highest- and lowest-paid groups across unadjusted and adjusted analyses. In the unadjusted model, patients with the highest level had a mean KCCQ-os score that was 15.3 points higher than those with the lowest income (95% CI: 10.4 to 20.1; p < 0.001). This variability was attenuated but still significantly different after adjustment for other patient characteristics in model 1 (5.6 points; 95% CI: 1.0 to 10.2; p = 0.02) (Online Table 2) and persisted after adjusting for medical therapies in model 2 (4.7 points; 95% CI: 0.1 to 9.2; p = 0.045) (Table 3). A test for trend across all income levels was significant for both unadjusted (p < 0.0001) and adjusted (p = 0.003) models. Both unadjusted and fully adjusted models for sex, race/ethnicity, and SES are shown in Figure 2.
FIGURE 2.
Unadjusted and Adjusted Mean KCCQ-os Score Disparities by Sex, Race/Ethnicity, and Socioeconomic Status
Candidate variables considered for multivariate analyses were age, sex, race, BMI, insurance status, highest level of education, house income, employment status, diabetes mellitus, CKD, COPD, depression, tobacco use/smoking, atrial fibrillation, CAD, hypertension, hyperlipidemia, ventricular tachycardia/fibrillation, CRT, number of prior HF hospitalizations, systolic blood pressure, heart rate, LVEF, ACEi/ARB, beta-blocker, MRA, ARNI, loop diuretic agent, hydralazine, digoxin, ivabradine, inotrope, and number of HF medications. Variables included in multivariate analysis after backward selection were age, sex, race, BMI, house income, employment status, CKD, COPD, depression, atrial fibrillation, number of prior HF hospitalizations, systolic blood pressure, heart rate, LVEF, ARNI, loop diuretic therapy, ivabradine, and inotrope. Reference category for sex was male. Reference category for race/ethnicity was white. Reference category for total household income was <$25,000 (annually). ACEi/ARB = angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; ARNI = angiotensin-receptor neprilysin inhibitor; BMI = body mass index; CAD = coronary artery disease; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; CRT = cardiac resynchronization therapy; HF = heart failure; LVEF = left ventricular ejection fraction; MRA = mineralocorticoid antagonist; other abbreviations as in Tables 1 and 2.
DISCUSSION
A primary goal for treating patients with HFrEF is to minimize their symptoms and optimize their function and quality of life (10). To accomplish this goal, clinicians have a range of established and emerging medical and device therapies (11–13), but whether these are applied with similar success to optimize the health status of patients with different sex, race/ethnicity, and SES is unknown. In the present study, we used data from CHAMP-HF to explore disparities in health status by sex, race/ethnicity, and SES. We found that, among a large population of patients with HFrEF in outpatient clinical practice, women, blacks and Hispanics, and lower-income patients had statistically significantly worse HF-specific health status in unadjusted analyses. Moreover, even after adjustment for numerous patient and treatment factors, a small but statistically significant worse KCCQ-os score remained in women, Hispanics, and poorer patients. CHAMP-HF is a contemporary registry that, for the first time, captures the care and outcomes of patients with HFrEF. Our findings describe significant disparities in the control of HF symptoms and optimization of function and quality of life between women and men, whites and Hispanics, and those with lower and higher SES that warrant further efforts to achieve the goals of equity in health care.
Our findings extend previous efforts to describe disparities in the care of patients with HF by documenting differences in patients’ health status across different sociodemographic groups. Thus, although prior efforts have described sociodemographic disparities in relation to cardiovascular mortality and routine implementation of guideline-directed HFrEF therapies, insights into health status disparities are limited (14–16). For example, in regard to sex, our results substantially extend several prior, smaller studies suggesting better (17,18), worse (19–26), or comparable (27) quality of life in women. Our analysis describes health status in a larger, more contemporary stable HFrEF population. When interpreting our findings, we believe it is important to focus upon the unadjusted, as opposed to only adjusted, effect sizes. Although adjusting for confounders is very important in observational research seeking to associate patient characteristics with outcomes, in this case, we do not believe that there is a biologically plausible reason why clinicians should be less capable of controlling the symptoms and optimizing the quality of life of women than that of men. Finding a clinically important difference in KCCQ-os scores in women from this large multicenter registry suggests that we are not being as effective in optimizing the health status of women with HFrEF and that more research is needed to better understand how to overcome these apparent sex-level disparities. For example, prior studies associating female sex with lower adherence to guideline-directed HFrEF therapies may be one possible mechanism of health status inequality, although we did account for differences in treatment in our analyses (17). Whether, as others have suggested, these differences are due to HF management knowledge, perceived control, self-care confidence (28), or competing demands between family responsibilities, sex roles, and self-care (29–31) is unknown and further studies are needed to identify how to deliver care that is more equitable between men and women with HFrEF (32).
Health disparities between whites and nonwhites are well known, but racial variability in HF-specific health status remains understudied (20,33,34). Our findings parallel prior research that has shown poorer perceived HF-related health (18) and steeper functional decline (34) in blacks, although other studies have failed to observe any racially driven associations with health status during outpatient follow-up (35). Although results of our fully adjusted model showed no significant differences in health status between whites and blacks, it remains noteworthy that unadjusted health status differences between those groups were statistically and clinically significant (~5 points lower in blacks). This highlights an important reality in the management of the HFrEF population, in that African Americans have worse health status, whether mediated by an underlying biological mechanism or other sociodemographic patient characteristics, and this should not be overlooked as part of routine outpatient care. Multiple reasons for this variability in health status have been postulated, including cultural differences in what constitutes health and factors considered during self-evaluation of health status (18), although there have been no reports suggesting that these observed differences might not be overcome with more aggressive therapy, an important future research priority.
Our results concerning Hispanics are novel in that they contradict earlier reports that Latin Americans experienced comparable health status, on initial evaluation, compared to other ethnic and racial groups (33,36). As our study is descriptive (and the first to report differences in the health status of Hispanics), we cannot provide causal insights into these observations.
Finally, our findings are most indicative of sizeable variability in HF-specific health status based on financial income, with similar patterns having been previously described (19,37–39). In our study, we leveraged annual household income as a proxy for SES, which coincides with definitions used previously in published reports (18). Overall, our results can be understood in the context of routine HF management, where chronic illness is predictably disabling and thereby forces patients to make significant lifestyle changes that have an impact on overall quality of life. By extension, an adequate financial income provides an uninterrupted layer of insulation against barriers to self-care created by inadequate resources (37).
One strategy that may help address these observed disparities would be to routinely capture and report patient-reported health status in clinical care, a means of transparently and reproducibly documenting the symptoms, function, and quality of life of patients with HF at each and every clinic visit. By consistently capturing and reporting patients’ health status, clinicians could readily identify those for whom additional therapeutic strategies may be needed to improve their management. Toward that end, a new Medicare framework (40,41) has been designed to reward providers for collecting patient-reported outcomes measurements through the Centers for Medicare and Medicaid Services Merit-Based Payment System. However, although the Centers for Medicare and Medicaid Services has created a mechanism to encourage the collection of patient-reported outcomes measurements, the means of feasibly collecting, scoring, and reporting these data at the time of a clinical visit will require further work. Although it is possible that the routine collection of patients’ health status can reduce these health status disparities, this will require further investigation after the implementation of patient-reported outcome-based performance measures.
STUDY LIMITATIONS
Our findings must be interpreted in context of the following limitations. First, although the CHAMP-HF registry is among the largest cross-sectional assessments of the health status of patients in routine clinical care, it was conducted in voluntary participating sites committed to clinical research. Whether these findings are generalizable throughout the United States is unknown, and our estimates of health status disparities may not accurately reflect the entire country. Second, our analysis assessed health status at a single point in time (enrollment), and further work will be needed to describe the trajectories of patients’ health status over time. Third, one-fourth of patients did not report annual household income, and this was treated as a separate category. Fourth, residual measured or unmeasured confounding might have influenced the associations observed. Fifth, the use of multiple comparisons might have influenced the statistical significance and interpretation of final p values. Sixth and finally, this initial, descriptive report was not able to formally test mediators of observed difference in health status across vulnerable groups nor define practice patterns that might support intervention to reduce these disparities.
CONCLUSIONS
In analyzing a unique, prospective observational registry of patients with chronic HFrEF, we found that women, blacks, and Hispanics and patients with lower socioeconomic status had worse symptoms, function, and health-related quality of life. After multivariate adjustment, clinically significant disparities remained across sex, race/ethnicity, and socioeconomic groups. Our findings indicate that previously reported disparities in survival and hospitalization rates extend to patients’ health status and underscore the need for novel strategies to reduce health status disparities as well as future work to better understand their complexity.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
In the first large assessment of health status in patients with HFrEF, we found that women, blacks, and Hispanics and patients with lower income signify unique populations that may benefit from more aggressive HF follow-up and treatment in the outpatient setting.
TRANSLATIONAL OUTLOOK
This analysis describes the association between HFrEF patients’ health status and medical and sociodemographic characteristics at enrollment in an observational registry in a contemporary clinical setting. Future studies will be needed to identify health status trajectories over time as well as detect practice patterns that might reduce health status disparities by sex, race/ethnicity, and socioeconomic status in this high-risk population.
Acknowledgments
The CHAMP-HF registry was funded by the Novartis Pharmaceuticals Corp. Drs. Khariton and Nassif are supported by National Heart, Lung, and Blood Institute of Health Under Aware grant T32HL110837. Dr. Spertus has received funding from National Institutes of Health (NIH), Patient-Centered Outcomes Research Institute (PCORI), and Abbott Vascular; and is a consultant for United Healthcare, V-wave, Corvia, Janssen, AstraZeneca, Novartis, Amgen, and Bayer AG Pharmaceuticals Co.; and holds intellectual property rights for the Kansas City Cardiomyopathy Questionnaire and equity interest in Health Outcomes Sciences. Dr. Thomas has received funding from Novartis Pharmaceuticals Corp. Dr. Fonarow has received research support from NIH; and is a consultant for Amgen, Janssen, Medtronic, Novartis, and St. Jude Medical; and has served on the Get With the Guidelines steering committee. Dr. DeVore has received research support from American Heart Association, Amgen, NIH, and Novartis; and is a consultant for Novartis. Dr. Hernandez has received research support from AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Luitpold Pharmaceuticals, Merck Sharpe and Dohme, and Novartis; and has received honoraria from Bayer AG, Boston Scientific, and Novartis. Dr. Butler has received research support from NIH, PCORI, and European Union; and is a consultant for Amgen, Array, AstraZeneca, Bayer AG, Boehringer Ingelheim, Bristol Myers-Squib, CVRx, G3 Pharmaceutical, Innolife, Janssen, Luitpold, Medtronic, Merck Sharpe and Dohme, Novartis, Relypsa, StealthPeptide, SC Pharma, Vifor, and ZS Pharma. Dr. Patterson has received research funding from Amgen, Bristol-Myers Squibb, Merck Sharpe and Dohme, and Novartis; and is a consultant for Amgen and Novartis. Drs. Sharma, McCague, and Duffy are employees of Novartis Pharmaceuticals Corp. Dr. Albert is a consultant for Novartis Pharmaceuticals Corp. The content of this paper is the sole responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ABBREVIATIONS AND ACRONYMS
- CI
confidence interval
- HFrEF
heart failure and reduced ejection fraction
- KCCQ-os
Kansas City Cardiomyopathy Questionnaire-overall summary score
- LVEF
left ventricular ejection fraction
- SES
socioeconomic status
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
APPENDIX For supplemental tables, please see the online version of this paper.
References
- 1.Toward Quality Measures for Population Health and the Leading Health Indicators. Washington, DC: National Academies Press; 2013. Institute of Medicine Committee on Quality Measures for the Healthy People Leading Health Indicators. [PubMed] [Google Scholar]
- 2.Ziaeian B, Kominski GF, Ong MK, Mays VM, Brook RH, Fonarow GC. National differences in trends for heart failure hospitalizations by sex and race/ethnicity. Circ Cardiovasc Qual Outcomes. 2017;10:e003552. doi: 10.1161/CIRCOUTCOMES.116.003552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Husaini BA, Mensah GA, Sawyer D, et al. Race, sex, and age differences in heart failure-related hospitalizations in a southern state: implications for prevention. Circ Heart Fail. 2011;4:161–9. doi: 10.1161/CIRCHEARTFAILURE.110.958306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spertus JA, Jones PG. Development and validation of a short version of the Kansas City Cardiomyopathy Questionnaire. Circ Cardiovasc Qual Outcomes. 2015;8:469–76. doi: 10.1161/CIRCOUTCOMES.115.001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeVore AD, Thomas L, Albert NM, et al. Change the management of patients with heart failure: Rationale and design of the CHAMP-HF registry. Am Heart J. 2017;189:177–83. doi: 10.1016/j.ahj.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Garin O, Herdman M, Vilagut G, et al. Assessing health-related quality of life in patients with heart failure: a systematic, standardized comparison of available measures. Heart Fail Rev. 2014;19:359–67. doi: 10.1007/s10741-013-9394-7. [DOI] [PubMed] [Google Scholar]
- 7.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–55. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 8.Dreyer RP, Jones PG, Kutty S, Spertus JA. Quantifying clinical change: discrepancies between patients’ and providers’ perspectives. Qual Life Res. 2016;25:2213–20. doi: 10.1007/s11136-016-1267-9. [DOI] [PubMed] [Google Scholar]
- 9.Soto GE, Jones P, Weintraub WS, Krumholz HM, Spertus JA. Prognostic value of health status in patients with heart failure after acute myocardial infarction. Circulation. 2004;110:546–51. doi: 10.1161/01.CIR.0000136991.85540.A9. [DOI] [PubMed] [Google Scholar]
- 10.Writing Committee M. Yancy CW, Jessup M, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2013;128:e240–327. [Google Scholar]
- 11.Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–66. doi: 10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 12.McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 13.Swedberg K, Komajda M, Bohm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–85. doi: 10.1016/S0140-6736(10)61198-1. [DOI] [PubMed] [Google Scholar]
- 14.Fonarow GC, Albert NM, Curtis AB, et al. Improving evidence–based care for heart failure in outpatient cardiology practices: primary results of the registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting (IMPROVE HF) Circulation. 2010;122:585–96. doi: 10.1161/CIRCULATIONAHA.109.934471. [DOI] [PubMed] [Google Scholar]
- 15.Chan PS, Oetgen WJ, Buchanan D, et al. Cardiac performance measure compliance in outpatients: the American College of Cardiology and National Cardiovascular Data Registry’s PINNACLE (Practice Innovation And Clinical Excellence) program. J Am Coll Cardiol. 2010;56:8–14. doi: 10.1016/j.jacc.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Opasich C, Tavazzi L, Lucci D, et al. Comparison of one-year outcome in women versus men with chronic congestive heart failure. Am J Cardiol. 2000;86:353–7. doi: 10.1016/s0002-9149(00)00934-6. [DOI] [PubMed] [Google Scholar]
- 17.Frankenstein L, Clark AL, Ribeiro JP. Influence of sex on treatment and outcome in chronic heart failure. Cardiovasc Ther. 2012;30:182–92. doi: 10.1111/j.1755-5922.2010.00253.x. [DOI] [PubMed] [Google Scholar]
- 18.Carlson B, Pozehl B, Hertzog M, Zimmerman L, Riegel B. Predictors of overall perceived health in patients with heart failure. J Cardiovasc Nurs. 2013;28:206–15. doi: 10.1097/JCN.0b013e31824987a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gott M, Barnes S, Parker C, et al. Predictors of the quality of life of older people with heart failure recruited from primary care. Age Ageing. 2006;35:172–7. doi: 10.1093/ageing/afj040. [DOI] [PubMed] [Google Scholar]
- 20.Clark DO, Tu W, Weiner M, Murray MD. Correlates of health-related quality of life among lower-income, urban adults with congestive heart failure. Heart Lung. 2003;32:391–401. doi: 10.1016/j.hrtlng.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Lesman–Leegte I, Jaarsma T, Coyne JC, Hillege HL, Van Veldhuisen DJ, Sanderman R. Quality of life and depressive symptoms in the elderly: a comparison between patients with heart failure and age- and gender-matched community controls. J Card Fail. 2009;15:17–23. doi: 10.1016/j.cardfail.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Friedman MM. Gender differences in the health related quality of life of older adults with heart failure. Heart Lung. 2003;32:320–7. doi: 10.1016/s0147-9563(03)00098-0. [DOI] [PubMed] [Google Scholar]
- 23.Riedinger MS, Dracup KA, Brecht ML Dysfunction SISoLV. Quality of life in women with heart failure, normative groups, and patients with other chronic conditions. Am J Crit Care. 2002;11:211–9. [PubMed] [Google Scholar]
- 24.Chin MH, Goldman L. Gender differences in 1-year survival and quality of life among patients admitted with congestive heart failure. Med Care. 1998;36:1033–46. doi: 10.1097/00005650-199807000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Hou N, Chui MA, Eckert GJ, Oldridge NB, Murray MD, Bennett SJ. Relationship of age and sex to health-related quality of life in patients with heart failure. Am J Crit Care. 2004;13:153–61. [PubMed] [Google Scholar]
- 26.Franzen K, Saveman BI, Blomqvist K. Predictors for health related quality of life in persons 65 years or older with chronic heart failure. Eur J Cardiovasc Nurs. 2007;6:112–20. doi: 10.1016/j.ejcnurse.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Riegel B, Moser DK, Carlson B, et al. Gender differences in quality of life are minimal in patients with heart failure. J Card Fail. 2003;9:42–8. doi: 10.1054/jcaf.2003.1. [DOI] [PubMed] [Google Scholar]
- 28.Heo S, Moser DK, Lennie TA, Riegel B, Chung ML. Gender differences in and factors related to self-care behaviors: a cross-sectional, correlational study of patients with heart failure. Int J Nurs Stud. 2008;45:1807–15. doi: 10.1016/j.ijnurstu.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nesbitt T, Doctorvaladan S, Southard JA, et al. Correlates of quality of life in rural patients with heart failure. Circ Heart Fail. 2014;7:882–7. doi: 10.1161/CIRCHEARTFAILURE.113.000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bennett SJ, Perkins SM, Lane KA, Deer M, Brater DC, Murray MD. Social support and health-related quality of life in chronic heart failure patients. Qual Life Res. 2001;10:671–82. doi: 10.1023/a:1013815825500. [DOI] [PubMed] [Google Scholar]
- 31.Stromberg A, Martensson J. Gender differences in patients with heart failure. Eur J Cardiovasc Nurs. 2003;2:7–18. doi: 10.1016/S1474-5151(03)00002-1. [DOI] [PubMed] [Google Scholar]
- 32.Rector TS, Anand IS, Cohn JN. Relationships between clinical assessments and patients’ perceptions of the effects of heart failure on their quality of life. J Card Fail. 2006;12:87–92. doi: 10.1016/j.cardfail.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Riegel B, Moser DK, Rayens MK, et al. Ethnic differences in quality of life in persons with heart failure. J Card Fail. 2008;14:41–7. doi: 10.1016/j.cardfail.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Vivo RP, Krim SR, Cevik C, Witteles RM. Heart failure in Hispanics. J Am Coll Cardiol. 2009;53:1167–75. doi: 10.1016/j.jacc.2008.12.037. [DOI] [PubMed] [Google Scholar]
- 35.Qian F, Parzynski CS, Chaudhry SI, et al. Racial differences in heart failure outcomes: evidence from the Tele-HF Trial (Telemonitoring to Improve Heart Failure Outcomes) J Am Coll Cardiol HF. 2015;3:531–8. doi: 10.1016/j.jchf.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riegel B, Carlson B, Glaser D, Romero T. Changes over 6-months in health-related quality of life in a matched sample of Hispanics and non-Hispanics with heart failure. Qual Life Res. 2003;12:689–98. doi: 10.1023/a:1025132623647. [DOI] [PubMed] [Google Scholar]
- 37.Close GR, Newton PJ, Fung SC, et al. Socioeconomic status and heart failure in Sydney. Heart Lung Circ. 2014;23:320–4. doi: 10.1016/j.hlc.2013.10.056. [DOI] [PubMed] [Google Scholar]
- 38.Hawkins NM, Jhund PS, McMurray JJ, Capewell S. Heart failure and socioeconomic status: accumulating evidence of inequality. Eur J Heart Fail. 2012;14:138–46. doi: 10.1093/eurjhf/hfr168. [DOI] [PubMed] [Google Scholar]
- 39.Will JC, Valderrama AL, Yoon PW. Preventable hospitalizations for congestive heart failure: establishing a baseline to monitor trends and disparities. Prev Chronic Dis. 2012;9:E85. [PMC free article] [PubMed] [Google Scholar]
- 40.Making Sense of MACRA: advancing care information and improvement activities. Fam Pract Manag. 2017;24:17–20. [PubMed] [Google Scholar]
- 41.Huston KK. MACRA: a new age for physician payments. J Clin Rheumatol. 2017;23:167–8. doi: 10.1097/RHU.0000000000000504. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.