Abstract
Background
Ovarian tissue cryopreservation is increasing as a preferred option for fertility preservation for prepubertal and young adolescent females facing a fertility threatening diagnosis or treatment.
Data Sources
Ovid MEDLINE and PubMed searches for terms related to ovarian tissue removal for fertility preservation revealed there is no current consensus on operative technique for surgical ovarian cortical tissue removal in adult females. Additionally, there are limited published reports of surgical approach and outcomes in the pediatric population. In total, 22 publications were reviewed for their operative approach, ovarian tissue harvesting techniques, complications and outcomes.
Conclusions
Reported operative approaches and techniques for ovarian tissue cryopreservation for pediatric and adolescent patients are variable. Further investigations into operative technique and tissue harvesting that maintains healthy ovarian follicles for transplant will help establish standard technical principles for surgery in pediatric and adolescent females undergoing fertility preservation.
Keywords: Ovarian tissue cryopreservation, Prepubertal females, Adolescent females, Laparoscopy, Operative technique, Fertility preservation
INTRODUCTION
Pediatric female patients with a variety of cancer, genetic, endocrine, and rheumatologic conditions may be candidates for fertility preservation as part of their comprehensive care. Currently, children who receive a new cancer diagnosis can anticipate a five-year survival of 80 % as compared to 60 % in the late 1970s due to continued advancement in medical therapies.1 This remarkable improvement in survivorship has prompted increased awareness of long-term quality of life concerns, including the risk of infertility and premature ovarian insufficiency amongst adult female survivors of childhood cancer.2
Many of the common pediatric cancer diagnoses require multimodal treatments that expose patients to gonadotoxic therapies such as alkylating agents, pelvic irradiation, and/or stem cell transplant that increase their risk for post- therapy infertility. Historically, oophoropexy was the only fertility preservation option for prepubertal girls. While shown to be an effective means of mitigating radiation exposure and preserving ovarian function post radiotherapy, it does not provide protection from systemic therapies.3–5 In addition, very young prepubertal girls have limited area for anatomic transposition, making oophoropexy less likely to be technically successful.6
Currently, embryo and oocyte cryopreservation are the only assisted reproductive techniques that are considered to be non-experimental by the American Society for Reproductive Medicine.7 Unfortunately, there are limitations to these modalities when they are applied to the young adolescent population. Both require 3-4 weeks of ovarian stimulation with gonadotropins for oocyte harvest and typically involve use of transvaginal ultrasound and needle oocyte retrieval techniques, which could require general anesthesia or be technically not feasible in a sexually immature patient. This delay is often not acceptable for females requiring urgent therapy. Young girls are typically not candidates for embryo cryopreservation as it requires the use of sperm for embryo development. Additionally, oocyte cryopreservation cannot be offered to prepubertal females due to their immature hypothalamic-pituitary axis and inability to produce mature eggs.
Ovarian tissue cryopreservation (OTC) was first described by Hovatta et al. in 1996 and involves surgically removing ovarian cortical tissue, independent of hormone stimulation, that is cryopreserved for potential future fertility and hormone restoration.8 While OTC remains experimental and requires prior approval by the healthcare institution’s Institutional Review Board (IRB), it has become a viable method for fertility preservation and is currently the only pre-treatment option for prepubertal girls.9 Currently, there are over 60 reported successful pregnancies resulting in live births through ovarian tissue transplant after OTC from adult patients,10 one live birth following transplant of tissue preserved when the patient was peri-pubertal,11 and news reports of one live birth from OTC performed in a prepubertal patient.12
Despite the increasing use of OTC, there is no current standard technique for surgical removal of ovarian tissue. Many operative techniques are described in the adult literature, but very few studies identify a technique that is applicable to the pediatric population. Therefore, this summary aims to review the currently described operative techniques for surgical removal of ovarian cortical tissue for cryopreservation and to discuss special considerations for prepubertal and young adolescent females undergoing surgical procedures for fertility preservation.
METHODS
Ovid MEDLINE and PubMed searches were performed to identify articles published in the English language with the keywords of “fertility preservation”, “cryopreservation”, “ovarian tissue”, “surgical technique”, “operative technique”, “ovarian biopsy”, and “oophorectomy”. Only human studies between January 2000 and December 2016 were reviewed. Three hundred and sixty-three articles resulted of which 133 had mention of operative approach after review of the title and/or abstract by one author (KC). The remaining manuscripts were reviewed for descriptive details of operative technique for OTC including operative approach, tissue harvest method, dissection technique, intraoperative complications, and postoperative outcomes and complications. Articles with the same first author that described similar operative techniques were included only once in the review. Overall, 22 manuscripts were found to report descriptive details in regards to the operative technique for OTC.
RESULTS
Of the 22 manuscripts reviewed, 4 described using ovarian cortical biopsy, 6 partial oophorectomy, and 8 unilateral oophorectomy as their technique for OTC. Four studies reported multiple techniques used at their institution. Four manuscripts specifically reported on pediatric cohorts, defined as younger than 20 years of age. Refer to Table 1 for manuscript review details.
Table 1.
Operative approach, ovarian tissue harvest methods, complications and outcomes of reviewed studies.
| Author (year of publication) |
Age (years) |
No. of Patients |
Operative Approach |
Ovarian Tissue Harvest Method |
Dissection Technique |
Complications | Outcomes/Special Considerations |
|---|---|---|---|---|---|---|---|
| Borgstrom (2009)* | 8-19.8 | 47 | laparoscopy | 25-50% ovarian cortex removed from 1 ovary | not described | none | OTC for Turner syndrome patients (15% had follicles identified in specimens) |
| Courbiere (2016) | 15-33 | 8 | single-site laparoscopy | unilateral oophorectomy | automated stapler (Endo-GIA) | none | primordial follicles identified in all specimens, no evidence of malignancy or ischemic changes |
| Demeestere (2015) | 13 | 1 | laparoscopy | unilateral oophorectomy | not described | none | successful spontaneous live birth at 27 years old after autograph transplantation |
| Donnez (2004) | 25 | 1 | laparoscopy | 5 ovarian cortical biopsies (12-15 mm long × 5 mm wide) | not described | none | successful live birth after orthotopic transplantation of cryopreserved ovarian tissue |
| Fabbri (2012)* | 1.6-17.9 | 45 | 3-port laparoscopy | bilateral partial oophorectomy | cold scissors | not described | follicles identified in both pre- and post-chemotherapy cohorts |
| Feigin (2007)* | 5-17.5 | 23 | 3-port laparoscopy | unilateral partial oophorectomy, unilateral oophorectomy | Ligaclips, Endoloop, Ligasure, Harmonic scalpel | none | no delays in initiation of medical therapy reported; one patient in adulthood undergoing fertility treatments |
| Hourvitz (2015) | 2 - 41 | 246 | laparoscopy | 2/3 ovarian cortex removed from 1 ovary | not described | none | performed oocyte aspirations just prior to OTC safely without complications |
| Huang (2008) | 25-37 | 26 | laparotomy, laparoscopy | multiple ovarian cortical biopsies | not described | not described | solid-surface vitrification and slow-freezing methods could maintain normal morphology of follicles and endocrine functions of ovarian cortex |
| Jadoul (2007) | not reported | not reported | 4-port laparoscopy | unilateral oophorectomy with vascular pedicle | cold scissors, vascular clips | not described | technical description of unilateral oophorectomy with vascular pedicle for whole ovary cryopreservation |
| Jenninga (2008) | 13.8- 33.7 | 24 | laparotomy, laparoscopy | unilateral oophorectomy | not described | none | performed in conjunction with another procedure when possible |
| Keros (2009) | 28-43 | 20 | laparoscopy | multiple ovarian cortical biopsies (2 mm × 3-5 mm × 5-8 mm) | not described | none | biopsies performed at time of cesarean section to use for evaluation of tissue vitrification versus slow programmed freezing |
| Kikuchi (2012) | 24-36 | 6 | 2-port laparoscopy, single-site laparoscopy | unilateral oophorectomy | Ligasure | none | suggest oophorectomy can be carried out safely using reduced port laparoscopy |
| Lima (2014)* | 13.4 (mean) | 54 | 3-port laparoscopy | 2/3 ovarian cortex removed from 2 ovaries | cold scissors | 1 incidence of intraoperative bleeding requiring red blood cell transfusion | use Argon plasma coagulator to gain hemostasis after partial oophorectomy |
| Martin (2007) | 30 | 1 | laparoscopy | unilateral oophorectomy | bipolar electrocautery | none | performed in conjunction with oophoropexy in a patient with recurrent lumbar spine ependymoma requiring pelvic irradiation |
| Mayerhofer (2016) | 14-42 | 85 | 3-port laparoscopy, laparotomy | 1/2 - 2/3 ovarian cortex removed from 1 ovary | cold scissors | 2 patients with hematologic malignancy developed postoperative fever requiring antibiotics | 30% of patients underwent additional procedures at time of OTC |
| Meirow (1999) | 11-34 | 40 | 3-port laparoscopy | 5-6 ovarian cortical biopsies (5 mm diameter) | round metal laparoscopic biopter | none | all specimens obtained contained primordial follicles |
| Mwesigwa (2016) | 6-28 | 11 | laparoscopy | unilateral salpingo- oophorectomy | not described | 1 incidence of umbilical port site cellulitis | cohort included patients with cancer and hematologic diseases (sickle cell anemia, thalassemia) |
| Nunez (2015) | 14-38 | 21 | 4-port laparoscopy, single-site laparoscopy | unilateral oophorectomy, 1/2 ovarian cortex removed from 1 ovary | bipolar electrocautery, cold scissor | none | no statistically significant differences in surgical and postoperative outcomes between conventional laparoscopy and single-site laparoscopy |
| Poirot (2002) | 2.7-34 | 31 | laparotomy, laparoscopy | unilateral oophorectomy, 1/2 ovarian cortex removed from 1 ovary | bipolar electrocautery | none | changed protocol from partial oophorectomy to unilateral oophorectomy secondary to electrocautery damage to tissue |
| Roux (2011) | 37 | 1 | laparoscopy | unilateral oophorectomy | Endo-GIA stapler, vascular clips | none | propose use of stapler to minimize ischemic time when dividing vasculature |
| Sanchez-Serrano (2009) | 19-39 | 63 | not described | unilateral right oophorectomy | not described | none | one patient underwent reimplantation 2.5 years after extraction with reported regular menses |
| von Wolff (2009) | Not reported | Not reported | laparoscopy | 5-10 cortical biopsies (5 mm3), 50% ovarian cortex removed from 1 ovary, unilateral oophorectomy | bipolar electrocautery, cold scissor | none | choice of technique dependent on fertility risk profile of expected medical therapy |
Pediatric cohort only (all patients < 20 years old)
Ovarian cortical tissue biopsy
Ovarian cortical tissue contains the primordial follicles, known as the follicle reserve, irrespective of the female patient age.13 Ovarian cortical tissue biopsies can be performed either laparoscopically or in conjunction with another open procedure such as a primary tumor resection.14,15 Regardless of technique, an area away from the hilum that is free of visible predominant follicles and/or luteal tissue is preferred as the site of biopsy. The goal is to maximize the number of primordial follicles per specimen without compromising vascular supply to the remaining ovarian tissue.16 Meirow et al. describe using a three-port laparoscopic approach in which they stabilize the utero- ovarian ligament with a grasper and obtain five or six pieces (5 mm × 3 mm) of cortical tissue using a laparoscopic biopter. Hemostasis was achieved with bipolar electrocautery after biopsy.17 Similarly, another group described using laparoscopy to obtain up to ten biopsies with 5 mm3 volume each depending on the volume and size of the ovary.18 Of those that included postoperative outcomes, there were no intraoperative or postoperative complications noted and an average length of stay less than 24 hours was reported. Patients did not experience any delays in anticipated medical therapy in either study.17,19
Partial Oophorectomy
Partial excisions of ovarian cortical tissue can also be performed by either laparotomy or laparoscopy, but was most commonly reported as an elective laparoscopic procedure in the studies reviewed. Both three-port and reduced/single-port surgeries are described.20,21 Unlike cortical tissue biopsies, the partial excision technique extracts a single block of cortical tissue from either one or both ovaries.22,23 Anywhere from 1/4 to 2/3 of ovarian tissue is removed for cryopreservation purposes leaving a partially intact ovary in situ.17,18,20,22,24,25 In many cases, the right ovary was selected due to its preferred anatomic location away from the sigmoid colon.26 Dissection was carried out with the use of sharp scissors out of fear for follicle damage secondary to electrocautery burn.
When reported, hemostasis was most often achieved using bipolar electrocautery.20,22,25 One report noted the use of a thrombin hemostatic matrix and another the use of argon beam coagulation to gain a more superficial level of hemostasis out of concern for cautery damaging to the remaining ovarian tissue.21,23 Data in the adult gynecology literature suggests that both bipolar and ultrasonic electrocautery have effects on the reserve of remaining ovarian tissue after laparoscopic ovarian cystectomy.27–29 Poirot et al. modified their approach after observing thermal injury of their partial oophorectomy specimens and proceeded with unilateral oophorectomy for the remainder of the study.30
Of the studies that included postoperative outcomes, the majority reported no complications and an average length of stay ranging from same-day surgery to two-day hospital admission.18,20,22,24 One incident of clinically significant intraoperative blood loss requiring transfusion was described in a pediatric patient.23
Unilateral Oophorectomy
Laparoscopic unilateral oophorectomy was carried out for the purposes of ovarian cortical tissue cryopreservation, as well as whole ovary cryopreservation. Both standard two to four-port and reduced/single-port laparoscopy were described.3,19,31–33 Division of the infundibulopelvic (suspensory) and utero-ovarian ligaments was carried out by a wide range of techniques and devices including bipolar electrocautery, Endo-GIA stapler, Ligasure, Harmonic scalpel, Endoloop, as well as vascular clips.3,19,21,31,33,34 No study addressed the superiority of one technique compared to another in regards to division of the vasculature. Feigin et al. reported institutional variability due to surgeon preference for which device to use for dissection and vascular ligation, but did not address or compare outcomes according to technique.19 Again, the right ovary was cited as preferable for oophorectomy due to its anatomic positioning.3,11 The specimen was removed with the use of an Endocatch in all cases that reported extraction methods.3,21,33
Of those that describe postoperative outcomes, one superficial surgical site infection requiring antibiotics was described, otherwise no postoperative complications were reported.19,33,35,36
When single-site was compared to conventional laparoscopy, there were no statistically significant differences in surgical or postoperative outcomes identified.21 Similar results were appreciated when using a reduced-port approach.33
Institutions that reported multiple techniques cited age, ovary size/volume, and overall risk for post-therapy infertility as reasons for choosing one method over another.18,19,21,30
Ovarian Cortical Tissue Preparation
While many authors described their respective methods of ovarian tissue removal and preparation, consensus was lacking in terms of how much ovarian tissue should be harvested and how it should be processed for cryopreservation.37 Both slow freeze and vitrification have been used as cryopreservation methods for cortical tissue and verified through successful autograft and pregnancy.10 Slow freezing techniques remain the standard of care for clinics. While the specific details about tissue processing and cryopreservation techniques are essential to ensure successful preservation of ovarian follicles, they are beyond the scope of this review.
Ovarian Cortical Tissue Removal in Pediatric Patients
Four studies specifically described operative techniques for a pediatric cohort of patients. Ages ranged from 5 to 20 years of age. The primary indications for OTC were Turner syndrome, hematologic disorder, solid tumor, and hematologic malignancy. All ovarian cortical tissue was harvested laparoscopically but the amount of tissue removed varied between authors. Borgstrom et al and Feign et al report removing 25-50% of ovarian cortical tissue from one ovary,19,24 while Lima et al and Fabbri et al report bilateral partial oophorectomies19,20,23,24. One patient was reported to have significant intraoperative hemorrhage requiring transfusion, otherwise no significant complications were documented.23 The pediatric population is most at need for a defined OTC procedure, as they have no other pre-treatment options to preserve their fertility at this time.
DISCUSSION
Regardless of the operative approach, laparoscopic ovarian tissue removal for OTC has been shown to be safe with minimal intraoperative and postoperative risks for both adults and children. The majority of the literature reviewed described operative approaches to OTC that have limited utility or application in the pediatric population. There are many anatomic and pediatric-specific risk factors to consider when approaching laparoscopy for OTC in prepubertal and young adolescent females.
A laparoscopic approach to surgical ovarian tissue removal is preferred in children but ovarian tissue may also be removed at the time of initial tumor resection as described in adult females. Although there were reports of similar outcomes with reduced/single-port as compared to conventional laparoscopy, this technique is unlikely to be applied to the prepubertal or young adolescent population solely due to patient size. Conventional laparoscopy can allow for flexibility in port placement which may be determined by the age and size of the child. For example, a 10-mm umbilical camera/extraction port with 2 additional 5-mm working ports placed in the left lower quadrant and suprapubic midline can be used for pre-adolescent and adolescent patients. For infants, one may consider placing both ports in the hemi-abdomen opposite of the ovary that is selected for cryopreservation.6
The size of a pediatric ovary must be considered when selecting an appropriate operative technique for ovarian tissue removal. Before 6 years of age the average ovarian volume is less than 1 cm3. After puberty, the average volume of an ovary is 8 cm3 but can range from 2.5 cm3 to 20 cm3 depending on the menstrual cycle.38 Because of the small size of the ovary, biopsy and partial oophorectomy become technically challenging, may not produce adequate tissue for follicle preservation, and may damage the ovarian tissue in the process, as described in the above references citing ovarian tissue damage with electrocautery.27–29 Therefore, laparoscopic unilateral oophorectomy is the preferred method for surgical ovarian tissue removal in the pediatric patient at our center.6 Care should be taken when performing the oophorectomy to avoid over manipulation of the ovary to preserve the ovarian cortex, where the highest density of primordial follicles are present.39 Another anatomic difference to consider when performing a laparoscopic oophorectomy for OTC on infants and young girls is the narrow mesovarium, resulting in the fallopian tube at close proximity to the ovary (Figure 1). In our center, it is an option to perform a salpingo-oophorectomy in these cases to minimize the potential thermal damage to the ovary. This operative approach is not described in the adult literature as the mesovarium widens over time providing adequate room to dissect the ovary without concern for collateral tissue damage.6
Figure 1.
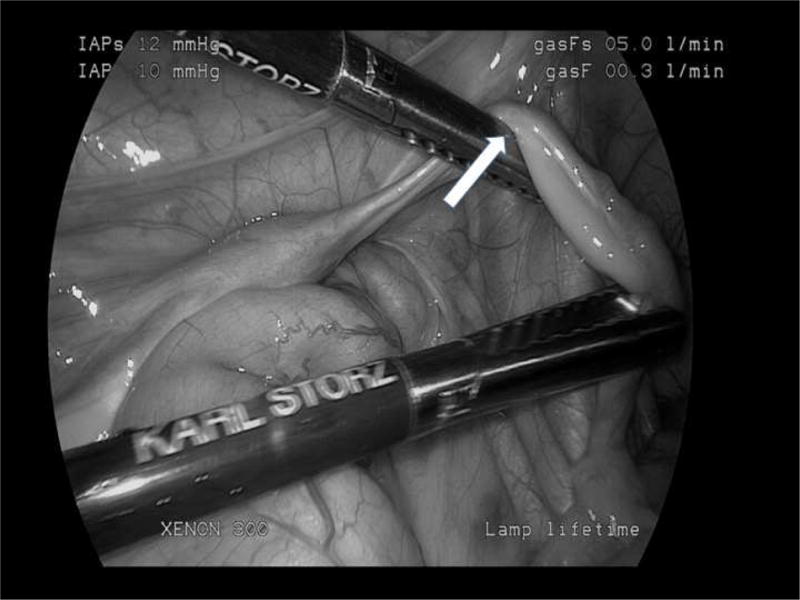
Intraoperative photo demonstrating ovarian anatomy in 2-year-old female with Ewing sarcoma. Arrow identifies the narrow mesovarium. (black and white)
There was no consensus in the reviewed literature on what instrument should be used to divide the vascular supply during oophorectomy and there are no studies to date that determine the superiority of one method versus another. Although multiple studies reported the successful use of an Endo-GIA stapler to divide the infundibulopevlic ligament without the use of electrocautery or ultrasonic energy, this approach could not be applied to prepubertal females due to small size of the pelvis which would not accommodate a stapler and presents a technical challenge for use in young adolescent females due to the need for larger port sizes than otherwise necessary.31,34
Because surgery for OTC is an elective fertility and hormone preservation procedure, the surgeon must ensure that they can perform the procedure with little to no-risk to the patient and minimal disruption to their planned oncological treatments. However, any surgical or postoperative complications including the need for transfusion or superficial surgical site infections could delay lifesaving treatment for the patient. Partial oophorectomy was carried out with sharp dissection in the majority of cases reviewed, which left a raw surface on the remaining ovary. Intervention was required to achieve hemostasis with either bipolar cautery, argon beam coagulation, or thrombin matrix. This technique could introduce a greater risk for clinically significant bleeding.23 Unfortunately, there are no large studies that specifically address the risk of postoperative complications between operative techniques at this time.
Another special consideration for pediatric patients is to limit their exposure to general anesthesia by minimizing the number of times the patient has to be anesthetized. Many patients need further invasive workup of their underlying disease process and/or may require central venous access for their anticipated medical therapy. When possible, coordination of ancillary procedures with surgery should be attempted to minimize anesthetic risks. This strategy was also reported in the adult literature.30,36 Common procedures that can be coordinated for pediatric cancer patients include lumbar puncture, bone marrow biopsy, central venous port/catheter placement, and MRI studies requiring anesthesia.
Lastly, while laparoscopic oophorectomy may be considered a straightforward procedure for surgeons who operate on adult female patients, performing the operation in prepubertal and young adolescent females may best be suited for a surgeon with pediatric operative experience because of the considerations mentioned in this review.
CONCLUSION
There is ample opportunity for further research to establish a standard operative approach for laparoscopic ovarian tissue extraction for cryopreservation in females electing to preserve their ovarian tissue, as there is no current consensus on technique. Model organisms could be used to evaluate exploratory methods and techniques with experimentation and analysis of harvested ovarian tissue. Special attention should be given to the pediatric and adolescent population, whose ovaries may be more susceptible to damage during the surgical procedure. Investigations need to be performed to identify the energy device that has the least amount of associated thermal damage to the adjacent tissues as it is paramount to avoid any capsular or follicle damage during dissection. It is also worthwhile to investigate the effects of timing of vascular division, specimen extraction, and warm ischemia time on the quality and quantity of primordial follicles for cryopreservation. Determining the optimal surgical and OTC parameters would lead to standard operating procedures and result in predictably successful ovarian tissue transplants that restore future fertility and hormone function.
HIGHLIGHTS.
Ovarian tissue cryopreservation is increasing as a preferred option for fertility preservation for prepubertal and young adolescent females facing a fertility threatening diagnosis or treatment. Reported operative approaches for ovarian tissue cryopreservation are variable, therefore further investigations into operative technique and tissue harvesting that maintains healthy ovarian follicles for transplant will help establish standard technical principles for surgery in pediatric and adolescent females undergoing fertility preservation.
Acknowledgments
none
Funding Sources: The National Physicians Cooperative and the Oncofertility Program at Ann & Robert H Lurie Children’s Hospital of Chicago, in collaboration with Northwestern University, was established under the P50HD076188 grant from the National Institutes of Health National Center for Translational Research in Reproduction and Infertility (NCTRI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Armenian SH, Landier W, Hudson MM, et al. Children’s Oncology Group’s 2013 blueprint for research: survivorship and outcomes. Pediatr Blood Cancer. 2013;60(6):1063–1068. doi: 10.1002/pbc.24422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barton SE, Najita JS, Ginsburg ES, et al. Infertility, infertility treatment, and achievement of pregnancy in female survivors of childhood cancer: a report from the Childhood Cancer Survivor Study cohort. The Lancet Oncology. 2013;14(9):873–881. doi: 10.1016/S1470-2045(13)70251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin JR, Kodaman P, Oktay K, Taylor HS. Ovarian cryopreservation with transposition of a contralateral ovary: a combined approach for fertility preservation in women receiving pelvic radiation. Fertil Steril. 2007;87(1):189 e185–187. doi: 10.1016/j.fertnstert.2006.04.051. [DOI] [PubMed] [Google Scholar]
- 4.Paradisi R, Fabbri R, Magnani V, Battaglia C, Venturoli S. A new simple technique of laparoscopic temporary ovarian suspension in addition to ovarian cryopreservation for women prior to posterior pelvic radiation. Gynecol Oncol. 2010;117(2):385–386. doi: 10.1016/j.ygyno.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 5.Tulandi T, Al-Took S. Laparoscopic ovarian suspension before irradiation. Fertil Steril. 1998;70(2):381–383. doi: 10.1016/s0015-0282(98)00155-1. [DOI] [PubMed] [Google Scholar]
- 6.Rowell EE. Optimal Technique for Laparoscopic Oophorectomy for Ovarian Tissue Cryopreservation in Pediatric Girls. In: Woodruff TK GY, editor. Pediatric and Adolescent Oncofertility. 2017. pp. 243–250. [Google Scholar]
- 7.Practice Committee of American Society for Reproductive M. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2013;100(5):1214–1223. doi: 10.1016/j.fertnstert.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Hovetta O, SR, Krausz T, et al. Cryopreservation of human ovarian tissue using dimethylsulphoxide and propanediol-sucrose as cryoprotectants. Human Reproduction. 1996;11(6):1268–1272. doi: 10.1093/oxfordjournals.humrep.a019370. [DOI] [PubMed] [Google Scholar]
- 9.Filippi F, Meazza C, Paffoni A, Raspagliesi F, Terenziani M, Somigliana E. Egg Freezing in Childhood and Young Adult Cancer Survivors. Pediatrics. 2016 doi: 10.1542/peds.2016-0291. [DOI] [PubMed] [Google Scholar]
- 10.Donnez J, Dolmans MM. Ovarian cortex transplantation: 60 reported live births brings the success and worldwide expansion of the technique towards routine clinical practice. J Assist Reprod Genet. 2015;32(8):1167–1170. doi: 10.1007/s10815-015-0544-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demeestere I, Simon P, Dedeken L, et al. Live birth after autograft of ovarian tissue cryopreserved during childhood. Hum Reprod. 2015;30(9):2107–2109. doi: 10.1093/humrep/dev128. [DOI] [PubMed] [Google Scholar]
- 12.Donnelly L. Woman gives birth to baby using ovary frozen in her childhood in ‘world first’. The Telegraph. 2016 [Google Scholar]
- 13.Duncan FE, Pavone ME, Gunn AH, et al. Pediatric and Teen Ovarian Tissue Removed for Cryopreservation Contains Follicles Irrespective of Age, Disease Diagnosis, Treatment History, and Specimen Processing Methods. J Adolesc Young Adult Oncol. 2015;4(4):174–183. doi: 10.1089/jayao.2015.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keros V, Xella S, Hultenby K, et al. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum Reprod. 2009;24(7):1670–1683. doi: 10.1093/humrep/dep079. [DOI] [PubMed] [Google Scholar]
- 15.Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364(9443):1405–1410. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 16.Huang L, Mo Y, Wang W, Li Y, Zhang Q, Yang D. Cryopreservation of human ovarian tissue by solid- surface vitrification. Eur J Obstet Gynecol Reprod Biol. 2008;139(2):193–198. doi: 10.1016/j.ejogrb.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Meirow D, Fasouliotis SJ, Nugent D, Schenker JG, Gosden RG, Rutherford AJ. A laparoscopic technique for obtaining ovarian cortical biopsy specimens for fertility conservation in patients with cancer. Fertil Steril. 1999;71(5):948–951. doi: 10.1016/s0015-0282(99)00067-9. [DOI] [PubMed] [Google Scholar]
- 18.von Wolff M, Donnez J, Hovatta O, et al. Cryopreservation and autotransplantation of human ovarian tissue prior to cytotoxic therapy--a technique in its infancy but already successful in fertility preservation. Eur J Cancer. 2009;45(9):1547–1553. doi: 10.1016/j.ejca.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 19.Feigin E, Abir R, Fisch B, et al. Laparoscopic ovarian tissue preservation in young patients at risk for ovarian failure as a result of chemotherapy/irradiation for primary malignancy. J Pediatr Surg. 2007;42(5):862–864. doi: 10.1016/j.jpedsurg.2006.12.041. [DOI] [PubMed] [Google Scholar]
- 20.Fabbri R, Vicenti R, Macciocca M, et al. Cryopreservation of ovarian tissue in pediatric patients. Obstet Gynecol Int. 2012;2012:910698. doi: 10.1155/2012/910698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nunez Valera MJ, Padilla Iserte P, Higueras Garcia G, et al. Single site laparoscopy for fertility preservation: a cohort study. J Minim Invasive Gynecol. 2015;22(2):291–296. doi: 10.1016/j.jmig.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Mayerhofer K, Ott J, Nouri K, et al. Laparoscopic ovarian tissue harvesting for cryopreservation: an effective and safe procedure for fertility preservation. Eur J Obstet Gynecol Reprod Biol. 2010;152(1):68–72. doi: 10.1016/j.ejogrb.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 23.Lima M, Gargano T, Fabbri R, Maffi M, Destro F. Ovarian tissue collection for cryopreservation in pediatric age: laparoscopic technical tips. J Pediatr Adolesc Gynecol. 2014;27(2):95–97. doi: 10.1016/j.jpag.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Borgstrom B, Hreinsson J, Rasmussen C, et al. Fertility preservation in girls with turner syndrome: prognostic signs of the presence of ovarian follicles. J Clin Endocrinol Metab. 2009;94(1):74–80. doi: 10.1210/jc.2008-0708. [DOI] [PubMed] [Google Scholar]
- 25.Hourvitz A, Yerushalmi GM, Maman E, et al. Combination of ovarian tissue harvesting and immature oocyte collection for fertility preservation increases preservation yield. Reprod Biomed Online. 2015;31(4):497–505. doi: 10.1016/j.rbmo.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez-Serrano M, Novella-Maestre E, Rosello-Sastre E, Camarasa N, Teruel J, Pellicer A. Malignant cells are not found in ovarian cortex from breast cancer patients undergoing ovarian cortex cryopreservation. Hum Reprod. 2009;24(9):2238–2243. doi: 10.1093/humrep/dep196. [DOI] [PubMed] [Google Scholar]
- 27.Chang HJ, Han SH, Lee JR, et al. Impact of laparoscopic cystectomy on ovarian reserve: serial changes of serum anti-Mullerian hormone levels. Fertil Steril. 2010;94(1):343–349. doi: 10.1016/j.fertnstert.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 28.Kim SS, Lee WS, Chung MK, Lee HC, Lee HH, Hill D. Long-term ovarian function and fertility after heterotopic autotransplantation of cryobanked human ovarian tissue: 8-year experience in cancer patients. Fertil Steril. 2009;91(6):2349–2354. doi: 10.1016/j.fertnstert.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 29.Song T, Kim WY, Lee KW, Kim KH. Effect on ovarian reserve of hemostasis by bipolar coagulation versus suture during laparoendoscopic single-site cystectomy for ovarian endometriomas. J Minim Invasive Gynecol. 2015;22(3):415–420. doi: 10.1016/j.jmig.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Poirot C, Vacher-Lavenu MC, Helardot P, Guibert J, Brugieres L, Jouannet P. Human ovarian tissue cryopreservation: indications and feasibility. Hum Reprod. 2002;17(6):1447–1452. doi: 10.1093/humrep/17.6.1447. [DOI] [PubMed] [Google Scholar]
- 31.Courbiere B, Crochet P, Marcelli M, Saias-Magnan J, Grillo JM, Agostini A. Laparoscopic ovariectomy by single-port access for ovarian cryopreservation. Arch Gynecol Obstet. 2016;293(3):591–594. doi: 10.1007/s00404-015-3839-2. [DOI] [PubMed] [Google Scholar]
- 32.Jadoul P, Donnez J, Dolmans MM, Squifflet J, Lengele B, Martinez-Madrid B. Laparoscopic ovariectomy for whole human ovary cryopreservation: technical aspects. Fertil Steril. 2007;87(4):971–975. doi: 10.1016/j.fertnstert.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 33.Kikuchi I, Kagawa N, Silber S, et al. Oophorectomy for fertility preservation via reduced-port laparoscopic surgery. Surg Innov. 2013;20(3):219–224. doi: 10.1177/1553350612449074. [DOI] [PubMed] [Google Scholar]
- 34.Roux I, Grynberg M, Linehan J, Messner A, Deffieux X. Ovarian cryopreservation after laparoscopic ovariectomy using the Endo-GIA stapling device and LAPRO-clip absorbable ligating clip in a woman: a case report. J Med Case Rep. 2011;5:48. doi: 10.1186/1752-1947-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mwesigwa P, Patel S, Patel V, Gomez-Lobo V. Case Series of Ovarian Tissue Preservation Cases in a Pediatric Hospital. Journal of Pediatric and Adolescent Gynecology. 2016;29(2):202–203. [Google Scholar]
- 36.Jenninga E, Hilders CG, Louwe LA, Peters AA. Female fertility preservation: practical and ethical considerations of an underused procedure. Cancer J. 2008;14(5):333–339. doi: 10.1097/PPO.0b013e31818860ac. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez-Wallberg KA, Oktay K. Recent advances in oocyte and ovarian tissue cryopreservation and transplantation. Best Pract Res Clin Obstet Gynaecol. 2012;26(3):391–405. doi: 10.1016/j.bpobgyn.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asavoaie C, Fufezan O, Cosarca M. Ovarian and uterine ultrasonography in pediatric patients. Pictorial essay. Med Ultrason. 2014;16(2):160–167. doi: 10.11152/mu.201.3.2066.162.ca1of2. [DOI] [PubMed] [Google Scholar]
- 39.Edwards RG, Fowler RE, Gore-Langton RE, et al. Normal and abnormal follicular growth in mouse, rat and human ovaries. J Reprod Fertil. 1977;51(1):237–263. doi: 10.1530/jrf.0.0510237. [DOI] [PubMed] [Google Scholar]


