Abstract
The blood-brain barrier (BBB) regulates the transport of ions, nutrients, and metabolites to help maintain proper brain function. This restrictive interface formed by brain microvascular endothelial cells excludes the majority of small and large molecule drugs from entering the brain, and blood-brain barrier dysfunction is a signature of many neurological diseases. Thus, in vitro models of the BBB based on brain endothelial cells have been developed to facilitate screening drugs for BBB permeability. However, while brain endothelial cells form the main interface, they work in concert with other brain-resident cells such as neural progenitor cells, pericytes, astrocytes, and neurons to form the neurovascular unit (NVU). Importantly, non-endothelial cells of the NVU play key roles in eliciting BBB phenotypes and in regulating the dynamic responses of the BBB to brain activity and disease. As a result, emerging in vitro BBB models have incorporated these NVU cell types in addition to endothelial cells. These multicellular BBB or NVU models have found increasing application not only in drug screening, but also in studying complex cellular and molecular mechanisms underlying BBB biology and disease.
Keywords: Blood-Brain Barrier, Neurovascular Unit, Brain Endothelial Cells, Pericytes, Astrocytes, Neurons
Introduction
The blood-brain barrier (BBB) comprises highly specialized brain microvascular endothelial cells (BMECs) that maintain the delicate balance of ions, nutrients, and other molecules essential for proper brain function, while also excluding toxins from the central nervous system (CNS). Among the specialized properties of BMECs are (i) lack of fenestrae, (ii) tight junctions between adjacent endothelial cells, (iii) presence of solute carriers that regulate ion and small molecule transport, (iv) expression of efflux transporters including P-glycoprotein (P-gp), Breast Cancer Resistance Protein (BCRP), and Multidrug Resistance Proteins (MRPs), (v) low levels of pinocytosis, and (vi) receptor-mediated processes for specific uptake of macromolecules (reviewed in [1–3]).
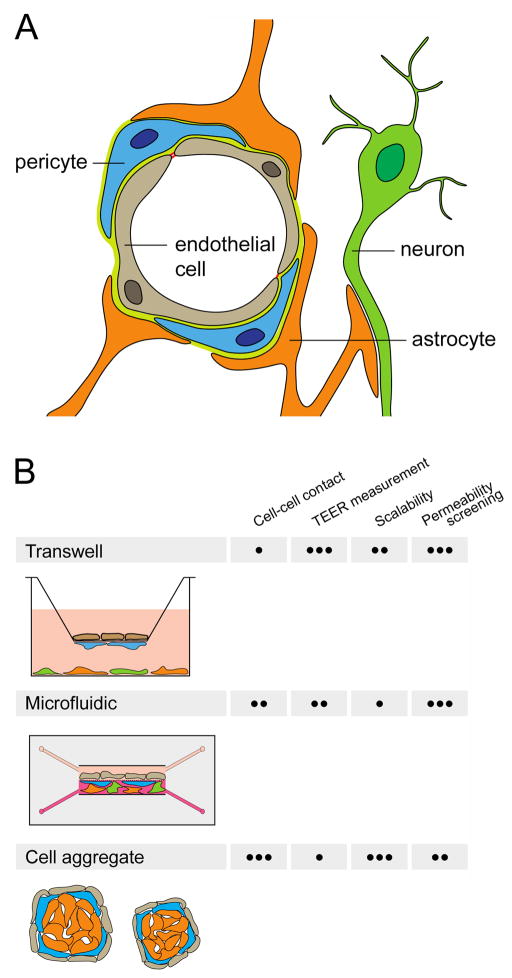
Although the microvascular endothelium constitutes this restrictive interface, other cell types present in the neurovascular microenvironment during development and adulthood including neural progenitor cells, pericytes, astrocytes, and neurons contribute significantly to the BBB phenotype. Increasing appreciation of the importance of multiple cell types in regulating dynamic BBB responses to physiological and disease stimuli has led to the concept of an integrated neurovascular unit (NVU), which minimally consists of BMECs, pericytes, astrocytes, and neurons (Figure 1A), and for some studies can extend to include neural stem cells or microglia.
Figure 1.
The NVU and multicellular BBB models. (a) Cross-section of a brain capillary, showing the organization of BMECs, pericytes, astrocytes, and neurons. (b) Transwell, microfluidic, and cell aggregate-based in vitro model systems incorporating BMEC monolayers along with other NVU cell types. General attributes of in vitro model systems, including: (i) the ability to achieve cell-cell contact, (ii) the ability to quantify barrier formation by transendothelial electrical resistance (TEER) measurement, (iii) the ease of scale up for high-throughput experiments and (iv) the ease of permeability screening, are characterized as • poor, •• moderate, or ••• excellent.
The development of in vitro BBB models has been driven by the desire to understand BBB function in development, health, and disease. Moreover, because the BBB excludes the vast majority of small molecule, protein, and gene therapeutics [4], in vitro BBB models also offer a platform for screening drug candidates for BBB permeability. To date, considerable effort has led to the generation of many BMEC-based models of the BBB (reviewed in [5–7]). Importantly, in vitro models that incorporate multiple NVU cell types can have advantages over BMEC-only models. First, the presence other NVU cell types can induce or improve barrier properties, such as the formation of continuous tight junctions to reduce paracellular diffusion or “leakiness”. When used for drug permeability screening, such models may therefore yield results that are more predictive of in vivo permeability. Second, multicellular models can provide a tool to interrogate paracrine and juxtacrine signaling that may underlie elements of BBB development and maintenance. Finally, given emerging knowledge about the roles of neurovascular dysfunction in many diseases of the CNS (reviewed in [3,8]), in vitro models of the NVU, including those derived from patient-specific induced pluripotent stem cells (iPSCs), may provide opportunities to better understand molecular and cellular mechanisms of CNS diseases.
We will first briefly discuss the roles of neural progenitor cells, pericytes, astrocytes, and neurons in regulating the development and maintenance of the BBB. We will then review recent advances in BBB modeling resulting from incorporation of NVU cells to form multicellular BBB models, and highlight several examples of the utility of such models in understanding BBB biology and disease.
Roles of non-endothelial NVU cells in BBB formation and function
Stewart and Wiley [9] used quail-chick transplantation studies to show that developing neural tissue was necessary for endothelial BBB development. Subsequent work established the ability of both astrocytes [10,11] and neurons [11,12] to induce BBB phenotypes in endothelial cells. In addition, during early embryogenesis the BBB initially forms in the presence of neural progenitor cells when astrocytes are not yet present. Studies have demonstrated the ability of embryonic neural progenitor cells (NPCs) to induce BBB properties such as decreased endothelial permeability and improved tight junction formation in vitro [13], and it was later determined that Wnt/β-catenin signaling driven by NPCs is required for CNS angiogenesis and contributes to barriergenesis during development [14]. In addition, signaling through retinoic acid secreted by radial glial cells [15], Hedgehog secreted by astrocytes [16], and GPR124 [17,18] have also been implicated in aspects of BBB development. Key roles for pericytes in barriergenesis have also been described, as pericytes regulate BBB endothelial tight junction morphology, transcytosis, and expression of leukocyte adhesion molecules [19]. Pericytes are also required for the maintenance of the BBB in adulthood, as demonstrated by pericyte-dependent endothelial gene expression, reduction in endothelial transcytosis, and astrocyte end-foot polarization [20]. Furthermore, given the ability of astrocytes to induce and maintain endothelial BBB properties in vitro, the close association of astrocytes with endothelial cells in vivo, and correlations between astrocyte pathologies and BBB breakdown (reviewed in [21]), it is likely that continued astrocyte-endothelial signaling is necessary for BBB maintenance. Neurons similarly have the ability to induce and maintain BBB properties in vitro [11,12,22], but currently a detailed picture of neuron-endothelial crosstalk is lacking. Taken together, there is a clear impact of non-BMEC cell types on BBB formation and function motivating the development and use of multicellular NVU-type models to continue to advance our understanding of these complex phenomena in neural health, disease, and therapy.
Advances in multicellular BBB models
Recently developed multicellular BBB models have incorporated neural progenitor cells, pericytes, astrocytes, and neurons. These models have employed both primary and immortalized cells from human, rodent, bovine, and porcine sources. NVU cells derived from pluripotent stem cell or neural stem cell sources have also been used (Table 1). Most models have been constructed using either Transwell culture inserts or microfluidic devices, and models based on cell aggregates are an emerging alternative (Figure 1B). Below we will summarize each of these configurations as they pertain to the contribution of NVU cells to the BBB model.
Table 1.
Summary of cell types and cell sources used in multicellular BBB models.
| Neurovascular unit cell type | Species and cell source | References |
|---|---|---|
| BMECs | Human primary | [31,36,37,40,41] |
| Human immortalized | [32,33,37] | |
| Human pluripotent stem cell-derived | [22,23,26–30,35,38,39] | |
| Porcine primary | [24,42] | |
| Rat primary | [13,22,33] | |
| Rat immortalized | [12] | |
| Mouse primary | [38] | |
| Mouse immortalized | [25,34,38] | |
| Neural progenitor/stem cells | Human primary | [23,28] |
| Human pluripotent stem cell-derived | [28] | |
| Rat primary | [13] | |
| Pericytes | Human primary | [23,27,28,36,37,40,41] |
| Human pluripotent stem cell-derived | [29] | |
| Porcine primary | [24,42] | |
| Rat primary | [33] | |
| Mouse immortalized | [34] | |
| Astrocytes | Human primary | [28,31,36,37,41] |
| Human pluripotent stem cell-derived | [22,27–29,38–40] | |
| Human neural progenitor cell-derived | [23] | |
| Porcine primary | [24,42] | |
| Rat primary | [13,26,32,33,35] | |
| Mouse primary | [38] | |
| Mouse immortalized | [25,34,40] | |
| Neurons | Human pluripotent stem cell-derived | [22,29,39,40] |
| Human neural progenitor cell-derived | [23] | |
| Rat primary | [12,32] |
Transwell models
Transwell-based BBB models typically consist of endothelial cells cultured on an extracellular matrix-coated permeable membrane of a cell culture insert, which is then suspended within a well of a 12- or 24-well plate (Figure 1B). Benefits of the Transwell platform include ease of use, moderate scalability, and the ability to rapidly and nondestructively quantify barrier integrity via measurement of transendothelial electrical resistance (TEER). Additionally, for permeability screening, molecules or cells can be added to the culture medium in the top (apical or “blood-side”) chamber and their accumulation in the bottom (basolateral or “brain-side”) chamber evaluated over time, or vice versa. Drawbacks of the Transwell system include the lack of fluid flow and the relatively large media volume, which may attenuate the effect of cell-cell signaling through soluble factors. Additionally, the permeable membrane prevents substantial contact between BMECs and other NVU cell types.
The Transwell system can be readily adapted to multicellular BBB models, and offers flexibility in the arrangement of different cell types depending on the intended application of the model. NVU cell types can be cultured on the bottom of the well, allowing the exchange of soluble factors with BMECs cultured on the insert. For example, human pluripotent stem cell (hPSC)-derived BMECs have been co-cultured sequentially with primary human pericytes and human neural progenitor cell-derived neurons and astrocytes in this manner, demonstrating robust increases in TEER up to 5,000 Ω × cm2 [23]. In addition to allowing sequential co-culture with different cell types, the Transwell platform also allows simultaneous co-culture of three distinct cell types while maintaining spatial separation of each cell type for subsequent molecular analysis. BMECs are typically cultured on the top surface of the membrane, a second cell type is cultured on the bottom surface of the membrane (sometimes referred to as “contact” co-culture, though the membrane prevents in vivo-like cell-cell contact), and the third cell type is cultured on the bottom of the well (Figure 1B). For example, Thomsen et al. developed a Transwell BBB model incorporating primary porcine brain endothelial cells, pericytes, and astrocytes [24]. Both pericytes and astrocytes increased TEER and decreased mannitol permeability compared to BMEC monoculture. “Contact” co-culture of pericytes and BMECs or astrocytes and BMECs increased expression of the gene encoding the tight junction protein claudin-5, an effect not observed in analogous “non-contact” co-cultures. Transwells are also amenable to alternative arrangements of cells. As a recent example, Hawkins et al. demonstrated that endothelial cells could be cultured on the bottom of the insert to facilitate a comparison of monolayer (two-dimensional, 2D) and collagen hydrogel (three-dimensional, 3D) astrocyte culture in the top chamber [25]. Addition of TGF-β1 to 3D astrocyte-endothelial co-cultures led to a larger decrease in TEER than in 2D, and this effect was not observed in the absence of astrocytes.
Several recently reported Transwell-based models incorporate one or more cell types derived from hPSCs. hPSCs are an attractive cell source for in vitro modeling since they are renewable and scalable, there are established protocols for their differentiation to many relevant NVU cell types including BMEC-like cells [23,26,27], and the resulting cells may have more relevance to human biology than immortalized cell lines or cells isolated from nonhuman animals, particularly for modeling human disease. For example, a quadruple-culture model encompassing hPSC-derived brain endothelial cells, hPSC-derived and primary astrocytes and neural stem cells, and primary pericytes exhibited increased TEER, decreased permeability to 40 kDa dextran, and increased expression of the glucose transporter Glut-1 (SLC2A1) compared to endothelial monoculture [28]. Another potentially powerful application of stem cell technology is the use of patient-derived iPSCs to create patient-specific multicellular BBB models wherein all cell types are derived from the same donor iPSC line. Recently, Canfield et al. demonstrated the differentiation of BMECs, neurons, and astrocytes from the same iPSC line [22]. Subsequent triple-culture of BMECs with a mixture of neurons and astrocytes in a physiologically-relevant 1:3 ratio increased TEER and improved tight junction continuity compared to BMECs cultured alone. As another example, co-differentiation of endothelial cells and pericytes from iPSCs by sequential treatment with several growth factors, and co-culture with iPSC-derived neurons and astrocytes induced BBB properties in the endothelial cells, including expression of nutrient and efflux transporters and development of tight junctions [29]. Subsequently, the resultant BMEC-like cells were purified via FACS and incorporated in a Transwell model in co-culture with astrocytes. Examples of the utility of such NVU models derived from patient-specific iPSCs in disease modeling are discussed below.
Microfluidic models
Microfluidic devices offer several benefits in multicellular BBB modeling. Compared to Transwells, the smaller relative medium volume in microfluidic systems minimizes dilution of secreted factors that may be important in modulating BBB properties. These systems also facilitate the application of shear stress by medium flow, mimicking the effect of blood flow in vivo, and therefore serve as a platform to investigate influences of shear stress on BBB properties [30,31]. Microfluidic models also permit a more physiologically-relevant arrangement of the different NVU cell types, including the possibility of legitimate cell-cell contacts (Figure 1B). Drawbacks of microfluidic BBB models include limited scalability and the requirement of specialized equipment and expertise for their construction.
As a recent example of a microfluidic device facilitating physiologically-relevant arrangement of cell types, Adriani et al. developed a BBB model employing primary rat NVU cells in four parallel channels [32]. The first channel contained a neural cell culture medium, the second and third contained hydrogels with neurons and astrocytes, respectively, and the fourth contained a tubular BMEC monolayer and endothelial cell culture medium. The authors demonstrated close association of astrocyte processes with endothelial cells, confirmed the formation of a barrier by evaluating dextran permeability from the lumen, and assayed the barrier’s restrictiveness to glutamate via neuronal calcium imaging.
Other microfluidic devices employ permeable membranes as a BMEC substrate. For example, primary rat BMECs and pericytes were cultured on opposite sides of a membrane and astrocytes were cultured on the bottom surface of the device, reminiscent of the common Transwell arrangement [33]. The device also incorporates transparent electrodes for TEER measurement and imaging during operation. Wang et al. similarly constructed a microfluidic device for the triple-culture of immortalized mouse BMECs, pericytes, and astrocytes, and showed a significant increase in P-gp activity in a model incorporating all three cell types compared to an endothelial-pericyte co-culture [34]. Furthermore, increasing the medium volume decreased TEER, likely as a result of the dilution of astrocyte- or pericyte-secreted factors.
Finally, microfluidic systems incorporating iPSC-derived BMECs are also emerging. Wang et al. co-cultured iPSC-derived BMECs with primary rat astrocytes in a microfluidic device where flow is driven by gravity and scaled to achieve a medium residence time similar to that observed in the brain microcirculation [35]. The multicellular BBB system maintained TEER above 3000 Ω × cm2 up to day 10 of operation, and permeabilities for six evaluated small and large molecules correlated well to in vivo transport across the BBB. In the future, microfluidic models incorporating multiple iPSC-derived NVU cell types, rather than from primary or immortalized sources, may offer improvements in fidelity and scalability.
Cell aggregate-based models
Self-assembled cell aggregates comprising BMECs, astrocytes, and pericytes are emerging as a possible alternative to Transwell and microfluidic models for certain applications. These “spheroid” models permit direct contact between different NVU cell types and yield an endothelial monolayer for permeability studies (Figure 1B). Furthermore, such models are highly scalable and simpler to fabricate and operate than microfluidic devices. Drawbacks of these models include the inability to measure TEER, and that they presently lack neuronal contributions.
Urich et al. first demonstrated that under low-attachment culture conditions, primary human brain endothelial cells, astrocytes, and pericytes self-assembled into organized spheroidal structures with an astrocyte core covered with pericytes surrounded by an outer monolayer of endothelial cells [36]. BMECs in the assembled spheroids possess tight junctions and P-gp activity, and proof-of-concept screening of a panel of fluorescently-labeled cell-penetrating peptides identified four peptides that appeared to enter the brain after intravenous injection [37]. Incorporating iPSC-derived cell types will be an important next step toward realizing the full utility of this emerging aggregate-based BBB model system.
Applications of NVU models
The significant technical advances in multicellular BBB models described above have facilitated recent applications in understanding neurovascular biology and disease. One exciting set of applications has leveraged patient-specific iPSCs for neurovascular disease modeling. For example, astrocytes were differentiated both from normal iPSCs and those carrying mutations in Amyotrophic Lateral Sclerosis linked genes SOD1 or FUS [38]. The authors showed that endothelial cells co-cultured with SOD1-mutant astrocytes had increased P-gp expression and activity compared to endothelial cells co-cultured with normal astrocytes, and that this effect was dependent on nuclear translocation of NF-κB and correlated with increased reactive oxygen species (ROS) in endothelial cells. They also demonstrated that FUS-mutant astrocytes induced similar effects on P-gp and NF-κB activity, but these effects correlated with TNF-α production rather than ROS [38]. As another example, BMECs, astrocytes, and neurons were differentiated from iPSCs carrying mutations in the thyroid hormone transporter monocarboxylate transporter 8 (MCT8), which has been linked to Allan-Herndon-Dudley syndrome (AHDS), a severe form of mental retardation [39]. Using the iPSC-derived cells to individually model the effects of MCT8-deficiency indicated that neural cell development proceeded normally in a T3 thyroid hormone-dependent fashion. However, MCT8-deficiency substantially reduced T3 thyroid hormone transport across BMECs in a Transwell model to suggest that AHDS may be a result of a BBB transport deficiency. Notably, through the use of genome editing tools, the phenotype could be rescued by correcting the MCT8 mutation in patient-derived iPSCs.
Other applications have sought to use multicellular BBB models to understand the response of the NVU to inflammatory stimuli. Brown et al. constructed a NVU model from primary human BMECs and pericytes, mouse astrocytes, and human iPSC-derived neurons in a two-chamber microfluidic device [40]. After exposure of the vascular (apical) chamber to lipopolysaccharide (LPS) or a cocktail of inflammatory cytokines, the authors harvested media from the vascular and brain chambers and used LC-MS-based metabolomics to identify metabolic pathways influenced by inflammatory stimuli, including several that were differentially-affected between the two chambers, indicating the impact of the multicellular configuration. Similarly, a microfluidic NVU model comprising a tubular monolayer of primary human BMECs surrounded by pericytes or astrocytes was employed to evaluate cytokine release upon TNF-α stimulation [41]. The authors demonstrated that astrocyte and pericyte co-cultures showed increased basal levels of the pro-survival cytokine granulocyte colony stimulating factor (G-CSF) compared to BMEC monoculture, and co-cultures also displayed increased induction of G-CSF upon TNF-α stimulation. They further showed that these effects were not detectable in an analogous Transwell model. Taken together, these examples demonstrate the unique ability of multicellular in vitro models of the NVU to provide novel biological insights that would be difficult or impossible to discern with BMEC-only models.
Conclusions
The shift from BMEC-centric in vitro BBB models to multicellular BBB models with one or more additional NVU cell types has greatly expanded their potential beyond drug permeability screening to the interrogation of molecular and cellular mechanisms underlying BBB physiology and disease. We therefore anticipate increasing application of multicellular BBB models incorporating iPSC-derived BMECs and other NVU cell types to the study of neurovascular contributions to diseases. As new models of the NVU are developed, additional elements that merit consideration include the composition of extracellular matrix [42] and inclusion of additional cell types such as microglia, especially in the context of inflammation [43]. Finally, we suggest that incorporating additional cell types present in vascular microenvironments may improve the utility of other in vitro models such as those related to the blood-nerve barrier or other organ-specific endothelia.
Highlights.
The BBB is formed by specialized brain microvascular endothelial cells (BMECs).
Diverse cell types of the neurovascular unit regulate the BBB.
Inclusion of these cell types in addition to BMECs improves in vitro BBB models.
These multicellular models are useful in studying BBB biology and disease.
Acknowledgments
This work was supported by National Institutes of Health grant NS083688 and National Science Foundation grant CBET-1703219. B.D.G. was supported by National Institutes of Health Biotechnology Training Program grant T32 GM008349.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hawkins BT, Davis TP. The Blood-Brain Barrier / Neurovascular Unit in Health and Disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 2.Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 3.Zlokovic BV. The Blood-Brain Barrier in Health and Chronic Neurodegenerative Disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Pardridge WM. The blood-brain barrier: Bottleneck in brain drug development. NeuroRX. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helms HC, Abbott NJ, Burek M, Cecchelli R, Couraud P-O, Deli MA, Förster C, Galla HJ, Romero IA, Shusta EV, et al. In vitro models of the blood–brain barrier: An overview of commonly used brain endothelial cell culture models and guidelines for their use. J Cereb Blood Flow Metab. 2016;36:862–890. doi: 10.1177/0271678X16630991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson HK, Shusta EV. Human-Based In Vitro Brain Endothelial Cell Models. In: Di L, Kerns EH, editors. Blood–Brain Barrier in Drug Discovery: Optimizing Brain Exposure of CNS Drugs and Minimizing Brain Side Effects for Peripheral Drugs. John Wiley & Sons; 2015. pp. 238–73. [Google Scholar]
- 7.Aday S, Cecchelli R, Hallier-Vanuxeem D, Dehouck MP, Ferreira L. Stem Cell-Based Human Blood-Brain Barrier Models for Drug Discovery and Delivery. Trends Biotechnol. 2016;34:382–393. doi: 10.1016/j.tibtech.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Stanimirovic DB, Friedman A. Pathophysiology of the Neurovascular Unit: Disease Cause or Consequence? J Cereb Blood Flow Metab. 2012;32:1207–1221. doi: 10.1038/jcbfm.2012.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart PA, Wiley MJ. Developing nervous tissue induces formation of blood-brain barrier characteristics in invading endothelial cells: A study using quail-chick transplantation chimeras. Dev Biol. 1981;84:183–192. doi: 10.1016/0012-1606(81)90382-1. [DOI] [PubMed] [Google Scholar]
- 10.Janzer RC, Raff MC. Astrocytes induce blood–brain barrier properties in endothelial cells. Nature. 1987;325:253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- 11.Tontsch U, Bauer HC. Glial cells and neurons induce blood-brain barrier related enzymes in cultured cerebral endothelial cells. Brain Res. 1991;539:247–253. doi: 10.1016/0006-8993(91)91628-e. [DOI] [PubMed] [Google Scholar]
- 12.Savettieri G, Di Liegro I, Catania C, Licata L, Pitarresi GL, D’Agostino S, Schiera G, De Caro V, Giandalia G, Giannola LI, et al. Neurons and ECM regulate occludin localization in brain endothelial cells. Neuroreport. 2000;11:1081–1084. doi: 10.1097/00001756-200004070-00035. [DOI] [PubMed] [Google Scholar]
- 13.Weidenfeller C, Svendsen CN, Shusta EV. Differentiating embryonic neural progenitor cells induce blood-brain barrier properties. J Neurochem. 2007;101:555–565. doi: 10.1111/j.1471-4159.2006.04394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A. 2009;106:641–6. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mizee MR, Wooldrik D, Lakeman KAM, van het Hof B, Drexhage JAR, Geerts D, Bugiani M, Aronica E, Mebius RE, Prat A, et al. Retinoic Acid Induces Blood-Brain Barrier Development. J Neurosci. 2013;33:1660–1671. doi: 10.1523/JNEUROSCI.1338-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, Terouz S, Sabbagh M, Wosik K, Bourbonniere L, Bernard M, et al. The Hedgehog Pathway Promotes Blood-Brain Barrier Integrity and CNS Immune Quiescence. Science. 2011;334:1727–1731. doi: 10.1126/science.1206936. [DOI] [PubMed] [Google Scholar]
- 17.Kuhnert F, Mancuso MR, Shamloo A, Wang H-T, Choksi V, Florek M, Su H, Fruttiger M, Young WL, Heilshorn SC, et al. Essential Regulation of CNS Angiogenesis by the Orphan G Protein-Coupled Receptor GPR124. Science. 2010;330:985–989. doi: 10.1126/science.1196554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cullen M, Elzarrad MK, Seaman S, Zudaire E, Stevens J, Yang MY, Li X, Chaudhary A, Xu L, Hilton MB, et al. GPR124, an orphan G protein-coupled receptor, is required for CNS-specific vascularization and establishment of the blood-brain barrier. Proc Natl Acad Sci. 2011;108:5759–5764. doi: 10.1073/pnas.1017192108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, et al. Pericytes regulate the blood–brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 21.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006 doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- •22.Canfield SG, Stebbins MJ, Morales BS, Asai SW, Vatine GD, Svendsen CN, Palecek SP, Shusta EV. An isogenic blood–brain barrier model comprising brain endothelial cells, astrocytes, and neurons derived from human induced pluripotent stem cells. J Neurochem. 2017;140:874–888. doi: 10.1111/jnc.13923. (Canfield et al.) The authors built a Transwell-based in vitro model of the NVU with BMECs, astrocytes, and neurons all generated efficiently from the same iPSC line, and showed that these astrocytes and neurons improve BMEC TEER and tight junction continuity. This work enables modeling of NVU disease using patient-derived iPSCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lippmann ES, Al-Ahmad A, Azarin SM, Palecek SP, Shusta EV. A retinoic acid-enhanced, multicellular human blood-brain barrier model derived from stem cell sources. Sci Rep. 2014;4:4160. doi: 10.1038/srep04160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomsen LB, Burkhart A, Moos T. A triple culture model of the blood-brain barrier using porcine brain endothelial cells, astrocytes and pericytes. PLoS One. 2015;10:1–16. doi: 10.1371/journal.pone.0134765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hawkins BT, Grego S, Sellgren KL. Three-dimensional culture conditions differentially affect astrocyte modulation of brain endothelial barrier function in response to transforming growth factor β1. Brain Res. 2015;1608:167–176. doi: 10.1016/j.brainres.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 26.Lippmann ES, Azarin SM, Kay JE, Nessler RA, Wilson HK, Al-Ahmad A, Palecek SP, Shusta EV. Derivation of Blood-Brain Barrier Endothelial Cells from Human Pluripotent Stem Cells. Nat Biotechnol. 2012;30:783–791. doi: 10.1038/nbt.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hollmann EK, Bailey AK, Potharazu AV, Neely MD, Bowman AB, Lippmann ES. Accelerated differentiation of human induced pluripotent stem cells to blood–brain barrier endothelial cells. Fluids Barriers CNS. 2017;14:9. doi: 10.1186/s12987-017-0059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Appelt-Menzel A, Cubukova A, Günther K, Edenhofer F, Piontek J, Krause G, Stüber T, Walles H, Neuhaus W, Metzger M. Establishment of a Human Blood-Brain Barrier Co-culture Model Mimicking the Neurovascular Unit Using Induced Pluri- and Multipotent Stem Cells. Stem Cell Reports. 2017;8:894–906. doi: 10.1016/j.stemcr.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamizu K, Iwasaki M, Takakubo H, Sakamoto T, Ikuno T, Miyoshi M, Kondo T, Nakao Y, Nakagawa M, Inoue H, et al. In Vitro Modeling of Blood-Brain Barrier with Human iPSC-Derived Endothelial Cells, Pericytes, Neurons, and Astrocytes via Notch Signaling. Stem Cell Reports. 2017;8:634–647. doi: 10.1016/j.stemcr.2017.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.DeStefano JG, Xu ZS, Williams AJ, Yimam N, Searson PC. Effect of shear stress on iPSC-derived human brain microvascular endothelial cells (dhBMECs) Fluids Barriers CNS. 2017;14:20. doi: 10.1186/s12987-017-0068-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cucullo L, Hossain M, Puvenna V, Marchi N, Janigro D. The role of shear stress in Blood-Brain Barrier endothelial physiology. BMC Neurosci. 2011;12:40. doi: 10.1186/1471-2202-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••32.Adriani G, Ma D, Pavesi A, Kamm RD, Goh ELK. A 3D neurovascular microfluidic model consisting of neurons, astrocytes and cerebral endothelial cells as a blood–brain barrier. Lab Chip. 2017;17:448–459. doi: 10.1039/c6lc00638h. (Adriani et al.) The authors developed a microfluidic NVU model comprising an immortalized human BMEC (hCMEC/D3) monolayer, rat astrocytes, and rat neurons. The device has four parallel channels to facilitate spatial organization of different media, matrices, and cell types. Notably, the authors did not restrict analyses to endothelial cells, quantifying neuronal morphology and activity in the presence or absence of astrocytes and endothelial cells. [DOI] [PubMed] [Google Scholar]
- 33.Walter FR, Valkai S, Kincses A, Petneházi A, Czeller T, Veszelka S, Ormos P, Deli MA, Dér A. A versatile lab-on-a-chip tool for modeling biological barriers. Sensors Actuators, B Chem. 2016;222:1209–1219. [Google Scholar]
- 34.Wang JD, Khafagy ES, Khanafer K, Takayama S, Elsayed MEH. Organization of Endothelial Cells, Pericytes, and Astrocytes into a 3D Microfluidic in Vitro Model of the Blood-Brain Barrier. Mol Pharm. 2016;13:895–906. doi: 10.1021/acs.molpharmaceut.5b00805. [DOI] [PubMed] [Google Scholar]
- •35.Wang YI, Abaci HE, Shuler ML. Microfluidic blood–brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol Bioeng. 2017;114:184–194. doi: 10.1002/bit.26045. (Wang et al.) The authors developed a microfluidic NVU model based on iPSC-derived endothelial cells and rat astrocytes, capable of maintaining TEER above 3,000 Ω × cm2 for several days. The device employs gravity-driven rather than pump-driven flow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urich E, Patsch C, Aigner S, Graf M, Iacone R, Freskgård P-O. Multicellular Self-Assembled Spheroidal Model of the Blood Brain Barrier. Sci Rep. 2013;3:1500. doi: 10.1038/srep01500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••37.Cho C-F, Wolfe JM, Fadzen CM, Calligaris D, Hornburg K, Chiocca EA, Agar NYR, Pentelute BL, Lawler SE. Blood-brain-barrier spheroids as an in vitro screening platform for brain-penetrating agents. Nat Commun. 2017;8:15623. doi: 10.1038/ncomms15623. (Cho et al.) The authors generated BBB spheroids, formed by self-assembly of human brain endothelial cells, astrocytes, and pericytes. They further showed that the barrier formed by an outer monolayer of endothelial cells allows these spheroids to be used for permeability screening of fluorescently-labeled cell-penetrating peptides. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •38.Qosa H, Lichter J, Sarlo M, Markandaiah SS, McAvoy K, Richard JP, Jablonski MR, Maragakis NJ, Pasinelli P, Trotti D. Astrocytes drive upregulation of the multidrug resistance transporter ABCB1 (P-Glycoprotein) in endothelial cells of the blood–brain barrier in mutant superoxide dismutase 1-linked amyotrophic lateral sclerosis. Glia. 2016;64:1298–1313. doi: 10.1002/glia.23003. (Qosa et al.) The authors co-cultured endothelial cells with astrocytes differentiated from iPSCs with mutations in the Amyotrophic Lateral Sclerosis-related genes SOD1 and FUS. They demonstrated that mutant astrocytes upregulate P-gp in endothelial cells, an effect driven in both cases by NF-κB nuclear translocation and correlated with reactive oxygen species (in SOD1-mutants) or TNF-α (in FUS-mutants) [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••39.Vatine GD, Al-Ahmad A, Barriga BK, Svendsen S, Salim A, Garcia L, Garcia VJ, Ho R, Yucer N, Qian T, et al. Modeling Psychomotor Retardation using iPSCs from MCT8-Deficient Patients Indicates a Prominent Role for the Blood-Brain Barrier. Cell Stem Cell. 2016 doi: 10.1016/j.stem.2017.04.002. (Vatine et al.) The authors differentiated BMECs, astrocytes, and neurons from normal iPSCs and those carrying a mutation in the thyroid hormone transporter MCT8 that causes Allan-Herndon-Dudley Syndrome. They demonstrated that MCT8-deficiency does not impair differentiation or thyroid hormone (T3)-dependent maturation of neurons, but does impair T3 transport across BMECs in a Transwell model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown JA, Codreanu SG, Shi M, Sherrod SD, Markov DA, Neely MD, Britt CM, Hoilett OS, Reiserer RS, Samson PC, et al. Metabolic consequences of inflammatory disruption of the blood-brain barrier in an organ-on-chip model of the human neurovascular unit. J Neuroinflammation. 2016;13:306. doi: 10.1186/s12974-016-0760-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herland A, Van Der Meer AD, FitzGerald EA, Park TE, Sleeboom JJF, Ingber DE. Distinct contributions of astrocytes and pericytes to neuroinflammation identified in a 3D human blood-brain barrier on a chip. PLoS One. 2016;11:1–21. doi: 10.1371/journal.pone.0150360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zobel K, Hansen U, Galla HJ. Blood-brain barrier properties in vitro depend on composition and assembly of endogenous extracellular matrices. Cell Tissue Res. 2016;365:233–245. doi: 10.1007/s00441-016-2397-7. [DOI] [PubMed] [Google Scholar]
- 43.da Fonseca ACC, Matias D, Garcia C, Amaral R, Geraldo LH, Freitas C, Lima FRS. The impact of microglial activation on blood-brain barrier in brain diseases. Front Cell Neurosci. 2014;8:1–13. doi: 10.3389/fncel.2014.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]