Abstract
Little evidence is available regarding outcomes of advanced digital technology (ADT) hearing aid wearers with mild sensorineural hearing loss (MSNHL). The purpose of this article is to report the characteristics of and outcomes for this population. A cross-sectional research design was employed with 56 participants from a private practice setting. The International Outcomes Inventory for Hearing Aids (IOI-HA), Satisfaction with Amplification in Daily Life (SADL), and the Measure of Audiologic Rehabilitation Self-Efficacy for Hearing Aids (MARS-HA) were completed, scored, and compared with normative data. Results revealed that ADT aids were worn 10.5 hours/day, were mostly advanced to premium (55%), had an average cost per aid of $2,138 (SD = $840), and provided significant benefit (IOI-HA overall score: mean = 4.1; SD = 0.6) and satisfaction (SADL global score: mean = 5.4; SD = 0.8) to users who had good overall self-efficacy (MARS-HA composite score: mean = 81.7; SD = 12.8). Patients were most dissatisfied with and had the least self-efficacy for managing background noise and advanced handling of their devices. ADT hearing aid users with MSNHL achieved excellent outcomes, but ongoing follow-up and counseling from hearing health care providers may be important for successful management of background noise and mastery of advanced handling skills.
Keywords: mild hearing loss, adults, advanced digital hearing aids
Learning Outcomes: As a result of this activity, the participant will be able to describe benefits from, satisfaction with, and self-efficacy for advanced digital hearing aids in users with mild sensorineural hearing loss.
Untreated sensorineural hearing loss (SNHL) can negatively impact health-related quality of life in individuals and their families 1 2 3 and often is associated with social isolation, increased rates of depression and anxiety, and lessened self-efficacy and mastery. 1 Recent research has shown that SNHL is associated with accelerated cognitive decline in elderly persons living independently 4 and with global brain atrophy, particularly in the temporal lobe. 5
Although hearing aids (HAs) are the most common treatment for SNHL, 1 many people wait about 10 years or more from the first time they notice that they have a hearing problem until they seek help for it. 6 Further, the HA uptake rate (i.e., the percentage of people who are candidates for HAs and who actually obtain them) shows that only about one in five candidates actually pursues amplification. 7 8 9 In other words, this low uptake rate indicates that around 70 to 80% of persons with hearing impairment remain at risk for the insidious effects of untreated SNHL. Unfortunately, approximately 67 to 86% of people with SNHL who could benefit from HAs do not obtain or use them. 10 It is difficult to know or understand the reasons why these large numbers of candidates do not pursue amplification, especially given the advances made in hearing aid technologies over the past decade. Perhaps, one way to motivate these individuals to try amplification would be to examine whether the 20 to 30% of persons who do acquire HAs actually use them, and if so, then document and promote what benefits and satisfaction they derive from the devices.
Current research on cochlear synaptopathy (a.k.a., hidden hearing loss) suggests that even persons with slight hearing loss may benefit from amplification. For some time now, audiologists who dispense HAs have questioned whether amplification should be provided to individuals with slight or mild hearing loss, and if so, whether advantages derived from fitting them early before the loss progresses would be similar to those (e.g., maintaining social involvement and participation in activities requiring communication, and possibly even thwarting cognitive issues) known to exist for persons with more severe losses. Our own research 11 and clinical experience has shown that many persons with mild sensorineural hearing loss (MSNHL) can benefit (both improved hearing and reduced tinnitus) from early intervention through amplification.
The prevalence of MSNHL varies according to how it is defined. 12 Recently, mild hearing loss has been defined as a four-frequency pure-tone average (FFPTA) at 500, 1,000, 2,000, and 4,000 Hz of 26 to 40 dB HL. 13 14 However, some individuals with FFPTAs of 25 dB HL or better may be considered to have normal hearing for those frequencies, but still have MSNHL in the high frequencies that may result in communication difficulties, particularly understanding speech in background noise. Many of these patients may benefit from mild-gain HAs. 11 The prevalence of MSNHL is approximately 9 to 15% of the population for persons older than 15 years, but this estimate may vary from country to country. 14 There are about 25.4 million persons in the United States aged 12 years or older who have mild hearing loss in their better ear. 15 The prevalence of MSNHL increases with age and is estimated to rise to around 39% of the population for persons older than 55 years. 16 However, patients with MSNHL, like their peers with more severe losses, have a low uptake of HAs. This was reflected in MarkeTrak VIII, 17 which reported that only 29% of persons sampled who reported having mild hearing loss said they had visited an audiologist. Astonishingly, that survey 17 reported that 43% of the respondents' hearing health care providers had told them to wait before seeking amplification and 25% said that they were told that HAs would not help them. If this is true, then inappropriate or inaccurate information and recommendations from hearing health care professionals are an odious reason for patients not to pursue amplification, especially given the high-quality HA technology available today for patients with any degree of loss.
Another reason why persons with MSNHL may not seek amplification is a lack of accessibility to and affordability of hearing health care. 13 Several years ago, the National Institute of Deafness and Other Communication Disorders/National Institutes of Health assembled a research working group on Accessible and Affordable Hearing Health Care for Adults with Mild to Moderate Hearing Loss for the purpose of developing a research agenda to increase access to hearing health services and HAs. 13 Similarly, in 2016, the National Academies of Sciences, Engineering, and Medicine (NASEM) assembled an expert committee to study the affordability and accessibility of hearing health care for adults in the United States. This group recommended evidence-based key institutional, technological, and regulatory changes to accomplish its goals, one of which was to increase accessibility to HAs. 10 For example, the expert committee, in addition to the President's Council of Advisors on Science and Technology (PCAST), 18 recommended that the U.S. Food and Drug Administration (FDA) develop a classification of over-the-counter (OTC) wearable hearing instruments for use by adults with mild to moderate hearing losses. Recently, the Over-the-Counter Hearing Aid Act of 2017 19 included recommendations from the NASEM 10 and PCAST. 18 The American Academy of Audiology (AAA) 20 published a position statement about OTC HAs and generally supported the notion of these devices, but only for patients with MSNHL. Unfortunately, there is little or no research supporting or refuting the recommendation of OTC devices for patients with MSNHL, and there are few findings regarding the outcomes of patients with MSNHL who use advanced digital technology (ADT) HAs.
Johnson and colleagues 11 found that only 10 studies were available in the peer-reviewed literature that had reported HA outcomes for patients with MSNHL, and most of those investigations only involved low levels of evidence, were rather dated, and reported findings for analog and early digital devices. Cox et al and Johnson et al, 21 22 23 however, reported outcomes from studies that compared entry- to premium-level ADT HAs for patients with mild and moderate SNHL. Although the findings for persons with MSNHL were similar to those with moderate losses, outcomes were not reported separately for the group with MSNHL. The purpose of this study was to describe the characteristics of and benefits from, satisfaction with, and self-efficacy for ADT HAs in users with MSNHL.
Methods
Practice Setting
The site used for data collection in the present study was a two-office private practice in Santa Barbara County, CA, in which HA selection, evaluation, fitting, and verification are performed by licensed hearing health care professionals using standard clinical procedures. 24 Prior to HA fittings in this practice, an electroacoustic analysis is performed on all hearing instruments to ensure that they are functioning according to manufacturers' specifications. 25 Briefly, the HA–fitting protocol included: assessment of the physical fit and comfort of the devices; verification of output of the HA for soft, average, and loud speech inputs that match NAL-NL2 targets within ± 5 dB from 250 to 4,000 Hz; instruction on the care and use of the devices; and information about the warranty. Follow-up procedures included a phone call within 48 hours of the fitting and weekly visits until the end of the 45-day (or longer if necessary) trial period during which final arrangements were made for purchase of the HAs.
Measures
The materials used in the present study were: (1) a patient information form (PIF); (2) the Visual Analog Scale for Daily Use of Hearing Aids (VASuse), 26 (3) the International Outcome Inventory for Hearing Aids (IOI-HA), 27 (4) the Measure of Audiologic Rehabilitation Self-Efficacy for Hearing Aids (MARS-HA), 28 and (5) the Satisfaction with Amplification in Daily Life (SADL). 29 These materials are described briefly here.
Patient Information Form
The PIF was designed for the specific needs of the present study and queried participants' age, gender, marital status, number of people in the household, hours per day of HA use, experience with current HAs, lifetime experience with hearing instruments, and self-reported degree of unaided hearing difficulty. Questions about experience with current and lifetime HA use were adapted from the SADL. 29
The Visual Analog Scale for Daily Use of Hearing Aids 26
The VASuse was included as Item 5 on the PIF and consisted of the question “How many hours per day do you wear your hearing aids?” As shown in Fig. 1 , an example of the visual analog scale was provided first with instructions and then participants were asked to mark an “X” to represent their answer on the second visual analog scale.
Figure 1.
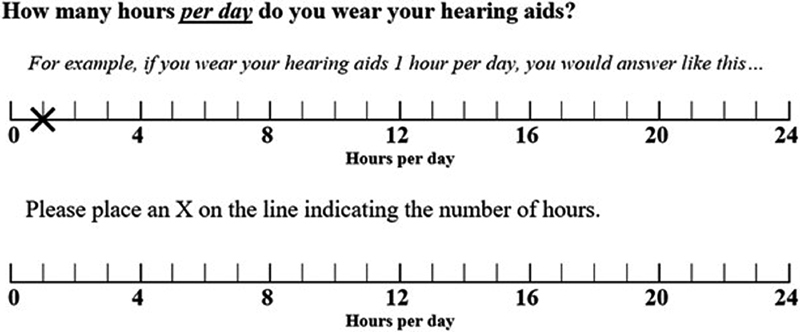
Question #5 on the patient information form, which queried how many hours per day participants wore their hearing aids.
The International Outcome Inventory for Hearing Aids 27
The IOI-HA is a post-HA-fitting questionnaire consisting of eight items that focus on self-reported: daily HA use (USE), benefit (BEN), residual activity limitations (RAL), satisfaction with devices and services (SAT), residual participation restriction (RPR), impact on others (IO), quality of life (QoL), and unaided hearing difficulty (UHD). Items are scored from 1 to 5, with higher scores indicating a positive impact of HAs on seven of these eight items; question #8 asks participants to rate unaided communication difficulty where higher scores indicate greater levels of difficulty. Cronbach's α value for overall internal consistency for the IOI-HA is 0.78. 30
The Measure of Audiologic Rehabilitation Self-Efficacy for Hearing Aids 28
The MARS-HA quantifies patients' HA self-efficacy (i.e., their confidence in being able to perform the skills needed to be successful amplification users). The MARS-HA is a 24-item questionnaire with embedded instructions and two practice items to help ensure that participants understand the task. Items are scored on an 11-point scale in increments of 10 from 0 to 100, with higher scores indicating greater self-efficacy with HAs. The MARSHA yields a global score and scores from the 24 items load onto four subscales: Basic Handling (BH), Advanced Handling (AH), Adjustment (ADJ), and Aided Listening (AL). Cronbach's a value for overall internal consistency for the MARS-HA is 0.92. 28
The Satisfaction With Amplification in Daily Life 29
The SADL is a 15-item self-assessment tool that measures patient satisfaction with HAs. Items are scored on a 7-point scale from 1 to 7, with higher scores indicating greater satisfaction with HAs. This form also has several reversed items (i.e., questions 2, 4, 7, and 13). The questionnaire yields a global score and scores for four subscales: Positive Effect (PE), Negative Features (NF), Service and Cost (SC), and Personal Image (PI). Cronbach's a value for overall internal consistency for the IOI-HA is 0.82. 29
Participant Recruitment and Inclusion/Exclusion Criteria
Participants were recruited from a convenience sample of HA users from a two-office private practice in Santa Barbara County, CA, who met the following inclusion criteria: (1) 18 to 90 years of age, (2) FFPTAs ≤ 40 dB HL in the better ear, (3) received hearing instruments within 6 weeks to 5 years from the start date of the investigation, and (4) able to complete outcome measures in the English language. Patients were excluded from participation if they: (1) were under contract from a Veteran's Affairs Medical Center, (2) returned or voided the order for the HAs prior to fitting, (3) had specialty CROS or BICROS amplification arrangements, and/or (4) were incarcerated at the time of the study (this practice sees patients from a local penitentiary).
Investigators consulted with an employee of Computers Unlimited that manages the Total Information Management System (TIMS) electronic medical records system (used by this practice) to aid in the querying and provision of a mailing list of patients who would meet inclusion criteria for the present study sample. The search of the patient database focused on the most recent HA(s) purchased by the potential participants. Patients with cancelled orders, returns, voided purchases, or those who purchased devices from providers at other service-delivery sites were excluded, as were deceased patients, those patients fit under Veterans Administration Medical Administration contracts, and minors younger than 18 years. The query process resulted in a potential sample of 838 patients who might be able to participate in the study.
From the 838 patients, 500 were randomly invited to participate in the present study and were mailed a packet that contained: (1) a letter from the owner of the private practice that briefly explained the study and sought their participation, (2) an informational sheet about the study approved by the University of Oklahoma Health Sciences Center's Institutional Review Board (IRB #: 5744), (3) a coupon redeemable for two packages of HA batteries; (4) outcome measures (i.e., PIF, IOI-HA, MARS-HA, and SADL), and (5) a stamped return envelope. The order of outcome measures was counterbalanced across participants to minimize order effects. Completed outcome measures were accepted for 3 months from the original send date. The packets were mailed to the potential participants on September 3, 2015, and the cutoff date was October 15, 2015.
Results and Discussion
Of the 500 invited to participate, 16 were returned to sender. Of the remaining 484, 153 surveys were completed, rendering a 31.2% overall response rate. Thus, the data from 153 patients were included as participants in the present study. This sample of 153 participants included 79 females and 74 males with a mean age of 73.7 years (SD = 12). The participants had varying degrees of SNHL, which was determined by their FFPTA in the better ear and were categorized as: mild (0–40 dB HL), moderate (41–60 dB HL), severe (61–80 dB HL), or profound (>81 dB HL). Most of the participants had mild ( N = 56; 36.8%) or moderate ( N = 71; 46.7%) hearing losses in their better ears, while a smaller portion of the sample had severe ( N = 20; 13.2%) or profound ( N = 5; 3.3%) losses.
What Were the Characteristics of the ADT HA Wearers with MSNHL?
Tables 1 , 2 , and 3 show the participants' demographic, audiometric, and amplification characteristics, respectively, for the 56 (fewer responded to some items) with MSNHL. These participants had a mean age of 72 years (SD = 10.3), and Table 1 shows that they were 28 males and 27 females, and that most of them had two or more individuals living in their households, and were or had been married.
Table 1. Demographic information for patients having MSNHL.
| Characteristics | Frequency | Percent |
|---|---|---|
| Gender ( N = 55) | ||
| Male | 28 | 50.9 |
| Female | 27 | 49.1 |
| Marital status ( N = 56) | ||
| Single | 4 | 7.1 |
| Married | 37 | 66.1 |
| Divorced | 4 | |
| Separated | 1 | 1.8 |
| Widowed | 10 | 17.9 |
| Number in the household ( N = 56) | ||
| 1 | 11 | 19.6 |
| 2 | 35 | 62.5 |
| 3 or more | 10 | 17.9 |
Table 2. Mean (M) and standard deviation (SD) data for audiometric thresholds in right and left ears of participants with MSNHL ( N = 56) .
| Right | Left | |||
|---|---|---|---|---|
| M | SD | M | SD | |
| 250 | 20.4 | 14.2 | 20.2 | 14.7 |
| 500 | 23.5 | 14.7 | 23.7 | 19.0 |
| 1,000 | 30.8 | 15.4 | 30.0 | 17.6 |
| 2,000 | 39.6 | 14.1 | 42.5 | 17.9 |
| 4,000 | 57.4 | 15.6 | 58.3 | 15.7 |
| 8,000 | 67.7 | 19.9 | 68.1 | 19.1 |
Table 3. Information about MSNHL participants' hearing aid ownership including time with current devices, lifetime HA use, monaural versus binaural fitting, level of technology, and method of payment.
| Characteristic | Frequency | Percentage |
|---|---|---|
| Fitting ( N = 56) | ||
| Monaural | 13 | 23.2 |
| Binaural | 43 | 76.8 |
| Level of technology ( N = 53) | ||
| Entry | 5 | 9.45 |
| Standard | 19 | 35.9 |
| Advanced | 12 | 22.6 |
| Premium | 17 | 32.1 |
| Method of payment ( N = 56) | ||
| Private pay only | 39 | 69.6 |
| Private pay + insurance | 9 | 16.1 |
| Insurance only | 8 | 14.3 |
| Time with current device ( N = 55) | ||
| < 6 mo | 5 | 9.1 |
| 7–12 mo | 7 | 12.7 |
| 13–18 mo | 10 | 18.2 |
| 19–24 mo | 6 | 10.9 |
| > 24 mo | 27 | 49.1 |
| Lifetime hearing aid use ( N = 53) | ||
| < 6 mo | 2 | 3.8 |
| 7–12 mo | 5 | 9.45 |
| 13–18 mo | 7 | 13.2 |
| 19–24 mo | 5 | 9.45 |
| 2–10 y | 28 | 52.8 |
| > 10 y | 6 | 11.3 |
Table 2 shows audiometric data for right and left ears for those with MSNHL. Recall that Donahue and colleagues' definition 13 of MSNHL was an FFPTA of 26 to 40 dB HL in the better ear. The definition of MSNHL used in the present study was modified to an FFPTA ≤ 40 dB HL in the better ear without setting a lower limit, because it is possible that patients could have a precipitous high-frequency SNHL with an FFPTA ≤ 20 dB HL.
The participants' mean audiometric thresholds collectively showed a downward-sloping configuration of hearing loss. It is important to acknowledge that a variety of configurations (e.g., downward or upward sloping, and flat) of hearing loss fit within our definition of MSNHL. In addition, although many of the losses were symmetric, a few were unilateral and asymmetric. It is important for hearing health care professionals to realize that MSNHL does not necessarily imply that the loss is easy to fit. Indeed, patients with one nonfunctional ear and a MSNHL in the other need counseling to inform them that obtaining amplification in the better ear will likely not solve all communication difficulties caused by the head shadow effect. Additionally, some patients with MSNHL who have clinically significant tinnitus will likely require a hearing health care professional who can provide necessary counseling and who possesses the skills to fit an ADT combination device (i.e., HAs with built-in sound generators) with strategic use of built-in sound generators. However, there is little evidence available which shows that combination devices are any more effective in reducing the complaints of tinnitus than conventional HAs. 31 32
Table 3 shows information about participants' HA ownership including time with current devices, lifetime HA use, monaural versus binaural fitting, level of technology, and method of payment. Participants' HAs were primarily binaural fittings of varying levels of ADT. Seventy percent of the participants with MSNHL had paid for their HAs out of pocket and more than half of them opted for advanced or premium level devices. This is an important finding compared with other studies that have incorporated clinical trials of devices where participants may have indicated that they “would” like to keep them 33 or where the devices were provided to them for free in return for their participation in the study. 34 The participants in the present study had purchased their HAs, which seems to be a stronger finding than simply saying that they “would” like to buy them in the future. This finding would seem to imply that these individuals were convinced of the benefits they received from their HAs in spite of the fact that they had mild losses. This has even greater impact considering that the policy of the practice in the present study is that patients do not pay for their HAs until they are satisfied with them at the end of the trial period and that patients can return their devices for any reason without question at the end of the trial if they are not satisfied. The mean cost per aid was $2,138 (SD = $840) with an average total expenditure of $3,797 (SD = $1,775). Styles were predominantly receiver-in-the canal (in-the-ear) (RIC/RITE) HAs with a few receiver-in-the-aid and receiver-in-the-ear devices. These participants had varying levels of lifetime experience with their HAs and nearly two-thirds of them had used amplification for 2 years or more.
What Was the Benefit and Satisfaction Achieved by These ADT HA Users with MSNHL?
Benefit
Table 4 shows the mean and standard deviation data for our participants with MSNHL for the individual items on the IOI-HA compared with those from Smith and colleagues' 34 patients who obtained their ADT devices for free from Veterans Administration medical centers. Two sets of values are provided for the Smith et al data because Cox and colleagues 30 recommended having different norms to separate patients who rated their unaided communication difficulty as mild or moderate from those whose self-ratings were moderately severe or worse. Our MSNHL participants' self-ratings of unaided communication difficulty ranged from mild to moderately severe, with at least 25% in the latter category. Further, Cox and colleagues 30 recommended that separate norms be used for private-pay patients versus those whose HAs are provided for free. We elected to compare our results to Smith and colleagues 34 because their norms were for multiple-channel, multiple-memory HAs with digital signal processing to provide comparison with the ADT devices in the current study.
Table 4. Mean (M) and standard deviation (SD) data on the IOI-HA items for the hours of daily use (USE), benefit (BEN), residual activity limitation (RAL), satisfaction (SAT), residual participation restriction (RPR), impact on others (IO), and quality of life (QoL) for participants in the present study compared with those in the Smith and colleagues 34 study ( N = 56) .
| Present study | Smith et al 34 | |||||
|---|---|---|---|---|---|---|
| None to moderate | Moderately severe + | |||||
| M | SD | M | SD | M | SD | |
| USE | 4.30 | 1.06 | 3.6 | 1.0 | 4.2 | 1.0 |
| BEN | 4.04 | 0.94 | 3.8 | 1.0 | 3.9 | 1.0 |
| RAL | 3.70 | 0.77 | 3.9 | 0.8 | 3.3 | 1.0 |
| SAT | 4.29 | 0.99 | 4.2 | 1.0 | 4.3 | 1.0 |
| RPR | 4.13 | 0.73 | 4.1 | 0.7 | 3.3 | 1.1 |
| IO | 3.96 | 0.80 | 4.2 | 0.9 | 3.2 | 1.2 |
| QoL | 4.00 | 0.92 | 4.0 | 0.8 | 3.9 | 0.8 |
On average, our participants' mean response on the USE item indicated that they wore their HAs between 9 and 16 hours per day, which is consistent with their responses on the VASuse from the PIF, which revealed a mean wearing time of 10.1 hours per day (SD = 4.82). This indicates that most of our patients with MSNHL used their HAs during most waking hours and their wearing time was comparable to the norms reported by Smith and colleagues. 34 Our findings are also in agreement with that of Timmer and colleagues, 35 who found that according to data logging, patients with MSNHL wore their HAs an average of 8.5 hours per day and did not significantly differ from their peers with moderate SNHL. Similarly, our participants' mean values for the BEN, RAL, SAT, RPR, IO, and QoL were comparable to those reported by Smith and colleagues, 34 which causes us to conclude that patients with MSNHL generally achieve significant benefit from ADT HAs. Persons with mild losses perceive that they derive benefit from their HAs; this seems to be true whether the devices are paid for by patients or provided for free.
Satisfaction
Table 5 shows the mean and standard deviation data for our participants with MSNHL on the PE, SC, NF, and PI subscales and the global score obtained on the SADL compared with descriptive statistics from Uriarte and colleagues 36 and Hosford-Dunn and Halpern. 37
Table 5. Means (M) and standard deviation (SD) data on SADL subscales and global SADL scores for participants in this study compared with those in Uriarte and colleagues 36 and Hosford-Dunn and Halpern 37 ( N = 56) .
| Present study | Uriarte and colleagues 36 | Hosford-Dunn and Halpern 37 | ||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |
| PE | 5.26 | 1.13 | 4.98 | 1.21 | 4.9 | 1.2 |
| SC | 5.27 | 0.99 | 5.70 | 1.14 | 5.0 | 1.0 |
| NF | 4.81 | 1.53 | 4.74 | 1.38 | 4.0 | 1.5 |
| PI | 6.20 | 0.87 | 5.86 | 0.93 | 5.8 | 1.0 |
| Global | 5.38 | 0.81 | 5.27 | 0.81 | 5.0 | 0.8 |
Abbreviations: NF, Negative Features; PE, Positive Effect; PI, Personal Image; SC, Service and Cost.
Uriarte and colleagues 36 presented data from the SADL for a group of 1,014 Australian HA wearers who had received government-funded devices, and Hosford-Dunn and Halpern 37 provided norms on the SADL for patients fit with digitally programmable devices in a private-practice setting. Recall that the patients with MSNHL in the present study paid for their HAs primarily out of pocket, but in some cases, a partial or complete insurance benefit was used to obtain the HAs.
The patients with MSNHL in the present study achieved a high level of satisfaction on the PE subscale of the SADL, which measures contentment with acoustic and psychological aspects of HAs. Although statistical comparisons are not appropriate due to the heterogeneity among the three studies, the patients in the present study achieved a higher mean score on the PE subscale than the HA wearers from the other two studies. It is most likely that the higher average PE subscale score in the present study was due to our patients being fit mostly with advanced or premium level ADT devices, whereas the other studies only included ratings from persons fit with digitally programmable, analog HAs. Our patients with MSNHL achieved a high level of satisfaction with the acoustic benefits provided by ADT HAs as reflected in the global scores. Also, SADL item #1 asks, “Compared to using no hearing aid at all, do your hearing aids help you understand the people you speak with most frequently?,” and patients in the present study had a mean rating of 5.4 (SD = 1.2), which corresponds to “Considerably” to “Greatly.” Similarly, SADL item #5 asks, “Do your hearing aids reduce the number of times you have to ask people to repeat?,” and patients in the present study had a mean rating of 5.0 (SD = 1.5), which corresponds to “Considerably.” In addition, SADL item #10 asks, “How natural is the sound from your hearing aids?,” and patients in the present study had a mean rating of 5.1 (SD = 1.2), which corresponds to “Considerably.” Indeed, our HA wearers with MSNHL who were fit with mostly advanced to premium level ADT HAs using best practices for returning audibility had, on average, a high level of satisfaction with the acoustic benefits provided through the use of their amplification.
Uriarte and colleagues' 36 participants achieved a significantly higher mean score on the SC subscale than both the participants in the present study and the Hosford-Dunn and Halpern 37 study. Recall that the SC subscale measures satisfaction with the services provided by hearing health care professionals and the cost of the HAs. It is most likely that the differences seen here lies in the fact that the HAs provided to participants in the Uriarte and colleagues' 36 study were government-funded through the Australian Health Service, whereas the devices were paid for primarily out of pocket in the present study and the Hosford-Dunn and Halpern 37 study. Even so, the patients with MSNHL in the present study were highly satisfied with the service and the cost of their ADT HAs.
The mean scores on the NF subscale of the SADL for our participants (mean = 4.8; SD = 1.5) and those in the Uriarte et al (mean = 4.7; SD = 1.4) study were significantly higher than those reported by Hosford-Dunn and Halpern 37 (mean = 4.0; SD = 1.5). These differences may be due to the technology of HAs dispensed. As previously stated, most of the patients with MSNHL in the present study were fit with advanced-to-premium devices, while the aids in the Uriarte et al study 36 were digitally programmable, although the authors stated that, “Specific hearing aid details were not provided to the researchers.” Interestingly, Hosford-Dunn and Halpern 37 reported that 31% of their patients' HAs were single-channel and compression instruments and that 40.4% were analog instruments with adjustable multichannel/memory via NOAH software, and 28.6% were early digital signal processing devices. Our participants with MSNHL achieved a moderate level of satisfaction with the reduction of noise and feedback provided by their ADT HAs. For example, SADL item #2 asks, “Are you frustrated when your hearing aids pick up sounds that keep you from hearing what you want to hear?,” and participants in the present study had a mean rating of 4.3 (SD = 2.0), which corresponds to “Medium.” Similarly, SADL item #7 asks, “Are you bothered by an inability to get enough loudness from your hearing aids without feedback (whistling)?” and the participants in the present study had a mean rating of 5.5 (SD = 1.9), which, because it is a reversed item, corresponds to “Somewhat” to “A little.” As indicated earlier, these ADT HA wearers with MSNHL seemed to benefit from follow-up with hearing health care providers during the trial periods to ensure that participants could maximize the full capability of ADT HAs in reducing noise and eliminating feedback.
HA wearers from all three studies had high mean scores on the PI subscale, which indicates a high degree of satisfaction with the way their devices look and how patients feel wearing them. Consistent findings across the three studies support the notion that the hearing health care industry has improved the cosmetic appeal of HAs. Indeed, the “Hearing Aid Effect” or the negative stigma that observers exhibit toward persons who wear amplification and the noticeability of HAs 38 has been reduced with new styles of aids such as the completely-in-the-canal (CIC) devices that were popular at the time that the Hosford and Halpern study was conducted and via the open-ear fittings that were mainly used in the present study. Hosford-Dunn and Halpern 37 and Uriarte and colleagues 36 each reportedly fit nearly equal proportions of behind-the-ear, in-the-ear, and CIC style HAs, while most of the participants fit in the present study obtained RIC/RITE HAs.
Rauterkus and Palmer 39 conducted a study involving the noticeability of a variety of listening devices, including HAs, and concluded that the Hearing Aid Effect was no longer present in the eyes of persons observing individuals who wear HAs. However, Johnson and Danhauer 40 noted that while the Hearing Aid Effect has been reduced in the eyes of those observing persons wearing HAs, it still exists in the minds of person who wear HAs. In other words, it may matter less to patients what the lay public thinks of persons wearing HAs, but more so how patients themselves feel about wearing amplification, particularly those with mild SNHL who might be able to “get by” otherwise without help. The high degree of satisfaction achieved by our ADT HA wearers with MSNHL on the PI subscale of the SADL may indicate that the Hearing Aid Effect is less of an issue to persons with mild hearing loss, at least in the sample used in the present study. For example, SADL item #4 asks, “Do you think people notice your hearing loss more when you wear your hearing aids?,” and our patients in the present study had a mean rating of 6.1 (SD = 1.2), which, because it is a reversed item, corresponds to “A little.” Similarly, SADL item #8 asks, “Do you think wearing your hearing aids makes you seem less capable?” and our patients in the present study had a mean rating of 6.7 (SD = 0.9), which, because it is a reversed item, corresponds to “Not at all.” Thus, in summary, not only were these patients with MSNHL pleased with the appearance of their HAs, but they also exhibited a strong sense of self-esteem when wearing them.
Self-Efficacy
Table 6 shows the mean and standard deviation data for our HA wearers with MSNHL for the BH, AH, ADJ, and AL subscales of the MARS-HA. 28 Scores on the MARS-HA may range from 0 to 100%, with those below 80% indicating that additional counseling is warranted from a hearing health care professional. For example, our patients with MSNHL, on average, were confident with the basic handling of and adjustment to their devices. Alternatively, on the AH subscale, these HA wearers with MSNHL had a mean score of 65.0% with a large standard deviation (SD = 23.5), which indicates that additional counseling may be needed because the participants lacked confidence on skills such as identifying different components of HAs, stopping feedback (i.e., “whistling”), troubleshooting problems with their devices, naming the make/model of their devices, and the appropriate size of battery. For instance, our patients were not confident in troubleshooting their HAs (mean = 57.0; SD = 33.8) and had the least confidence in naming the make/model of their HAs (mean = 35.0; SD = 37.4). The large standard deviations obtained on all of the items loading onto the AH subscale indicated that some of these patients with MSNHL would need additional counseling to increase their self-efficacy in this area. Furthermore, it is important to note that these are satisfied HAs wearers who have achieved significant benefit from their devices, with nearly 90% of them having more than 1 year of experience with amplification. The finding that many lack confidence indicates a need for counseling and that the HA wearers' relationships with their hearing health care providers are critical. These data also indicate that development of OTC models for use with patients with MSNHL should seriously consider the importance of including of therapeutic relationships with hearing health care providers to help insure that consumers become confident HA wearers.
Table 6. Mean (M) and standard deviation (SD) data on the MARS-HA subscales and composite scores for users of ADT HAs with MSNHL ( N = 56) .
| M | SD | |
|---|---|---|
| BH | 94.4 | 9.4 |
| AH | 65.0 | 23.5 |
| ADJ | 90.6 | 14.5 |
| AL | 78.0 | 27.8 |
| Composite | 81.7 | 12.8 |
Abbreviations: ADJ, Adjustment; AH, Advanced Handling; AL, Aided Listening; BH, Basic Handling.
Another area of concern was the average score of 78% (SD = 15.8) on the AL subscale, which asks about patients' confidence in understanding conversations with another person, in small groups, and on telephones in quiet and in noise. Additionally, some items loading onto this subscale ask wearers about their confidence in understanding the television, a lecture, public service announcements over loudspeakers, and conversations in a car. In particular, average responses on the items asking about their confidence understanding lectures (mean = 61.2; SD = 27.0), understanding one-on-one (mean = 56.6; SD = 25.5) or small group conversations (mean = 70.5; SD = 25.8) in a noisy place, and understanding public service announcements over a loudspeaker (mean = 75.9; SD = 20.9) indicated a need for further counseling. Additionally, the large standard deviations indicated that patients with MSNHL vary in their confidence in being able to understand in noisy situations. Indeed, these results tended to agree with these patients' mean ratings on SADL #2, which indicated only a moderate degree of satisfaction in the noise reduction capabilities of their ADT instruments. These findings indicated that when patients with MSNHL are fit with ADT HAs (with the majority of them being advanced and premium devices), they need extensive counseling on how, when, and why to use their different available programs to their advantage so that they will have confidence in difficult listening situations. Moreover, it is important to ask these patients about any difficulties they are having on an ongoing basis to determine whether additional hearing assistive technology or compensatory communication strategies are warranted.
The MARS-HA 28 only queries patients about their perceived confidence in handling HAs, not their actual ability to understand specific information or to perform specific skills with their instruments. The degree of benefit from and satisfaction with ADT HAs as measured by the IOI-HA and the SADL indicated that the outcomes achieved by these patients with MSNHL with these devices exceeded their confidence levels. The private practice dispensing these devices provides informational and personal adjustment counseling to all of their HA wearers. The fact that even successful ADT HA wearers reported a lack of confidence indicates that they may need additional counselling and ongoing support from their hearing health care providers. These findings indicate that future delivery models with OTC devices should ensure that adequate counseling is available for patients with MSNHL, particularly about the importance of care and maintenance of their devices. For example, the item receiving the lowest average score for these MSNHL participants on the entire MARS-HA was the one asking patients about their certainty about their ability to clean and care for a HA regularly. Regardless of whether patients with MSNHL lack confidence due to inadequate HA knowledge and skills or not being able to find enough time to care for their HAs, they need to know that changing a wax trap, for example, may make all the difference in being able to use their devices. In addition, proper care and maintenance can extend the life of ADT HAs, indirectly protecting patients' investments. Unfortunately, there will likely be no one (let alone an audiologist) to provide counseling in a OTC model for consumers.
Summary
This study presented preliminary findings about the characteristics of and outcomes for a cohort of patients with MSNHL who were fit with ADT HAs and primarily paid out of pocket for their devices. To date, no study has reported on patients with MSNHL who have obtained ADT HAs despite the mandate that the U.S. FDA develop a classification of OTC wearable hearing instruments for use by adults with mild to moderate hearing losses. In fact, the AAA 20 and the ASHA 41 published position statements about OTC HAs and generally supported the notion of OTC devices, but only for patients with MSNHL. Unfortunately, there is a paucity of data available indicating the needs of and outcomes for HA wearers with MSNHL, whether purchased OTC or via traditional audiologic dispensing protocols. Careful consideration must be given to the design of the classification of OTC devices and with the delivery models available for dispensing the devices. It is important for the hearing health care industry to realize that mild hearing loss does not imply “easy” cases in which patients' needs can necessarily be met with simple OTC wearable amplification, only worn in select situations. The definition of MSNHL used in this investigation indicates a wide variety of possibly complicated patient scenarios, many of which may require evidence-based fitting with ADT technology that insures audibility. These solutions may need to be coupled with hearing assistive technology and some individuals may require directed auditory rehabilitation programs.
HA uptake and use is low for a variety of reasons. Positive experiences with HAs and hearing health care providers are critical for patients to use and receive benefit from and achieve satisfaction with ADT. We believe that the early experiences of patients with MSNHL are critical in setting the stage for lifelong successful HA use. Our patients with MSNHL fit with ADT HAs using best practices to achieve audibility received significant benefit from, satisfaction with, and had a high degree of self-efficacy for their amplification. They reportedly wore their ADT HAs an average of 10.5 hours per day, which was likely enhanced by the comprehensive follow-up program used by the private practice that dispensed the HAs. Nevertheless, it was surprising that some of the participants still lacked confidence in advanced handling skills and in their ability to understand in difficult listening situations. Although satisfaction was high on most of the SADL subscales, only moderate levels of satisfaction were achieved on individual items that inquired about whether their HAs picked up sounds that kept them from hearing what they wanted to hear. These findings indicated that adequate follow-up and counseling by hearing health care professionals is critical for patients with MSNHL to have confidence in their ability to use the noise reduction capabilities of ADT HAs. At the same time, these results call into question whether similar outcomes could be achieved via OTC devices that are sold as a commodity without professional assistance.
Future research is needed to determine the needs of and possible outcomes for patients of different ages, communication demands, and various configurations of MSNHL that are fit with ADT HAs by service-delivery models that vary from that used here. It is unknown whether these results would generalize to models that differ from the one used in the present study. Nevertheless, the present study demonstrated that patients with mild hearing loss, who mainly purchased their ADT HAs out of pocket, derived significant benefits from and satisfaction with their devices. These results for patients with mild hearing losses were similar to other studies where patients did not have to pay for the HAs themselves. Collectively, these are findings that audiologists, physicians, and interested third parties should know and communicate to prospective patients so that they may be more likely to avail themselves of hearing help before their losses progress along with the insidious negative comorbidities of untreated SNHL. In current studies, we are addressing tinnitus issues associated with this population of patients having MSNHL and whether HAs can help reduce the negative effects of tinnitus as well as hearing loss.
References
- 1.Chisolm T H, Johnson C E, Danhauer J L et al. A systematic review of health-related quality of life and hearing aids: final report of the American Academy of Audiology Task Force On the Health-Related Quality of Life Benefits of Amplification in Adults. J Am Acad Audiol. 2007;18(02):151–183. doi: 10.3766/jaaa.18.2.7. [DOI] [PubMed] [Google Scholar]
- 2.Keller B K, Morton J L, Thomas V S, Potter J F. The effect of visual and hearing impairments on functional status. J Am Geriatr Soc. 1999;47(11):1319–1325. doi: 10.1111/j.1532-5415.1999.tb07432.x. [DOI] [PubMed] [Google Scholar]
- 3.Kramer S E, Goverts S T, Dreschler W A, Boymans M, Festen J M. International Outcome Inventory for Hearing Aids (IOI-HA): results from The Netherlands. Int J Audiol. 2002;41(01):36–41. doi: 10.3109/14992020209101310. [DOI] [PubMed] [Google Scholar]
- 4.Lin F R, Albert M. Hearing loss and dementia - who is listening? Aging Ment Health. 2014;18(06):671–673. doi: 10.1080/13607863.2014.915924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin F R, Yaffe K, Xia J et al. Hearing loss and cognitive decline in older adults. JAMA Intern Med. 2013;173(04):293–299. doi: 10.1001/jamainternmed.2013.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institute on Deafness and Other Communication Disorders.Hearing loss and older adults. 2016Available at:https://www.nidcd.nih.gov/health/hearing-loss-older-adults. Accessed June 12, 2017
- 7.Abrams H B, Kihm J. An Introduction to MarkeTrak IX: A new baseline for the hearing aid market. Hearing Review. 2015;22(06):16. [Google Scholar]
- 8.Bainbridge K E, Ramachandran V. Hearing aid use among older U.S. adults; the national health and nutrition examination survey, 2005-2006 and 2009-2010. Ear Hear. 2014;35(03):289–294. doi: 10.1097/01.aud.0000441036.40169.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis A, Smith P, Ferguson M, Stephens D, Gianopoulos I. Acceptability, benefit and costs of early screening for hearing disability: a study of potential screening tests and models. Health Technol Assess. 2007;11(42):1–294. doi: 10.3310/hta11420. [DOI] [PubMed] [Google Scholar]
- 10.Blazer D G, Domnitz S, Liverman C T. Washington, DC: National Academies Press; 2016. Hearing Health Care for Adults: Priorities for Improving Access and Affordability. [PubMed] [Google Scholar]
- 11.Johnson C E, Danhauer J L, Ellis B B, Jilla A M. Hearing aid benefit in patients with mild sensorineural hearing loss: a systematic review. J Am Acad Audiol. 2016;27(04):293–310. doi: 10.3766/jaaa.14076. [DOI] [PubMed] [Google Scholar]
- 12.Timmer B H, Hickson L, Launer S. Adults with mild hearing impairment: Are we meeting the challenge? Int J Audiol. 2015;54(11):786–795. doi: 10.3109/14992027.2015.1046504. [DOI] [PubMed] [Google Scholar]
- 13.Donahue A, Dubno J R, Beck L. Guest editorial: accessible and affordable hearing health care for adults with mild to moderate hearing loss. Ear Hear. 2010;31(01):2–6. doi: 10.1097/AUD.0b013e3181cbc783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization.WHO Global Estimates on Prevalence of Hearing Loss: Mortality and Burden of Diseases and Prevention of Blindness and Deafness Geneva: WHO; 2012 [Google Scholar]
- 15.Goman A M, Lin F R. Prevalence of hearing loss by severity in the United States. Am J Public Health. 2016;106(10):1820–1822. doi: 10.2105/AJPH.2016.303299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sindhusake D, Mitchell P, Smith W et al. Validation of self-reported hearing loss. The Blue Mountains Hearing Study. Int J Epidemiol. 2001;30(06):1371–1378. doi: 10.1093/ije/30.6.1371. [DOI] [PubMed] [Google Scholar]
- 17.Kochkin S. MarkeTrak VIII: The key influencing factors in hearing aid purchase intent. Hear Rev. 2012;19(03):12–25. [Google Scholar]
- 18.Holdren J P, Lander E, Press W, Savitz M. Washington, DC: President's Council of Advisors on Science and Technology; 2015. Aging America and Hearing Loss: Imperative of Improved Hearing Technologies; pp. 1–15. [Google Scholar]
- 19.U.S. Congress.H.R. 2430: FDA Reauthorization Act of 2017. August 29, 2017Available at:https://www.govtrack.us/congress/bills/115/hr2430/summary. Accessed November 19, 2017
- 20.Over-the-Counter (OTC) Hearing Devices.Issue Statement. January 26, 2017Available at:https://www.audiology.org/publications/over-counter-otc-hearing-devices. Accessed November 8, 2017
- 21.Cox R M, Johnson J A, Xu J. Impact of hearing aid technology on outcomes in daily life I: The patients' perspective. Ear Hear. 2016;37(04):e224–e237. doi: 10.1097/AUD.0000000000000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson J A, Xu J, Cox R M. Impact of hearing aid technology on outcomes in daily life II: Speech understanding and listening effort. Ear Hear. 2016;37(05):529–540. doi: 10.1097/AUD.0000000000000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson J A, Xu J, Cox R M. Impact of hearing aid technology on outcomes in daily life III. Ear Hear. 2017;38(06):746–759. doi: 10.1097/AUD.0000000000000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Academy of Audiology.Guidelines for the Audiologic Management of Adult Hearing Impairment Reston, VA: American Academy of Audiology; 2007 [Google Scholar]
- 25.American National Standards Institute - Acoustical Society of America.Specification of Hearing Aid Characteristics Melville, NY: American National Standards Institute - Acoustical Society of America; 2009 [Google Scholar]
- 26.Jilla A M. Oklahoma City, OK: Jilla AM; 2016. The Visual Analog Scale of Hearing Aid Use (VASuse) [Google Scholar]
- 27.Cox R M, Alexander G C. The International Outcome Inventory for Hearing Aids (IOI-HA): psychometric properties of the English version. Int J Audiol. 2002;41(01):30–35. doi: 10.3109/14992020209101309. [DOI] [PubMed] [Google Scholar]
- 28.West R L, Smith S L. Development of a hearing aid self-efficacy questionnaire. Int J Audiol. 2007;46(12):759–771. doi: 10.1080/14992020701545898. [DOI] [PubMed] [Google Scholar]
- 29.Cox R M, Alexander G C. Measuring Satisfaction with Amplification in Daily Life: the SADL scale. Ear Hear. 1999;20(04):306–320. doi: 10.1097/00003446-199908000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Cox R M, Alexander G C, Beyer C M. Norms for the international outcome inventory for hearing aids. J Am Acad Audiol. 2003;14(08):403–413. [PubMed] [Google Scholar]
- 31.Henry J A, Frederick M, Sell S, Griest S, Abrams H. Validation of a novel combination hearing aid and tinnitus therapy device. Ear Hear. 2015;36(01):42–52. doi: 10.1097/AUD.0000000000000093. [DOI] [PubMed] [Google Scholar]
- 32.Parazzini M, Del Bo L, Jastreboff M, Tognola G, Ravazzani P. Open ear hearing aids in tinnitus therapy: An efficacy comparison with sound generators. Int J Audiol. 2011;50(08):548–553. doi: 10.3109/14992027.2011.572263. [DOI] [PubMed] [Google Scholar]
- 33.Humes L E, Rogers S E, Quigley T M, Main A K, Kinney D L, Herring C. The effects of service-delivery model and purchase price on hearing-aid outcomes in older adults: a randomized double-blind placebo-controlled clinical trial. Am J Audiol. 2017;26(01):53–79. doi: 10.1044/2017_AJA-16-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith S L, Noe C M, Alexander G C. Evaluation of the International Outcome Inventory for Hearing Aids in a veteran sample. J Am Acad Audiol. 2009;20(06):374–380. doi: 10.3766/jaaa.20.6.5. [DOI] [PubMed] [Google Scholar]
- 35.Timmer B HB, Hickson L, Launer S. Hearing aid use and mild hearing impairment: Learnings from big data. J Am Acad Audiol. 2017;28(08):731–741. doi: 10.3766/jaaa.16104. [DOI] [PubMed] [Google Scholar]
- 36.Uriarte M, Denzin L, Dunstan A, Sellars J, Hickson L. Measuring hearing aid outcomes using the Satisfaction with Amplification in Daily Life (SADL) questionnaire: Australian data. J Am Acad Audiol. 2005;16(06):383–402. doi: 10.3766/jaaa.16.6.6. [DOI] [PubMed] [Google Scholar]
- 37.Hosford-Dunn H, Halpern J. Clinical application of the Satisfaction with Amplification in Daily Life scale in private practice I: statistical, content, and factorial validity. J Am Acad Audiol. 2000;11:523–539. [PubMed] [Google Scholar]
- 38.Johnson C E, Danhauer J L, Gavin R B, Karns S R, Reith A C, Lopez I P. The “hearing aid effect” 2005: a rigorous test of the visibility of new hearing aid styles. Am J Audiol. 2005;14(02):169–175. doi: 10.1044/1059-0889(2005/019). [DOI] [PubMed] [Google Scholar]
- 39.Rauterkus E P, Palmer C V. The hearing aid effect in 2013. J Am Acad Audiol. 2014;25(09):893–903. doi: 10.3766/jaaa.25.9.10. [DOI] [PubMed] [Google Scholar]
- 40.Johnson C E, Danhauer J L. Comments regarding Rauterkus and Palmer (2014) J Am Acad Audiol. 2015;26(08):741–743. doi: 10.3766/jaaa.15044. [DOI] [PubMed] [Google Scholar]
- 41.ASHA.ASHA Position Statement on Policy Related to Over-the-Counter Hearing Aids. February 14, 2017Available at:https://www.asha.org/News/2017/ASHA-Position-Statement-on-Policy-Related-to-Over-the-Counter-Hearing-Aids/. Accessed November 8, 2017


