Abstract
Standard audiometric evaluations are not sensitive enough to identify hidden hearing loss (HHL) and/or cochlear synaptopathy (CS). Patients with either of these conditions frequently present with difficulty understanding speech in noise or other complaints such as tinnitus. The purpose of this systematic review is to identify articles in peer-reviewed journals that assessed the sensitivity of audiologic measures for detecting HHL and/or CS, and which showed potential for use in a clinical test battery for these disorders. A reference librarian submitted specific boolean terminology to MEDLINE, Embase, and Web of Science. The authors used a consensus approach with specially designed score sheets for the selection of titles, abstracts, and then articles for inclusion in the systematic review and for quality assessment. Fifteen articles were included in the systematic review. Seven articles involved humans; seven involved animals, and one study used both humans and animals. Results showed that pure-tone audiometry to 20 kHz, otoacoustic emissions, electrocochleography, auditory brainstem response (ABR), electrophysiological tests, speech recognition in noise with and without temporal distortion, interviews, and self-report measures have been used to assess HHL and/or CS. For HHL, ultra-high-frequency audiometry may help identify persons with sensory hair cell loss that does not show up on standard audiograms. Promising nonbehavioral measures for CS included ABR wave I amplitude, the summating potential-to-action potential ratio, and speech recognition in noise with and without temporal distortion. Self-report questionnaires also may help identify auditory dysfunction in persons with normal hearing.
Keywords: hidden hearing loss, cochlear synaptopathy, King–Kopetzky syndrome, assessment
Learning Outcomes: As a result of this activity, the participant will be able to: (1) discuss symptoms associated with hidden hearing loss and with cochlear synaptopathy; (2) describe audiologic tests which have been used to investigate hidden hearing loss and/or cochlear synaptopathy in studies involving human and animal models of hearing.
Sensorineural hearing loss (SNHL) resulting from damage to the cochlea has traditionally been associated with elevation of hearing thresholds above 20 dB HL as assessed using pure-tone audiometry in the range from 0.25 to 8 kHz. These elevated thresholds have been presumed to represent dysfunction or loss of inner and/or outer hair cells, with outer hair cells demonstrating the greatest initial susceptibility to damage. Pure-tone audiometry has been used for monitoring hearing sensitivity in hearing conservation programs that are based on the premise that if audiometric thresholds are ≤ 20 dB HL, no damage to the auditory system is suspected. However, audiologists often conduct evaluations on patients who report auditory symptoms such as difficulty hearing in noise, tinnitus, and misunderstanding spoken messages, but who present with audiometrically normal hearing on standard hearing tests. This can be frustrating for patients who experience limitations in the ability to participate in daily activities, but find that formal testing does not validate their complaints. Unfortunately, these patients frequently receive counseling that they have normal hearing while their other auditory symptoms and complaints go undiagnosed and/or untreated. While audiologists may be sympathetic to these patients' complaints, they do not routinely have or use diagnostic tools to evaluate complaints effectively. Further, patients' confidence in audiologists and audiologic measures may become questioned when patient complaints are not adequately addressed and confirmed in audiologic evaluations. This class of auditory disorder now has a name: hidden hearing loss (HHL).
The idea that some patients with apparently normal hearing sensitivity report difficulties in understanding speech and difficulties tolerating tinnitus has been reported extensively. 1 2 This phenomenon has variously been labeled King–Kopetzky syndrome, obscure auditory dysfunction, auditory disability with normal hearing, or a subcategory of central auditory processing disorder. 3 4 5 More recently, the term HHL has been used to describe patients whose hearing loss is undetectable by pure-tone audiometry between 0.25 and 8 kHz, but which may potentially be revealed through patient report or some physiologic measures. 6 7
Recent evidence from animal models has revealed that cochlear damage related to noise exposure can exist in the presence of normal hearing thresholds in the conventional audiometric range. For instance, Kujawa and Liberman found that mice that were exposed to mild acoustic trauma demonstrated only a temporary shift in hearing thresholds, but a permanent deafferentation (i.e., loss of afferent ribbon synapses at the base of inner hair cells) of more than 50% when measured at the base of the cochlea (i.e., the area corresponding to high-frequency sensitivity). 7 Similarly, abnormal spontaneous neuronal activity and neuropathy have been observed in structures in the auditory brainstem of rodents that received mild noise exposure. 8 9 10 If these findings are generalizable to humans, then the findings may support the existence of auditory physiologic changes in noise-exposed individuals that may not be detectable using common clinical measures. The literature reveals that HHL is sometimes used synonymously with the term cochlear synaptopathy (CS); however, not all HHL may be attributable to loss of cochlear ribbon synapses. 11 12
In addition to these documented links between CS and noise exposure, there is a growing body of evidence relating ribbon synapse loss to normal aging. 13 14 Abdala and Dhar have found that changes from aging can begin in early adulthood even for those without significant noise exposure. 15 Sergeyenko and colleagues showed that aging decreases the number of spiral ganglion cells, synaptic structures, and cochlear nerve terminal boutons in an animal model. 13 Evidence from Fernandez and colleagues compared control animals to noise-exposed animals over time to describe differences between aging without synaptopathic noise exposure and the combined effects of aging with synaptopathic noise exposure. 11 Collectively, those studies provide evidence that although aging alone may be associated with CS, noise exposure may cause the inner ear aging process to accelerate. 11 13
Given the recent increase in awareness of HHL and CS, and the shortcomings of the standard audiologic test battery for detection of HHL/CS, it would behoove audiologists to develop and validate tests that are sensitive to early changes in the auditory system. Such tests could be valuable in understanding the effects of damage to and aging of the auditory system, and aid in the prevention, diagnosis, and intervention for preclinical auditory dysfunction. Enhancing the standard audiologic test battery may assist hearing health care professionals in identifying early auditory dysfunction so that measures may be taken to protect hearing or treat subtle damage in the inner ear more quickly. Liberman and colleagues found that young musicians had significantly different results on a test battery for CS than young low-risk noise exposure participants, suggesting that such a battery could be used to help identify individuals in need of hearing conservation programs. 16 In addition, basic science research teams are assessing CS in animal models using different noise exposure protocols and tests of auditory dysfunction, some of which are routinely used in clinical audiology. 11 13 17 18 19 20 21 22 Some preclinical studies are in the process of transitioning to phase II clinical trials of compounds that might prevent or reverse CS in humans, which requires a test battery with clinical end points that are sensitive to CS in humans.
Effective test batteries are those that not only are sensitive and specific for the disorders being assessed, but also are feasible for use in both clinical trials and in audiology clinical test protocols. Development of new test protocols must begin with a careful look at investigations assessing HHL and/or CS in humans and in animal models. The purpose of the present systematic review was to determine: (1) what audiologic measures have been used to assess HHL and CS in both human and animal models, and (2) whether there is a sufficient evidence base to recommend certain clinical tests for detection of HHL and CS. Results of this review will: (1) assist in identifying techniques that can be used for the early detection of auditory dysfunction; (2) augment understanding of standard audiologic assessment of patients with normal hearing sensitivity, but who present with suprathreshold auditory dysfunction; and (3) identify tests most likely to serve as clinical end points in phase II clinical trials.
Methods
Study Selection Criteria
The systematic review team (C. M. B., J. J., C. E. J., J. H. P., E. M. S.) agreed on the following a priori criteria for types of studies, participants, and diagnostic tools that had to be satisfied for articles to be included in the present systematic review.
Types of studies: The literature search focused on experimental studies of HHL and/or CS, which included audiological measures as diagnostic tools, histology, and which were published in peer-reviewed journals.
Types of participants: Articles with either animal and/or human participants were included. Studies in animals could include mammals of all ages and genders. Studies in humans could include participants of all ages and genders.
Types of diagnostic tools: The literature search sought outcomes for diagnostic tools including, but not limited to: otoacoustic emissions (OAEs), auditory brainstem response (ABR), electrocochleography (ECochG), tone detection with noise, speech in noise, frequency following response (FFR), high-frequency audiometry, interaural time difference detection (ITDD), questionnaires, and other tools identified in the reviewed studies.
Search and Retrieval Process for Identifying Studies
Members of the systematic review team (C. M. B., J. J., C. E. J., J. H. P., E. M. S.) developed a full search strategy to identify potential studies to include in the present systematic review. A reference librarian used search strategies shown in Appendix I for the MEDLINE, Embase, and Web of Science databases. The literature search was completed on June 15, 2017.
Appendix I. Search engines with their respective boolean strategies used by the reference librarian.
| MEDLINE search strategy: |
|---|
| 1 synaptopath$.mp. (212) |
| 2 cochlea$.mp. (47248) |
| 3 1 and 2 (54) |
| 4 remove duplicates from 3 (51) |
| 5 ..l/4 lg = en (47) |
| 6 electrocochleograph$.mp. (967) |
| 7 ecog.mp. (7002) |
| 8 tenhl.mp. (0) |
| 9 ten$.mp. (942620) |
| 10 otoacoustic$.mp. (5536) |
| 11 audiometry.mp. (28390) |
| 12 speech$.mp. (98819) |
| 13 nois$.mp. (132804) |
| 14 (listen$ adj2 effort$).mp. (203) |
| 15 or/6–14 (1180896) |
| 16 1 and 15 (47) |
| 17 remove duplicates from 16 (44) |
| 18 ..l/17 lg = en (41) |
| 19 18 not 5 (5) |
| Embase search strategy: |
| 1 synaptopath$.mp. (264) |
| 2 cochlea$.mp. (56319) |
| 3 1 and 2 (49) |
| 4 remove duplicates from 3 (44) |
| 5 ..l/4 lg = en (42) |
| 6 electrocochleography/(1408) |
| 7 (electrocochleogr$ or cochleo$).mp. (4275) |
| 8 ecog.mp. (19945) |
| 9 tenhl.mp. (0) |
| 10 ten$.mp. (1336065) |
| 11 otoacoustic$.mp. (6517) |
| 12 audiometr$.mp. (41240) |
| 13 speech$.mp. (141685) |
| 14 nois$.mp. (155180) |
| 15 (listen$ adj2 effort$).mp. (165) |
| 16 or/6–15 (1656319) |
| 17 1 and 16 (44) |
| 18 remove duplicates from 17 (40) |
| 19 ..l/18 lg = en (38) |
| 20 19 not 5 (6) |
| Web of Science search strategy: |
| 1 synaptopath* (239) |
| 2 cochle* (38,823) |
| 3 #2 and #1 (42) |
| 4 #2 and #1 AND language: (English) (44) |
| 5 Electrocochleogr* or cochlea*, or tenhl*, or otoacoustic*, or audiometr*, or speech*, or nois*, or listen* or effort* and language (English) (936,363) |
| 6 #5 and #1 (42) |
| 7 #6 not #3 and language (English) (6) |
Quality Assessment
The members of the systematic review team (listed above) independently used the same rubric to assess the quality of the included studies and answered the following questions:
Was the paradigm used appropriate for the experimental question(s)?
Did the study account for other possibilities for CS and/or HHL results?
Were the experimental conditions assessed unconfounded?
Were the dependent variables clearly defined?
Were the parameters for measurements described completely?
Were measurements made with judges blinded to the experimental conditions?
Was interjudge reliability assessed?
Was intrajudge reliability assessed?
Were dropouts accounted for?
Results
Study Flow
Fig. 1 shows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) chart detailing the search and retrieval process. In total, 150 records were identified through the database search. Sixty-three articles remained after removal of duplicates. The team members independently reviewed titles and abstracts, and, lastly, selected full articles that met inclusion criteria for use in the systematic review. A consensus approach determined that from 63 potential titles, 22 abstracts were reviewed, which resulted in 15 articles being retrieved for full-text review because a priori criteria were met and the articles were suitable for inclusion in the present systematic review.
Figure 1.
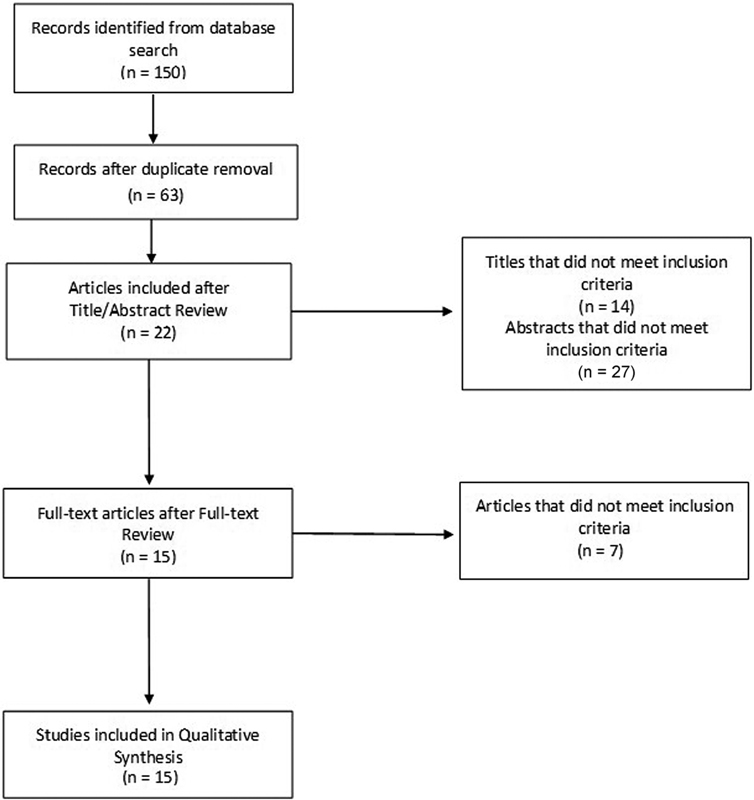
PRISMA chart of the search and retrieval process.
Study Characteristics
Appendices II and III provide information about the experimental questions, participant characteristics, noise exposure paradigm (if applicable), test protocol, and study outcomes of human and animal studies, respectively. Eight articles involved humans and eight were conducted with animals.
Appendix II. Summary of studies of hidden hearing loss and cochlear synaptopathy involving humans.
| Author(s) (year) Study Design |
Question | Participant characteristics | Noise protocol | Test protocol | Outcome |
|---|---|---|---|---|---|
| Bramhall et al (2017) 23 | Are higher lifetime noise exposure histories in young adults with normal pure-tone thresholds associated with lower ABR wave I amplitudes? | 19–35 years of age Pure-tone thresholds of ≤20 dB HL from 0.25–8 kHz 29 veterans 35 nonveterans Assigned to one of four groups based on reported noise exposure and veteran status 11 veterans with high noise exposure 7 veterans with low noise exposure |
Questionnaire: Lifetime Exposure of Noise and Solvents Questionnaire (LENS-Q) Interview |
ABR
Stimulus type : tone burst at 1, 3, 4, and 6 kHz Stimulus amplitude : @ 1 kHz—70, 80, 90, 100, 110 dB p-peSPL @4 kHz—60, 70, 80, 90, 100, 110 dB p-peSPL @ 6 kHz—110 dB p-peSPL Stimulus rate : 11.1 Hz Stimulus polarity : alternating Repetitions : 1,024 presentations for two replications Stimulus duration : rise/fall time of 0.5 ms with Blackman envelope; 4 ms for 1 kHz; 2.5 ms for 3 kHz; 2 ms for 4 kHz; 1.5 ms for 6 kHz Electrode montage : tiptrode ear canal and high forehead High frequency audiometry (HFA) 9–16 kHz OAE ER-10 B+ probe microphone; EMAV software Frequency ratio f 1 /f 2 = 1.2; L 1 = 55 dB SPL; L 2 = 65 |
ABR: Wave I amplitude reduced in group with higher reported noise exposure; decrease in amplitude of 29% in veteran high noise group compared with nonveterans control group |
| Guest et al (2017) 24 | Is tinnitus along with a normal audiogram (TNA) associated with greater lifetime noise exposure? Is TNA associated with ABR effects consistent with CS? Is TNA associated with temporal coding deficits consistent with CS? |
25.7 ± 1.3 years of age for tinnitus group; 25.5 ± 1.3 years of age for control group Pure-tone thresholds of 0.25–8 kHz, normal middle ear pressure and compliance 20 subjects in tinnitus group; 20 subjects in control group No history of head trauma, middle ear surgery, neurologic disorder, and ototoxic exposure |
Interview |
ABR
Stimulus type : high-pass filtered click Stimulus amplitude : 102 dB peSPL Stimulus rate : 7.05 Hz Repetitions : 7,040 per ear Transducer : ER3A insert Electrode montage : Cz and ipsilateral mastoid EFR Stimulus type : transposed tones with a 4-kHz carrier and 0.1-Hz modulator Stimulus duration : 400 ms plus 15 ms onset and offset ramps S/N : 20-dB broadband RMS Modulation depth and polarity : 0 dB and −6 dB re:100% modulation; two polarities Repetitions : 630 Interstimulus interval: 400 ms |
ABR: no association evident between noise exposure and amplitude of wave I; no association between noise exposure and wave I/V ratio Tinnitus was not associated with reduced ABR wave I amplitude between groups EFR: no association between noise exposure and EFR amplitude at shallow modulation depth |
| Liberman et al (2016) 16 | Are ECochG, OAE, HFA, and speech perception sensitive to differences in groups of high-risk versus low-risk noise exposure? | 18–41 years of age, native English speakers, pure-tone thresholds of <20 dB HL at 0.25–8 kHz 22 subjects in high-risk group 12 subjects in low-risk group No history of ear or hearing problems, or neurologic disorders |
Self-report via questionnaire |
Degraded speech recognition:
Stimulus: NU-6, 50 word lists Condition: in quiet, ipsilateral S/N +5 dB, ipsilateral S/N 0 dB Stimulus: Time compressed Condition: 45 or 65% both with 0.3 second reverberation added ECochG: Stimulus type : 100 μs clicks Stimulus amplitude : 94.5 dB nHL stimulus and 55 dB nHL contralateral masking Stimulus polarity : alternating Stimulus rate : 9.1 or 40.1 Hz Repetitions : up to 2 k Filter : 0.01–3 kHz bandpass filter Artifact reject : enabled Transducer : ER3A earphones Electrode montage : horizontal with forehead ground Impedance : N/R; between electrodes were <2 k ohms HFA: 9–16 kHz using Circumaural HDA200 high-frequency headset OAE: DPOAE; amplitude versus frequency sweeps; frequency ratio f 1 /f 2 = 1.2; L 1 =65 dB SPL, L 2 = 55 dB SPL; frequencies from 0.5–12 kHz |
Degraded speech recognition (speech in noise, time compressed, time compressed with reverberation); performance was significantly worse in the high-risk group HFA thresholds were significantly worse for high-risk group ECochG SP/AP ratio high-risk nearly twice low-risk group OAE amplitudes were not significantly different between groups |
| Mehraei et al (2017) 26 | Does auditory nerve fiber deafferentation increase forward masking thresholds and does forward masking increase wave V latency? | 20–30 years of age; pure-tone thresholds of 0.25–8 kHz < 20 dB HL | None |
Forward masking ABR:
Masker stimulus type : 100 ms long broadband noise Probe stimulus type : flat-spectrum, broadband chirp (0.08–20 kHz) Masker stimulus amplitude : 35 and 70 dB SPL Probe stimulus amplitude : 90 dB peSPL Stimulus polarity : not stated Stimulus rate : 2 Hz Repetitions : 1.5 k trials per stimulus condition Filter : 0.1–2 kHz Artifact reject : not stated Transducer : not stated Electrode montage : 5 channel: Pz, Cz, Fz, M1, M2 Impedance : not stated MPI : 20, 40, 201 ms Forward masking behavioral experiment: Masker stimulus amplitude : 35 and 70 dB SPL Probe stimulus: flat-spectrum, broadband chirp (0.08–20 kHz) Offset of masker to onset of probe (MPI ): 20, 40, 72, 132, 168, 201 ms Measure : chirp threshold in quiet and for each condition |
Masked ABR may be a useful tool in identifying cochlear synaptopathy |
| Mehraei et al (2016) 19 | Does noise affect latency of ABR wave V? Does the effect of noise on wave V latency predict perceptual temporal sensitivity? | 20–40 years of age; pure-tone thresholds of ≤ 15 dB HL 0.25–8 kHz | None for human (see animal table for animal data from this study) |
ABR wave I and V
Stimulus type : 80 μs click Stimulus amplitude : 50–90 dB peSPL in 10 dB steps (wave V) and 60–100 dB peSPL (wave I) Stimulus polarity : alternating Stimulus rate : 10 Hz Repetitions : 3 k Filter : bandpass 0.1–2 kHz Artifact reject : N/R Transducer : ER-1 insert phones Electrode montage : Ear canal, Cz, Fz Impedance : N/R ABR masked Stimulus type : 80 μs click Stimulus amplitude : 80 dB peSPL with broadband noise varying from 42–82 dB SPL in 1-dB steps Stimulus polarity : alternating Stimulus rate : 10 Hz Repetitions : 3,000 Filter : bandpass 0.1–2 kHz Artifact reject : N/R Transducer : ER-10C earphones Electrode montage : Ear canal, Cz, Fz Impedance : N/R Interaural time difference detection: transposed tone; carrier frequency either 2 or 4 kHz; modulation frequency of 50 Hz Carrier phase same for both ears; ITDD applied to the 50 Hz envelope OAE Click-evoked; bandpass filtered 0.25–6 kHz; growth function; f 2 /f 1 = 1.2 |
ABR: Wave V latency decreased with intensity increase; wave V latency in background noise increased with increase in intensity Perceptual temporal sensitivity can be predicted by wave V latency (accounting for effects of noise) No correlation between wave V latency shift and OAE results |
| Prendergast et al (2017) 28 | Is cochlear synaptopathy prevalent in young adults with normal hearing and is it measurable by suprathreshold electrophysiologic measures? | 18–36 years of age; pure-tone thresholds of ≤ 25 dB HL 0.25–8 kHz | Structured interview |
ABR
Stimulus type : 100 μs diotic clicks high-pass filtered at 1.5 kHz Stimulus amplitude : 80 and 100 dB peSPL Stimulus polarity : alternating Stimulus rate : 11.1 Hz Repetitions : 7,480 Filter : no online filtering Artifact reject : no online rejection criteria set Transducer : ER3A inserts Electrode montage : C7, Fz, M1, M2 Impedance : N/R FFR Stimulus type : 4 tones presented simultaneously with low frequency tone (.24 to .285 kHz) transposed to 4 kHz; 220 ms in duration including 10 ms ramps Stimulus amplitude : 80 dB SPL Stimulus polarity : alternating Stimulus rate : Interstimulus interval randomly selected within 85–95 ms range Repetitions : 4,000 (2,000 per polarity) Filter : no online filtering Artifact reject : no online rejection criteria set Transducer : ER3A inserts Electrode montage : Fz, C7 Impedance : N/R HFA 16 kHz using Sennheiser HAD-200 circumaural headphones OAE Transient evoked; six frequencies in range of 1.5–4 kHz in .5 kHz steps using narrow band clicks presented at 83 dB peSPL |
ABR: No evidence of ABR or FFR changes based on reported noise exposure in young adults; 16 kHz thresholds increased with noise exposure for females, but not males Total recovery of DPOAEs suggested recovery of outer hair cell function |
| Stuermer et al (2015) 27 | Can AS/AN be differentiated from SNHL by differences in threshold, latency, or amplitude ratio between AP and SP? | Age 6 months to 11 years; moderate to profound hearing loss; 10 with absent or severely abnormal ABRs with preserved CMs (cochlear microphonics); OAEs detected in 6 of these participants 10 with elevated ABR thresholds, but clearly detectable ABR waveforms with no signs of asynchrony; absent OAEs |
N/A |
ECochG
State of arousal : anesthetized Stimulus type : 2 kHz tone burst 2 ms rise fall time with 6 ms duration Stimulus amplitude : 10–20 dB above individual threshold level between 70 and 102 dB nHL Stimulus polarity : condensation and rarefaction Stimulus rate : 21.3 Hz Repetitions : 500 Filter : bandpass 0.15–3 kHz Artifact reject : N/R Transducer : ER-2A inserts Electrode montage : promontory Impedance : N/R |
Significant differences in CM, SP thresholds were lower than CAP thresholds in AS/AN patients; CM, SP thresholds were comparable to CAP thresholds in SNHL patients |
| Verhulst et al (2016) 25 | Does a functional model designed to predict the slope of ABR wave V latency–intensity function and amplitude intensity function compare with measured latency–intensity function and amplitude–intensity function measured from 30 participants with normal or high-frequency sloping audiograms? | 12 listeners with normal hearing; 18 listeners with high-frequency sloping audiograms | N/A |
ABR
Stimulus type : 100 μs clicks Stimulus amplitude : 70, 80, 90, and 100 dB peSPL Stimulus polarity : condensation Stimulus rate : 33.3 Hz Repetitions : 7,000 Filter : bandpass 0.7–1.5 kHz Artifact reject : N/R Transducer : ER-2 inserts Electrode montage : Cz, earlobes Impedance : N/R OAE Distortion product threshold at 4 kHz; frequency ratio f 1 /f 2 = 1.2 over a ⅓ octave range around 4 kHz; growth function |
Latency–intensity function and amplitude–intensity function may help differentiate CS from other sensorineural loss |
Abbreviations: ABR, auditory brainstem response; DPOAE, distortion product otoacoustic emissions; ECochG, electrocochleograpy; EFR, envelope following response; FFR, frequency following response; HFA, high frequency audiometry; ITDD, interaural time difference detection; N/A, not applicable; NR, not reported; OAE, otoacoustic emission; TEOAE, transient evoked otoacoustic emissions.
Appendix III. Summary of studies of hidden hearing loss and cochlear synaptopathy involving animals.
| Author(s) (year) Study Design |
Question | Participant characteristics | Noise protocol | Test protocol | Outcome |
|---|---|---|---|---|---|
| Fernandez et al (2015) 11 | How does temporary noise damage change the aging process in the cochlea? | CBA/CaJ male mice Entered at 16 weeks old; 138 animals reported by group assignment |
For all groups, animals were tested and then euthanized at varying postexposure times ranging from 1 hour to 20 months
Group 1:
8–16 kHz broadband noise at 100 dB SPL for 2-hour exposure
Group 2: 8–16 kHz broadband noise at 91 dB SPL for 2-hour exposure Group 2b: same noise as group 2, but for 8-hour exposure Group 3: age-only controls Testing post noise exposure time : preexposure, 1 hour, 24 hours, 2 weeks, 16 weeks, 32 weeks, 48 weeks, 64 weeks, 80 weeks, 96 weeks, 112 weeks. Animals were euthanized and histology was completed after examination at each designated testing postexposure time |
ABR
Anesthesia : ketamine and xylazine Stimulus type : tone burst 5.6–45.2 kHz Stimulus duration: rise/fall time of 0.5 ms Stimulus amplitude : 5-dB step size Stimulus polarity : alternating Stimulus rate : 30 Hz Repetitions : 1,024 Filter : 0.3–3 kHz Artifact reject : N/A Transducer : CDMF15008–03A earphones Electrode montage : subdermal needle electrode at vertex and ventrolateral to pinna; ground electrode at base of tail Histology Cochlear mapping, hair cell and synaptic count; spiral ganglion cell quantification OAE DPOAE; Frequency ratio f 1 /f 2 = 1.2; L1–L2 = 10; growth function; threshold |
Immediately after exposure, the 91 dB, 2-hour exposure group showed no synaptic loss, but did show up to 40 dB threshold shift. Ears with 91 dB SPL, 8-hour exposure group did show evidence of acute synaptopathy as evidenced by histology. Ears exposed to 100 dB did show immediate reduction in synapse count. Key differences at 88 weeks for the 100 dB 2-hour exposed group when compared with age-matched animals. |
| Jensen et al (2015) 17 | What are the immediate and delayed effects of cochlear synaptopathy in mice with prepubescent noise exposure? | CBA/CaJ male mice Entered at 6 weeks of age; participated in the study up to 18 months of age |
8–16 kHz broadband noise at 94–100 dB SPL for 2 hours (neuropathic) Age, gender, and stain-matched controls Testing post noise exposure time: 6 hours, 2 weeks, 4 weeks, 10 months, 16 months Animals were euthanized and histology was completed after examination at each designated testing postexposure time |
ABR
Anesthesia : ketamine and xylazine Stimulus type : only reported as frequencies of 11.3 and 32.0 kHz Stimulus amplitude : 80 dB SPL Stimulus polarity : N/R Stimulus rate : N/R Repetitions : 512 Filter : 0.3–3 kHz Artifact reject : N/A Transducer : N/R Electrode montage : subdermal ipsilateral pinna, vertex, ground on tail Histology Synaptic ribbon counts, stereological counts of peripheral axons and cell bodies OAE DPOAE; Frequency ratio f 1 /f 2 = 1.2; f 2 = 5.66–45.25 kHz 5 dB intensity increments from 15–80 dB SPL |
Synaptic loss was noted at 6 hours post noise exposure; but did not spread to spiral ganglion loss until 8–16 months after synaptic loss ABR threshold shifts of 20–50 dB were highly significant above 10 kHz at 6 hours post exposure, but returned to normal 2 weeks postexposure |
| Lobarinas et al (2017) 18 | Does noise exposure that produces robust TTS and ABR wave I amplitude reduction, but not PTS reduce hearing in noise performance? | Adult Sprague Dawley rats (9–12 months of age) | Under ketamine/xylene anesthesia; 8–16 kHz broadband noise at 106 or 109 dB SPL for 2 hours Testing post noise exposure time : preexposure; 24 hours, 2 weeks |
ABR
Anesthesia : ketamine and xylazine Stimulus type : only reported as frequencies at 8, 16, 24, 32 kHz Stimulus amplitude : 90 dB SPL decreasing in 10-dB steps to 10 dB below wave I threshold Stimulus polarity : N/R Stimulus rate : N/R Repetitions : 1,052 Filter : 0.3–3 kHz Artifact reject : N/A Transducer : N/R Electrode montage : subdermal needle electrodes vertex-ventrolateral to pinna Behavioral Startle reflex; ongoing background noise, narrowband burst startle response measured at 8, 12, 16, 20, and 24 kHz OAE DPOAE; Frequency ratio 2f 1 − f 2 and f 2 = 8, 16, 24, 36 kHz; L1 = 30–70 dB SPL in 10-dB steps |
No relationship between performance on hearing in noise testing and ABR wave I amplitude was seen. A TTS greater than 30 dB, 24 hours post noise exposure was a predictor of functional deficit. No amplitude reduction in DPOAE or ABR wave I response 2 weeks postexposure |
| Mehraei et al (2016) 19 | Does noise-induced cochlear synaptopathy affect latency shift of ABR wave IV with increasing background noise? | 63 CBA/CaJ male mice; 16–18 weeks of age |
Group 1
: 8–16 kHz broadband noise at 100 dB SPL for 2 hours (neuropathic noise; expected to cause PTS)
Group 2 : 8–16 kHz at 94 dB SPL for 2 hours (nonneuropathic noise; expected to cause TTS) Group 3 : Control Testing post noise exposure time: 6–10 weeks 9 Animals (3 from each group) were euthanized and histology was completed after examination at each designated testing post exposure time |
ABR
Anesthesia : ketamine and xylazine Stimulus type : 4 ms 32 kHz tone pips Stimulus amplitude : 15–80 dB SPL in 5-dB steps Stimulus polarity : alternating Stimulus rate : 40 Repetitions : 1,024 Filter : 0.3–3 kHz band pass Artifact reject : N/A Transducer : custom Electrode montage : subdermal needle electrodes at vertex and ventral edge of left pinna with ground on tail ABR masked Stimulus type : 4 ms 32 kHz tone pips Masker : broadband extending from 4–64 kHz; swept from −5 to 85 dB SPL in 5-dB steps Stimulus amplitude : 60 and 80 dB SPL Stimulus polarity : N/R Stimulus rate : N/R Repetitions : 1,024 Filter : 0.3–3 kHz band pass Artifact reject : N/A Transducer : custom Electrode montage : subdermal needle electrodes at vertex and ventral edge of left pinna with ground on tail Histology Synaptic ribbon counts OAE DPOAE; Frequency ratio f 1 /f 2 = 1.2 and f 2 = 8–45.3 kHz in half-octave steps; L1–L2 = 10; swept from L2 = 10–80 dB SPL in 5-dB steps |
Wave IV latency increased with increasing noise level; slope of latency versus noise was smaller in neuropathic noise exposed group relative to control and nonneuropathic noise group |
| Möhrle et al (2016) 20 | How does central sound responsiveness change after acoustic trauma in young, middle-aged, and older rats? | Female Wistar rats; young (2–3 months old); middle-aged (6–10 months old); and old (19–22 months old) with normal ABR responses | 8–16 kHz broadband noise at 100 dB SPL RMS for 2 hours Baseline testing data were obtained before noise exposure Testing time: Preexposure, directly after (15–30 minutes), and 2 or 4 weeks |
ABR
Slope of ABR wave latency growth function ASSR Amplitude modulated sinusoidal stimuli (carrier frequency 8 kHz) at 20 dB above threshold (SL); amplitude modulated 100% between 0.128 and 1.024 kHz in half octave steps Histology Number of CtBP2/RIBEYE-immunopositive dots quantified as indicators for ribbon synapses OAE DPOAE; 2f 1 –f 2 ; growth function |
Elevated ABR threshold in last weeks of life; more synchronous auditory processing possible in young and middle-aged rats with normal hearing thresholds despite progressing auditory cochlear neuropathy |
| Paquette et al (2016) 21 | How does synaptopathic noise exposure and nonsynaptopathic noise exposure affect changes in the cochlea of the FVB/nJ mouse strain? | FVB/nJ mice, male and female | 8–16 kHz gaussian noise at 105 dB SPL for either 30 or 60 minutes Testing post noise exposure time: 14 days post |
ABR
Anesthesia : ketamine and acepromazine/saline Stimulus type : 5 ms tone pips at 8, 12, 16, 24, and 32 kHz, or 5 ms full tone spectrum clicks Stimulus amplitude : 15–75 dB SPL Stimulus polarity : N/R Stimulus rate : N/R Repetitions : 512 Filter : N/R Artifact reject : N/A Transducer : 10B+ Electrode montage : needle electrodes at vertex (ground), near each pinna Histology Synaptic ribbon count OAE DPOAE; Frequency ratio f 1 /f 2 = 1.2 and f 2 = 8–45.3 kHz in half-octave steps; L1–L2 = 10; swept from L1 = 20–75 dB SPL in 5-dB steps |
DPOAE measures recovered completely after 14 days post exposure despite ribbon synapse loss for both noise exposure groups; ABR wave I amplitudes recovered by the 14th day post noise exposure for the shorter time of noise exposure; ABR wave I amplitude showed only partial recovery for the longer exposure time |
| Sergeyenko et al (2013) 13 | What are the age-related cochlear synaptic and neural degeneration changes in CBA/CaJ mice never exposed to noise? | CBA/CaJ male mice; 4–144 weeks of age |
None
Laboratory environment: sound levels between 50 and 60 dB SPL > 95% of the time and > 80 dB SPL < 1% of the time |
ABR
Anesthesia : ketamine and xylazine Stimulus type : tone burst 0.5 ms rise-fall; 5.6–45.2 kHz Stimulus amplitude : below threshold to 90 dB SPL in 5-dB steps Stimulus polarity : alternating Stimulus rate : N/R Repetitions : N/R Filter : 0.3–3 kHz bandpass Artifact reject : N/A Transducer: Custom Electrode montage : needle electrodes at vertex, ventrolateral to pinna with ground near tail Histology Quantify spiral ganglion cells, synaptic structures, and cochlear nerve terminal boutons below inner hair cells OAE DPOAE; 2f 1 –f 2 ; with f 2 = ABR test frequencies; 5 dB steps from below threshold to f 2 = 80 dB SPL; growth function |
Age-related test results were similar between CBA/CaJ mice and male humans when calculated as lifespan age based on male human behavioral thresholds and animal ABR thresholds Cochlear synaptic loss occurred from youth to old age throughout the cochlea well before threshold changes or hair cell loss |
|
Song et al (2016)
22
Design: Prospective cohort |
What are the dynamic changes of ribbon synapses and the coding function of auditory nerve fiber single units 1 month after brief noise exposure which caused massive damage to ribbon synapses but no permanent threshold shift? | 64 albino guinea pigs with red eyes, both genders; 2 to 3 months of age | Gaussian broadband noise at 105 dB SPL for 2 hours Testing post noise exposure time: 1 month post exposure |
ABR
Anesthesia : phenobarbital Stimulus type : 2–48 kHz tone bursts; 10 ms duration; 0.5 ms rise-fall time Stimulus amplitude: 90 dB SPL to below threshold in 5 dB steps Stimulus polarity : N/R Stimulus rate : 21.1 Repetitions : 1,000 Filter : N/R Artifact reject : N/A Transducer : Broadband speaker Electrode montage: Subdermal needle electrodes; vertex, reference and ground on two earlobes CAP Stimulus type : 2–48 kHz tone bursts; 10 ms duration; 0.5 ms rise-fall time Stimulus amplitude : 90 dB reSPL to below threshold in 5 dB steps Stimulus polarity : N/R Stimulus rate : N/R Repetitions : 100 Filter : N/R Artifact reject : N/R Transducer : broadband speaker Electrode montage : silver ball electrode on round window Histology Ribbon synapse count OAE DPOAE; 65 dB SPL; f 1 /f 2 = 1.2 Means of the two tones were spread from 1 to 16 kHz |
Recovery of ABR and DPOAE responses despite ribbon synapse loss; a huge decrease in the CAP amplitude at 1 day post noise with incomplete recovery later; CAP amplitude showed some recovery at 1 month post noise, but did not return to preexposure amplitudes |
Abbreviations: ABR, auditory brainstem response; CAP, cochlear action potential; DPOAE, distortion product otoacoustic emissions; ECochG, electrocochleography; HFA, high-frequency audiometry; N/A, not applicable; NR, not reported; OAE, otoacoustic emissions; S/N, signal-to-noise ratio; TEOAE, transient evoked otoacoustic emissions.
Experimental Questions
Six of the eight studies involving human participants focused on testing for HHL and/or CS with participants having normal hearing sensitivity, but differing noise exposure histories. 16 23 Additionally, some studies compared adults with normal hearing sensitivity with and without tinnitus or high-frequency SNHL. 24 25 One study enrolled young adults with normal hearing sensitivity and assessed the effect of background noise on nonbehavioral measures of auditory function. 26 Another study investigated whether auditory neuropathy/auditory dyssynchrony (AN/AD) could be differentiated from SNHL via measures involving aspects of the summating potential and action potential (e.g., threshold, latency, or ratio). 27
Unlike the human studies, those involving animals assessed the effect of controlled noise exposure and measured impacts on auditory function and histology (e.g., cochlear mapping, hair cell/synaptic count, spiral ganglion cell quantification). 11 13 18 19 20 21 22 In addition, most of the studies assessed possible synergistic effects of age, gender, and noise exposure on auditory function and/or histology. 11 13 17 20 21 22
Participant Characteristics
The majority of the human studies enrolled young adults with normal hearing, although the upper age limit for inclusion was 36 years in two studies 23 28 and extended to early middle-age (e.g., 40 years) in two others. 16 26 One study compared young adults' performance to peers with tinnitus 24 and another to those with high-frequency SNHL. 25 One study assessed children with moderate to profound hearing loss. 27 Several of the studies compared participants with and without a history of significant noise exposure determined through interview and/or self-report questionnaires. 16 23 28 Bramhall and colleagues recruited veterans and nonveterans with and without prior histories of noise exposure. 23 29 Similarly, Liberman and colleagues included musicians and nonmusicians who were high- versus low-risk for CS. 16
The animal studies used a variety of mice (CBA/Caj, 11 13 17 FVB/nJ, 21 ) and rats (Wistar, 20 Sprague Dawley 18 ), and one study used albino guinea pigs with red eyes. 22 Several of these studies looked at young, middle-aged, and old animals to help understand progression of damage from a single noise exposure.
Noise Exposure Protocols
None of the human studies exposed participants to noise, but rather several of the investigations were retrospective cohort investigations comparing young adults with and without histories of noise exposure on various auditory measures. 16 23 28 Amount of previous noise exposure was determined by interviews and questionnaires. Bramhall and colleagues used the Lifetime Exposure of Noise and Solvents Questionnaire (LENS-Q). 23 Similarly, Liberman and colleagues used the following three questionnaires to determine noise exposure history and presence of other auditory symptomology: (1) Hearing Health and Personal Characteristics, (2) Experiences and Abilities in Different Listening Situations, and (3) Loudness/Annoyance of Sounds and Hyperacusis. 16 Mehraei and colleagues did not expose participants to excessive noise, but assessed whether ABRs with masking affected the latency of wave V and if those values predicted temporal sensitivity. 19
Most of the animal studies exposed rodents to 8 to 16 kHz broadband stimuli at presentation levels varying from 91 to 109 dB SPL for durations ranging from 30 minutes to 8 hours. Generally, the higher SPL levels were matched with shorter duration times. Sergeyenko and colleagues did not have a noise protocol; instead, this study focused on investigating CS due to age. 13 Extracting and fixating the cochlea and cochlear sections occurred directly following audiologic testing for seven of the eight studies that used histologic investigation to quantify cochlear damage or cochlear change. 11 13 17 19 20 21 22
Audiologic Test Protocols
Tests used to assess HHL or CS in the studies included in this systematic review were: pure-tone audiometry from 0.25 to 8 kHz in 7 studies; 18 19 23 24 25 26 28 high-frequency audiometry in 3 studies; 16 23 28 tone detection in noise in 1 study; 18 speech perception in noise in 1 study; 16 OAEs in 12 studies; 11 13 16 17 18 19 20 21 22 23 25 28 ECochG in 3 studies; 16 22 27 ABR in 11 studies; 11 13 17 19 20 21 22 23 24 25 28 forward masking ABR in 1 study; 26 masked ABR in 1 study; 19 auditory steady-state response (ASSR) in 1 study; 20 FFR in 1 study; 28 envelope following response (EFR) in 1 study; 24 and ITDD in 1 study. 19
Quality Assessment
A consensus approach was used in completing the quality assessment of studies included in the present systematic review as to whether studies met criteria. Tables 1 and 2 show the results for the quality assessment for human and animal studies, respectively. All studies were judged to have used appropriate paradigms to address the experimental questions, accounted for other possibilities regarding the results of their investigations, used unconfounded experimental conditions, and had clearly defined dependent variables. However, only one animal study 11 defined all the electrophysiologic parameters for measurements. Replication of studies depends on the clear description and inclusion of all test parameters. None of the human studies reported blinding for experimental conditions when making audiologic measurements, which may have introduced bias. 17 As an example, when marking amplitudes for ABR waves, knowledge of the experimental condition may have biased investigators' judgment, particularly for difficult-to-measure waveforms. Lastly, none of the studies described intra- or interjudge reliability for measurements.
Table 1. Quality assessment of human studies on selected criteria.
| Criteria | Author(s) (year) | |||||||
|---|---|---|---|---|---|---|---|---|
| Bramhall et al (2017) 23 | Guest et al (2017) 24 | Liberman et al (2016) 16 | Mehraei et al (2017) 26 | Mehraei et al (2016) 19 | Prendergast et al (2017) 28 | Stuermer et al (2015) 27 | Verhulst et al (2016) 25 | |
| Was the paradigm used appropriate for the experimental question(s)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Did the study account for other possibilities for CS and/or HHL results? | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Were the experimental conditions assessed unconfounded? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Were the dependent variables clearly defined? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Were the parameters for measurements described completely? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Were measurements made with judges blinded to the experimental conditions? | No | No | No | No | No | No | No | No |
| Was interjudge reliability assessed? | No | No | No | No | No | No | No | No |
| Was intrajudge reliability assessed? | No | No | No | No | No | No | No | No |
| Were “dropouts” accounted for? | Yes | No | No | No | Yes | No | No | No |
Abbreviations: CS, cochlear synaptopathy; HHL, hidden hearing loss.
Table 2. Quality assessment of animal studies on selected criteria.
| Criteria | Author(s) (year) | |||||||
|---|---|---|---|---|---|---|---|---|
| Fernandez et al (2015) 11 | Jensen et al (2015) 17 | Lobarinas et al (2017) 18 | Mehraei et al (2016) 19 | Möhrle et al (2016) 20 | Paquette et al (2016) 21 | Sergeyenko et al (2013) 13 | Song et al (2016) 22 | |
| Was the paradigm used appropriate for the experimental question(s)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Did the study account for other possibilities for CS and/or HHL results? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Were the experimental conditions assessed unconfounded? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Were the dependent variables clearly defined? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Were the parameters for measurements described completely? | Yes | No | No | Yes | No | Yes | Yes | Yes |
| Were measurements made with judges blinded to the experimental conditions? | No | Yes | No | No | No | No | No | No |
| Was interjudge reliability assessed? | No | No | No | No | No | No | No | No |
| Was intrajudge reliability assessed? | No | No | No | No | No | No | No | No |
| Were “dropouts” accounted for? | No | No | No | No | No | No | No | No |
Abbreviations: CS, cochlear synaptopathy; HHL, hidden hearing loss.
Discussion
Below, the results for various audiologic measures used to assess HHL and/or CS from the studies included in the present systematic review are discussed. The tests examined included pure-tone audiometry, high-frequency pure-tone audiometry, OAEs, ECochG, ABR, listening in noise, and self-report questionnaires.
Pure-Tone Audiometry
Pure-tone audiometry in the standard frequency range from 0.25 to 8 kHz has been used to define and determine whether a person has normal hearing. As stated earlier, evidence from human studies (and supported by animal studies) has indicated that some people with normal pure-tone thresholds of hearing have complaints of auditory dysfunction (e.g., understanding speech in noise and tinnitus). 1 2 All eight human studies included in the present systematic review conducted pure-tone audiometry at 0.25 to 8 kHz and several studies used these data for participant inclusion criteria to determine normal hearing sensitivity. 16 19 23 24 25 26 27 28 For example, normal hearing was defined as participant thresholds of ≤15 dB HL in one study; 19 ≤20 dB HL in three studies; 23 24 26 and ≤25 dB HL in one study. 28 In those studies, all participants had to have normal hearing as evidenced by pure-tone thresholds, but were grouped by either high or low risk for CS due to their history of noise exposure. Overall, pure-tone thresholds for those participants with normal audiograms were similar for those with extensive histories of noise exposure compared with control groups. 23 Alternatively, two studies measured hearing thresholds, but did not require all subjects to have hearing within normal limits. 25 27 Stuermer and colleagues enrolled children with moderate to profound hearing loss; Verhulst and colleagues compared participants with normal hearing to their peers with high-frequency SNHL.
Pure-tone audiometry may help identify temporary threshold shifts (TTS) versus permanent threshold shifts (PTS) using repeated measures over time. 30 One of the animal studies reviewed conducted behavioral hearing thresholds and found changes that were consistent with a TTS rather than a PTS after exposure to either 106 or 109 dB SPL of 8 to 16 kHz broadband noise for 2 hours. 18
High-Frequency Audiometry
High-frequency audiometry has been assessed for use in identifying early signs of noise-induced hearing loss and for ototoxicity monitoring. 31 The present systematic review identified three human studies, which investigated high-frequency audiometry in the 9 to 16 kHz range. 16 23 28 Bramhall and colleagues showed no significant differences in high-frequency pure-tone thresholds among four different noise exposure groups of: (1) nonveterans with no significant noise exposure, (2) nonveterans with a history of firearms usage, (3) veterans with a history of low noise exposure, and (4) veterans with a history of high noise exposure. 23 Alternatively, Liberman and colleagues found statistically significant group differences in high-frequency pure-tone thresholds between young adults at low versus high risk for CS. 16 Prendergast and colleagues found that females showed a significant increase in high-frequency pure-tone thresholds with increasing noise exposure, but did not find the same relationship in males. 28 High-frequency pure-tone audiometry may be a promising measure to include in a test battery for identifying HHL and/or CS. Indeed, Liberman and colleagues stated that high-frequency pure-tone audiometry was included as a measure because animal studies have shown that the earliest damage from noise exposure occurs at the very base of the cochlea or in the area tuned to very high frequencies. 16 In addition, it has been suggested that monitoring programs should define individual patients' sensitive ranges for ototoxicity as the highest frequency at which a threshold is obtained at or below 100 dB SPL followed by the next six lower adjacent frequencies in 1/6-octave steps. 32 Furthermore, high-frequency pure-tone audiometry is routinely conducted on patients who report tinnitus and/or hyperacusis, which are possible complaints by persons with CS. 33 Therefore, high-frequency pure-tone audiometry has shown great potential as a useful tool within a clinical test battery for HHL and/or CS.
Otoacoustic Emissions
Some objective assessments of auditory function may provide insight into HHL or CS that may be related to both noise exposure and aging. Clinically, OAEs are routinely used to assess cochlear outer hair cell biomechanics, 34 and may be one of the earliest tests to indicate damage from noise, even in the presence of a normal audiogram. 35 36 Further, there is evidence that OAEs are more sensitive to noise exposure than pure-tone threshold measures. 37 However, OAEs may still recover to within normal limits within 24 hours after damaging noise exposure, 11 which could cause the effects of noise on cochlear physiology to be missed when measuring OAEs more than a day after a noise exposure.
Two of the retrospective cohort studies showed that distortion product otoacoustic emission (DPOAE) thresholds were similar across noise groups for studies using participants with normal hearing. 16 23 Recall that Bramhall and colleagues found no significant differences between DPOAE thresholds among four groups of young adults comprised of veteran and nonveterans with varying histories of noise exposure. 23 Additionally, Liberman and colleagues found no significant differences in DPOAE thresholds between two groups of young adults composed of musicians and nonmusicians who were at high and low risk for CS. 16
Two studies found no significant relationship between history of noise exposure and amplitude of transient evoked otoacoustic emissions (TEOAE) responses. 19 28 The Prendergast et al study found no significant difference in TEOAE amplitudes or on OAE amplitude growth functions between young adults with and without histories of noise exposure. 28 Verhulst and colleagues found that amplitude growth functions corresponded to the slope of hearing loss in listeners with a measurable loss in hearing sensitivity using standard pure-tone thresholds from 0.25 to 8 kHz. 25 Overall, a significant difference in TEOAE responses was not found between high- and low-risk noise exposure groups.
All of the animal studies included in the present systematic review measured amplitude growth functions for OAEs. 11 13 17 18 19 20 21 Overall, several of the included animal studies showed that noise exposure caused DPOAE threshold shifts, which recovered after 24 hours; 11 18 21 22 however, one study showed a PTS in the high frequencies with a higher DPOAE threshold in mice. 17 Sergeyenko and colleagues investigated DPOAE amplitude changes related to age and found that, in mice, changes were gradual and mild as the animals aged until 96 weeks, and then changes grew more rapidly. 13 Möhrle and colleagues demonstrated that hearing threshold elevations in DPOAE input/output functions in old animals were caused by a loss of outer hair cells. 20 Overall, these studies showed a positive association between HHL and/or CS with noise and/or aging, and short-term DPOAE abnormality, suggesting that DPOAE may be sensitive to loss of cochlear synapses if measured within 24 hours of exposure.
Aging is a cause of CS. 38 Uchida and colleagues conducted a retrospective longitudinal study and found changes in DPOAEs as a function of age in adults with normal hearing. 38 Poling and colleagues have developed equipment that can measure DPOAEs from 0.125 to 20 kHz, which extends into frequency regions not typically assessed by OAEs. 39 That study reported one barrier to assessing OAEs in frequencies > 6 kHz was the inability to use stimuli that can deliver calibrated signals for higher frequencies. Poling and colleagues analyzed responses from over 1,100 participants with pure-tone thresholds < 20 dB HL that ranged in age from 10 to 55 years. 39 This study found that aging of the auditory system was seen in 25- to 29-year-olds with non–drug or noise-exposed ears and that the greatest change in auditory function due to age occurred between 25 and 45 years. 39 High-frequency DPOAEs show promise as a future tool in a test battery for HHL and/or CS. Future research is needed to develop clinical equipment with ultra-high-frequency test protocols and normative data that may be used for clinic purposes and in research laboratories.
Electrocochleography
ECochG is an electrophysiologic response to sound which measures: (1) the cochlear summating potential (SP), which is direct-current, cochlear electrical activity arising primarily from the outer hair cells; and (2) the action potential (AP), which is equivalent to wave I of the ABR and produced by the auditory nerve. Initially, the ECochG response was used as a measure of hearing, but was replaced by the more easily obtained ABR. Subsequently, ECochG has primarily been used to assess endolymphatic hydrops by measuring the SP/AP ratio. In addition, a newer method of ECochG analysis, called “area under the curve,” uses the onset and offset timing of the SP and the AP rather than the peak to generate a ratio, which more accurately defines the differences between the SP and AP timing information. Because ECochG evaluates potentials within the cochlea, 30 31 32 this test may be able to show subtle differences to synaptic changes.
Two human studies investigated use of ECochG. 16 27 Liberman and colleagues showed a significant difference between high- and low-risk groups using the SP/AP ratio. The SP/AP ratio of the high-risk group was nearly twice that for the low-risk group for CS. 16 It is important to note that their study measured SP/APs using gold-foiled tiptrodes to reduce variability in the measurement process instead of earlobe or mastoid electrodes. The study reported no difficulties with the measurement process, which indicated that the use of tiptrodes may be a viable way of measuring ECochG with an electrode placement peripheral to the tympanic membrane. 16 Stuermer and colleagues found significant differences between participants with auditory synaptopathy/neuropathy compared with participants with SNHL specifically in the amplitude of the cochlear microphonic and summating potential compared with the cochlear action potential. 27 However, more evidence is needed and further research using ECochG for SP/AP and area under the curve are needed before a clear determination can be made regarding inclusion in a test battery for HHL and/or CS. One animal study investigated the compound action potential (CAP). 22 Results from Song and colleagues demonstrated that the CAP amplitude decreased 1 month post noise exposure. 22 Histologic evidence showed some recovery of synapses 1 month post noise exposure compared with 1 day post noise exposure; however, recovery was not enough to explain the decrease in amplitude indicating that some auditory nerve fibers were likely damaged. 22
Auditory Brainstem Response and Other Electrophysiological Tests
The ABR technique has been used to assess auditory neural synchrony for decades, 40 41 and has been investigated as a measure for CS. Five of the studies found that only a temporary ABR threshold shift was present even when noise exposure caused permanent loss of cochlear synapses; however, suprathreshold ABR wave I amplitudes at high frequencies were reduced. 11 13 18 19 21 Additionally, one study reported age-related changes in ABR amplitudes that were reduced in middle age and older rats. 20 Those results suggest that measuring ABR suprathreshold wave I with a near-field placement of electrodes may be useful in identifying CS and/or HHL.
Bramhall and colleagues found that ABR wave I amplitudes at suprathreshold levels were smaller for participants with higher noise exposure. 23 Mehraei and colleagues found a significant relationship between ABR wave I amplitude to high-level clicks and wave V latency shifts. 19 Additionally, that study assessed the use of a masking noise while measuring wave V and found an increasing wave V latency with masking. 19 Further, Mehraei and colleagues found a forward masking effect in both behavioral forward masking and ABR forward masking paradigms. 26 Verhulst and colleagues created a model to test whether the ABR growth ratio of latency versus amplitude could distinguish between CS versus cochlear gain loss, and determined that ABR growth ratio predictions held true for participants with high-frequency hearing losses. 25 Overall, electrode placement seemed to play a role in whether a significant difference was found between high- and low-risk groups. Definitive evidence of change in animal models may be more easily identified due to measurement conditions which include needle electrode placement and anesthetized subjects. In other words, the use of needle electrodes placed at the site of the generator measures a larger response, and therefore, variability related to individual anatomical differences among animals may be reduced compared with human variability with externally placed electrodes. Other parameter differences were unable to be identified and compared between studies as most of the animal articles included in this review did not state all parameters.
Other electrophysiologic measures in both human and animal studies that were investigated included: ITDD, FFR, EFR, and ASSR. Briefly, ITDD uses modulated transposed tones as stimuli within a background of notched noise to reduce frequency cues in assessing fine temporal coding precision and did so in one study. 19 25 FFR investigates frequency-specific coding of timing and was used to assess temporal fine-structure and envelope coding in one study. 28 EFR is an objective measure of temporal acuity and assesses whether synaptopathic ears would show an increase in response amplitude with an increase in signal modulation. 42 ASSR can be used to estimate hearing sensitivity through measurement of the ability of auditory neurons to phase lock. 43 ASSR algorithms have been used to automate analysis of the auditory response in place of measuring peaks on ABR waves; ASSR provides a more frequency-specific response at high intensities than tone-burst ABRs. 44
Each of the following responses was reported as an investigative tool in human studies: ITDD, FFR, and EFR. Mehraei and colleagues assessed use of ITTD and found that performance improved during an ITDD task for normal hearing threshold listeners with larger wave V latency shift during masked ABR. 19 Prendergast and colleagues assessed use of FFR and found no evidence of differences in young adults categorized by noise exposure. 28 Guest and colleagues assessed use of EFR and found no association between noise exposure reported and amplitude of EFR response measured at a shallow modulation depth. 24 The current systematic review did not identify ITDD, FFR, or EFR as useful tools in human studies to measure differences between high- and low-risk noise exposure participants. 19 25 28
Only a single animal study included in this review used an electrophysiologic response other than ECochG and ABR. Möhrle and colleagues used ASSR as a way to assess temporal resolution of sound processing. That study did not find a significant link between ASSR and HHL and/or CS. 20 This review did not identify ASSR as a useful tool in the animal model; further, no human studies included in this review used ASSR as an investigative tool for identifying HHL and/or CS.
Hearing in Noise
One common complaint from patients suspected of HHL and/or CS has been difficulty hearing in noise. One study investigated speech perception in noise for words with temporal distortion of compression and reverberation to compare participants at high versus low risk for CS. The results of that study showed statistically significant group differences for signal-to-noise ratios (S/N) of +5 and 0 dB and for time compressed speech with and without reverberation. 16 Speech perception testing in noise and temporal distortion through time compression and/or reverberation is another promising way of assessing HHL and/or CS. 16
Regarding hearing in noise tests in animal studies, Lobarinas and colleagues assessed the S/N needed to hear the presence of a tone in background noise to assess suprathreshold behavioral response changes to tones in noise after CS noise damage. 18 That study found significant changes only at the poorest (lowest) S/N when the TTS was greater than 30 dB at 24 hours post noise exposure, which provided support for using suprathreshold signal-in-noise detection as a way to evaluate CS. 18
Self-Report Questionnaires and Interviews
Three of the retrospective cohort studies involving humans used self-report questionnaires and interviews for either identifying level of risk for HHL and/or CS or for measuring surpathreshold auditory symptoms of these two conditions. 16 23 28 Recall that Bramhall and colleagues used the LENS-Q to gauge veterans' and nonveterans' degree of noise exposure. 23 Similarly, Liberman and colleagues used three different questionnaires: (1) Hearing Health and Personal Characteristics, (2) Experiences and Abilities in Different Listening Situations, and (3) Loudness/Annoyance of Sounds and Hyperacusis for two reasons. 16 Initially, the Liberman et al study used the questionnaires to assign young adults into low- versus high-risk groups for CS. Then, the researchers found that those in the high-risk group with larger SP/AP had greater sound annoyance and avoidance than their peers at lower risk for CS. No differences were found, however, between the groups in terms of self-reported tinnitus. As the field of audiology expands its traditional test battery, self-report questionnaires and patient interview will likely become important for the identification, diagnosis, and treatment of HHL and/or CS. For instance, patients with normal hearing on an audiogram may identify problem areas on self-assessment scales such as the Hearing Handicap Inventory for Adults or the Tinnitus Handicap Inventory. 45 46
Summary
The purpose of the present systematic review was to search and retrieve articles from peer-reviewed journals that used audiologic measures to evaluate HHL and/or CS. To be included in the systematic review, articles had to involve either human and/or animal participants, and assess auditory function to evaluate HHL and/or CS. All of the studies involving animals that included histologic examination provided confirmation of the presence of CS. Obviously, for the studies enrolling human participants, histologic examination was not possible. CS is deafferentation or the loss of ribbon synapses at the base of inner hair cells. Inner hair cells innervate 95% of the afferent eighth nerve fibers. CS can be caused by a variety of factors, but the two most commonly discussed in the literature are noise and age. Studies included in the present systematic review that involved humans used self-report questionnaires and interviews for assignment of young adults into low- versus high-risk groups for use in retrospective cohort studies that submitted participants to audiologic test batteries which may be sensitive to CS. For example, high-frequency pure-tone audiometry and, although not specifically used in any of the studies included in this systematic review, high-frequency OAEs show promise for identifying HHL and early changes in the auditory systems due to noise.
In routine hearing evaluations, thresholds are obtained for 0.25 to 8 kHz. To evaluate frequencies above 8 kHz, audiometers must be calibrated for those frequencies above 8 kHz and special high-frequency circumaural headphones need to be used. 47 High-frequency pure-tone audiometry is routinely used in ototoxicity monitoring 32 and tinnitus assessment. 33 Liberman and colleagues found that high-frequency thresholds were significantly poorer for participants who indicated higher risk of CS from more excessive noise exposure than a group of nonexposed peers. 16 Moreover, this latter group had SP/APs that were nearly twice that of the low-risk group, which indicated the presence of CS. The fact that those with larger SP/APs also had significantly higher high-frequency pure-tone thresholds mandates further evaluation of these measures in their utility for identifying early CS. Further research is needed to determine whether high-frequency pure-tone audiometry will be useful in evaluation of patients with normal audiograms from 0.25 to 8 kHz who complain of difficulty hearing.
Most of the studies included in the systematic review included various measures of OAE in their test batteries. OAE amplitudes have been shown to be temporarily reduced during a TTS, and the amplitude of OAE responses recovers at approximately the same rate as threshold recovery. Long believed to be an early predictor of damage within the cochlea, and specifically the outer hair cells, evidence accumulated during this systematic review suggested that OAE amplitude recovered when TTS recovered, typically within 24 hours despite cochlear ribbon synaptopathy. 35 36 After recovery of OAEs in animal models, histopathology demonstrated damage to cochlear ribbon synapses. 11 13 17 19 20 21 22 In human studies, OAEs have not been shown to be a good predictor of high- versus low-risk noise exposure participants. 16 19 23 28 However, the work of Poling and colleagues suggested that high-frequency DPOAEs may show promise for use in test batteries aimed at detecting early changes in cochlear function and for HHL and/or CS. 39
The animal research has indicated that suprathreshold electrophysiologic measures, specifically ECochG and ABR wave I, with near-field placement of the electrode, show significant differences between animals in control groups versus those with noise-induced CS, even after the recovery of auditory thresholds. 11 17 20 21 22 Indeed, this difference seems to progressively worsen over time. Both SP/AP and area-under-the-curve measurements may be valuable clinical tools, particularly in phase II clinical trials measuring the efficacy of pharmaceuticals in the prevention, treatment, and reversal of CS. Overall, human studies involving ECochG using mastoid placement did not show significant differences between high-risk groups versus low-risk groups. 24 However, when a tiptrode placement was used, a significant difference was found. 16 23 Although normative data are not yet available for use of ECochG as an early measure of noise-induced damage, this method of evaluation would be feasible in clinics where an auditory evoked system is available.
In the animal model, ABR wave I amplitudes can be measured consistently, intersubject and intrasubject. 48 The animal studies included in this systematic review primarily used needle electrodes close to the site of generation. Not all adjustable parameters were listed in the animal studies; for example, stimulus repetition rate and stimulus polarity were only listed in one study. Other parameters, such as impedance and artifact reject, were likely irrelevant in the animal studies where needle electrodes were used on anesthetized subjects. In human studies, amplitude of wave I can be manipulated based on location of electrode as well as intensity of sound. 49 To identify repeatable, reliable differences across human subjects with and without high-risk noise exposure, normative data for wave I amplitudes should be investigated at each electrode site to find the variance and normal range for the amplitude. Then, it can be determined whether and how this measure is clinically useful for identifying HHL and/or CS.
Further research in this population using questionnaires to measure handicap, and possible development of a self-report tool specific to HHL may be useful. Questionnaires being used in the reviewed studies were primarily to assess noise history, and tinnitus handicap. A questionnaire such as the Hearing Handicap Inventory for Adults (HHIA) would be an interesting measure for this population that displays a normal audiogram with complaints or other symptoms of hearing loss. 45 The Tinnitus Handicap Inventory or other questionnaire tools that measure hyperacusis and/or sound annoyance 46 may be critical for this patient population. Patients' self-reported perception of their hearing problems will be important in the clinic and in research laboratories for defining the impact of HHL and/or CS on their health-related quality of life. However, it is important to keep in mind that usefulness of such a questionnaire or measure would be determined based on objective measures which can identify HHL and/or CS.
Development of sensitive and specific test batteries for HHL and/or CS will assist in: identifying techniques for the early detection of auditory dysfunction; augmenting understanding of standard audiologic assessment of patients with normal hearing sensitivity, but who present with suprathreshold auditory dysfunction; and in identifying tests most likely to serve as clinical end points in phase II clinical trials. The results of the present systematic review indicated that there will probably be no single test for HHL and/or CS, but rather a test battery approach with an opportunity to use the cross-check principle based on what aspects of HHL and/or CS are being investigated. Animal studies have verified the existence of HHL and/or CS through histological procedures which reveal the cochlear damage even after TTS and recovery of thresholds seen in objective measures such as the ABR.
Conclusions
The present systematic review included 15 articles; 7 articles involved humans, 7 involved animals, and 1 study used both humans and animals. Tests used to assess HHL and/or CS have included pure-tone audiometry to 20 kHz, OAEs, ECochG, ABR, and other electrophysiological tests, in addition to interviews and self-report measures. For HHL, ultra-high-frequency audiometry may help identify persons with sensory hair cell loss that does not show up on the standard audiogram. Promising nonbehavioral measures for HHL and/or CS include amplitude of wave I of the ABR and the SP/AP ratio in ECochG. Self-report questionnaires also may be helpful in identifying or confirming the effects of auditory dysfunction.
References
- 1.Tremblay K L, Pinto A, Fischer M E et al. Self-reported hearing difficulties among adults with normal audiograms: the beaver dam offspring study. Ear Hear. 2015;36(06):e290–e299. doi: 10.1097/AUD.0000000000000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao F, Stephens D. A critical review of King-Kopetzky syndrome: hearing difficulties but normal hearing. Audiol Med. 2007;5(02):119–124. [Google Scholar]
- 3.Hinchcliffe R, Coles R R, King P F. Occupational noise induced vestibular malfunction? Br J Ind Med. 1992;49(01):63–65. doi: 10.1136/oem.49.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saunders G H, Haggard M P. The clinical assessment of obscure auditory dysfunction--1. Auditory and psychological factors. Ear Hear. 1989;10(03):200–208. doi: 10.1097/00003446-198906000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Stephens S, Rendell R J. Auditory disability with normal hearing. Quaderni de Audiologia. 1998;4:233–238. [Google Scholar]
- 6.Schaette R, McAlpine D. Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. J Neurosci. 2011;31(38):13452–13457. doi: 10.1523/JNEUROSCI.2156-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kujawa S G, Liberman M C. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29(45):14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mulders W H, Robertson D. Hyperactivity in the auditory midbrain after acoustic trauma: dependence on cochlear activity. Neuroscience. 2009;164(02):733–746. doi: 10.1016/j.neuroscience.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 9.Finlayson P G, Kaltenbach J A.Alterations in the spontaneous discharge patterns of single units in the dorsal cochlear nucleus following intense sound exposure Hear Res 2009256(1–2):104–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maison S F, Usubuchi H, Liberman M C. Efferent feedback minimizes cochlear neuropathy from moderate noise exposure. J Neurosci. 2013;33(13):5542–5552. doi: 10.1523/JNEUROSCI.5027-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez K A, Jeffers P W, Lall K, Liberman M C, Kujawa S G. Aging after noise exposure: acceleration of cochlear synaptopathy in “recovered” ears. J Neurosci. 2015;35(19):7509–7520. doi: 10.1523/JNEUROSCI.5138-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kujawa S G, Liberman M C.Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss Hear Res 2015330(Pt B):191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sergeyenko Y, Lall K, Liberman M C, Kujawa S G. Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J Neurosci. 2013;33(34):13686–13694. doi: 10.1523/JNEUROSCI.1783-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viana L M, O'Malley J T, Burgess B J et al. Cochlear neuropathy in human presbycusis: Confocal analysis of hidden hearing loss in post-mortem tissue. Hear Res. 2015;327:78–88. doi: 10.1016/j.heares.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdala C, Dhar S. Maturation and aging of the human cochlea: a view through the DPOAE looking glass. J Assoc Res Otolaryngol. 2012;13(03):403–421. doi: 10.1007/s10162-012-0319-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liberman M C, Epstein M J, Cleveland S S, Wang H, Maison S F. Toward a differential diagnosis of hidden hearing loss in humans. PLoS One. 2016;11(09):e0162726. doi: 10.1371/journal.pone.0162726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen J B, Lysaght A C, Liberman M C, Qvortrup K, Stankovic K M. Immediate and delayed cochlear neuropathy after noise exposure in pubescent mice. PLoS One. 2015;10(05):e0125160. doi: 10.1371/journal.pone.0125160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lobarinas E, Spankovich C, Le Prell C G. Evidence of “hidden hearing loss” following noise exposures that produce robust TTS and ABR wave-I amplitude reductions. Hear Res. 2017;349:155–163. doi: 10.1016/j.heares.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Mehraei G, Hickox A E, Bharadwaj H M et al. Auditory brainstem response latency in noise as a marker of cochlear synaptopathy. J Neurosci. 2016;36(13):3755–3764. doi: 10.1523/JNEUROSCI.4460-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Möhrle D, Ni K, Varakina K et al. Loss of auditory sensitivity from inner hair cell synaptopathy can be centrally compensated in the young but not old brain. Neurobiol Aging. 2016;44:173–184. doi: 10.1016/j.neurobiolaging.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Paquette S T, Gilels F, White P M. Noise exposure modulates cochlear inner hair cell ribbon volumes, correlating with changes in auditory measures in the FVB/nJ mouse. Sci Rep. 2016;6:25056. doi: 10.1038/srep25056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song Q, Shen P, Li X et al. Coding deficits in hidden hearing loss induced by noise: the nature and impacts. Sci Rep. 2016;6:25200. doi: 10.1038/srep25200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bramhall N F, Konrad-Martin D, McMillan G P, Griest S E. Auditory brainstem response altered in humans with noise exposure despite normal outer hair cell function. Ear Hear. 2017;38(01):e1–e12. doi: 10.1097/AUD.0000000000000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guest H, Munro K J, Prendergast G, Howe S, Plack C J. Tinnitus with a normal audiogram: relation to noise exposure but no evidence for cochlear synaptopathy. Hear Res. 2017;344:265–274. doi: 10.1016/j.heares.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verhulst S, Jagadeesh A, Mauermann M, Ernst F. Individual differences in auditory brainstem response wave characteristics: relations to different aspects of peripheral hearing loss. Trends Hear. 2016;20:11. doi: 10.1177/2331216516672186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehraei G, Gallardo A P, Shinn-Cunningham B G, Dau T. Auditory brainstem response latency in forward masking, a marker of sensory deficits in listeners with normal hearing thresholds. Hear Res. 2017;346:34–44. doi: 10.1016/j.heares.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stuermer K J, Beutner D, Foerst A, Hahn M, Lang-Roth R, Walger M. Electrocochleography in children with auditory synaptopathy/neuropathy: diagnostic findings and characteristic parameters. Int J Pediatr Otorhinolaryngol. 2015;79(02):139–145. doi: 10.1016/j.ijporl.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 28.Prendergast G, Guest H, Munro K J et al. Effects of noise exposure on young adults with normal audiograms I: electrophysiology. Hear Res. 2017;344:68–81. doi: 10.1016/j.heares.2016.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plack C J, Léger A, Prendergast G, Kluk K, Guest H, Munro K J. Toward a diagnostic test for hidden hearing loss. Trends Hear. 2016;20:7. doi: 10.1177/2331216516657466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dobie R A, Clark W W. Exchange rates for intermittent and fluctuating occupational noise: a systematic review of studies of human permanent threshold shift. Ear Hear. 2014;35(01):86–96. doi: 10.1097/AUD.0b013e3182a143ec. [DOI] [PubMed] [Google Scholar]
- 31.Mehrparvar A H, Mirmohammadi S J, Davari M H et al. Conventional audiometry, extended high-frequency audiometry, and DPOAE for early diagnosis of NIHL. Iran Red Crescent Med J. 2014;16(01):e9628. doi: 10.5812/ircmj.9628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konrad-Martin D G, Gordon J S, Reavis K M, Wilmington D J, Helt W J, Fausti S A. Audiological monitoring of patients receiving ototoxic drugs. Perspec Hear Hear Dis Diagn. 2005;9(01):17–22. [Google Scholar]
- 33.Henry J A, Jastreboff M M, Jastreboff P J, Schechter M A, Fausti S A. Assessment of patients for treatment with tinnitus retraining therapy. J Am Acad Audiol. 2002;13(10):523–544. [PubMed] [Google Scholar]
- 34.Kemp D T, Ryan S, Bray P. A guide to the effective use of otoacoustic emissions. Ear Hear. 1990;11(02):93–105. doi: 10.1097/00003446-199004000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Desai A, Reed D, Cheyne A, Richards S, Prasher D. Absence of otoacoustic emissions in subjects with normal audiometric thresholds implies exposure to noise. Noise Health. 1999;1(02):58–65. [PubMed] [Google Scholar]
- 36.Prasher D, Sułkowski W. The role of otoacoustic emissions in screening and evaluation of noise damage. Int J Occup Med Environ Health. 1999;12(02):183–192. [PubMed] [Google Scholar]
- 37.Attias J, Horovitz G, El-Hatib N, Nageris B. Detection and clinical diagnosis of noise-induced hearing loss by otoacoustic emissions. Noise Health. 2001;3(12):19–31. [PubMed] [Google Scholar]
- 38.Uchida Y, Ando F, Shimokata H, Sugiura S, Ueda H, Nakashima T. The effects of aging on distortion-product otoacoustic emissions in adults with normal hearing. Ear Hear. 2008;29(02):176–184. doi: 10.1097/aud.0b013e3181634eb8. [DOI] [PubMed] [Google Scholar]
- 39.Poling G L, Siegel J H, Lee J, Lee J, Dhar S. Characteristics of the 2f(1)-f(2) distortion product otoacoustic emission in a normal hearing population. J Acoust Soc Am. 2014;135(01):287–299. doi: 10.1121/1.4845415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hood L J. A review of objective methods of evaluating auditory neural pathways. Laryngoscope. 1999;109(11):1745–1748. doi: 10.1097/00005537-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Krause C M, Boman P A, Sillanmäki L, Varho T, Holopainen I E. Brain oscillatory EEG event-related desynchronization (ERD) and -sychronization (ERS) responses during an auditory memory task are altered in children with epilepsy. Seizure. 2008;17(01):1–10. doi: 10.1016/j.seizure.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 42.Purcell D W, John S M, Schneider B A, Picton T W. Human temporal auditory acuity as assessed by envelope following responses. J Acoust Soc Am. 2004;116(06):3581–3593. doi: 10.1121/1.1798354. [DOI] [PubMed] [Google Scholar]
- 43.Venail F, Artaud J P, Blanchet C, Uziel A, Mondain M. Refining the audiological assessment in children using narrow-band CE-Chirp-evoked auditory steady state responses. Int J Audiol. 2015;54(02):106–113. doi: 10.3109/14992027.2014.935496. [DOI] [PubMed] [Google Scholar]
- 44.Cone-Wesson B, Dowell R C, Tomlin D, Rance G, Ming W J.The auditory steady-state response: comparisons with the auditory brainstem response J Am Acad Audiol 20021304173–187., quiz 225–226 [PubMed] [Google Scholar]
- 45.Newman C W, Weinstein B E, Jacobson G P, Hug G A. The Hearing Handicap Inventory for Adults: psychometric adequacy and audiometric correlates. Ear Hear. 1990;11(06):430–433. doi: 10.1097/00003446-199012000-00004. [DOI] [PubMed] [Google Scholar]
- 46.Newman C W, Jacobson G P, Spitzer J B. Development of the Tinnitus Handicap Inventory. Arch Otolaryngol Head Neck Surg. 1996;122(02):143–148. doi: 10.1001/archotol.1996.01890140029007. [DOI] [PubMed] [Google Scholar]
- 47.Poulsen T. Equivalent threshold sound pressure levels (ETSPL) for Interacoustics DD 45 supra-aural audiometric earphones. Int J Audiol. 2010;49(11):850–855. doi: 10.3109/14992027.2010.500625. [DOI] [PubMed] [Google Scholar]
- 48.Zhou X, Jen P HS, Seburn K L, Frankel W N, Zheng Q Y. Auditory brainstem responses in 10 inbred strains of mice. Brain Res. 2006;1091(01):16–26. doi: 10.1016/j.brainres.2006.01.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bauch C D, Olsen W O. Comparison of ABR amplitudes with TIPtrode and mastoid electrodes. Ear Hear. 1990;11(06):463–467. doi: 10.1097/00003446-199012000-00010. [DOI] [PubMed] [Google Scholar]


