Abstract
Objective
To assess patient-reported symptoms and burden of disease in relapsing polychondritis (RP).
Methods
Patients with RP completed a disease-specific online survey to identify symptoms attributed to illness. Patients were divided into subgroups based upon presence or absence of ear/nose, airway, or joint involvement. Pathway to diagnosis, treatment, and disease-related complications were assessed within each subgroup.
Results
Data from 304 respondents were included in this analysis. Prior to diagnosis, most patients with RP went to the emergency room (54%), saw > 3 physicians (54%), and had symptoms for >5 years (64%). A concomitant diagnosis of fibromyalgia and absence of ear/nose or joint involvement was associated with diagnostic delay >1 year. Common diagnoses prior to RP diagnosis included asthma in patients with airway involvement (35% vs 22%, p=0.03) and ear infection in patients with ear/nose involvement (51% vs 6%, p<0.01). Patients with joint involvement were more likely to receive a glucocorticoid-sparing agent (85% vs 13%, p<0.01). Most patients reported a major complication including disability (25%), tracheomalacia (16%) or hearing loss (34%). Patients with airway involvement reported more tracheomalacia (20% vs 4%, p<0.01). Disability (24% vs 7%, p<0.01) and hearing loss (39% vs 11%, p<0.01) were prevalent in the joint involvement subgroup.
Conclusion
Patient-reported data in RP highlight a significant burden of disease. Patterns of organ involvement may lead to diagnostic delay and influence treatment decisions, ultimately impacting the development of disease-related complications. Timely diagnosis, standardization of treatment approaches, and prevention of disease-related complications are major unmet needs in RP.
Keywords: relapsing polychondritis, tracheomalacia, quality of life, survey, illness perceptions
Relapsing polychondritis (RP) is a systemic and in some cases, fatal condition initially described by Jacksch-Waternhorst in 1923(1). RP is defined by inflammation of cartilaginous structures and the disease course and clinical manifestations are highly variable. End organ complications due to inflammation of cartilage and proteoglycan rich structures are diverse and include deformity of the ears(2), nose(3) and chest(4), subglottic stenosis (5), obstructive lung disease, tracheomalacia(6), bronchomalacia(7), recurrent pneumonia(8), mitral and aortic regurgitation(9, 10), large and medium vessel aneurysms(11), blindness(12), deafness(13), renal insufficiency(14), chronic pain, and sudden death(15–17). The protean aspects of RP pose diagnostic challenges. Participants can have transient and isolated involvement of cartilaginous structures including the ear, nose, larynx, and tracheobronchial tree. Complications may vary depending on the disease pattern and severity of organ involvement.
Delay in diagnosis ranges from 2.9–14 years (18, 19). The reasons for diagnostic delay have not been well characterized but are likely due in part to the clinical heterogeneity of the disease, lack of markers for early signs of the disease, lack of a diagnostic biomarker, over-reliance on physician-based observation of tissue damage to establish the diagnosis, and the relapsing nature of the illness. Overlap with other conditions and misattribution of signs and symptoms may also contribute to delay in diagnosis and adversely affect outcomes.
In many chronic rheumatologic conditions, patient-perceived burden of illness can differ substantially from physician-based perception of disease. Large epidemiologic studies of RP are non-existent due to the rare nature of the illness and similarly no study has focused upon the patient-reported experience in RP. As a result, information is lacking regarding patient-perceived symptoms of illness, the impact of RP on quality of life, or the potential for environmental triggers to cause disease flares. The objective of this study was to evaluate patient-reported perception of disease-related symptoms, complications, patterns of organ involvement, and treatment in RP.
PATIENT AND METHODS
Study Population
Patients were recruited through the Relapsing Polychondritis Awareness and Support Foundation (RPASF) to participate in an online survey. RPASF advertised the survey to participants with self-identified RP via emails to subjects who had previously agreed to answer questionnaires, by a direct link to the survey on their website (www.polychondritis.org), and by promotion on other related support group websites. This anonymous survey was considered exempt from IRB review by the National Institutes of Health (NIH) Office of Human Subjects Research Protections (OHSRP). The survey was open to the public on February 1, 2016 and closed on August 7, 2016. Adult and juvenile patients with RP were invited to complete the survey; however, for this study, only responses from adult participants ≥ 18 years were considered for analysis.
Survey Elements
Patients were asked to detail symptoms attributed to RP prior to diagnosis and throughout the course of disease. A list of potential symptoms was generated based upon literature review of clinical features associated with RP, including symptoms represented in the various published diagnostic criteria (16, 20–22). Patients were asked about the length of time between symptom onset and time of diagnosis, alternative diagnoses, and the number and type of physicians seen in order to establish the diagnosis. Major complications related to RP were queried, including disability, tracheomalacia, and hearing loss. Patients were asked about current and prior therapies including maximum doses of daily prednisone required to control disease activity. Information about flares of disease activity and potential specific triggers of disease relapse were assessed. A copy of the complete survey can be found in the Supplementary Table.
Categories of Organ Involvement
Patients were divided into overlapping subgroups based upon presence of ear/nose, airway, or joint involvement. Ear/nose involvement was defined as presence of symptoms related to ear or sinonasal disease (ear pain, nasal pain, nasal redness). Airway involvement was defined as presence of shortness of breath, pain with breathing, or voice changes. Joint involvement was defined as swelling or pain affecting at least one peripheral joint.
Statistical analysis
Categorical variables were compared using Fisher’s exact test. SAS v9.4 (SAS Institute, Inc, Cary, NC) was used for general analyses, and ClustVis (biit.cs.ut.ee) was used to identify potentially novel subgroups of participants with RP. Briefly, principal component (PC) analysis of patient-reported symptoms of disease was performed. Potential clusters were visualized by plotting the first two PCs on a scatterplot. Putative associations of PC analysis results and clinical features of RP were explored based upon patterns of organ involvement and cumulative number of reported symptoms across different organ systems. Multivariable logistic regression was used to identify variables associated with diagnostic delay >1 year, including gender, age, race, concomitant medical conditions, and patterns of organ system involvement. Data are reported as frequencies and percentages, means and standard deviations (SD), or odds ratios (OR) and 95% Confidence Intervals (CI). A two-sided p-value less than 0.05 was considered statistically significant.
RESULTS
Patient characteristics
Survery respondent characteristics are detailed in Table 1. 320 patients completed the survey. Results from 16 patients were excluded from further analysis because the age of the respondent was <18 years or not reported. Thus, 304 surveys were included in the analysis. The majority of patients were female (87%), Caucasian (88%), and born in the USA (71%). The mean age of respondents was 48.6 (SD 11.2 years). The mean age at diagnosis was 43.2 years (SD 12.2). Ninety-four percent of patients reported ear/nose involvement, 74% reported airway involvement, and 82% reported joint involvement (Table 1).
Table 1.
Characteristics of Survey Respondents Grouped by Organ System Involvement
| All Patients | Ear/nose Involvement | Airway Involvement | Joint Involvement | |
|---|---|---|---|---|
| n=304 | n=287 | n=225 | n=250 | |
| Age mean (SD) | 48 (11.2) | 49 (11) | 47.9 (13) | 47.4 (12.9) |
| Age at diagnosis mean (SD) | 42 (12.2) | 43.6 (11.9) | 43.9 (12.2) | 43.2 (12.1) |
| Gender | n (%) | n (%) | n (%) | n (%) |
| Female | 263 (87) | 253 (88) | 197 (87) | 230 (92) |
| Race | n (%) | n (%) | n (%) | n (%) |
| White | 268 (88) | 255 (89) | 203 (90) | 222 (89) |
| Hispanic | 15 (5) | 13 (4) | 8 (3) | 10 (4) |
| Other | 13 (4) | 11 (4) | 6 (2) | 7 (3) |
| More than one | 8 (3) | 8 (3) | 8 (3) | 11 (4) |
| Country of origin | n (%) | n (%) | n (%) | n (%) |
| United States | 222 (73) | 205 (71) | 173 (77) | 186 (74) |
| Canada | 13 (4) | 11 (4) | 8 (4) | 10 (4) |
| Latin America | 4 (1) | 4 (1) | 2 (1) | 2 (1) |
| Central America | 7 (2) | 3 (1) | 4 (2) | 3 (1) |
| Europe | 52 (17) | 49 (17) | 31 (14) | 44 (18) |
Initial and overall symptoms
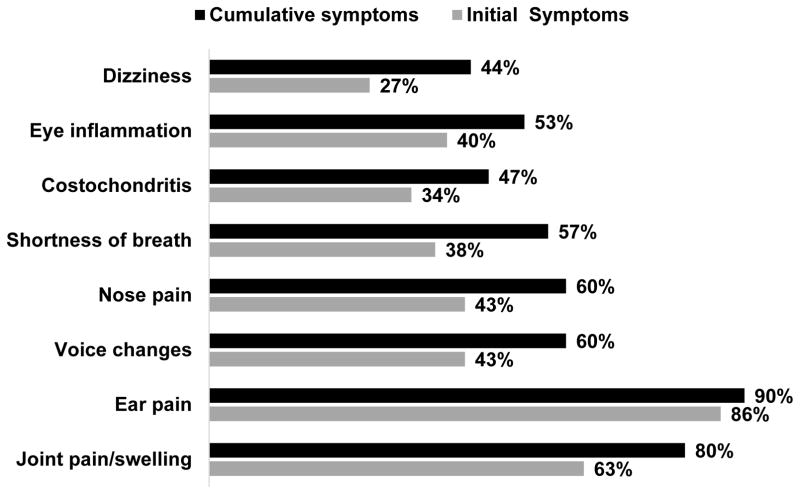
The most common initial symptom prior to diagnosis was ear pain (86%). Other common initial symptoms prior to diagnosis included voice changes (43%), ocular inflammation (40%), shortness of breath (38%), costochondritis (34%), and dizziness (27%). Some patients reported constitutional symptoms, including fatigue (6%) and flu-like symptoms and fever (2.6%), as initial symptoms. Although ear involvement is a hallmark feature of RP, 30 out of 304 participants (10%) denied a history of ear pain. Common symptoms prior to diagnosis and cumulative are displayed in Figure 1.
Figure 1.
Characteristics of study participants relative to published literature
Based on patient-reported symptoms, all but 16 patients (5%) fulfilled either McAdam’s or Damiani’s criteria. Prevalence of specific disease-related features are detailed in Table 2 in relationship to similar data from other published cohorts in RP. The prevalence of several symptoms, such as Raynaud’s syndrome, are detailed for the first time in RP in this cohort. Raynaud’s phenomenon was more prevalent in patients with airway involvement (24.4% vs 11.4%, p=0.01) and in patients with joint involvement (24.8% vs 3.7%, p<0.01)
Table 2.
Prevalence of Overall Symptoms in Multiple Cohorts of Relapsing Polychondritis
| Current study | Dion21 | Hazra22 | Trentham18 | Michet16 | McAdam20 | Sharma17 | |
|---|---|---|---|---|---|---|---|
| n of patients | 304 | 142 | 50 | 66 | 112 | 23 | 26 |
| Auricular chondritis | 262 (86) | 126 (89) | 35 (70) | 62 (95) | 95 (85) | 20 (89) | 24(96) |
| Nasal chondritis | 193 (63) | 89 (63) | 13 (26) | 43(66) | 60 (54) | 16 (72) | 3 (11) |
| Nose pain | 193 (63) | - | - | - | - | - | - |
| Nose redness | 126 (41) | - | - | - | - | - | - |
| Tracheobronchial tree | 225 (74) | 71 (50) | 6 (12) | 31(48) | 53 (48) | 12 (23) | 3 (11) |
| Voice changes | 180 (60) | - | - | - | - | - | - |
| Pleuritic chest pain | 109 (36) | - | - | - | - | - | - |
| Shortness of breath | 171 (56) | - | - | - | - | - | - |
| Joint involvement | 250 (82) | 97 (69) | 18 (36) | 56 (85) | 58 (52) | 18 (23) | 14(53) |
| Costochondritis | 143 (47) | 57 (40) | - | - | - | - | - |
| Knee pain | 166 (54) | - | - | - | - | - | - |
| Ankle pain | 130 (43) | - | - | - | - | - | - |
| Elbow pain | 106 (35) | - | - | - | - | - | - |
| Wrist pain | 144 (47) | - | - | - | - | - | - |
| Finger pain | 153 (50) | - | - | - | - | - | - |
| Knee swellling | 88 (29) | - | - | - | - | - | - |
| Ankle swelling | 82 (27) | - | - | - | - | - | - |
| Elbow swelling | 46 (15) | - | - | - | - | - | - |
| Wrist swelling | 53 (17) | - | - | - | - | - | - |
| Finger swelling | 110 (36) | - | - | - | - | - | - |
| Eye inflammation | 151 (53) | 79 (56) | 10 (20) | 37 (57) | 57 (51) | 15 (65) | 11(42) |
| Inner Ear involvement | 240 (79) | 48 (34) | - | - | - | - | - |
| Dizziness | 136 (45) | 29 (20) | - | 35 (53) | NR | 3 (13) | NR |
| hearing loss | 104 (34) | 39 (27) | - | 27 (42) | 33 (29) | 3 (14) | 12 (46) |
| Raynaud’s | 64 (21) | 11 (8) | - | - | - | - | - |
Subsets of participants with RP based on reported symptoms
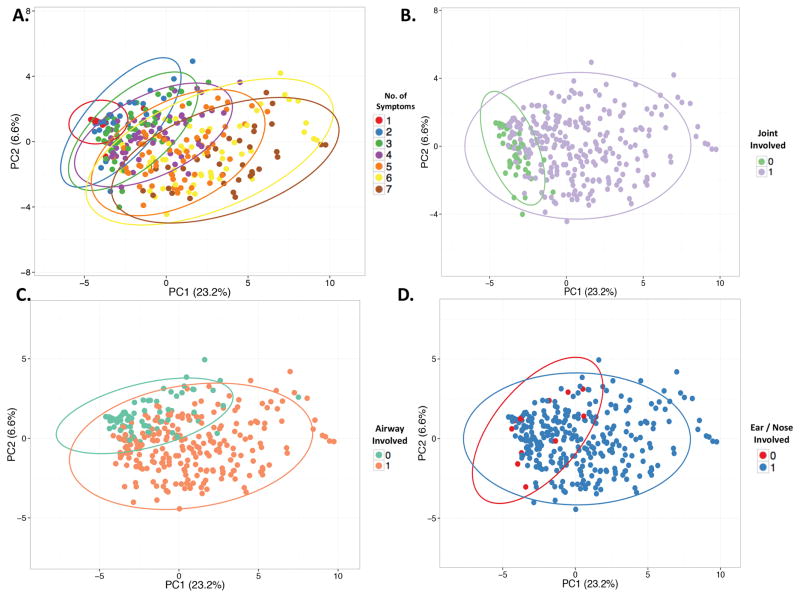
Cluster analysis was performed using patient-reported data on presence or absence of the following 7 symptoms: ear pain, nose pain, shortness of breath, voice changes, hearing loss or dizziness, costochondritis, and joint pain. The first two PCs explained 23.3% and 6.6% of variability in the dataset. Increasing number of symptoms was the strongest source of variability within the first and second PCs, suggesting that patients with RP can have localized or systemic forms of the disease (Figure 2a). Single organ involvement was reported in 18 out of 304 patients (6%) with isolated ear involvement reported by 16 patients and isolated airway involvement reported by 2 patients. Subsets of patients could be visualized within the scatterplots based upon presence of joint pain (Figure 2b) or presence of airway involvement (Figure 2c). Presence of ear/nose involvement was not useful to identify subsets of patients as the majority of patients (94%) reported these symptoms (Figure 2d).
Figure 2.
Triggers of disease activity
A number of items were reported as potential triggers of disease activity in RP. A majority of patients (52%) reported worsening of symptoms in relationship to vigorous physical activity. Lack of sleep (45%), upper respiratory infections (40%), change in weather (33%), and airplane travel (12%) were identified as potential triggers of disease activity. Many patients reported worsening of symptoms in relationship to specific dietary factors (37%), including intake of food containing high sugar content (12%), gluten (10%), or consumption of alcohol (8%). Little information is known about pregnancy outcomes or hormonal influence on disease activity in RP. A substantial number of patients (96% of 77 who answered the question) reported disease flare associated with menses, and 20% of 203 respondents who answered the question reported a disease flare during pregnancy.
Establishment of diagnosis
Prior to diagnosis, the majority of patients were evaluated by >3 physicians (55%), reported symptoms for >5 years (64%), and 54% went to the emergency room for RP-related symptoms. The main reasons prompting ER visits included ear pain (28%), chest pain/costochondritis (27%), shortness of breath (26%), and pain during respiration (13%). Patients with airway involvement were more likely to go to the ER and to see >3 physicians prior to diagnosis (57% vs 43%, p=0.03; 60% vs 40%, p<0.01). The majority of patients were diagnosed by a rheumatologist (55%); however, other specialties that established the diagnosis included otolaryngology (20%), primary care (7%), dermatology (4%), emergency physicians (3%), and pulmonology (2%).
Symptoms of relapsing polychondritis may be attributable to other conditions. Prior to diagnosis, the most common alternative diagnoses were ear infections (48%), sinusitis (39%), asthma (31%), and fibromyalgia (24%). A diagnosis of asthma was more frequently reported in patients with airway involvement (p=0.03) (Table 3). Patients with ear/nose involvement were more likely to have a previous diagnosis of ear infection (p<0.01). Patients with airway or joint involvement, but not ear/nose involvement, were more likely to report a diagnosis of fibromyalgia (p<0.01). Only 18% of patients underwent biopsy of cartilaginous tissue to support the diagnosis. Several autoimmune diseases have been associated with RP, and the majority of study participants (56%) endorsed presence of another concurrent autoimmune disorder, including rheumatoid arthritis (13%), vasculitis (10%), thyroiditis (10%), and Sjögren’s syndrome (5%).
Table 3.
Differences Among Patients with Relapsing Polychondritis Grouped by Organ System Involvement
| All Patients |
Ear/Nose Inolvment |
Ear/Nose not Involved |
P value | Airway Involvment |
Airway not Involved |
P value | Joint Involvement |
Joint not Involved |
P value | |
|---|---|---|---|---|---|---|---|---|---|---|
| n=304 | n=287 | n=17 | n=225 | n=79 | n=250 | n=54 | ||||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||||
| Age at Diagnosis | ||||||||||
| Age of diagnosis > 40 years old | 188 (62) | 182 (63) | 6 (35) | 0.04 | 143 (64) | 45 (57) | 0.3 | 162 (65) | 26 (48) | 0.03 |
| Pathway to Diagnosis | ||||||||||
| ER visits prior to diagnosis | 164 (54) | 153(53) | 10 (59) | 0.8 | 128 (57) | 34 (43) | 0.04 | 131 (52) | 32 (59) | 0.4 |
| Biopsy | 55 (18) | 49 (17) | 6 (35) | 0.1 | 38 (17) | 17 (22) | 0.4 | 45 (18) | 10 (19) | 0.8 |
| Saw > 3 physicians prior RP diagnosis | 168 (55) | 171(60) | 7 (41) | 0.2 | 134 (60) | 32 (41) | <0.01 | 142 (57) | 25 (46) | 0.2 |
| Other Diagnoses | ||||||||||
| Asthma | 95 (31) | 85 (30) | 10 (59) | 0.02 | 78 (34) | 17 (22) | 0.03 | 77 (31) | 18 (33) | 0.7 |
| Ear Infection | 145 (48) | 145 (51) | 1 (6) | <0.01 | 107 (48) | 38 (48) | >0.9 | 126 (50) | 19 (35) | 0.05 |
| Sinusitis | 118 (41) | 113 (42) | 5 (30) | 0.4 | 94 (45) | 24 (31) | 0.04 | 104 (45) | 14 (28) | 0.02 |
| Fibromyalgia | 71 (23) | 70 (25) | 1 (6) | 0.08 | 63 (28) | 9 (11) | <0.01 | 67 (27) | 4 (7) | <0.01 |
| Treatment | ||||||||||
| DMARDS or Biologics | 253 (83) | 237 (82) | 12 (71) | 0.2 | 182 (76) | 58 (24) | 0.1 | 213 (86) | 33 (61) | <0.01 |
| DMARDs | 206 (68) | 199 (69) | 9 (53) | 0.1 | 152 (67) | 51 (65) | 0.7 | 173 (69) | 30 (55) | 0.06 |
| Biologics | 47 (15) | 38 (13) | 3 (17) | 0.7 | 30 (13) | 7 (9) | 0.4 | 40 (16) | 3 (5) | 0.05 |
| Prednisone >60 mg/day during flare | 134 (44) | 124 (53) | 9 (53) | 0.4 | 112 (49) | 23 (29) | <0.01 | 113 (45) | 20 (37) | 0.2 |
| Major Complications | 181 (60) | 173 (60) | 8 (47) | 0.3 | 140 (63) | 41 (52) | 0.1 | 152 (60) | 29 (54) | 0.3 |
| Disability | 63 (21) | 59 (21) | 4 (25) | 0.7 | 53 (24) | 10 (13) | 0.05 | 59 (24) | 4 (7) | <0.01 |
| Tracheomalacia | 47 (15) | 41 (14) | 5 (29) | 0.1 | 44 (20) | 3 (4) | <0.01 | 41 (17) | 6 (11) | 0.4 |
| Hearing loss | 104 (34) | 100 (35) | 4 (24) | 0.4 | 76 (34) | 28 (35) | 0.7 | 98 (39) | 6 (11) | <0.01 |
Percent’s may not be based on total denominators if there were missing responses
Conditions associated with diagnostic delay
Multivariable logistic regression analysis was used to identify several clinical features associated with a diagnostic delay >1 year from the time of symptom onset. In multivariable modeling, absence of ear/nose involvement was most strongly associated with diagnostic delay (Table 4). Patients with RP who did not report ear/nasal involvement had 5.6 times increased odds for diagnostic delay of >1 year compared to patients with RP who reported earl/nose involvement as a feature of disease (95% CI: 1.4 - 22.4; p=0.01). A concomitant diagnosis of fibromyalgia (OR=5.3, 95% CI: 1.8–15.3; p<0.01) and lack of joint involvement (OR=2.3, 95% CI: 1.2–4.6; p=0.02) were also significantly associated with increased odds for diagnostic delay in a multivariable regression model.
Table 4.
Regression Analysis: Predictors of diagnostic delay of >1 year from symptom onset
| Univariable Model | Multivariable Model | |||
|---|---|---|---|---|
|
| ||||
| Variable | Odds Ratio (95% Confidence Interval) | P Value | Odds Ratio (95% Confidence Interval) | P Value |
| Absence of Ear/Nose Involvement | 10.16 (2.79–40.40) | <0.01 | 5.61 (1.40–22.44) | 0.01 |
| Fibromyalgia | 7.16 (2.52–20.43) | <0.01 | 5.28 (1.82–15.33) | <0.01 |
| Absence of Joint Involvement | 3.61 (1.94–6.73) | <0.01 | 2.29 (1.15–4.59) | 0.02 |
| Female Gender | 2.32 (1.14–4.74) | 0.02 | 1.33 (0.58–3.02) | 0.50 |
| Absence of Airway Involvement | 1.89 (1.07–3.35) | 0.03 | 1.23 (0.66–2.32) | 0.51 |
|
|
||||
| Age at diagnosis | 1.01 (0.99–1.04) | 0.20 | Not included in multivariable model. | |
| Caucasian Race | 1.41 (0.64–3.12) | 0.40 | ||
Treatment differences and disease-related complications in RP subgroups
Patients reported that glucocorticoids were the most commonly used treatment for RP. The majority of patients (81%) reported use of prednisone at some point during the disease. Among patients treated with glucocorticoids, 54% reported that high doses of prednisone (60->100mg per day) were required to control symptoms of disease activity. Other frequently used treatments included methotrexate (30%) and azathioprine (14%). Forty-seven (15%) patients reported use of a biologic agent, and the most common class of biologic agent reported was TNF-inhibitors (94%). Other reported biologic therapies included tocilizumab (8%, n=4), rituximab (8%, n=4), and anakinra (4%, n=2). Cyclophosphamide was reportedly used in 3 patients.
RP-related complications and treatment approaches in patients with RP grouped based upon ear/nose, airway, or joint involvement are presented in Table 3. Patients with airway involvement reported use of a daily dose of prednisone >60mg to control flares (p<0.01). Patients with joint involvement were more likely to be treated with a DMARD or a biologic (p<0.01). There was no significant difference of use of a glucocorticoid-sparing agent in patients with or without ear/nose or airway involvement (Table 3). The majority of patients with RP (60%) reported at least one major complication of disease. Prevalence of specific complications of RP included disability (21%), tracheomalacia (15%), and hearing loss (34%). Underlying reasons for disability included extreme fatigue and difficulty concentrating (38%), treatment refractory disease (27%), and difficulty breathing (17%). Tracheomalacia was more frequently reported by patients with airway involvement (p<0.01). Patients with joint involvement were more likely to report disability (p<0.01), to be older than 40 years at the time of diagnosis (p=0.03) and have hearing loss (p<0.01). Although not statistically significant patients with airway involvement may be more likely to have disability (p= 0.05). (Table 3).
DISCUSSION
This international online study provides unique information about RP from the patient’s perspective. The survey findings demonstrate that RP is a multisystem disease that may significantly impact quality of life and result in disability in a substantial number of patients, especially patients with airway involvement. Diagnostic delay is common, and other diagnoses are frequently entertained prior to establishing a diagnosis of RP. Glucocorticoids are most commonly used to treat RP, often at very high doses during disease flares. Although use of glucocorticoid-sparing agents was frequently reported in this cohort, specific therapeutic approaches varied considerably.
Identification of subgroups of patients with RP based upon patterns of disease involvement has clinical benefit. A recent retrospective study of 142 patients with RP used cluster analysis to identify 3 subgroups of patients with RP, including those with tracheobronchial involvement, associated myelodysplastic syndrome, or absence of airway or hematologic disease. Compared to the remainder of the cohort, patients with tracheobronchial involvement were younger, had a lower prevalence of auricular chondritis, were more likely to receive immunosuppressive or biologic agents, were more frequently admitted to an intensive care unit, and were more susceptible to infections (21). In the current study, patients with airway involvement were also more likely to endure major disease-related complications, including tracheomalacia, and may be more likely to have disability. These data, taken together, suggest that patterns of organ involvement contribute to clinical outcomes in RP.
Findings from this study demonstrate a need for classification criteria in RP and should inform their development. Currently, there are no validated classification criteria for RP. Given the clinical heterogeneity of the disease, accurate case definition for recruitment into clinical trials is a major unmet need in RP. The survey findings detail a diversity both of symptoms and patterns of organ involvement with auricular symptoms being the most prevalent feature of RP at the time of diagnosis and over the course of disease. While most patients with RP report symptoms of auricular chondritis, 10% of patients in this cohort did not report a history of ear pain. Moreover, some patients with RP (6%) may not endorse symptoms of ear or nose involvement at any point in the disease. These individuals may be diagnosed on the basis of airway, ocular, and musculoskeletal involvement. In this study, cluster analysis of patient-reported symptoms revealed that the number of symptoms reported by a patient was the greatest source of variability among survey respondents. Some patients reported symptoms of auricular chrondritis as the only feature of disease, and others reported involvement of multiple organ systems. These results highlight the existence of both limited and systemic forms of RP. Future classification criteria should represent the clinical spectrum of RP, including airway-predominant and localized forms of the disease.
The study results demonstrate that establishing a diagnosis of RP is challenging, and long periods of diagnostic delay are common. Patients with RP report multiple visits to different physicians and to the emergency department prior to establishment of the diagnosis. Although rheumatologists most commonly assigned the diagnosis of RP, physicians from a spectrum of subspecialties may be the first to encounter such patients, highlighting a need for disease awareness across a range of health care providers. The survey findings also suggest a potential reliance on ear/nose involvement to make a diagnosis of RP. Absence of ear/nose involvement was significantly associated with diagnostic delay and complications including tracheomalacia. Further, patients with airway and joint involvement more commonly reported a previous diagnosis of fibromyalgia compared to patients with ear/nose involvement, and a concomitant diagnosis of fibromyalgia was also an independent factor associated with diagnostic delay. Whether a diagnosis of fibromyalgia represents a misattribution of signs and symptoms of RP or reflects a disease-associated comorbidity remains unclear.
The survey also highlights considerable variability in therapeutic approaches to RP. Despite therapeutic advancements in a number of rheumatologic diseases, patients with RP report substantial glucocorticoid requirements at high doses during times of disease activity. The need for high doses of glucocorticoids to control flares have been reported by others (23, 24). While the majority of patients in this study report use of glucorticoid-sparing therapies, multiple different medications were detailed, highlighting a need for clinical trials and subsequent treatment guidelines in RP. The current analysis shows that patients with joint involvement are more likely to be treated with a glucocorticoid-sparing agent. In contrast, airway involvement in RP was not associated with significantly greater use of DMARDs or biologic therapies despite the fact that patients with airway involvement are likely to have major complications from the disease.
This study demonstrates that most patients with RP report major disease-related complications, emphasizing a need for improved disease surveillance and early treatment strategies to minimize morbidity in RP. Lack of recognition of symptoms of airway involvement, such as voice changes or shortness of breath, or misattribution of these symptoms as features of asthma, may contribute to diagnostic delay in RP and to the development of permanent airway damage.
This study has some potential limitations. The process of advertising and conducting a study online is subject to selection bias, including predominantly female participation. This report may not be generalizable to all patients with RP. However, this type of study design enables recruitment of a larger number of participants with RP and allows for the opportunity to collect information for purposes of thorough disease characterization. Second, the psychometric properties of the survey were not validated. Rather, the survey was designed to be specifically applicable to patients with RP based upon literature review. Third, study patients self-reported the diagnosis and these medical attributions were not confirmed through independent medical record audit. However, the frequency of specific clinical features of disease were consistent with published reports of physician-observed cohorts of RP, 95% of the study participants met either the McAdams or Damiani’s diagnostic criteria for RP based upon reported symptoms, and the majority of patients enrolled in this study endorsed the use of DMARDs or biologic therapy improving confidence in the accuracy of self-reported diagnosis. Finally, inherent in the nature of the study design is the possibility of recall bias. Specifically, the tendency for survey respondents to inflate the significance of pre-conceived risk factors or associations, including identification of potential triggers of disease activity, may result in ascertainment bias.
The study has several important strengths. Use of an anonymous web-based strategy enabled recruitment of a large and geographically diverse population of patients with RP. Additionally, this report is the first of its kind to inquire about a broad range of disease symptoms, triggers of disease activity, and disease-related complications from the patient’s perspective in RP.
In conclusion, this study represents the largest patient-based study in RP. These survey findings highlight several major unmet needs in RP, namely a need for improved diagnostic strategies, standardized treatment approaches, classification criteria, and awareness of the complete clinical spectrum of disease by a wide range of physician subspecialties. Furthermore, the findings demonstrate substantial disease-related complications that affect most patients with RP, particularly patients with airway involvement. Taken as a whole, these data provide strong evidence for the need for continued research efforts in RP to improve clinical outcomes in this potentially devastating disease.
Supplementary Material
SIGNIFICANCE AND INNOVATIONS.
This study is the first of its kind to assess relapsing polychondritis from the patient’s perspective in an international cohort and identifies major unmet needs in relapsing polychondirits including need for improved diagnostic strategies, standardized treatment approaches to minimize disease-related complications, development of classification criteria, and physician awareness of the complete clinical spectrum of disease.
The majority of patients with relapsing polychondritis report diagnostic delay of >5 years from symptom onset, and a concomitant diagnosis of fibromyalgia or lack of ear, nose, or joint involvement is associated with diagnostic delay.
The majority of patients with relapsing polychondritis report development of serious disease-related complications including disability.
Acknowledgments
We thank the patients with Relapsing polychondritis for their participation. We also thank Thomas Christie, Carol Giordano, Lissete Arriola-Gardea, Susan Dale Ross, and Michael Linn from the Relapsing Polychondritis Awareness and Support Foundation for their support.
Footnotes
Financial supports of conflicts disclosure:
This research was supported through the Intramural Research Program at the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) and the NIH Clinical Center. The authors do not have any conflicts of interest related to this work.
References
- 1.Jackch-Worthenhorst R. Polychondropathia. Arch Intern Med. 1923;6:93–100. [Google Scholar]
- 2.Thurston CS, Curtis AC. Relapsing polychondritis. Report of a patient with “beefy” red ears and severe polyarthritis. Arch Dermatol. 1966;93(6):664–9. doi: 10.1001/archderm.93.6.664. [DOI] [PubMed] [Google Scholar]
- 3.Tobisawa Y, Shibata M. A case of saddle nose deformity caused by relapsing polychondritis: a long-term follow-up report after iliac bone grafting. J Plast Reconstr Aesthet Surg. 2013;66(11):1621–2. doi: 10.1016/j.bjps.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Lim MC, Chan HL. Relapsing polychondritis--a report on two Chinese patients with severe costal chondritis. Ann Acad Med Singapore. 1990;19(3):396–403. [PubMed] [Google Scholar]
- 5.Lee CC, Singer AJ. Respiratory failure due to subglottic stenosis from relapsing polychondritis. Am J Emerg Med. 2006;24(6):750–2. doi: 10.1016/j.ajem.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 6.Adliff M, Ngato D, Keshavjee S, Brenaman S, Granton JT. Treatment of diffuse tracheomalacia secondary to relapsing polychondritis with continuous positive airway pressure. Chest. 1997;112(6):1701–4. doi: 10.1378/chest.112.6.1701. [DOI] [PubMed] [Google Scholar]
- 7.Mezghani Ben Salah S, Harzallah MF, Marouen F, Aissa S, Hayouni A, Khlifa M, et al. Tracheomalacia bronchomalacia: a severe complication of relapsing polychondritis. Tunis Med. 2013;91(1):84–5. [PubMed] [Google Scholar]
- 8.Bin-Sagheer ST, Pema K, Verghese A. Recurrent pneumonia in relapsing polychondritis. West J Med. 1994;161(2):171–2. [PMC free article] [PubMed] [Google Scholar]
- 9.Sohi GS, Desai AM, Ward WW, Flowers NC. Aortic cusp involvement causing severe aortic regurgitation in a case of relapsing polychondritis. Cathet Cardiovasc Diagn. 1981;7(1):79–86. doi: 10.1002/ccd.1810070111. [DOI] [PubMed] [Google Scholar]
- 10.Otasevic P, Pavlovski K, Popovic AD. Isolated mitral regurgitation complicating relapsing polychondritis. Int J Cardiol. 1997;60(2):213–5. doi: 10.1016/s0167-5273(97)00093-4. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs CE, March RJ, Hunt PJ, Rivera AG, Cavanagh S, McCarthy WJ. Repair of a complex thoracic aneurysm from relapsing polychondritis. Vasc Endovascular Surg. 2013;47(5):387–9. doi: 10.1177/1538574413488459. [DOI] [PubMed] [Google Scholar]
- 12.Loeffler KU, McLean IW. Bilateral necrotizing scleritis and blindness in the myelodysplastic syndrome presumably due to relapsing polychondritis. Acta Ophthalmol Scand. 2000;78(2):228–31. doi: 10.1034/j.1600-0420.2000.078002228.x. [DOI] [PubMed] [Google Scholar]
- 13.Bollet AJ, Smith JG, Mushet GR, Austin F. Arthritis, deafness, and saddle nose(relapsing polychondritis). Medical Grand Rounds Medical College of Georgia. J Med Assoc Ga. 1969;58(7):315–9. [PubMed] [Google Scholar]
- 14.Neild GH, Cameron JS, Lessof MH, Ogg CS, Turner DR. Relapsing polychondritis with crescentic glomerulonephritis. Br Med J. 1978;1(6115):743–5. doi: 10.1136/bmj.1.6115.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winstanley S, Boyde A, Attanoos R. Fatal relapsing tracheobronchial polychondritis diagnosed at autopsy. BMJ Case Rep. 2015;2015 doi: 10.1136/bcr-2015-209483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michet CJ, Jr, McKenna CH, Luthra HS, O’Fallon WM. Relapsing polychondritis. Survival and predictive role of early disease manifestations. Ann Intern Med. 1986;104(1):74–8. doi: 10.7326/0003-4819-104-1-74. [DOI] [PubMed] [Google Scholar]
- 17.Sharma A, Law AD, Bambery P, Sagar V, Wanchu A, Dhir V, et al. Relapsing polychondritis: clinical presentations, disease activity and outcomes. Orphanet J Rare Dis. 2014;9:198. doi: 10.1186/s13023-014-0198-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trentham DE, Le CH. Relapsing polychondritis. Ann Intern Med. 1998;129(2):114–22. doi: 10.7326/0003-4819-129-2-199807150-00011. [DOI] [PubMed] [Google Scholar]
- 19.Lin DF, Yang WQ, Zhang PP, Lv Q, Jin O, Gu JR. Clinical and prognostic characteristics of 158 cases of relapsing polychondritis in China and review of the literature. Rheumatol Int. 2016;36(7):1003–9. doi: 10.1007/s00296-016-3449-8. [DOI] [PubMed] [Google Scholar]
- 20.McAdam LP, O’Hanlan MA, Bluestone R, Pearson CM. Relapsing polychondritis: prospective study of 23 patients and a review of the literature. Medicine (Baltimore) 1976;55(3):193–215. [PubMed] [Google Scholar]
- 21.Dion J, Costedoat-Chalumeau N, Sène D, Cohen-Bittan J, Leroux G, Dion C, et al. Relapsing Polychondritis Can Be Characterized by Three Different Clinical Phenotypes: Analysis of a Recent Series of 142 Patients. Arthritis and Rheumatology. 2016;68(12):2992–3001. doi: 10.1002/art.39790. [DOI] [PubMed] [Google Scholar]
- 22.Hazra N, Dregan A, Charlton J, Gulliford MC, D’Cruz DP. Incidence and mortality of relapsing polychondritis in the UK: a population-based cohort study. Rheumatology (Oxford) 2015;54(12):2181–7. doi: 10.1093/rheumatology/kev240. [DOI] [PubMed] [Google Scholar]
- 23.Jeon CH. Relapsing Polychondritis with Central Nervous System Involvement: Experience of Three Different Cases in a Single Center. J Korean Med Sci. 2016;31(11):1846–50. doi: 10.3346/jkms.2016.31.11.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirayama K, Iwanaga N, Izumi Y, Yoshimura S, Kurohama K, Yamashita M, et al. A Case of Relapsing Polychondritis Initiating with Unexplained Fever. Case Rep Med. 2016;2016:9462489. doi: 10.1155/2016/9462489. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.