Abstract
Objective
Aging is associated with muscle fiber hypotrophy and decreased percentages of rapidly-contracting myosin heavy chain (MyHC) type IIb muscle fibers. Tongue exercise programs used to treat dysphagia target age related decline in tongue muscle function, but the impact of exercise on the intrinsic tongue muscles is unknown. We hypothesized that exercise would induce muscle fiber hypertrophy and increase the percentage of MyHC IIa fibers in the intrinsic tongue.
Methods
Eight old and 8 young-adult rats underwent 8-weeks of tongue exercise training and 8 animals of each age group served as controls. Longitudinal, transverse, and verticalis muscle samples from the anterior, middle, and posterior regions of the tongue were sectioned and stained to determine muscle fiber diameter and MyHC composition.
Results
MyHC fiber type distribution was altered by exercise, and the effects differed by muscle and region of the tongue. In the exercise groups, as compared to the control groups, the anterior transverse and middle superior longitudinal muscles had significantly reduced percentages of MyHC IIx positive fibers and higher percentages of rapidly-contracting fatigable MyHC IIb positive muscle fibers, whereas the middle transverse and posterior longitudinal muscles had increased percentages of the less rapidly-contracting and more fatigue-resistant MyHC IIa fibers. The impact of exercise did not differ with age as there was no significant interaction between age and exercise. Tongue exercise had no significant effect on muscle fiber diameter.
Conclusion
The impact of exercise varied among the tongue muscles, which may indicate different functional contributions to the tongue exercise task.
Keywords: Lingual, Tongue, Muscle, Intrinsic, Exercise
Introduction
Healthy swallowing function is critical for airway safety, nutrition, and quality of life, yet aged individuals frequently develop difficulty swallowing, with dysphagia occurring in 15-40% of individuals over age 60.1 Tongue weakness, associated with both old age and dysphagia,2,3 has been identified as a therapeutic target. Tongue strengthening exercises increase maximum isometric tongue pressures and improve patient-reported measures of swallowing.4–7 However, the biological mechanisms accounting for changes in tongue force with exercise are unclear. In this vein, a better understanding of the impact of exercise on the tongue muscles and swallowing function is needed, with the ultimate goal of optimizing current therapies.
A primary determinant of skeletal muscle contractile properties is muscle fiber cross-sectional area, which affects force generation capacity. In addition, the myosin heavy chain (MyHC) composition of a muscle, or MyHC fiber type, affects contraction properties by influencing contraction velocity and fatigue.8,9 Age is typically associated with reductions in muscle fiber cross-sectional area,10 and increased percentages of slower-contracting, fatigue-resistant muscle fiber types,11 although the impact of age may vary by muscle.12,13 The influence of exercise on MyHC fiber type distribution and fiber size also differs by muscle, exercise program, and age of participants.14 Aerobic exercise increased the prevalence of slowly-contracting, fatigue-resistant muscle fibers and increased fiber size in the vastus lateralus,15 yet endurance training had the opposite effect on the diaphragm with reduced muscle fiber size, and conversion to more rapidly-contracting muscle fiber types.16,17 Resistance exercise has frequently been found to increase the proportion of slowly-contracting fatigue-resistant fibers.14,18,19 Therefore, it is necessary to study the impact of a given exercise protocol on each muscle of interest to understand musculoplastic adaptation.
Healthy aged individuals with no swallowing complaints swallow more slowly than young adults and have weaker maximum isometric tongue pressures.1,2,20 With health challenges, an exacerbation of these factors can compromise swallowing.2 Age-related changes in the intrinsic tongue muscles21 may contribute to manifestations of altered swallowing. These muscles have an active role in swallowing, particularly bolus containment and propulsion.22,23 A prior study found that with age, the intrinsic tongue muscles had increased percentages of slowly-contracting fatigue-resistant fibers. Additionally, in the protrusive transverse and verticalis muscles, muscle fiber sizes were reduced.21 EMG activity in the intrinsic tongue has been shown to increase with protrusive tongue force generation,24 indicating that these muscles are likely targeted by tongue exercise. However, the direct impact of tongue exercise on the intrinsic tongue muscles has not been studied.
To better understand the impact of exercise on the tongue muscles, an aging rat model of tongue exercise was developed in which an 8-week tongue exercise program significantly increased protrusive tongue force.25 In the genioglossus, a protrusive extrinsic tongue muscle, both age and tongue exercise increased the percentage of slower-contracting MyHC isoforms,26,27 and exercise resulted in a trend towards larger fiber cross-sectional areas.25,27 We hypothesized that, following 8 weeks of tongue exercise, the muscle fiber diameter and percentage of slowly-contracting fatigue-resistant fibers in the intrinsic tongue muscles would increase.
Materials and Methods
Animals
Experiments were approved by the University of Wisconsin School of Medicine and Public Health Animal Care and Use Committee. Thirty-two male Fischer 344/Brown Norway rats were obtained from the National Institute on Aging colony four weeks prior to tongue exercise training to allow acclimation to the reversed light cycle, water restriction (3hrs per day), and training operandum. This rat strain, used in aging research, has a median life span of 34 months.28,29 At study completion, animals were aged 9 months (young adult) or 32 months (old). In each age group, 8 rats were assigned to tongue exercise and 8 served as sham-exercise controls.
Tongue Exercise
The clinically-based, progressive 8-week tongue exercise training paradigm has previously been described in detail.25 Rats were trained to press an instrumented disk with their tongue for a water reward. Estimated maximum press (EMP, mN) was determined for each animal, averaging the 10 largest forces obtained over 3 days. Tongue exercise occured 5 days/week with a reward threshold of 50% EMP for weeks 1-2, increased to 60% for weeks 3-4. New EMPs were calculated at the end of week 4 and training progressed at 70% EMP for weeks 5-6 and 80% for weeks 7-8. Using the same method as EMP, post-treatment assessment of maximum voluntary tongue force (MVTF) was collected at the end of week 8. Control rats learned the exercise task and were placed in the training operandum 5 days/week, but did not perform tongue exercises.
Muscle Samples and Fiber Typing
After completion of training, rats were euthanized, tongue muscles were removed, snap frozen in OCT (Tissue Tek), and stored at −80°C. Longitudinal, transverse, and verticalis muscle samples were later dissected from anterior, middle, and posterior regions of the tongue (Figure 1) and sectioned at 10 µm on a cryostat (Leica). A detailed description of intrinsic tongue muscle sampling and staining for MyHC types has been published previously.21
Figure 1. Schematic of the intrinsic tongue muscles.
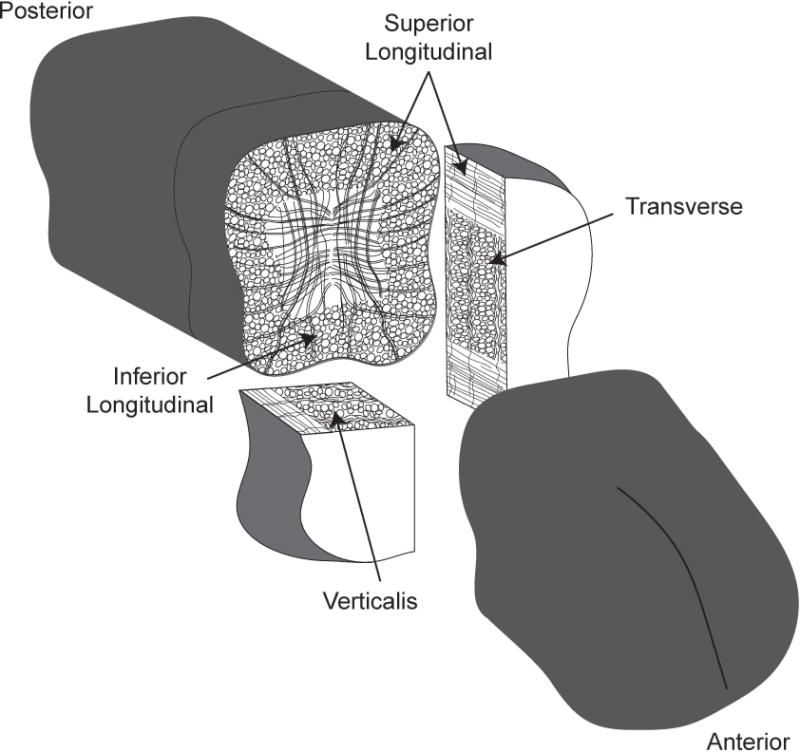
Two 0.25cm thick sections of the tongue were collected from the anterior, middle, and posterior of the intrinsic tongue, centered at 0.25cm, 1cm, and 1.75cm from the tongue tip, respectively. Middle samples shown. Of each sample pair, one was sectioned coronally with the longitudinal muscles in cross section and the second sample was split and sectioned in the transverse plane with the verticalis muscle in cross section and midsagittal sections with the transverse muscle in cross section as shown.
Primary antibodies (DSHB) were used to identify muscle fibers positive for MyHC type I (BA-F8 RRID:AB_10572253, 1:50), IIa (SC-71 RRID:AB_2147165, 1:600), IIx (6H1 RRID:AB_1157897, 1:100), and IIb (BF-F3 RRID:AB_2266724, 1:200.)30–34 Because antibodies for MyHC IIx and IIb fibers bind the same secondary antibodies, two sequential slides were prepared for each muscle, one stained for MyHC I, IIa, and IIb, and the other MyHC IIa and IIx. Additionally, samples were stained for laminin (Sigma-Aldrich, 1:1000). MyHC 1 fibers were very rare and were not included in any analyses.
Each section was imaged at 20× (Nikon N-STORM, Andor iXon 897 EMCCD camera) with three randomly sampled fields of view, for 18 total images per muscle (2 sections × 3 regions × 3 images). A detailed description of intrinsic tongue muscle sampling and staining for MyHC types has been published previously.21 Image analysis was performed in the MATLAB application SMASH.35 The percent of muscle fibers positive for each MyHC type was determined. A hybrid fiber positive for more than one MyHC type was counted for each positive MyHC type. Fiber size was measured by minimum Feret’s diameter, the smallest possible distance of two parallel lines tangential to the muscle fiber, which is robust to fiber angle as an obliquely cut fiber has approximately the same minimum ferrets diameter as if the fiber were cut at a right angle.36
Statistical Analysis
A multivariate repeated measures ANOVA was used to analyze MyHC fiber type percentages. Between subjects factors were age and exercise. Within-subject factors were muscle and region. The three dependent measures were the percent of MyHC IIb, IIa, and IIx. Significance was determined using α < 0.05 as the critical value. Test statistics are reported using Pillai’s trace (V).37
A linear mixed effects model was used to analyze muscle fiber size. Age, exercise, muscle and region were fixed effects, with individual subjects as random effects. All interactions between age, exercise, muscle, and region were included. The dependent variable was mean minimum Feret’s diameter for each muscle fiber type (IIb, IIx, IIa).
A repeated measures ANOVA was used to analyze the maximum voluntary tongue force before and after treatment, with age and exercise as the between group factors.
Pearson’s correlation coefficient was calculated to quantify the relationship between maximum voluntary tongue force and average intrinsic tongue muscle fiber size.
Results
Tongue Force
As previously reported,25,27,38–40 tongue exercise training significantly increased maximum voluntary tongue force. The time-treatment group interaction was significant (F(1,28) = 78.731, p < 0.001). Control and exercise groups were not significantly different at baseline, but the exercise group achieved significantly higher forces than the control group after the 8-week experimental period (Figure 2). There were no significant main effects of age or interactions with age.
Figure 2. Maximum voluntary tongue force increased with exercise.
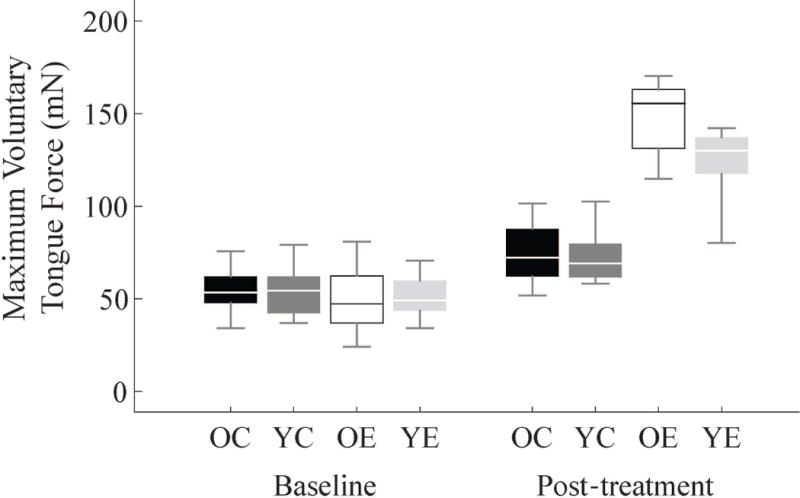
Tongue forces are shown at baseline and after 8 weeks of tongue exercise or sham-exercise treatment (OC = old control group, YC = young adult control, OE = old exercise, YE = young adult exercise). The groups were not significantly different at baseline, but after treatment the exercise groups for both ages had significantly higher voluntary tongue forces (YC = 72.77±14.9mN, YE = 123.99±20.58mN p < 0.001; OC = 74.73±16.66mN, OE = 148.14±20.58mN, p < 0.001, ±Standard Deviations).
MyHC Fiber Type
A significant multivariate region-muscle-exercise interaction (V = 0.904, F(18,11) = 5.728, p = 0.003), indicated that shifts in MyHC fiber type percentages occurred only in specific muscles and regions. The multivariate simple effects of exercise indicated significant changes in MyHC fiber type composition in the anterior transverse (F(3,26) = 5.426, p = 0.005) and middle superior longitudinal muscles (F(3,26) = 3.484, p = 0.03,), which had increased percentages of more rapidly-contracting MyHC fiber types with exercise. A significant change in MyHC fiber type composition following exercise was also noted in middle transverse (F(3,26) = 4.078, p = 0.017) and posterior inferior longitudinal (F(3,26) = 4.637, p = 0.010) muscles, which had increased percentages of more slowly-contracting fiber types (Figures 3 and 4). These significant effects were further broken down by univariate posthoc analysis of each MyHC fiber type individually: the percentage of Type IIx muscle fibers significantly decreased in all but the middle transverse muscles and Type IIa fibers increased in the middle transverse and posterior inferior longitudinal muscles (Figure 3).
Figure 3. Fiber type shifts with exercise.
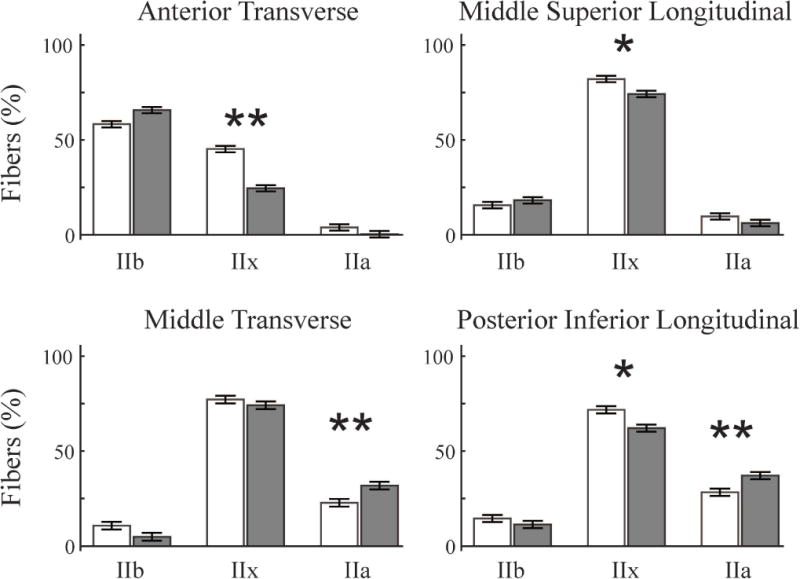
Significant changes in fiber type composition occurred with exercise in four different muscle-regions (MANOVA). Two muscles, the anterior transverse (AT) and middle superior longitudinal (MSL), shifted towards faster-contracting fibers with increased MyHC IIb fibers and less IIx and IIa fibers (White = control groups, Gray = exercise groups, * p < 0.01, **p < 0.001). The middle transverse (MT) and posterior inferior longitudinal (PIL) shifted in the opposite directions with increased percentages of the slower fatigue-resistant IIb fibers and less IIx and IIb fibers. Univariate ANOVA was used to analyze the fiber type percentages individually, which determined significant reductions in IIx fibers in the AT (p = 0.003), MSL (p = 0.017), and PIL (p = 0.031), as well as increased IIa fibers in the MT (p = 0.004), and PIL(p = 0.003).
Figure 4. Fiber type staining examples.
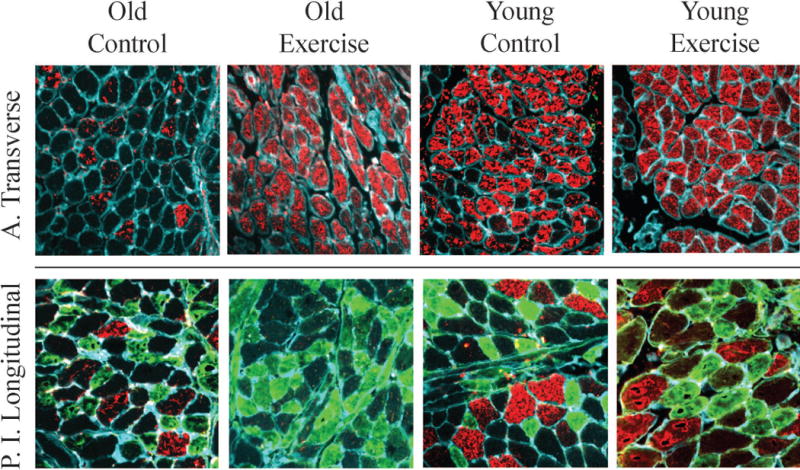
Muscle sections were stained for MyHC IIb (Red), MYHC IIa (Green), laminin (Cyan, outlines muscle fibers). MyHC IIx fibers are unstained in these images (MyHC IIx data were collected from serial sections stained for MyHC IIx). The MyHC muscle fiber type composition of the anterior transverse muscle (top) and posterior inferior longitudinal muscle (bottom) shifted differentially with exercise. The AT increased in the percentage of the fastest-contracting IIb fibers and a decrease in IIx, while the PIL also decreased in IIx, but increased in the percentage of slower fatigue-resistant IIa fibers. Changes in fiber type with exercise were not significantly different between age groups.
As previously reported with age,21 there was a significant multivariate interaction between age and region (V = 0.738, F (6,23) = 10.81, p < 0.001). Old animals had significantly less MyHC IIb fibers and more fibers positive for MyHC IIx in all regions, as well as significantly more IIa positive fibers in the middle and posterior regions (Table 1). There were no significant age-exercise interactions, which indicated the effects of exercise did not differ between young adult and old animals.
Table 1. Impact of age on fiber type by tongue region.
The estimated marginal means of the percent muscle fibers positive for each myosin heavy chain type reported by age and region with the estimated standard error. Exercise and control group results are combined, as there was no significant age-region-exercise interaction.
| Fiber Type | Age Group | Anterior | Middle | Posterior |
|---|---|---|---|---|
|
| ||||
| MyHC IIb | Old | 54.3±3.5% | 14.3±1.5% | 4.0±1.0% |
| Young Adult | 76.0±3.5% | 21.0±1.5% | 8.0±1.0% | |
| p < 0.001* | p = 0.004* | p = 0.009* | ||
|
| ||||
| MyHC IIx | Old | 41.9±3.5% | 73.7±1.5% | 75.2±1.7% |
| Young Adult | 22.9±3.5% | 69.0±1.5% | 80.3±1.7% | |
| p = 0.001* | p = 0.035* | p = 0.041* | ||
|
| ||||
| MyHC IIa | Old | 2.8±1.3% | 21.2±1.0% | 29.2±1.3% |
| Young Adult | 0.6±1.3% | 1.3±1.0% | 16.2±1.3% | |
| p = 0.235 | p < 0.001* | p < 0.001* | ||
Muscle Fiber Size
There were no significant main effects or interactions with exercise for muscle fiber size (minimum Feret’s diameter) whether analyzed by MyHC fiber type (IIb, IIx, and IIa) or with all MyHC fiber types pooled. Within treatment groups, final maximum voluntary tongue force increased with overall mean muscle fiber diameter (Figure 5, Pearson’s correlation coefficient, one-tailed: r = 0.56, p = 0.011; r = 0.42, p = 0.051, control and exercise groups respectively. Old and young animals were combined within treatment groups as mean fiber size did not differ between age groups: old vs young control p = 0.25, old vs young exercise p = 0.52).
Figure 5. Muscle fiber size and voluntary tongue pressing force.
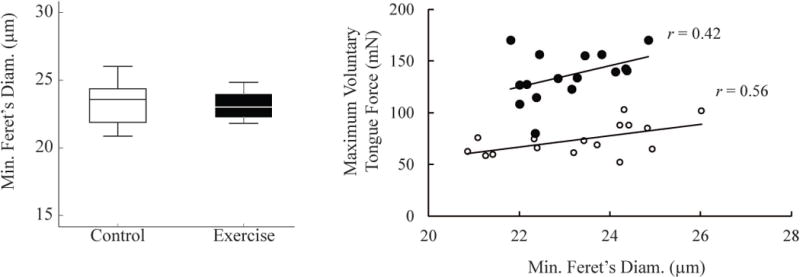
There were no significant effects of exercise on fiber size in any muscle or region for any fiber type or for all fiber types combined. Muscle fiber diameter was moderately correlated with maximum voluntary tongue force. A. The overall average intrinsic tongue muscle fiber sizes are shown for control (white) and exercise (black) animal groups. B. Within treatment groups, overall average intrinsic tongue muscle fiber size was correlated with maximum voluntary tongue pressing force (Pearson’s correlation coefficient, one sided: Control (White circles) r = 0.56, p = 0.011; Exercise group (Black dots) r = 0.42, p = 0.051).
There was a significant age-region interaction for MyHC IIb fiber size (F(2,299) = 4.26, p = 0.015). For all muscles, old animals had significantly smaller IIb fiber diameters in the posterior region (old = 24.93±0.65µm, young adult = 27.38±0.51µm, p = 0.003).
Discussion
We hypothesized that 8 weeks of tongue exercise would increase the percentage of fatigue-resistant MyHC fiber types and increase muscle fiber diameter of the intrinsic tongue muscles. Our findings allowed us to accept our hypothesis only in part and led to new interpretations and hypotheses about how differential changes in muscle structure throughout the tongue may impact function, both with aging and exercise treatment. That is, the clinically based tongue exercise program induced a significant change in MyHC fiber type distribution, but the directionality of these changes – towards fast-fatiguable or slower fatigue-resistant fibers, varied by muscle and location (Figures 3 and 4). Tongue forces increased with exercise (Figure 2), but were not associated with intrinsic muscle fiber hypertrophy (Figure 5).
Muscle Fiber Type
In the middle transverse and posterior inferior longitudinal muscles, exercise increased the percentage of MyHC IIa fibers, the most slowly-contracting and fatigue-resistant of the fiber types common in the rat intrinsic tongue. Similar results have previously been reported in the genioglossus, in which MyHC type 1 isoform prevalence increased with tongue exercise.27 Shifts in muscle composition to fibers with greater fatigue resistance may contribute to the improved fatigue indices found with tongue exercise in previous studies of rat tongue muscle contractile properties.27 Because this study did not examine physiological properties of muscle contraction, this hypothesis should be examined in future research. In contrast, following exercise, the anterior transverse and middle superior longitudinal muscles had a greater percentage of MyHC IIb fibers, which have a faster contraction velocity and greater force generation capacity due to larger fiber cross-sectional areas.8 With exercise, the MyHC profiles of aged animal in these muscle-regions were more similar to those seen in younger animals as compared to the aged control group (Figure 4).21 Age is associated with slower oral transit times,1 slower bolus velocity,41 and longer time to develop peak swallowing tongue pressures.42 More rapidly-contracting MyHC muscle fibers in the middle and anterior regions of the tongue may allow peak bolus driving forces to be reached more rapidly, leading to improvements in bolus transit times. Future studies of the impact of exercise on intrinsic tongue muscle contractile properties and the translation to swallowing function are needed to examine the physiological and functional significance of these mixed findings.
The mixed effects of exercise on MyHC fiber type among the intrinsic tongue muscles is not without precedent—the impact of exercise in the quadriceps has been shown to vary among this muscle group as well as along the length of each muscle,14,43 depending on the loading and activation each muscle experiences during the task. Thus, varied impact of exercise among the tongue muscles may indicate differential contributions to the tongue exercise task. For example, EMG studies have indicated that the extrinsic genioglossus positions the tongue while the intrinsic tongue muscles generate protrusive force.24 It may be that among the intrinsic muscles, the middle transverse and posterior inferior longitudinal muscles provide a positioning or stabilizing function similar to the genioglossus, which undergoes a similar transformation in muscle composition to more slowly-contracting fiber types with tongue exercise.27 In contrast, the anterior transverse and middle superior longitudinal muscles, which transitioned to more rapidly-contracting MyHC fiber types, may have a more dynamic role in tongue force generation. Additionally, regional localization of motor units along the length of the tongue allows for segmental activation of the intrinsic tongue muscles,44 which may allow for regional differences in response to exercise within an individual muscle.
The protrusive transverse and verticalis muscles elongate the tongue by contracting to shorten the diameter.45,46 Significant exercise effects were found in the transverse but not the verticalis. It is possible the transverse muscle has a greater role in protrusive force generation; the horizontal fibers may have a mechanical advantage due to the wide flat shape of the anterior tongue.
Muscle Fiber Size
Muscle fiber hypertrophy is considered the primary method by which exercise increases force generation.14 In our study, despite an increase in protrusive tongue force, no significant increase in intrinsic muscle fiber diameter was found. Previous results in the rat genioglossus muscle with tongue exercise reported a trend towards increasing fiber cross-sectional area, suggesting longer exercise durations may have resulted in significant muscle fiber hypertrophy.25
Changes in fiber type distribution that occurred with exercise in the present study may be a confounding factor on fiber size. Rapidly-contracting muscle fibers are characterized by the largest diameters and transitions between fiber types as well as hybrid fibers may contribute to fiber diameter variability.
Within each treatment group, intrinsic tongue muscle fiber diameter was moderately correlated with tongue force (Figure 5). Accordingly, some of the variation in force between animals can be attributed to differences in intrinsic tongue muscle fiber size. However, these data suggest that mechanisms of increased tongue force with exercise other than muscle fiber hypertrophy should be considered.
Increases in force without muscle fiber hypertrophy may occur through several different mechanisms. First, a muscle fiber’s ability to generate force may change independently of fiber diameter. Single muscle fiber experiments have determined that the force a muscle fiber can generate per unit of cross-sectional area, known as specific force, can be modulated by both age and exercise.47 Age has been associated with decreased specific force,48 and exercise has been shown to increase muscle fiber specific force.49 Changes in specific force have been attributed to the fiber’s MyHC concentation.47 Second, neural adaptations have also been shown to increase force output without hypertrophy of muscle fibers or overall muscle, particularly for a new task and early in training.14,50,51 These force improvements were attributed to improved muscle coordination and activation.50 The tongue force increase with exercise may have been influenced by learning the tongue pressing task in that a slight increase from baseline occurred in the control groups (Figure 2). Third, overall muscle size may increase by increasing the number of muscle fibers rather than size of individual fibers. The evidence for increased muscle fiber numbers due to exercise remains inconclusive,14 yet the loss of muscle fiber number and overall muscle size with age is well established.10,52 Thus, muscle metrics other than fiber diameter must be examined in seeking a definitive explanation for increases in tongue force with exercise.
Muscle fiber diameter may represent a tradeoff between force and fatigue, such that development of larger muscle fiber diameters, and thus greater force capacity, may be accomplished at the expense of limiting fatigue resistance. Endurance exercise decreased muscle fiber cross-sectional area in the diaphragm, which was thought to reduce fatigue by improving oxygen transfer within the muscle fibers.16,17 Endurance may be critical for the tongue to meet the demands of breathing, eating, and speaking. Unique features of tongue muscles that may maximize endurance include high capillarization and mitochondrial enzyme activity relative to limb muscles.53,54 The tongue exercise protocol used featured progressive resistance training, yet rats averaged 141 tongue presses per day above their target force, which may have resulted in a hybrid of endurance and resistance training. Muscle fiber size may also be limited by the tightly packed and interwoven anatomy of the intrinsic muscles. Therefore, it may be necessary for the tongue muscles to increase force generation through pathways other than muscle fiber hypertrophy.
Limitations
A limitation of this study is that a behavioral swallowing assessment, such as videofluoroscopy, was not included. More detailed measures of tongue and bolus movement during swallowing may yield an increased understanding of how age- and exercise-related changes in the intrinsic tongue muscles relate to swallowing function. Another limitation was that potential mechanisms of tongue force increases with exercise, such as the number of muscle fibers and specific force of individual fibers, were not addressed. An accurate assessment of muscle fiber number was not possible with the methods used in this study because a complete muscle cross section was required and our tongues were subdivided to sample each intrinsic lingual muscle within the same animal (Figure 1).
Conclusions
This study found that tongue exercise altered the biochemical composition of the intrinsic lingual muscles, such that the MyHC composition of some aged tongue muscles were more similar to those of young adults while other muscles transitioned towards more fatigue-resistant MyHC fiber types. However, the impact of these changes on swallowing function has yet to be determined. The animal model of tongue exercise used in this study consisted of a protrusive tongue motion to press a disk, and significant changes occurred in 4 of 12 sampled muscle-regions. Clinical tongue training protocols are often more complex, including both anterior and posterior tongue pressing,5,6 lateral tongue movements, or effortful swallows,4 and may induce a wider range of changes among the intrinsic tongue muscles. A better understanding of how the intrinsic lingual muscles meet the demands of healthy swallowing may be necessary to determine the most effective and efficient tongue strengthening protocol to improve swallowing function.
Acknowledgments
The authors gratefully acknowledge the assistance of Austin Potrue in the completion of this work. This work was funded from the following sources: NIH grants T32DC009401, R01DC008149, R01DC005935, and R01DC014358.
Footnotes
Conflicts of Interest: None of the authors has any conflict of interest to disclose
Level of Evidence: N/A
References
- 1.Robbins J, Bridges AD, Taylor A. Oral, pharyngeal and esophageal motor function in aging. GI Motil Online. 2006 [Google Scholar]
- 2.Robbins J, Levine R, Wood J, Roecker EB, Luschei E. Age effects on Lingual Pressure Generation as a Risk Factor for Dysphagia. J Gerontol Med Sci. 1995;50A(5):M257–M262. doi: 10.1093/gerona/50a.5.m257. [DOI] [PubMed] [Google Scholar]
- 3.Lee JH, Kim H-S, Yun DH, et al. The Relationship Between Tongue Pressure and Oral Dysphagia in Stroke Patients. Ann Rehabil Med. 2016;40(4):620. doi: 10.5535/arm.2016.40.4.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park T, Kim Y. Effects of tongue pressing effortful swallow in older healthy individuals. Arch Gerontol Geriatr. 2016;66:127–133. doi: 10.1016/j.archger.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Rogus-Pulia N, Rusche N, Hind JA, et al. Effects of Device-Facilitated Isometric Progressive Resistance Oropharyngeal Therapy on Swallowing and Health-Related Outcomes in Older Adults with Dysphagia. J Am Geriatr Soc. 2016;64(2):417–424. doi: 10.1111/jgs.13933. [DOI] [PubMed] [Google Scholar]
- 6.Yeates EM, Molfenter SM, Steele CM. Improvements in tongue strength and pressure-generation precision following a tongue-pressure training protocol in older individuals with dysphagia: three case reports. Clin Interv Aging. 2008;3(4):735. doi: 10.2147/cia.s3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robbins J, Gangnon RE, Theis SM, Kays SA, Hewitt AL, Hind JA. The Effects of Lingual Exercise on Swallowing in Older Adults. J Am Geriatr Soc. 2005;53(9):1483–1489. doi: 10.1111/j.1532-5415.2005.53467.x. [DOI] [PubMed] [Google Scholar]
- 8.Bottinelli R, Schiaffino S, Reggiani C. Force-velocity relations and myosin heavy chain isoform compositions of skinned fibres from rat skeletal muscle. J Physiol. 1991;437:655. doi: 10.1113/jphysiol.1991.sp018617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pette D, Starton RS. Myosin isoforms, muscle fiber types, and trasitions. Microsc Res Tech. 2000;50(6):500–509. doi: 10.1002/1097-0029(20000915)50:6<500::AID-JEMT7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 10.Porter MM, Vandervoort AA, Lexell J. Aging of human muscle: structure, function and adaptability. Scand J Med Sci Sports. 1995;5(3):129–142. doi: 10.1111/j.1600-0838.1995.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 11.Larsson L, Sjödin B, Karlsson J. Histochemical and biochemical changes in human skeletal muscle with age in sedentary males, age 22–65 years. Acta Physiol. 1978;103(1):31–39. doi: 10.1111/j.1748-1716.1978.tb06187.x. [DOI] [PubMed] [Google Scholar]
- 12.Monemi M, Eriksson P-O, Eriksson A, Thornell L-E. Adverse changes in fibre type composition of the human masseter versus biceps brachii muscle during aging. J Neurol Sci. 1998;154:35–48. doi: 10.1016/s0022-510x(97)00208-6. [DOI] [PubMed] [Google Scholar]
- 13.Holloszy JO, Chen M, Cartee GD, Young JC. Skeletal muscle atrophy in old rats: Differential changes in the three fiber types. Mech Aging Dev. 1991;60:199–213. doi: 10.1016/0047-6374(91)90131-i. [DOI] [PubMed] [Google Scholar]
- 14.Folland JP, Williams AG. The Adaptations to Strength Training. Sports Med. 2007;37(2):145–168. doi: 10.2165/00007256-200737020-00004. [DOI] [PubMed] [Google Scholar]
- 15.Konopka AR, Trappe TA, Jemiolo B, Trappe SW, Harber MP. Myosin Heavy Chain Plasticity in Aging Skeletal Muscle With Aerobic Exercise Training. J Gerontol A Biol Sci Med Sci. 2011;66A(8):835–841. doi: 10.1093/gerona/glr088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green HJ, Plyley MJ, Smith DM, Kile JG. Extreme endurance training and fiber type adaptation in rat diaphragm. J Appl Physiol. 1989;66(4):1914–1920. doi: 10.1152/jappl.1989.66.4.1914. [DOI] [PubMed] [Google Scholar]
- 17.Powers SK, Criswell D, Lieu F-K, Dodd S, Silverman H. Diaphragmatic fiber type specific adaptation to endurance exercise. Respir Physiol. 1992;89(2):195–207. doi: 10.1016/0034-5687(92)90050-7. [DOI] [PubMed] [Google Scholar]
- 18.Wilson JM, Loenneke JP, Jo E, Wilson GJ, Zourdos MC, Kim J-S. The effects of endurance, strength, and power training on muscle fiber type shifting. J Strength Cond Res. 2012;26(6):1724–1729. doi: 10.1519/JSC.0b013e318234eb6f. [DOI] [PubMed] [Google Scholar]
- 19.Fry AC. The role of resistance exercise intensity on muscle fibre adaptations. Sports Med. 2004;34(10):663–679. doi: 10.2165/00007256-200434100-00004. [DOI] [PubMed] [Google Scholar]
- 20.Crow HC, Ship JA. Tongue strength and endurance in different aged individuals. J Gerontol A Biol Sci Med Sci. 1996;51(5):M247–M250. doi: 10.1093/gerona/51a.5.m247. [DOI] [PubMed] [Google Scholar]
- 21.Cullins MJ, Connor NP. Alterations of intrinsic tongue muscle properties with aging. Muscle Nerve. 2017 doi: 10.1002/mus.25605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Napadow VJ, Chen Q, Wedeen VJ, Gilbert RJ. Biomechanical basis for lingual muscular deformation during swallowing. Am J Physiol-Gastrointest Liver Physiol. 1999;277(3):G695–G701. doi: 10.1152/ajpgi.1999.277.3.G695. [DOI] [PubMed] [Google Scholar]
- 23.Kayalioglu M, Shcherbatyy V, Seifi A, Liu Z-J. Roles of intrinsic and extrinsic tongue muscles in feeding: electromyographic study in pigs. Arch Oral Biol. 2007;52(8):786–796. doi: 10.1016/j.archoralbio.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pittman LJ, Bailey EF. Genioglossus and Intrinsic Electromyographic Activities in Impeded and Unimpeded Protrusion Tasks. J Neurophysiol. 2008;101(1):276–282. doi: 10.1152/jn.91065.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Connor NP, Russell JA, Wang H, Jackson MA, Mann L, Kluender K. Effect of tongue exercise on protrusive force and muscle fiber area in aging rats. J Speech Lang Hear Res. 2009;52(3):732–744. doi: 10.1044/1092-4388(2008/08-0105). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaser AJ, Wang H, Volz LM, Connor NP. Biochemistry of the Anterior, Medial, and Posterior Genioglossus in the Aged Rat. Dysphagia. 2011;26(3):256–263. doi: 10.1007/s00455-010-9297-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kletzien H, Russell JA, Leverson GE, Connor NP. Differential effects of targeted tongue exercise and treadmill running on aging tongue muscle structure and contractile properties. J Appl Physiol. 2013;114(4):472–481. doi: 10.1152/japplphysiol.01370.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54(11):B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- 29.Conn PM. Handbook of Models for Human Aging. Academic Press; 2011. [Google Scholar]
- 30.Borrione AC, Zanellato AMC, Saggin L, Mazzoli M, Azzarello G, Sartore S. Neonatal myosin heavy chains are not expressed in Ni-induced rat rhabdomyosarcoma. Differentiation. 1988;38(1):49–59. doi: 10.1111/j.1432-0436.1988.tb00591.x. [DOI] [PubMed] [Google Scholar]
- 31.Schiaffino S, Gorza L, Sartore S, et al. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil. 1989;10(3):197–205. doi: 10.1007/BF01739810. [DOI] [PubMed] [Google Scholar]
- 32.Lucas CA, Kang LHD, Hoh JFY. Monospecific Antibodies against the Three Mammalian Fast Limb Myosin Heavy Chains. Biochem Biophys Res Commun. 2000;272(1):303–308. doi: 10.1006/bbrc.2000.2768. [DOI] [PubMed] [Google Scholar]
- 33.Azzarello G, Sartore S, Saggin L, et al. Myosin isoform expression in rat rhabdomyosarcoma induced by Moloney murine sarcoma virus. J Cancer Res Clin Oncol. 1987;113(5):417–429. doi: 10.1007/BF00390035. [DOI] [PubMed] [Google Scholar]
- 34.Bloemberg D, Quadrilatero J. Rapid Determination of Myosin Heavy Chain Expression in Rat, Mouse, and Human Skeletal Muscle Using Multicolor Immunofluorescence Analysis. In: Berdeaux R, editor. PLoS ONE. 4. Vol. 7. 2012. p. e35273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith LR, Barton ER. SMASH–semi-automatic muscle analysis using segmentation of histology: a MATLAB application. Skelet Muscle. 2014;4(1):1. doi: 10.1186/2044-5040-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Briguet A, Courdier-Fruh I, Foster M, Meier T, Magyar JP. Histological parameters for the quantitative assessment of muscular dystrophy in the mdx-mouse. Neuromuscul Disord. 2004;14(10):675–682. doi: 10.1016/j.nmd.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 37.Field A. Discovering Statistics Using IBM Statistics. 4th. Sage Publications; 2013. Multivariate Analysis of Variance (MANOVA) pp. 643–653. [Google Scholar]
- 38.Behan M, Moeser AE, Thomas CF, et al. The Effect of Tongue Exercise on Serotonergic Input to the Hypoglossal Nucleus in Young and Old Rats. J Speech Lang Hear Res. 2012;55(3):919. doi: 10.1044/1092-4388(2011/11-0091). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krekeler BN, Connor NP. Age-related changes in mastication are not improved by tongue exercise in a rat model: Age-Related Changes in Mastication. The Laryngoscope. 2017;127(1):E29–E34. doi: 10.1002/lary.26045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaser AJ, Ciucci MR, Connor NP. Cross-activation and detraining effects of tongue exercise in aged rats. Behav Brain Res. 2016;297:285–296. doi: 10.1016/j.bbr.2015.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russell JA, Ciucci MR, Hammer MJ, Connor NP. Videofluorographic Assessment of Deglutitive Behaviors in a Rat Model of Aging and Parkinson Disease. Dysphagia. 2013;28(1):95–104. doi: 10.1007/s00455-012-9417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nicosia MA, Hind JA, Roecker EB, et al. Age effects on the temporal evolution of isometric and swallowing pressure. J Gerontol A Biol Sci Med Sci. 2000;55(11):M634–M640. doi: 10.1093/gerona/55.11.m634. [DOI] [PubMed] [Google Scholar]
- 43.Housh DJ, Housh TJ, Johnson GO, Chu W-K. Hypertrophic response to unilateral concentric isokinatic resistance training. J Appl Physiol. 1992;73(1):65–70. doi: 10.1152/jappl.1992.73.1.65. [DOI] [PubMed] [Google Scholar]
- 44.Sokoloff AJ. Localization and contractile properties of intrinsic longitudinal motor units of the rat tongue. J Neurophysiol. 2000;84(2):827–835. doi: 10.1152/jn.2000.84.2.827. [DOI] [PubMed] [Google Scholar]
- 45.McClung JR, Goldberg SJ. Functional anatomy of the hypoglossal innervated muscles of the rat tongue: a model for elongation and protrusion of the mammalian tongue. Anat Rec. 2000;260(4):378–386. doi: 10.1002/1097-0185(20001201)260:4<378::AID-AR70>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 46.Kier WM, Smith KK. Tongues, tentacles and trunks: the biomechanics of muvement in muscular-hydrostats. Zool J Linn Soc. 1985;83:307–324. [Google Scholar]
- 47.Canepari M, Pellegrino MA, D’Antona G, Bottinelli R. Single muscle fiber properties in aging and disuse. Scand J Med Sci Sports. 2010;20(1):10–19. doi: 10.1111/j.1600-0838.2009.00965.x. [DOI] [PubMed] [Google Scholar]
- 48.D’Antona G. The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol. 2003;552(2):499–511. doi: 10.1113/jphysiol.2003.046276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parente V, D’Antona G, Adami R, et al. Long-term resistance training improves force and unloaded shortening velocity of single muscle fibres of elderly women. Eur J Appl Physiol. 2008;104(5):885–893. doi: 10.1007/s00421-008-0845-0. [DOI] [PubMed] [Google Scholar]
- 50.Gabriel DA, Kamen G, Frost G. Neural adaptations to resistive exercise. Sports Med. 2006;36(2):133–149. doi: 10.2165/00007256-200636020-00004. [DOI] [PubMed] [Google Scholar]
- 51.Sale DG. Strength and Power in Sport, Second Edition. 2nd. Blackwell Science Ltd; Oxford, UK: 2008. Neural Adaptation to Strength Training; pp. 281–314. [Google Scholar]
- 52.Brunner F, Schmid A, Sheikhzadeh A, Nordin M, Yoon J, Frankel V. Effects of aging on Type Il muscle fibers: a systematic review of the literature. J Aging Phys Act. 2007;15(3):336–348. doi: 10.1123/japa.15.3.336. [DOI] [PubMed] [Google Scholar]
- 53.Stål P, Marklund S, Thornell L-E, De Paul R, Eriksson P-O. Fibre Composition of Human Intrinsic Tongue Muscles. Cells Tissues Organs. 2003;173(3):147–161. doi: 10.1159/000069470. [DOI] [PubMed] [Google Scholar]
- 54.Granberg I, Lindell B, Eriksson P-O, Pedrosa-Domellöf F, Stål P. Capillary Supply in Relation to Myosin Heavy Chain Fibre Composition of Human Intrinsic Tongue Muscles. Cells Tissues Organs. 2010;192(5):303–313. doi: 10.1159/000318645. [DOI] [PubMed] [Google Scholar]


