Abstract
Background
Weight loss in response to the long-acting GLP-1 receptor (GLP1R) analog, liraglutide, is correlated with delay in gastric emptying (GE). The aim of this pilot study was to assess whether specific genetic variants in GLP1R or TCF7L2 are associated with delayed GE and weight loss in obese patients treated with liraglutide or the short-acting GLP-1 agonist, exenatide.
Methods
We evaluated in obese individuals the associations of genetic variations of GLP1R (rs6923761) and TCF7L2 (rs7903146) on GE T1/2 and weight from two trials that evaluated separately exenatide, 5μg BID for 30 days, or liraglutide, 3mg daily for 5 weeks. Data were analyzed using the dominant genetic model and intention to treat analysis.
Key Results
There was a significant correlation between changes in weight and GE T1/2 (rs=−0.382, p=0.004). GLP1R rs6923761 minor allele A (AA_AG) carriers who received either exenatide or liraglutide had greater delay in GE T1/2 relative to baseline [117.9±27.5 (SEM) minutes and 128.9±38.32 minutes] compared to GG genotype (95.8±30.4 minutes and 61.4±21.4 minutes, respectively) (p 0.11). There was a nonsignificant difference in weight loss based on GLP1R rs6923761 genotype after 5 weeks of treatment. There were no significant correlations with TCF7L2 (rs7903146) genotype.
Conclusions & Inferences
The minor A allele of GLP1R (rs6923761) is associated with greater delay in GE T1/2 in response to liraglutide and exenatide. These studies provide data to plan pharmacogenetics testing of the hypothesis that GLP1R (rs6923761) influences weight loss in response to GLP1R agonists.
Keywords: glucagon-like peptide 1 receptor, rs6923761, liraglutide, gastric emptying, obesity
Abbreviated Abstract
The minor A allele of GLP1R (rs6923761) is associated with greater delay in gastric emptying T1/2 in response to liraglutide and exenatide. These studies provide data to plan pharmacogenetics testing of the hypothesis that GLP1R (rs6923761) influences weight loss in response to GLP1R agonists.
INTRODUCTION
A subset of obese individuals has significantly increased calorie intake, larger fasting gastric volume, and accelerated gastric emptying of solids1. Glucagon-like peptide 1 (GLP-1) is a hormone secreted by the enteroendocrine intestinal L cells; GLP-1 results in insulin secretion, glucagon inhibition 2, and inhibition of gastric emptying 3,4. Morbidly obese patients (BMI=42±4 kg/m2) had faster gastric emptying and lower GLP-1 levels compared to normal weight controls5; this was replicated among overweight and obese class I or class II/III individuals1. There is also evidence of variation in GLP-1 secretion in obesity after oral and small intestinal nutrient administration6; the precise mechanism(s) associated with this difference is unclear. GLP-1 receptor (GLP1R) agonists include short-acting, such as exenatide, and long-acting analogs, such as liraglutide. Participants treated with liraglutide experienced average weight loss of 8% after 6 months of treatment, with >10% body weight loss in 33% of participants; factors associated with greater weight loss are unclear 7.
In a randomized, placebo-controlled trial of liraglutide, 3.0mg daily, in obese individuals, we recently reported that liraglutide delayed gastric emptying, and this was associated with weight loss with liraglutide over a 16-week treatment period 8. Similarly, in a prior randomized, placebo-controlled trial of exenatide, 5μg BID for 30 days, obese\participants developed significant delay in gastric emptying, with non-significant higher weight loss with exenatide than placebo (p=0.23) 9. Studies with liraglutide have shown that ~15% of patients lose >15% of body weight, and this is maintained over 1 or 3 years 7. The reason(s) for the marked variation of weight loss in response to liraglutide treatment is unclear.
Our hypothesis was that the effect of GLP-1 agonists, such as exenatide and liraglutide, on weight loss may be affected by genetic variances of the GLP1R gene or the TCF7L2 gene. The rationale for selecting these genes and single nucleotide polymorphisms (SNP) is provided in the methods section. The aim of this pilot study was to assess whether specific genetic variants in GLP1R (rs6923761) or TCF7L2 (rs7903146) are associated with prolongation of gastric emptying and weight loss in obese patients treated with exenatide or liraglutide.
MATERIALS AND METHODS
Overall Design of Trial, Participants, and Treatment
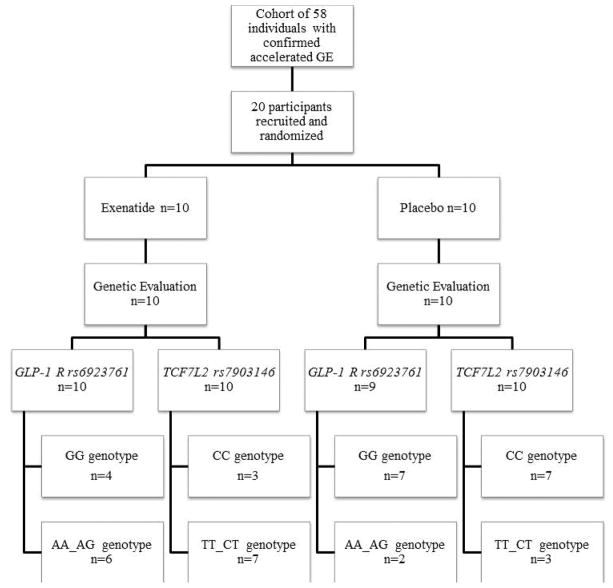
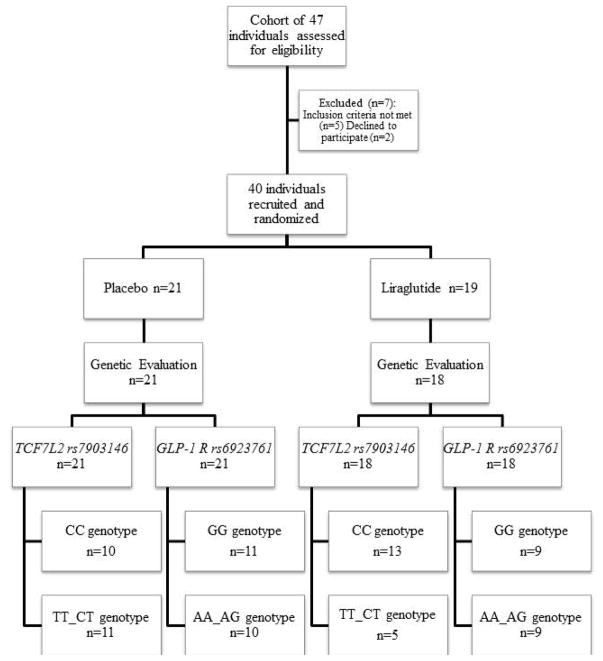
We evaluated the genetic variations of GLP1R and TCF7L2 from two different published studies 8,9 involving single-center, double-blinded, placebo-controlled, randomized clinical trials: the first (ClinicalTrials.gov #NCT02160990) utilized exenatide, 5μg BID administered for 30 days, in 20 obese individuals with rapid gastric emptying of solids (GE T1/2 <90 min) 9, and the second (ClinicalTrials.gov #NCT02647944) studied liraglutide, 3mg/day, administered over 16 weeks in 40 obese individuals with normal or accelerated gastric emptying of solids 8. Further details of these trials are provided in the relevant publications and in Figures 1A and 1B.
Figure 1.
Figure 1A. CONSORT flow chart of participants in the exenatide study
Figure 1B. CONSORT flow chart of participants in the liraglutide study
Gastric Emptying Measurement
Both studies utilized the same 296kcal test meal and scintigraphic method to measure gastric emptying. Details are available elsewhere8,9,10, 11.
Rationale for Selection of Genes and SNPs
GLP1R is the therapeutic target of GLP-1 and its agonists, such as exenatide, and analogs, such as liraglutide. Genetic variants in GLP1R have been associated with altered GLP1R function12. Of these SNPs, GLP1R (rs6923761) is the most frequent missense variant that has been studied in obesity and type 2 diabetes13. In prior studies in the same geographic region, a genetic evaluation of GLP1R (rs6923761) demonstrated that 51 patients (56.7%) had GG genotype and 39 (43.3%) had either AA or AG genotypes. Based on an analysis using a dominant genetic model in 90 overweight patients with type 2 diabetes, participants with the variant A allele (AA_AG) demonstrated greater effects on body mass index (BMI), body weight, and fat mass in response to liraglutide, 1.8mg/day, than participants carrying only the G allele (GG) 13.
TCF7L2 is involved in the secretion of GLP-1 from enteroendocrine L cells and, therefore, it impacts the levels of endogenous GLP-1. TCF7L2 (rs7903146) gene variants have been associated with defects in insulin secretion and type 2 diabetes14–16. In a study by Vazquez-Roque et al,, reduced fasting gastric volume and more rapid gastric emptying of liquids were associated with the T allele of the TCF7L2 (rs7903146) gene 17. Accelerated gastric emptying is associated with obesity1. Based on a similar population of 3,609 participants, TCF7L2 (rs7903146) allelic frequency consisted of 53.4% with CC and 46.6% with TT_CT18.
Genotyping GLP1R rs6923761 and TCF7L2 rs7903146
DNA was extracted from venous blood, and candidate genotype analysis was conducted on stored DNA by established PCR-based methods using TaqMan® SNP Genotyping Assays rs6923761 [GLP-1 (catalog no. C__25615272_20)] and rs7903146 [TCF7L2 (catalog no. C_29347861_10)] (Applied Biosystems, Foster City, CA) in accordance with the manufacturer’s instructions. Following polymerase chain reaction amplification, end reactions were analyzed with an ABI ViiA-7 Real-Time PCR System using QuantStudio™ Real-Time PCR software (Applied Biosystems, Foster City, CA).
Statistical Analysis
The genes were grouped into dominant (AA) and minor alleles (Aa/aa) based on reported prevalence in the population from the NCBI database 19. We used an intention to treat analysis with multiple imputations for any missing data. Our major outcomes were the changes in gastric emptying and weight loss at 5 weeks for liraglutide and at 30 days for exenatide studies. First, we combined data from the two studies to assess effects of GLP-1 agonist or analog versus placebo.
In the genotype by treatment analysis, data from the two studies were assessed using three groups (liraglutide, exenatide, and the combined placebo group). Transformation to rank scale was used for change in weight (TCF7L2), but not for GLP1. For post-gastric emptying (e.g. GE T½ at 30 days), a proportional hazards regression analysis was used to accommodate “censored” values. Analyses of the “interaction effects” of treatment by genotype were used for both changes in weight and GE T½. Data were reported as mean ± standard error of the mean (SEM).
RESULTS
Disposition
Figure 1 shows the CONSORT flow charts and genotypes by treatment group for both exenatide and liraglutide studies. Genotyping for GLP1R rs6923761 was unavailable for one participant in the placebo group in the exenatide cohort. One participant in the liraglutide group refused genotyping. In our study, the overall allelic frequencies for GLP1R (GG 53.5%, AA_AG 46.5%) and TCF7L2 (CC 55.9%, TT_CT 44.1%) were similar to those reported in the literature.
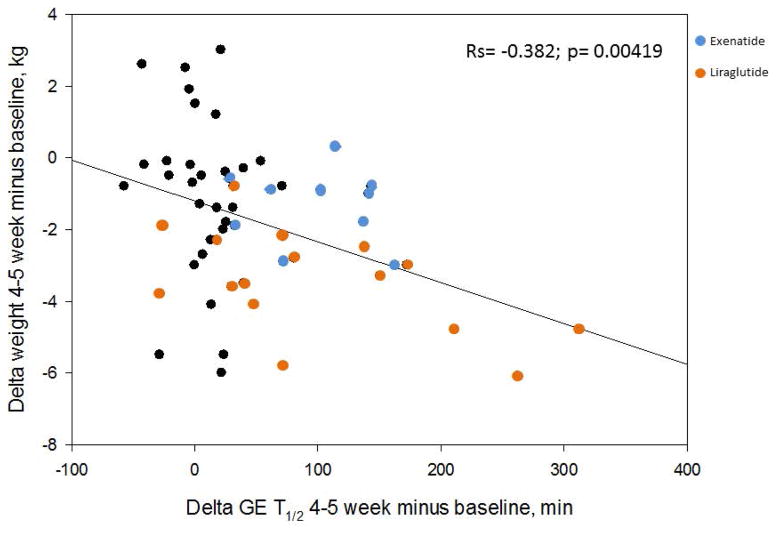
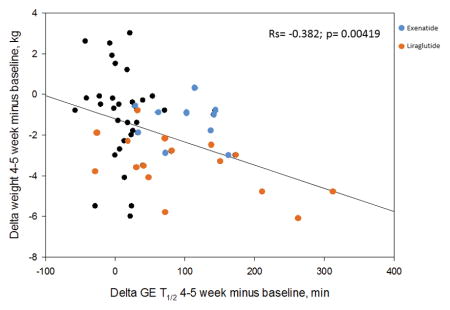
The baseline demographics in the three groups (placebo vs. liraglutide vs. exenatide) were not significantly different (Table 1). The mean baseline gastric emptying T1/2 for the 31 participants in the placebo arm was 103.7±6.2 (SEM) minutes and for the liraglutide group it was 121.2±58 minutes, which is consistent with the reported range for normal controls [median 120 minutes (5–95th percentile, 78.4–174)] 20. In the exenatide group, we had preselected participants with accelerated gastric emptying (GE T1/2 82.4±5.1 minutes). The effects of these GLP-1 agents are published elsewhere 8,9. There was a significant correlation between the change in weight and gastric emptying at 30 days or 5 weeks (rs=−0.382, p=0.004) (Figure 2).
Table 1.
Demographics of participants in the studies, with participants in the two trials assigned to placebo grouped together
| Mean ± SEM | Drug | Placebo (n=31) | |
|---|---|---|---|
| Liraglutide (n=18) | Exenatide (n=10) | ||
| Gender (% Female) | 89 % | 60 % | 80.6 % |
| Age (years) | 41.4 ± 2.6 | 39.0 ± 3.6 | 38.4 ± 2.2 |
| Baseline BMI (Kg/m2) | 37.4 ± 1.0 | 34.6 ± 0.9 | 35.7 ±0.7 |
Figure 2.
Spearman correlation between change in weight (y axis) and change in gastric emptying (x axis) at 5 weeks. The orange circles represent participants who received liraglutide, and the blue circles those who received exenatide.
Effect of Genetic Variants on Gastric Emptying in Response to Exenatide and Liraglutide
Data obtained by subdividing the groups by GLP1R (rs6923761) and TCF7L2 (rs7903146) genotypes are available in the Appendix table.
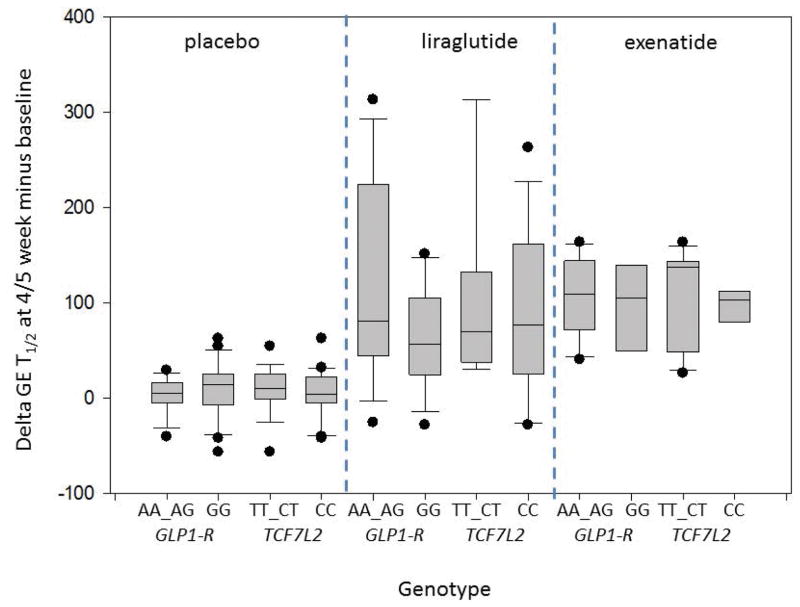
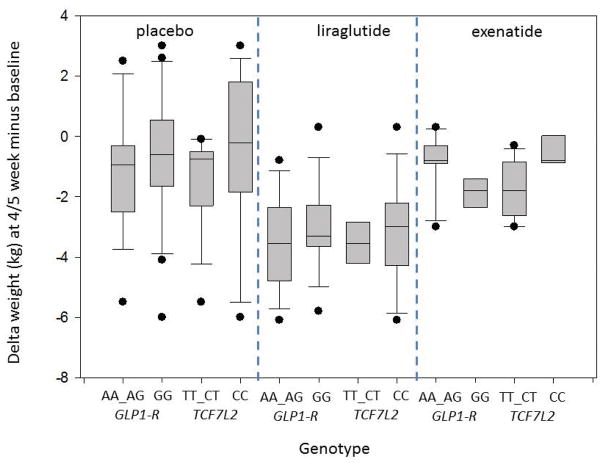
Within GLP1R (rs6923761), patients carrying the minor allele A (AA_AG) who received either exenatide or liraglutide had a numerically larger mean retardation in gastric emptying relative to baseline, 117.9±27.5 minutes and 128.9±38.3 minutes, respectively, compared to those with the dominant allele G (GG), 98.5±30.4 minutes and 61.4±21.4 minutes, respectively (p=0.11). As seen in Figure 3A, the delay in gastric emptying was greatest in response to liraglutide in patients with the AA_AG genotype of GLP1R compared to the GG genotype. There was a nonsignificant mean change in weight (Δ weight) at 5 weeks in the AA_AG group (−.9±0.5kg in the exenatide group and −3.5±0.6kg in the liraglutide group) compared to the GG group (−1.9±0.4kg in the exenatide group and −3.0±0.5kg in the liraglutide group) (p=0.62) (Figure 3B).
Figure 3.
Box plots comparing the changes in gastric emptying and weight loss at 5 weeks compared to baseline among the various genotypes and drug vs. placebo.
A. Effects of liraglutide vs. placebo on gastric emptying at 5 weeks based on genetic variants of GLP1 R (rs6923761) and TCF7L2 (rs7903146) genes. Note that the median prolongation of GE T1/2 was 80.0 (48.0, 211.0) in the AA_AG genotype and 55.5 (24.0, 105.0) in the GG genotype group for GLP-1 R (rs6923761).
B. Effects of liraglutide vs. placebo on weight loss at 5 weeks based on genetic variants of GLP1R (rs6923761) and TCF7L2 (rs7903146) genes. There were no significant associations of genotypes tested with weight loss.
For the TCF7L2 (rs7903146) genotype (major vs. minor allele), assessment, changes in gastric emptying at 5 weeks (p=0.93) and weight loss at 5 weeks (p=0.72) were not different. The box-and-whiskers plot (Figure 3B) highlights these results.
DISCUSSION
In this study, we evaluated the potential impact of functional genetic variants of GLP1R (rs6923761) and TCF7L2 rs7903146 on the response to exenatide or liraglutide. Our data represent a 67.5 minute (~50%) difference in the mean change in gastric emptying in (AA_AG) and (GG) subgroups based on the GLP1R (rs6923761) variants on treatment with liraglutide. Hence, we observed a numerically greater delay in gastric emptying in carriers of the minor variant (AA_AG) of GLP1R (rs6923761) in response to liraglutide. Although this difference was not statistically significant, a mean difference of 65 minutes (and median 25 minutes) is biologically relevant from the perspective of the association of delayed gastric emptying with satiation and, therefore, caloric intake, since a 25 minute difference in gastric emptying T1/2 would be associated with 80kcal greater calorie intake in a buffet meal or nutrient drink test 23. There was also a greater delay in the change in gastric emptying in the AA_AG compared to the GG group for exenatide (mean difference, 19.4 min), but not to the same degree observed with liraglutide. Although we did not observe a substantial difference in weight loss at 5 weeks between AA-AG and GG GLP1R (rs6923761) genotype groups, this may reflect a type II error and the relatively short duration of the treatment trial.
Genetic variants of GLP-1R have been associated with changes in GLP1R function 12, response to hypocaloric diets 24, as well as response to treatment with liraglutide 13,25. Specifically, the minor variant allele (A) of GLP1R (rs6923761) has been associated with better anthropometric parameters after a hypocaloric diet for 3 months 26 and 9 months 24 compared to a standard diet. A study by de Luis et al. in diabetic patients with obesity treated with liraglutide, 1.8mg/day, showed that the minor variant allele (A) carriers had a greater decrease in weight loss and fat mass13. Our study demonstrated a potential effect of genetic variability in GLP1R (rs6923761) on gastric emptying in response to treatment with liraglutide and provided pilot data for planning a future hypothesis-testing pharmacogenetics trial.
In this study, genotype variants in TCF7L2 (rs7903146) gene were associated with no difference in the mean change in gastric emptying of solids in response to either exenatide or liraglutide. Similar to our findings, Vazquez-Roque et al. showed that there was no effect of genotype variants in TCF7L2 (rs7903146) gene on gastric emptying of solids17.
Limitations
While we demonstrated a greater delay in gastric emptying in the minor variant allele (A) in GLP1R (rs6923761), our study was not powered to detect a pharmacogenetics association, and the observations were not statistically significant (p=0.11). Based on the minor allele (A) frequency of ~36% in the population 19, which is similar to the minor allele frequency in our patient population, we used the current data and calculated that a future study would conservatively require 70 patients with the minor variant (AA_AG) and 160 patients with the dominant variant (GG) to detect the clinically important difference in the effect of liraglutide on gastric emptying of 25 minutes.
Lastly, the exenatide cohort was preselected for those with accelerated gastric emptying. In theory, these patients should have greater delay in gastric emptying after exposure to a GLP-1 agonist, but, in our cohort, the degree of change in gastric emptying among those with accelerated gastric emptying at baseline was similar to the delay observed in participants with normal gastric emptying at baseline.
In summary, the current pilot study supports the need for further studies to test the hypothesis that the minor variant allele (A) of GLP1R (rs6923761) plays a role in the response to GLP-1 agonists by delaying gastric emptying. This study also has provided the preliminary data on the coefficient of variation in responses in order to plan further evaluation of genetic variants of the GLP1R (rs6923761), gastric emptying and weight loss in response to both exenatide and liraglutide. We believe this could have important implications in patient selection of who will respond to the relatively expensive GLP-1 agonists, exenatide and liraglutide, based on GLP1R variants, supporting the general objective to provide individualized or precision medicine.
Supplementary Material
KEY POINTS.
The aim of this pilot study was to explore whether specific genetic variants in GLP1R (rs6923761) or TCF7L2 (rs7903146) are associated with prolongation of gastric emptying and weight loss in obese patients treated with GLP-1 agonists (liraglutide and exenatide).
A allele of GLP1R (rs6923761) is associated with a greater delay in gastric emptying in response to treatment with GLP-1 agonists.
This study has acquired pilot data to plan a future study to assess the effect of genetic variability in GLP1R (rs6923761) on gastric emptying and weight loss in response to treatment with GLP-1 agonists.
Acknowledgments
The authors thank Mrs. Cindy Stanislav for excellent secretarial assistance.
Funding: Dr. Camilleri was supported by grant R56-DK67071 from National Institutes of Health. Some of the medication supplies were provided by Novo Nordisk Inc., Plainsboro, NJ, USA. The study was conducted in the Clinical Research Unit [supported by Mayo Clinic Center for Clinical and Translational Science (CCaTS) grant UL1-TR000135].
Footnotes
Disclosures: The authors have no conflicts of interest.
Authors’ contributions:
Victor Chedid – acquiring and interpreting data, co-authorship of manuscript
Priya Vijayvargiya - acquiring and interpreting data, co-authorship of manuscript
Paula Carlson - genotype assays
Kathleen Van Malderen - co-authorship of manuscript
Andres Acosta - co-investigator, acquiring and interpreting data, co-authorship of manuscript
Alan Zinsmeister - study statistician; author of statistical analysis plan, interim and final analyses
Michael Camilleri - PI; project concept and design; senior author of manuscript
References
- 1.Acosta A, Camilleri M, Shin A, et al. Quantitative gastrointestinal and psychological traits associated with obesity and response to weight-loss therapy. Gastroenterology. 2015;148(3):537–546. e534. doi: 10.1053/j.gastro.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shigeto M, Ramracheya R, Tarasov AI, et al. GLP-1 stimulates insulin secretion by PKC-dependent TRPM4 and TRPM5 activation. The Journal of Clinical Investigation. 2015;125(12):4714–4728. doi: 10.1172/JCI81975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nauck MA, Niedereichholz U, Ettler R, et al. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. American Journal of Physiology - Endocrinology And Metabolism. 1997;273(5):E981–E988. doi: 10.1152/ajpendo.1997.273.5.E981. [DOI] [PubMed] [Google Scholar]
- 4.Deane AM, Nguyen NQ, Stevens JE, et al. Endogenous glucagon-like peptide-1 slows gastric emptying in healthy subjects, attenuating postprandial glycemia. The Journal of clinical endocrinology and metabolism. 2010;95(1):215–221. doi: 10.1210/jc.2009-1503. [DOI] [PubMed] [Google Scholar]
- 5.Naslund E, Gryback P, Backman L, et al. Distal small bowel hormones: correlation with fasting antroduodenal motility and gastric emptying. Digestive diseases and sciences. 1998;43(5):945–952. doi: 10.1023/a:1018806129102. [DOI] [PubMed] [Google Scholar]
- 6.Seimon RV, Brennan IM, Russo A, et al. Gastric emptying, mouth-to-cecum transit, and glycemic, insulin, incretin, and energy intake responses to a mixed-nutrient liquid in lean, overweight, and obese males. American journal of physiology Endocrinology and metabolism. 2013;304(3):E294–300. doi: 10.1152/ajpendo.00533.2012. [DOI] [PubMed] [Google Scholar]
- 7.Pi-Sunyer X, Astrup A, Fujioka K, et al. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. New England Journal of Medicine. 2015;373(1):11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 8.Halawi H, Khemani D, Eckert D, et al. Effects of liraglutide on weight, satiation, and gastric functions in obesity: a randomised, placebo-controlled pilot trial. Lancet Gastroenterol Hepatol. 2017;2(12):890–899. doi: 10.1016/S2468-1253(17)30285-6. [DOI] [PubMed] [Google Scholar]
- 9.Acosta A, Camilleri M, Burton D, et al. Exenatide in obesity with accelerated gastric emptying: a randomized, pharmacodynamics study. Physiological Reports. 2015;3(11):e12610. doi: 10.14814/phy2.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vazquez Roque MI, Camilleri M, Stephens DA, et al. Gastric Sensorimotor Functions and Hormone Profile in Normal Weight, Overweight, and Obese People. Gastroenterology. 2006;131(6):1717–1724. doi: 10.1053/j.gastro.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 11.Cremonini F, Mullan BP, Camilleri M, Burton DD, Rank MR. Performance characteristics of scintigraphic transit measurements for studies of experimental therapies. Alimentary Pharmacology & Therapeutics. 2002;16(10):1781–1790. doi: 10.1046/j.1365-2036.2002.01344.x. [DOI] [PubMed] [Google Scholar]
- 12.Sathananthan A, Man CD, Micheletto F, et al. Common genetic variation in GLP1R and insulin secretion in response to exogenous GLP-1 in nondiabetic subjects: a pilot study. Diabetes care. 2010;33(9):2074–2076. doi: 10.2337/dc10-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Luis DA, Diaz Soto G, Izaola O, Romero E. Evaluation of weight loss and metabolic changes in diabetic patients treated with liraglutide, effect of RS 6923761 gene variant of glucagon-like peptide 1 receptor. Journal of diabetes and its complications. 2015;29(4):595–598. doi: 10.1016/j.jdiacomp.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Knop FK. Comment on: Villareal et al. (2009) TCF7L2 variant rs7903146 affects the risk of type 2 diabetes by modulating incretin action. Diabetes;59:479–485. Diabetes. 2010;59(6):e4. doi: 10.2337/db09-1169. author reply e5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pilgaard K, Jensen CB, Schou JH, et al. The T allele of rs7903146 TCF7L2 is associated with impaired insulinotropic action of incretin hormones, reduced 24 h profiles of plasma insulin and glucagon, and increased hepatic glucose production in young healthy men. Diabetologia. 2009;52(7):1298–1307. doi: 10.1007/s00125-009-1307-x. [DOI] [PubMed] [Google Scholar]
- 16.Villareal DT, Robertson H, Bell GI, et al. TCF7L2 variant rs7903146 affects the risk of type 2 diabetes by modulating incretin action. Diabetes. 2010;59(2):479–485. doi: 10.2337/db09-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vazquez-Roque MI, Camilleri M, Vella A, Carlson P, Laugen J, Zinsmeister AR. Association of TCF7L2 allelic variations with gastric function, satiation, and GLP-1 levels. Clinical and translational science. 2011;4(3):183–187. doi: 10.1111/j.1752-8062.2011.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papadopoulou A, Lynch KF, Shaat N, et al. Gestational diabetes mellitus is associated with TCF7L2 gene polymorphisms independent of HLA-DQB1*0602 genotypes and islet cell autoantibodies. Diabetic medicine : a journal of the British Diabetic Association. 2011;28(9):1018–1027. doi: 10.1111/j.1464-5491.2011.03359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Center for Biotechnology Information. [Accessed May 23, 2017];2017 https://www.ncbi.nlm.nih.gov/
- 20.Camilleri M, Iturrino J, Bharucha AE, et al. Performance characteristics of scintigraphic measurement of gastric emptying of solids in healthy participantS. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2012;24(12):1076–e1562. doi: 10.1111/j.1365-2982.2012.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buse JB, Nauck M, Forst T, et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet (London, England) 2013;381(9861):117–124. doi: 10.1016/S0140-6736(12)61267-7. [DOI] [PubMed] [Google Scholar]
- 22.Horowitz M, Aroda VR, Han J, Hardy E, Rayner CK. Upper and/or lower gastrointestinal adverse events with glucagon-like peptide-1 receptor agonists: Incidence and consequences. Diabetes, obesity & metabolism. 2017;19(5):672–681. doi: 10.1111/dom.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halawi H, Camilleri M, Acosta A, et al. Relationship of Gastric Emptying or Accommodation with Satiation, Satiety and Postprandial Symptoms in Health. American journal of physiology Gastrointestinal and liver physiology. 2017 doi: 10.1152/ajpgi.00190.2017. ajpgi.00190.02017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Luis DA, Aller R, Izaola O, Romero E. Effects of a high-protein/low-carbohydrate versus a standard hypocaloric diet on adipocytokine levels and cardiovascular risk factors during 9 months, role of rs6923761 gene variant of glucagon-like peptide 1 receptor. Journal of endocrinological investigation. 2015;38(11):1183–1189. doi: 10.1007/s40618-015-0304-9. [DOI] [PubMed] [Google Scholar]
- 25.Jensterle M, Pirs B, Goricar K, Dolzan V, Janez A. Genetic variability in GLP-1 receptor is associated with inter-individual differences in weight lowering potential of liraglutide in obese women with PCOS: a pilot study. European journal of clinical pharmacology. 2015;71(7):817–824. doi: 10.1007/s00228-015-1868-1. [DOI] [PubMed] [Google Scholar]
- 26.de Luis DA, Aller R, Izaola O, et al. Effect of rs6923761 gene variant of glucagon-like peptide 1 receptor on metabolic response and weight loss after a 3-month intervention with a hypocaloric diet. Journal of endocrinological investigation. 2014;37(10):935–939. doi: 10.1007/s40618-014-0117-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.