Abstract
Background
Endoanal MRI and MR defecography are used to identify anal sphincter injury and disordered defecation. However, few studies have evaluated findings in asymptomatic healthy people. The effects of BMI and parity on rectoanal motion and evacuation are unknown.
Methods
In 113 asymptomatic females (age 50 ± 17 years, Mean ± SD) without risk factors for anorectal trauma, anal sphincter appearance, anorectal motion, and pelvic organ prolapse were evaluated with MRI. The relationship between age, BMI, and parity and structural findings were evaluated with parametric and non-parametric tests.
Results
The anal sphincters and puborectalis appeared normal in over 90% of women. During dynamic MRI, the anorectal angle was 100 ± 1° (Mean ± SEM) at rest, 70 ± 2° at squeeze, and 120 ± 2° during defecation. The change in anorectal angle during squeeze (r = −0.25, P<0.005), but not during evacuation (r = 0.13, P=0.25) was associated with age. In the multivariable models, BMI (P<0.01) and parity (P<0.01) were respectively independently associated with the intersubject variation in the anorectal angle at rest and the angle change during squeeze. Ten percent or fewer women had had descent of the bladder base or uterus 4 cm or more below the pubococcygeal line or a rectocele measuring 4 cm or larger. Only 5% had a patulous anal canal.
Conclusions
In addition to age, BMI and parity also affect anorectal motion in asymptomatic women. These findings provide age-adjusted normal values for rectoanal anatomy and pelvic floor motion.
Graphical Abstract
Compared to age, BMI and parity have lesser effects on anorectal motion in asymptomatic women. These findings provide age-adjusted normal values for rectoanal anatomy and pelvic floor motion.
BACKGROUND
Magnetic resonance imaging (MRI) is used to identify damage to the anal sphincters and pelvic floor muscles and to assess anorectal and pelvic floor motion in real time during defecation and pelvic floor contraction.(1–3) In contrast to barium defecography, MRI is generally conducted in the supine position, avoids radiation exposure, and visualizes the pelvic floor muscles, the urinary bladder and uterus without adding contrast to these organs.
Anal manometry, the rectal balloon expulsion test, and defecography, which can be conducted with fluoroscopy or MRI, are used to diagnose defecatory disorders.(4) In one controlled study, 94% of constipated women, including some women with a normal rectal balloon expulsion test, had features of impaired evacuation.(2) The American Gastroenterological Association and American College of Gastroenterology guidelines for identifying defecatory disorders (DD) recommend anal manometry and the rectal balloon expulsion test, followed, if necessary, by MR or fluoroscopic defecography, i.e., defecography as a second-line test.(3, 5) However, recent studies have highlighted the limited utility of anal manometry for identifying defecatory disorders.(6) Hence, the recently revised Rome 4 criteria require at least 2 of 4 abnormal tests for diagnosing DD.(4)
Several reasons underscore the need for a robust characterization of anal sphincter morphology and anorectal motion in asymptomatic people with MRI. First, anorectal functions are affected by aging.(7) For example, anal resting, and less so, squeeze pressures are lower in older than younger asymptomatic people. With fluoroscopic and MR defecography, the anorectal junction is lower in older than younger asymptomatic women.(2, 7, 8) Second, some healthy people may find it challenging to evacuate in the supine position, as is required during MRI. Third, besides age, the effects of other putative factors (e.g., parity and BMI) that may affect anorectal functions even in asymptomatic people are poorly understood. Parity has also been associated with reduced squeeze pressure increment in asymptomatic women.(9) Additionally, while age and parity are associated,(10) it is unclear if the effects of parity are independent of age. While obesity is an independent risk factor for FI,(11) the effects of BMI on anorectal motion in asymptomatic women is unknown. Last, some findings (e.g., rectocele, excessive perineal descent) are common in asymptomatic women.(12, 13) Hence, while MR defecography is useful for diagnosing DD particularly when other tests are normal, a comparison with healthy people is necessary to determine the utility of MR defecography for diagnosing DD. However, to our knowledge, only a few studies that evaluated the utility of MR defecography in defecatory disorders were conducted with a control population.(2, 14)
A literature search with the terms “defecography” and “MRI” identified several small studies that evaluated anorectal motion during pelvic floor contraction and rectal evacuation with MR defecography in healthy people.(1, 7, 15–23) Only some studies(1, 7, 22, 24) excluded women with a history of 3rd and 4th degree anal sphincter injury or assessed for bowel symptoms (e.g., constipation and diarrhea), which may be associated with pelvic floor dysfunction, with a questionnaire.
The aims of this study were to evaluate the appearance of the anal sphincters and puborectalis, anorectal motion during pelvic floor contraction and rectal evacuation, and pelvic organ prolapse in asymptomatic women, and the effects of age, parity, and BMI on these parameters. In particular, a better understanding of age-specific normal values is needed to establish the utility of MRI for diagnosing defecatory disorders and pelvic organ prolapse.
MATERIALS AND METHODS
Participants
After approval by the Institutional Review Board at Mayo Clinic, 113 asymptomatic women participated in these studies. They were recruited by public advertisement. Some results from these studies have been presented elsewhere.(2, 7, 25) Only one of these earlier reports, published over 10 years ago, provided age-specific normal values for anorectal MRI, i.e. in 38 healthy women.(7)
In this cohort, 20 females were aged between 20–29 years, 16 were aged 30–39 years, 20 were aged 40–49 years, 21 were aged 50–59 years, 20 were aged 60–69 years, and 16 were aged ≥ 70 years. All participants underwent a clinical interview and physical examination. Exclusion criteria included: significant cardiovascular, respiratory, neurological, psychiatric or endocrine disease; functional bowel disorders or fecal incontinence identified with a validated bowel disease questionnaire;(26) medications that can affect gastrointestinal motility or sensation; and abdominal surgery (other than appendectomy, cholecystectomy or hysterectomy). Participants who had sustained anorectal trauma during delivery (i.e. grade 3 or 4 laceration), which was the only one of several obstetric risk factors that was associated with anal sphincter injury documented by MRI in our previous study, were excluded from this report.(22)
Pelvic MR imaging
Procedure
A disposable endorectal coil (MRInnervu®, Medrad, Inc., Indianola, PA, USA) prior to dynamic MR proctography was used to image the anal sphincters.(1, 2, 27) For imaging the anal sphincters, axial T2-weighted fast spin-echo images[(field of view (FOV) 12 cm, repetition time (TR) = 4000mS, echo time (TE) = 105 mS, 3.5 mm slice thickness/0 mm skip, 256 × 192 matrix, 2 NEX)] and corresponding T1-weighted spin-echo images[(FOV 12 cm, TE/TR minimum/400, 3.0 mm thickness, 0.5 mm skip, 256 × 256 matrix, 1 NEX)] were acquired with additional fast spin-echo images acquired in the coronal plane with similar parameters and a 14 cm FOV.
The disposable endorectal coil was removed. Then, 120 cc of ultrasound gel was instilled into the rectum, and a four-element phased-array coil was placed around the pelvis. The volume of ultrasound gel was similar to that of barium paste used for fluoroscopic defecography. An interactive single-shot, fast spin-echo (SSFSE) imaging technique was employed for dynamic MR defecography. Images were acquired with a field of view (FOV) of 24 to 32 cm, 5 mm slice, repetition time (TR) of 1400 to 2000 ms, echo time (TE) of 90 ms, and a matrix size of 256 × 160 (half-nex). An oblique sagittal plane bisecting the anorectum was defined by three points that were selected from axial images during real-time imaging. Images were acquired every 1 to 1.4 seconds during rest, squeeze, and defecation in the supine position and reconstructed in real-time, allowing patients to be coached during maneuvers. Anal canal integrity was evaluated at rest in the midsagittal plane. The canal was deemed patulous when the anterior and posterior anal canal was not coapted and the lumen was filled with ultrasound gel for the entire length of the anal canal. Patients were removed from the magnet after the defecation sequence and asked to empty the bladder and remaining rectal contents. Subsequently, they were then placed back in the magnet in the same position, and dynamic mid-sagittal SSFSE images were acquired during the Valsalva maneuver. Using a standardized approach, the same radiologist (JGF) analyzed anal sphincter appearance and pelvic floor motion in all patients.
Data Analysis
Anal Sphincter Appearance
Sphincters were characterized by their appearance (i.e., normal, mild focal thinning, marked focal thinning or tear, scar, or atrophy), location of abnormalities around the anal canal circumference and along its longitudinal axis (i.e., from the most superficial aspect of the subcutaneous external anal sphincter to anorectal junction).(1, 2, 22)
Anorectal Motion and Rectal Evacuation
Anorectal and pelvic floor motion from rest to squeeze and rest to defecation was recorded by archiving sequential images during these maneuvers; anorectal motion was analyzed by comparing single images at rest, and during maximum excursion during squeeze, defecation, and the post-defecation Valsalva maneuver. Established definitions were used to measure the anorectal angle, motion of the anorectal junction, rectocoele, and enterocoele, bladder, and uterine prolapse. The anorectal angle was the angle between the central axis of the anal canal, and the tangent to the posterior wall of the rectum. Vertical motion of the anorectal junction (i.e., perineal motion) during squeeze, and defecation was measured as the perpendicular distance from the pubococcygeal line; descent below the line was represented as a positive value. The same landmark, albeit during a Valsalva maneuver, was used to measure descent of the uterus (i.e., uterocervical junction) and bladder base.(28) A rectocele was defined as a bulge of the anterior rectal wall beyond the expected and extrapolated line of the anterior rectal wall. A peritoneocele referred to protrusion of the peritoneal fat or fluid crossing the junction of the upper one third and distal two thirds of the vagina, with separation of the rectovaginal septum. An enterocele was diagnosed when this pouch also contained small intestinal loops.(27) Rectal evacuation was evaluated by measuring the change in area ie, after versus before defecation, on sagittal slices with a software program.
Statistical Analysis
The relationship between age, BMI, and parity on anorectal functions (i.e., anal pressures measured with manometry as well as anorectal and pelvic floor motion measured with MRI) were evaluated with Spearman’s correlation coefficient. Wilcoxon’s rank-sum test analyzed the association between anorectal parameters and age and separately the BMI. Multivariable linear regression models evaluated the extent to which predictor variables (i.e., age, BMI, and parity and appropriate interaction terms) explained inter-individual variability in anorectal motion parameters. Statistical analyses were conducted with SAS software. Except where stated otherwise, all data are Mean ± SEM.
RESULTS
The participants were aged from 19 to 86 years (50 ± 17 years, Mean ± SD). The BMI was 27 ± 0.6 kg/m2; 29 subjects had a BMI > 30 kg/m2. 39 females had none, 12 had one, 33 had two, and 13 had three, 16 had four or more vaginal deliveries. Nine women had one or more cesarean sections but no vaginal deliveries. Women younger than 51 years of age, which was the median age of all participants, were more likely to be nulliparous (P=0.002) and less likely to have had a vaginal delivery (P=0.002). Older women were more likely (P<0.002) to have had a hysterectomy.
Anal Sphincter and Pelvic Floor Morphology by MRI
The anal sphincters were imaged with endoanal MRI in 112/113 women. One woman was uncomfortable with the endoanal coil. Of 112 women, 103 (91 percent), had normal appearing internal and external anal sphincters and 107 (95%) had a normal puborectalis sling. Focal thinning (mild or marked) or, scarring were found in internal and external anal sphincters respectively of 7 and 9 women. Two women each had external anal sphincter atrophy and global thickening of the internal anal sphincters. Five women had unilateral or bilateral atrophy of the puborectalis muscle, of whom one had 9 vaginal deliveries. These abnormalities were not associated with age.
Rectal Evacuation and Pelvic Floor Motion
During dynamic MRI, the anorectal angle was 100 ± 1° at rest, 70 ± 2° at squeeze, and 120 ± 2° during defecation.(Table 1) During evacuation, healthy women evacuated a median of 57% (IQ range 18–82%) of the ultrasound gel. Rectal evacuation was lower in women aged under 51 years (i.e., 47% [10–78%]) than older women (i.e., 64% [19–85%]) but differences were not statistically significant.
Table 1.
Demographic variables and pelvic floor motion in study participants
| Age < 51 years (n = 56) | Age ≥ 51 years (n = 57) | P value by Chi Square or Wilcoxon rank | Spearman correlations (P value) | |||
|---|---|---|---|---|---|---|
| Values a | 10th, 90th percentile values | Values a | 10th, 90th percentile values | |||
| Demographic and Clinical Variables | ||||||
| Body mass index (BMI) (kg/m2) | 25 | (22, 31) | 27 | (23, 30) | 0.3 | |
| Nulliparous women (n) | 27 (58%) | NA | 12 (21%) | NA | 0.002 | |
| Vaginal delivery (n) | 24 (43%) | NA | 41 (72%) | NA | 0.002 | |
| Hysterectomy (n) | 2 (4%) | NA | 19 (33%) | NA | < 0.001 | |
| Anorectal Angle | ||||||
| At Rest (°) | 99 (92, 107) | 87, 120 | 99 (92, 107) | 86, 119 | 1.0 | 0.13 |
| Change during Squeeze (°) | −26 (−37, −20) | −48.5, −12 | −32 (−41, −26) | −57.6, −12 | 0.07 | −0.25 b |
| Change during defecation (°) | 16 (8, 31) | −1.5, 37 | 20 (9, 35) | −0.6, 49 | 0.2 | 0.13 |
| Location of anorectal junction b | ||||||
| At rest (cm) | 2.1 (1.4, 2.7) | 0.6, 3.4 | 2.6 (1.9, 3.2) | 1.3, 3.9 | 0.03 | 0.34 c |
| Change during squeeze (cm) | −1.6 (−2.1, −1.2) | −2.6, −0.7 | −1.3 (−1.5, −0.8) | −2.3, − 0.4 | 0.002 | 0.23 b |
| Change during defecation (cm) | 3.1 (1.9, 4.2) | 1.2, 5.5 | 3 (2.2, 3.9) | 1.4, 4.6 | 0.6 | −0.04 |
Values are Median (IQ Range) unless stated otherwise
Negative and positive values reflect location above and below the pubococcygeal line respectively
P value < 0.05 = a, P value < 0.01 = b, P value < 0.001= c
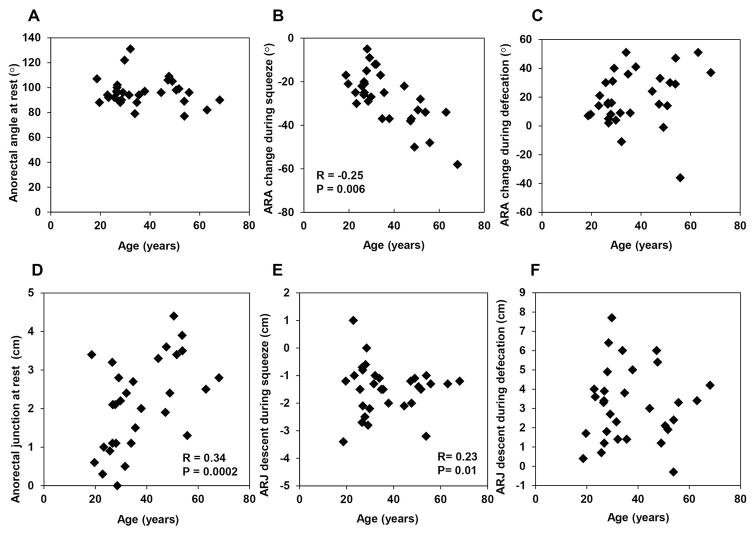
The change in anorectal angle during squeeze (r = −0.25, P<0.005), but not during defecation (r = 0.13, P=0.25) was associated with age. Similarly the location of the anorectal junction at rest (r = 0.34, P<0.001), and change in anorectal motion during squeeze (r = 0.23, P<0.003), but not during defecation (r = −0.04, P=0.58) was associated with age (Figures 1 and 2, Table 1).
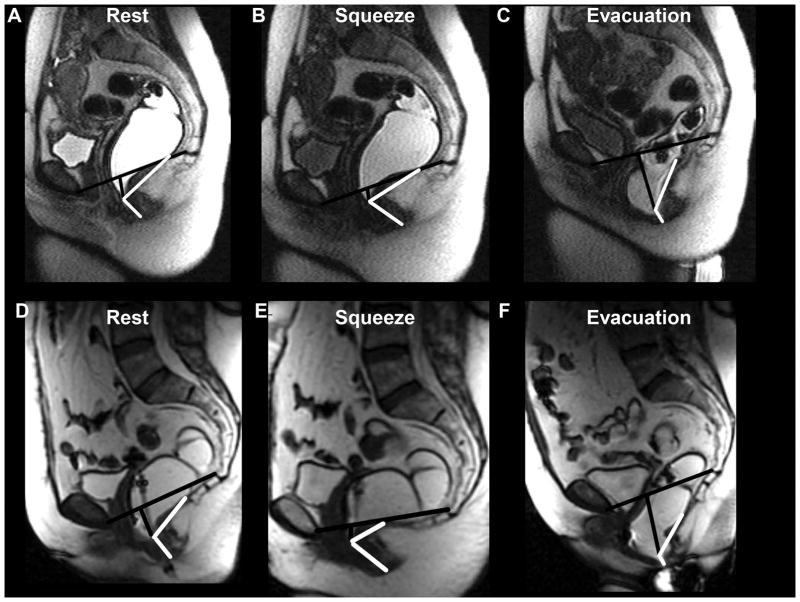
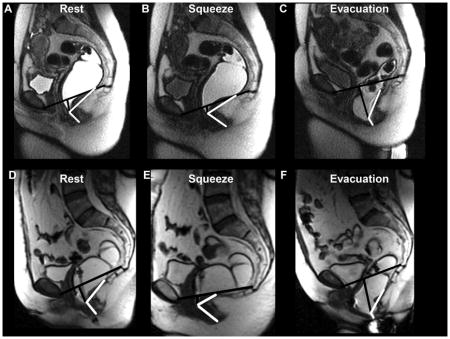
Figure 1. Representative examples of anorectal motion during rest, squeeze and defecation in a younger women aged 34 years (upper panel) and an older women aged 63 years (lower panel).
The pubococcygeal line and the perpendicular extending from this line to the anorectal junction are marked in black. The boundaries of the anorectal angle are shown in white. Compared to the younger woman, the anorectal junction at rest and during squeeze was lower in the older woman, in whom the angle change during squeeze was also more pronounced.
Figure 2. Relationship between age, anorectal angle, and location of anorectal junction.
The angle change during squeeze, location of the anorectal junction at rest and motion during squeeze were significantly correlated with age.
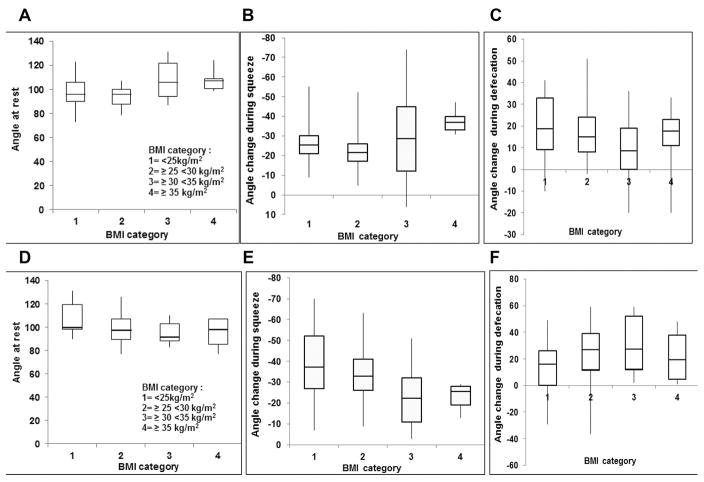
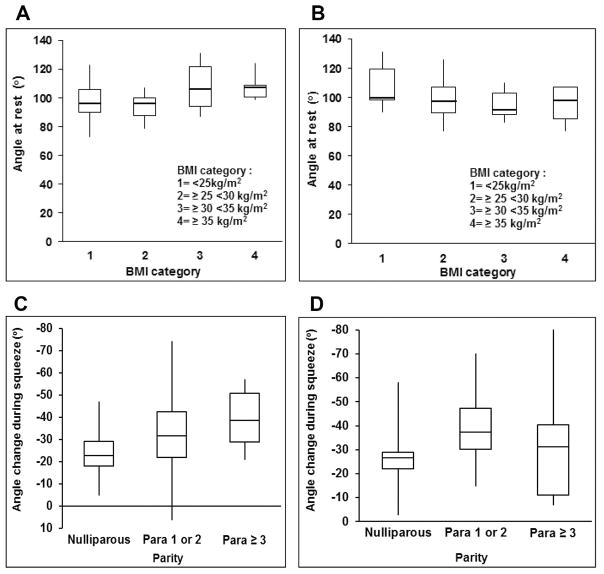
In the multivariable linear regression model, age, BMI, parity and interaction terms between these variables independently explained 24% and 17% of the intersubject variation in the anorectal angle at rest and the angle change during squeeze respectively (Table 2). The interaction terms – age*BMI and age*parity – were respectively significant in the models for angle at rest and angle change during squeeze, suggesting that the relationship between age and these parameters were influenced by BMI and parity. Figure 3 shows that in younger women, the median anorectal resting angle was greater (average of 108°) in women with a BMI ≥ 30 kg/m2 than a BMI between 25–29.9 (average of 95°) or < 25 kg/m2 (98°). By contrast, among older women, the median anorectal resting angle was greater in women with a lower than a higher BMI. Thus, obesity has different effects on the anorectal resting angle in younger and older women. Among younger but not older women, the angle change during squeeze was associated with parity (Figure 3). By contrast, age, BMI, and parity were not associated with the angle change during defecation.
Table 2.
Multivariable Model for Effects of Age, BMI and Parity
| Angle at rest (R2) | Angle change during squeeze (R2) | Angle change during defecation (R2) | |
|---|---|---|---|
| Age | 0.07 b | 0.06 b | 0.01 |
| BMI | 0.09 b | 0.01 | 0.02 |
| Parity | 0.001 | 0.1b | 0.00003 |
| Interactions | |||
| Age and BMI | 0.09 b | 0.03 | 0.02 |
| Age and parity | 0.009 | 0.1 c | 0.002 |
| Total variance (R2) | 0.24 | 0.17 | 0.04 |
Values are Parameter Estimate and Standard Error
P value < 0.05 = a, P value < 0.01 = b, P value < 0.001= c
Figure 3. Relationship between BMI, parity, and age with anorectal motion.
In younger women (Panel 2A), the median anorectal resting angle was 97° in women with a BMI less than 25 kg/m2 and 107° with a BMI ≥ 35 kg/m2. By contrast, in older women (Panel 2B), this angle was similar across BMI values. Among younger (fig. 2C) but not older women (fig. 2D), the angle change during squeeze was associated with parity.
Pelvic organ prolapse
Of 113 women, 6 (5%) had a patulous anal canal and 4 (4%) had rectal prolapse (Table 3). Twelve (11%) had rectal intussusception of any degree. A patulous canal was more common in women with a BMI < 25.9 kg/m2, with other abnormalities were not associated with age or BMI.
Table 3.
Relationship between Pelvic Organ Prolapse, Age, and Parity.
| Age | BMI | |||
|---|---|---|---|---|
| < 51 years | ≥ 51 years | ≤ 25.9 kg/m2 | >25.9 kg/m2 | |
| Patulous anal canal | 2% | 9% | 11% a | 0% |
| Rectal prolapse | 4% | 4% | 4% | 4% |
| Intussusception | 9% | 13% | 11% | 11% |
p = 0.01
Of 113 women, 70 (62%) had a rectocele larger than 2cm, 54 (48%) had a bladder base descent by more than 2cm, and 32 (28%) had uterine descent greater than 2cm. (Figure 4) However, ≤ 10% of women had descent of the bladder base or uterus for 4 cm or more below the pubococcygeal line or a rectocele measuring 4 cm or larger, which suggests that this cutoff is useful for identifying pathological descent or rectoceles. While bladder base descent was greater and rectoceles were more prevalent in women 51 years and older, these associations with age were not statistically significant. Descent of the bladder base and uterus greater than 4 cm below the pubococcygeal line was respectively observed in 9% and 5% women.
Figure 4. Severity of pelvic organ prolapse.
Figures demonstrate visceral descent relative to the pubococcygeal line and rectocele size.
DISCUSSION
These findings provide normal values for anal sphincter and pelvic floor anatomy and function in asymptomatic healthy women. All MR images were reviewed by a single radiologist. The internal or external anal sphincters or puborectalis muscle were each abnormal in less than 10% of asymptomatic women. Some women had pelvic organ prolapse; 6 (5%) had a patulous anal canal, 4 (4%) had rectal prolapse and 12 (11%) had rectal intussusception. A rectocele, bladder base descent, and uterine descent each greater than 2 cm were respectively observed in 70 (62%), 54 (48%), and 32 (28%) of women. MRI-based functional assessments disclosed that the location of the anorectum and pelvic floor rest and motion during squeeze but not during evacuation were associated with age. After adjusting for age, increased BMI and parity were independently associated respectively with the anorectal angle at rest and the angle change during squeeze.
These findings provide age-appropriate normal values for anorectal motion measured with MRI. Similar to a previous study with barium evacuating proctography (8), the perineum was lower at rest and during maneuvers in older than in younger females, reflecting perineal laxity. For some parameters, such as the location of the anorectal junction at rest or motion change during squeeze, the normal values for younger and older women are different. For other parameters (eg, motion during evacuation), age-specific normal values are unnecessary.
Absent normal values for MR defecography, normal values from barium defecography are often used to interpret anorectal motion imaged with MRI in clinical practice. Despite differences in the age distribution and parity, amount of rectal filling, and techniques for measuring anorectal parameters, it is informative to compare findings from MR defecography and the 2 largest studies with barium defecography (Table 4).(12, 13) For MR defecography, the rectum was filled with 120 ml of ultrasound gel. The barium defecography studies filled the rectum until patients reported the desire to defecate; however some barium refluxed into the sigmoid colon. With both barium and MR defecography, the resting anorectal angle was approximately 100°; the standard deviation for parameters of motion (i.e, angle change, descent) during squeeze and defecation was high, which suggests considerable variability among participants.
Table 4.
Comparison of anorectal motion parameters measured with MRI in this study and defecating proctography in previous studies
| Variable | MR Proctography (this study) | Defecating Proctography, study 1 (12) | Defecating Proctography, study 2 (13) |
|---|---|---|---|
| No of women (n) | 113 | 23 | 28 |
| Age (years) | 50 ± 17 | 21 ± 1.6 | 43a |
| Nulliparous (%) | 39 | 100 | 39 |
| Rectal filling (ml) | 120 | 80 – 200 b | 221 ± 72 |
| Posterior anorectal angle (°) | |||
| At rest | 100 ± 12 c | 95 ± 16 | 110 ± 12 |
| Change from rest – squeeze | −31 ± 17 | −24 ± NA | −13 ± 9 |
| Change from rest – defecation | 19 ± 18.6 | 4 ± NA | 23 ± 16 |
| Distance between anorectal junction and bony landmarks | |||
| At rest | 23 ± 10c | 4 ± 13 d | |
| Change from rest – squeeze | −14 ± 8 | −10 ± 6 e | |
| Change from rest – defecation | 31 ± 16 | 20 ± 14 e | |
| Intussusception (%) | 11 | 43 | 18 |
| Rectocele (%) | 71 (any size) 62 (>2 cm) |
81 (any size), 5 (> 2 cm) |
93 (any size) NA (> 2 cm) |
Data are Mean ± SD unless mentioned otherwise
Median
Range
Perpendicular distance from pubococcygeal line
Distance from inferior margin of ischial tuberosity
Estimated from data provided in paper
NA – not available
Similar to barium defecography,(12, 13) rectoceles were frequently observed during MR defecography. In the study by Shorvon et al, (12) rectoceles were small, probably because the women were younger and nulliparous. By comparison, in the study by Palit et al, the rectoceles were larger, measuring 2.5 cm on average.(13) In this study, 10% or fewer women had descent of the bladder base or uterus for 4 cm or more below the pubococcygeal line or a rectocele measuring 4 cm or larger, which suggests that this cutoff is useful for identifying pathological descent or rectoceles. However, even smaller rectoceles may cause symptoms, particularly if they do not empty during defecation.(29)
Globally, aging affects muscle mass and strength, nerve function, and connective tissue. Fortuitously, the median age in this cohort was 51 years, which is the average age of menopause in the United States (30) and also when muscle mass begins to decline substantially.(31) The loss of muscle strength exceeds that of muscle mass,(32, 33) which may explain why pelvic floor contraction was impaired in older women despite normal appearing external anal sphincter and pelvic floor muscles. Impaired strength is attributed to changes in muscle architecture, loss of sarcomeres, and intramuscular fibrosis.(30) Aging is also accompanied by remodeling of motor units, which is characterized by denervation and reinnervation of muscle fibers as spinal motoneurons fail or are lost.(34) As a result there are fewer functioning motor units coupled with incomplete compensatory reinnervation of muscle fibers.(34) Indeed, needle electromyography documented neurogenic injury affecting the external anal sphincter in older asymptomatic nulliparous women.(35) The substantial decrease in predicted muscle force production and increase in pelvic floor muscle collagen content i.e., fibrosis with aging, which was not altered by parity represent likely mechanisms for the pelvic floor muscle dysfunction in older women.(30) To speculate, these effects of age may be clinically significant when they progress over time and/or are combined with additional risk factors (e.g., obstetric trauma), Perhaps this explains, at least partly, why FI in females generally begins in the seventh decade.(36)
Obesity is a risk factor for fecal (11, 22) and urinary incontinence.(37) Parity is a risk factor for urinary and defecatory symptoms and anal weakness.(10, 38) However, previous studies have not evaluated the effects of obesity or parity on anorectal functions. In this study, BMI and parity affected anorectal functions, albeit differently, and independent of age even in asymptomatic women. In the multivariable model, BMI explained 9% of the variations in the anorectal resting angle among women. Moreover, BMI had different effects on the resting angle in younger and older women. In younger women, a greater BMI was associated with a more obtuse angle, perhaps because increased BMI is associated with increased abdominal pressure that may predispose to increased perineal descent at rest. In older women, the converse effect was observed, perhaps because the effects of BMI were overshadowed by the effects of age. Increasing parity was associated with greater motion during squeeze in younger but not in older women, which suggests that parity has a more pronounced effect on pelvic floor motion in younger women. Consistent with epidemiologic data, aging is the strongest risk factor for progression of pelvic floor disorders after menopause; many older women experience pelvic floor disorders independent of parity.(38)
There are some limitations of this study. Because normal values for rectal evacuation are different in men and women, these normal values are only applicable to women.(13) Forceps-assisted or multiple deliveries were not excluded from the dataset because these risk factors are not always associated with anal sphincter and pelvic injury and the risk is mostly explained by the first delivery.(10) Indeed, only 2 of the 21 women with 4 or more vaginal or forceps-assisted deliveries in this study had pelvic floor injury (i.e., mild focal thinning of the external sphincter alone in 1 woman, and external sphincter and puborectalis injury in 1 woman). For the same reason, we did not exclude women with a history of anorectal procedures. Indeed, endoanal MRI did not demonstrate anal sphincter or puborectalis injury in any of the 7 women with a history of minor procedures for hemorrhoids (5 women), incision and drainage of a perirectal abscess (2 women), and anal sphincterotomy (1 woman). Some women had undergone a simple hysterectomy, which has relatively modest effects on rectal stiffness and sensation but not on anal resting and squeeze pressures.(39) Measurements of anorectal motion during barium and MR defecography, are prone to intra- and inter- observer errors.(21, 40) A validated semi-automated program to measure anorectal parameters reduces observer errors was not used in this study because we sought to provide normal values for the research community.(25) All images were reviewed by a single experienced radiologist. The lower end of the normal range for rectal emptying during defecation was approximately 20%, probably because MRI was performed in the supine position. Since an enema was not administered before the MR proctogram, it is conceivable that residual stool hindered the evacuation of ultrasound gel in some participants.(41) Hence, only patients who empty less than 20% during defecation are abnormal.
KEY POINTS.
While MRI is used to identify anal sphincter injury and diagnose impaired defecation, there are limited normal values. The effects of age, BMI, and parity on rectoanal motion and evacuation are unknown.
In addition to age, BMI and parity also affect anorectal motion in asymptomatic women.
These findings provide age-adjusted normal values for rectoanal anatomy and pelvic floor motion.
Acknowledgments
This work was supported in part by Grants R01 DK 78924 and General Clinical Research Center grant M01 RR00585 from the National Institutes of Health, U.S. Public Health Service. This work was partly presented at the 118th Annual Meeting of the American Gastroenterological Association, 2017.
References
- 1.Bharucha AE, Fletcher JG, Harper CM, et al. Relationship between symptoms and disordered continence mechanisms in women with idiopathic fecal incontinence. Gut. 2005;54:546–555. doi: 10.1136/gut.2004.047696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bharucha AE, Fletcher JG, Seide B, Riederer SJ, Zinsmeister AR. Phenotypic Variation in Functional Disorders of Defecation. Gastroenterology. 2005;128:1199–1210. doi: 10.1053/j.gastro.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 3.Wald A, Bharucha AE, Cosman BC, Whitehead W. ACG Clinical Guidelines:Management of Benign Anorectal Disorders. Am J Gastroenterol. 2014;109:1141–1157. doi: 10.1038/ajg.2014.190. [DOI] [PubMed] [Google Scholar]
- 4.Rao S, Bharucha AE, Chiarioni G, et al. Functional anorectal disorders. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.02.009. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bharucha AE, Locke GR, Pemberton JH. AGA Practice Guideline on Constipation: Technical Review. Gastroenterology. 2013;144:218–238. doi: 10.1053/j.gastro.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee TH, Bharucha AE. How to Perform and Interpret a High-resolution Anorectal Manometry Test. J Neurogastroenterol Motil. 2016;22:46–59. doi: 10.5056/jnm15168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox JC, Fletcher JG, Zinsmeister AR, Seide B, Riederer SJ, Bharucha AE. Effect of aging on anorectal and pelvic floor functions in females. Dis Colon Rectum. 2006;49:1726–1735. doi: 10.1007/s10350-006-0657-4. [DOI] [PubMed] [Google Scholar]
- 8.Bannister JJ, Abouzekry L, Read NW. Effect of aging on anorectal function. Gut. 1987;28:353–357. doi: 10.1136/gut.28.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jameson JS, Chia YW, Kamm MA, Speakman CT, Chye YH, Henry MM. Effect of age, sex and parity on anorectal function. Br J Surg. 1994;81:1689–1692. doi: 10.1002/bjs.1800811143. [DOI] [PubMed] [Google Scholar]
- 10.Boyle DJ, Knowles CH, Murphy J, et al. The effects of age and childbirth on anal sphincter function and morphology in 999 symptomatic female patients with colorectal dysfunction. Diseases of the Colon & Rectum. 2012;55:286–293. doi: 10.1097/DCR.0b013e31823fe7f1. [DOI] [PubMed] [Google Scholar]
- 11.Bharucha AE, Zinsmeister AR, Schleck CD, Melton LJ., 3rd Bowel disturbances are the most important risk factors for late onset fecal incontinence: a population-based case-control study in women. Gastroenterology. 2010;139:1559–1566. doi: 10.1053/j.gastro.2010.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shorvon PJ, McHugh S, Diamant NE, Somers S, Stevenson GW. Defecography in normal volunteers: results and implications. Gut. 1989;30:1737–1749. doi: 10.1136/gut.30.12.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palit S, Bhan C, Lunniss PJ, et al. Evacuation proctography: a reappraisal of normal variability. Colorectal Disease. 2014;16:538–546. doi: 10.1111/codi.12595. [DOI] [PubMed] [Google Scholar]
- 14.Healy JC, Halligan S, Reznek RH, et al. Magnetic resonance imaging of the pelvic floor in patients with obstructed defaecation. Br J Surg. 1997;84:1555–1558. [PubMed] [Google Scholar]
- 15.Kruyt RH, Delemarre JB, Doornbos J, Vogel HJ. Normal anorectum: dynamic MR imaging anatomy. Radiology. 1991;179:159–163. doi: 10.1148/radiology.179.1.2006269. [DOI] [PubMed] [Google Scholar]
- 16.Lienemann A, Anthuber C, Baron A, Kohz P, Reiser M. Dynamic MR colpocystorectography assessing pelvic-floor descent. Eur Radiol. 1997;7:1309–1317. doi: 10.1007/s003300050294. [DOI] [PubMed] [Google Scholar]
- 17.Schoenenberger AW, Debatin JF, Guldenschuh I, Hany TF, Steiner P, Krestin GP. Dynamic MR defecography with a superconducting, open-configuration MR system. Radiology. 1998;206:641–646. doi: 10.1148/radiology.206.3.9494480. [DOI] [PubMed] [Google Scholar]
- 18.Goh V, Halligan S, Kaplan G, Healy JC, Bartram CI. Dynamic MR imaging of the pelvic floor in asymptomatic subjects. Am J Roentgenol. 2000;174:661–666. doi: 10.2214/ajr.174.3.1740661. [DOI] [PubMed] [Google Scholar]
- 19.Law PA, Danin JC, Lamb GM, Regan L, Darzi A, Gedroyc WM. Dynamic imaging of the pelvic floor using an open-configuration magnetic resonance scanner. Journal of Magnetic Resonance Imaging. 2001;13:923–929. doi: 10.1002/jmri.1132. [DOI] [PubMed] [Google Scholar]
- 20.Lienemann A, Sprenger D, Janssen U, Grosch E, Pellengahr C, Anthuber C. Assessment of pelvic organ descent by use of functional cine-MRI: which reference line should be used? 2004;23:33–37. doi: 10.1002/nau.10170. [DOI] [PubMed] [Google Scholar]
- 21.Morren GL, Balasingam AG, Wells JE, Hunter AM, Coates RH, Perry RE. Triphasic MRI of pelvic organ descent: sources of measurement error. Eur J Radiol. 2005;54:276–283. doi: 10.1016/j.ejrad.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Bharucha AE, Fletcher JG, Melton LJ, 3rd, Zinsmeister AR. Obstetric Trauma, Pelvic Floor Injury And Fecal Incontinence: A Population-Based Case-Control Study. Am J Gastroenterol. 2012;107:902–911. doi: 10.1038/ajg.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schreyer AG, Paetzel C, Furst A, et al. Dynamic magnetic resonance defecography in 10 asymptomatic volunteers. World journal of gastroenterology. 2012;18:6836–6842. doi: 10.3748/wjg.v18.i46.6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai XR, Qiu L, Wu HJ, Liu SR. Assessment of levator ani morphology and function in asymptomatic nulliparous women via static and dynamic magnetic resonance imaging. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2013;121:233–239. doi: 10.1016/j.ijgo.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 25.Noelting J, Bharucha AE, Lake DS, et al. Semi-automated vectorial analysis of anorectal motion by magnetic resonance defecography in healthy subjects and fecal incontinence. Neurogastroenterol Motil. 2012;24:e467–475. doi: 10.1111/j.1365-2982.2012.01962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bharucha AE, Locke GR, Seide B, Zinsmeister AR. A New Questionnaire for Constipation and Fecal Incontinence. Alimentary Pharmacology & Therapeutics. 2004;20:355–364. doi: 10.1111/j.1365-2036.2004.02028.x. [DOI] [PubMed] [Google Scholar]
- 27.Fletcher JG, Busse RF, Riederer SJ, et al. Magnetic resonance imaging of anatomic and dynamic defects of the pelvic floor in defecatory disorders. Am J Gastroenterol. 2003;98:399–411. doi: 10.1111/j.1572-0241.2003.07235.x. [DOI] [PubMed] [Google Scholar]
- 28.Healy JC, Halligan S, Reznek RH, Watson S, Phillips RK, Armstrong P. Patterns of prolapse in women with symptoms of pelvic floor weakness: assessment with MR imaging. Radiology. 1997;203:77–81. doi: 10.1148/radiology.203.1.9122419. [DOI] [PubMed] [Google Scholar]
- 29.Palit S, Thin N, Knowles CH, Lunniss PJ, Bharucha AE, Scott SM. Diagnostic disagreement between tests of evacuatory function: a prospective study of 100 constipated patients. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2016 doi: 10.1111/nmo.12859. In press. [DOI] [PubMed] [Google Scholar]
- 30.Alperin M, Cook M, Tuttle LJ, Esparza MC, Lieber RL. Impact of vaginal parity and aging on the architectural design of pelvic floor muscles. American Journal of Obstetrics & Gynecology. 2016;215:312e311–319. doi: 10.1016/j.ajog.2016.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dennison EM, Sayer AA, Cooper C. Epidemiology of sarcopenia and insight into possible therapeutic targets. Nature Reviews Rheumatology. 2017;13:340–347. doi: 10.1038/nrrheum.2017.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morse CI, Thom JM, Davis MG, Fox KR, Birch KM, Narici MV. Reduced plantarflexor specific torque in the elderly is associated with a lower activation capacity. European Journal of Applied Physiology. 2004;92:219–226. doi: 10.1007/s00421-004-1056-y. [DOI] [PubMed] [Google Scholar]
- 33.Macaluso A, Nimmo MA, Foster JE, Cockburn M, McMillan NC, De Vito G. Contractile muscle volume and agonist-antagonist coactivation account for differences in torque between young and older women. Muscle & Nerve. 2002;25:858–863. doi: 10.1002/mus.10113. [DOI] [PubMed] [Google Scholar]
- 34.Hepple RT, Rice CL. Innervation and neuromuscular control in ageing skeletal muscle. Journal of Physiology. 2016;594:1965–1978. doi: 10.1113/JP270561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bharucha AE, Daube J, Litchy W, et al. Anal sphincteric neurogenic injury in asymptomatic nulliparous women and fecal incontinence. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2012;303:G256–262. doi: 10.1152/ajpgi.00099.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bharucha AE, Zinsmeister AR, Locke GR, et al. Prevalence and burden of fecal incontinence: A population based study in women. Gastroenterology. 2005;129:42–49. doi: 10.1053/j.gastro.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Mishra GD, Hardy R, Cardozo L, Kuh D. Body weight through adult life and risk of urinary incontinence in middle-aged women: results from a British prospective cohort. Int J Obes. 2008;32:1415–1422. doi: 10.1038/ijo.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kepenekci I, Keskinkilic B, Akinsu F, et al. Prevalence of pelvic floor disorders in the female population and the impact of age, mode of delivery, and parity. Diseases of the Colon & Rectum. 2011;54:85–94. doi: 10.1007/DCR.0b013e3181fd2356. [DOI] [PubMed] [Google Scholar]
- 39.Bharucha AE, Klingele CJ, Seide BM, Gebhart JB, Zinsmeister AR. Effects of vaginal hysterectomy on anorectal sensorimotor functions--a prospective study. Neurogastroenterol Motil. 2012;24:235–241. doi: 10.1111/j.1365-2982.2011.01825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diamant NE, Kamm MA, Wald A, Whitehead WE. AGA technical review on anorectal testing techniques. Gastroenterology. 1999;116:735–760. doi: 10.1016/s0016-5085(99)70195-2. [DOI] [PubMed] [Google Scholar]
- 41.Di Palma L, Coletta M, Tomba C, Forzenigo LV, Biondetti P, Basilisco G. Magnetic resonance imaging of rectal volume in patients with irritable bowel syndrome. Digestive & Liver Disease. 2011;43:529–534. doi: 10.1016/j.dld.2011.01.004. [DOI] [PubMed] [Google Scholar]