Abstract
Social engagement is associated with healthy aging and preserved cognition. Two dimensions of engagement, verbal interactions and perceived support, likely impact cognition via distinct mechanistic pathways. We explored the cognitive benefit of each construct among enrollees (N=1,052, mean age=60.2 years) in the Wisconsin Registry for Alzheimer’s Prevention (WRAP) study, who provide neuropsychological and sociobehavioral data at two-year intervals. Outcomes included six cognitive factor scores representing key domains of executive function and memory. Key predictors included self-reported perceived social support and weekly verbal interaction. Results indicated that after adjusting for lifestyle covariates, social support was positively associated with Speed and Flexibility, and that verbal interactions were associated with Verbal Learning and Memory. These findings suggest that support, which may buffer stress, and verbal interaction, an accessible, aging-friendly form of environmental enrichment, are uniquely beneficial. Both are integral in the design of clinical and community interventions and programs that promote successful aging.
Keywords: Social networks, social activity, environmental enrichment, cognition
Introduction
Increasing prevalence of age-related cognitive dysfunction creates societal and individual burden; accordingly, much research has focused on risk factors for Alzheimer’s disease (AD) and cognitive aging. However, consensus on truly modifiable risk or protective factors is elusive and there are still gaps in our knowledge, particularly concerning sociobehavioral ameliorative factors such as social engagement. Social engagement across the lifespan may be associated with better cognitive function and reduced risk for AD (Barnes, Mendes de Leon, Wilson, Bienias, & Evans, 2004; Bennett, Schneider, Tang, Arnold, & Wilson, 2006; Glei et al., 2005; James, Wilson, Barnes, & Bennett, 2011; Kuiper et al., 2015; Kuiper et al., 2016; Lee et al., 2007; Lee et al., 2013; Litwin & Stoeckel, 2016; Seeman, Lusignolo, Albert, & Berkman, 2001; Tomioka, Kurumatani, & Hosoi, 2016; Wang et al., 2013), but the mechanisms underlying this relationship are not well documented. Here, we consider two dimensions of the engagement construct, social support and verbal interaction; both may, through distinct pathways, protect cognitive health. Some studies of social networks and participation have explained the relationship through models of stress and coping. According to this psychologically and biologically plausible explanation, social support provides a “buffer” for stressful events (Aslund, Larm, Starrin, & Nilsson, 2014; Cohen & Wills, 1985; Paykel, 1994; Schoevers et al., 2000) and mitigates cognitively detrimental effects of stress such as inflammation (Kiecolt-Glaser et al., 2005; Kiecolt-Glaser et al., 2003; Lutgendorf et al., 1999; Miller, Rohleder, & Cole, 2009; Rohleder, 2014; Steptoe et al., 2003) and depressive symptomatology (Dautovich, Dzierzewski, & Gum, 2014; Dean, Kolody, & Wood, 1990; Glass, Kasl, & Berkman, 1997; Kahana, Kelley-Moore, & Kahana, 2012; Virtanen et al., 2015). Others have suggested an additional role for social interactions in stimulating brain networks (Barnes et al., 2004; Bassuk, Glass, & Berkman, 1999; Bennett et al., 2006). If this is true, conversational stimulation as a form of environmental enrichment benefits cognitive health via mechanisms, such as neurogenesis, that are conceptually and biologically distinct from stress-and-coping processes. However, few studies have attempted to methodologically explore this enrichment pathway (Hultsch, Hertzog, Small, & Dixon, 1999; James et al., 2012; Seeman et al., 2011; Ybarra et al., 2008; Ybarra & Winkielman, 2012). We focus here on subclinical levels of cognitive dysfunction during midlife and older age. Such research is relevant and useful in considering both healthy and ailing older populations, as maintenance and protection of cognitive ability at any age is desirable. Self-reported quality of life is higher if cognitive abilities remain intact (Missotten et al., 2008; Pan et al., 2015; Teng, Tassniyom, & Lu, 2012). Further, neuropathological processes responsible for eventual clinical presentation of AD are implicated in milder cognitive changes across the lifespan (Doherty et al., 2015; Salthouse, 2009). Prevention or delay of sublinical changes, therefore, is likely to delay AD onset as well.
Using data from the Wisconsin Registry for Alzheimer’s Prevention (WRAP), we examined relationships between perceived social support, quantity of verbal interaction, and cognition. We hypothesized, based on theorized benefits of stress buffering and environmental enrichment, that greater levels of both perceived support and reported verbal interaction would be independently associated with better cognitive performance.
Design and Methods
Participants
Data were drawn from WRAP, a longitudinal study of cognitive function in adults enriched for a parental history of AD (Sager, Hermann, & La Rue, 2005). The original WRAP study design is described in detail elsewhere (La Rue et al., 2008). Briefly, the study is comprised of two subsamples: a parental history-positive and a parental history-negative group. Participants in the parental history-positive group, representing two-thirds of the total WRAP sample, have at least one biological parent with dementia due to AD. This was determined by review of medical records of the parent, including in some cases autopsy records, or by administering a dementia questionnaire to the adult child. Many in WRAP were recruited while accompanying a parent to an evaluative visit in a University of Wisconsin-Madison or satellite Memory Assessment Clinic. Others, including most parental history-negative participants, learned of the study via statewide educational presentations or word of mouth. The parental history-negative group consists of persons who do not have a first degree relative diagnosed with AD; to be eligible, they must have a mother who survived to age 75 and a father who survived to age 70. Most participants were between the age of 40 and 65 at enrollment, are English-speaking, and were cognitively intact at that time as determined by their scores on neuropsychological testing at the first study visit. Baseline enrollment began in late 2001 and is ongoing, with recent efforts targeted toward increasing the ethnic diversity of the sample. Participants come in approximately every two years for a full study visit. Due to the rolling enrollment design of WRAP, the number of evaluations (and accompanying data) for each participant varies depending on when they enrolled. The WRAP protocol for Visit 1 does not include social data; social engagement and other lifestyle questionnaires are currently completed by participants at Visit 2 and all subsequent visits. A novel verbal interaction questionnaire (Zuelsdorff et al., 2016) was introduced as a complement to the WRAP lifestyle measures in 2010.
Data for the current study thus came from Visits 2, 3, and 4, and only participants with complete social support and verbal interaction data from at least one of those visits were included in the analysis (N=1,052). Participants with a history of conditions that may influence cognitive function such as multiple sclerosis, Parkinson’s disease, stroke, epilepsy, or meningitis were excluded. Participants who were determined by clinical consensus to have met diagnostic criteria for Mild Cognitive Impairment, AD, or another dementia at any follow-up WRAP study visit were also excluded. This study was conducted with the approval of the University of Wisconsin Health Sciences Institutional Review Board and all participants provided signed informed consent prior to enrollment.
Measures
WRAP visits are approximately three hours in duration. At each visit, participants complete questionnaires on health history, psychosocial and sociobehavioral factors, and lifestyle; a nurse collects a blood sample and clinical data including height, weight, and blood pressure; and a trained psychometrist administers a comprehensive battery of commonly used clinical neuropsychological tests, described in detail below. Additional details on the WRAP study protocol and sample characteristics are available in a recent WRAP publication (Clark et al., 2016).
Neuropsychological assessment
Key outcome variables included six cognitive factor scores from each WRAP visit, determined previously (Dowling, Hermann, La Rue, & Sager, 2010; Koscik et al., 2014) based on WRAP data. Briefly, factor analysis using promax rotation and maximum likelihood estimation (Grice, 2001) was used to reduce the set of cognitive measures to a smaller number of factors and obtain weights used to combine the measures within each factor. The resulting weighted factor scores were then standardized [~N (0,1)] into z-scores, using means and standard deviations obtained from the whole baseline sample. The six cognitive factors represent domains of episodic memory and executive function. These cognitive factors were Verbal Learning & Memory, Immediate Memory, Visual Learning & Memory, Story Recall, Speed & Flexibility, and Working Memory. Verbal Learning & Memory was derived from the Rey Auditory Verbal Learning Test (RAVLT), specifically RAVLT Learning Trials 3–5 and the RAVLT Delayed Recall Trial (Schmidt, 1996). Immediate Memory was also derived from the RAVLT, specifically from RAVLT Learning Trials 1–2. Visual Learning & Memory was derived from the three learning trials and delayed recall trial of the Brief Visuospatial Memory Test – Revised (Benedict, 1997). Story Recall is derived from the Logical Memory immediate and delayed recall subtests of the Weschler Memory Scale – Revised (Weschler, 1987). Speed & Flexibility is derived from time to completion on the Trailmaking Test A & B (Heaton, Miller, Taylor, & Grant, 2004), and number of items completed on the Stroop Neuropsychological Screening Test Color-Word Interference condition (Trenerry, Crosson, Deboe, & Leber, 1989). Working Memory is derived from number of correct items on the Digit Span Forward, Digit Span Backward, and Letter-Number Sequencing subtests of the Wechsler Adult Intelligence Scale-III (Wechsler, 1997).
Social support
Perceived social support was assessed via nine items taken from the Medical Outcomes Survey (Sherbourne & Stewart, 1991). Participants were asked how often different kinds of support were available to them if they needed it (e.g., “Someone you can count on to listen to you when you need to talk”). Response options ranged from 0 (none of the time) to 4 (all of the time) (Sherbourne & Stewart, 1991). Responses from all nine items were summed to create a support index score (possible range, 0–36). Prior to analyses, the support index score was standardized across all data points [~N (0, 1)].
Verbal interaction
A novel verbal interaction questionnaire was implemented in 2010 in order to explore our dual-pathways hypothesis. Reliability of the instrument is substantial, with weighted kappa values ranging from 0.49 to 0.79 (Zuelsdorff et al., 2016). Participants reported quantity of verbal interaction in seven distinct social domains (spouse/partner, other family, friends, colleagues, club/hobbies, religious meeting attendance, and interactions with strangers) and one “other” inquiry designed to capture interactions that were not included in a previous domain. Responses, based on time per day or time per week depending on the domain in question, were on a six-point scale and ranged from a “none” response (e.g., “I don’t have/talk with other family members”) to four different time ranges between “Less than 30 minutes” and “Over two hours” (with write-in capability). To simplify analyses, assess the quantity of verbal interaction as a whole, and account for the possibility of substitution or tradeoff (for example, a decrease in time spent interacting with friends if time spent interacting with colleagues increased), a summed total time index score, using either midpoint of time range in a given response or written-in quantity, was created to represent the average number of minutes per week spent verbally interacting with others. The total time index score was standardized across all data points [~N (0, 1)].
In order to analytically distinguish the benefits of affect-neutral stimulation arising from conversation-related brain processes from the potential stress-mitigating or stress-generating effect of positive or negative social exchanges, a valence question inquiring on the overall quality of the interactions in question was included for each social domain (“How pleasant or unpleasant do you find these interactions to be most of the time?”). Response options for each ranged from −2 (very unpleasant) to 2 (very pleasant). Responses were weighted by quantity of interaction in the given domain and summed to create an important covariate, our overall quality of interaction index score.
APOE genotyping
Because the presence of the APOE ε4 allele is one of the most well-established risk factors for AD and early cognitive decline (Scarabino, Gambina, Broggio, Pelliccia, & Corbo, 2016), and prevalence of the risk allele in our family history-enriched sample is high relative to the population as a whole, ε4 carrier status is included as a key covariate in all analyses. Genotyping for the two APOE single nucleotide polymorphisms that determine the ε2, ε3, and ε4 alleles, rs429358 and rs7412, was done previously by WRAP and has been described in detail (Johnson et al., 2011).
Other potential covariates
Several health and lifestyle covariates were considered. Participants were coded as never, ever, or current smokers and as abstinent, moderate, or heavy drinkers (abstinent=0 drinks in the past month, moderate=<1 or 1–2 drinks per day, heavy=3–5 or ≥6 drinks per day). Caffeine consumption was coded dichotomously; participants were categorized as heavy or not-heavy users of caffeine (heavy caffeine use = 3 or more caffeinated beverages per day). Height and weight were measured and body mass index (BMI) was calculated (kg/m2). Self-reported physical activity was converted to metabolic equivalent of task (MET) hours per week. Partner status was dichotomous, with those reporting having a spousal or partner relationship considered partnered regardless of legal marital status.
Statistical analysis
Included participants had between one and three visits’ worth of data available for analysis. Although there were too few participants with social data spanning three timepoints (N=3) to make a longitudinal analysis of cognitive change over time possible, a significant portion of participants (N=410) did have data from two observations available, and in order to make use of that data, we conducted all analyses using mixed effects models in SAS, version 9.2 for Windows. To account for within-subject and within-sibling group correlations, random intercepts for participant and family were included in all models.
A base model was chosen based on established demographic and genetic risk factors. Health and lifestyle covariates were retained if they changed the sociobehavioral predictor-cognitive outcome relationship by ≥10%, independently improved the fit of the predictor-outcome regression model as measured by Akaike information criterion (AIC), or demonstrated a significant association at the p<.05 level with at least two cognitive outcome variables. Based on these criteria, BMI, alcohol consumption, and caffeine use were dropped. Ultimately, our six cognitive factor scores were regressed on our key predictor variables, social support and quantity of verbal interaction, in a set of nested models: (1) a base model controlling for age, gender, race, education, APOE ε4 carrier status, parental history of AD, and WRAP clinic site; (2) a model that added two lifestyle factors (smoking history and physical activity); and (3) a model that added an important social confounder, partner status. In the assessment of social support, set (3) represented our fullest model. In final assessments of verbal interaction quantity, however, we added (4) a model that included our valence control variable, quality of interaction. In consideration of possible non-linear relationships between key predictor variables and cognitive outcomes, contribution of squared terms for each sociobehavioral predictor was also assessed.
Potential multicollinearity was assessed with Pearson correlation analyses (not shown) of all variables included in the fullest models; further, tolerance values indicated that collinearity was not a concern.
Results
Participant characteristics from their earliest complete visit (representing a “baseline” for this cohort) are presented in Table 1. Participants ranged in age from 40 to 78 years old at the first included visit and a majority was female and partnered. Education levels for the sample were high; nearly two-thirds had completed a bachelor’s degree and over 40% had at least some postcollege education. Prestandardization social support scores were high, with a mean of 28.9 out of 36 possible points. Quantity of interaction data was winsorized at the 99th percentile (81.3 hours per week of face-to-face verbal interaction) to account for improbability of self-reported quantities above that value. In Table 2, we present the adjusted associations of health and lifestyle covariates, as well as our quality of interaction control variable, with all six outcome measures; these models do not include our key social predictor variables. When models incorporating social support and total weekly quantity of verbal interaction were fitted, a squared term for verbal interaction showed a statistically significant association with at least one cognitive factor score. To ease interpretation and more precisely identify what levels of interaction were significantly related to performance, we divided quantity of interaction into quartiles and created indicator variables representing the following levels of weekly verbal interaction: Low (<9 hours/week), Moderate (9–15 hours/week), High (15–25 hours/week), and Very High (>25 hours/week). The “Low” group was used as a reference group in models incorporating these verbal interaction indicator variables. In Table 3, we present the coefficients for social support index scores as well as moderate, high, and very high quartiles of verbal interaction in four nested models.
Table 1.
Descriptive statistics for study participants (N =1052) at first sociobehavioral assessment.
| Variable | Percent or M (SD) | Range |
|---|---|---|
| Age, years | 60.2 (6.7) | 40–78 |
| Site | ||
| Madison | 72% | |
| La Crosse | 22% | |
| Milwaukee | 6% | |
| Gender, female | 69% | |
| Race, non-white | 5% | |
| Parental history of AD | 72% | |
| Education | ||
| High school/GED | 9% | |
| Some college | 28% | |
| College graduate | 20% | |
| Postcollege | 43% | |
| BMI, kg/m2 | 29.0 (6.3) | 17.1–57.6 |
| APOE ε4 carrier | 39% | |
| Smoking | ||
| Never | 57% | |
| Past | 38% | |
| Current | 5% | |
| Alcohol use | ||
| Abstinent | 20% | |
| Moderate | 68% | |
| Heavy | 12% | |
| Caffeine consumption, heavy | 32% | |
| Physical activity, MET hrs/wk | 17.7 (15.0) | 0–81.3 |
| Partner, yes | 77% | |
| Support index | 28.9 (6.8) | 0–36 |
| Quantity of interaction, hrs/wk* | 19.7 (15.6) | 0.25–81.3 |
| Speed & Flexibility | 0.05 (1.04) | −4.17–3.59 |
| Immediate Memory | −0.04 (1.10) | −3.40–3.31 |
| Working Memory | 0.08 (1.01) | −2.54–3.30 |
| Verbal Learning & Memory | 0.02 (1.06) | −3.56–1.83 |
| Visual Learning & Memory | 0.03 (1.03) | −2.59–2.07 |
| Story Recall | −0.08 (1.00) | −3.02–2.70 |
Reported quantity of interaction was winsorized at the 99th %.
Table 2.
Adjusted regression coefficients ± standard errors (p-values) for covariates by cognitive domain.
| Covariates | Verbal Learning & Memory | Immediate Memory | Visual Learning & Memory | Story Recall | Speed & Flexibility | Working Memory |
|---|---|---|---|---|---|---|
|
| ||||||
| β (se) | β (se) | β (se) | β (se) | β (se) | β (se) | |
| Age | −0.03±0.004 (<.001) | −0.03±0.005 (<.001) | −0.03±0.005 (<.001) | −0.02±0.004 (<.001) | −0.07±0.004 (<.001) | −0.02±0.004 (<.001) |
| Female | 0.75±0.06 (<.001) | 0.64±0.07 (<.001) | 0.29±0.06 (<.001) | 0.26±0.06 (<.001) | 0.33±0.06 (<.001) | −0.02±0.06 (.71) |
| Non-white | −0.21±0.19 (.27) | −0.13±0.20 (.52) | −0.05±0.19 (.79) | −0.21±0.19 (.27) | −0.49±0.18 (.007) | −0.35±0.19 (.07) |
| Education | 0.17±0.03 (<.001) | 0.10±0.03 (<.001) | 0.14±0.03 (<.001) | 0.20±0.03 (<.001) | 0.08±0.03 (.006) | 0.19±0.03 (<.001) |
| APOE ε4 carrier | −0.05±0.06 (.45) | −0.06±0.07 (.35) | −0.10±0.06 (.14) | −0.11±0.06 (.09) | −0.12±0.07 (.05) | −0.08±0.06 (.20) |
| Parental history of AD | −0.04±0.07 (.54) | −0.05±0.07 (.53) | 0.04±0.07 (.57) | −0.10±0.07 (.17) | 0.07±0.07 (.29) | −0.03±0.07 (.70) |
| Site | ||||||
| Madison | (ref) | (ref) | (ref) | (ref) | (ref) | (ref) |
| La Crosse | −0.09±0.08 (.24) | 0.03±0.08 (.67) | −0.09±0.08 (.27) | −0.16±0.08 (.04) | −0.11±0.07 (.12) | −0.07±0.08 (.39) |
| Milwaukee | −0.05±0.18 (.79) | 0.04±0.19 (.84) | −0.47±0.18 (.01) | −0.19±0.18 (.29) | −0.60±0.17 (<.001) | −0.32±0.18 (.08) |
| METs (10 hr/week) | −0.02±0.02 (.21) | 0.002±0.02 (.93) | −0.04±0.02 (.05) | −0.01±0.02 (.63) | 0.003±0.02 (.83) | −0.01±0.02 (.67) |
| Smoking status | ||||||
| Never | (ref) | (ref) | (ref) | (ref) | (ref) | |
| Past | −0.01±0.06 (.84) | −0.08±0.06 (.24) | −0.13±0.06 (.03) | 0.07±0.06 (.24) | −0.009±0.06 (.88) | 0.13±0.06 (.04) |
| Current | −0.30±0.13 (.02) | −0.42±0.14 (.003) | −0.23±0.13 (.08) | −0.25±0.14 (.07) | −0.30±0.12 (.01) | 0.07±0.13 (.61) |
| Partner | 0.27±0.07 (<.001) | 0.33±0.07 (<.001) | 0.24±0.07 (<.001) | 0.07±0.07 (.30) | 0.16±0.06 (.01) | 0.15±0.07 (.03) |
| Quality of interaction | −0.003±0.02 (.89) | −0.03±0.03 (.32) | −0.01±0.03 (.64) | −0.03±0.03 (.18) | 0.06±0.02 (.008) | −0.03±0.02 (.16) |
Note: Table S1 models include all covariates simultaneously but do not include key sociobehavioral predictor variables.
Table 3.
Regression coefficients ± standard errors (p-values) for social support and verbal interaction quantity by model and cognitive domain
| Verbal Learning & Memory | Immediate memory | Visual Learning & Memory | Story Recall | Speed & Flexibility | Working Memory | |
|---|---|---|---|---|---|---|
| Model 1:Base demographic variables | ||||||
|
| ||||||
| Social support index | 0.05±0.03 (.06) | 0.07±0.03 (.01) | 0.04±0.03 (.12) | 0.04±0.03 (.15) | 0.09±0.02 (<.001) | 0.05±0.02 (.06) |
| Verbal Interaction | ||||||
| Moderate | 0.10±0.06 (.10) | 0.06±0.07 (.41) | 0.13±0.07 (.06) | 0.03±0.07 (.63) | 0.07±0.06 (.19) | 0.002±0.06 (.98) |
| High | 0.14±0.06 (.02) | 0.13±0.08 (.09) | 0.02±0.07 (.79) | −0.04±0.07 (.52) | 0.12±0.06 (.04) | −0.09±0.06 (.15) |
| Very high | 0.08±0.06 (.22) | 0.01±0.08 (.87) | −0.01±0.07 (.93) | −0.09±0.07 (.23) | 0.08±0.06 (.20) | −0.07±0.06 (.30) |
|
| ||||||
| Model 2: Base + Lifestyle variables | ||||||
|
| ||||||
| Social support index | 0.04±0.03 (.14) | 0.06±0.03 (.04) | 0.03±0.03 (.24) | 0.03±0.03 (.23) | 0.08±0.02 (<.001) | 0.04±0.03 (.13) |
| Verbal Interaction | ||||||
| Moderate | 0.10±0.06 (.10) | 0.06±0.07 (.44) | 0.13±0.07 (.06) | 0.03±0.07 (.63) | 0.08±0.06 (.15) | −0.002±0.06 (.98) |
| High | 0.15±0.06 (.02) | 0.14±0.08 (.06) | 0.04±0.07 (.59) | −0.05±0.07 (.51) | 0.14±0.06 (.02) | −0.09±0.06 (.13) |
| Very high | 0.10±0.07 (.15) | 0.03±0.08 (.73) | 0.01±0.07 (.93) | −0.09±0.07 (.21) | 0.09±0.06 (.14) | −0.07±0.06 (.31) |
|
| ||||||
| Model 3: Base + Lifestyle + Partner status | ||||||
|
| ||||||
| Social support index | 0.02±0.03 (.50) | 0.03±0.03 (.29) | 0.01±0.03 (.72) | 0.03±0.03 (.31) | 0.07±0.03 (.008) | 0.03±0.03 (.33) |
| Verbal Interaction | ||||||
| Moderate | 0.07±0.06 (.22) | 0.02±0.07 (.82) | 0.10±0.07 (.17) | 0.03±0.07 (.70) | 0.07±0.06 (.22) | −0.02±0.06 (.77) |
| High | 0.12±0.07 (.06) | 0.10±0.08 (.19) | 0.01±0.07 (.94) | −0.05±0.07 (.46) | 0.12±0.06 (.04) | −0.12±0.06 (.08) |
| Very high | 0.07±0.07 (.31) | −0.01±0.08 (.92) | −0.02±0.08 (.75) | −0.10±0.07 (.18) | 0.08±0.06 (.19) | −0.08±0.07 (.21) |
|
| ||||||
| Model 4: Base + Lifestyle + Partner + Quality of Interaction | ||||||
|
| ||||||
| Social support index | N/A | N/A | N/A | N/A | N/A | N/A |
| Verbal Interaction | ||||||
| Moderate | 0.09±0.06 (.15) | 0.04±0.07 (.21) | 0.11±0.07 (.12) | 0.03±0.07 (.61) | 0.05±0.06 (.40) | −0.01±0.06 (.84) |
| High | 0.16±0.07 (.02) | 0.16±0.08 (.05) | 0.02±0.08 (.79) | −0.05±0.07 (.49) | 0.08±0.06 (.22) | −0.09±0.07 (.15) |
| Very high | 0.12±0.09 (.20) | 0.09±0.11 (.37) | −0.03±0.10 (.75) | −0.10±0.10 (.29) | −0.03±0.08 (.68) | −0.06±0.09 (.52) |
Note: Model 1 adjusts for age, gender, race, education, APOE ε4 carrier status, parental history of AD, and WRAP clinic site. Model 2 additionally adjusts for physical activity and smoking.
In a base model adjusting for key demographic and sampling characteristics only, higher social support index score was associated with higher Speed & Flexibility and Immediate Memory scores, β=0.09, p<0.001, and β=0.07, p=0.01, respectively; these relationships were attenuated but remained significant when two lifestyle factors, smoking status and physical activity, were added to the base model. While the relationship with Immediate Memory was attenuated to the point of non-significance when partner status was included, the relationship between social support and Speed & Flexibility remained significant, β=0.07, p=0.01.
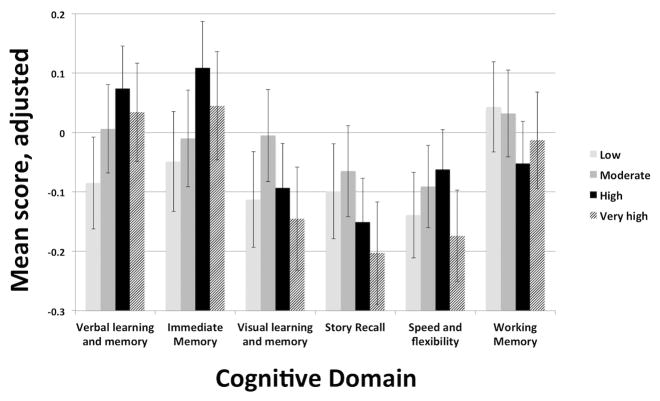
Verbal interaction showed positive associations with both Speed & Flexibility and Verbal Learning & Memory in our base model, but these associations were seen only in a sub-range of reported quantity of interaction. The “high” quantity of interaction quartile, representing 15–25 hours of reported interaction per week, was the only quartile to demonstrate a relationship in both Speed & Flexibility, β=0.12, p=0.04, and Verbal Learning & Memory, β=0.14, p=0.02, domains. The relationship between high quantity of interaction and SF became non-significant when quality of interactions was controlled for, while the relationship between high quantity of interaction and Verbal Learning & Memory remained significant, β=0.16, p=0.02. A relationship between high quantity of interaction and Immediate Memory approached statistical significance, β=0.16, p=0.05; in fact, a non-significant trend wherein cognitive test performance peaked at moderate or high levels of verbal interaction, but declined at very high levels was seen across several cognitive domains (Figure 1).
Figure 1. Cognitive factor scores by verbal interaction quartile.
Note: Cognitive factor scores are adjusted for age, gender, race, education, APOE ε4 carrier status, parental history of AD, WRAP clinic site, physical activity, smoking, partner status, perceived social support, and quality of interaction
Discussion
In this study, we examined the role of two dimensions of social activity, perceived social support and quantity of face-to-face verbal interaction, in cognitive test performance among a population of middle-aged and older adults at risk for AD. There was some support for our hypothesis that each social dimension would be positively associated with cognitive function independent of benefit from the other. In fact, social support and verbal interaction showed positive associations with distinct cognitive domains: Speed & Flexibility and Verbal Learning & Memory, respectively. Interestingly, the relationship between verbal interaction and Verbal Learning & Memory was quadratic rather than linear, with a diminishing of returns as reported interaction rose to very high levels.
The WRAP research group has reported a preliminary cross-sectional association between perceived availability of social support and SF in a subsample of parental history-positive WRAP participants (Zuelsdorff et al., 2013). The current study incorporates a much larger sample including persons both with and without a parental history of AD, and utilizes data from repeated visits for a large portion of participants. Crucially, this analysis replicates and expands upon the previous finding with the incorporation of novel verbal interaction data, including a quality-of-interaction covariate designed to control for positive versus negative valence of verbal interactions in each social domain. Our nested models demonstrate the importance of accounting for partner status in the assessment of social support and cognitive benefit: attenuation of coefficients was seen in five of six cognitive domains with the inclusion of the variable. Of the two cognitive domains showing a significant association with social support in a model including demographics and lifestyle factors, Immediate Memory showed attenuation to the point of non-significance after adjusting for partner status.
The observed association between social support and Speed & Flexibility, a key component of executive function, is consistent with previous studies that specifically measured a support construct. Positive associations with global function (Gow, Mortensen, & Avlund; Pillemer & Holtzer, 2016; Seeman et al., 2001) and with executive function or processing speed in particular have been seen in populations of differing age, ethnicity, and nationality (Dickinson, Potter, Hybels, McQuoid, & Steffens, 2011; Gow et al.; Seeman et al., 2011; Sims, Levy, Mwendwa, Callender, & Campbell, 2011). Conversely, social isolation and social conflict have been associated with diminished executive function (Liao et al., 2014; Seeman et al., 2011), and depression potentially mediates such relationships (Gow et al.). Within this context, our findings provide additional evidence for the hypothesis that social support contributes to cognitive function via pathways of stress, buffering, and positive or negative affect.
Our original research question and motivation for measuring verbal interaction as directly as possible was driven by earlier social engagement research suggesting a separate beneficial role for conversation-related stimulation: observational studies of frequency of social contact (James et al., 2012; Seeman et al., 2011; Ybarra et al., 2008) and experimental studies showing immediate cognitive performance increases following social engagement with peers (Ybarra et al., 2008). Meta-analyses of previous work on social engagement and cognitive aging affirm that “structural” components of engagement including network size and participation frequency operate as lifestyle-based determinants of cognitive health trajectories (Kuiper et al., 2015; Kuiper et al., 2016). Finally, very recent study findings have provided additional evidence. In a study characterizing independent and joint benefits of social resources and all-type activity participation for later-life cognition, specifically for word recall, investigators concluded not only that each was cognitively beneficial, but that the social component of activity participation stood out as yielding the strongest positive influence (Litwin & Stoeckel, 2016). And, recent WRAP findings provide neuroimaging evidence for social interaction as environmental enrichment: in a study of occupational complexity and cognitive reserve, more complex work with people, but not with data or things, protected cognition in the face of hippocampal and whole brain atrophy (Boots et al., 2015).
Our discovery that the relationship between quantity of verbal interaction and cognitive test performance was parabolic rather than linear in shape, with lower outcome scores at the very low and very high ends of the interaction range, was borne out in greater detail with the quartile-based indicator variables. There are a few potential explanations for diminished test performance seen among those reporting the highest quantity of interpersonal interactions. First, very extensive interaction may introduce cognitive or emotional demand that outweighs the benefits seen for lower levels. Recent research exploring potential cognitively stimulating effects of grandparenting found that the relationship between days of childcare and cognitive function is parabolic, suggesting that beneficial engagement may reach a critical threshold, with additional activity representing a cognitively detrimental stressor (Burn & Szoeke, 2015; Burn, Henderson, Ames, Dennerstein, & Szoeke, 2014). Reverse causality is another possibility, with people experiencing declines in cognition possibly spending increased time interacting with family members or other caregivers. Alternately, there may be unmeasured confounders in the group reporting very high levels of interaction. Comparing the highest quartile (“very high” interacters) to the third quartile (“high” interacters) revealed several modest differences between the two groups (data not shown). Those in the highest quartile were not only younger, but also more likely to be non-white, and less likely to have a parental history of AD than those in the third quartile (High). The education levels also varied between the two upper quartiles; in the Very High group, fewer participants reported having at least a college degree. While these factors were included as covariates in analytical models, and do not point irrefutably toward obvious additional confounding, the differences do raise the possibility that the Very High group is likely to be unique in other ways. For example, unmeasured confounders such as socioeconomic disadvantage could represent competing risk factors that mitigate the cognitive benefits of interaction. Finally, lower test scores at the highest levels of reported interaction could be due to differential misclassification, whereby a subgroup of individuals who have trouble performing on cognitive tests also have difficulty accurately and plausibly reporting the time spent in face-to-face interactions each week, perhaps due to unfamiliarity with, or anxiety related to, the research environment. This explanation seems especially worthy of consideration given the arguable implausibility of the upper range of reported time spent interacting with others.
Once all covariates were included, significant verbal interaction relationships were only seen in Verbal Learning & Memory, though the relationship with Immediate Memory also approached significance. Verbal Learning & Memory and Immediate Memory scores are determined by performance on a test of word recall (Lezak, Howieson, Loring, Hannay, & Fischer, 2004), and in that sense we echo others’ findings (Litwin & Stoeckel, 2016). Interestingly, the relationship between verbal interaction and Speed & Flexibility was significant until our quality of interaction covariate was introduced to the model. While many more complex interactions are likely to require and promote executive functions (Ybarra et al., 2008; Ybarra & Winkielman, 2012), we believe this attenuation reaffirms the importance of relationship quality, stress, and affect in this domain of cognition. The null findings for other cognitive domains are difficult to fully explain, though there is evidence that some of those domains are quite strongly influenced by genetic risk factors (Darst et al., 2015).
In addition to the uncertainty regarding the non-linear relationship between our verbal interaction predictor and Verbal Learning & Memory, there are a few limitations related to this study. First, though repeated measures were available for approximately 40% of our study sample, additional within-subject data points are needed before we can assess the potentially bidirectional relationship between sociobehavioral factors and cognitive aging, and explore the impact of these factors on rates of change over time. Second, the WRAP sample may not be representative of the broader aging population: women and the highly educated are overrepresented, the sample was mostly white, and while all participants were cognitively intact at baseline, WRAP by design oversamples for genetic vulnerability to earlier cognitive decline. Finally, the available measure of perceived social support cannot be conflated with amount of support actually received (Haber, Cohen, Lucas, & Baltes, 2007). Though our conceptual modeling for stress and coping processes places support as a moderator of stress and stress-related mood changes, our analytical models do not account for the potential role that stressful contexts and negative affect have been shown to play in the perception, and reporting, of available support (Cohen, Towbes, & Flocco, 1988).
Nonetheless, we believe this to be a strong, unique contribution to a nascent body of research establishing the pathways between social engagement and cognition. We hope that our findings will additionally be of interest to physicians guiding older patients in the “healthy aging” quest. While patients and their caregivers are often instructed in the benefit of staying socially engaged and cognitively active, traditional examples of such activities – forging new social relationships, joining groups or activities, puzzles or word games – can be unappealing, intimidating, or simply impossible for many adults. The addition of “everyday” activities such as casual conversation to the roster of beneficial activities may be perceived as more accessible, and offer more options to patients of varying personalities, abilities, and resources.
Acknowledgments
This work was supported by the University of Wisconsin – Madison Graduate School, by a Center for Demography of Health and Aging training grant (T32AG00129) from the National Institute on Aging, and in part by a core grant to the Center for Demography and Ecology at the University of Wisconsin-Madison [grant number P2C HD047873]. The WRAP program is funded by the Helen Bader Foundation, Northwestern Mutual Foundation, Extendicare Foundation, Clinical and Translational Science Award (CTSA) program through the NIH National Center for Advancing Translational Sciences (NCATS) [grant number UL1-TR000427], and a National Institute on Aging grant [grant number 5R01-AG27161-2, Wisconsin Registry for Alzheimer’s Prevention: Biomarkers of Preclinical AD].
References
- Aslund C, Larm P, Starrin B, Nilsson KW. The buffering effect of tangible social support on financial stress: Influence on psychological well-being and psychosomatic symptoms in a large sample of the adult general population. International Journal of Equity in Health. 2014;13(1):85. doi: 10.1186/s12939-014-0085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes LL, Mendes de Leon CF, Wilson RS, Bienias JL, Evans DA. Social resources and cognitive decline in a population of older African Americans and whites. Neurology. 2004;63(12):2322–2326. doi: 10.1212/01.wnl.0000147473.04043.b3. [DOI] [PubMed] [Google Scholar]
- Bassuk SS, Glass TA, Berkman LF. Social disengagement and incident cognitive decline in community-dwelling elderly persons. Annals of Internal Medicine. 1999;131(3):165–173. doi: 10.7326/0003-4819-131-3-199908030-00002. [DOI] [PubMed] [Google Scholar]
- Benedict RH. Brief visuospatial memory test - revised. Odessa, FL: Psychological Assessment Resources; 1997. [Google Scholar]
- Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS. The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: A longitudinal cohort study. Lancet Neurology. 2006;5(5):406–412. doi: 10.1016/S1474-4422(06)70417-3. [DOI] [PubMed] [Google Scholar]
- Boots EA, Schultz SA, Almeida RP, Oh JM, Koscik RL, Dowling MN, … Okonkwo OC. Occupational complexity and cognitive reserve in a middle-aged cohort at risk for Alzheimer’s disease. Archives of Clinical Neuropsychology. 2015;30(7):634–642. doi: 10.1093/arclin/acv041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burn K, Szoeke C. Grandparenting predicts late-life cognition: Results from the Women’s Healthy Ageing Project. Maturitas. 2015;81(2):317–322. doi: 10.1016/j.maturitas.2015.03.013. [DOI] [PubMed] [Google Scholar]
- Burn KF, Henderson VW, Ames D, Dennerstein L, Szoeke C. Role of grandparenting in postmenopausal women’s cognitive health: Results from the Women’s Healthy Aging Project. Menopause. 2014;21(10):1069–1074. doi: 10.1097/GME.0000000000000236. [DOI] [PubMed] [Google Scholar]
- Clark LR, Koscik RL, Nicholas CR, Okonkwo OC, Engelman CD, Bratzke LC, … Johnson SC. Mild cognitive impairment in late middle age in the Wisconsin Registry for Alzheimer’s Prevention Study: Prevalence and characteristics using robust and standard neuropsychological normative data. Archives of Clinical Neuropsychology. 2016 doi: 10.1093/arclin/acw024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LH, Towbes LC, Flocco R. Effects of induced mood on self-reported life events and perceived and received social support. Journal of Personal and Social Psychology. 1988;55(4):669–674. doi: 10.1037//0022-3514.55.4.669. [DOI] [PubMed] [Google Scholar]
- Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychological Bulletin. 1985;98(2):310–357. [PubMed] [Google Scholar]
- Darst BF, Koscik RL, Hermann BP, La Rue A, Sager MA, Johnson SC, Engelman CD. Heritability of cognitive traits among siblings with a parental history of Alzheimer’s disease. Journal of Alzheimer’s Disease. 2015;45(4):1149–1155. doi: 10.3233/JAD-142658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautovich ND, Dzierzewski JM, Gum AM. Older adults display concurrent but not delayed associations between life stressors and depressive symptoms: A microlongitudinal study. American Journal of Geriatric Psychiatry. 2014;22(11):1131–1139. doi: 10.1016/j.jagp.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean A, Kolody B, Wood P. Effects of social support from various sources on depression in elderlypersons. Journal of Health and Social Behavior. 1990;31(2):148–161. [PubMed] [Google Scholar]
- Dickinson WJ, Potter GG, Hybels CF, McQuoid DR, Steffens DC. Change in stress and social support as predictors of cognitive decline in older adults with and without depression. International Journal of Geriatric Psychiatry. 2011;26(12):1267–1274. doi: 10.1002/gps.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty BM, Schultz SA, Oh JM, Koscik RL, Dowling NM, Barnhart TE, … Okonkwo OC. Amyloid burden, cortical thickness, and cognitive function in the Wisconsin Registry for Alzheimer’s Prevention. Alzheimer’s and Dementia. 2015;1(2):160–169. doi: 10.1016/j.dadm.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling NM, Hermann B, La Rue A, Sager MA. Latent structure and factorial invariance of a neuropsychological test battery for the study of preclinical Alzheimer’s disease. Neuropsychology. 2010;24(6):742–756. doi: 10.1037/a0020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass TA, Kasl SV, Berkman LF. Stressful life events and depressive symptoms among the elderly: Evidence from a prospective community study. Journal of Aging and Health. 1997;9(1):70–89. doi: 10.1177/089826439700900104. [DOI] [PubMed] [Google Scholar]
- Glei DA, Landau DA, Goldman N, Chuang YL, Rodriguez G, Weinstein M. Participating in social activities helps preserve cognitive function: an analysis of a longitudinal, population-based study of the elderly. International Journal of Epidemiology. 2005;34(4):864–871. doi: 10.1093/ije/dyi049. [DOI] [PubMed] [Google Scholar]
- Gow AJ, Mortensen EL, Avlund K. Activity participation and cognitive aging from age 50 to 80 in the Glostrup 1914 Cohort. Journal of the American Geriatrics Society. 2012;60(10):1831–1838. doi: 10.1111/j.1532-5415.2012.04168.x. [DOI] [PubMed] [Google Scholar]
- Grice JW. Computing and evaluating factor scores. Psychological Methods. 2001;6(4):430–450. [PubMed] [Google Scholar]
- Haber MG, Cohen JL, Lucas T, Baltes BB. The relationship between self-reported received and perceived social support: A meta-analytic review. American Journal of Community Psychology. 2007;39(1–2):133–144. doi: 10.1007/s10464-007-9100-9. [DOI] [PubMed] [Google Scholar]
- Heaton RK, MIller SW, Taylor MJ, Grant I. Revised comprehensive norms for an expanded Halstead-Reitan battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults. Lutz, FL: Psychological Assessment Resources Inc; 2004. [Google Scholar]
- Hultsch DF, Hertzog C, Small BJ, Dixon RA. Use it or lose it: Engaged lifestyle as a buffer of cognitive decline in aging? Psychology of Aging. 1999;14(2):245–263. doi: 10.1037//0882-7974.14.2.245. [DOI] [PubMed] [Google Scholar]
- James BD, Glass TA, Caffo B, Bobb JF, Davatzikos C, Yousem D, Schwartz BS. Association of social engagement with brain volumes assessed by structural MRI. Journal of Aging Research. 2012;2012:512714. doi: 10.1155/2012/512714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James BD, Wilson RS, Barnes LL, Bennett DA. Late-life social activity and cognitive decline in old age. Journal of the International Neuropsychological Society. 2011;17(6):998–1005. doi: 10.1017/S1355617711000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, La Rue A, Hermann BP, Xu G, Koscik RL, Jonaitis EM, … Sager MA. The effect of TOMM40 poly-T length on gray matter volume and cognition in middle-aged persons with APOE epsilon3/epsilon3 genotype. Alzheimer’s and Dementia. 2011;7(4):456–465. doi: 10.1016/j.jalz.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana E, Kelley-Moore J, Kahana B. Proactive aging: A longitudinal study of stress, resources, agency, and well-being in late life. Aging and Mental Health. 2012;16(4):438–451. doi: 10.1080/13607863.2011.644519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Loving TJ, Stowell JR, Malarkey WB, Lemeshow S, Dickinson SL, Glaser R. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Archives of General Psychiatry. 2005;62(12):1377–1384. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(15):9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koscik RL, La Rue A, Jonaitis EM, Okonkwo OC, Johnson SC, Bendlin BB, … Sager MA. Emergence of mild cognitive impairment in late middle-aged adults in the Wisconsin Registry for Alzheimer’s Prevention. Dementia and Geriatric Cognitive Disorders. 2014;38(1–2):16–30. doi: 10.1159/000355682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper JS, Zuidersma M, Oude Voshaar RC, Zuidema SU, van den Heuvel ER, Stolk RP, Smidt N. Social relationships and risk of dementia: A systematic review and meta-analysis of longitudinal cohort studies. Ageing Research Review. 2015;22:39–57. doi: 10.1016/j.arr.2015.04.006. [DOI] [PubMed] [Google Scholar]
- Kuiper JS, Zuidersma M, Zuidema SU, Burgerhof JG, Stolk RP, Oude Voshaar RC, Smidt N. Social relationships and cognitive decline: A systematic review and meta-analysis of longitudinal cohort studies. International Journal of Epidemiology. 2016;45(4):1169–1206. doi: 10.1093/ije/dyw089. [DOI] [PubMed] [Google Scholar]
- La Rue A, Hermann B, Jones JE, Johnson S, Asthana S, Sager MA. Effect of parental family history of Alzheimer’s disease on serial position profiles. Alzheimer’s and Dementia. 2008;4(4):285–290. doi: 10.1016/j.jalz.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BK, Glass TA, McAtee MJ, Wand GS, Bandeen-Roche K, Bolla KI, Schwartz BS. Associations of salivary cortisol with cognitive function in the Baltimore memory study. Archives of General Psychiatry. 2007;64(7):810–818. doi: 10.1001/archpsyc.64.7.810. [DOI] [PubMed] [Google Scholar]
- Lee T, Lipnicki DM, Crawford JD, Henry JD, Trollor JN, Ames D, … Team OR. Leisure activity, health, and medical correlates of neurocognitive performance among monozygotic twins: The Older Australian Twins Study. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2013 doi: 10.1093/geronb/gbt031. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW, Hannay HJ, Fischer JS. Neuropsychological Assessment. Oxford University Press; USA: 2004. [Google Scholar]
- Liao J, Head J, Kumari M, Stansfeld S, Kivimaki M, Singh-Manoux A, Brunner EJ. Negative aspects of close relationships as risk factors for cognitive aging. American Journal of Epidemiology. 2014;180(11):1118–1125. doi: 10.1093/aje/kwu236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin H, Stoeckel KJ. Social network, activity participation, and cognition: A complex relationship. Research on Aging. 2016;38(1):76–97. doi: 10.1177/0164027515581422. [DOI] [PubMed] [Google Scholar]
- Lutgendorf SK, Garand L, Buckwalter KC, Reimer TT, Hong SY, Lubaroff DM. Life stress, mood disturbance, and elevated interleukin-6 in healthy older women. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 1999;54(9):M434–439. doi: 10.1093/gerona/54.9.m434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Rohleder N, Cole SW. Chronic interpersonal stress predicts activation of pro- and anti-inflammatory signaling pathways 6 months later. Psychosomatic Medicine. 2009;71(1):57–62. doi: 10.1097/PSY.0b013e318190d7de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missotten P, Squelard G, Ylieff M, Di Notte D, Paquay L, De Lepeleire J, Fontaine O. Quality of life in older Belgian people: Comparison between people with dementia, mild cognitive impairment, and controls. International Journal of Geriatric Psychiatry. 2008;23(11):1103–1109. doi: 10.1002/gps.1981. [DOI] [PubMed] [Google Scholar]
- Pan CW, Wang X, Ma Q, Sun HP, Xu Y, Wang P. Cognitive dysfunction and health-related quality of life among older Chinese. Science Reports. 2015;5:17301. doi: 10.1038/srep17301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paykel ES. Life events, social support and depression. Acta Psychiatrica Scandinavica Supplement. 1994;377:50–58. doi: 10.1111/j.1600-0447.1994.tb05803.x. [DOI] [PubMed] [Google Scholar]
- Pillemer SC, Holtzer R. The differential relationships of dimensions of perceived social support with cognitive function among older adults. Aging and Mental Health. 2016;20(7):727–735. doi: 10.1080/13607863.2015.1033683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohleder N. Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosomatic Medicine. 2014;76(3):181–189. doi: 10.1097/PSY.0000000000000049. [DOI] [PubMed] [Google Scholar]
- Sager MA, Hermann B, La Rue A. Middle-aged children of persons with Alzheimer’s disease: APOE genotypes and cognitive function in the Wisconsin Registry for Alzheimer’s Prevention. Journal of Geriatric Psychiatry and Neurology. 2005;18(4):245–249. doi: 10.1177/0891988705281882. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. When does age-related cognitive decline begin? Neurobiology of Aging. 2009;30(4):507–514. doi: 10.1016/j.neurobiolaging.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarabino D, Gambina G, Broggio E, Pelliccia F, Corbo RM. Influence of family history of dementia in the development and progression of late-onset Alzheimer’s disease. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2016;171B(2):250–256. doi: 10.1002/ajmg.b.32399. [DOI] [PubMed] [Google Scholar]
- Schmidt M. Rey Auditory Verbal Learning Test: A Handbook. Los Angeles, CA: Western Psychological Services; 1996. [Google Scholar]
- Schoevers RA, Beekman AT, Deeg DJ, Geerlings MI, Jonker C, Van Tilburg W. Risk factors for depression in later life: Results of a prospective community based study (AMSTEL) Journal of Affective Disorders. 2000;59(2):127–137. doi: 10.1016/s0165-0327(99)00124-x. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Lusignolo TM, Albert M, Berkman L. Social relationships, social support, and patterns of cognitive aging in healthy, high-functioning older adults: MacArthur Studies of Successful Aging. Health Psychology. 2001;20(4):243–255. doi: 10.1037//0278-6133.20.4.243. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Miller-Martinez DM, Stein Merkin S, Lachman ME, Tun PA, Karlamangla AS. Histories of social engagement and adult cognition: Midlife in the U.S. Study. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2011;66B(Supplement 1):i141–i152. doi: 10.1093/geronb/gbq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherbourne CD, Stewart AL. The MOS social support survey. Social Science & Medicine. 1991;32(6):705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- Sims RC, Levy SA, Mwendwa DT, Callender CO, Campbell AL., Jr The influence of functional social support on executive functioning in middle-aged African Americans. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology, and Cognition. 2011;18(4):414–431. doi: 10.1080/13825585.2011.567325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Kunz-Ebrecht S, Owen N, Feldman PJ, Rumley A, Lowe GD, Marmot M. Influence of socioeconomic status and job control on plasma fibrinogen responses to acute mental stress. Psychosomatic Medicine. 2003;65(1):137–144. doi: 10.1097/01.psy.0000039755.23250.a7. [DOI] [PubMed] [Google Scholar]
- Teng E, Tassniyom K, Lu PH. Reduced quality-of-life ratings in mild cognitive impairment: analyses of subject and informant responses. American Journal of Geriatric Psychiatry. 2012;20(12):1016–1025. doi: 10.1097/JGP.0b013e31826ce640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka K, Kurumatani N, Hosoi H. Social participation and cognitive decline among community-dwelling older adults: A community-based longitudinal study. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2016 doi: 10.1093/geronb/gbw059. [DOI] [PubMed] [Google Scholar]
- Trenerry M, Crosson B, Deboe J, Leber L. Stroop Neuropsychological Screening Test. Odessa, FL: Psychological Assessment Resources; 1989. [Google Scholar]
- Virtanen M, Ferrie JE, Batty GD, Elovainio M, Jokela M, Vahtera J, … Kivimaki M. Socioeconomic and psychosocial adversity in midlife and depressive symptoms post retirement: A 21-year follow-up of the Whitehall II study. American Journal of Geriatric Psychiatry. 2015;23(1):99–109. doi: 10.1016/j.jagp.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HX, Jin Y, Hendrie HC, Liang C, Yang L, Cheng Y, … Gao S. Late life leisure activities and risk of cognitive decline. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2013;68(2):205–213. doi: 10.1093/gerona/gls153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Weschler Adult Intelligence Scale. 3. San Antonio: Psychological Corporation; 1997. [Google Scholar]
- Weschler D. Weschler Memory Scale - Revised (WMS-R) New York: Psychological Corporation; 1987. [Google Scholar]
- Ybarra O, Burnstein E, Winkielman P, Keller MC, Manis M, Chan E, Rodriguez J. Mental exercising through simple socializing: Social interaction promotes general cognitive functioning. Personality and Social Psychology Bulletin. 2008;34(2):248–259. doi: 10.1177/0146167207310454. [DOI] [PubMed] [Google Scholar]
- Ybarra O, Winkielman P. On-line social interactions and executive functions. Frontiers in Human Neuroscience. 2012;6:75. doi: 10.3389/fnhum.2012.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuelsdorff M, Koscik RL, Okonkwo OC, Peppard PE, Hermann BP, Sager MA, … Engelman CD. Reliability of a novel social activity questionnaire: Perceived social support and verbal interaction in the Wisconsin Registry for Alzheimer’s Prevention. Journal of Aging and Health. 2016 Oct; doi: 10.1177/0898264316674812. 2016 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuelsdorff ML, Engelman CD, Friedman EM, Koscik RL, Jonaitis EM, Rue AL, Sager MA. Stressful events, social support, and cognitive function in middle-aged adults with a family history of Alzheimer’s disease. Journal of Aging and Health. 2013;25(6):944–959. doi: 10.1177/0898264313498416. [DOI] [PMC free article] [PubMed] [Google Scholar]