Abstract
Corneal transplantation has been proven effective for returning the gift of sight to those affected by corneal disorders such as opacity, injury, and infections that are a leading cause of blindness. Immune privilege plays an important role in the success of corneal transplantation procedures; however, immune rejection reactions do occur, and they, in conjunction with a shortage of corneal donor tissue, continue to pose major challenges.
Corneal immune privilege is important to the success of corneal transplantation and closely related to the avascular nature of the cornea. Corneal avascularity may be disrupted by the processes of angiogenesis and lymphangiogenesis, and for this reason, these phenomena have been a focus of research in recent years. Through this research, therapies addressing certain rejection reactions related to angiogenesis have been developed and implemented. Corneal donor tissue shortages also have been addressed by the development of new materials to replace the human donor cornea. These advancements, along with other improvements in the corneal transplantation procedure, have contributed to an improved success rate for corneal transplantation.
We summarize recent developments and improvements in corneal transplantation, including the current understanding of angiogenesis mechanisms, the anti-angiogenic and anti-lymphangiogenic factors identified to date, and the new materials being used. Additionally, we discuss future directions for research in corneal transplantation.
Keywords: corneal transplantation, corneal neovascularization, angiogenesis, lymphangiogenesis, VEGF, VEGFR, biomaterial, stem cell
INTRODUCTION
The cornea, the avascular, transparent, dome-shaped anterior surface of the eye, serves as a mechanical barrier to foreign particles and as a refractive surface. The avascularity of the cornea, its uniform structure, and its state of relative dehydration all contribute to its transparency, which is crucial for normal vision.
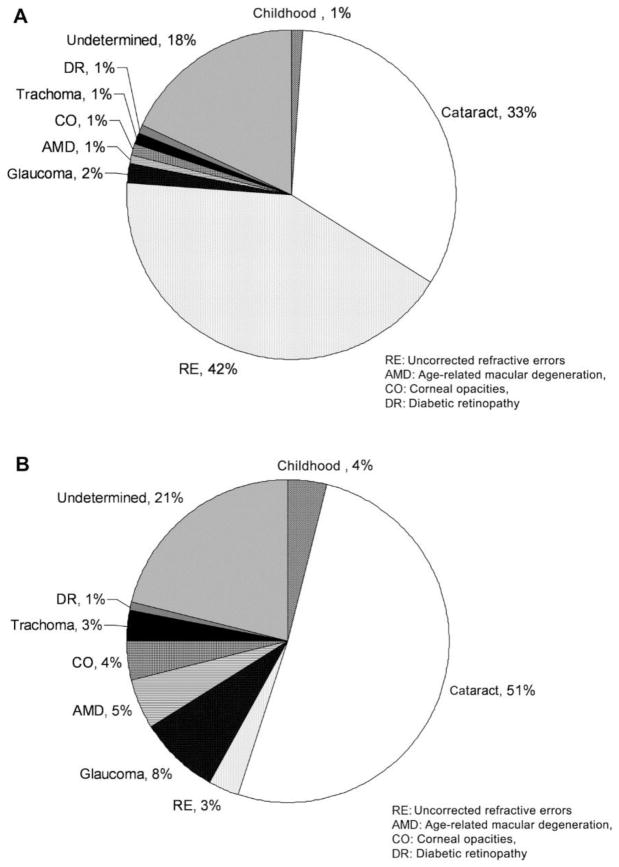
Loss of corneal avascularity and integrity leads to corneal opacity, a leading cause of diminished visual acuity [Fig. 1].142 Studies have shown that a decrease in visual acuity has widespread effects on daily life, leading to reduced participation in social activities, limited mobility, -restricted ability to perform daily chores,44; 60; 90; 100; 115; 192 and increased risks of falls, hip fractures, need for nursing home admission, and ultimately mortality.38 Also some studies have shown that a reduction in visual acuity also puts patients at an increased risk for emotional distress, particularly depression.60; 63; 90; 100; 115
Figure 1.
(A) Global causes of visual impairment, inclusive of blindness, as percentage of global visual impairment in 2010. (B) Global causes of blindness as percentage of global blindness in 2010. AMD, age-related macular degeneration; CO, corneal opacities; DR, diabetic retinopathy; RE, uncorrected refractive errors.142 Adapted from Pascolini et al with permission from BJM)
The avascularity of the cornea also plays a role in immune privilege,37; 130; 131; 145; 177 a concept in which certain transplanted tissues undergo less destructive immunological rejection processes upon transplantation. Barker and coworkers demonstrated this by showing that grafts placed in certain anatomical sites such as the brain and eye survive for longer periods of time as compared to other transplanted tissues.20
Angiogenesis is the process of new blood vessel growth from pre-existing vascular structures. Corneal angiogenesis can compromise corneal transparency and, in transplanted corneas, threaten the prognosis of donor graft tissue by causing inflammation, corneal scarring, and edema.16; 176 Corneal angiogenesis can occur from excessive levels of pro-angiogenic factors--vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), matrix metalloproteinases (MMPs), and others.2; 5; 93; 125; 133; 188 Members of the VEGF family (VEGFA, C, and D) specifically have been recognized as thoroughly involved in both corneal angiogenesis and lymphangiogenesis. 2; 5; 31; 46; 188 Corneal angiogenesis can also occur secondary to a relative paucity of anti-angiogenic factors.9; 69 Examples of anti-angiogenic/lymphangiogenic factors are soluble VEGF receptor (VEGFR)-1, -2, -3, pigment epithelium–derived factor, angiostatin (created by the proteolytic cleavage of plasminogen), and endostatin (a type XVIII collagen proteolytic product).54; 84; 132; 191
PATHOLOGY
Rapid development of the corneal transplantation technique in the recent years, from full-thickness to lamellar keratoplasty, has allowed specific replacement of layers of the cornea that have been damaged secondary to non-inflammatory causes (keratoconus, pellucid marginal degeneration, and post refractive surgery ectasia), inherited corneal conditions (corneal dystrophies), and scarring from infectious keratitis.Fuchs dystrophy (39%), keratoconus (27%), and the sequellae of infectious keratitis (20%) represent the major pathologies leading to surgical intervention.67
SUCCESS RATE OF CORNEAL TRANSPLANTATION
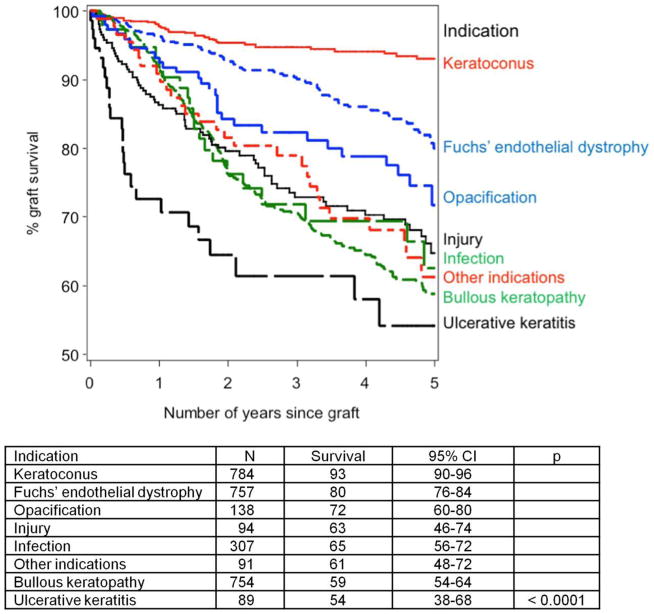
A new cornea lasts for 10 years in the majority of patients with keratoconus (89%), Fuchs dystrophy (73%), and corneal scarring (70% for non-herpetic scar and 60% for herpetic scar).181 There are various reports regarding the outcome of corneal graft survival rates. According to Vail and coworkers in the Corneal Transplant Follow-up Study, graft survival at 1 year was 88%.187 In addition, the Australia Corneal Graft Registry reported survival rates of 91% at 1 year.194 Beckingsale and coworkers reported a survival rate of 66% at 5 years of follow-up specifically for penetrating keratoplasty.22 Armitage et al also compiled data for corneal graft survival over approximately 5 years (April 1, 1999, to March 31, 2005) from the National Health Service Blood and Transplant database (Fig. 2).13 Notably, for patients with other pre-existing conditions such as glaucoma, amblyopia, or cataract, the visual result might be unsatisfactory even if the surgery is successful.203
Figure 2. Kaplan–Meier plot showing the effects of different indications on 5-year estimated graft survival of first corneal transplants. 13.
(Adapted from Armitage et al with permission from IOVS)
TECHNIQUES OF CORNEAL TRANSPLANTATION
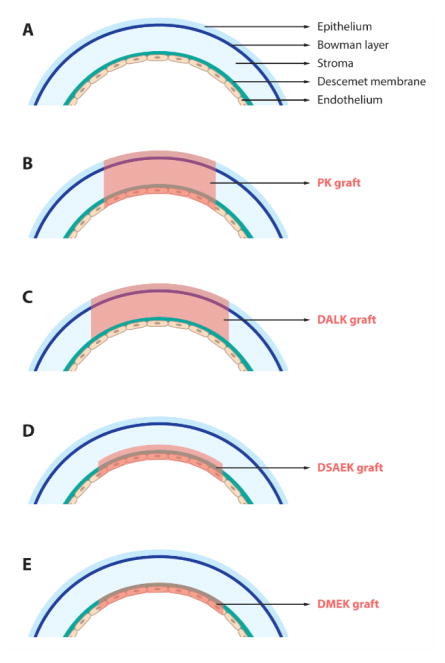
Corneal allotransplantation is the most widely recognized and effective form of transplantation in humans. There are three major types of surgical transplantation procedures: (i) penetrating keratoplasty (PK, full-thickness), (ii) anterior lamellar keratoplasty (ALK, partial-thickness), and (iii) endothelial keratoplasty (EK) (Fig. 5).
Figure 5. Schematic overview displaying.
(A) a virgin cornea and (B–E) different keratoplasty procedures: (B) PK, (C) DALK, (D) DSAEK, and (E) DMEK
Penetrating keratoplasty (PK) is a transplantation procedure in which a trephine of an appropriate diameter is utilized to make a full-thickness resection of the patient's cornea, then a full-thickness donor corneal graft is placed.14
Anterior lamellar keratoplasty (ALK) is an alternative treatment specifically to replace the anterior part of the cornea when it is scarred or distorted. ALK is preferred when the endothelial layer of the recipient is healthy or needs to be preserved. It is also less intrusive than PK and associated with virtually no endothelial immune graft rejection.128 Deep ALK (DALK) is one of the major techniques of ALK in which the epithelium and at least three quarters, if not all, of the corneal stroma are replaced.99; 160; 180
Endothelial keratoplasty (EK) is the surgical procedure in which diseased endothelial tissue is removed and replaced with viable endothelium from a donor.64; 199 EK is preferred for patients with advanced visual disability related to endothelial dysfunction.26; 199 Keratoconus, hypotony, or corneal opacity and scarring serve as contraindications owing to limited visual outcomes.152
The two main types of EK are:
Descemet stripping endothelial keratoplasty (DSEK)
Descemet membrane endothelial keratoplasty (DMEK)
DSEK is the most widely recognized EK technique. In this type of EK, the surgeon implants the posterior parts of the donor cornea comprising the Descemet membrane, endothelial layer, and the stroma with variable thickness into the recipient’s eye. The stroma acts as an adhesion layer to the recipient’s cornea.64; 79; 156 In Descemet stripping automated endothelial keratoplasty (DSAEK) a microkeratome device is used in the corneal tissue dissection to produce a smoother surface.26; 70
DMEK involves a very thin donor tissue, which consists of the Descemet membrane and endothelial layer without the stromal layer. DMEK is associated with better vision outcomes and reduced risk of graft rejection.{Heinzelmann, 2016 #41
Several innovations in technique have been made to improve the outcome of corneal transplantation procedures, for example, the use of a microkeratome or femtosecond laser to increase the precision in cutting,{Lee, 2009 #329;Lee, 2008 #330;Azar, 2002 #336;Azar, 2000 #339} new suture techniques or suture-less surgery,24; 28; 91; 149; 150; 153; 172; 201 and specific layer transplantation (lamellar keratoplasty).64; 92; 99; 128; 152; 160; 180; 199
RISKS OF CORNEAL TRANSPLANTATION
Stechschulte and Azar175 have classified several postoperative complications after PK:
early postoperative complications: wound leaks, epithelial defects, suture complications, filamentary keratopathy, immediate pressure elevation, postoperative inflammation, choroidal complications (effusions, detachments, and hemorrhage), microbial keratitis, endophthalmitis, retrocorneal membrane, anterior synechiae, and primary graft failure.
late postoperative complications: graft rejection, glaucoma, refractive error, epithelial downgrowth, and disease transmission.
A retrospective study by Wagoner and coworkers also reported that, in addition to rejection, there are other postoperative complications for adult PK. These include glaucoma, infections (keratitis and endophthalmitis), persistent epithelial defects, wound dehiscence, and primary graft failure.189
FACTORS LEADING TO CORNEAL TRANSPLANTATION REJECTION
The rejection reaction involves a complex immune process that recognizes the donor cornea as foreign tissue and attacks it. This reaction subsequently causes graft cell destruction and eventually may lead to graft failure. Thus, graft rejection is a major factor for graft failure.108; 124; 129
Corneal rejection rates are comparatively low given the tissue’s strong immune privilege. This immune privilege is primarily due to the lack of blood and lymphatic vessels in the cornea, the phenomenon of anterior chamber-associated immune deviation (ACAID), and immunosuppressive molecules inside the eye.83; 184 ACAID is a phenomenon where, upon being introduced to an antigen, the immune system generates systemic tolerance instead of a hypersensitivity immune response. Additionally, immune responses are further suppressed by the presence of various immunosuppressive factors, such as α-melanocyte–stimulating hormone, vasoactive intestinal peptide, calcitonin gene–related peptide, transforming growth factor (TGF)-β2, thrombospondin (TSP)-1, and cell surface molecules of the B7 family. These three aspects contribute to the lowest rejection rate of cornea among all organ transplants.
Located on the ocular surface is eye-associated lymphoid tissue (EALT). The role of this tissue in corneal transplantation is not fully understood, but the EALT seems to play a role in the process of immune tolerance.105
Graft rejection is due to major histocompatibility antigens and the degree of vascularization of the host cornea.103; 141 The diagnosis of graft rejection is based on clinical findings, and occasionally the signs and symptoms are subtle, especially in the early stages of epithelial rejection. According to Inoue and coworkers89, the general rates of rejection-free graft survival and graft survival at 10 years after PK were 77.9% and 79.3%, respectively. They also showed that the relative risk of graft failure and rejection is positively associated with the degree of corneal vascularization, but found no relation between graft failure or allograft rejection and graft size or suture technique, respectively. In contrast, Jonas and coworkers94 reported that the immunologic graft reaction increases significantly with the incidence of loosening of corneal sutures. As suture loosening occurs more often with interrupted sutures, it can be surmised that a graft reaction occurs more often with interrupted sutures. Furthermore, Boisjoly and coworkers in 1989 analyzed 348 adult recipients of corneal transplants and found that a transplant size of 8 mm or larger is an important risk factor.23 Large grafts extend near to the host limbus, which is the border between the cornea and sclera, a source of corneal epithelial stem cells, and is known to have more antigen-presenting cells, making these grafts more susceptible to rejection.57; 103 High-risk cases include those with vascularization in more than two quadrants, herpes simplex keratitis, previously rejected grafts, active inflammation, young recipient age, and several surgical procedures performed at the time of grafting.
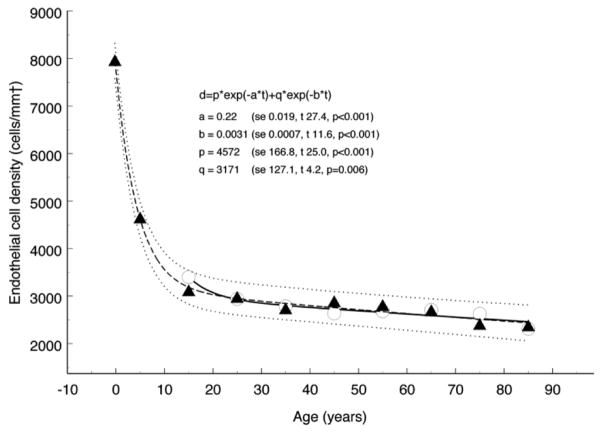
Another important factor in selecting the most appropriate procedure for a patient is the number of endothelial cells available, both in the recipient and donor. Endothelial cell depletion may threaten graft survival, as these cells are responsible for maintaining corneal stromal hydration and clarity. Although decreased numbers of these cells are normal with aging, surgical procedures such as cataract surgery and PK may enhance this process. Late endothelial failure (LEF) happens when the corneal stroma swells (edema) and becomes opaque.25; 26 Thus, it is necessary to utilize a specific procedure that causes less disruption of the endothelial cells, for example anterior lamellar keratoplasty over PK. Armitage and coworkers compiled data for endothelial cell number in the non-diseased aging eye (Fig. 3) 12 versus that after PK (Fig. 4).12 Anshu et al found that DMEK is associated with a significantly lower risk of graft rejection compared to DSEK and PK. They hypothesized that this is due to a reduction in rejections stemming from the donor epithelium and stromal layer, and that the use of fewer cell layers in corneal transplantations may result in a weaker immune reaction.10 Lamellar keratoplasty (DALK) may also be associated with less susceptibility to graft rejection compared to PK, based on preservation of the endothelium.129; 135; 160; 178
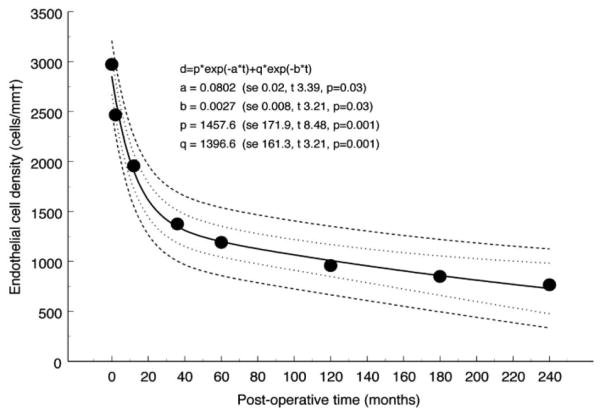
Figure 3. Endothelial cell loss with aging in nondiseased eyes.
Biexponential model fitted to data from cadaveric eyes (Møller-Pedersen)126 showing 95% prediction interval. The coefficients are shown with their respective standard error (se) and the corresponding values for t and p. The half times for the fast and slow components of the model, calculated from the relevant exponential rate constants, are 3.1 and 224 years, respectively. The residual standard deviation was 113.9 cells/mm2. Also shown for comparison are data from live subjects (Yee et al)198. The corresponding half times for the fast and slow components of the decay are 3.5 and 277 years, respectively.12 (Adapted from Armitage et al with permission from IOVS)
Figure 4. Endothelial cell loss after PK.
Bi-exponential model fitted to data from Bourne25 showing 95% confidence interval (inner dotted lines) and 95% prediction interval (outer dashed lines). The coefficients are shown with their respective standard error (se) and the corresponding values for t and p. The half times for the fast and slow components of the model, calculated from the relevant exponential rate constants, are 8.6 and 257 months, respectively. The residual standard deviation was 109.6 cells/mm2. 12 (Adapted from Armitage et al with permission from IOVS)
MANAGEMENT OF CORNEAL TRANSPLANT REJECTION
The imperative objective in the management of corneal transplant rejection is to prolong the corneal graft survival, either by preventative courses or treatments. Since corneal transplant rejection is closely related to the host immune response and corneal neovascularization, the use of immunosuppressive and anti-angiogenic agents eventually will also reduce the rejection reaction. Patients also need to be informed of the signs and symptoms of graft rejection, as early detection and aggressive therapy lead to a better prognosis.33; 141
Panda and coworkers extensively reviewed several preventative strategies141 , including the reduction of antigenic load of donor tissue by using the central part of the cornea, minimizing antigenic differences between the host and donor through HLA compatibility matching, and suppressing the host immune responses using immunosuppressive and anti-angiogenic agents.
To date, corticosteroids, both topical and systemic, have been the principal management for corneal transplant rejection.80; 81; 103; 141; 151; 157; 171 Panda and coworkers compiled a list of corneal graft management strategies based on the use of corticosteroids, as shown in Table 1.141 If corticosteroid-induced glaucoma is suspected, corticosteroid use may be stopped or tapered to the minimum dose.106; 158 Utilizing a steroid with a lower tendency to increase the intraocular pressure, such as fluorometholone, loteprednol, or rimexolone, may also be considered.158 Taravella and coworkers reported that topical steroid phosphate preparations may also induce band keratopathy, which warrants steroid discontinuation.183
Table 1.
| Guidelines for Prevention of Corneal Graft Rejection152 | ||
|---|---|---|
| Preoperative | Postoperative | |
| 1. Normal-risk cornea | - | Topical corticosteroid |
| 2. High-risk host | Topical corticosteroid | Topical Corticosteroid (longer duration) |
| 3. High-risk host (previous | Topical corticosteroid | Topical + systemic |
| graft rejection) | cyclosporine A | corticosteroid (longer duration) |
| Guideline for Management 152 | ||
|---|---|---|
| Topical | Systemic | |
| 1. Epithelial rejection | Frequent corticosterioid | |
| 2. Sub-epithelial infiltrate | Frequent corticosteroid | |
| 3. Endothelial rejection | Aggressive corticosteroid cyclosporine A + cycloplegics |
IV pulse corticosteroid maintenance |
| 4. Combined rejection | Aggressive corticosteroid cycloplegics | IV pulse corticosteroid. cyclosporine A + maintenance |
| 5. Rejection in a rejected graft | Aggressive corticosteroid cycloplegics | IV pulse corticosteroid cyclosporine A + maintenance |
| 6. Miscellaneous: | ||
| a) Only acute edema | Aggressive corticosteriod | |
| b) Only gradual edema | Nonaggressive corticosteriod | |
(Adapted from Panda at el with permission from Elsevier)
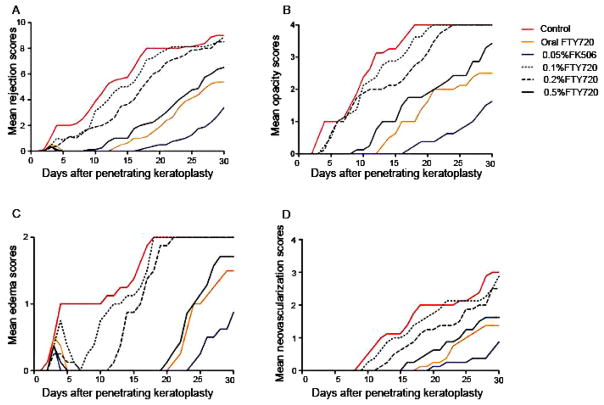
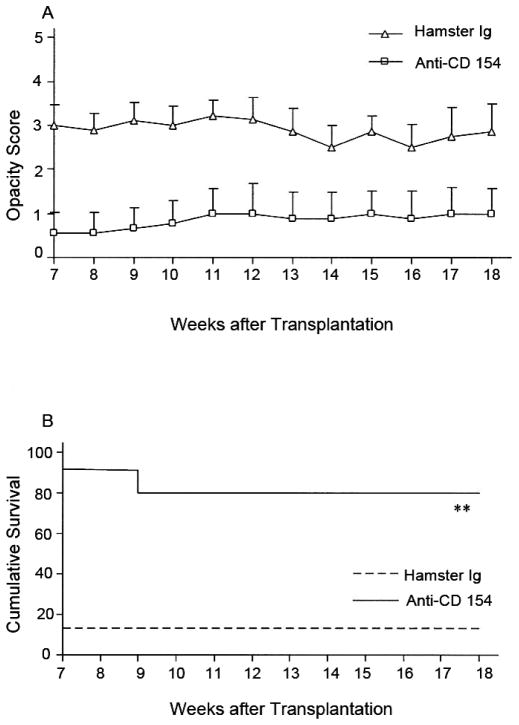
Other reported non-steroid immunosuppressive agents used include cyclosporine A, azathioprine, and tacrolimus.141 Immunosuppressive treatment is sometimes used for severe cases. In particular, cyclosporine is used when graft rejection happens despite corticosteroid therapy or in high-risk patients who have bilateral disease.80; 157 These treatments are rarely used because of more possibility of serious side effects and the higher cost of treatment. Some researchers have explored using other immunosuppressive agents. For example, Liu and coworkers investigated the use of fingolimod (FTY720) (Fig. 6)119 and Qian and coworkers studied outcomes with anti-CD154 (Figs. 7–9).155
Figure 6. Average clinical finding scores for corneal rejection in all groups throughout the entire follow-up period.
(a) Mean rejection scores; (b) mean opacity scores; (c) mean edema scores; and (d) mean neovascularization scores. The results obtained for the 0.5% FTY720 (Fingolimod) group, the oral FTY720 group, and the 0.05% FK506 (Tacrolimus) group were significantly lower than those obtained for the control group (all p < 0.05). 119 Adapted from Liu et al with permission from NPG)
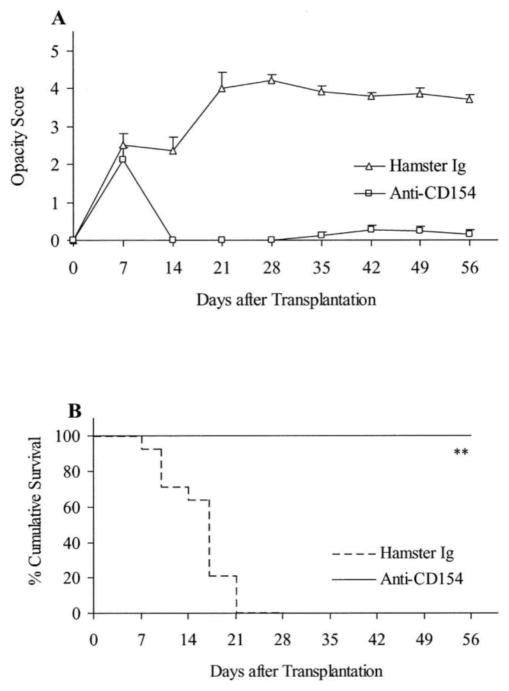
Figure 7. Fate of high-risk minor H–disparate corneal transplants in BALB/c mice (n = 14/group) that received corneal grafts from B10.D2 mice and were randomized to receive anti-CD154 or hamster Ig. Graft rejection was scored clinically.
(A), and graft survival data are presented as Kaplan–Meier survival curves (B). Anti-CD154 therapy enhanced the survival of minor H–disparate grafts in high-risk transplantation (**P = 0.0001).155 (Adapted from Qain et al with permission from IOVS)
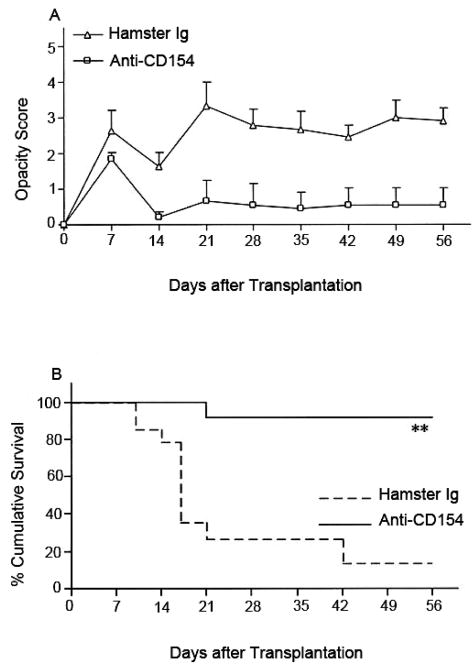
Figure 9. Fate of high-risk MHC-disparate corneal transplants in BALB/c mice (n = 14/group) that received corneal grafts from BALB.b mice and were randomized to receive anti-CD154 or hamster Ig.
Graft rejection was scored clinically (A), and graft survival data are presented as Kaplan–Meier survival curves (B). Anti-CD154 therapy enhanced the survival of minor H–disparate grafts in high-risk transplantation (**P = 0.0002).155 (Adapted from Qain et al with permission from IOVS)
ROLE OF CORNEAL NEOVASCULARIZATION IN CORNEAL DISORDERS
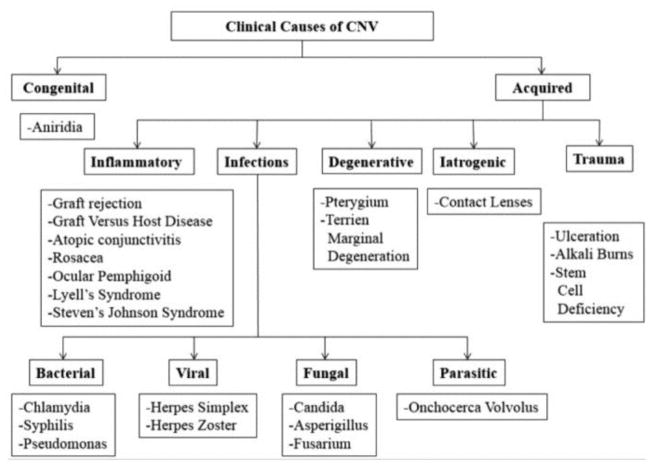
Neovascularization (NV) is the development of new corneal vasculature in a previously avascular area.16 NV includes both angiogenesis and lymphangiogenesis. Highly regulated NV is crucial for many physiologic processes, such as wound healing, growth, and development. Unregulated NV, on the contrary, may drive various pathologic conditions.66 NV in the eye is almost always pathological, as the new vessels generally lack structural integrity and leak fluid that may result in hemorrhage, exudates, and fibrosis.112 The presence of NV in the cornea specifically mediates various visual morbidities, for example blindness, general visual impairment, and abnormal wound healing. Thus, for the cornea to remain transparent, the layers must be devoid from any form of NV. Corneal angiogenic privilege is an important factor that preserves this corneal clarity via a balance of pro-angiogenic and anti-angiogenic factors.16; 59 Corneal pathologies that involve NV are classified by Abdelfattah and coworkers in Figure 10.3
Figure 10.
Causes of corneal NV (CNV).
A wide variety of pro-angiogenic factors that promote corneal NV as well as anti-angiogenic factors are summarized in Table 2 reproduced from Qazi and coworkers.154
Table 2.
Angiogenic and anti-angiogenic factors in corneal neovascularization165 (Pending Approval)
| Angiogenic molecules | Anti-angiogenic molecules |
|---|---|
| Vascular endothelial growth factors (VEGFs) | Pigment epithelium-derived factor (PEDF) |
| Fibroblast growth factor (FGF) | Endostatin |
| Phosphatidylinositol-glycan biosynthesis class F or Placental growth factor (PlGF) | Angiostatin |
| Transforming growth factor alpha (TGF-α), TGF-β | Prolactin (PRL) |
| Insulin-like growth factor (IGF) | Tissue inhibitor metalloproteinases (TIMPs) |
| Platelet-derived growth factor (PDGF) | Thrombospondin (TSP)-1, TSP-2 |
| Matrix metalloproteinases (MMPs) | Interleukin (IL)-4, -12, -13, -18 |
| Hepatocyte growth factor (HGF)/Scatter factor (SF) | Arresten |
| Tumor necrosis factor alpha(TNF-α) | Canstatin |
| Connective tissue growth factor(CTGF) | Tumstatin |
| IL-1, -8 | TNF-α |
| Monocyte chemoattractant protein (MCP)-1 | MMPs |
| Leptin | Interferon gamma (IFN-γ) |
| Integrins (αV β3) | |
| Angiogenin | |
| Thromboxane (TXA)-2, Cyclooxygenase (COX)-2 | |
| Nitric Oxide (NO) | |
| Platelet activating factor (PAF) |
Corneal immune privilege is a phenomenon that is tightly bound to angiogenic privilege because the blood and lymphatic vessels constitute the respective afferent and efferent arms of an immune reflex arc.47; 55; 98 As an afferent arm, lymph vessels enable the transport of antigenic materials and antigen-presenting cells (APCs) between the donor and recipient, accelerating the sensitization process in the regional lymph nodes.118 This T cell–activating process consequently induces an inflammatory and rejection response that is magnified by the inflow of inflammatory cells through the efferent arm, which is the blood vessel.
The presence of NV in a corneal graft is one sign of a rejection process. Additionally, a recipient cornea with preoperative corneal NV is regarded as a ‘high risk’, in which transplant rejection may occur faster and more frequently.17 Other than corneal NV, uncontrolled glaucoma, decreased corneal sensation, the presence of persistent active intraocular inflammation, and associated ocular abnormalities, such as eyelid disease, abnormal conjunctiva, or dry eye syndromes also confer a higher risk of rejection.43; 167
For many years it was believed that angiogenesis plays the major role in many pathological processes, especially in graft rejection, as corneal lymphangiogenesis is generally invisible with conventional imaging and difficult to appreciate.36 Through studies using recently discovered lymphatic endothelial cell-specific markers,19; 49; 193however, it is now recognized that lymphangiogenesis is equally as important as angiogenesis in graft rejection. Cursiefen and coworkers reported that angiogenesis and lymphangiogenesis may be triggered by an identical pathophysiological process, grow in parallel, but regress over different time scales. They suggested that the regression of pathologic lymphatic vessels can be used to predict the probability of corneal graft survival in high-risk eyes over an extended period after an inflammatory insult.48 Ecoiffier and coworkers demonstrated in a murine model of suture placement that parallel outgrowth of blood and lymph vessels occurs in the cornea, though they behave independently under certain circumstances.58
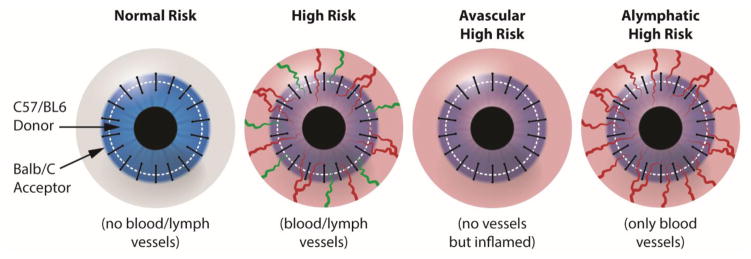
Dietrich and coworkers transplanted donor corneas from C57BL/6 mice into uninflamed or inflamed avascular, prehemvascularized only (alymphatic) or prehemvascularized and prelymphvascularized recipient BALB/C mice (Fig. 12).55 Donor corneas transplanted to prehemvascularized alymphatic beds (lymphangiogenesis inhibition using VEGFR-3 antibody mF4-31C1 and integrin α5β1 inhibitor) showed better survival rates than those transplanted into prehemvascularized and prelymphvascularized beds. They then proposed that corneal lymphangiogenesis sensitization is the most important mediator of immune rejection after corneal transplantation.55 Regenfuss and coworkers and Cursiefen and coworkers also reported that the presence of lymphatic vasculature suggests a high-risk status of a recipient bed.49; 159Graft failure may not be immune related; however, graft rejection normally suggests an immunologic reaction of the host to the donor corneal tissue.51
Figure 12. Generation of different transplantation models. Schematic diagram showing the generation of normal-risk (avascular), high-risk (inflamed and hemvascularized and lymphvascularized), avascular high-risk (inflamed, but avascular, recipient), and alymphatic high-risk recipient beds (inflamed and hemvascularized, but no lymphatic vessels) as transplantation models.55.
(Adapted from Dietrich et al with permission)
ANIMAL MODELS OF CORNEAL TRANSPLANTATION
It is of major importance to understand the mechanisms that mediate corneal transplant rejection for the testing of various therapies to prevent rejection. It is impossible to comprehend the significance of different factors in any in vitro model since transplantation responses include a wide range of factors working in concert that will ultimately determine whether the transplanted tissue survives or fails. Consequently, studies utilizing animals are needed to identify the factors that are vital to either the success or failure of transplanted tissue. Murine models show that a tilt in the balance of corneal angiogenic/lymphangiogenic and anti-angiogenic/anti-lymphangiogenic factors results in spontaneous corneal blood and lymphatic vessel formation.6; 16; 48 Ambati’s group showed that the cornea expresses soluble VEGF receptor-1 (sVEGFR-1) and that suppression of this endogenous VEGF-A trap prompts angiogenesis in the mouse cornea.8; 56 Similarly, deletion of soluble VEGFR-2 (sVEGFR-2) in corneal stromal cells results in spontaneous lymphatic vessel formation in the mouse cornea.6
Disrupted corneal epithelial, stromal and limbal stem cell architecture and metaplasia of corneal epithelium towards a skin-like form (such as keratinization, sub-epithelial NV, loss of transparency) are important factors associated with corneal stromal angiogenesis and lymphangiogenesis in transgenic mouse models.101; 114 Corn1 mice have corneal opacity and NV. Hence they are useful for studies of neovascularization and corneal disease.174 Kelch-like ECT2 interacting protein (KLEIP)-deficient mice have been shown to have corneal neovascular dystrophy.72 Mechanisms of phenotype development may be shared between diverse transgenic mouse models of corneal NV. Spontaneous corneal NV models will be beneficial in angiogenesis research for examining several intertwined signaling pathways that trigger corneal angiogenesis and lymphangiogenesis.101
ADVANTAGES AND LIMITATIONS OF TRANSGENIC MODELS OF CORNEAL ANGIOGENESIS AND LYMPHANGIOGENESIS
Mouse models are used in research related to numerous forms of transplantation including corneal transplantation. The main advantage of murine models is the availability of various strains that express certain transgenes or have been gene-targeted to lack expression of particular immunological factors whose role in transplantation is to be explored. Murine models have been utilized to characterize factors that facilitate the survival of corneal allografts during acute rejection in the absence of immunosuppressive therapy and to characterize the factors that are most critical in rejection of such allografts. Moreover, multiple factors involved in corneal transplantation are also known to be applicable in transplantation of other tissues. The discovery and understanding of the mechanisms of both corneal allograft acceptance and rejection have been mainly achieved via studies using murine models of corneal transplantation. 30; 36; 58; 95; 109; 147; 161; 168; 174; 184; 196; 200; 205
CORNEAL TRANSPLANTATION IN THE PRESENCE OF ANTI-ANGIOGENIC AND ANTI- LYMPHANGIOGENIC FACTORS
Transplantation is one of the therapeutic choices for reviving tissue function; however, graft failure caused by immune rejection continues to be a major barrier to the success of all transplantations.57 The induction and expression of the alloimmune response are two key factors that influence the immunologic fate of an allograft. The lymphatic vessels facilitate trafficking of APCs from the graft site to regional draining lymphoid tissues where APCs can then present alloantigens to undeveloped host T cells. On the other hand, blood vessels facilitate the homing of primed effector T cells back to the graft site where the cells mediate allo-rejection.130 Experimental attempts to subdue the afferent and efferent arms of the allo-immune response have both exhibited potential in anticipating allo-rejection, but the relative efficacy of focusing on angiogenesis and lymphangiogenesis in corneal transplantation remains to be explored.33; 34; 46; 47; 50; 111; 143
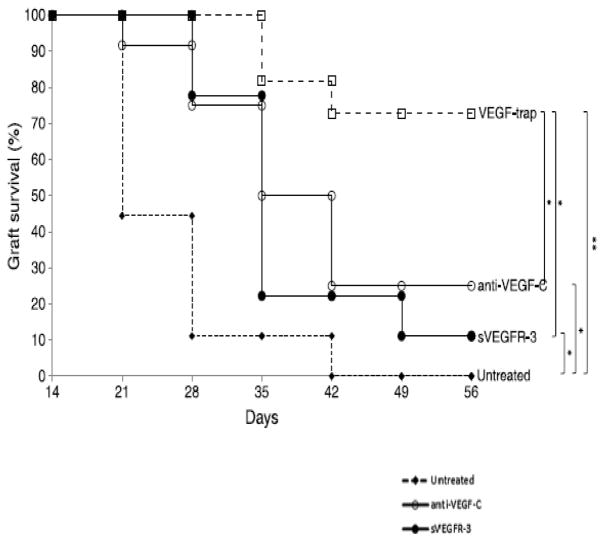
Both angiogenesis and lymphangiogenesis are mainly driven by the VEGF family of receptors and ligands. VEGF-A and VEGF-B binding to VEGFR-1 and VEGFR-2 drives angiogenesis, while VEGF-C and VEGF-D binding to VEGFR-2 and VEGFR-3 drives lymphangiogenesis. 46; 59; 74; 139 These complex interactions between VEGFs and VEGF receptors exhibit sophisticated control over angiogenesis and lymphangiogenesis. Research has shown that modulating both VEGFs and their receptors in both low-risk and high-risk corneal transplantation can reduce angiogenesis and lymphangiogenesis, thereby reducing the potential for NV and increasing the chance of transplant survival. Furthermore, many anti-lymphangiogenesis-specific methodologies have demonstrated success in enhancing graft survival.46; 55; 96; 123 VEGF-trap is decidedly proving to be more fruitful in advancing long-term graft survival in comparison to anti-VEGF-C and sVEGFR-3 (Fig. 13).56
Figure 13.
Effect of VEGF neutralization on high-risk corneal transplant survival. Animals underwent high-risk allogeneic corneal transplantation and received treatment with anti-VEGF-C, sVEGFR-3, or VEGF-trap at the time of transplantation, at 3, 7, 10, and 14 days after transplantation, and then once per week for an additional 6 weeks, or they remained untreated. The VEGF-trap treatment was most effective in increasing allograft survival (72%), though treatment with anti-VEGF-C (25%) and sVEGFR3 (11%) also significantly improved survival compared to that in the untreated control group. To create the Kaplan-Meier survival curve, graft opacity was evaluated according to an established 0 to 5+ scale by slit-lamp biomicroscopy. Scores greater than or equal to 2+ are considered rejected. Each group consisted of n=9–12 mice. **P < 0.005, *P < 0.05, error bars represent standard error of the mean (SEM). Data from one experiment of two are shown. 56 (Adapted from Dohlman et al with permission from Wolters Kluwer)
Neutralization of VEGF-A
VEGF-A has clearly emerged as the family member principally responsible for HA. The direct effects of VEGF-A on angiogenesis are mediated principally via VEGFR-2 signaling.139 VEGF-A also plays a crucial role in pathological angiogenesis, such as in the rapid growth of solid tumors. Many antiangiogenic agents currently in development for the treatment of cancers target VEGF-A or VEGFR-2 for this reason. VEGF-A neutralization may directly or indirectly exert anti-angiogenic effects. VEGF-A neutralization also may hinder the recruitment of APCs to the graft bed as VEGF-A has also been implicated to be a powerful chemoattractant for monocytes/macrophages.18; 42; 46; 116
One of the methods for neutralizing VEGF-A action is by trapping, which refers to a phenomenon where VEGF-A is inactivated by the effect of VEGFR-1 binding. Naturally, it occurs through interactions with other molecules such as MMP-14. 35,75 MMP-14 also regulates VEGFR-373, but only cleaves VEGFR-1, and this cleaved VEGFR-1 with reduced angiogenic potential creates the trapping effect.
Lymphangiogenesis has been considered to be activated mainly by VEGF-C and -D attachment to their high-affinity receptor VEGFR-3. Pharmacological neutralization of VEGF-A, which is for the most part considered an angiogenic growth factor, decreases lymphangiogenesis as well, suggesting a new pathway for inhibiting lymphangiogenesis and sensitization to donor antigens.46
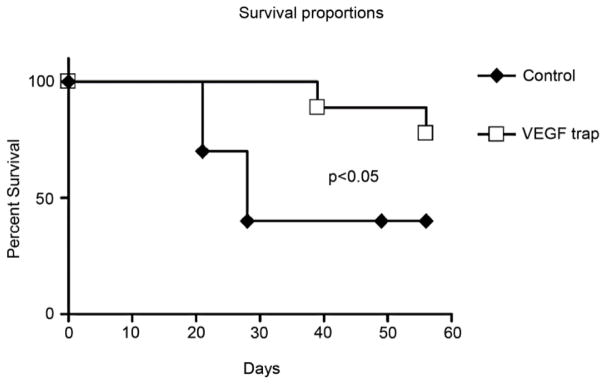
Additionally, Cursiefen and coworkers found that using VEGF TrapR1R2 to neutralize VEGF-A promotes graft survival compared to treatment with Fc-fragments only in murine models (Fig. 14).45 Aflibercept is one drug that is based on this mechanism.
Figure 14. Effect of pharmacologic neutralization of VEGF-A on survival of allogeneic cornea grafts. Panels of BALB/c mice received orthotopic transplants from C57BL/6 donors in one low-risk eye.
The recipients in one panel were treated with VEGF TrapR1R2, whereas the other panel (control) received Fc-fragments only. Survival of grafts in mice treated with VEGF Trap was significantly greater than in control animals (78% vs. 40%; P < 0.05; n = 22 mice in both groups).45 (Adapted from Cursiefen et al with permission from IOVS)
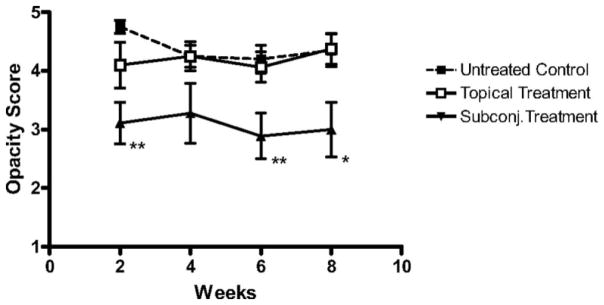
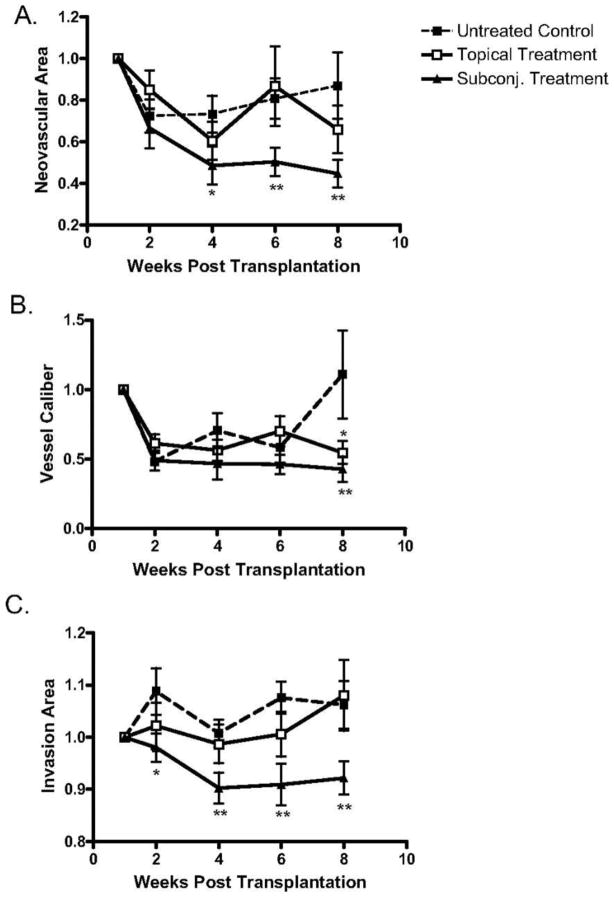
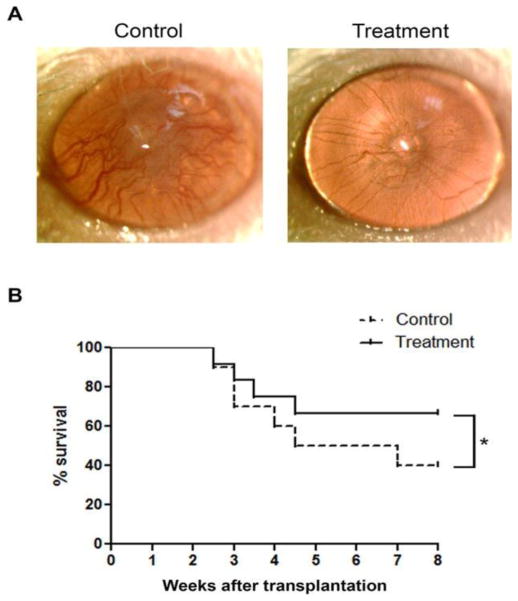
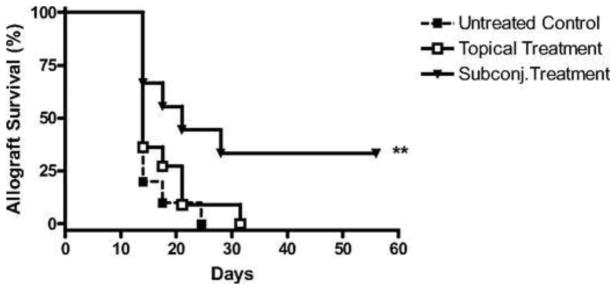
Bevacizumab, an FDA-approved antiangiogenic for cancer treatment, binds and neutralizes all VEGF-A isoforms. Bevacizumab, as an off-label use, has also reported to be successful in treating choroidal, iris, and retinal NV in macular degeneration and diabetic retinopathy.21; 116; 138 These reports promote bevacizumab exploration for corneal NV treatment. Dastjerdi and coworkers transplanted C57BL/6 mice corneas into high-risk graft beds in BALB/c mice and investigated the use of topical or subconjunctival bevacizumab for inhibiting corneal NV, compared to untreated control (Figs. 15–17).53 They showed that subconjunctival bevacizumab significantly diminished corneal graft opacity, prolonged graft survival, and reduced neovascular area (NA), vessel caliber (VC), and invasion area (IA) compared to topical treatment and no treatment (control). Harooni et al also reported a successful trial of subconjunctival injection of bevacizumab in a patient with corneal NV post PK (Fig. 18).76
Figure 15.
Subconjunctival delivery of bevacizumab diminishes opacity of corneal allografts in the high-risk setting. High-risk graft beds in BALB/c mice were transplanted with C57BL/6 cornea, and mice were left untreated (n = 10) or were treated topically (n = 10) or subconjunctivally (n = 10) with bevacizumab. Corneal allografts were examined regularly to 8 weeks after transplantation by slit lamp, and graft opacity was scored using a standard grading scheme. Student's t-test was performed to evaluate statistical significance (*P < 0.05; **P < 0.01).53 (Adapted from Dastjerdi et al with permission from IOVS)
Figure 17.
Analysis of topical versus subconjunctival bevacizumab on the corneal NA, VC, and IA. High-risk graft beds in BALB/c mice were transplanted with C57BL/6 cornea, and mice were left untreated (n = 10) or were treated topically (n = 10) or subconjunctivally (n = 10) with bevacizumab. (A) Total area of blood vessels in each cornea was calculated and normalized to the baseline to yield the mean NA at the indicated time points to 8 weeks after transplantation. Although topical bevacizumab treatment mildly reduced NA in high-risk corneal transplantation, subconjunctival treatment resulted in a significant and marked reduction in NA at weeks 4, 6, and 8. (B) Normalized mean values for estimated blood vessel caliber at the indicated times to 8 weeks after transplantation. Although the subconjunctival treatment significantly reduced VC at week 8 (P = 0.03), topical bevacizumab appeared to have a marginal statistical difference from the control group (P = 0.05). (C) The total area of each given cornea invaded by blood vessels was calculated and normalized to yield the mean IA at the indicated times to 8 weeks after transplantation. Subconjunctival bevacizumab treatment appeared to be the only effective method to reduce IA. Student's t-test was performed to evaluate statistical significance (*P ≤ 0.05; **P < 0.01).53 (Adapted from Dastjerdi et al with permission from IOVS)
Figure 18.
A, Slit-lamp image from the patient demonstrating corneal NV in the stroma crossing into the transplant at time of presentation; B, at 1 week after treatment; and C, at 1 month after treatment.76 (Adapted from Harooni et al with permission from Elsevier)
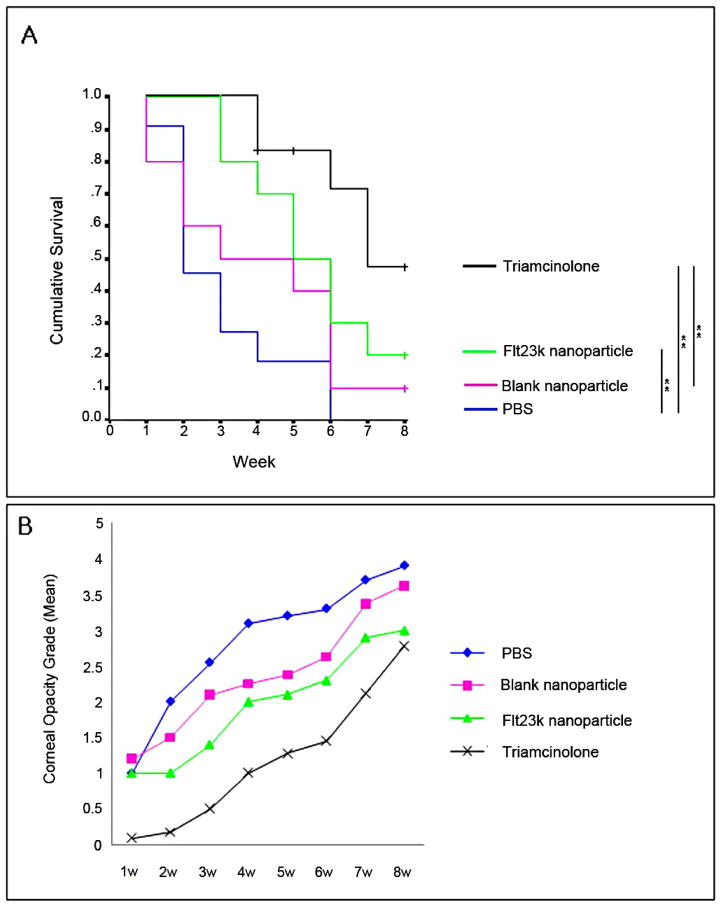
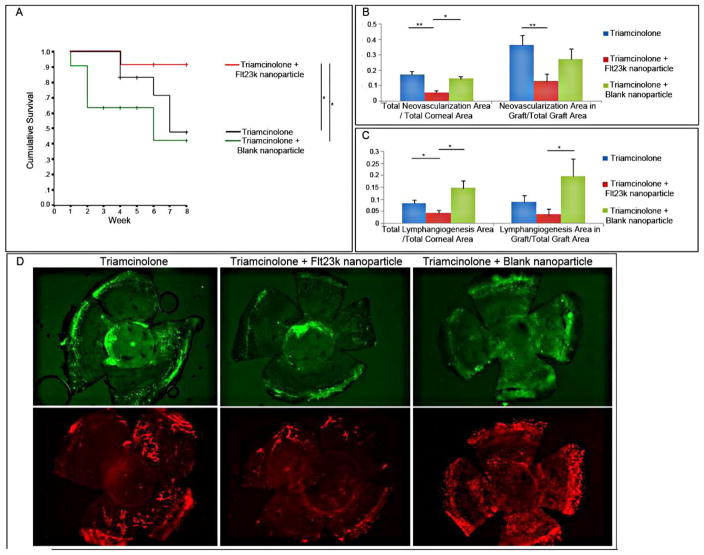
The anti-VEGF intraceptor, Flt23k, is a recombinant construct of the VEGF-binding domains 2 and 3 of VEGFR-1 that binds VEGF intracellularly and sequesters it within the endoplasmic reticulum, thereby inhibiting VEGF secretion. Cho and coworkers investigated the impact of Flt23k on corneal NV in murine models, either as a stand-alone treatment or combination treatment together with triamcinolone (Figs. 19–20).40 As a standalone treatment, Flt23k treatment led to better graft survival and the corneal opacity grade than treatment with PBS or blank nanoparticles. Given together with triamcinolone, Flt23k treatment resulted in an even better graft survival rate than triamcinolone alone.
Figure 19.
(A) Comparison of graft survival through 8 weeks postoperatively. The Flt23k nanoparticle group showed better survival than the PBS group (P = 0.009) until 8 weeks postoperatively and better survival than the blank nanoparticle group until 3 weeks postoperatively (P = 0.029). (B) Comparison of graft opacity grade for each week. Through 2 to 5 weeks postoperatively, the Flt23k nanoparticle group showed decreased opacification compared with the PBS group (P < 0.05).40 (Adapted from Cho et al with permission from IOVS) )
Figure 20. (A) Treatment with Flt23k nanoparticle plus triamcinolone increased graft survival compared with triamcinolone and triamcinolone plus blank nanoparticles.
(P = 0.048, P = 0.020, respectively). +Sensored data, *P < 0.05, **P < 0.01. (B) The Flt23k nanoparticle plus triamcinolone group showed less total NV compared with the triamcinolone group (P = 0.000) and triamcinolone plus blank nanoparticle group (P = 0.028). The Flt23k nanoparticle plus triamcinolone group showed less graft NV compared with the triamcinolone group (P = 0.008). (C) The Flt23k nanoparticle plus triamcinolone group showed significantly less total lymphangiogenesis compared with the triamcinolone group (P = 0.043) and triamcinolone plus blank nanoparticle group (P = 0.014). The Flt23k nanoparticle plus triamcinolone group showed less graft lymphangiogenesis compared with the triamcinolone plus blank nanoparticle group (P = 0.028). (D) Representative images of NV (upper row) and lymphangiogenesis (lower row) in each group.40 (Adapted from Cho et al with permission from IOVS)
Inhibition of VEGFR-1/VEGFR-2
It is known that VEGF-A drives angiogenesis principally by binding to VEGFR-2 and concurrently acts as a chemoattractant for monocytes/macrophages through binding with VEGFR-1. This chemotaxis consequently results in lymphangiogenesis as monocytes/macrophages release lymphangiogenic growth factors. 46 Hong and coworkers also reported that VEGF-A may induce lymphangiogenesis through VEGFR-2 binding. 82 Hence, by inhibiting VEGFR-1 and VEGFR-2, both HA and LA could also be averted.46; 59; 122
In earlier experiments sVEGFR-1 was shown to be a natural potent inhibitor of angiogenesis,8 and sVEGFR-2 to be an inhibitor of lymphangiogenesis.6 Hayashi and coworkers then conducted an experiment in which they administered sVEGFR-1/Fc chimera protein and sVEGFR-2/Fc chimera protein in a mouse model of corneal transplantation.77 They discovered that no NV occurred in mice given sVEGFR-1/Fc chimera protein but the graft failed due to wound rupture. Though sVEGFR-1/Fc chimera protein administration was successful in inhibiting corneal NV, it may impede corneal wound healing, as was reported in previous experiments.53; 104 On the other hand, mice treated with sVEGFR-2/Fc chimera protein exhibited reduced corneal NV without wound healing disruption, suggesting that preserving angiogenesis is important for delivering nutrients and promoting corneal wound healing. Furthermore, VEGFR-2/Fc chimera protein decreased macrophage infiltration, further downregulating the immune response, and finally increased corneal graft survival rates.
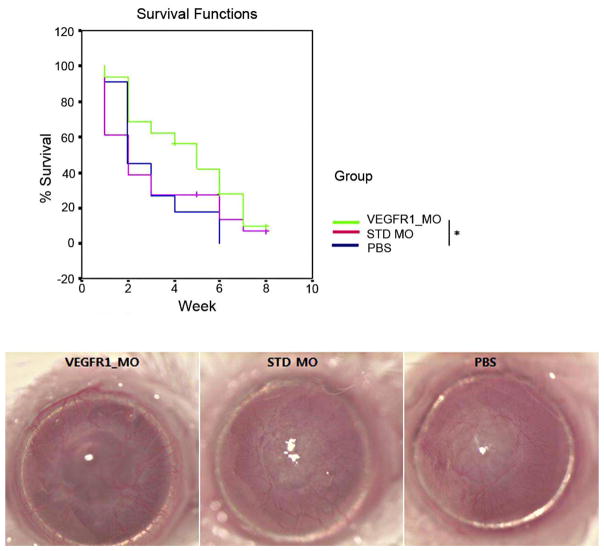
Morpholinos are synthetic molecules similar to DNA oligonucleotides that can bind RNA to block translation or alternative splicing. According to a study by Cho and coworkers, VEGFR-1–specific morpholino (VEGFR-1_MO) can inhibit NV and inflammation in a murine corneal suture model.39 They continued their investigation and showed that VEGFR-1_MO may also promote graft survival in a murine PK model (Fig. 21).41
Figure 21. Survival curve for NR PK and biomicroscopic pictures from each group at 8 weeks postoperatively.
VEGFR-1_MO increased graft survival compared to the PBS control treatment (P = 0.043). *P < 0.05 41 (Adapted from Cho et al with permission from IOVS)
Administration of Endostatin
Various investigators have also found that administration of endostatin protein inhibits tumor growth in many animal models, as well as in humans.65; 113; 190 Specifically, endostatin is one of the many angiostatic factors that inhibit angiogenesis by interfering with the mechanisms that govern endothelial cell growth.162 Corneal NV can be suppressed by administering exogenous endostatin through an assessment of endostatin levels in a setting in which allografts are not rejected.204 Tan and coworkers showed that endogenous endostatin upregulation is part of the mechanism that prevents graft failure (Fig. 22).182 Using an enzyme-linked immunosorbent assays (ELISA), they demonstrated upregulation of endostatin production in normal BALB/c murine corneas after corneal transplantation, regardless of the donor tissue; however, they observed that endostatin production starts to decline at postoperative day 10 in allogeneic corneal transplants and reaches the level in normal corneas by postoperative day 20, generally faster than in syngeneic corneal transplants. Maximal production of endostatin occurs around postoperative day 10 in the distal stroma of the allografts, and this was confirmed by immunochemistry. The number of T cells increases as the early infiltrating allospecific T cells enclosed the endostatin-producing cells by postoperative day 10, causing a reduction in the number of endostatin-producing cells. On the contrary, in syngeneic corneal grafts there is no early recruitment of T cells and endostatin levels remain consistently high, maintaining corneal clarity and survival.182
Figure 22. Administration of exogenous endostatin promoted corneal allograft survival. (.
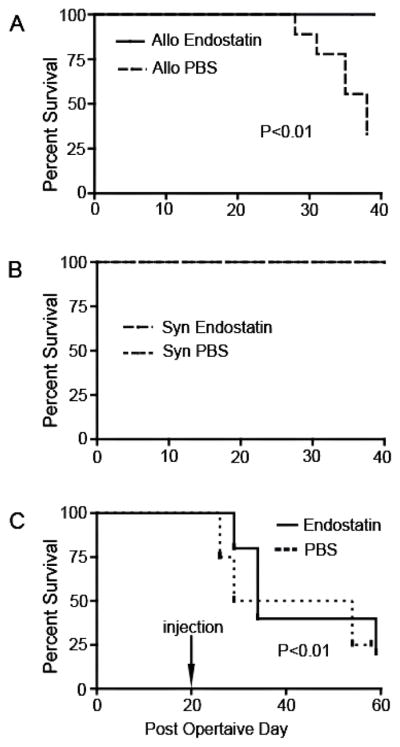
A) Mice with corneal transplants were treated with subconjunctival injections of either endostatin or PBS (control) from postoperative day (POD) 0 to POD30. In the PBS-treated group, the allografts started to be rejected at POD27. By POD40, 6 of 10 allografts in the PBS group had been rejected. In contrast, all allografts in the endostatin-treated group survived through POD40 (p < 0.01, n = 10). (B) All syngeneic grafts survived in both treatment groups. (C) Exogenous endostatin or PBS was administered to mice from POD20, when the allografts were vascularized, to POD50. By POD60, 75% of the allografts had been rejected, and there was no significant difference between the PBS-treated group and the endostatin-treated group (p > 0.05).182 (Adapted from Tan et al with permission)
VEGF, which is known to have a pro-angiogenic role, is expressed at higher levels in allogeneic grafts than in syngeneic grafts. In addition, a high level of endostatin is maintained in syngeneic grafts, but not allogeneic grafts. 182
LYMPHANGIOGENESIS-SPECIFIC INHIBITION
Blocking of VEGFR-3
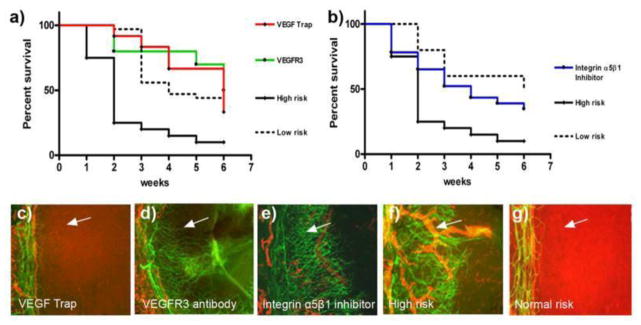
A dysfunctional lymphatic network can promote inflammation, cancer metastasis, infections, and transplant rejection. These avenues for pathologic involvement mark the importance of lymphangiogenesis and have led to the discovery of lymphatic markers such as lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1), transcription factor prospero homeobox protein-1 (Prox-1), and podoplanin.19; 27; 193 With the discovery of these markers, lymphatic vessels have been established as significant arbitrators of immune processes, as they provide the repaired tissue with foreign antigenic material and APCs, such as dendritic cells. Dietrich and coworkers used VEGFR-3 antibody and integrin α5β1 small molecules inhibitor specifically to suppress lymphangiogenesis in murine models to observe the importance of lymphangiogenesis in corneal graft survival rates (Fig. 23).55 They discovered that graft survival rates are equal for both cases of lymphatic vascularized high-risk recipient beds and low-risk recipient beds. On the contrary, survival is worse with pre-existing lymphatic vessels and blood vessels. Although such cases are considered high risk, those with alymphatic beds have good survival rates because lymphangiogenesis inhibition severed the afferent arm of the immune response reflex, rendering it unable to induce a transplantation rejection reaction.55 Additionally, Singh and coworkers investigated the ability of soluble VEGFR-3 (sVEGFR-3) that is expressed in the cornea to act like a trap that binds and sequesters VEGF-C and to inhibit lymphangiogenesis and corneal avascularity by inhibiting the VEGFR-2 phosphorylation effects of VEGF-C (Fig. 24).173
Figure 23. Lymphatic vessels in the recipient bed prior to transplantation determine graft survival.
In the 2 weeks prior to transplantation (when corneal suture placement was used to induce pathologic corneal NV in the recipient bed), mice were treated with VEGF-TrapR1R2 (a [red line] and c; resulting in no blood or lymphatic vessels, but reduced inflammation in the recipient bed at the time of transplantation), the VEGFR-3 Ab mF4-31C1 (a [green line] and d; resulting in no lymphatic vessels, but only blood vessels present in the recipient bed at the time of transplantation), or the JSM6427 integrin α5β1 inhibitor (b [blue line] and e; resulting in no lymphatic vessels, but only blood vessels, present in the recipient bed at the time of transplantation). Graft survival was compared with prehemvascularized and prelymphvascularized controls (a and b [black line], f: “high-risk” recipient bed) and avascular recipient controls (a and b [dotted line], g: “low-risk” recipient bed). The graft survival was significantly better when transplants were placed into recipient beds lacking lymphatic vessels compared with beds with lymphatic vessels present at the time of transplantation (VEGF-Trap versus high-risk: p < 0.0001; VEGFR-3 versus high-risk: p < 0.0002; n = 10; JSM6427 versus high-risk: p < 0.032, n = 23; Kaplan–Meyer survival curve). (c–g) Representative images of recipient corneal beds at the time of transplantation after corneas were treated with VEGF-TrapR1R2 (c), mF4-31C1 (VEGFR-3 Ab) (d), JSM6427 (e), or untreated high-risk (f) and normal-risk (g) recipient beds (original magnification ×100). Green, blood vessels; red, lymphatic vessels; arrow, prevascularized cornea.55 (Adapted from Dietrich at el with permission)
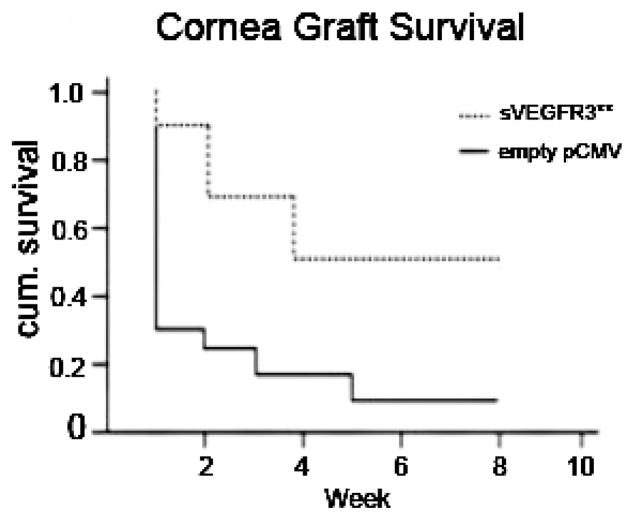
Figure 24. Overexpression of sVEGFR-3 is protective of transplant graft survival.
Corneal transplant graft survival was 40.0% with subconjunctival injection of sVEGFR-3–overexpressing plasmid (pCMV.sVEGFR-3) compared with 8.3% with empty pCMV in BALB/c recipient mice (n = 9-12). **P < .05 Kaplan-Meier survival analysis.173
Blocking of Integrins
Integrins are a group of heterodimeric cell surface transmembrane glycoproteins that are involved in cell–cell and cell–matrix interactions, although their functions are not fully understood.4; 68; 134 Kang and coworkers reported that integrins, specifically ITGA-9, are highly expressed in newly formed lymphatic valves in the cornea. Using an in vivo murine model of corneal inflammatory lymphangiogenesis with valve formation, they demonstrated that ITGA-9 has a key role in corneal lymphatic valve formation (valvulogenesis).96 Huang and coworkers also reported that, during development, ITGA-9 knockout mice died of chylothorax because of compromised lymphatic vessel integrity.86 This lethal condition happened as inhibition of lymph valvulogenesis caused disruption in lymphatic vessel maturity and ultimately its integrity. Kang and coworkers then demonstrated that corneal graft survival rate was increased when the murine model was treated with ITGA-9 antibody, subsequently preventing the formation of lymphatic valves and suggesting a disruption of the immune reflex arc in which the role of the afferent arm is played by the lymphatic pathway (Fig. 25).96 Dietrich et al also utilized integrin α5β1 antibody specifically to ssuppress lymphangiogenesis and demonstrated the importance of lymphangiogenesis in the transplantation rejection process in murine models.55
Figure 25. Integrin alpha 9 blockade promoted corneal graft survival.
(A) Representative images from slit-lamp examination of rejected and surviving grafts in control and treatment conditions, respectively. (B) Kaplan-Meier survival curves showing significantly higher survival rate in the treatment group. *P < 0.05.96 (Adapted from Kang et al with permission from IOVS)
Neutralization of Podoplanin
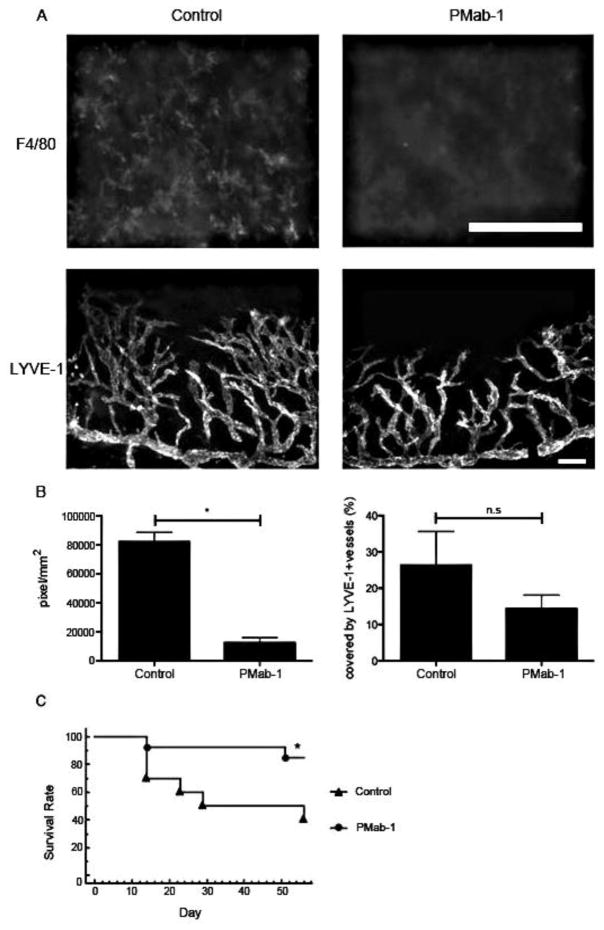
Podoplanin has been recognized as a specific marker of the lymphatic endothelium, yet its specific function is unknown.27; 123 Maruyama and coworkers investigated the function of podoplanin in lymphangiogenesis and inflammation using podoplanin antibody (PMab-1) (Fig. 26). They discovered that podoplanin inhibition using PMab-1 inhibited lymphangiogenesis and may have the potential to increase graft survival rates.123Table 3 summarizes the anti-angiogenic and anti-lymphangiogenic factors used in corneal transplantation.
Figure 26. Effect of PMab-1 on graft survival in a corneal transplantation model.
(A) Fluorescence micrograph indicating mps (F4/80) and lymphangiogenesis (LYVE-1) in the PMab-1– and PBS-treated mouse corneas 7 days after corneal transplantation. (B) Quantification of the number of mps (*P = 0.0286) and of lymphangiogenesis in the corneal transplantation model assay (n = 5, each group). n.s., no significant difference. (C) Graft survival rate in mice treated with PMab-1 (n = 13) or PBS as control (n = 12). *P = 0.0259.123 (Adapted from Maruyama et al with permission from IOVS)
Table 3.
Prospective Angiogenic Therapies
Hsu et al compiled a list of current and potential therapies, as shown in Table 4.85
Table 4.
Corneal antiangiogenesis target therapies91
| Targets | Methods | Therapeutics |
|---|---|---|
| Vascular endothelial growth factor | Anti-VEGF-A antibodies | Bevacizumab |
| Ranibizumab | ||
| FD006 | ||
| Soluble or modified VEGF receptors | Recombinant dimeric VEGFR-2-Fc | |
| sVEGFR-1 overexpression gene therapy | ||
| sVEGFR-3 overexpression gene therapy | ||
| VEGFR-1 morpholino | ||
| Aflibercept/VEGF-Trap(R1R2) | ||
| VEGFR intraceptor gene therapy | ||
| (Flt23k, Flt24k) | ||
| VEGF-A aptamer | Pegaptanib | |
| Pigment epithelium-derived factor | PEDF direct effect | PEDF |
| PEDF-derived peptide | ||
| PEDF gene therapy | ||
| Platelet-derived growth factor | PDGF receptor inhibitor | AG 1296 |
| Multitargeted receptor tyrosine | Sunitinib | |
| kinase inhibitor | ||
| Angiostatin | Angiostatin direct effect | Angiostatin pump |
| Hypoxia-inducible factors | shRNA for hypoxia-inducible factors | HIF-1α shRNA gene therapy (HIF- 1α RNAi-A) |
| 12-Hydroxyeicosatrienoic acid | siRNA for cytochrome P450 mono-oxygenase | CYP4B1 siRNA gene therapy |
| Vascular adhesion protein | VAP-1/SSAO inhibitor | U–V002 |
| LJP1207 | ||
| Decorin | Decorin direct effect | Decorin gene therapy |
| Vasohibin-1 | Vasohibin-1 directly effect | Vasohibin-1 gene therapy |
| Cannabinoid receptor CB1 | CB1 antagonist | Rimonabant |
CYP = cytochrome P450 mono-oxygenase; HIF-1α = hypoxia-inducible factor 1α; PDGF = platelet- derived growth factor; PEDF = pigment epithelium-derived factor; SSAO = semicarbazide-sensitive amine oxidase; sVEGFR = soluble form of vascular endothelial growth factor receptor; VAP- 1 = vascular adhesive protein-1; VEGF = vascular endothelial growth factor; VEGFR = vascular endothelial growth factor receptor.
Other potential therapies: steroid (dexamethasone, prednisolone, fluorometholone), rapamycin, cyclosporine A, thalidomide analogue (CC-3052). (Adapted from Hsu at el. with permission from Elsevier)
THERAPEUTIC ALTERNATIVES FOR CORNEAL TRANSPLANTATION
Keratocytes, which are the typically quiescent cells of the corneal stroma, transform into fibroblasts to produce scar tissue to repair corneal damage. The presence of fibrotic extracellular matrix (ECM) segments in stromal scars, in turn, scatters light, resulting in reduction of visual capacity.144 Visual debilitation and blindness attributable to corneal scarring influence millions worldwide and represent the most common indication for corneal transplantation (PK) in China and India52; 140 and likely in the developing world. Given the global shortage of donor corneal grafts, there is expanding enthusiasm for the advancement of corneal substitutes to help satisfy the global demand for donor corneal tissue.
Keratoprosthesis
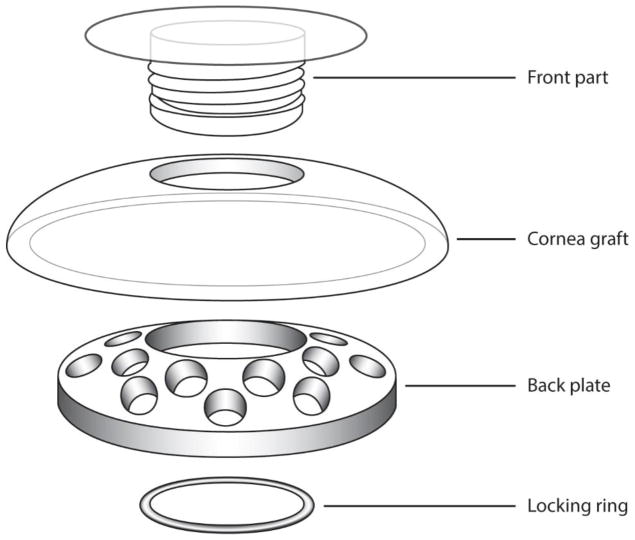
The Dohlman-Doane type I (Boston) (Fig. 27), AlphaCor, osteo-odonto-keratoprosthesis (OOKP), and Fyodorov-Zuev are keratoprosthesis (KPro) implants currently on the market. KPro continues to be the technique utilized in patients with a poor prognosis for keratoplasty. KPro devices have decreased the overall rate of complications and improved the prognosis for visual rehabilitation for more than 30 years.15
Figure 27. Assembly of the Boston Type I KPro device.
(http://webeye.ophth.uiowa.edu/eyeforum/tutorials/Cornea-Transplant-Intro/6-kprosth.htm)
The most widely used KPro device is the Boston KPro (BKPro). There are basically two types of BKPro devices.11; 87; 120 In the United States, the most commonly used is type I, which is composed of a front plate that accommodates the refractive portion and a second plate that is a detachable perforated back plate. The donor corneal tissue is placed between the two plates.
Recently, a newer generation type I BKPro has been developed, which has a threadless design and titanium back plate (vs. polymethylmetacrylate). This model does not need a locking c-ring, is easier to assemble, and induces less inflammation and retroprosthetic membrane (RPM) complication.185
Creating a Tissue-Engineered Cornea
Some researchers have tried to recreate corneal tissue from cells. By using the information contained in cells, researchers have tried to mimic the structure and function of the cornea.169 Such efforts have used fibroblasts, epithelial cells, and endothelial cells. Guo and coworkers were able to culture human corneal fibroblasts inside ascorbic acid where they created their own ECM. The architecture of resultant tissue showed similarity with human corneal architecture, but the fiber diameter (38.1 ± 7.4 nm) was thicker than that in the natural cornea (30.1 ± 2.5 nm).71 Others have attempted to use mechanical stimuli to develop the tissue.137 Epithelial and endothelial cells can be made into cell sheets simply by using temperature-responsive culture dishes.179; 195 Hayashida and workers78 transplanted epithelial sheets into rabbit after excimer laser photoablation and observed a significant increase in corneal clarity. Sumide and coworkers used endothelial cell sheets in rabbit eyes and found less corneal swelling compared to control animals (496 ± 111.6 μm vs. 887.5 ± 69.0 μm).179 Finally, Zieske and coworkersl cultured all three cell types together (fibroblasts, epithelial cells, and endothelial cells), and their results indicate that interactions between different tissues will affect how the cell layers grow. 206
Scaffolds Used for Tissue-Engineered Cornea
In addition to the cell layers, a frame is also needed as a place for the cells to grow. This frame is called a scaffold. Various types of biomaterials have been used in attempts to create the best scaffold possible. The two main materials used for creating a scaffold have been collagen and polymers.
The composition of a collagen scaffold resembles that of the human cornea, but it has weaker mechanical properties. To compensate for this, researchers crosslinked the collagen gel with other materials. Unfortunately, the rejection reaction is another problem that is encountered when using a scaffold. Rejection reactions occur when allogenic cells in a standard corneal transplant are targeted by the recipient’s immune system. Buznyk and coworkers29 showed that this can be avoided by using cell-free recombinant human collagen type III (RHCIII)-based corneal implants crosslinked with 2-methacryloyloxyethyl phosphorylcholine (MPC) polymer to create structures that roughly mimic the shape of the human cornea. Furthermore, MPC polymer has anti-inflammatory properties and could decrease the immune reactions. Fagerholm and coworkers62 also cross-linked RHC with carbodiimide (RHCIII-EDC/NHS) and showed that the revitalized neo-corneas utilizing RHC implants were accepted without rejection as, in comparison to donor grafts, inflammatory dendritic cells were not recruited into the implant area. Silk fibroin may be another effective substrate for cell proliferation. 1; 117
Synthetic polymers may offer another way to fabricate an effective scaffold for a corneal equivalent. This type of scaffold can be designed to be more mechanically similar to the natural cornea. In addition, some of the limitations of collagen can be overcome by these scaffolds as they do not contract in the presence of cells and can be fabricated with more stable and tunable degradation properties.169 Despite that, synthetic polymer scaffolds seem to elicit a stronger immune reaction than collagen scaffolds. For example, Hu and coworkers investigated the use of poly-glycolic acid (PGA) scaffolds in rabbit cornea and observed corneal thickening despite the corneas being transparent after 8 weeks. Further research is required before this material can be used in humans.
Biosynthetic implants may become an excellent substitute in high-risk cases for restoring corneal integrity. They may also alleviate the social and religious ethical dilemmas that stop patients from pursuing transplantation with allograft corneas and have the potential to become feasible long-term options to overcome the shortage of donor corneas in many parts of the world.29
STEM CELL THERAPY
Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs), multipotent cells embedded in different tissues, such as bone marrow, umbilical cord, amniotic membrane, cartilage, adipose tissue, cornea, and conjunctiva, can be extracted and expanded in vitro. MSCs are self-renewing and can differentiate into other types of connective tissue cells under specific conditions. Generally, MSCs are harvested from the bone marrow (BMSCs), yet the umbilical cord (UMSCs) may have more advantages due to greater availability and potency in cell division and differentiation capacity.97; 197 MSCs also show low expression of major histocompatibility class II (MHC-II) and can evade immune system monitoring, thus allowing allogeneic transplantation without rejection.110; 163
One of the disadvantages of MSCs is that, although they express many cell surface markers including stem cell markers, no unique markers are available for their detection.97 MSCs share many similar markers with keratocytes, and without a unique marker, some researchers believe that there is little evidence that MSCs can differentiate into corneal epithelial cells.197 They further suggested that MSCs exert their therapeutic potential not only by differentiating into corneal epithelial cells, but also through their own anti-inflammatory and anti-angiogenic functions.121; 136
A study by Ma and coworkers demonstrated recovery of damaged rat cornea treated with hMSCs and suggested that hMSCs inhibit both inflammation and inflammation-related angiogenesis. Expression of both CD45, a lymphocyte common antigen 7, and interleukin 2 (IL-2) was significantly depressed as a sign of anti-inflammation; and MMP-2, an inflammation-related angiogenesis modulator, was also undetected in hMSC-treated damaged rat cornea.121
Oh et al also reported upregulation of thrombospondin-1 (TSP-1), a potent anti-angiogenic factor, in MSC-treated damaged rat corneal epithelium. TSP-1 reduces angiogenesis by promoting endothelial apoptosis, inhibiting VEGF, and down-regulating MMPs and subsequently restores corneal angiogenic privilege.136
Limbal Stem Cells
Adult stem cells at the human corneoscleral limbus, called limbal stem cells, are responsible for producing a steady and constant supply of corneal epithelial cells throughout life, and these cells maintain a stable and uniformly refractive corneal surface.170; 186; 202 Blinding conditions such as ocular burns thatwere previously considered incurable have been addressed successfully by therapeutic approaches based on association between ocular surface disease and limbal stem cell dysfunction.
In recent times, many innovations from research of ocular surface biology have made the surgical technique for limbal stem cell transplantation simpler and more predictable.32; 102; 146; 156; 164; 165
Autologous limbal transplantation, either simple limbal epithelial transplantation (SLET) or cultivated limbal epithelial transplantation (CLET) may be possible if at least a 1-hour clock of healthy limbus tissue is available in the unaffected eye. This is an advantage over conjunctival-limbal autologous transplant (CLAU) that require more than 1-hour clock of tissue, thus bearing the risk of scarring. SLET is prescribed as the favored surgical technique over CLET. This is because SLET does not require cell culture, which needs advanced and clean facilities, and thus is less complicated, more cost effective, and more practically viable in a setting with limited resources.156; 164; 166
When both eyes have total LSCD, however, the preference for cell-based therapy is between allogeneic-limbal transplantation (either from living related or cadaveric) or autologous cultivated oral mucosa epithelial transplantation (COMET).32; 61; 88; 127; 148 This is a selection between long-term immunosuppression (allogeneic limbal transplantation) and visual outcomes (COMET). Efforts must be continued by major cell therapy centers to publish standardized clinical outcome data that aid the surgeon's ability to decide on which treatment to offer. Yet, there is no consensus among ocular surface surgeons regarding the favored therapy for bilateral LSCD.32
FUTURE DIRECTIONS
Despite many biomolecular discoveries, much research is still needed in the field of angiogenesis and lymphangiogenesis to explain the process in detail. By knowing the mechanisms in detail, prevention strategies and new treatments can be developed.
The development of an artificial cornea is a field of much interest, and many more studies are needed to develop more effective materials, to identify the factors involved in integration of the artificial tissue, to test the system in animal studies, and finally to examine its potential for use in humans. The ability to artificially engineer a corneal tissue would potentially solve the issue of the worldwide donor cornea shortage.
Various degrees of success in limbal stem cell transplantation warrant the development of an in vitro model of limbal stem cell transplantation that permits testing of various scenarios to further investigate the best strategy for use in clinical settings.166
CONCLUSION
Demand for donor corneal tissues remains high, whereas the supply remains low. These problems serve as the motivation for important research to reduce rejection rates of corneal transplants and find alternatives to human corneal tissue. A successful outcome of corneal transplantation is attributable to corneal immune privilege. Recent and ongoing research continues to show that immune-mediated corneal graft rejection depends on both angiogenesis and lymphangiogenesis, both of which are principally coordinated by the VEGF family of receptors and ligands. Studies show that several anti-lymphangiogenic strategies have exhibited success in improving graft survival. Thus, a thorough understanding of the roles played by angiogenesis and lymphangiogenesis in corneal transplantations has the potential to reduce the rejection rates for corneal grafts.
Method of Literature Search
In order to prepare this review, we conducted Google, Medline, and PubMed searches of the medical literature for the period between 1963 and 2017 using the following key words in various combinations: angiogenesis, lymphangiogenesis, neovascularization, and corneal transplant rejection. In addition, reference lists from the selected articles were used to identify additional articles not included in the electronic databases. Articles were appraised critically, and pertinent information was included in this review and cited accordingly.
Figure 8. Fate of high-risk MHC-disparate corneal transplants in mice (n= 14/group) after anti-CD154 treatment was discontinued at week 8 after transplantation.
Graft rejection was scored clinically (A), and graft survival data are presented as Kaplan–Meier survival curves (B). Only one rejection occurred after cessation of anti-CD154 therapy (**P = 0.0002).155 (Adapted from Qain et al with permission from IOVS)
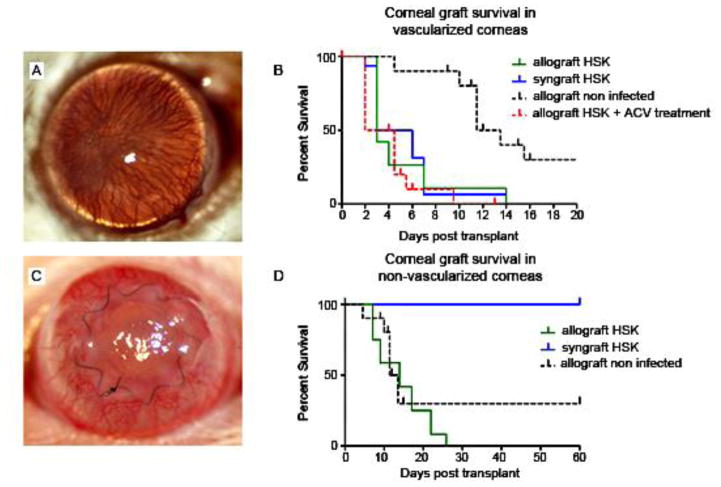
Figure 11. Rejection of corneal grafts placed into WT HSV-1–infected high-risk hosts. Wild-type BALB/c mice were infected with HSV-1 6 weeks (42 days) prior to placement of corneal grafts, which were secured with a continuous suture in this set of experiments.
(a) Clinical appearance of the vascularized corneal graft bed at 6 week post HSV-1 infection; (b) corneal graft survival in vascularized corneal beds in syngraft HSK (n = 15), allograft HSK (n = 20), and allograft HSK treated with ACV (n = 10) groups compared with WT, noninfected BALB/c recipients transplanted with allograft (n = 20); (c) clinical appearance of rejected corneal allograft placed into vascularized corneal graft bed (day 3 post allograft); (d) corneal graft survival in previously infected but not vascularized (at 6 weeks post infection [p.i.]) graft beds for both allo- and syngrafts compared with WT, noninfected BALB/c recipient transplanted with allograft.107 (Adapted from Kuffova et al with permission from IOVS)
Figure 16.
Subconjunctival bevacizumab promotes corneal allograft survival in the high-risk setting. High-risk graft beds in BALB/c mice were transplanted with C57BL/6 cornea, and mice were left untreated (n = 10) or were treated topically (n = 10) or subconjunctivally (n = 10) with bevacizumab.53 (Adapted from Dastjerdi et al with permission from IOVS)
Acknowledgments
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article. Publication for this article was supported by National Institutes of Health grants EY10101 (D.T.A.), EY023691, EY021886, I01 BX002386, Eversight, Midwest Eye Bank Award (J.H.C), and EY01792, EY027912 (MIR) and an unrestricted grant from Research to Prevent Blindness, New York, NY.
Abbreviations
- bFGF
Basic fibroblast growth factor
- BM
Basement membrane
- ECM
Extracellular matrix
- EGF
Epidermal growth factor
- EGFR
Epidermal growth factor receptor
- FGF
Fibroblast growth factor
- FGFR
Fibroblast growth factor receptor
- HPC
Hematopoietic progenitor cell
- KI
Knock-in
- KO
Knockout
- MMP
Matrix metalloproteinase
- NV
Neovascularization
- PDGF
Platelet-derived growth factor
- PDGFR
Platelet-derived growth factor receptor
- PTEN
Phosphatase and tensin homolog
- RECK
Reversion-inducing cysteine-rich protein with Kazal motifs
- ROCK
Rho-associated kinase
- TLR
Toll-like receptor
- VEGF
Vascular endothelial growth factor
- VEGFR
Vascular endothelial growth factor receptor
Footnotes
Disclosures
No conflicts of interest, financial or otherwise, are declared by the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abdel-Naby W, Liu A, Wey N, Rosenblatt MI. Silk fibroin substrates support corneal epithelial cellular adhesion under shear stress. Invest Ophthalmol Vis Sci. 2014;55:4633–4633. [Google Scholar]
- 2.Abdelfattah NS, Amgad M, Zayed AA, et al. Molecular underpinnings of corneal angiogenesis: advances over the past decade. International Journal of Ophthalmology. 2016;9:768–779. doi: 10.18240/ijo.2016.05.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdelfattah NS, Amgad M, Zayed AA, et al. Clinical correlates of common corneal neovascular diseases: a literature review. Int J Ophthalmol. 2015;8:182–193. doi: 10.3980/j.issn.2222-3959.2015.01.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abram CL, Lowell CA. The ins and outs of leukocyte integrin signaling. Annu Rev Immunol. 2009;27:339–362. doi: 10.1146/annurev.immunol.021908.132554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Debasi T, Al-Bekairy A, Al-Katheri A, et al. Topical versus subconjunctival anti-vascular endothelial growth factor therapy (Bevacizumab, Ranibizumab and Aflibercept) for treatment of corneal neovascularization. Saudi Journal of Ophthalmology. 2017;31:99–105. doi: 10.1016/j.sjopt.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albuquerque RJ, Hayashi T, Cho WG, et al. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat Med. 2009;15:1023–1030. doi: 10.1038/nm.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altin JG, Sloan EK. The role of CD45 and CD45-associated molecules in T cell activation. Immunol Cell Biol. 1997;75:430–445. doi: 10.1038/icb.1997.68. [DOI] [PubMed] [Google Scholar]
- 8.Ambati BK, Nozaki M, Singh N, et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993–997. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson C, Zhou Q, Wang S. An alkali-burn injury model of corneal neovascularization in the mouse. J Vis Exp. 2014 doi: 10.3791/51159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anshu A, Price MO, Price FW., Jr Risk of corneal transplant rejection significantly reduced with Descemet's membrane endothelial keratoplasty. Ophthalmology. 2012;119:536–540. doi: 10.1016/j.ophtha.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Aquavella JV, Qian Y, McCormick GJ, Palakuru JR. Keratoprosthesis: the Dohlman-Doane device. Am J Ophthalmol. 2005;140:1032–1038. doi: 10.1016/j.ajo.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 12.Armitage WJ, Dick AD, Bourne WM. Predicting endothelial cell loss and long-term corneal graft survival. Invest Ophthalmol Vis Sci. 2003;44:3326–3331. doi: 10.1167/iovs.02-1255. [DOI] [PubMed] [Google Scholar]
- 13.Armitage WJ, Jones MN, Zambrano I, et al. The suitability of corneas stored by organ culture for penetrating keratoplasty and influence of donor and recipient factors on 5-year graft survival. Invest Ophthalmol Vis Sci. 2014;55:784–791. doi: 10.1167/iovs.13-13386. [DOI] [PubMed] [Google Scholar]
- 14.Arnalich-Montiel F, Alio Del Barrio JL, Alio JL. Corneal surgery in keratoconus: which type, which technique, which outcomes? Eye Vis (Lond) 2016;3:2. doi: 10.1186/s40662-016-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avadhanam VS, Smith HE, Liu C. Keratoprostheses for corneal blindness: a review of contemporary devices. Clin Ophthalmol. 2015;9:697–720. doi: 10.2147/OPTH.S27083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azar DT. Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2006;104:264–302. [PMC free article] [PubMed] [Google Scholar]
- 17.Bachmann B, Taylor RS, Cursiefen C. Corneal neovascularization as a risk factor for graft failure and rejection after keratoplasty: an evidence-based meta-analysis. Ophthalmology. 2010;117:1300–1305e1307. doi: 10.1016/j.ophtha.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 18.Bachmann BO, Bock F, Wiegand SJ, et al. Promotion of graft survival by vascular endothelial growth factor a neutralization after high-risk corneal transplantation. Arch Ophthalmol. 2008;126:71–77. doi: 10.1001/archopht.126.1.71. [DOI] [PubMed] [Google Scholar]
- 19.Banerji S, Ni J, Wang SX, et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barker CF, Billingham RE. Immunologically privileged sites. Adv Immunol. 1977;25:1–54. [PubMed] [Google Scholar]
- 21.Bashshur ZF, Bazarbachi A, Schakal A, et al. Intravitreal bevacizumab for the management of choroidal neovascularization in age-related macular degeneration. Am J Ophthalmol. 2006;142:1–9. doi: 10.1016/j.ajo.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 22.Beckingsale P, Mavrikakis I, Al-Yousuf N, et al. Penetrating keratoplasty: outcomes from a corneal unit compared to national data. Br J Ophthalmol. 2006;90:728–731. doi: 10.1136/bjo.2005.086272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boisjoly HM, Bernard PM, Dube I, et al. Effect of factors unrelated to tissue matching on corneal transplant endothelial rejection. Am J Ophthalmol. 1989;107:647–654. doi: 10.1016/0002-9394(89)90262-6. [DOI] [PubMed] [Google Scholar]
- 24.Bonfadini G, Moreira H, Jun AS, et al. Modified femtosecond laser-assisted sutureless anterior lamellar keratoplasty. Cornea. 2013;32:533–537. doi: 10.1097/ICO.0b013e31826e828c. [DOI] [PubMed] [Google Scholar]
- 25.Bourne WM. Cellular changes in transplanted human corneas. Cornea. 2001;20:560–569. doi: 10.1097/00003226-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Boynton GE, Woodward MA. Evolving Techniques in Corneal Transplantation. Current Surgery Reports. 2015;3:2. doi: 10.1007/s40137-014-0079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breiteneder-Geleff S, Soleiman A, Horvat R, et al. Podoplanin--a specific marker for lymphatic endothelium expressed in angiosarcoma. Verh Dtsch Ges Pathol. 1999;83:270–275. [PubMed] [Google Scholar]
- 28.Busin M, Arffa RC. Deep suturing technique for penetrating keratoplasty. Cornea. 2002;21:680–684. doi: 10.1097/00003226-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Buznyk O, Pasyechnikova N, Islam MM, et al. Bioengineered Corneas Grafted as Alternatives to Human Donor Corneas in Three High-Risk Patients. Clin Transl Sci. 2015;8:558–562. doi: 10.1111/cts.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bysterska P, Svozilkova P, Farghali H. Effect of certain immunosuppressants on non-specific immunity cells in murine corneal grafts: study on early phases after transplantation. Physiol Res. 2007;56:603–610. doi: 10.33549/physiolres.931081. [DOI] [PubMed] [Google Scholar]
- 31.Cao R, Ji H, Feng N, et al. Collaborative interplay between FGF-2 and VEGF-C promotes lymphangiogenesis and metastasis. Proc Natl Acad Sci U S A. 2012;109:15894–15899. doi: 10.1073/pnas.1208324109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cauchi PA, Ang GS, Azuara-Blanco A, Burr JM. A systematic literature review of surgical interventions for limbal stem cell deficiency in humans. Am J Ophthalmol. 2008;146:251–259. doi: 10.1016/j.ajo.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 33.Chang JH, Gabison EE, Kato T, Azar DT. Corneal neovascularization. Curr Opin Ophthalmol. 2001;12:242–249. doi: 10.1097/00055735-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Chang JH, Garg NK, Lunde E, et al. Corneal neovascularization: an anti-VEGF therapy review. Surv Ophthalmol. 2012;57:415–429. doi: 10.1016/j.survophthal.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang JH, Huang YH, Cunningham CM, et al. Matrix metalloproteinase 14 modulates signal transduction and angiogenesis in the cornea. Surv Ophthalmol. 2016;61:478–497. doi: 10.1016/j.survophthal.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang JH, Putra I, Huang YH, et al. Limited versus total epithelial debridement ocular surface injury: Live fluorescence imaging of hemangiogenesis and lymphangiogenesis in Prox1-GFP/Flk1::Myr-mCherry mice. Biochim Biophys Acta. 2016;1860:2148–2156. doi: 10.1016/j.bbagen.2016.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chauhan SK, Dohlman TH, Dana R. Corneal Lymphatics: Role in Ocular Inflammation as Inducer and Responder of Adaptive Immunity. Journal of clinical & cellular immunology. 2014;5:1000256. doi: 10.4172/2155-9899.1000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chia EM, Wang JJ, Rochtchina E, et al. Impact of bilateral visual impairment on health-related quality of life: the Blue Mountains Eye Study. Invest Ophthalmol Vis Sci. 2004;45:71–76. doi: 10.1167/iovs.03-0661. [DOI] [PubMed] [Google Scholar]
- 39.Cho YK, Uehara H, Young JR, et al. Vascular endothelial growth factor receptor 1 morpholino decreases angiogenesis in a murine corneal suture model. Invest Ophthalmol Vis Sci. 2012;53:685–692. doi: 10.1167/iovs.11-8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cho YK, Uehara H, Young JR, et al. Flt23k nanoparticles offer additive benefit in graft survival and anti-angiogenic effects when combined with triamcinolone. Invest Ophthalmol Vis Sci. 2012;53:2328–2336. doi: 10.1167/iovs.11-8393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cho YK, Zhang X, Uehara H, et al. Vascular Endothelial Growth Factor Receptor 1 morpholino increases graft survival in a murine penetrating keratoplasty model. Invest Ophthalmol Vis Sci. 2012;53:8458–8471. doi: 10.1167/iovs.12-10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clauss M, Weich H, Breier G, et al. The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J Biol Chem. 1996;271:17629–17634. doi: 10.1074/jbc.271.30.17629. [DOI] [PubMed] [Google Scholar]
- 43.The collaborative corneal transplantation studies (CCTS) Effectiveness of histocompatibility matching in high-risk corneal transplantation. The Collaborative Corneal Transplantation Studies Research Group. Arch Ophthalmol. 1992;110:1392–1403. [PubMed] [Google Scholar]
- 44.Coyne KS, Margolis MK, Kennedy-Martin T, et al. The impact of diabetic retinopathy: perspectives from patient focus groups. Fam Pract. 2004;21:447–453. doi: 10.1093/fampra/cmh417. [DOI] [PubMed] [Google Scholar]
- 45.Cursiefen C, Cao J, Chen L, et al. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Invest Ophthalmol Vis Sci. 2004;45:2666–2673. doi: 10.1167/iovs.03-1380. [DOI] [PubMed] [Google Scholar]
- 46.Cursiefen C, Chen L, Borges LP, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cursiefen C, Chen L, Dana MR, Streilein JW. Corneal lymphangiogenesis: evidence, mechanisms, and implications for corneal transplant immunology. Cornea. 2003;22:273–281. doi: 10.1097/00003226-200304000-00021. [DOI] [PubMed] [Google Scholar]
- 48.Cursiefen C, Maruyama K, Jackson DG, et al. Time course of angiogenesis and lymphangiogenesis after brief corneal inflammation. Cornea. 2006;25:443–447. doi: 10.1097/01.ico.0000183485.85636.ff. [DOI] [PubMed] [Google Scholar]
- 49.Cursiefen C, Schlotzer-Schrehardt U, Kuchle M, et al. Lymphatic vessels in vascularized human corneas: immunohistochemical investigation using LYVE-1 and podoplanin. Invest Ophthalmol Vis Sci. 2002;43:2127–2135. [PubMed] [Google Scholar]
- 50.Dana MR. Angiogenesis and lymphangiogenesis-implications for corneal immunity. Semin Ophthalmol. 2006;21:19–22. doi: 10.1080/08820530500509358. [DOI] [PubMed] [Google Scholar]
- 51.Dandona L, Naduvilath TJ, Janarthanan M, Rao GN. Causes of corneal graft failure in India. Indian J Ophthalmol. 1998;46:149–152. [PubMed] [Google Scholar]
- 52.Dandona L, Ragu K, Janarthanan M, et al. Indications for penetrating keratoplasty in India. Indian J Ophthalmol. 1997;45:163–168. [PubMed] [Google Scholar]
- 53.Dastjerdi MH, Saban DR, Okanobo A, et al. Effects of topical and subconjunctival bevacizumab in high-risk corneal transplant survival. Invest Ophthalmol Vis Sci. 2010;51:2411–2417. doi: 10.1167/iovs.09-3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dawson DW, Volpert OV, Gillis P, et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 55.Dietrich T, Bock F, Yuen D, et al. Cutting edge: lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J Immunol. 2010;184:535–539. doi: 10.4049/jimmunol.0903180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dohlman TH, Omoto M, Hua J, et al. VEGF-trap Aflibercept Significantly Improves Long-term Graft Survival in High-risk Corneal Transplantation. Transplantation. 2015;99:678–686. doi: 10.1097/TP.0000000000000512. [DOI] [PubMed] [Google Scholar]
- 57.Dua HS, Azuara-Blanco A. Corneal allograft rejection: risk factors, diagnosis, prevention, and treatment. Indian J Ophthalmol. 1999;47:3–9. [PubMed] [Google Scholar]
- 58.Ecoiffier T, Yuen D, Chen L. Differential distribution of blood and lymphatic vessels in the murine cornea. Invest Ophthalmol Vis Sci. 2010;51:2436–2440. doi: 10.1167/iovs.09-4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ellenberg D, Azar DT, Hallak JA, et al. Novel aspects of corneal angiogenic and lymphangiogenic privilege. Prog Retin Eye Res. 2010;29:208–248. doi: 10.1016/j.preteyeres.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elyashiv SM, Shabtai EL, Belkin M. Correlation between visual acuity and cognitive functions. British Journal of Ophthalmology. 2013;98:129. doi: 10.1136/bjophthalmol-2013-304149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Espana EM, Di Pascuale M, Grueterich M, et al. Keratolimbal allograft in corneal reconstruction. Eye (Lond) 2004;18:406–417. doi: 10.1038/sj.eye.6700670. [DOI] [PubMed] [Google Scholar]
- 62.Fagerholm P, Lagali NS, Ong JA, et al. Stable corneal regeneration four years after implantation of a cell-free recombinant human collagen scaffold. Biomaterials. 2014;35:2420–2427. doi: 10.1016/j.biomaterials.2013.11.079. [DOI] [PubMed] [Google Scholar]
- 63.Fenwick EK, Lamoureux EL, Keeffe JE, et al. Detection and Management of Depression in Patients with Vision Impairment. Optometry and Vision Science. 2009;86:948–954. doi: 10.1097/OPX.0b013e3181b2f599. [DOI] [PubMed] [Google Scholar]
- 64.Fernandez MM, Afshari NA. Endothelial Keratoplasty: From DLEK to DMEK. Middle East Afr J Ophthalmol. 2010;17:5–8. doi: 10.4103/0974-9233.61210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Folkman J. Antiangiogenesis in cancer therapy--endostatin and its mechanisms of action. Exp Cell Res. 2006;312:594–607. doi: 10.1016/j.yexcr.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 66.Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267:10931–10934. [PubMed] [Google Scholar]
- 67.Gain P, Jullienne R, He Z, et al. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016;134:167–173. doi: 10.1001/jamaophthalmol.2015.4776. [DOI] [PubMed] [Google Scholar]
- 68.Ganguly KK, Pal S, Moulik S, Chatterjee A. Integrins and metastasis. Cell Adh Migr. 2013;7:251–261. doi: 10.4161/cam.23840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goel S, Duda DG, Xu L, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91:1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gorovoy MS. Descemet-stripping automated endothelial keratoplasty. Cornea. 2006;25:886–889. doi: 10.1097/01.ico.0000214224.90743.01. [DOI] [PubMed] [Google Scholar]
- 71.Guo X, Hutcheon AE, Melotti SA, et al. Morphologic characterization of organized extracellular matrix deposition by ascorbic acid-stimulated human corneal fibroblasts. Invest Ophthalmol Vis Sci. 2007;48:4050–4060. doi: 10.1167/iovs.06-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hahn N, Dietz CT, Kuhl S, et al. KLEIP deficiency in mice causes progressive corneal neovascular dystrophy. Invest Ophthalmol Vis Sci. 2012;53:3260–3268. doi: 10.1167/iovs.12-9676. [DOI] [PubMed] [Google Scholar]
- 73.Han KY, Chang JH, Azar DT. MMP14 Regulates VEGFR3 Expression on Corneal Epithelial Cells. Protein Pept Lett. 2016;23:1095–1102. doi: 10.2174/0929866523666161024142824. [DOI] [PubMed] [Google Scholar]
- 74.Han KY, Chang JH, Dugas-Ford J, et al. Involvement of lysosomal degradation in VEGF-C-induced down-regulation of VEGFR-3. FEBS Lett. 2014;588:4357–4363. doi: 10.1016/j.febslet.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Han KY, Dugas-Ford J, Lee H, et al. MMP14 Cleavage of VEGFR1 in the Cornea Leads to a VEGF-Trap Antiangiogenic Effect. Invest Ophthalmol Vis Sci. 2015;56:5450–5456. doi: 10.1167/iovs.14-16248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Harooni H, Reddy V, Root T, Ambati B. Bevacizumab for graft rejection. Ophthalmology. 2007;114:1950. doi: 10.1016/j.ophtha.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 77.Hayashi T, Usui T, Yamagami S. Suppression of Allograft Rejection with Soluble VEGF Receptor 2 Chimeric Protein in a Mouse Model of Corneal Transplantation. Tohoku J Exp Med. 2016;239:81–88. doi: 10.1620/tjem.239.81. [DOI] [PubMed] [Google Scholar]
- 78.Hayashida Y, Nishida K, Yamato M, et al. Transplantation of tissue-engineered epithelial cell sheets fter excimer laser photoablation reduces postoperative corneal haze. Invest Ophthalmol Vis Sci. 2006;47:552–557. doi: 10.1167/iovs.05-0995. [DOI] [PubMed] [Google Scholar]
- 79.Heinzelmann S, Bohringer D, Eberwein P, et al. Outcomes of Descemet membrane endothelial keratoplasty, Descemet stripping automated endothelial keratoplasty and penetrating keratoplasty from a single centre study. Graefes Arch Clin Exp Ophthalmol. 2016;254:515–522. doi: 10.1007/s00417-015-3248-z. [DOI] [PubMed] [Google Scholar]
- 80.Hill JC. Immunosuppression in corneal transplantation. Eye (Lond) 1995;9( Pt 2):247–253. doi: 10.1038/eye.1995.48. [DOI] [PubMed] [Google Scholar]
- 81.Holland EJ, Mogilishetty G, Skeens HM, et al. Systemic immunosuppression in ocular surface stem cell transplantation: results of a 10-year experience. Cornea. 2012;31:655–661. doi: 10.1097/ICO.0b013e31823f8b0c. [DOI] [PubMed] [Google Scholar]
- 82.Hong YK, Lange-Asschenfeldt B, Velasco P, et al. VEGF-A promotes tissue repair-associated lymphatic vessel formation via VEGFR-2 and the alpha1beta1 and alpha2beta1 integrins. FASEB J. 2004;18:1111–1113. doi: 10.1096/fj.03-1179fje. [DOI] [PubMed] [Google Scholar]
- 83.Hori J. Mechanisms of immune privilege in the anterior segment of the eye: what we learn from corneal transplantation. J Ocul Biol Dis Infor. 2008;1:94–100. doi: 10.1007/s12177-008-9010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hornig C, Weich HA. Soluble VEGF receptors. Angiogenesis. 1999;3:33–39. doi: 10.1023/a:1009033017809. [DOI] [PubMed] [Google Scholar]
- 85.Hsu CC, Chang HM, Lin TC, et al. Corneal neovascularization and contemporary antiangiogenic therapeutics. J Chin Med Assoc. 2015;78:323–330. doi: 10.1016/j.jcma.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 86.Huang XZ, Wu JF, Ferrando R, et al. Fatal bilateral chylothorax in mice lacking the integrin alpha9beta1. Mol Cell Biol. 2000;20:5208–5215. doi: 10.1128/mcb.20.14.5208-5215.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ilhan-Sarac O, Akpek EK. Current concepts and techniques in keratoprosthesis. Curr Opin Ophthalmol. 2005;16:246–250. doi: 10.1097/01.icu.0000172829.33770.d3. [DOI] [PubMed] [Google Scholar]
- 88.Inatomi T, Nakamura T, Kojyo M, et al. Ocular surface reconstruction with combination of cultivated autologous oral mucosal epithelial transplantation and penetrating keratoplasty. Am J Ophthalmol. 2006;142:757–764. doi: 10.1016/j.ajo.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 89.Inoue K, Amano S, Oshika T, Tsuru T. Risk factors for corneal graft failure and rejection in penetrating keratoplasty. Acta Ophthalmol Scand. 2001;79:251–255. doi: 10.1034/j.1600-0420.2001.790308.x. [DOI] [PubMed] [Google Scholar]
- 90.Ishii K, Kabata T, Oshika T. The Impact of Cataract Surgery on Cognitive Impairment and Depressive Mental Status in Elderly Patients. American Journal of Ophthalmology. 2008;146:404–409. doi: 10.1016/j.ajo.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 91.Jabbarvand M, Hashemian H, Khodaparast M, et al. Femtosecond laser–assisted sutureless anterior lamellar keratoplasty for superficial corneal opacities. J Cataract Refract Surg. 2014;40:1805–1812. doi: 10.1016/j.jcrs.2014.02.044. [DOI] [PubMed] [Google Scholar]
- 92.Jain S, Azar DT. New lamellar keratoplasty techniques: posterior keratoplasty and deep lamellar keratoplasty. Curr Opin Ophthalmol. 2001;12:262–268. doi: 10.1097/00055735-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 93.Johnson KE, Wilgus TA. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv Wound Care (New Rochelle) 2014;3:647–661. doi: 10.1089/wound.2013.0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jonas JB, Rank RM, Budde WM. Immunologic graft reactions after allogenic penetrating keratoplasty. Am J Ophthalmol. 2002;133:437–443. doi: 10.1016/s0002-9394(01)01426-x. [DOI] [PubMed] [Google Scholar]
- 95.Joo CK, Pepose JS, Stuart PM. T-cell mediated responses in a murine model of orthotopic corneal transplantation. Invest Ophthalmol Vis Sci. 1995;36:1530–1540. [PubMed] [Google Scholar]
- 96.Kang GJ, Truong T, Huang E, et al. Integrin Alpha 9 Blockade Suppresses Lymphatic Valve Formation and Promotes Transplant Survival. Invest Ophthalmol Vis Sci. 2016;57:5935–5939. doi: 10.1167/iovs.16-20130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kao WW, Coulson-Thomas VJ. Cell Therapy of Corneal Diseases. Cornea. 2016;35(Suppl 1):S9–S19. doi: 10.1097/ICO.0000000000001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kaplan HJ, Streilein JW, Stevens TR. Transplantation immunology of the anterior chamber of the eye. II. Immune response to allogeneic cells. J Immunol. 1975;115:805–810. [PubMed] [Google Scholar]
- 99.Karimian F, Feizi S. Deep anterior lamellar keratoplasty: indications, surgical techniques and complications. Middle East Afr J Ophthalmol. 2010;17:28–37. doi: 10.4103/0974-9233.61214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Karp A, Paillard-Borg S, Wang HX, et al. Mental, Physical and Social Components in Leisure Activities Equally Contribute to Decrease Dementia Risk. Dementia and Geriatric Cognitive Disorders. 2006;21:65–73. doi: 10.1159/000089919. [DOI] [PubMed] [Google Scholar]
- 101.Kather JN, Kroll J. Transgenic mouse models of corneal neovascularization: new perspectives for angiogenesis research. Invest Ophthalmol Vis Sci. 2014;55:7637–7651. doi: 10.1167/iovs.14-15430. [DOI] [PubMed] [Google Scholar]
- 102.Kenyon KR, Tseng SC. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989;96:709–722. doi: 10.1016/s0161-6420(89)32833-8. discussion 722–703. [DOI] [PubMed] [Google Scholar]
- 103.Kharod-Dholakia B, Randleman JB, Bromley JG, Stulting RD. Prevention and treatment of corneal graft rejection: current practice patterns of the Cornea Society (2011) Cornea. 2015;34:609–614. doi: 10.1097/ICO.0000000000000403. [DOI] [PubMed] [Google Scholar]
- 104.Kim TI, Chung JL, Hong JP, et al. Bevacizumab application delays epithelial healing in rabbit cornea. Invest Ophthalmol Vis Sci. 2009;50:4653–4659. doi: 10.1167/iovs.08-2805. [DOI] [PubMed] [Google Scholar]
- 105.Knop E, Knop N. The role of eye-associated lymphoid tissue in corneal immune protection. J Anat. 2005;206:271–285. doi: 10.1111/j.1469-7580.2005.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kornmann HL, Gedde SJ. Glaucoma management after corneal transplantation surgeries. Curr Opin Ophthalmol. 2016;27:132–139. doi: 10.1097/ICU.0000000000000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kuffova L, Knickelbein JE, Yu T, et al. High-Risk Corneal Graft Rejection in the Setting of Previous Corneal Herpes Simplex Virus (HSV)-1 Infection. Invest Ophthalmol Vis Sci. 2016;57:1578–1587. doi: 10.1167/iovs.15-17894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lam H, Dana MR. Corneal graft rejection. Int Ophthalmol Clin. 2009;49:31–41. doi: 10.1097/IIO.0b013e3181924e23. [DOI] [PubMed] [Google Scholar]
- 109.Lau CH, Nicholls SM, Easty DL. A murine model of interlamellar corneal transplantation. Br J Ophthalmol. 1998;82:294–299. doi: 10.1136/bjo.82.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Le Blanc K, Tammik C, Rosendahl K, et al. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 111.Lee HS, Hos D, Blanco T, et al. Involvement of corneal lymphangiogenesis in a mouse model of allergic eye disease. Invest Ophthalmol Vis Sci. 2015;56:3140–3148. doi: 10.1167/iovs.14-16186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee P, Wang CC, Adamis AP. Ocular neovascularization: an epidemiologic review. Surv Ophthalmol. 1998;43:245–269. doi: 10.1016/s0039-6257(98)00035-6. [DOI] [PubMed] [Google Scholar]
- 113.Li L, Huang JL, Liu QC, et al. Endostatin gene therapy for liver cancer by a recombinant adenovirus delivery. World J Gastroenterol. 2004;10:1867–1871. doi: 10.3748/wjg.v10.i13.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lim P, Fuchsluger TA, Jurkunas UV. Limbal stem cell deficiency and corneal neovascularization. Semin Ophthalmol. 2009;24:139–148. doi: 10.1080/08820530902801478. [DOI] [PubMed] [Google Scholar]
- 115.Lin MY, Gutierrez PR, Stone KL, et al. Vision Impairment and Combined Vision and Hearing Impairment Predict Cognitive and Functional Decline in Older Women. Journal of the American Geriatrics Society. 2004;52:1996–2002. doi: 10.1111/j.1532-5415.2004.52554.x. [DOI] [PubMed] [Google Scholar]
- 116.Lipp M, Bucher F, Parthasarathy A, et al. Blockade of the VEGF isoforms in inflammatory corneal hemangiogenesis and lymphangiogenesis. Graefe's Archive for Clinical and Experimental Ophthalmology. 2014;252:943–949. doi: 10.1007/s00417-014-2626-2. [DOI] [PubMed] [Google Scholar]
- 117.Liu J, Lawrence BD, Liu A, et al. Silk Fibroin as a Biomaterial Substrate for Corneal Epithelial Cell Sheet GenerationSF as a Biomaterial Substrate. Invest Ophthalmol Vis Sci. 2012;53:4130–4138. doi: 10.1167/iovs.12-9876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu Y, Hamrah P, Zhang Q, et al. Draining lymph nodes of corneal transplant hosts exhibit evidence for donor major histocompatibility complex (MHC) class II-positive dendritic cells derived from MHC class II-negative grafts. J Exp Med. 2002;195:259–268. doi: 10.1084/jem.20010838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu Z, Lin H, Huang C, et al. Development and Effects of FTY720 Ophthalmic Solution on Corneal Allograft Survival. Sci Rep. 2015;5:16468. doi: 10.1038/srep16468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ma JJ, Graney JM, Dohlman CH. Repeat penetrating keratoplasty versus the Boston keratoprosthesis in graft failure. Int Ophthalmol Clin. 2005;45:49–59. doi: 10.1097/01.iio.0000176365.71016.28. [DOI] [PubMed] [Google Scholar]
- 121.Ma Y, Xu Y, Xiao Z, et al. Reconstruction of chemically burned rat corneal surface by bone marrow-derived human mesenchymal stem cells. Stem Cells. 2006;24:315–321. doi: 10.1634/stemcells.2005-0046. [DOI] [PubMed] [Google Scholar]
- 122.Maruyama K, Ii M, Cursiefen C, et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest. 2005;115:2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maruyama Y, Maruyama K, Kato Y, et al. The effect of podoplanin inhibition on lymphangiogenesis under pathological conditions. Invest Ophthalmol Vis Sci. 2014;55:4813–4822. doi: 10.1167/iovs.13-13711. [DOI] [PubMed] [Google Scholar]
- 124.Maumenee AE. Clinical aspects of the corneal homograft reaction. Invest Ophthalmol. 1962;1:244–252. [PubMed] [Google Scholar]
- 125.Mizia-Malarz A, Sobol G, Wos H. Proangiogenic factors: vascular-endothelial growth factor (VEGF) and basic fibroblast growth factor--the characteristics and function. Przegl Lek. 2008;65:353–357. [PubMed] [Google Scholar]
- 126.Moller-Pedersen T. A comparative study of human corneal keratocyte and endothelial cell density during aging. Cornea. 1997;16:333–338. [PubMed] [Google Scholar]
- 127.Nakamura T, Takeda K, Inatomi T, et al. Long-term results of autologous cultivated oral mucosal epithelial transplantation in the scar phase of severe ocular surface disorders. Br J Ophthalmol. 2011;95:942–946. doi: 10.1136/bjo.2010.188714. [DOI] [PubMed] [Google Scholar]
- 128.Nguyen A, Azar D. Lamellar keratoplasty revisited. Int Ophthalmol Clin. 2004;44:83–91. doi: 10.1097/00004397-200404410-00010. [DOI] [PubMed] [Google Scholar]
- 129.Niederkorn JY. Immune mechanisms of corneal allograft rejection. Curr Eye Res. 2007;32:1005–1016. doi: 10.1080/02713680701767884. [DOI] [PubMed] [Google Scholar]
- 130.Niederkorn JY. High-risk corneal allografts and why they lose their immune privilege. Curr Opin Allergy Clin Immunol. 2010;10:493–497. doi: 10.1097/ACI.0b013e32833dfa11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Niederkorn JY. Corneal Transplantation and Immune Privilege. International reviews of immunology. 2013;32 doi: 10.3109/08830185.08832012.08737877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nishida N, Yano H, Nishida T, et al. Angiogenesis in cancer. Vasc Health Risk Manag. 2006;2:213–219. doi: 10.2147/vhrm.2006.2.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Niu G, Chen X. Vascular endothelial growth factor as an anti-angiogenic target for cancer therapy. Curr Drug Targets. 2010;11:1000–1017. doi: 10.2174/138945010791591395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Niu G, Chen X. Why integrin as a primary target for imaging and therapy. Theranostics. 2011;1:30–47. doi: 10.7150/thno/v01p0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Noble BA, Agrawal A, Collins C, et al. Deep Anterior Lamellar Keratoplasty (DALK): visual outcome and complications for a heterogeneous group of corneal pathologies. Cornea. 2007;26:59–64. doi: 10.1097/01.ico.0000240080.99832.f3. [DOI] [PubMed] [Google Scholar]
- 136.Oh JY, Kim MK, Shin MS, et al. The anti-inflammatory and anti-angiogenic role of mesenchymal stem cells in corneal wound healing following chemical injury. Stem Cells. 2008;26:1047–1055. doi: 10.1634/stemcells.2007-0737. [DOI] [PubMed] [Google Scholar]
- 137.Orwin E, Shah A, Voorhees A, Ravi V. Bioreactor design for cornea tissue engineering: Material-cell interactions. Acta Biomater. 2007;3:1041–1049. doi: 10.1016/j.actbio.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 138.Oshima Y, Sakaguchi H, Gomi F, Tano Y. Regression of iris neovascularization after intravitreal injection of bevacizumab in patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2006;142:155–158. doi: 10.1016/j.ajo.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 139.Otrock ZK, Makarem JA, Shamseddine AI. Vascular endothelial growth factor family of ligands and receptors: review. Blood Cells Mol Dis. 2007;38:258–268. doi: 10.1016/j.bcmd.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 140.Pan Q, Li X, Gu Y. Indications and outcomes of penetrating keratoplasty in a tertiary hospital in the developing world. Clin Exp Ophthalmol. 2012;40:232–238. doi: 10.1111/j.1442-9071.2011.02598.x. [DOI] [PubMed] [Google Scholar]
- 141.Panda A, Vanathi M, Kumar A, et al. Corneal graft rejection. Surv Ophthalmol. 2007;52:375–396. doi: 10.1016/j.survophthal.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 142.Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96:614–618. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- 143.Patel SP, Dana R. Corneal lymphangiogenesis: implications in immunity. Semin Ophthalmol. 2009;24:135–138. doi: 10.1080/08820530902801320. [DOI] [PubMed] [Google Scholar]
- 144.Patel SV, McLaren JW, Hodge DO, Baratz KH. Scattered light and visual function in a randomized trial of deep lamellar endothelial keratoplasty and penetrating keratoplasty. Am J Ophthalmol. 2008;145:97–105. doi: 10.1016/j.ajo.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 145.Paunicka KJ, Mellon J, Robertson D, et al. Severing Corneal Nerves in One Eye Induces Sympathetic Loss of Immune Privilege and Promotes Rejection of Future Corneal Allografts Placed in Either Eye. American Journal of Transplantation. 2015;15:1490–1501. doi: 10.1111/ajt.13240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pellegrini G, Traverso CE, Franzi AT, et al. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990–993. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- 147.Plskova J, Kuffova L, Holan V, et al. Evaluation of corneal graft rejection in a mouse model. Br J Ophthalmol. 2002;86:108–113. doi: 10.1136/bjo.86.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Prabhasawat P, Ekpo P, Uiprasertkul M, et al. Long-term result of autologous cultivated oral mucosal epithelial transplantation for severe ocular surface disease. Cell Tissue Bank. 2016;17:491–503. doi: 10.1007/s10561-016-9575-4. [DOI] [PubMed] [Google Scholar]
- 149.Prakash G, Agarwal A, Jacob S, et al. Femtosecond-assisted descemet stripping automated endothelial keratoplasty with fibrin glue-assisted sutureless posterior chamber lens implantation. Cornea. 2010;29:1315–1319. doi: 10.1097/ICO.0b013e3181cb4120. [DOI] [PubMed] [Google Scholar]
- 150.Prakash G, Jacob S, Ashok Kumar D, et al. Femtosecond-assisted keratoplasty with fibrin glue-assisted sutureless posterior chamber lens implantation: new triple procedure. J Cataract Refract Surg. 2009;35:973–979. doi: 10.1016/j.jcrs.2008.12.049. [DOI] [PubMed] [Google Scholar]
- 151.Price FW, Jr, Price DA, Ngakeng V, Price MO. Survey of steroid usage patterns during and after low-risk penetrating keratoplasty. Cornea. 2009;28:865–870. doi: 10.1097/ICO.0b013e318197ef07. [DOI] [PubMed] [Google Scholar]
- 152.Price MO, Price FW., Jr Endothelial keratoplasty - a review. Clin Exp Ophthalmol. 2010;38:128–140. doi: 10.1111/j.1442-9071.2010.02213.x. [DOI] [PubMed] [Google Scholar]
- 153.Proano CE, Azar DT, Mocan MC, et al. Photochemical keratodesmos as an adjunct to sutures for bonding penetrating keratoplasty corneal incisions. J Cataract Refract Surg. 2004;30:2420–2424. doi: 10.1016/j.jcrs.2004.02.068. [DOI] [PubMed] [Google Scholar]
- 154.Qazi Y, Maddula S, Ambati BK. Mediators of ocular angiogenesis. J Genet. 2009;88:495–515. doi: 10.1007/s12041-009-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Qian Y, Boisgerault F, Benichou G, Dana MR. Blockade of CD40-CD154 costimulatory pathway promotes survival of allogeneic corneal transplants. Invest Ophthalmol Vis Sci. 2001;42:987–994. [PubMed] [Google Scholar]
- 156.Ramachandran C, Basu S, Sangwan VS, Balasubramanian D. Concise review: the coming of age of stem cell treatment for corneal surface damage. Stem Cells Transl Med. 2014;3:1160–1168. doi: 10.5966/sctm.2014-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Randleman JB, Stulting RD. Prevention and treatment of corneal graft rejection: current practice patterns (2004) Cornea. 2006;25:286–290. doi: 10.1097/01.ico.0000178731.42187.46. [DOI] [PubMed] [Google Scholar]
- 158.Razeghinejad MR, Katz LJ. Steroid-induced iatrogenic glaucoma. Ophthalmic Res. 2012;47:66–80. doi: 10.1159/000328630. [DOI] [PubMed] [Google Scholar]
- 159.Regenfuss B, Bock F, Parthasarathy A, Cursiefen C. Corneal (lymph)angiogenesis--from bedside to bench and back: a tribute to Judah Folkman. Lymphat Res Biol. 2008;6:191–201. doi: 10.1089/lrb.2008.6348. [DOI] [PubMed] [Google Scholar]
- 160.Reinhart WJ, Musch DC, Jacobs DS, et al. Deep anterior lamellar keratoplasty as an alternative to penetrating keratoplasty a report by the american academy of ophthalmology. Ophthalmology. 2011;118:209–218. doi: 10.1016/j.ophtha.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 161.Reis A, Megahed M, Reinhard T, et al. RAD, a new immunosuppressive macrolide in murine corneal transplantation. Graefes Arch Clin Exp Ophthalmol. 2001;239:689–692. doi: 10.1007/s004170100258. [DOI] [PubMed] [Google Scholar]
- 162.Ribatti D. Endogenous inhibitors of angiogenesis: a historical review. Leuk Res. 2009;33:638–644. doi: 10.1016/j.leukres.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 163.Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond) 2005;2:8. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sangwan VS, Basu S, MacNeil S, Balasubramanian D. Simple limbal epithelial transplantation (SLET): a novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br J Ophthalmol. 2012;96:931–934. doi: 10.1136/bjophthalmol-2011-301164. [DOI] [PubMed] [Google Scholar]
- 165.Sangwan VS, Basu S, Vemuganti GK, et al. Clinical outcomes of xeno-free autologous cultivated limbal epithelial transplantation: a 10-year study. Br J Ophthalmol. 2011;95:1525–1529. doi: 10.1136/bjophthalmol-2011-300352. [DOI] [PubMed] [Google Scholar]
- 166.Sangwan VS, Jain R, Basu S, et al. Transforming ocular surface stem cell research into successful clinical practice. Indian J Ophthalmol. 2014;62:29–40. doi: 10.4103/0301-4738.126173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sano Y, Ksander BR, Streilein JW. Fate of orthotopic corneal allografts in eyes that cannot support anterior chamber-associated immune deviation induction. Invest Ophthalmol Vis Sci. 1995;36:2176–2185. [PubMed] [Google Scholar]
- 168.Sano Y, Ksander BR, Streilein JW. Murine orthotopic corneal transplantation in high-risk eyes. Rejection is dictated primarily by weak rather than strong alloantigens. Invest Ophthalmol Vis Sci. 1997;38:1130–1138. [PubMed] [Google Scholar]
- 169.Shah A, Brugnano J, Sun S, et al. The development of a tissue-engineered cornea: biomaterials and culture methods. Pediatr Res. 2008;63:535–544. doi: 10.1203/PDR.0b013e31816bdf54. [DOI] [PubMed] [Google Scholar]
- 170.Shapiro MS, Friend J, Thoft RA. Corneal re-epithelialization from the conjunctiva. Invest Ophthalmol Vis Sci. 1981;21:135–142. [PubMed] [Google Scholar]
- 171.Shimmura-Tomita M, Shimmura S, Satake Y, et al. Keratoplasty postoperative treatment update. Cornea. 2013;32(Suppl 1):S60–64. doi: 10.1097/ICO.0b013e3182a2c937. [DOI] [PubMed] [Google Scholar]
- 172.Shousha MA, Yoo SH, Kymionis GD, et al. Long-term results of femtosecond laser-assisted sutureless anterior lamellar keratoplasty. Ophthalmology. 2011;118:315–323. doi: 10.1016/j.ophtha.2010.06.037. [DOI] [PubMed] [Google Scholar]
- 173.Singh N, Tiem M, Watkins R, et al. Soluble vascular endothelial growth factor receptor 3 is essential for corneal alymphaticity. Blood. 2013;121:4242–4249. doi: 10.1182/blood-2012-08-453043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Smith RS, Hawes NL, Kuhlmann SD, et al. Corn1: a mouse model for corneal surface disease and neovascularization. Invest Ophthalmol Vis Sci. 1996;37:397–404. [PubMed] [Google Scholar]
- 175.Stechschulte SU, Azar DT. Complications after penetrating keratoplasty. Int Ophthalmol Clin. 2000;40:27– 43. doi: 10.1097/00004397-200040010-00005. [DOI] [PubMed] [Google Scholar]
- 176.Stevenson W, Cheng SF, Dastjerdi MH, et al. Corneal neovascularization and the utility of topical VEGF inhibition: ranibizumab (Lucentis) vs bevacizumab (Avastin) Ocul Surf. 2012;10:67–83. doi: 10.1016/j.jtos.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Streilein JW. New thoughts on the immunology of corneal transplantation. Eye (Lond) 2003;17:943–948. doi: 10.1038/sj.eye.6700615. [DOI] [PubMed] [Google Scholar]
- 178.Sugita J, Kondo J. Deep lamellar keratoplasty with complete removal of pathological stroma for vision improvement. Br J Ophthalmol. 1997;81:184–188. doi: 10.1136/bjo.81.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sumide T, Nishida K, Yamato M, et al. Functional human corneal endothelial cell sheets harvested from temperature-responsive culture surfaces. FASEB J. 2006;20:392–394. doi: 10.1096/fj.04-3035fje. [DOI] [PubMed] [Google Scholar]
- 180.Tan DT, Anshu A. Anterior lamellar keratoplasty: 'Back to the Future'- a review. Clin Exp Ophthalmol. 2010;38:118–127. doi: 10.1111/j.1442-9071.2009.02180.x. [DOI] [PubMed] [Google Scholar]
- 181.Tan DT, Dart JK, Holland EJ, Kinoshita S. Corneal transplantation. Lancet. 2012;379:1749–1761. doi: 10.1016/S0140-6736(12)60437-1. [DOI] [PubMed] [Google Scholar]
- 182.Tan Y, Cruz-Guilloty F, Medina-Mendez CA, et al. Immunological disruption of antiangiogenic signals by recruited allospecific T cells leads to corneal allograft rejection. J Immunol. 2012;188:5962–5969. doi: 10.4049/jimmunol.1103216. [DOI] [PubMed] [Google Scholar]
- 183.Taravella MJ, Stulting RD, Mader TH, et al. Calcific band keratopathy associated with the use of topical steroid-phosphate preparations. Arch Ophthalmol. 1994;112:608–613. doi: 10.1001/archopht.1994.01090170052021. [DOI] [PubMed] [Google Scholar]
- 184.Taylor AW. Ocular Immune Privilege and Transplantation. Front Immunol. 2016;7:37. doi: 10.3389/fimmu.2016.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Todani A, Ciolino JB, Ament JD, et al. Titanium back plate for a PMMA keratoprosthesis: clinical outcomes. Graefes Arch Clin Exp Ophthalmol. 2011;249:1515–1518. doi: 10.1007/s00417-011-1684-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Townsend WM. The limbal palisades of Vogt. Trans Am Ophthalmol Soc. 1991;89:721–756. [PMC free article] [PubMed] [Google Scholar]
- 187.Vail A, Gore SM, Bradley BA, et al. Conclusions of the corneal transplant follow up study. Collaborating Surgeons. Br J Ophthalmol. 1997;81:631–636. doi: 10.1136/bjo.81.8.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Voiculescu OB, Voinea LM, Alexandrescu C. Corneal neovascularization and biological therapy. Journal of Medicine and Life. 2015;8:444–448. [PMC free article] [PubMed] [Google Scholar]
- 189.Wagoner MD, Ba-Abbad R, Al-Mohaimeed M, et al. Postoperative complications after primary adult optical penetrating keratoplasty: prevalence and impact on graft survival. Cornea. 2009;28:385–394. doi: 10.1097/ICO.0b013e31818d3aef. [DOI] [PubMed] [Google Scholar]
- 190.Walia A, Yang JF, Huang YH, et al. Endostatin's emerging roles in angiogenesis, lymphangiogenesis, disease, and clinical applications. Biochim Biophys Acta. 2015;1850:2422–2438. doi: 10.1016/j.bbagen.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang Z, Dabrosin C, Yin X, et al. Broad targeting of angiogenesis for cancer prevention and therapy. Semin Cancer Biol. 2015;35(Suppl):S224–243. doi: 10.1016/j.semcancer.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.West SK, Rubin GS, Broman AT, et al. How does visual impairment affect performance on tasks of everyday life? The SEE Project. Salisbury Eye Evaluation. Arch Ophthalmol. 2002;120:774–780. doi: 10.1001/archopht.120.6.774. [DOI] [PubMed] [Google Scholar]
- 193.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 194.Williams KA, Roder D, Esterman A, et al. Factors predictive of corneal graft survival. Report from the Australian Corneal Graft Registry. Ophthalmology. 1992;99:403–414. doi: 10.1016/s0161-6420(92)31960-8. [DOI] [PubMed] [Google Scholar]
- 195.Yang J, Yamato M, Nishida K, et al. Corneal epithelial stem cell delivery using cell sheet engineering: not lost in transplantation. J Drug Target. 2006;14:471–482. doi: 10.1080/10611860600847997. [DOI] [PubMed] [Google Scholar]
- 196.Yang JF, Walia A, Huang YH, et al. Understanding lymphangiogenesis in knockout models, the cornea, and ocular diseases for the development of therapeutic interventions. Surv Ophthalmol. 2016;61:272– 296. doi: 10.1016/j.survophthal.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yao L, Bai H. Review: mesenchymal stem cells and corneal reconstruction. Mol Vis. 2013;19:2237–2243. [PMC free article] [PubMed] [Google Scholar]
- 198.Yee RW, Matsuda M, Schultz RO, Edelhauser HF. Changes in the normal corneal endothelial cellular pattern as a function of age. Curr Eye Res. 1985;4:671–678. doi: 10.3109/02713688509017661. [DOI] [PubMed] [Google Scholar]
- 199.Yeh PC, Azar DT, Colby K. Selective endothelial transplantation: novel surgical techniques for the treatment of endothelial dysfunction. Int Ophthalmol Clin. 2004;44:51–66. doi: 10.1097/00004397-200404410-00007. [DOI] [PubMed] [Google Scholar]
- 200.Yin XT, Tajfirouz DA, Stuart PM. Murine corneal transplantation: a model to study the most common form of solid organ transplantation. J Vis Exp. 2014:e51830. doi: 10.3791/51830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yoo SH, Kymionis GD, Koreishi A, et al. Femtosecond laser-assisted sutureless anterior lamellar keratoplasty. Ophthalmology. 2008;115:1303–1307. 1307 e1301. doi: 10.1016/j.ophtha.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 202.Yoon JJ, Ismail S, Sherwin T. Limbal stem cells: Central concepts of corneal epithelial homeostasis. World J Stem Cells. 2014;6:391–403. doi: 10.4252/wjsc.v6.i4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yorston D, Garg P. Corneal grafting: what eye care workers need to know. Community Eye Health. 2009;22:44–45. [PMC free article] [PubMed] [Google Scholar]
- 204.Yoshida J, Wicks RT, Zambrano AI, et al. Inhibition of Corneal Neovascularization by Subconjunctival Injection of Fc-Endostatin, a Novel Inhibitor of Angiogenesis. J Ophthalmol. 2015;2015:137136. doi: 10.1155/2015/137136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhu J, Dugas-Ford J, Chang M, et al. Simultaneous in vivo imaging of blood and lymphatic vessel growth in Prox1-GFP/Flk1::myr-mCherry mice. FEBS J. 2015;282:1458–1467. doi: 10.1111/febs.13234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zieske JD, Mason VS, Wasson ME, et al. Basement membrane assembly and differentiation of cultured corneal cells: importance of culture environment and endothelial cell interaction. Exp Cell Res. 1994;214:621–633. doi: 10.1006/excr.1994.1300. [DOI] [PubMed] [Google Scholar]