Abstract
Chemokines are named and best known for their chemotactic cytokine activity in the hematopoietic system; however, their importance extends far beyond leukocytes, cell movement and immunoregulation. CXCL12, the most protean of chemokines, regulates development in multiple systems, including the hematopoietic, cardiovascular and nervous systems, and regulates diverse cell functions, including differentiation, distribution, activation, immune synapse formation, effector function, proliferation and survival in the immune system alone. The broad importance of CXCL12 is revealed by the complex lethal developmental phenotypes in mice lacking either Cxcl12 or either one of its two known 7-transmembrane domain receptors Cxcr4 and Ackr3, as well as by gain-of-function mutations in human CXCR4, which cause WHIM syndrome, a multisystem and combined immunodeficiency disease and the only Mendelian condition caused by a chemokine system mutation. In addition, wild type CXCR4 is important in the pathogenesis of HIV/AIDS and cancer. Thus, CXCL12 and its receptors CXCR4 and ACKR3 provide extraordinary examples of multisystem multitasking in the chemokine system in both health and disease.
Keywords: chemokine, WHIM syndrome, HIV, development, hematopoiesis, leukocyte
1. Introduction
Darwin’s theory of the origin of species by natural selection of variation in heritable traits is the foundation of modern biology, although it predated identification of the gene as the heritable unit. Had he known about the gene, Darwin might have predicted that genomic complexity would increase with increased phenotypic complexity, and across the major taxonomic groupings of viruses, prokaryotes and eukaryotes he would be right. However, the pattern breaks down spectacularly among multicellular organisms. For example, the number of annotated protein-encoding genes in C. elegans and man is estimated to differ by only 1–2% and is only about 20% greater than in Drosophila. Impressively, the human genome contains only twice the number of genes found in even single cell protozoans. The paradox of genetic parsimony in multicellular eukaryotes may be explained by diverse genomic and post-genomic mechanisms, including diversification of the genetic repertoire, differential gene expression, epigenetic and environmental factors, non-coding RNA genes, RNA splicing and editing, post-translational protein processing, and protein multitasking. In the chemokine system, there are now many examples of multisystem protein multitasking, the subject of this special issue of Cytokine, but CXCL12 is the richest and will be the primary focus of this review. There is a vast literature on CXCL12 and its receptors that cannot be comprehensively summarized in any one review article. Emphasis will be placed here on non-canonical, non-chemotactic functions of CXCL12 signaling as applied to basic principles and the strongest examples from the study of human disease. The importance of CXCL12 signaling in leukocyte trafficking has been reviewed previously [1,2] and its role in cancer is reviewed in an accompanying article by Peled et al in this issue [3].
2. The CXCL12 signaling system
Despite a shared ancestry with all other chemokines, CXCL12 has an atypical evolutionary history. Chemokine genes are first found in vertebrates, where they have evolved rapidly through duplication, extinction and non-synonymous mutation [4–6]. In this way, during vertebrate evolution chemokine repertoires have diversified in size and chemokine orthologues have diversified in sequence. CXCL12 deviates markedly from this pattern since it lacks paralogs in most vertebrates and has extremely highly conserved orthologues. For example, there is only one amino acid difference, a conserved valine for isoleucine at position 18, among the 68 amino acids in the mature forms of human and mouse CXCL12α [7], the best-studied of six isoforms of CXCL12, whereas for other chemokine orthologues from these species there is usually much greater than 20% amino acid sequence divergence [6].
The six human CXCL12 isoforms are generated by identical splicing of the first 3 exons and differential splicing of a fourth exon, resulting in identical sequence from amino acids 1–68, and an additional 4, 20, 51, 1 and 11 amino acids at the C-terminus for isoforms β, γ, δ, ε and ϕ, respectively. Only CXCL12α, β and γ are conserved in mouse. There is evidence of differential expression and some evidence of differential function for these variants. For example, the C-terminus of the γ-isoform contains a positively charged glycosaminoglycan (GAG) binding site, which enhances CXCL12 gradient formation [8,9]. Differential splicing such as that described for CXCL12 is not a common general mechanism for generating diversity among chemokines, and CXCL12 is the only CXC chemokine that undergoes differential mRNA splicing. CXCL12 is also exceptional among chemokines in being regulated more by posttranslational than by transcriptional mechanisms.
CXCL12 has two known receptors, CXCR4 and ACKR3 [10,11], both of which are also unusual in being highly conserved members of the large and otherwise rapidly evolving chemokine receptor family [1]. Consistent with their exceptionally highly conserved structures, CXCL12, CXCR4 and ACKR3 are the only chemokine system genes that are essential for life, as revealed by perinatal lethal developmental phenotypes in the corresponding gene knockout mice [12–15]. Gain-of-function mutations in CXCR4 are also poorly tolerated. In particular, almost all known germline mutations in human CXCR4 increase receptor signaling capacity and cause warts-hypogammaglobulinemia-infections-myelokathexis (WHIM) syndrome, a multisystem developmental and immunodeficiency disease (see below) [16,17]. In addition, all known acquired somatic mutations in human CXCR4 have been found in cancerous plasma cells in patients with Waldenstrom’s Macroglobulinemia, where they are associated with poor prognosis [18]. Interestingly, some of the CXCR4 mutations found in Waldenstrom’s Macroglobulinemia also independently cause WHIM syndrome [19,20].
The fact that Cxcl12 and Cxcr4 knockout mice have the same perinatal lethal multisystem phenotypes was originally interpreted to mean that CXCL12 and CXCR4 form a monogamous ligand-receptor pair; however, perinatal lethality in these mice precluded identification of concordant and discordant phenotypes in adult animals. Subsequent discovery of ACKR3 as a high affinity CXCL12 receptor as well as functional interactions among CXCR4, ACKR3 and other signaling receptors has pointed to the possibility of far greater complexity in this signaling system. CXCR4 is a G protein-coupled receptor specific for the Gi family of heterotrimeric G proteins. ACKR3 is an atypical chemokine receptor that signals predominantly in a G protein-independent and β-arrestin-dependent manner, but may also functionally downregulate CXCR4 signaling by either scavenging CXCL12 or by heterodimerizing with CXCR4 [21]. ACKR3 was originally identified as an orphan receptor named RDC1 in 1990 by cloning of cDNA from a canine thyroid library [22]. In 1991, a human orthologue of RDC1 named GNR1 was cloned from leukemic pre-B cells but was misidentified as a vasoactive intestinal polypeptide receptor [23]. In 1995, RDC1 was reported to be a functional receptor for CGRP, adrenomedullin and amylin, which were all able to bind to and induce cAMP accumulation in RDC1-transfected cells [24]. CXCL12 was later identified as a chemokine ligand for human RDC1 and was originally reported to mediate T cell chemotaxis as well as cell survival and adhesion [11,25]. Accordingly, RDC1 was renamed CXCR7 then ACKR3 when the ACKR nomenclature system was established for a small group of 7-transmembrane domain chemokine receptors that do not signal through G proteins, if at all [26]. The other members of this family are ACKR1, previously known as the Duffy antigen or DARC (Duffy antigen receptor for chemokines), ACKR2 (previously known as D6) and ACKR4 (previously known as CCX CKR and CCRL1, among other aliases).
CXCR4 and ACKR3 are differentially expressed with some overlap, including in marginal zone B cells. Interactions between ACKR3 and CXCR4 that fine tune cell migration may occur either in cis through heterodimerization [14,27] or in trans by exclusive expression on neighboring cells. An excellent example of interaction in trans is provided by elegant genetic and imaging studies in zebrafish in which gonadal germ cell positioning was shown to be dependent on activation of Cxcr4b (one of two CXCR4 receptor subtypes in zebrafish) by Cxcl12, whose concentration is shaped dynamically into gradients by Ackr3 acting as a Cxcl12 sink on somatic cells [28–30]. ACKR3 binds CXCL12 with higher affinity than CXCR4 and, unlike CXCR4, continually recycles in a ligand-independent but β-arrestin- and ubiquitin-dependent manner without being degraded [31].
CXCL12 and its splice variants are the only endogenous chemokine agonists known for CXCR4, whereas ACKR3 has one other known chemokine agonist, CXCL11 (previously known as I-TAC), which together with CXCL9 and CXCL10 is also an agonist at the T cell-specific chemokine receptor CXCR3. Many DNA viruses have pirated chemokine and chemokine receptor genes from their hosts, and one of these, human herpesvirus-8, encodes an exogenous chemokine ligand for CXCR4 named vMIP-II; however, it acts as an antagonist [32]. Non-chemokine ligands have also been identified for both CXCL12 receptors [2], including MIF (macrophage migration inhibitory factor) [33,34], extracellular ubiquitin [35], and the envelope glycoprotein gp120 from T cell-tropic strains of HIV [36], which are all agonists at CXCR4; human β defensin-3, which is an endogenous antagonist at CXCR4 [37]; and adrenomedullin, which was originally reported to be an agonist at ACKR3 and may be scavenged by it [38]. In addition, the highly conserved chromatin protein HMGB1 (high mobility group box 1) can act as a damage-associated molecular pattern (DAMP) when it is released from dying cells, and then form a complex with CXCL12, enhancing the chemokine’s ability to bind to and activate CXCR4 [39]. This multitude of potential ligand-receptor interactions is rendered even more complex since CXCR4 and ACKR3 ligands may interact differentially with different states of CXCR4 and ACKR3, as well as with additional receptors, including other chemokine receptors (e.g. MIF is also an agonist at CD74 [40] and CXCR2 [34]).
Structures of CXCR4 complexed to small ligands (small molecule antagonist IT1t and cyclic peptide antagonist CVX15 derived from Limulus polyphemus) as well as to vMIP-II have been solved, resolving the receptor as a dimer with hydrophobic contact sites in helices V and VI [41]. The vMIP-II-CXCR4 structure revealed 1:1 chemokine-receptor stoichiometry and a surprisingly broad chemokine-receptor interface prompting a revision of the classic 2-site model for chemokine ligand-receptor binding [42]. Unlike many other G protein-coupled receptors, CXCR4 lacks a short 8th alpha helix and a site in the C-tail for palmitoylation [41]. Biochemical and genetic studies have revealed that the receptor may undergo N-terminal domain sulfation on three tyrosine residues, which may contribute to site 1 for ligand binding, and C-terminal ubiquitinylation, which is important for degradation after receptor internalization [43].
Key functional domains in CXCL12 include the CXCR4 site 1-docking domain from amino acids 12–17, the CXCR4 site 2-activating domain in the N-terminus, especially Asp1 and Pro2, which are essential for activity, and the BBXB domain from amino acids 24–27, which is important for glycosaminoglycan (GAG) binding. GAG binding by CXCL12 appears to be important in regulating in vivo presentation and activity [44,45]. Post-translational processing of CXCL12 by proteolytic enzymes such as diprolylpeptidase 4 rapidly produces N-terminal truncation forms with reduced or absent activity [46]. The crystal structure of CXCL12 is a dimer; however, its state upon binding to CXCR4 could vary depending on the local concentration of the ligand as well as the state of the receptor, which might exist as a monomer, homodimer or higher order homomultimer, or as a heteromer. CXCR4 has been shown to form heterodimers with ACKR3 as well as with several G protein-coupled chemokine receptors and other G protein-coupled receptors [47], and some evidence suggests that in some cases this may be functionally significant in primary cells [48]. CXCR4 has also been reported to act as a coreceptor, including with CD74 to form a MIF receptor [40], with CD4 to form an HIV entry factor [36], with the T cell receptor to promote T cell activation in the immune synapse [49], and with TLR2 to modulate angiogenesis [50].
CXCL12 was first identified as a product of bone marrow stromal cells and therefore was originally named ‘stromal cell-derived factor-1’ (SDF-1) [51]. The first function identified for CXCL12 was its mitogenic activity for B-cell progenitors in vitro, thus the competing alias given to it at the time of “pre-B cell growth-stimulating factor” (PBSF) [52]. It is now known that CXCL12 is constitutively expressed in many tissues, including primary and secondary immune organs, lung, liver, thymus, uterus and gut, including in the embryo, so that it is considered a homeostatic chemokine, regulating normal developmental and non-emergency functions. Endothelial cells in bone marrow sinusoids and bone marrow stromal cells are rich constitutive cell sources of CXCL12, and hematopoietic stem cell homing to and positioning and retention in bone marrow stem cell niches is mediated in part by CXCL12 signaling through CXCR4 [53,54]. However, CXCL12 may also be upregulated in the context of infection and inflammation, and should be more properly classified as a dual-use homeostatic/inflammatory chemokine [55]. CXCR4 is expressed on high percentages of most subsets of mature and immature hematopoietic cells, neurons of the central and peripheral nervous system, microglia and astrocytes, many cancer cells of both hematopoietic and non-hematopoietic origin, and many other undifferentiated and differentiated cell types, consistent with the multifunctional potential of CXCL12 [56,57]. CXCR4 is the only chemokine receptor besides CCR7 that is constitutively expressed on naïve T cells.
CXCL12-CXCR4 functions are unusually broad compared to other chemokines and include cell trafficking and positioning, neovascularization, and cell survival and growth. Nevertheless, CXCR4 signaling involves widely shared G protein-coupled signaling pathways, involving pertussis toxin-sensitive Gi-proteins, phospholipase C activation, calcium mobilization, and activation of Akt, MAP-kinases and PI-3 kinase, as well as arrestin-dependent signaling [58]. Factors that may explain how these common signals are integrated to produce diverse and unique cell response profiles after CXCL12 stimulation include the cell context, CXCL12 signal strength, possible CXCR4 homo- and heterodimerization and novel signaling pathways recruited to the dimerized receptor [48,59]. ACKR3 signaling through β-arrestin may involve activation of Akt and the MAP kinases ERK1 and ERK2 [60].
3. Exploitation of CXCR4 by HIV as a coreceptor for cell entry
Historically, CXCR4 was first identified as an orphan receptor by multiple groups under various names. It was then rediscovered as the first HIV coreceptor and briefly named ‘fusin’ to convey its HIV envelope-target cell membrane fusogen activity during the process of cell entry by the virus [36,61]. It is specific for HIV strains that efficiently infect certain cultured T cell lines and PBMCs, but not primary macrophages. Thus, its first known function was unrelated to leukocyte chemotaxis or any other physiologic function, but instead involved its pathologic exploitation by a virus resulting in acquired immunodeficiency. HIV strains that exploit CXCR4 are now eponymously called X4 strains and bind via the HIV envelope glycoprotein gp120 to both CD4 and CXCR4 on the target cell. Soon after CXCR4 was identified as an HIV coreceptor, CXCL12 was identified as its first chemokine agonist [62,63], and the known HIV-inhibiting bicyclam AMD3100 (Mozobil, plerixafor; marketed by Sanofi-Aventis) was shown to work by acting as a specific small molecule antagonist at CXCR4 [64]. Many other naturally occurring and synthetic CXCR4 binding agents can also block entry of target cells by X4 strains of HIV [65].
The importance of CXCR4 in HIV pathogenesis is suggested in part by 1) the identification of HIV strains from patients that require CXCR4 for cell entry in vitro; and 2) the ability of AMD3100 to suppress X4 HIV viral load in patients. Development of AMD3100 for clinical use was abandoned in HIV/AIDS 1) because it must be given at high doses subcutaneously, 2) because of some limited evidence of toxicity at the high doses needed for efficacy, and 3) because X4 strains typically do not transmit and initiate infection, but rather appear at late stages of infection in a small subset of patients associated with the onset of AIDS. Nevertheless, potent hematopoietic stem cell (HSC)-mobilizing activity was noticed during clinical trials of AMD3100 in HIV/AIDS [66], and it was eventually repurposed and approved by the FDA in combination with G-CSF for peripheral blood HSC collection for autologous bone marrow transplantation in patients receiving myeloablative chemotherapy for multiple myeloma or non-Hodgkins lymphoma [67,68].
Most of the many other HIV coreceptors that have been identified by in vitro assays are also chemokine receptors [61]. CCR5, which is used by eponymous R5 strains of HIV for cell entry, is the only one besides CXCR4 that is clearly important in vivo, as supported by an abundance of genetic and pharmacologic evidence, including 1) genetic resistance to HIV infection in individuals lacking CCR5 due to homozygous inheritance of the complete loss-of-function allele CCR5Δ32, which encodes a massively truncated protein that does not traffic to the plasma membrane [69–73]; 2) the clinical antiretroviral efficacy of the specific CCR5 antagonist maraviroc, developed and marketed by Pfizer [74,75]; and 3) the remarkable and fortuitous case of the “Berlin patient”, an HIV+ individual who was functionally cured of HIV infection by bone marrow transplantation from a homozygous CCR5Δ32 donor after myeloablative chemotherapy for acute myelogenous leukemia [76,77]. CCR5 is the coreceptor used by the majority of primary HIV isolates including the disease-transmitting strains.
Pharmacotherapy directed at CCR5 and CXCR4 in HIV/AIDS requires foreknowledge of the strain or strains present in the patient. This and the fact that many HIV strains can use both CXCR4 and CCR5 for cell entry have limited the use of maraviroc in the treatment of HIV/AIDS. Still, the critical importance of these coreceptors has inspired investigation of HIV cure strategies involving gene editing in patient leukocytes. Proof of principle for this approach has been published for a zinc finger nuclease targeting the CCR5 gene [78,79] and is now being expanded to CRISPR/Cas9 editing.
Important unanswered questions about CXCR4 in HIV/AIDS include 1) why X4 strains do not appear to efficiently transmit the disease even when X4 virus is transmitted congenitally or by blood transfusion; 2) why X4 viruses do not efficiently infect primary macrophages; and 3) whether X4 viruses or defective X4 viral-like particles contribute to immune hyperactivation and accelerated CD4+ T cell depletion by gp120 activation of CXCR4. The role of constitutive expression of CXCL12 and other endogenous CXCR4 ligands, as well as the ratio of defective to infectious virus are important considerations in resolving these questions.
4. Insights into CXCL12-CXCR4 multitasking from WHIM syndrome immunodeficiency
WHIM syndrome is an extremely rare congenital multisystem combined immunodeficiency disease caused by autosomal dominant gain-of-function mutations in the C-tail of CXCR4 [16]. It is the only Mendelian condition caused by a mutation in the chemokine system, and it provides an important opportunity to define the biologic importance of CXCR4 signaling in humans. “WHIM” is an acronym for the most common clinical manifestations in the disease: treatment-refractory Warts, Hypogammaglobulinemia, recurrent Infections and Myelokathexis. WHIM syndrome is a subtype of severe congenital neutropenia, and myelokathexis is a neologism meaning “bone marrow retention”, which was coined to convey the mechanism originally proposed to explain the unusual type of neutropenia in the disease: normal production but abnormal retention of neutrophils in the bone marrow [80]. To our knowledge, all humans identified so far with germline CXCR4 mutations affecting the C-tail have had congenital neutropenia and almost all eventually develop one or more other features of WHIM syndrome; conversely, only ~2% of patients clinically diagnosed with WHIM syndrome lack a CXCR4 mutation [81]. Severe deficiencies in other leukocyte subsets in the blood and mild thrombocytopenia are often present in WHIM patients, whereas anemia is usually absent. The disease has been reported in all major racial groups, but most reported patients have been from the USA, France and Italy.
WHIM mutations may be inherited or appear de novo, and include frame shifts, nonsense and non-synonymous point mutations affecting the C-tail of CXCR4. This region of the receptor is even more highly conserved than the overall protein sequence, with 100% identity, for example, in the terminal 19 amino acids between human and chicken CXCR4 [82]. Truncation of even small portions of the C-tail results in loss of multiple serine residues resulting in potential loss of ligand-induced G protein-coupled receptor kinase (GRK)-mediated phosphorylation of the receptor, β-arrestin binding to the activated receptor, and negative regulation (desensitization, receptor downregulation) [83]. This provides a molecular explanation for how a loss of structure may paradoxically result in a CXCL12-stimulated gain-of-function for WHIM receptors in calcium flux, chemotaxis, Akt and Erk phosphorylation, and other functional assays [16]. However, since CXCL12-induced downregulation of wild type CXCR4 normally occurs much later than when the gain of function is first apparent after stimulation of WHIM receptors (within seconds in real-time signaling assays), other mechanisms are also likely to contribute to increasing WHIM receptor signaling, including for example enhancement of G protein coupling by loss of inhibitory determinants in the mutated C-tail [58,84].
Although immunodeficiency in WHIM syndrome may be broad and include severe panleukopenia and hypogammaglobulinemia, the spectrum of infections is paradoxically narrow, mainly HPV and recurrent but non-life threatening non-invasive otosinopulmonary and skin infections caused by common extracellular bacterial pathogens. Since hypogammaglobulinemia is the least penetrant feature, neutropenia may be the most important antibacterial host defense component of WHIM immunodeficiency that drives the recurrent infection phenotype. Two normal, non-canonical, non-chemotactic CXCR4 activities are thought to be exaggerated in WHIM syndrome to explain neutropenia. The first involves positioning and anchoring of neutrophils in the bone marrow in response to constitutively high local concentrations of CXCL12 [85,86]. This mechanism initially seemed clear from analysis of neutrophil dynamics in wild type mice, but has subsequently been called into question by analysis of a WHIM mouse model in which neutropenia was not associated with an excess of neutrophils in bone marrow [87]. Moreover, plerixafor was reported to mobilize neutrophils to the blood from the bone marrow in mice [88]. The second mechanism involves apoptosis. CXCR4 signaling is known to induce apoptosis of many cell types, and, consistent with this, high frequencies of apoptotic neutrophils have been reported in the blood and bone marrow of patients with WHIM syndrome [89,90]. Apoptosis drives upregulation of CXCR4 expression in neutrophils, which may normally force the cells in the blood to home back to bone marrow for destruction in response to high local concentrations of CXCL12.
Importantly, WHIM neutrophils have been reported to produce normal levels of superoxide anion after stimulation with PMA and to kill S. aureus normally ex vivo, and they have enhanced chemotactic responses to CXCL12 [91]. Therefore, they should be effective in host defense if they are able to access the peripheral blood. In fact, acute bacterial infection can induce neutrophilia in WHIM patients, as it does in non-immunocompromised individuals, which may resolve the clinical paradox of why most WHIM patients may have extremely severe neutropenia without suffering from invasive life-threatening infections [92]. This may also explain why diagnosis of WHIM syndrome is often delayed for years or even decades in de novo cases: even when infections are frequent and severe, neutropenia may be masked at the time of clinical presentation. Ultimately, the diagnosis of WHIM syndrome requires a bone marrow evaluation to demonstrate myelokathexis, which is typically considered only when chronic neutropenia or other evidence of immunodeficiency is documented in the blood between infections. Clinical studies showing that very low doses of G-CSF or plerixafor can markedly increase the absolute neutrophil count in the bloodstream of WHIM patients within 3 hours after administration suggest that the WHIM-dependent mechanisms driving neutropenia can be fairly easily overcome [93,94]. Thus, infection-induced neutrophilia appears to be a critical protective mechanism for WHIM patients, making WHIM syndrome a relatively “benign” immunodeficiency. The mechanism underlying this phenomenon is an important unanswered question.
Nevertheless, severe morbidity may still occur in WHIM patients if there is recurrent infection in the same organ (e.g., hearing loss, bronchiectasis). Moreover, there are a few case reports of premature mortality resulting from invasive infection, which are much less common in WHIM patients, and HPV- or herpesvirus-associated cancer. One of the most interesting and unexplained questions in WHIM syndrome is why HPV is the signature pathogen and why other viruses usually cause only sporadic and limited illness. Lymphopenia and monocytopenia per se might be expected to increase susceptibility to viral infections more broadly, even if adaptive immune cell development and the capacity for antigen-driven responsiveness were normal, particularly if trafficking through secondary lymphoid organs were affected by the WHIM mutation. There is insufficient clinical material available to draw any firm conclusions about this in WHIM patients; however, a mouse model of WHIM syndrome is providing insight [87]. Myelopoiesis was reported to be normal in this mouse, whereas B and T lymphopoiesis was impaired. Lymph nodes had a paucity or absence of B cell follicles and an expansion of T cell zones and follicular hypoplasia was detected in the spleen. These defects in immune organs were associated with mild neutropenia unlike the severe neutropenia that occurs in patients. Both lymphoid follicular hyperplasia and hypoplasia have been documented in rare histopathologic studies of WHIM syndrome in patients [80,95]. Severe B lymphopenia occurs in patients and in the WHIM mouse, yet hypogammaglobulinemia is absent in the mouse and variable in patients. Thus, the WHIM mouse model recapitulates some but not all WHIM phenotypes.
Regarding T cell responses, the WHIM mutation has been reported to weaken immunologic synapse formation in vitro [49,96], and in limited anecdotal studies patients have been reported to have weak, delayed or aborted antibody responses to vaccines, including the HPV vaccine [92,95,97–100]. This highlights a third non-canonical non-chemotactic function mediated by CXCR4 signaling in leukocytes; however, it still does not explain HPV exceptionalism in the disease. One intriguing possibility is that CXCR4 may also multitask outside the immune system directly in keratinocytes to drive HPV replication. In this regard, there is evidence that keratinocytes can directly express both CXCL12 and CXCR4 in warts in response to expression of the HPV E6 and E7 oncogene products [101]. CXCR4 signaling in keratinocytes expressing the WHIM receptor may enhance keratinocyte proliferation and possibly HPV replication, and conversely CXCR4 blockade is able to decrease oncogene expression in keratinocytes. Numerous CXCR4+ immune cell types, including monocytes, dendritic cells, cytotoxic T lymphocytes (CTLs), plasmacytoid dendritic cells and natural killer (NK) cells, all of which are often deficient in the blood in WHIM patients, may play roles in HPV immunity [102]. The remarkable case of patient WHIM-09, in whom there was spontaneous resolution of warts, myelokathexis, neutropenia and monocytopenia, but not lymphopenia, associated with chromothriptic deletion of the WHIM allele and 163 other genes on chromosome 2 in the myeloid lineage, has suggested an important role for monocyte-derived cells in WHIM-associated HPV pathogenesis [103].
Plerixafor can rapidly increase all leukocyte subsets tested in the blood, suggesting the possibility that it may be an ideal mechanism-based personalized medicine for WHIM patients to restore infection susceptibility to normal [104]. The situation may be more complicated for adaptive immunity, however, since CXCR4 plays a more complex and nuanced role for adaptive immune cells than for neutrophils outside of the bone marrow in orchestrating trafficking itineraries, positioning in tissue and immune synapse formation. Nevertheless, in a six-month Phase 1 clinical trial of twice daily low-dose plerixafor in three WHIM patients, there was evidence of reduced wart burden and preliminary evidence of decreased susceptibility to infection [93].
Current treatment options in WHIM syndrome include wart ablation, HPV vaccination, G-CSF for neutropenia, prophylactic antibiotics and antibody replacement. G-CSF works in part by stimulating degradation of CXCL12 [105,106], presumably releasing cells from their WHIM anchor in bone marrow and other potential storage sites. Three WHIM patients have been reported to be cured: two by bone marrow allotransplantation and patient WHIM-09, mentioned previously, by chromothripsis (chromosome shattering) involving fortuitous deletion of the disease allele in one hematopoietic stem cell (HSC) that gained a relative growth advantage and clonally replaced HSCs and the myeloid lineage, but not the lymphoid lineage, with chromothriptic cells purged of the WHIM allele [103,107,108]. This patient has inspired research and development of a preferred cure strategy involving WHIM allele inactivation rather than WHIM allele repair by gene editing in HSCs to take advantage of enhanced bone marrow engraftment potential of CXCR4 haploinsufficient HSCs, thereby potentially obviating genotoxic recipient conditioning. As such, WHIM syndrome may have multiple strategic advantages as a disease indication for gene editing over loss-of-function type genetic diseases of the blood. Engineering CXCR4 haploinsufficiency might also be imagined as an adjunct to specific gene therapy for other inherited diseases of the blood to enhance engraftment. Meanwhile, plerixafor given subcutaneously and X4P-001-LD (AMD11070; under development by X4-Pharma) given orally are small molecule CXCR4 antagonists that are both currently undergoing Phase 2/3 clinical trials in the treatment of patients with WHIM syndrome (ClinicalTrials.gov NCT00967785, NCT02231879, NCT03005327). There are currently over 100 registered clinical trials testing CXCR4 antagonists, mostly for plerixafor in cancer and autoimmunity (www.clinicaltrials.gov).
5. CXCL12/CXCR4/ACKR3 in the cardiovascular system
A major life-threatening non-immunologic phenotype in a small minority of WHIM patients (<5%) is Tetralogy of Fallot, a severe and uncommon congenital cardiovascular developmental abnormality [109]. The mechanism is unknown; however, it is noteworthy that other cardiovascular phenotypes have also been found in WHIM patients, including ventricular septal defect in one case [110]. Moreover, ventricular septal defects and defective gastric and renal vascularization have been reported in both Cxcl12 and Cxcr4 knockout mice [111,112]. Consistent with this, CXCL12/CXCR4 signaling has been reported to stimulate angiogenesis, including in the context of ischemia. This has been related at the cellular level to endothelial cell migration and proliferation, at the molecular level to hypoxia-inducible factor (HIF)-1α-dependent ligand/receptor upregulation and CXCL12-dependent vascular endothelial growth factor (VEGF) upregulation, and at the disease level to reduced infarct size after myocardial infarction [113–115].
Fatal post-natal cardiac failure has been reported as the major phenotype in three independent lines of Ackr3 knockout mice; however, the causes of death differed [14,15,38]. In two of the lines, cardiomyocyte hyperplasia and increased heart weight in neonates were reported [15,38], whereas in the third line profound semilunar valve atresia as well as ventricular septal defects in a subset of animals were found [14]. The valve phenotype was recapitulated in mice selectively lacking Ackr3 on endothelial cells. Genetic depletion of adrenomedullin, which has been reported to be scavenged by ACKR3, was able to reverse the cardiovascular developmental phenotype and mortality in one study, suggesting that Ackr3 may act as a molecular rheostat by scavenging and shaping gradients of adrenomedullin to fine-tune its action at a signaling adrenomedullin receptor, such as CLR/RAMP [38]. Importantly, ACKR3 RNA was detected in the heart in endothelial cells and cardiomyocytes. Ackr3 has also been found in lymphatic endothelial cells, and lymphatic sac enlargement has been reported in Cxcr7 knockout mice. This was also reversible with genetic depletion of adrenomedullin [38].
6. CXCL12/CXCR4 in the nervous system
Cxcl12 and Cxcr4 are important for spatial and temporal developmental control of the nervous system. Mechanisms involved in neuronal cell migration in the cerebellar cortex have been the most thoroughly studied [13,116,117]. This is a complex process involving the balance of multiple chemoattractant and repulsive factors separated in space between the meninges and the developing neuroepithelium and in time between the embryonic and early post-natal cerebellum. Cxcl12 is thought to be the key attractive factor produced by the cerebellar meninges [116,118]. It is directly chemotactic for proliferating Cxcr4-expressing premature granule cells preventing their migration away from the proliferative external granule layer in the embryo. Cxcr4 expression is switched off in these cells in the early post-natal cerebellum, releasing them to migrate into the inner granule cell layer, where they become quiescent, differentiate and migrate through the Purkinje cell layer to their final position. Cxcl12 and Cxcr4 knockout mice both have abnormal migration of granule cells, ectopic Purkinje cells and malformation of the external granule cell layer [12,13]. In another example of its non-chemotactic actions, Cxcl12 enhances cerebellar granule cell proliferation induced by the mitogen sonic hedgehog, which is produced by Purkinje cells [119].
Cxcl12 signaling has been reported to modulate many other aspects of nervous system development and function. It appears to be important in the mature nervous system both for homeostasis (e.g. axon guidance, maintenance of neural progenitor cells, and for modulation of neurogenesis, neurotransmission, neurotoxicity and neuroglial interactions) [120,121] and for the response to stress (e.g. infection, cancer, stroke, autoimmunity and modulation of pain pathways) [122].
8. Conclusion
CXCL12 and its receptors CXCR4 and ACKR3 comprise a signaling system that is ancient, highly conserved and essential and provide clear examples of multisystem protein multitasking, which helps explain genetic parsimony. There are many examples of other chemokines and chemokine receptors that are likely to have broad functionality. As just one example, Ackr1 knockout mice have cerebellar and behavioral phenotypes associated with extremely high expression of Ackr1 in Purkinje cells of the cerebellum, yet the precise cellular function of ACKR1 remains elusive [123]. In addition, Ackr1 on nucleated erythroid cells in the bone marrow has recently been reported to regulate hematopoiesis [124]. Future investigations of the chemokine system are likely to uncover many other important roles beyond their eponymous role as chemotactic cytokines in leukocyte migration.
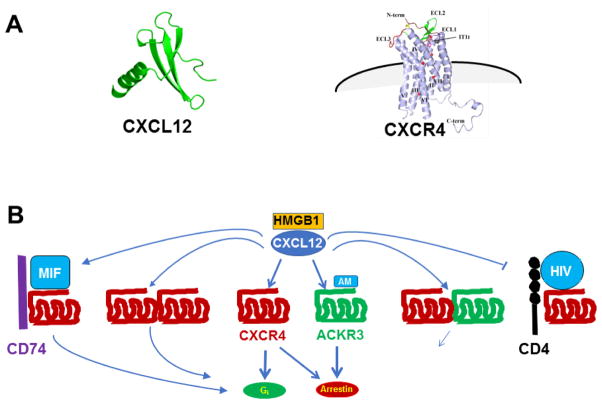
Figure 1.
CXCL12 and CXCR4 structure and molecular interactions. A) Structure. Crystal structure of the monomer forms are shown for CXCL12 and CXCR4 complexed with the small molecular weight antagonist IT1t [41]. B) Molecular interactions. Some demonstrated binding interactions are shown for CXCL12, CXCR4, ACKR3 and several non-chemokine ligands for CXCR4 and ACKR3. See text for details and references. Abbreviations: MIF, macrophage migration inhibitory factor; AM, adrenomedullin; HMGB1, high mobility group-box 1.
Table 1.
Properties of CXCL12 and its receptors at a glance.
| Molecule | |||
|---|---|---|---|
| CXCL12 | CXCR4 | ACKR3 | |
| Aliases | SDF-1, PBSF | CD184, Fusin, LESTR, HUMSTR | RDC1, CXCR7, GPR159 |
| Isoforms | CXCL12α,β,γ,δ,ε and ϕ | na | na |
| Structure | 68 aa homodimer (α) | 352 aa homodimer (hu) | 362 aa (hu) |
| Coligands | HMGB1 | na | na |
| Receptors | CXCR4, ACKR3 | na | na |
| Coreceptors | na | CD4, CD74, ACKR3 | CXCR4, CD4 |
| Natural Agonists | na | CXCL12, MIF, ubiquitin, HIV gp120 | CXCL11, CXCL12, MIF, adrenomedullin, Proenkephalin A |
| Natural Antagonists | na | HHV8 vMIP-II, HBD-3 | na |
| Signal transducer | CXCR4, ACKR3 | Gi, β-arrestin 2 | β-arrestin 2 |
| Intracellular signaling mediators | na | PLC, PI3K, Akt, Erk1/2 | Akt, MAP kinase |
| Cell responses | Movement Adhesion Proliferation Apoptosis Gene expression |
Movement Adhesion Proliferation Apoptosis Gene expression HIV entry |
Ligand scavenging Shaping chemoattractant gradients |
| Hematopoietic cell expression | na | Ubiquitous | Subsets of neutrophils, basophils and B, T and dendritic cells |
| Non-hematopoietic cell expression | Widespread: e.g. Bone marrow stromal and endothelial cells, inflamed microvascular endothelial cells, meninges | Widespread: e.g. Lung, liver, kidney, GI tract, adrenal gland, ovary, brain (e.g. cerebellar granule cells, neuroblasts, microglia), many cancers | endothelial cells (e.g. endocardium, lymphatics, brain, airway), cardiomyocytes, Kidney tubule cells, microglia, thyroid, placenta |
| Proven importance in humans | na | HIV/AIDS WHIM syndrome HSC mobilization |
na |
| FDA-approved drugs | na | Plerixafor (Mozobil, AMD3100) for HSC mobilization | na |
Abbreviations: na, not available/applicable; SDF, stromal cell-derived factor; PBSF, pre-B cell growth-stimulating factor; HSC, hematopoietic stem cell; HMGB1, high-mobility group box 1; ACKR, atypical chemokine receptor; MIF, macrophage migration inhibitory factor; HIV, human immunodeficiency virus; HHV8, human herpesvirus 8; vMIP-II, viral macrophage inflammatory protein-II; HBD-3, human bacterial defensin-3; PLC, phospholipase C; PI3K, phosphatidylinositol trisphosphate kinase; Erk, extracellular signal-related kinase; MAP, mitogen-activated protein
Table 2.
Some important phenotypes revealed by deficiency in CXCL12 and its receptors in model organisms (gene knockout for mouse; RNA knockdown for zebrafish).
| Targeted Gene | Phenotype | |||||
|---|---|---|---|---|---|---|
| Mouse | Zebrafish | |||||
| Mortality | Immune System | Cardiovascular System | Cerebellar Development | Gonadal development | Nervous system development | |
| Cxcl12 | 100% | Impaired BM myelopoiesis; Impaired B cell lymphopoiesis | VSD Abnormal gastric and renal vascularization |
Disorganized granule cell layer | Abnormal primordial germ cell guidance and survival | Abnormal guidance of trigeminal sensory neurons, neural progenitor cells and cranial neural crest cells |
| Cxcr4 | 100% | Impaired BM myelopoiesis; Impaired B cell lymphopoiesis | VSD Abnormal gastric and renal vascularization |
Disorganized granule cell layer | Abnormal primordial germ cell guidance and survival | Abnormal guidance of trigeminal sensory neuron and cranial neural crest cells |
| Ackr3 | 70–95% | normal | Cardiomegaly, VSD, semilunar valve atresia, lymphatic sac dilatation | Normal | Abnormal primordial germ cell guidance and survival | Abnormal guidance of trigeminal sensory neuron and neural progenitor cells |
Abbreviations: BM, bone marrow; VSD, ventricular septal defect
Acknowledgments
This work was supported by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Conflict of interest
The authors have no conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Bachelerie F, Ben-Baruch A, Burkhardt AM, Combadiere C, Farber JM, Graham GJ, et al. International Union of Basic and Clinical Pharmacology. [corrected]. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol Rev. 2014;66:1–79. doi: 10.1124/pr.113.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pawig L, Klasen C, Weber C, Bernhagen J, Noels H. Diversity and Inter-Connections in the CXCR4 Chemokine Receptor/Ligand Family: Molecular Perspectives. Front Immunol. 2015;6:429. doi: 10.3389/fimmu.2015.00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.To be added: Peled X. CXCL12 cancer review. Cytokine. 2017
- 4.Nomiyama H, Osada N, Yoshie O. Systematic classification of vertebrate chemokines based on conserved synteny and evolutionary history. Genes Cells. 2013;18:1–16. doi: 10.1111/gtc.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zlotnik A, Yoshie O, Nomiyama H. The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome Biol. 2006;7:243. doi: 10.1186/gb-2006-7-12-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeVries ME, Kelvin AA, Xu L, Ran L, Robinson J, Kelvin DJ. Defining the origins and evolution of the chemokine/chemokine receptor system. J Immunol. 2006;176:401–15. doi: 10.4049/jimmunol.176.1.401. [DOI] [PubMed] [Google Scholar]
- 7.Shirozu M, Nakano T, Inazawa J, Tashiro K, Tada H, Shinohara T, et al. Structure and chromosomal localization of the human stromal cell-derived factor 1 (SDF1) gene. Genomics. 1995;28:495–500. doi: 10.1006/geno.1995.1180. [DOI] [PubMed] [Google Scholar]
- 8.Laguri C, Sadir R, Rueda P, Baleux F, Gans P, Arenzana-Seisdedos F, et al. The novel CXCL12gamma isoform encodes an unstructured cationic domain which regulates bioactivity and interaction with both glycosaminoglycans and CXCR4. PLoS ONE. 2007;2:e1110. doi: 10.1371/journal.pone.0001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rueda P, Balabanian K, Lagane B, Staropoli I, Chow K, Levoye A, et al. The CXCL12gamma chemokine displays unprecedented structural and functional properties that make it a paradigm of chemoattractant proteins. PLoS ONE. 2008;3:e2543. doi: 10.1371/journal.pone.0002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier JL, Arenzana-Seisdedos F, et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–5. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 11.Balabanian K, Lagane B, Infantino S, Chow KYC, Harriague J, Moepps B, et al. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280:35760–6. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- 12.Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A. 1998;95:9448–53. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–9. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 14.Sierro F, Biben C, Martínez-Muñoz L, Mellado M, Ransohoff RM, Li M, et al. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc Natl Acad Sci U S A. 2007;104:14759–64. doi: 10.1073/pnas.0702229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerrits H, van Ingen Schenau DS, Bakker NEC, van Disseldorp AJM, Strik A, Hermens LS, et al. Early postnatal lethality and cardiovascular defects in CXCR7-deficient mice. Genesis. 2008;46:235–45. doi: 10.1002/dvg.20387. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez PA, Gorlin RJ, Lukens JN, Taniuchi S, Bohinjec J, Francois F, et al. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat Genet. 2003;34:70–4. doi: 10.1038/ng1149. [DOI] [PubMed] [Google Scholar]
- 17.Heusinkveld LE, Yim E, Yang A, Azani AB, Liu Q, Gao J-L, et al. Pathogenesis, diagnosis and therapeutic strategies in WHIM syndrome immunodeficiency. Expert Opinion on Orphan Drugs. 2017;5:813–25. doi: 10.1080/21678707.2017.1375403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Treon SP, Cao Y, Xu L, Yang G, Liu X, Hunter ZR. Somatic mutations in MYD88 and CXCR4 are determinants of clinical presentation and overall survival in Waldenstrom macroglobulinemia. Blood. 2014;123:2791–6. doi: 10.1182/blood-2014-01-550905. [DOI] [PubMed] [Google Scholar]
- 19.Liu Q, Pan C, Lopez L, Gao J, Velez D, Anaya-O’Brien S, et al. WHIM Syndrome Caused by Waldenström’s Macroglobulinemia-Associated Mutation CXCR4 (L329fs) J Clin Immunol. 2016;36:397–405. doi: 10.1007/s10875-016-0276-3. [DOI] [PubMed] [Google Scholar]
- 20.Xu L, Hunter ZR, Tsakmaklis N, Cao Y, Yang G, Chen J, et al. Clonal architecture of CXCR4 WHIM-like mutations in Waldenström Macroglobulinaemia. Br J Haematol. 2016;172:735–44. doi: 10.1111/bjh.13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajagopal S, Kim J, Ahn S, Craig S, Lam CM, Gerard NP, et al. Beta-arrestin- but not G protein-mediated signaling by the “decoy” receptor CXCR7. Proc Natl Acad Sci U S A. 2010;107:628–32. doi: 10.1073/pnas.0912852107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Libert F, Parmentier M, Lefort A, Dumont JE, Vassart G. Complete nucleotide sequence of a putative G protein coupled receptor: RDC1. Nucleic Acids Res. 1990;18:1917. doi: 10.1093/nar/18.7.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sreedharan SP, Robichon A, Peterson KE, Goetzl EJ. Cloning and expression of the human vasoactive intestinal peptide receptor. Proc Natl Acad Sci U S A. 1991;88:4986–90. doi: 10.1073/pnas.88.11.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapas S, Clark AJ. Identification of an orphan receptor gene as a type 1 calcitonin gene-related peptide receptor. Biochem Biophys Res Commun. 1995;217:832–8. doi: 10.1006/bbrc.1995.2847. [DOI] [PubMed] [Google Scholar]
- 25.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–13. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bachelerie F, Graham GJ, Locati M, Mantovani A, Murphy PM, Nibbs R, et al. New nomenclature for atypical chemokine receptors. Nat Immunol. 2014;15:207–8. doi: 10.1038/ni.2812. [DOI] [PubMed] [Google Scholar]
- 27.Levoye A, Balabanian K, Baleux F, Bachelerie F, Lagane B. CXCR7 heterodimerizes with CXCR4 and regulates CXCL12-mediated G protein signaling. Blood. 2009;113:6085–93. doi: 10.1182/blood-2008-12-196618. [DOI] [PubMed] [Google Scholar]
- 28.Knaut H, Werz C, Geisler R, Nüsslein-Volhard C Tübingen 2000 Screen Consortium. A zebrafish homologue of the chemokine receptor Cxcr4 is a germ-cell guidance receptor. Nature. 2003;421:279–82. doi: 10.1038/nature01338. [DOI] [PubMed] [Google Scholar]
- 29.Doitsidou M, Reichman-Fried M, Stebler J, Köprunner M, Dörries J, Meyer D, et al. Guidance of primordial germ cell migration by the chemokine SDF-1. Cell. 2002;111:647–59. doi: 10.1016/S0092-8674(02)01135-2. [DOI] [PubMed] [Google Scholar]
- 30.Boldajipour B, Mahabaleshwar H, Kardash E, Reichman-Fried M, Blaser H, Minina S, et al. Control of chemokine-guided cell migration by ligand sequestration. Cell. 2008;132:463–73. doi: 10.1016/j.cell.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 31.Naumann U, Cameroni E, Pruenster M, Mahabaleshwar H, Raz E, Zerwes H-G, et al. CXCR7 functions as a scavenger for CXCL12 and CXCL11. PLoS ONE. 2010;5:e9175. doi: 10.1371/journal.pone.0009175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kledal TN, Rosenkilde MM, Coulin F, Simmons G, Johnsen AH, Alouani S, et al. A broad-spectrum chemokine antagonist encoded by Kaposi’s sarcoma-associated herpesvirus. Science. 1997;277:1656–9. doi: 10.1126/science.277.5332.1656. [DOI] [PubMed] [Google Scholar]
- 33.Rajasekaran D, Gröning S, Schmitz C, Zierow S, Drucker N, Bakou M, et al. Macrophage Migration Inhibitory Factor-CXCR4 Receptor Interactions: EVIDENCE FOR PARTIAL ALLOSTERIC AGONISM IN COMPARISON WITH CXCL12 CHEMOKINE. J Biol Chem. 2016;291:15881–95. doi: 10.1074/jbc.M116.717751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med. 2007;13:587–96. doi: 10.1038/nm1567. [DOI] [PubMed] [Google Scholar]
- 35.Saini V, Marchese A, Majetschak M. CXC chemokine receptor 4 is a cell surface receptor for extracellular ubiquitin. J Biol Chem. 2010;285:15566–76. doi: 10.1074/jbc.M110.103408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–7. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 37.Feng Z, Dubyak GR, Lederman MM, Weinberg A. Cutting edge: human beta defensin 3--a novel antagonist of the HIV-1 coreceptor CXCR4. J Immunol. 2006;177:782–6. doi: 10.4049/jimmunol.177.2.782. [DOI] [PubMed] [Google Scholar]
- 38.Klein KR, Karpinich NO, Espenschied ST, Willcockson HH, Dunworth WP, Hoopes SL, et al. Decoy receptor CXCR7 modulates adrenomedullin-mediated cardiac and lymphatic vascular development. Dev Cell. 2014;30:528–40. doi: 10.1016/j.devcel.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schiraldi M, Raucci A, Muñoz LM, Livoti E, Celona B, Venereau E, et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med. 2012;209:551–63. doi: 10.1084/jem.20111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwartz V, Lue H, Kraemer S, Korbiel J, Krohn R, Ohl K, et al. A functional heteromeric MIF receptor formed by CD74 and CXCR4. FEBS Lett. 2009;583:2749–57. doi: 10.1016/j.febslet.2009.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu B, Chien EYT, Mol CD, Fenalti G, Liu W, Katritch V, et al. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science. 2010;330:1066–71. doi: 10.1126/science.1194396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qin L, Kufareva I, Holden LG, Wang C, Zheng Y, Zhao C, et al. Structural biology. Crystal structure of the chemokine receptor CXCR4 in complex with a viral chemokine. Science. 2015;347:1117–22. doi: 10.1126/science.1261064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marchese A, Benovic JL. Agonist-promoted ubiquitination of the G protein-coupled receptor CXCR4 mediates lysosomal sorting. J Biol Chem. 2001;276:45509–12. doi: 10.1074/jbc.C100527200. [DOI] [PubMed] [Google Scholar]
- 44.Proudfoot AEI, Uguccioni M. Modulation of chemokine responses: synergy and cooperativity. Front Immunol. 2016;7:183. doi: 10.3389/fimmu.2016.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Janssens R, Struyf S, Proost P. The unique structural and functional features of CXCL12. Cell Mol Immunol. 2017 doi: 10.1038/cmi.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mortier A, Gouwy M, Van Damme J, Proost P, Struyf S. CD26/dipeptidylpeptidase IV-chemokine interactions: double-edged regulation of inflammation and tumor biology. J Leukoc Biol. 2016;99:955–69. doi: 10.1189/jlb.3MR0915-401R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thelen M, Muñoz LM, Rodríguez-Frade JM, Mellado M. Chemokine receptor oligomerization: functional considerations. Curr Opin Pharmacol. 2010;10:38–43. doi: 10.1016/j.coph.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 48.Sohy D, Yano H, de Nadai P, Urizar E, Guillabert A, Javitch JA, et al. Hetero-oligomerization of CCR2, CCR5, and CXCR4 and the protean effects of “selective” antagonists. J Biol Chem. 2009;284:31270–9. doi: 10.1074/jbc.M109.054809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kallikourdis M, Trovato AE, Anselmi F, Sarukhan A, Roselli G, Tassone L, et al. The CXCR4 mutations in WHIM syndrome impair the stability of the T-cell immunologic synapse. Blood. 2013;122:666–73. doi: 10.1182/blood-2012-10-461830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wagner N-M, Bierhansl L, Nöldge-Schomburg G, Vollmar B, Roesner JP. Toll-like receptor 2-blocking antibodies promote angiogenesis and induce ERK1/2 and AKT signaling via CXCR4 in endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33:1943–51. doi: 10.1161/ATVBAHA.113.301783. [DOI] [PubMed] [Google Scholar]
- 51.Tashiro K, Tada H, Heilker R, Shirozu M, Nakano T, Honjo T. Signal sequence trap: a cloning strategy for secreted proteins and type I membrane proteins. Science. 1993;261:600–3. doi: 10.1126/science.8342023. [DOI] [PubMed] [Google Scholar]
- 52.Nagasawa T, Kikutani H, Kishimoto T. Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc Natl Acad Sci U S A. 1994;91:2305–9. doi: 10.1073/pnas.91.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–8. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 54.Dar A, Kollet O, Lapidot T. Mutual, reciprocal SDF-1/CXCR4 interactions between hematopoietic and bone marrow stromal cells regulate human stem cell migration and development in NOD/SCID chimeric mice. Exp Hematol. 2006;34:967–75. doi: 10.1016/j.exphem.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 55.Shachar I, Karin N. The dual roles of inflammatory cytokines and chemokines in the regulation of autoimmune diseases and their clinical implications. J Leukoc Biol. 2013;93:51–61. doi: 10.1189/jlb.0612293. [DOI] [PubMed] [Google Scholar]
- 56.Murphy PM, Baggiolini M, Charo IF, Hébert CA, Horuk R, Matsushima K, et al. International union of pharmacology. XXII,. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145–76. [PubMed] [Google Scholar]
- 57.Balkwill F. The significance of cancer cell expression of the chemokine receptor CXCR4. Semin Cancer Biol. 2004;14:171–9. doi: 10.1016/j.semcancer.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 58.Busillo JM, Benovic JL. Regulation of CXCR4 signaling. Biochim Biophys Acta. 2007;1768:952–63. doi: 10.1016/j.bbamem.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thelen M, Stein JV. How chemokines invite leukocytes to dance. Nat Immunol. 2008;9:953–9. doi: 10.1038/ni.f.207. [DOI] [PubMed] [Google Scholar]
- 60.Torossian F, Anginot A, Chabanon A, Clay D, Guerton B, Desterke C, et al. CXCR7 participates in CXCL12-induced CD34+ cell cycling through β-arrestin-dependent Akt activation. Blood. 2014;123:191–202. doi: 10.1182/blood-2013-05-500496. [DOI] [PubMed] [Google Scholar]
- 61.Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 62.Nagasawa T, Nakajima T, Tachibana K, Iizasa H, Bleul CC, Yoshie O, et al. Molecular cloning and characterization of a murine pre-B-cell growth-stimulating factor/stromal cell-derived factor 1 receptor, a murine homolog of the human immunodeficiency virus 1 entry coreceptor fusin. Proc Natl Acad Sci U S A. 1996;93:14726–9. doi: 10.1073/pnas.93.25.14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, et al. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–33. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 64.Donzella GA, Schols D, Lin SW, Esté JA, Nagashima KA, Maddon PJ, et al. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat Med. 1998;4:72–7. doi: 10.1038/nm0198-072. [DOI] [PubMed] [Google Scholar]
- 65.Wilen CB, Tilton JC, Doms RW. Molecular mechanisms of HIV entry. Adv Exp Med Biol. 2012;726:223–42. doi: 10.1007/978-1-4614-0980-9_10. [DOI] [PubMed] [Google Scholar]
- 66.De Clercq E. The bicyclam AMD3100 story. Nat Rev Drug Discov. 2003;2:581–7. doi: 10.1038/nrd1134. [DOI] [PubMed] [Google Scholar]
- 67.DiPersio JF, Stadtmauer EA, Nademanee A, Micallef INM, Stiff PJ, Kaufman JL, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113:5720–6. doi: 10.1182/blood-2008-08-174946. [DOI] [PubMed] [Google Scholar]
- 68.DiPersio JF, Micallef IN, Stiff PJ, Bolwell BJ, Maziarz RT, Jacobsen E, et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin’s lymphoma. J Clin Oncol. 2009;27:4767–73. doi: 10.1200/JCO.2008.20.7209. [DOI] [PubMed] [Google Scholar]
- 69.Liu R, Paxton WA, Choe S, Ceradini D, Martin SR, Horuk R, et al. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–77. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 70.Zimmerman PA, Buckler-White A, Alkhatib G, Spalding T, Kubofcik J, Combadiere C, et al. Inherited resistance to HIV-1 conferred by an inactivating mutation in CC chemokine receptor 5: studies in populations with contrasting clinical phenotypes, defined racial background, and quantified risk. Mol Med. 1997;3:23–36. [PMC free article] [PubMed] [Google Scholar]
- 71.Dean M, Carrington M, Winkler C, Huttley GA, Smith MW, Allikmets R, et al. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science. 1996;273:1856–62. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 72.Michael NL, Chang G, Louie LG, Mascola JR, Dondero D, Birx DL, et al. The role of viral phenotype and CCR-5 gene defects in HIV-1 transmission and disease progression. Nat Med. 1997;3:338–40. doi: 10.1038/nm0397-338. [DOI] [PubMed] [Google Scholar]
- 73.Samson M, Libert F, Doranz BJ, Rucker J, Liesnard C, Farber CM, et al. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–5. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 74.Gulick RM, Lalezari J, Goodrich J, Clumeck N, DeJesus E, Horban A, et al. Maraviroc for previously treated patients with R5 HIV-1 infection. N Engl J Med. 2008;359:1429–41. doi: 10.1056/NEJMoa0803152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fätkenheuer G, Pozniak AL, Johnson MA, Plettenberg A, Staszewski S, Hoepelman AIM, et al. Efficacy of short-term monotherapy with maraviroc, a new CCR5 antagonist, in patients infected with HIV-1. Nat Med. 2005;11:1170–2. doi: 10.1038/nm1319. [DOI] [PubMed] [Google Scholar]
- 76.Allers K, Hütter G, Hofmann J, Loddenkemper C, Rieger K, Thiel E, et al. Evidence for the cure of HIV infection by CCR5Δ32/Δ32 stem cell transplantation. Blood. 2011;117:2791–9. doi: 10.1182/blood-2010-09-309591. [DOI] [PubMed] [Google Scholar]
- 77.Hütter G, Nowak D, Mossner M, Ganepola S, Müssig A, Allers K, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360:692–8. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 78.Perez EE, Wang J, Miller JC, Jouvenot Y, Kim KA, Liu O, et al. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat Biotechnol. 2008;26:808–16. doi: 10.1038/nbt1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tebas P, Stein D, Tang WW, Frank I, Wang SQ, Lee G, et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med. 2014;370:901–10. doi: 10.1056/NEJMoa1300662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zuelzer WW. “MYELOKATHEXIS”--A NEW FORM OF CHRONIC GRANULOCYTOPENIA. REPORT OF A CASE N Engl J Med. 1964;270:699–704. doi: 10.1056/NEJM196404022701402. [DOI] [PubMed] [Google Scholar]
- 81.Balabanian K, Levoye A, Klemm L, Lagane B, Hermine O, Harriague J, et al. Leukocyte analysis from WHIM syndrome patients reveals a pivotal role for GRK3 in CXCR4 signaling. J Clin Invest. 2008;118:1074–84. doi: 10.1172/JCI33187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liang TS, Hartt JK, Lu S, Martins-Green M, Gao JL, Murphy PM. Cloning, mRNA distribution, and functional expression of an avian counterpart of the chemokine receptor/HIV coreceptor CXCR4. J Leukoc Biol. 2001;69:297–305. [PubMed] [Google Scholar]
- 83.Haribabu B, Richardson RM, Fisher I, Sozzani S, Peiper SC, Horuk R, et al. Regulation of human chemokine receptors CXCR4. Role of phosphorylation in desensitization and internalization. J Biol Chem. 1997;272:28726–31. doi: 10.1074/jbc.272.45.28726. [DOI] [PubMed] [Google Scholar]
- 84.Lagane B, Chow KYC, Balabanian K, Levoye A, Harriague J, Planchenault T, et al. CXCR4 dimerization and beta-arrestin-mediated signaling account for the enhanced chemotaxis to CXCL12 in WHIM syndrome. Blood. 2008;112:34–44. doi: 10.1182/blood-2007-07-102103. [DOI] [PubMed] [Google Scholar]
- 85.Martin C, Burdon PCE, Bridger G, Gutierrez-Ramos JC, Williams TJ, Rankin SM. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003;19:583–93. doi: 10.1016/S1074-7613(03)00263-2. [DOI] [PubMed] [Google Scholar]
- 86.Strydom N, Rankin SM. Regulation of circulating neutrophil numbers under homeostasis and in disease. J Innate Immun. 2013;5:304–14. doi: 10.1159/000350282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Balabanian K, Brotin E, Biajoux V, Bouchet-Delbos L, Lainey E, Fenneteau O, et al. Proper desensitization of CXCR4 is required for lymphocyte development and peripheral compartmentalization in mice. Blood. 2012;119:5722–30. doi: 10.1182/blood-2012-01-403378. [DOI] [PubMed] [Google Scholar]
- 88.Devi S, Wang Y, Chew WK, Lima R, A-González N, Mattar CNZ, et al. Neutrophil mobilization via plerixafor-mediated CXCR4 inhibition arises from lung demargination and blockade of neutrophil homing to the bone marrow. J Exp Med. 2013;210:2321–36. doi: 10.1084/jem.20130056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aprikyan AA, Liles WC, Park JR, Jonas M, Chi EY, Dale DC. Myelokathexis, a congenital disorder of severe neutropenia characterized by accelerated apoptosis and defective expression of bcl-x in neutrophil precursors. Blood. 2000;95:320–7. [PubMed] [Google Scholar]
- 90.Liu Q, Li ZY, Yang A, Gao J-LS, Velez DJ, Cho E, et al. Mechanisms of Sustained Neutrophilia in Patient WHIM-09, Cured of WHIM Syndrome by Chromothripsis. J Clin Immunol. 2017 doi: 10.1007/s10875-017-0457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McDermott DH, Lopez J, Deng F, Liu Q, Ojode T, Chen H, et al. AMD3100 is a potent antagonist at CXCR4(R334X), a hyperfunctional mutant chemokine receptor and cause of WHIM syndrome. J Cell Mol Med. 2011;15:2071–81. doi: 10.1111/j.1582-4934.2010.01210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Beaussant Cohen S, Fenneteau O, Plouvier E, Rohrlich P-S, Daltroff G, Plantier I, et al. Description and outcome of a cohort of 8 patients with WHIM syndrome from the French Severe Chronic Neutropenia Registry. Orphanet J Rare Dis. 2012;7:71. doi: 10.1186/1750-1172-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McDermott DH, Liu Q, Ulrick J, Kwatemaa N, Anaya-O’Brien S, Penzak SR, et al. The CXCR4 antagonist plerixafor corrects panleukopenia in patients with WHIM syndrome. Blood. 2011;118:4957–62. doi: 10.1182/blood-2011-07-368084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dale DC, Bolyard AA, Kelley ML, Westrup EC, Makaryan V, Aprikyan A, et al. The CXCR4 antagonist plerixafor is a potential therapy for myelokathexis, WHIM syndrome. Blood. 2011;118:4963–6. doi: 10.1182/blood-2011-06-360586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mentzer WC, Johnston RB, Baehner RL, Nathan DG. An unusual form of chronic neutropenia in a father and daughter with hypogammaglobulinaemia. Br J Haematol. 1977;36:313–22. doi: 10.1111/j.1365-2141.1977.tb00654.x. [DOI] [PubMed] [Google Scholar]
- 96.Kallikourdis M, Viola A, Benvenuti F. Human immunodeficiencies related to defective APC/T cell interaction. Front Immunol. 2015;6:433. doi: 10.3389/fimmu.2015.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tarzi MD, Jenner M, Hattotuwa K, Faruqi AZ, Diaz GA, Longhurst HJ. Sporadic case of warts, hypogammaglobulinemia, immunodeficiency, and myelokathexis syndrome. J Allergy Clin Immunol. 2005;116:1101–5. doi: 10.1016/j.jaci.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 98.Tassone L, Notarangelo LD, Bonomi V, Savoldi G, Sensi A, Soresina A, et al. Clinical and genetic diagnosis of warts, hypogammaglobulinemia, infections, and myelokathexis syndrome in 10 patients. Journal of Allergy and Clinical Immunology. 2009;123:1170–3e3. doi: 10.1016/j.jaci.2008.12.1133. [DOI] [PubMed] [Google Scholar]
- 99.Liu Q, Chen H, Ojode T, Gao X, Anaya-O’Brien S, Turner NA, et al. WHIM syndrome caused by a single amino acid substitution in the carboxy-tail of chemokine receptor CXCR4. Blood. 2012;120:181–9. doi: 10.1182/blood-2011-12-395608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Handisurya A, Schellenbacher C, Reininger B, Koszik F, Vyhnanek P, Heitger A, et al. A quadrivalent HPV vaccine induces humoral and cellular immune responses in WHIM immunodeficiency syndrome. Vaccine. 2010;28:4837–41. doi: 10.1016/j.vaccine.2010.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chow KYC, Brotin É, Ben Khalifa Y, Carthagena L, Teissier S, Danckaert A, et al. A pivotal role for CXCL12 signaling in HPV-mediated transformation of keratinocytes: clues to understanding HPV-pathogenesis in WHIM syndrome. Cell Host Microbe. 2010;8:523–33. doi: 10.1016/j.chom.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 102.Sasagawa T, Takagi H, Makinoda S. Immune responses against human papillomavirus (HPV) infection and evasion of host defense in cervical cancer. J Infect Chemother. 2012;18:807–15. doi: 10.1007/s10156-012-0485-5. [DOI] [PubMed] [Google Scholar]
- 103.McDermott DH, Gao J-L, Liu Q, Siwicki M, Martens C, Jacobs P, et al. Chromothriptic cure of WHIM syndrome. Cell. 2015;160:686–99. doi: 10.1016/j.cell.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ustwani O, Al Kurzrock R, Wetzler M. Genetics on a WHIM. Br J Haematol. 2014;164:15–23. doi: 10.1111/bjh.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–94. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 106.Lévesque J-P, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003;111:187–96. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Moens L, Frans G, Bosch B, Bossuyt X, Verbinnen B, Poppe W, et al. Successful hematopoietic stem cell transplantation for myelofibrosis in an adult with warts-hypogammaglobulinemia-immunodeficiency-myelokathexis syndrome. J Allergy Clin Immunol. 2016;138:1485–9e2. doi: 10.1016/j.jaci.2016.04.057. [DOI] [PubMed] [Google Scholar]
- 108.Kriván G, Erdos M, Kállay K, Benyó G, Tóth A, Sinkó J, et al. Successful umbilical cord blood stem cell transplantation in a child with WHIM syndrome. Eur J Haematol. 2010;84:274–5. doi: 10.1111/j.1600-0609.2009.01368.x. [DOI] [PubMed] [Google Scholar]
- 109.Badolato R, Dotta L, Tassone L, Amendola G, Porta F, Locatelli F, et al. Tetralogy of fallot is an uncommon manifestation of warts, hypogammaglobulinemia, infections, and myelokathexis syndrome. J Pediatr. 2012;161:763–5. doi: 10.1016/j.jpeds.2012.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Taniuchi S, Yamamoto A, Fujiwara T, Hasui M, Tsuji S, Kobayashi Y. Dizygotic twin sisters with myelokathexis: mechanism of its neutropenia. Am J Hematol. 1999;62:106–11. doi: 10.1002/(sici)1096-8652(199910)62:2<106::aid-ajh8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 111.Takabatake Y, Sugiyama T, Kohara H, Matsusaka T, Kurihara H, Koni PA, et al. The CXCL12 (SDF-1)/CXCR4 axis is essential for the development of renal vasculature. J Am Soc Nephrol. 2009;20:1714–23. doi: 10.1681/ASN.2008060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–4. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 113.Döring Y, Pawig L, Weber C, Noels H. The CXCL12/CXCR4 chemokine ligand/receptor axis in cardiovascular disease. Front Physiol. 2014;5:212. doi: 10.3389/fphys.2014.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–64. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 115.Saxena A, Fish JE, White MD, Yu S, Smyth JWP, Shaw RM, et al. Stromal cell-derived factor-1alpha is cardioprotective after myocardial infarction. Circulation. 2008;117:2224–31. doi: 10.1161/CIRCULATIONAHA.107.694992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhu Y, Yu T, Zhang X-C, Nagasawa T, Wu JY, Rao Y. Role of the chemokine SDF-1 as the meningeal attractant for embryonic cerebellar neurons. Nat Neurosci. 2002;5:719–20. doi: 10.1038/nn881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hillmer RE, Boisvert JP, Cucciare MJ, Dwinell MB, Joksimovic M. Generation and characterization of mice harboring a conditional CXCL12 allele. Int J Dev Biol. 2015;59:205–9. doi: 10.1387/ijdb.140348mj. [DOI] [PubMed] [Google Scholar]
- 118.Reiss K, Mentlein R, Sievers J, Hartmann D. Stromal cell-derived factor 1 is secreted by meningeal cells and acts as chemotactic factor on neuronal stem cells of the cerebellar external granular layer. Neuroscience. 2002;115:295–305. doi: 10.1016/s0306-4522(02)00307-x. [DOI] [PubMed] [Google Scholar]
- 119.Klein RS, Rubin JB, Gibson HD, DeHaan EN, Alvarez-Hernandez X, Segal RA, et al. SDF-1 alpha induces chemotaxis and enhances Sonic hedgehog-induced proliferation of cerebellar granule cells. Development. 2001;128:1971–81. doi: 10.1242/dev.128.11.1971. [DOI] [PubMed] [Google Scholar]
- 120.Williams JL, Holman DW, Klein RS. Chemokines in the balance: maintenance of homeostasis and protection at CNS barriers. Front Cell Neurosci. 2014;8:154. doi: 10.3389/fncel.2014.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li M, Ransohoff RM. Multiple roles of chemokine CXCL12 in the central nervous system: a migration from immunology to neurobiology. Prog Neurobiol. 2008;84:116–31. doi: 10.1016/j.pneurobio.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ransohoff RM, Liu L, Cardona AE. Chemokines and chemokine receptors: multipurpose players in neuroinflammation. Int Rev Neurobiol. 2007;82:187–204. doi: 10.1016/S0074-7742(07)82010-1. [DOI] [PubMed] [Google Scholar]
- 123.Schneider EH, Fowler SC, Lionakis MS, Swamydas M, Holmes G, Diaz V, et al. Regulation of motor function and behavior by atypical chemokine receptor 1. Behav Genet. 2014;44:498–515. doi: 10.1007/s10519-014-9665-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Duchene J, Novitzky-Basso I, Thiriot A, Casanova-Acebes M, Bianchini M, Etheridge SL, et al. Atypical chemokine receptor 1 on nucleated erythroid cells regulates hematopoiesis. Nat Immunol. 2017;18:753–61. doi: 10.1038/ni.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]