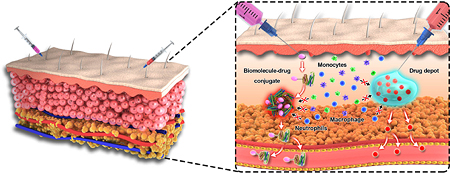
Abstract
Subcutaneous long-acting release (LAR) formulations have been extensively developed in the clinic to increase patient compliance and reduce treatment cost. Despite preliminary success for some LAR systems, a major obstacle limiting the therapeutic effect remains on their interaction with surrounding tissues. In this review, we summarize how living bodies respond to injected or implanted materials, and highlight some typical strategies based on smart material design, which may significantly improve long-term subcutaneous drug delivery. Moreover, possible strategies to achieve ultra-long (months, years) subcutaneous drug delivery systems are proposed. Based on these discussions, we believe the well-designed subcutaneous long-acting formulations will hold great promise to improve patient quality of life in the clinic.
Keywords: long-acting release (LAR), foreign body reaction, drug retention, immunoisolation, non-fouling materials
Graphical Abstract
Introduction
With the rapid advancement of the pharmaceutical industry, more and more biologics have played significant roles in the treatment of various diseases [1]. To improve the therapeutic efficacy and patient compliance, diverse routes of administration have been developed, including oral, nasal, subcutaneous, intravenous pathways and so on [2]. Among these methods, subcutaneous drug delivery is one of the most frequent choices because it is a relatively low-cost, self-administered, safe and highly effective route [3]. For example, proteins or peptides delivered by the subcutaneous route are more robust relative to oral delivery that may easily destabilize or degrade the protein or peptide structures due to the high proteolytic activity and low pH of the stomach [4, 5]. Moreover, compared to the intravenous route, subcutaneous administration is more economical as it can be conducted by patients at home. For example, switching 200 patients from hospital-based intravenous to self-administered subcutaneous injection of cancer drug trastuzumab saved more than $2 billion [6]. In addition, the physiological barrier in the subcutaneous administration is relatively easier to penetrate (routine injection) than the intranasal pathway as mucus layer and nasal epithelial cells provide a strong physical and biological barrier to obstruct drug adhesion and induce enzymatic degradation [7]. Given the advantages of subcutaneous drug delivery, major efforts have been made to develop and optimize more convenient and effective subcutaneous delivery strategies, which may exhibit large potential in the clinical practice and commercial market.
In order to improve subcutaneous drug delivery, long-acting formulations have attracted increasing attention, which may significantly decrease patient effort and increase their compliance [8–12]. For example, long-acting glucagon-like peptide 1 (GLP-1) delivery systems have been well developed, which can be administered around once per week to control blood glucose level (BGL) in type 2 diabetes [13–21]. Somatostatin analog depots including long-acting octreotide acetate and lanreotide formulations have been applied once-monthly to treat acromegaly with satisfactory effects [22–24]. Long-acting risperidone release systems have also been administered once every 2 weeks to treat schizophrenia and symptoms of bipolar disorder (manic depression) in the clinic [25, 26]. Moreover, various bio-responsive materials have been designed to achieve closed-loop, feedback-controlled drug release, which may extensively improve the utilization efficiency of the drugs by releasing payloads in response to biological stimuli [27–32]. However, critical barriers that are responsible for the failure of long-acting subcutaneous delivery systems are the foreign body reactions (FBR), which result in the formation of a vascular collagenous fibrous layer to restrict the interaction of the materials with the bioenvironment [33–36]. In this way, the longevity and functionality of the delivery systems may be compromised. Moreover, the high FBR may not only destroy the delivery systems, but also bring a high risk to patients if a severe inflammatory response occurs [37]. Hence, improving biocompatibility and immunoisolation through smart system design has been extensively studied in recent years [38], which may help significantly improve current long-acting drug delivery strategies.
In this review, we first introduce how foreign body reactions occur and their influence on subcutaneously injected or implanted materials. Then, we summarize how to design a system to enhance long-term subcutaneous drug delivery and discuss the mechanism. Finally, we propose future perspectives to develop ultra-long drug release formulations.
2 Foreign body reaction
During the evolution, mammals have developed a complicated mechanism to deal with exogenous substances [39–41]. When foreign bodies including injected or implanted materials remain in the body, a dense, hypocellular, collagen-rich sheath will be constructed around the materials due to a prolonged and aberrant wound healing, which acts as one of the most insurmountable barriers that is responsible for the failure of long-term subcutaneous drug delivery [33–36, 39–41].
2.1 General processes for FBR
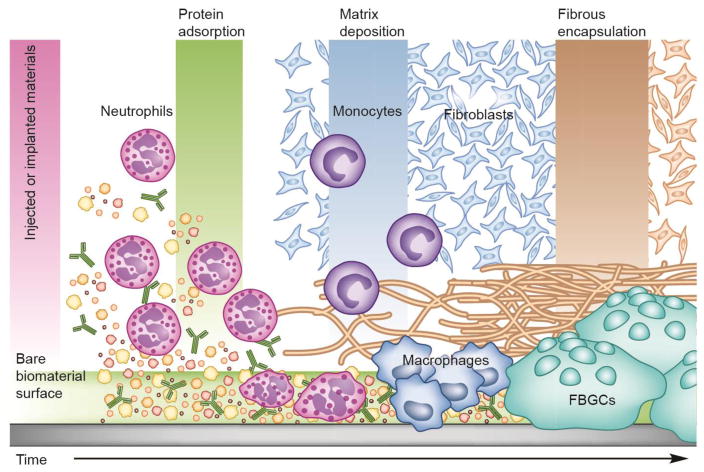
In general, FBR is a relatively complicated process, which involves the participation of numerous bio-systems including various host proteins, inflammatory cells, immune cells and so on [42–44]. At the beginning, instantaneous adsorption of many host proteins (e.g. fibrinogen) to the surface of injected or implanted materials occurs [45], followed by rapid neutrophil infiltration [46]. After activation, neutrophils release abundant soluble factors on the surface, which recruit and activate tissue-resident macrophages and circulating monocyte precursor cells [42–44, 46]. As the foreign materials designed for long-acting drug delivery cannot be easily removed by those cells through a phagocytic process, the cells fuse with each other to generate foreign-body giant cells (FBGCs) at the material surface. Moreover, macrophages secrete various factors including transforming growth factor β and other inflammatory cytokines to induce transformation of quiescent fibroblasts into myofibroblasts [47]. After SMAD mediator activation, the myofibroblasts synthesize the procollagen and secrete it into the extracellular matrix, which is crosslinked to facilitate the generation of a dense and hypocelluar capsule (Fig. 1) [42–44].
Fig. 1.
Schematic diagram of the FBR leading to the encapsulation of injected or implanted materials. Adapted with permission from reference [43].
2.2 Features of FBR-based capsule
When FBR occurs, it takes several weeks to gradually form a distinct, collagenous capsule around the materials [48]. Typical features of the capsules include [42–44]: (i) The thickness of the resulting capsules can be up to several hundred micrometers (50–200 μm) with a primary component of fibrotic collagens. The thick and robust sheath has proven to be able to physically and physiologically separate the materials and host tissues, which is impermeable or hypopermeable to cells and metabolites, significantly limiting material-bioenvironment interactions. (ii) The continual retention of an exogenous object induces chronic inflammation, which can be characterized by the presence of macrophages, monocytes, lymphocytes, and so on. Interestingly, the fibrin clot generated from an acute inflammatory process is transformed into highly vascularized tissues as a result of vascular endothelial cell and fibroblast proliferation. Although the neovascularization can partially improve nutrient transport and wound healing, most of the vessels are unusual and immature, which is inadequate to significantly accelerate substrate exchange. In such a way, the injected or implanted materials are always exposed to an inflammation-mediated environment without any appropriate contact with surrounding tissues. (iii) It has been confirmed that various cells are involved in the capsule formation. Among them, macrophages have been observed to engulf various large foreign particles and degrade them via enzyme-rich lysosomes [34]. In this way, the injected or implanted materials can be easily destroyed during the FBR process. Collectively, all the features of the FBR-based capsule indicate that it may serve as a natural barrier for long-term subcutaneous drug delivery systems, thus new strategies that can overcome FBR may hold great promise to improve long-term drug treatment in clinical practices.
3 Strategies to improve long-term subcutaneous drug delivery through smart design
With advances in material and life sciences, researchers have realized that rational material design can meet complex biological needs [38, 49–51]. As for long-acting drug delivery, reducing host recognition and aggression (overcoming FBR), prolonging subcutaneous drug retention, improving sustained drug release, and extending blood circulation of drugs are the most useful approaches. In this section, we discuss the recent strategies based on the material design to improve the performance of long-acting formulations.
3.1 Direct modification
3.1.1. Biomolecule binding
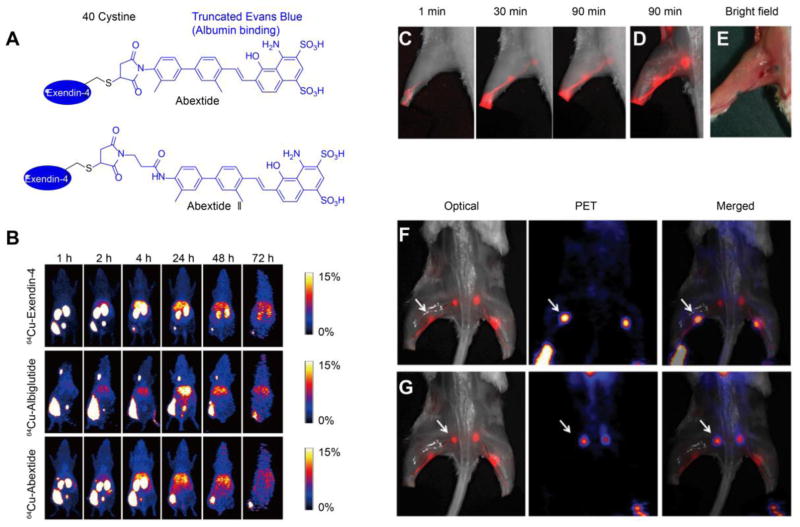
It has been confirmed that there are two potential routes for subcutaneously injected drugs. Lower molecular weight drugs (< 20 kD) mainly enter the general circulation through subcutaneous blood capillaries, while larger molecular weight drugs (> 20 kD) traffic through the interstitial matrix to the peripheral lymphatic system before entering the general circulation at the subclavian vein [3, 52]. Hence the size or aggregation form may significantly influence the fate and onset of the drug. To adjust the property of original drugs, the simplest and most straightforward method is the direct modification of the drug molecules [53]. One effective strategy to improve drug duration time is to develop some linkers to help the drug bind to biomolecules [53]. Among various biological species, human serum albumin (HSA) and immunoglobulin G (IgG) are ideal candidates due to their long half-life (approximately three weeks) and high abundance [54]. No matter in serum or interstitial fluid, the concentrations of albumin and IgG are both quite high (serum concentration: albumin is 4.58 ± 0.35 g/100 ml, IgG is 1.27 ± 0.23 g/100ml; interstitial fluid concentration: albumin is 2.92 ± 0.32 g/100ml, IgG is 0.65 ± 0.18 g/100ml) [55, 56]. Therefore, developing albumin or IgG-binding complexes has emerged as one of the most promising strategies to improve subcutaneous drug retention and blood circulation [53, 57, 58]. Typical U.S. Food and Drug Administration (FDA)-approved albumin or IgG-binding drugs are listed in Table 1, which have been applied subcutaneously to treat various diseases including diabetes, rheumatoid arthritis, cancers and so on [53]. With the help of albumin or IgG binding, all the conjugates significantly extend the duration of drug action, remarkably improving their therapeutic effects. Compared to artificial macromolecule modification (e.g. PEGylation), the small-molecule conjugation serves as a more biocompatible strategy without significant activity loss induced by the steric hindrance effect [53, 59–62]. One of the most successful strategies using small-molecule binders has been achieved by lipid modification (lipidation) [53]. Intriguingly, albumin contains nine different binding sites for fatty acids or similar molecules, facilitating conjugation with drug molecules [63]. The strategy has proven recently successful in anti-diabetic drugs [64–66], immune drugs [67], antithrombotic drugs [68], and so on, suggesting large potential in future applications. Similarly, our group has developed another albumin binder, a truncated Evens blue (tEB) derivative to improve drug performance [69–73]. One interesting example involves the modification of exendin-4 (Fig. 2A). After conjugating exendin-4 with tEB through a thiol group on Cys40 and maleimide group on the linker, the product (Abextide) significantly prolongs subcutaneous retention and blood circulation of exendin-4 due to albumin binding [71]. Moreover, minor adjustment of molecular structures by introducing an amide group in the linker creates a more stable conjugate (Abextide II), which lowers BGL and prolongs the normoglycemia duration in a type 2 diabetes mouse model (more effective than FDA-approved Albiglutide) [69]. It should be emphasized that the improvement of subcutaneous drug delivery as a result of biomolecule binding could be directly observed from positron emission tomography (PET) imaging [71]. Clearly, results showed that tEB modification could significantly prolong subcutaneous drug diffusion and absorption (~10%ID retaining after 24 h) compared to free exendin-4 (less than 10%ID only after 2 h) (Fig. 2B). In addition, the formation of complexes by EB derivatives and endogenous albumin in the interstitial fluid, and its slow migration and diffusion into the lymphatic node (LN) have been extensively observed by optical and PET imaging (Fig. 2C–G) [73]. Interestingly, no severe inflammation or FBR is observed as biomolecule binding renders some biomimetic properties, which significantly alleviate host recognition and rejection to the drugs. Besides the aforementioned binders, synthetic molecules including bile acid derivatives [74], phosphate esters [75], and some halogen-containing linkers [76, 77] have also been developed for biomolecule binding. Those well-designed structures have shown a negligible effect on drug functionality and act as ideal candidates to improve current therapeutic strategies that are limited by rapid drug clearance. We believe many of those potential conjugates will be quickly translated into clinical practices in the near future.
Table 1.
Currently approved subcutaneous long-acting formulations
| Brand name | International nonproprietary name | Strategy | Indication | Approval year |
|---|---|---|---|---|
| Ozempic | Semaglutide | Lipidation | Type 2 diabetes | 2017 |
| Xultophy 100/3.6 | Insulin degludec and liraglutide injection | Lipidation | Type 2 diabetes | 2016 |
| Tresiba | Insulin degludec | Lipidation | Type 1 and type 2 diabetes | 2015 |
| Tanzeum, Eperzan | Albiglutide | Albumin fusion | Type 2 diabetes | 2014 |
| Trulicity | Dulaglutide | Fc fusion | Type2 diabetes | 2014 |
| Saxenda | Liraglutide | Lipidation | Obesity | 2014 |
| Eloctate | Antihemophilic factor (recombinant) | Fc fusion | Hemophilia A | 2014 |
| Alprolix | Coagulation Factor IX (recombinant) | Fc fusion | Hemophilia B | 2014 |
| Eylea | Aflibercept | Fc fusion | Wet macular degeneration | 2011 |
| Nulojix | Belatacept | Fc fusion | Kidney transplant rejection | 2011 |
| Victoza | Liraglutide | Lipidation | Type 2 diabetes | 2010 |
| Arcalyst | Rilonacept | Fc fusion | Cryopyrin-associated periodic syndromes | 2008 |
| Nplate | Romiplostim | Fc fusion | Immune thrombocytopenic purpura | 2008 |
| Somatuline Depot | Lanreotide | Subcutaneous depot | Acromegaly | 2007 |
| Omnitrope | Somatropin (rDNA origin) | Subcutaneous depot | Growth hormone deficiency | 2006 |
| Orencia | Abatacept | Fc fusion | Rheumatoid arthritis | 2005 |
| Levemir | insuline detemir (rDNA origin) | Lipidation | Type 1 diabetes | 2005 |
| Bydureon | Exenatide | Subcutaneous depot | Type 2 diabetes | 2005 |
| Risperdal Consta | Risperidone | Subcutaneous depot | Schizophrenia and symptoms of bipolar disorder | 2003 |
| Enbrel | Etanercept | Fc fusion | Rheumatoid arthritis, psoriasis | 2002 |
| Nutropin AQ | Somatropin (rDNA origin) | Subcutaneous depot | Growth hormone deficiency | 1999 |
| Sandostatin Lar Depot | acetate | Subcutaneous depot | Acromegaly | 1998 |
Fig. 2.
(A) Typical structures of tEB modified exendin-4 (Adapted with permission from reference [69] and reference [71]). (B) Whole body PET images of BALB/c mice at different time points after subcutaneous injection of 64Cu-Abextide, 64Cu-Albiglutide and 64Cu-Exendin-4, (Adapted with permission from reference [71]). (C) Fluorescence images of the lymphatic system in BALB/c mice treated with fluorine-18 aluminum fluoride-labeled NOTA (1,4,7-triazacyclononane-N,N′,N″-triacetic acid)-conjugated truncated Evans blue (18F-AlF-NEB) and normal EB following a chronological order. (D) Ex vivo optical imaging of LNs at 90 min postinjection (skin removed). (E) Bright field image showed the blue color in the lymphatic system. (F) Optical and PET imaging of popliteal LNs (white arrow). (G) Optical and PET imaging of the sciatic LNs (white arrow). Fig. 2C–G were adapted with permission from reference [73]).
3.1.2. Direct immobilization
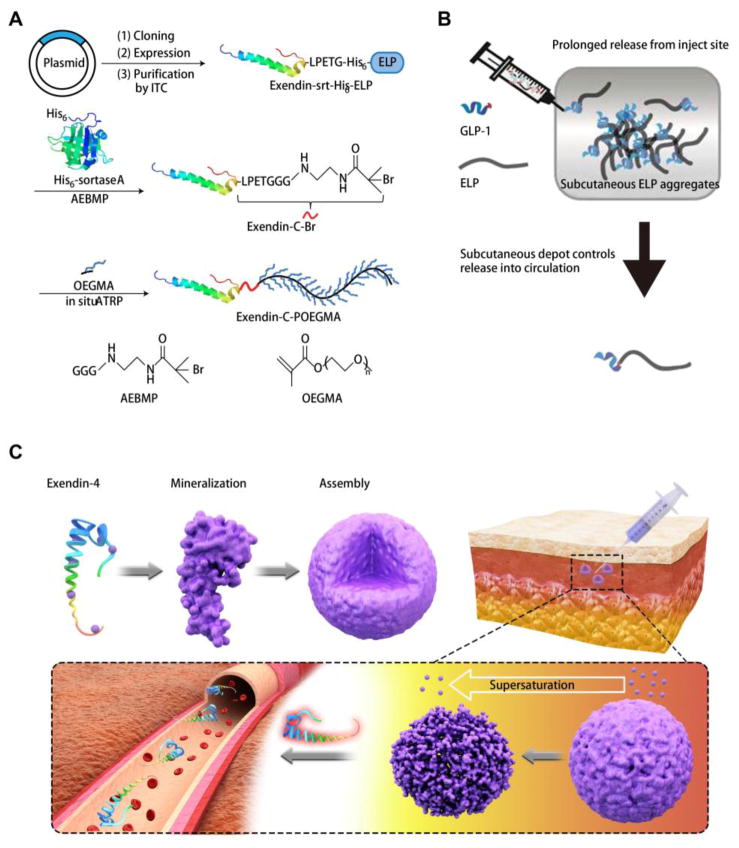
In addition to introducing simple molecular linkers for biomolecule binding (indirect immobilization), drugs could be directly immobilized through forming hydrogel or inorganic-organic hybrid.. It has been confirmed that many drugs with specific functional groups (e.g. amino, carboxyl, thiol groups) can be conjugated with natural or synthetic macromolecules (polymers or peptides) to construct largely sized depots and facilitate gradual release [9, 20, 78]. Recently, Chilkoti et al. conjugated GLP-1 or exendin-4 to a POEGMA polymer (Fig. 3A) or elastin-like polypeptide (ELP) (Fig. 3B) through protein fusion-based approaches, which have been proven to significantly extend the subcutaneous drug retention due to aggregation [17–19, 79]. Evidently, the depot-forming GLP-1 fusion protein can reduce BGL for at least five days with a single injection [17–19, 79]. This should be attributed to the zero-order release kinetics, which is ideal for both minimizing classic peak and valley pharmacokinetics and reducing dosing frequency [17]. Moreover, this kind of modification has been confirmed to trigger weaker antigenicity compared to PEG modification, suggesting high biocompatibility [18]. Besides, another solidification strategy lies in the inorganic-organic hybrid generation (e.g. mineralization reaction) [80, 81]. As we know, when the protein or peptide contains adequate acidic amino acid residues, such as glutamic acid (Glu) (pKa = 4.25) and aspartic acid (Asp) (pKa = 3.86), it may easily chelate calcium ions from supersaturated metastable solutions [82–84]. After nucleation, tiny crystals are generated around the protein or peptide, which tend to assemble into orderly structured spherical aggregates via a “bricks and mortar” self-assembly mechanism [85]. Moreover, the mineralized particles respond to physiological supersaturation (0.5–2.5 mM Ca) to release the payloads [83]. While the local supersaturation increases due to the calcium-based particle dissolution, the release rate spontaneously slows down, indicating a leverage-based release profile (Fig. 3C). It should be emphasized that no matter whether the drugs are under aforementioned polymer conjugation or mineralization modification, the final dissociated products are only biological absorbable components, which can be completely absorbed by surrounding tissues, inducing negligible inflammation, and achieving safe and controllable long-term drug release [17–20, 79, 83, 84]. Collectively, direct introduction of a bio-friendly modification on the drug molecule can significantly improve the performance of the therapeutic agent by enhancing subcutaneous retention and circulation time with mild immune rejection. Moreover, the modification method is simple, time-saving, and economical by just introducing a minor alteration on the original drugs. In such a way, we believe direct chemical modification can be an effective potential strategy to significantly improve long-acting drug releasing systems.
Fig. 3.
(A) Synthesis of exendin-C-POEGMA. Adapted with permission from reference [18]. (B) A fusion of ELP and GLP-1 can form an extended release subcutaneous depot and prolong peptide circulation time. Adapted with permission from reference [19]. (C) Schematic of mineralized exendin-4 generation and its disassembly in response to physiological supersaturation. Adapted with permission from reference [83].
3.2 Depot surface engineering
Although direct modification has proven to be a simple and effective strategy, it results in some alterations to the original drugs, which may lead to an unpredictable change to its pharmacological activity [53]. Therefore, another popular strategy to develop long-acting systems is to encapsulate the original drug in a hydrogel or macro-sized particles, which can be injected or implanted subcutaneously to release the payloads due to gradual degradation (Table 1). These strategies mainly focus on some natural or synthetic polymers such as poly(lactide-co-glycolide) (PLGA), alginate, hyaluronic acid, chitosan, and so on [4, 8, 12, 79, 86–94]. Despite preliminary success for those materials, ultra-long drug release (months, years) is still unattainable (the FBR is mainly responsible for the failure). In order to regulate the material-bioenvironment regulation, artificial engineering on the surface of the hydrogels or macro-particles has emerged as one of the most effective approaches, which may significantly extend the longevity and functionality of subcutaneous depots.
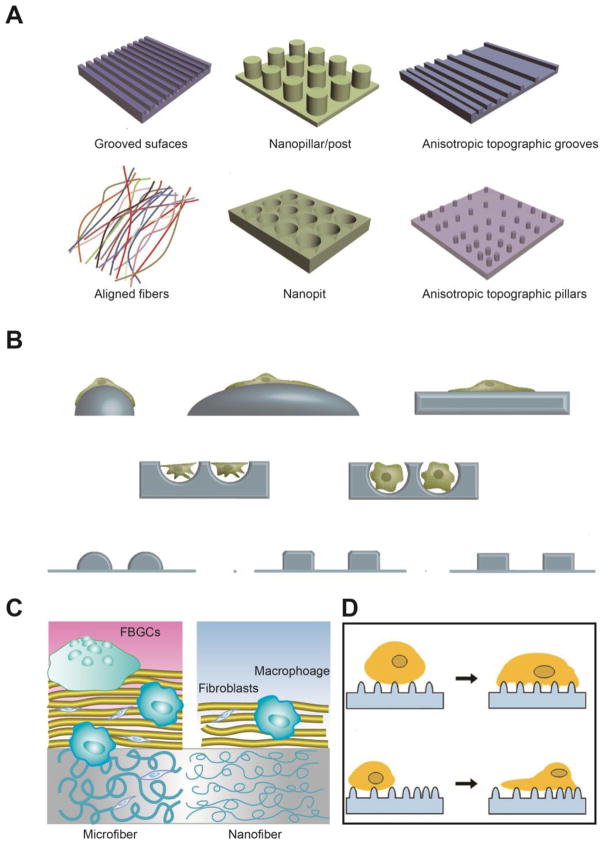
As for the surface modification, the most straightforward method is to coat the surface of the subcutaneous depots with biocompatible materials based on their masking ability [99–104]. The masking layer can provide a hydrophilic interface between the tissue fluids and depots, significantly reducing protein adsorption and cell attachment. For instance, mussel adhesive protein mimetic polymer coating has proven to be able to provide a non-fouling surface, which can resist cell attachment for at least 14 days [105]. A smart coating by using PLGA microspheres that are dispersed in poly(vinyl alcohol) (PVA) hydrogel can remarkably reduce FBR at the tissue-device interface to extend lifetime of the biosensor [99]. Besides, alternative coating by positive and negative polyelectrolyte multilayer thin film through a layer by layer process can significantly alleviate the host recognition to improve immunoisolation of implanted islets [106–108]. All these coatings are confirmed to reduce protein and cell infiltration, but offer a porous structure for nutrient and biomolecule diffusion, guaranteeing long-term material-bioenvironmental communication. Interestingly, this kind of immunoisolation effect is well controlled by the nanotopography of the coatings, which has shown a dramatic effect on the protein layer composition and cellular behaviors, owing to their influence on protein conformation and cell contact [95, 96, 109–112]. Typical topographic structures are listed in Fig. 4A, including grooves, nanopillars, fibers, nanopits and so on. When cells adhere to those structured surfaces, they change the shape according to the surface curvature, and highly curved surfaces may induce a larger extent of deformation of the cytoskeleton compared to the relatively flatter surface, leading to an acute response with high cytokine and chemokine secretion (Fig. 4B) [96]. Moreover, in the same topographical surface, the dimension of microstructures plays a significant role in cellular behaviors. For example, Luo et al. developed a fibrous membrane to combat FBR for long-acting devices [97]. It has been verified that the diameter of the fibers may remarkably influence the shielding effect of the coatings with the same thickness [113, 114]. Compared to microsized fibers, the nanosized fibers have greater potential in mitigating FBR (Fig. 4C). This phenomenon could be attributed to the structural homology of nanosized fibrous structures to the natural extracellular matrices (ECM). It has been confirmed that many of the pseudopodial processes of macrophages are oriented along the microfibers [97, 115]. The response of spreading is closely related to the macrophage activation, followed by the regulation of proinflammatory cytokines and chemokines secretion [113, 115]. Besides the dimension, the distribution of microstructures on a topographical surface also exhibits a significant effect on the cellular activities (Fig. 4D). The results indicate that cells tend to migrate to a dense topographical surface over a sparse area [98], in this way the orientation, extension and secretion of the cells are largely affected [96]. All these results suggest intricate surface engineering to alter nanotopography structure holds great promise to mediate the biological system attachment and response.
Fig. 4.
(A) Schematics of representative nanotopography geometries commonly used as cell culture substrates. Adapted with permission from reference [95]. (B) The effect of surface curvature on cell shape. Adapted with permission from reference [96]. (C) The influence of fiber size on FBR. Adapted with permission from reference [97]. (D) Schematic for guiding cell migration on isotropic and anisotropic topographies. Adapted with permission from reference [98].
In addition to nanotopography structures, the functional groups on the macroparticles or hydrogel surfaces are also highly associated with FBR inhibition [116]. It has been observed that the exposure of specific functional groups including carboxyl, hydroxyl, amine or methyl groups show a pronounced effect on protein adsorption, cell behavior, and tissue response [116]. Therefore, various biocompatible polymers with different functional groups have been applied to modify the surface of the biosensors or subcutaneous depots, significantly improving their in vivo performance (Table 2). Besides the broadly used polyelectrolytes and hydrogels, scientists have recently developed some new structures, which have shown large potential to serve as highly non-fouling surface groups in clinical applications. For instance, Anderson et al. screened 774 alginate analogs with different functional groups to evaluate their compatibility after subcutaneous implantation [117]. Interestingly, among all the analogs, they identified three triazole-containing candidates that substantially reduce FBR in both rodents and non-human primates for at least 6 months (Table 2) [117, 118]. The introduction of triazole-based functional groups creates a unique surface which can inhibit macrophage recognition and fibrous deposition [117]. Although the exact mechanism is still under investigation, the phenomenon clearly indicates that this kind of functional group may regulate immune cell population at the material surface through macrophage activation and fibrotic process disruption. Moreover, besides triazole groups, some other interesting functional surfaces have also been proven to significantly reduce FBR. For instance, Jiang et al. reported that zwitterionic hydrogels implanted in mice resist the FBR for at least three months (Table 2) [119–121]. The molecular mechanism of this non-fouling property could be ascribed to zwitterions with a minimized dipole and balanced charge that possess strong hydration capacity via electrostatic interaction, which highly resists protein adsorption [122]. The similar zwitterion-based strategy has also been applied by Loose et al. who modified a catheter with non-leaching polymer sulfobetaine, which contains equal amount of sulfonate (SO3−) and quaternary amine groups (NR4+) to coordinate with water molecules on the surface, significantly reducing protein, microbial, and mammalian cell attachment for a long period (at least 60 days) [123]. Obviously, simple surface functional group regulation can largely mediate the material-environment interaction. Despite the fact that not all the mechanisms are well elucidated, this kind of modification may significantly improve the performance of various subcutaneous injections or implants, such as tissue scaffolds, artificial organs, glucose sensors, and drug-releasing depots, where fibrotic reactions are undesirable.
Table 2.
Typical chemical structures that can reduce FBR
| Name/Description | Structure | Application |
|---|---|---|
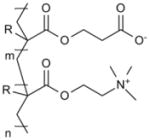
| Poly(styrene sulfonate) (PSS) |
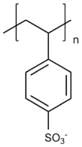
|
Islet implantation [108] |
| Poly(β-amino alcohols) (PBAA) |
|
Cell encapsulation [100] |
| Poly(L-lysine)-g-poly(ethylene glycol) (PLL-g-PEG) |
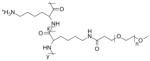
|
Islet implantation [106, 107] |
| Poly(diallyl dimethyl ammonium chloride) (PDADMAC) |
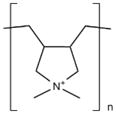
|
Islet implantation [108] |
| Poly(allylamine hydrochloride) (PAH) |
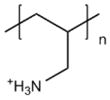
|
Islet implantation [108] |
| Poly(lactic-co-glycolic) acid (PLGA) |
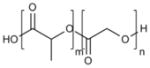
|
Biosensor implantation [99] |
| Poly(vinyl alcohol) (PVA) |
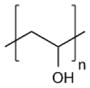
|
Biosensor implantation [99] Neural prostheses implantation [124] |
| Hyaluronic acid (HA) |
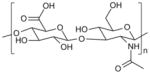
|
Cell encapsulation [125] |
| Alginate (ALG) |
|
Islet implantation [106, 107] |
| Poly(acrylic acid) (PAA) |
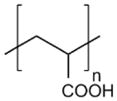
|
Neural prostheses implantation [124] |
| Poly(carboxybetaine) (pCB) |
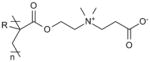
|
Biomedical device implantation [119, 120] |
| Poly(sulfobetaine) (pSB) |
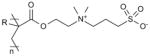
|
Catheter insertion [123] Biomedical device implantation [119, 120] |
| Phosphorylcholine (PC) |
|
Surface plasmon resonance sensor implantation [122] |
| Mixed charge polymer |
|
Biomedical device implantation [119] |
| Triazole-containing derivative |
|
Cell encapsulation [117, 118, 126] |
| Triazole-containing derivative |
|
Cell encapsulation [117, 118, 126] |
| Triazole-containing derivative |
|
Cell encapsulation [117, 118, 126] |
3.3 Size and shape effect
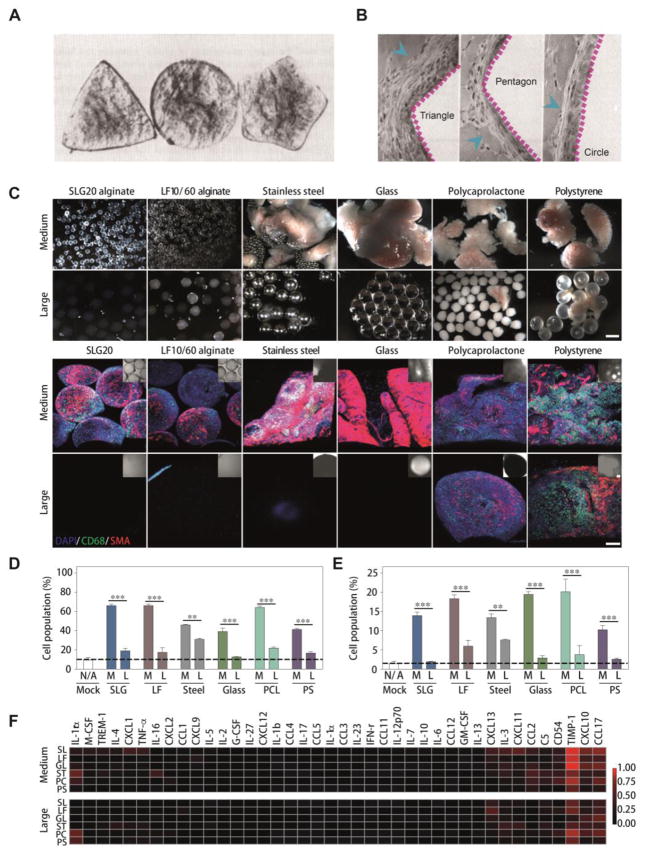
In long-term subcutaneous drug delivery, the injections or implants are built up to the micrometer, millimeter, or even centimeter scale, which represents a large foreign presence to biological systems [94, 127, 128]. In this way, alterations in size and shape always play a significant role in their interaction with surroundings, followed by the influence on FBR. As for the shape effect, it has been observed that triangle-shaped implants show a relatively higher immune response, while pentagon shapes present less, and circular structures induce the least reactions (Fig. 5A and B) [129]. This interesting phenomenon may be attributed to the sharper angles in the triangle or pentagon shapes, which may induce more acute injuries during their movements compared to circular structures [129]. Moreover, the shape difference has also been confirmed to profoundly affect cell behavior at the interface between the materials and tissue fluids.[130], with smooth, well-contoured structures (no sharp angles) demonstrating greater biocompatibility [130]. Obviously, we can get some clues from these observations that materials with smooth surfaces and no sharp edges are more bio-friendly to reduce FBR during long-term subcutaneous retention.
Fig. 5.
Size and shape effects of injected or implanted materials. (A) Three typical shapes of implants that are used to evaluate the FBR. Adapted with permission from reference [129] (B) Photomicrographs of H&E-stained sections after treatments by different shaped implants for 14 days. Blue arrows indicate the accumulated cells. Adapted with permission from reference [129]. (C) Bright-field images of different materials with distinct sizes after implantation for 14 days (upper panels). Immunofluorescence Z-stacked confocal images of retrieved materials. Blue (DAPI) indicates cell nuclei, green (CD68) indicates macrophages, and red (α-SMA) indicates fibrosis-associated activated myofibroblasts (lower panels). Scale bar, 300 μm. (D) Flow analysis using specific markers for the host macrophage. (E) Flow analysis using specific markers for the host neutrophils. (F) Cytokine array profiling of inflammatory cytokine protein production in response to implanted materials. Fig. 5C–F were adapted with permission from reference [126].
To avoid introducing sharp angles, sphere structures are frequently applied in various long-acting systems (e.g. alginate spheres, PLGA depots) [117, 118, 126]. Intriguingly, regardless of material type, the size difference leads to remarkably distinct FBR (Fig. 5C). Clearly, increased size resulted in significant decrease in the thickness of the FBR-based capsule.[126]. After implantation for 14 days, the macrophage (CD68) and myofibroblast (αSMA) deposition in retrieved materials showed a marked reduction in large materials (1.5 cm) compared to medium sized implants (0.5 cm) (Fig. 5C) [126]. Moreover, further evaluation of the innate immune response of different materials revealed that both host macrophages (Fig. 5D) and neutrophils (Fig. 5E) accumulated less in peripheral tissues upon larger sphere treatments, and a multiplexed inflammatory mouse cytokine profile indicated the same tendency (Fig. 5F). Considering all these materials showed a range of mechanical stiffness varying from soft (alginate) to hard (stainless steel), suggesting the size effect dominates biocompatibility over the stiffness. This phenomenon should be attributed to distinct macrophage activation in the different sized alginate depots [131, 132]. The expression of macrophage markers indicated that classical and wound-healing phenotypes are enriched in 1.5-mm spheres while regulatory macrophage markers were not significantly up-regulated, which were highly detected in 0.5-mm spheres [126]. These data suggest that a lack of regulatory macrophage cell accumulation on large-sized spheres correlates with truncated fibrosis formation. Although there are still some unclear details on geometry-based FBR regulation, researchers have observed that the size and shape of designed materials lead to a significant influence on their interaction with surroundings. As an easily controlled factor, geometric properties can be well regulated during fabrication processes, which may combine with surface chemistry and nanotopography to synergistically overcome FBR during long-term treatments.
4 Future perspectives
Currently, the most successful long-acting drug release formulations have extended the drug administration frequency up to 4 to 6 weeks in the clinic [22–24, 133]. However, this is not satisfactory as most of the diseases that need long-acting formulations last for tens of years or persist for the whole life of the patient. Therefore, ultra-long (months, years) formulations are under development, which may extensively alleviate patient sufferings and improve their quality of life. Based on this goal, there are still many directions worth studying in depth to improve current long-acting formulations.
4.1 Combinative strategies
In order to develop ultra-long subcutaneous drug delivery systems, simultaneously slowing drug release and reducing FBR are both necessary. The simplest way to maximize long-term effect lies in combining molecular level and macro-sized level strategies, which may achieve a synergistic effect by providing long subcutaneous retention and blood circulation (Fig. 6). For instance, after introducing an albumin-binding linker (tEB) on exendin-4, the tEB-modified exendin-4 drug can be further employed to construct inorganic-organic hybrid particles or be conjugated to polymer matrix as the linker is covalently bound to a specific site (Cys40) [69, 71], which is unrelated to the subsequent reactions.. In this way, the combinative products may serve as a biomimetic species to escape from host reaction and sustainably release the payloads upon gradual degradation. Moreover, the released payload will migrate slowly through the interstitial matrix upon albumin binding and enhance blood retention [3, 52]. It should be noted that in combinative strategies, many factors could be respectively adjusted with fewer confounding effects. Introducing biomolecule-binding linkers on drug molecules exerts a negligible influence on depot fabrication, while composition, geometry, and surface regulation on the depot has a minor effect on the biological activity of encapsulated drugs. In this way, a high degree of freedom to optimize related factors to maximize long-term drug release effects is permitted. Considering a single strategy has successfully extended the drug release to several weeks, we believe the combinative strategies will hold even greater promise to prolong the drug treatments to several months or even years by significantly and synergistically enhancing drug retention, reducing FBR, and improving blood circulation.
Fig. 6.
Combining molecular level and macro-sized level strategies to extend long-term subcutaneous drug treatments.
4.2 Smart release
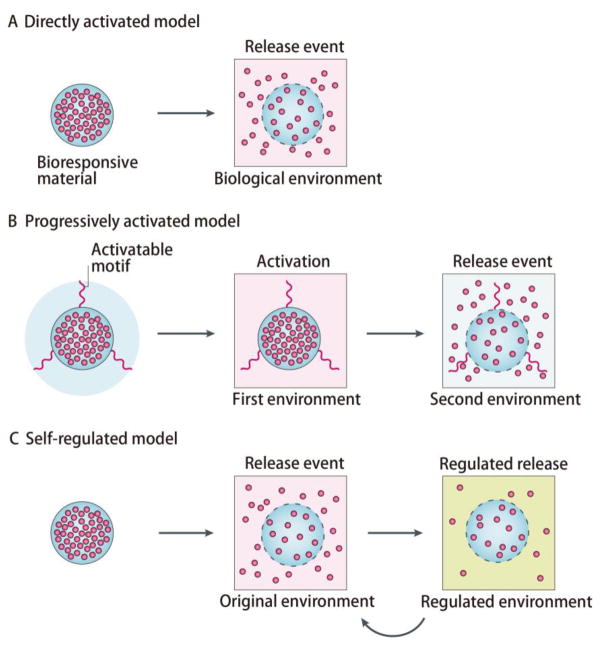
In most treatments, the effective concentrations of drugs in blood are often not high [134, 135], suggesting that a small amount of drug release at an indicated time point is adequate. Therefore, it is not necessary to release the drug during the whole treatment process. A self-regulated system, also known as a signal feedback-controlled system may specifically release the payloads in response to pathological stimuli, and self-regulate to slow down drug release in normal bioenvironments [27–30]. Compared to classic directly activated models and progressively activated models, the self-regulated model can provide a smart and on-demand drug treatment, maximizing the utilization efficiency of the drugs (Fig. 7) [27–30]. In this manner, it is supposed that self-regulated systems may exhibit remarkably prolonged drug release compared to others with the same loading amount. These kinds of formulations have recently been applied to treat various diseases such as diabetes [136–138] and blood coagulation [139, 140], extensively prolonging the drug duration time. Based on all the successes, we believe that those smart systems will open new windows to create more ultra-long drug release formulations by providing an accurate, on-demand drug treatment.
Fig. 7.
Bio-responsive modalities for controlled drug release (Adapted with permission from reference [27]). (A) Directly activated model. (B) Progressively activated model. (C) Self-regulated model.
4.3 Living-species involvement
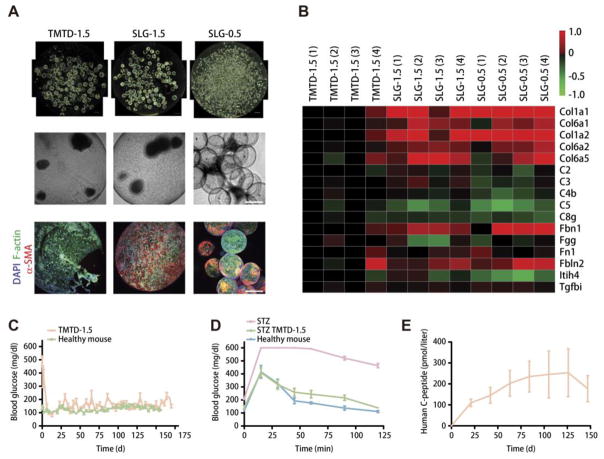
It should be noted that there is always an upper limit to the release time of traditional drug loading strategies, as the loaded drugs will be completely depleted. In order to achieve “permanent” drug treatments, biological species are always involved, which may provide an ultra-long metabolism-based drug exocytosis without the necessity of drug loading or refilling [106–108]. For instance, Anderson et al. encapsulated glucose-responsive mature β cells (derived from human embryonic stem cells, referred to as SC-β cells) in triazole–thiomorpholine dioxide (TMTD)-modified 1.5 mm alginate spheres (TMTD-1.5) to treat diabetes (Fig. 8) [118]. Similar to the previous study [117, 126], larger and triazole-modified particles (TMTD-1.5) indicated a lower FBR according to dark-field, bright-field, and z-stacked confocal immunofluorescence images from implants compared to smaller (SLG-0.5) and unmodified (SLG-1.5) species (Fig. 8A). Proteomic analysis of lysates from implants showed similar results (Fig. 8B). Moreover, in vivo tests revealed that SC-β cells encapsulated within TMTD-1.5 lowered the BGL of STZ-treated C57BL/6 mice for half a year (Fig. 8C), which meant that patients only need two injections within one year. Intravenous glucose tolerance test (IVGTT) showed that after 174-day implantation, the acute BGL elevation could still be controlled (Fig. 8D). Human C-peptide, a surrogate endpoint for insulin production, was detected during the course of study (Fig. 8E), which reflected that the transplanted SC-β cells remained alive and functional, suggesting that the cells escaped from attack by host immune systems. In addition, they also tested the systems on non-human primates and detected negligible FBR for 6 months [118]. This ultra-long drug release may be attributed to the non-fouling property of well-designed depots and unlimited insulin secretion of SC-β cells. This success inspires that the combination of biological species and smart materials may act as a highly promising approach to develop ultra-long formulations, which are expected to result in a revolution in drug administration strategies in the near future.
Fig. 8.
Long-term response of cell encapsulated alginate depots (Adapted with permission from reference [118]). (A) Representative dark-field, bright-field, and z-stacked confocal immunofluorescence images of SC-β cell implants. (B) Proteomic analysis of lysates from SC-β cell cluster implants retrieved from the STZ-treated C57BL/6J mice after 90 d implantation. (C) BGL in healthy mice or diabetic mice implanted with SC-β cell-encapsulated TMTD-1.5 spheres. (D) BGL of the STZ-treated C57BL/6 mice with or without TMTD alginate implantation that were subjected to an IVGTT after 174 d. (E) Blood human C-peptide levels of diabetic mice implanted with TMTD-1.5 spheres.
4.4 Electronic/mechanical device application
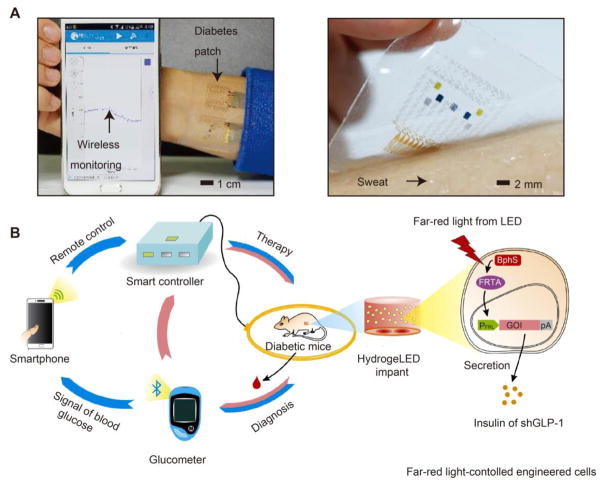
To achieve long-term drug release, monitoring the pathological signal or drug concentration always plays a significant role as it can help adjust drug dose and release kinetics, even in some cases pause drug release as needed. To realize accurate monitoring and control of drug release, introducing electronic/mechanical devices is a good choice as it can transform the pathological signal or drug concentration to readable digital data, and provide an artificially controllable profile. For example, Kim et al. constructed an efficient electrochemical interface based on the gold-doped graphene for stable transfer of electrical signals [141]. They fabricated a smart patch that consists of temperature, humidity, glucose and pH sensors to monitor the physiological signals and transferred them to a smartphone, providing a real-time monitoring (Fig. 9A). Besides, Ye et al. designed a smartphone-assisted semiautomatic system to treat diabetes [142]. They implanted a hydrogel capsule carrying both optically engineered cells and wirelessly powered far-red light LEDs, and used a glucometer to detect the BGL and a smartphone to artificially turn on insulin or short variant of human GLP-1 (shGLP-1) release (Fig. 9B). A Smartcontroller can also be used to provide a feedback controlled drug release. In such a way, highly accurate signal monitoring and semiautomatic drug treatment can be achieved. It is believed that the combination of those smart electronic/mechanical devices with highly non-fouling materials will be able to realize well-controlled long-term subcutaneous drug release for various clinical applications.
Fig. 9.
Electronic/mechanical device-based long-acting systems. (A) Wearable diabetes monitoring and therapy system based on a smartphone and a microneedle-array patch. Adapted with permission from reference [141]. (B) Abstract diagram showing smartphone-controlled engineered cells enabling semiautomatic point of care for combating diabetes. Adapted with permission from reference [142].
5 Conclusions
Whether in the clinical practice or the commercial market, subcutaneous drug delivery is considered one of the most popular routes of administration due to its low-cost, self-administration, safety, and high efficiency. To maximize the effect of subcutaneous drug delivery and increase patient compliance, long-acting formulations have been developed. During long-term drug treatment, FBR frequently limit the drug retention and lead to health risks. Therefore, various smart designs have been employed to combat FBR through reduction of host recognition and aggression responses. Some well-developed immunoisolation strategies including direct drug modification, depot surface engineering, size and shape regulation, and so on are discussed in depth in this review. To further improve longevity and functionality of long-acting formulations, we believe that combining molecular level and macro-sized level strategies, introducing smart self-regulated release systems, applying cell-based secretion strategy and introducing electronic/mechanical devices are promising approaches. Based on all these discussions, long-acting subcutaneous drug delivery has been confirmed to be one of the most effective strategies to improve patient lives in numerous diseases, and with the advance of material and life sciences, more strategies that can further extend drug release time and reduce dosing frequency are expected to be achieved. In this regard, we believe more effective ultra-long formulations will be realized upon smart material-bioenvironment interaction regulation in future.
Acknowledgments
This work was supported in part, by the China Postdoctoral Science Foundation (2015M570745 and 2016T90815), Natural Science Foundation of Guangdong Province (2016A030310229), National Natural Science Foundation of China (81602634, 31525009, and 31771096), and the Intramural Research Program, National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health (ZIA EB000073).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mullard A. 2014 FDA drug approvals. Nat Rev Drug Discov. 2015;14:77–81. doi: 10.1038/nrd4545. [DOI] [PubMed] [Google Scholar]
- 2.Jin J, Zhu L, Chen M, Xu H, Wang H, Feng X, Zhu X, Zhou Q. The optimal choice of medication administration route regarding intravenous, intramuscular, and subcutaneous injection. Patient Prefer Adherence. 2015;9:923–942. doi: 10.2147/PPA.S87271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones GB, Collins DS, Harrison MW, Thyagarajapuram NR, Wright JM. Subcutaneous drug delivery: An evolving enterprise. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aaf9166. [DOI] [PubMed] [Google Scholar]
- 4.Vermonden T, Censi R, Hennink WE. Hydrogels for protein delivery. Chem Rev. 2012;112:2853–2888. doi: 10.1021/cr200157d. [DOI] [PubMed] [Google Scholar]
- 5.Frokjaer S, Otzen DE. Protein drug stability: a formulation challenge. Nat Rev Drug Discov. 2005;4:298–306. doi: 10.1038/nrd1695. [DOI] [PubMed] [Google Scholar]
- 6.Tetteh EK, Morris S. Evaluating the administration costs of biologic drugs: development of a cost algorithm. Health Econ Rev. 2014;4:26. doi: 10.1186/s13561-014-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Illum L. Nasal drug delivery--possibilities, problems and solutions. J Control Release. 2003;87:187–198. doi: 10.1016/s0168-3659(02)00363-2. [DOI] [PubMed] [Google Scholar]
- 8.Ansary RH, Awang MB, Rahman MM. Biodegradable poly (D, L-lactic-co-glycolic acid)-based micro/nanoparticles for sustained release of protein drugs-A review. Trop J Pharm Res. 2014;13:1179–1190. [Google Scholar]
- 9.Uhrich KE, Cannizzaro SM, Langer RS, Shakesheff KM. Polymeric systems for controlled drug release. Chem Rev. 1999;99:3181–3198. doi: 10.1021/cr940351u. [DOI] [PubMed] [Google Scholar]
- 10.Freiberg S, Zhu X. Polymer microspheres for controlled drug release. Int J Pharm. 2004;282:1–18. doi: 10.1016/j.ijpharm.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Washington C. Drug release from microdisperse systems: a critical review. Int J Pharm. 1990;58:1–12. [Google Scholar]
- 12.Shive MS, Anderson JM. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv Rev. 1997;28:5–24. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 13.Drucker DJ, Buse JB, Taylor K, Kendall DM, Trautmann M, Zhuang D, Porter L DURATION-1 Study Group. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: A randomised, open-label, non-inferiority study. Lancet. 2008;372:1240–1250. doi: 10.1016/S0140-6736(08)61206-4. [DOI] [PubMed] [Google Scholar]
- 14.Diamant M, Van Gaal L, Guerci B, Stranks S, Han J, Malloy J, Boardman MK, Trautmann ME. Exenatide once weekly versus insulin glargine for type 2 diabetes (DURATION-3): 3-year results of an open-label randomised trial. Lancet Diabetes Endocrinol. 2014;2:464–473. doi: 10.1016/S2213-8587(14)70029-4. [DOI] [PubMed] [Google Scholar]
- 15.Bergenstal RM, Wysham C, MacConell L, Malloy J, Walsh B, Yan P, Wilhelm K, Malone J, Porter LE DURATION-2 Study Group. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet. 2010;376:431–439. doi: 10.1016/S0140-6736(10)60590-9. [DOI] [PubMed] [Google Scholar]
- 16.Diamant M, Van Gaal L, Stranks S, Northrup J, Cao D, Taylor K, Trautmann M. Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomised trial. Lancet. 2010;375:2234–2243. doi: 10.1016/S0140-6736(10)60406-0. [DOI] [PubMed] [Google Scholar]
- 17.Luginbuhl KM, Schaal JL, Umstead B, Mastria EM, Li X, Banskota S, Arnold S, Feinglos M, D’Alessio D, Chilkoti A. One-week glucose control via zero-order release kinetics from an injectable depot of glucagon-like peptide-1 fused to a thermosensitive biopolymer. Nat Biomed Eng. 2017;1:0078. doi: 10.1038/s41551-017-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qi Y, Simakova A, Ganson NJ, Li X, Luginbuhl KM, Ozer I, Liu W, Hershfield MS, Matyjaszewski K, Chilkoti A. A brush-polymer/exendin-4 conjugate reduces blood glucose levels for up to five days and eliminates poly (ethylene glycol) antigenicity. Nat Biomed Eng. 2016;1:0002. doi: 10.1038/s41551-016-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amiram M, Luginbuhl KM, Li X, Feinglos MN, Chilkoti A. A depot-forming glucagon-like peptide-1 fusion protein reduces blood glucose for five days with a single injection. J Control Release. 2013;172:144–151. doi: 10.1016/j.jconrel.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong J-H, Oh EJ, Chae SY, Lee KC, Hahn SK. Long acting hyaluronate–exendin 4 conjugate for the treatment of type 2 diabetes. Biomaterials. 2010;31:4121–4128. doi: 10.1016/j.biomaterials.2010.01.091. [DOI] [PubMed] [Google Scholar]
- 21.Kim JY, Lee H, Oh KS, Kweon S, Jeon O-c, Byun Y, Kim K, Kwon IC, Kim SY, Yuk SH. Multilayer nanoparticles for sustained delivery of exenatide to treat type 2 diabetes mellitus. Biomaterials. 2013;34:8444–8449. doi: 10.1016/j.biomaterials.2013.07.040. [DOI] [PubMed] [Google Scholar]
- 22.Stewart PM, Kane KF, Stewart SE, Lancranjan I, Sheppard MC. Depot long-acting somatostatin analog (Sandostatin-LAR) is an effective treatment for acromegaly. J Clin Endocr Metab. 1995;80:3267–3272. doi: 10.1210/jcem.80.11.7593436. [DOI] [PubMed] [Google Scholar]
- 23.Melmed S. New therapeutic agents for acromegaly. Nat Rev Endocrinol. 2016;12:90–98. doi: 10.1038/nrendo.2015.196. [DOI] [PubMed] [Google Scholar]
- 24.Caron P, Beckers A, Cullen D, Goth M, Gutt B, Laurberg P, Pico A, Valimaki M, Zgliczynski W. Efficacy of the new long-acting formulation of lanreotide (lanreotide Autogel) in the management of acromegaly. J Clin Endocr Metab. 2002;87:99–104. doi: 10.1210/jcem.87.1.8153. [DOI] [PubMed] [Google Scholar]
- 25.Shen J, Choi S, Qu W, Wang Y, Burgess DJ. In vitro-in vivo correlation of parenteral risperidone polymeric microspheres. J Control Release. 2015;218:2–12. doi: 10.1016/j.jconrel.2015.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kane JM, Eerdekens M, Lindenmayer J-P, Keith SJ, Lesem M, Karcher K. Long-acting injectable risperidone: efficacy and safety of the first long-acting atypical antipsychotic. Am J Psychiatry. 2003;160:1125–1132. doi: 10.1176/appi.ajp.160.6.1125. [DOI] [PubMed] [Google Scholar]
- 27.Lu Y, Aimetti AA, Langer R, Gu Z. Bioresponsive materials. Nat Rev Mater. 2016;2:16075. [Google Scholar]
- 28.Ulijn RV, Bibi N, Jayawarna V, Thornton PD, Todd SJ, Mart RJ, Smith AM, Gough JE. Bioresponsive hydrogels. Mater Today. 2007;10:40–48. [Google Scholar]
- 29.Kim J, Nayak S, Lyon LA. Bioresponsive hydrogel microlenses. J Am Chem Soc. 2005;127:9588–9592. doi: 10.1021/ja0519076. [DOI] [PubMed] [Google Scholar]
- 30.Purcell BP, Lobb D, Charati MB, Dorsey SM, Wade RJ, Zellars KN, Doviak H, Pettaway S, Logdon CB, Shuman JA, Freels PD, Gorman JH, 3rd, Gorman RC, Spinale FG, Burdick JA. Injectable and bioresponsive hydrogels for on-demand matrix metalloproteinase inhibition. Nat Mater. 2014;13:653–661. doi: 10.1038/nmat3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu J, Zhang Y, Kahkoska AR, Gu Z. Bioresponsive transcutaneous patches. Curr Opin Biotechnol. 2017;48:28–32. doi: 10.1016/j.copbio.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu J, Zhang Y, Ye Y, DiSanto R, Sun W, Ranson D, Ligler FS, Buse JB, Gu Z. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc Natl Acad Sci USA. 2015;112:8260–8265. doi: 10.1073/pnas.1505405112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Onuki Y, Bhardwaj U, Papadimitrakopoulos F, Burgess DJ. A review of the biocompatibility of implantable devices: current challenges to overcome foreign body response. J Diabetes Sci Technol. 2008;2:1003–1015. doi: 10.1177/193229680800200610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ward WK. A review of the foreign-body response to subcutaneously-implanted devices: the role of macrophages and cytokines in biofouling and fibrosis. J Diabetes Sci Technol. 2008;2:768–777. doi: 10.1177/193229680800200504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franz S, Rammelt S, Scharnweber D, Simon JC. Immune responses to implants–a review of the implications for the design of immunomodulatory biomaterials. Biomaterials. 2011;32:6692–6709. doi: 10.1016/j.biomaterials.2011.05.078. [DOI] [PubMed] [Google Scholar]
- 36.Williams DF. On the mechanisms of biocompatibility. Biomaterials. 2008;29:2941–2953. doi: 10.1016/j.biomaterials.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 37.Malik AF, Hoque R, Ouyang X, Ghani A, Hong E, Khan K, Moore LB, Ng G, Munro F, Flavell RA, Shi Y, Kyriakides TR, Mehal WZ. Inflammasome components Asc and caspase-1 mediate biomaterial-induced inflammation and foreign body response. Proc Natl Acad Sci USA. 2011;108:20095–20100. doi: 10.1073/pnas.1105152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanchez C, Arribart H, Guille MMG. Biomimetism and bioinspiration as tools for the design of innovative materials and systems. Nat Mater. 2005;4:277–288. doi: 10.1038/nmat1339. [DOI] [PubMed] [Google Scholar]
- 39.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang L, Eaton JW. Natural responses to unnatural materials: A molecular mechanism for foreign body reactions. Mol Med. 1999;5:351. [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson JM. Biological responses to materials. Annu Rev Mater Res. 2001;31:81–110. [Google Scholar]
- 42.Dondossola E, Holzapfel BM, Alexander S, Filippini S, Hutmacher DW, Friedl P. Examination of the foreign body response to biomaterials by nonlinear intravital microscopy. Nat Biomed Eng. 2016;1:0007. doi: 10.1038/s41551-016-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grainger DW. All charged up about implanted biomaterials. Nat Biotechnol. 2013;31:507–509. doi: 10.1038/nbt.2600. [DOI] [PubMed] [Google Scholar]
- 44.Hu W-J, Eaton JW, Ugarova TP, Tang L. Molecular basis of biomaterial-mediated foreign body reactions. Blood. 2001;98:1231–1238. doi: 10.1182/blood.v98.4.1231. [DOI] [PubMed] [Google Scholar]
- 45.Swartzlander MD, Barnes CA, Blakney AK, Kaar JL, Kyriakides TR, Bryant SJ. Linking the foreign body response and protein adsorption to PEG-based hydrogels using proteomics. Biomaterials. 2015;41:26–36. doi: 10.1016/j.biomaterials.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luttikhuizen DT, Harmsen MC, Luyn MJV. Cellular and molecular dynamics in the foreign body reaction. Tissue Eng. 2006;12:1955–1970. doi: 10.1089/ten.2006.12.1955. [DOI] [PubMed] [Google Scholar]
- 47.Sheikh Z, Brooks PJ, Barzilay O, Fine N, Glogauer M. Macrophages, foreign body giant cells and their response to implantable biomaterials. Materials. 2015;8:5671–5701. doi: 10.3390/ma8095269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ratner BD. Reducing capsular thickness and enhancing angiogenesis around implant drug release systems. J Control Release. 2002;78:211–218. doi: 10.1016/s0168-3659(01)00502-8. [DOI] [PubMed] [Google Scholar]
- 49.Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428:487–492. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 50.Wegst UG, Bai H, Saiz E, Tomsia AP, Ritchie RO. Bioinspired structural materials. Nat Mater. 2015;14:23–36. doi: 10.1038/nmat4089. [DOI] [PubMed] [Google Scholar]
- 51.Morais JM, Papadimitrakopoulos F, Burgess DJ. Biomaterials/tissue interactions: possible solutions to overcome foreign body response. AAPS J. 2010;12:188–196. doi: 10.1208/s12248-010-9175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Römgens AM, Bader DL, Bouwstra JA, Baaijens FP, Oomens CW. Diffusion profile of macromolecules within and between human skin layers for (trans) dermal drug delivery. J Mech Behav Biomed Mater. 2015;50:215–222. doi: 10.1016/j.jmbbm.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 53.van Witteloostuijn SB, Pedersen SL, Jensen KJ. Half-life extension of biopharmaceuticals using chemical methods: Alternatives to pegylation. ChemMedChem. 2016;11:2474–2495. doi: 10.1002/cmdc.201600374. [DOI] [PubMed] [Google Scholar]
- 54.Kontermann RE. Half-life extended biotherapeutics. Expert Opin Biol Ther. 2016;16:903–915. doi: 10.1517/14712598.2016.1165661. [DOI] [PubMed] [Google Scholar]
- 55.Poulsen HL. Subcutaneous interstitial fluid albumin concentration in long-term diabetes mellitus. Scand J Clin Lab Invest. 1973;32:167–173. doi: 10.3109/00365517309084345. [DOI] [PubMed] [Google Scholar]
- 56.Poulsen HL. Interstitial fluid concentrations of albumin and immunoglobulin G in normal men. Scand J Clin Lab Invest. 1974;34:119–122. [PubMed] [Google Scholar]
- 57.Liu Z, Chen X. Simple bioconjugate chemistry serves great clinical advances: albumin as a versatile platform for diagnosis and precision therapy. Chem Soc Rev. 2016;45:1432–1456. doi: 10.1039/c5cs00158g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koch-Weser J, Sellers EM. Binding of drugs to serum albumin. N Engl J Med. 1976;294:311–316. doi: 10.1056/NEJM197602052940605. [DOI] [PubMed] [Google Scholar]
- 59.Pan CQ, Buxton JM, Yung SL, Tom I, Yang L, Chen H, MacDougall M, Bell A, Claus TH, Clairmont KB, Whelan JP. Design of a long acting peptide functioning as both a glucagon-like peptide-1 receptor agonist and a glucagon receptor antagonist. J Biol Chem. 2006;281:12506–12515. doi: 10.1074/jbc.M600127200. [DOI] [PubMed] [Google Scholar]
- 60.Kim TH, Jiang HH, Lee S, Youn YS, Park CW, Byun Y, Chen X, Lee KC. Mono-PEGylated dimeric exendin-4 as high receptor binding and long-acting conjugates for type 2 anti-diabetes therapeutics. Bioconjug Chem. 2011;22:625–632. doi: 10.1021/bc100404x. [DOI] [PubMed] [Google Scholar]
- 61.Kim TH, Jiang HH, Lim SM, Youn YS, Choi KY, Lee S, Chen X, Byun Y, Lee KC. Site-specific PEGylated Exendin-4 modified with a high molecular weight trimeric PEG reduces steric hindrance and increases type 2 antidiabetic therapeutic effects. Bioconjug Chem. 2012;23:2214–2220. doi: 10.1021/bc300265n. [DOI] [PubMed] [Google Scholar]
- 62.Sun Z, Tong G, Kim TH, Ma N, Niu G, Cao F, Chen X. PEGylated exendin-4, a modified GLP-1 analog exhibits more potent cardioprotection than its unmodified parent molecule on a dose to dose basis in a murine model of myocardial infarction. Theranostics. 2015;5:240–250. doi: 10.7150/thno.10226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fanali G, di Masi A, Trezza V, Marino M, Fasano M, Ascenzi P. Human serum albumin: from bench to bedside. Mol Asp Med. 2012;33:209–290. doi: 10.1016/j.mam.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 64.Chou DH, Webber MJ, Tang BC, Lin AB, Thapa LS, Deng D, Truong JV, Cortinas AB, Langer R, Anderson DG. Glucose-responsive insulin activity by covalent modification with aliphatic phenylboronic acid conjugates. Proc Natl Acad Sci USA. 2015;112:2401–2406. doi: 10.1073/pnas.1424684112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zaykov AN, Mayer JP, DiMarchi RD. Pursuit of a perfect insulin. Nat Rev Drug Discov. 2016;15:425–439. doi: 10.1038/nrd.2015.36. [DOI] [PubMed] [Google Scholar]
- 66.Hirsch IB. Insulin analogues. N Engl J Med. 2005;352:174–183. doi: 10.1056/NEJMra040832. [DOI] [PubMed] [Google Scholar]
- 67.Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, Huang B, Van Egeren DS, Park C, Irvine DJ. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 2014;507:519–522. doi: 10.1038/nature12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zorzi A, Middendorp SJ, Wilbs J, Deyle K, Heinis C. Acylated heptapeptide binds albumin with high affinity and application as tag furnishes long-acting peptides. Nat Commun. 2017;8:16092. doi: 10.1038/ncomms16092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu Y, Wang G, Zhang H, Ma Y, Lang L, Jacobson O, Kiesewetter DO, Zhu L, Gao S, Ma Q, Chen X. Stable evans blue derived exendin-4 peptide for type 2 diabetes treatment. Bioconjug Chem. 2015;27:54–58. doi: 10.1021/acs.bioconjchem.5b00625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jacobson O, Kiesewetter DO, Chen X. Albumin-binding evans blue derivatives for diagnostic imaging and production of long-acting therapeutics. Bioconjug Chem. 2016;27:2239–2247. doi: 10.1021/acs.bioconjchem.6b00487. [DOI] [PubMed] [Google Scholar]
- 71.Chen H, Wang G, Lang L, Jacobson O, Kiesewetter DO, Liu Y, Ma Y, Zhang X, Wu H, Zhu L, Niu G, Chen X. Chemical conjugation of evans blue derivative: a strategy to develop long-acting therapeutics through albumin binding. Theranostics. 2016;6:243–253. doi: 10.7150/thno.14322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang F, Zhu G, Jacobson O, Liu Y, Chen K, Yu G, Ni Q, Fan J, Yang Z, Xu F. Transformative nanomedicine of an amphiphilic camptothecin prodrug for long circulation and high tumor uptake in cancer therapy. ACS Nano. 2017;11:8838–8848. doi: 10.1021/acsnano.7b03003. [DOI] [PubMed] [Google Scholar]
- 73.Wang Y, Lang L, Huang P, Wang Z, Jacobson O, Kiesewetter DO, Ali IU, Teng G, Niu G, Chen X. In vivo albumin labeling and lymphatic imaging. Proc Natl Acad Sci USA. 2015;112:208–213. doi: 10.1073/pnas.1414821112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jonassen I, Havelund S, Ribel U, Plum A, Loftager M, Hoeg-Jensen T, Volund A, Markussen J. Biochemical and physiological properties of a novel series of long-acting insulin analogs obtained by acylation with cholic acid derivatives. Pharm Res. 2006;23:49–55. doi: 10.1007/s11095-005-9047-1. [DOI] [PubMed] [Google Scholar]
- 75.Zobel K, Koehler MF, Beresini MH, Caris LD, Combs D. Phosphate ester serum albumin affinity tags greatly improve peptide half-life in vivo. Bioorg Med Chem Lett. 2003;13:1513–1515. doi: 10.1016/s0960-894x(03)00209-9. [DOI] [PubMed] [Google Scholar]
- 76.Trussel S, Dumelin C, Frey K, Villa A, Buller F, Neri D. New strategy for the extension of the serum half-life of antibody fragments. Bioconjug Chem. 2009;20:2286–2292. doi: 10.1021/bc9002772. [DOI] [PubMed] [Google Scholar]
- 77.Penchala SC, Miller MR, Pal A, Dong J, Madadi NR, Xie J, Joo H, Tsai J, Batoon P, Samoshin V, Franz A, Cox T, Miles J, Chan WK, Park MS, Alhamadsheh MM. A biomimetic approach for enhancing the in vivo half-life of peptides. Nat Chem Biol. 2015;11:793–798. doi: 10.1038/nchembio.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yoo HS, Oh JE, Lee KH, Park TG. Biodegradable nanoparticles containing doxorubicin-PLGA conjugate for sustained release. Pharm Res. 1999;16:1114–1118. doi: 10.1023/a:1018908421434. [DOI] [PubMed] [Google Scholar]
- 79.Gilroy CA, Luginbuhl KM, Chilkoti A. Controlled release of biologics for the treatment of type 2 diabetes. J Control Release. 2016;240:151–164. doi: 10.1016/j.jconrel.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hagrman PJ, Hagrman D, Zubieta J. Organic–inorganic hybrid materials: From “simple” coordination polymers to organodiamine-templated molybdenum oxides. Angew Chem Int Ed. 1999;38:2638–2684. doi: 10.1002/(sici)1521-3773(19990917)38:18<2638::aid-anie2638>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 81.Wernike E, Hofstetter W, Liu Y, Wu G, Sebald HJ, Wismeijer D, Hunziker EB, Siebenrock KA, Klenke FM. Long-term cell-mediated protein release from calcium phosphate ceramics. J Biomed Mater Res A. 2010;92:463–474. doi: 10.1002/jbm.a.32411. [DOI] [PubMed] [Google Scholar]
- 82.Mann S. Molecular tectonics in biomineralization and biomimetic materials chemistry. Nature. 1993;365:499–505. [Google Scholar]
- 83.Chen W, Wang G, Yung BC, Liu G, Qian Z, Chen X. Long-acting release formulation of exendin-4 based on biomimetic mineralization for type 2 diabetes therapy. ACS Nano. 2017;11:5062–5069. doi: 10.1021/acsnano.7b01809. [DOI] [PubMed] [Google Scholar]
- 84.Chen W, Tian R, Xu C, Yung BC, Wang G, Liu Y, Ni Q, Zhang F, Zhou Z, Wang J, Niu G, Ma Y, Fu L, Chen X. Microneedle-array patches loaded with dual mineralized protein/peptide particles for type 2 diabetes therapy. Nat Commun. 2017;8:1777. doi: 10.1038/s41467-017-01764-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Boal AK, Ilhan F, DeRouchey JE, Thurn-Albrecht T, Russell TP, Rotello VM. Self-assembly of nanoparticles into structured spherical and network aggregates. Nature. 2000;404:746–748. doi: 10.1038/35008037. [DOI] [PubMed] [Google Scholar]
- 86.Zhang WF, Chen XG, Li PW, He QZ, Zhou HY. Chitosan and chitosan/β-cyclodextrin microspheres as sustained-release drug carriers. J Appl Polym Sci. 2007;103:1183–1190. [Google Scholar]
- 87.Yu L, Ding J. Injectable hydrogels as unique biomedical materials. Chem Soc Rev. 2008;37:1473–1481. doi: 10.1039/b713009k. [DOI] [PubMed] [Google Scholar]
- 88.Martinez M, Rathbone M, Burgess D, Huynh M. In vitro and in vivo considerations associated with parenteral sustained release products: a review based upon information presented and points expressed at the 2007 Controlled Release Society Annual Meeting. J Control Release. 2008;129:79–87. doi: 10.1016/j.jconrel.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 89.Oh EJ, Park K, Kim KS, Kim J, Yang JA, Kong JH, Lee MY, Hoffman AS, Hahn SK. Target specific and long-acting delivery of protein, peptide, and nucleotide therapeutics using hyaluronic acid derivatives. J Control Release. 2010;141:2–12. doi: 10.1016/j.jconrel.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 90.Stockwell A, Davis S, Walker S. In vitro evaluation of alginate gel systems as sustained release drug delivery systems. J Control Release. 1986;3:167–175. [Google Scholar]
- 91.Bhattarai N, Ramay HR, Gunn J, Matsen FA, Zhang M. PEG-grafted chitosan as an injectable thermosensitive hydrogel for sustained protein release. J Control Release. 2005;103:609–624. doi: 10.1016/j.jconrel.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 92.Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Adv Drug Deliv Rev. 2001;53:321–339. doi: 10.1016/s0169-409x(01)00203-4. [DOI] [PubMed] [Google Scholar]
- 93.Bhattarai N, Gunn J, Zhang M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv Drug Deliv Rev. 2010;62:83–99. doi: 10.1016/j.addr.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 94.Agnihotri SA, Mallikarjuna NN, Aminabhavi TM. Recent advances on chitosan-based micro-and nanoparticles in drug delivery. J Control Release. 2004;100:5–28. doi: 10.1016/j.jconrel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 95.Kim D-H, Provenzano PP, Smith CL, Levchenko A. Matrix nanotopography as a regulator of cell function. J Cell Biol. 2012;197:351–360. doi: 10.1083/jcb.201108062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Harvey AG, Hill EW, Bayat A. Designing implant surface topography for improved biocompatibility. Expert Rev Med Devices. 2013;10:257–267. doi: 10.1586/erd.12.82. [DOI] [PubMed] [Google Scholar]
- 97.Wang K, Hou WD, Wang X, Han C, Vuletic I, Su N, Zhang WX, Ren QS, Chen L, Luo Y. Overcoming foreign-body reaction through nanotopography: biocompatibility and immunoisolation properties of a nanofibrous membrane. Biomaterials. 2016;102:249–258. doi: 10.1016/j.biomaterials.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 98.Kim SM, Lee SH, Suh KY. Cell research with physically modified microfluidic channels: a review. Lab Chip. 2008;8:1015–1023. doi: 10.1039/b800835c. [DOI] [PubMed] [Google Scholar]
- 99.Wang Y, Papadimitrakopoulos F, Burgess DJ. Polymeric “smart” coatings to prevent foreign body response to implantable biosensors. J Control Release. 2013;169:341–347. doi: 10.1016/j.jconrel.2012.12.028. [DOI] [PubMed] [Google Scholar]
- 100.Ma M, Liu WF, Hill PS, Bratlie KM, Siegwart DJ, Chin J, Park M, Guerreiro J, Anderson DG. Development of cationic polymer coatings to regulate foreign-body responses. Adv Mater. 2011;23:H189–H194. doi: 10.1002/adma.201100513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu L, Chen G, Chao T, Ratner BD, Sage EH, Jiang S. Reduced foreign body reaction to implanted biomaterials by surface treatment with oriented osteopontin. J Biomater Sci Polym Ed. 2008;19:821–835. doi: 10.1163/156856208784522083. [DOI] [PubMed] [Google Scholar]
- 102.Ikada Y. Surface modification of polymers for medical applications. Biomaterials. 1994;15:725–736. doi: 10.1016/0142-9612(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 103.Bridges AW, García AJ. Anti-inflammatory polymeric coatings for implantable biomaterials and devices. J Diabetes Sci Technol. 2008;2:984–994. doi: 10.1177/193229680800200628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim YK, Que R, Wang SW, Liu WF. Modification of biomaterials with a self-protein inhibits the macrophage response. Adv Healthc Mater. 2014;3:989–994. doi: 10.1002/adhm.201300532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dalsin JL, Hu B, Lee BP, Messersmith PB. Mussel adhesive protein mimetic polymers for the preparation of nonfouling surfaces. J Am Chem Soc. 2003;125:4253–4258. doi: 10.1021/ja0284963. [DOI] [PubMed] [Google Scholar]
- 106.Wilson JT, Cui W, Kozlovskaya V, Kharlampieva E, Pan D, Qu Z, Krishnamurthy VR, Mets J, Kumar V, Wen J, Song Y, Tsukruk VV, Chaikof EL. Cell surface engineering with polyelectrolyte multilayer thin films. J Am Chem Soc. 2011;133:7054–7064. doi: 10.1021/ja110926s. [DOI] [PubMed] [Google Scholar]
- 107.Wilson JT, Krishnamurthy VR, Cui W, Qu Z, Chaikof EL. Noncovalent cell surface engineering with cationic graft copolymers. J Am Chem Soc. 2009;131:18228–18229. doi: 10.1021/ja908887v. [DOI] [PubMed] [Google Scholar]
- 108.Krol S, del Guerra S, Grupillo M, Diaspro A, Gliozzi A, Marchetti P. Multilayer nanoencapsulation. New approach for immune protection of human pancreatic islets. Nano Lett. 2006;6:1933–1939. doi: 10.1021/nl061049r. [DOI] [PubMed] [Google Scholar]
- 109.Taylor S, Gibbons D. Effect of surface texture on the soft tissue response to polymer implants. J Biomed Mater Res A. 1983;17:205–227. doi: 10.1002/jbm.820170202. [DOI] [PubMed] [Google Scholar]
- 110.Chen S, Jones JA, Xu Y, Low H-Y, Anderson JM, Leong KW. Characterization of topographical effects on macrophage behavior in a foreign body response model. Biomaterials. 2010;31:3479–3491. doi: 10.1016/j.biomaterials.2010.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cao H, Mchugh K, Chew SY, Anderson JM. The topographical effect of electrospun nanofibrous scaffolds on the in vivo and in vitro foreign body reaction. J Biomed Mater Res A. 2010;93:1151–1159. doi: 10.1002/jbm.a.32609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Madden LR, Mortisen DJ, Sussman EM, Dupras SK, Fugate JA, Cuy JL, Hauch KD, Laflamme MA, Murry CE, Ratner BD. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc Natl Acad Sci USA. 2010;107:15211–15216. doi: 10.1073/pnas.1006442107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Saino E, Focarete ML, Gualandi C, Emanuele E, Cornaglia AI, Imbriani M, Visai L. Effect of electrospun fiber diameter and alignment on macrophage activation and secretion of proinflammatory cytokines and chemokines. Biomacromolecules. 2011;12:1900–1911. doi: 10.1021/bm200248h. [DOI] [PubMed] [Google Scholar]
- 114.Sanders J, Stiles C, Hayes C. Tissue response to single-polymer fibers of varying diameters: evaluation of fibrous encapsulation and macrophage density. J Biomed Mater Res. 2000;52:231–237. doi: 10.1002/1097-4636(200010)52:1<231::aid-jbm29>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 115.Paul NE, Skazik C, Harwardt M, Bartneck M, Denecke B, Klee D, Salber J, Zwadlo-Klarwasser G. Topographical control of human macrophages by a regularly microstructured polyvinylidene fluoride surface. Biomaterials. 2008;29:4056–4064. doi: 10.1016/j.biomaterials.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 116.Tang L, Thevenot P, Hu W. Surface chemistry influences implant biocompatibility. Curr Top Med Chem. 2008;8:270–280. doi: 10.2174/156802608783790901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vegas AJ, Veiseh O, Doloff JC, Ma M, Tam HH, Bratlie K, Li J, Bader AR, Langan E, Olejnik K, Fenton P, Kang JW, Hollister-Locke J, Bocheneck MA, Chiu A, Siebert S, Tang K, Jhunjhunwala S, Aresta-Dasilva S, Dholakia N, Thakrar R, Vietti T, Chen M, Cohen J, Siniakowicz K, Qi M, McGarrigle J, Graham AC, Lyle S, Harlan DM, Greiner DL, Oberholzer J, Weir GC, Langer R, Anderson DG. Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates. Nat Biotechnol. 2016;34:345–352. doi: 10.1038/nbt.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vegas AJ, Veiseh O, Gürtler M, Millman JR, Pagliuca FW, Bader AR, Doloff JC, Li J, Chen M, Olejnik K, Tam HH, JhunJhunwala S, Langan E, Aresta-Dasilva S, Gandham S, McGarrigle JJ, Bochenek MA, Hollister-Lock J, Oberholzer J, Greiner DL, Weir GC, Melton DA, Langer R, Anderson DG. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat Med. 2016;22:306–311. doi: 10.1038/nm.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jiang S, Cao Z. Ultralow-fouling, functionalizable, and hydrolyzable zwitterionic materials and their derivatives for biological applications. Adv Mater. 2010;22:920–932. doi: 10.1002/adma.200901407. [DOI] [PubMed] [Google Scholar]
- 120.Zhang L, Cao Z, Bai T, Carr L, Ella-Menye J-R, Irvin C, Ratner BD, Jiang S. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat Biotechnol. 2013;31:553–556. doi: 10.1038/nbt.2580. [DOI] [PubMed] [Google Scholar]
- 121.Hung HC, Jain P, Zhang P, Sun F, Sinclair A, Bai T, Li B, Wu K, Tsao C, Liu EJ, Sundaram HS, Lin X, Farahani P, Fujihara T, Jiang S. A Coating-Free Nonfouling Polymeric Elastomer. Adv Mater. 2017;29 doi: 10.1002/adma.201700617. [DOI] [PubMed] [Google Scholar]
- 122.Chen S, Zheng J, Li L, Jiang S. Strong resistance of phosphorylcholine self-assembled monolayers to protein adsorption: insights into nonfouling properties of zwitterionic materials. J Am Chem Soc. 2005;127:14473–14478. doi: 10.1021/ja054169u. [DOI] [PubMed] [Google Scholar]
- 123.Smith RS, Zhang Z, Bouchard M, Li J, Lapp HS, Brotske GR, Lucchino DL, Weaver D, Roth LA, Coury A, Biggerstaff J, Sukavaneshvar S, Langer R, Loose C. Vascular catheters with a nonleaching poly-sulfobetaine surface modification reduce thrombus formation and microbial attachment. Sci Transl Med. 2012;4:153ra132. doi: 10.1126/scitranslmed.3004120. [DOI] [PubMed] [Google Scholar]
- 124.Lu Y, Wang D, Li T, Zhao X, Cao Y, Yang H, Duan YY. Poly(vinyl alcohol)/poly(acrylic acid) hydrogel coatings for improving electrode-neural tissue interface. Biomaterials. 2009;30:4143–4151. doi: 10.1016/j.biomaterials.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 125.Hsieh CYC, Hu F-W, Chen W-S, Tsai W-B. Reducing the foreign body reaction by surface modification with collagen/hyaluronic acid multilayered films. ISRN Biomaterials. 2014;2014 718432. [Google Scholar]
- 126.Veiseh O, Doloff JC, Ma M, Vegas AJ, Tam HH, Bader AR, Li J, Langan E, Wyckoff J, Loo WS, Jhunjhunwala S, Chiu A, Siebert S, Tang K, Hollister-Lock J, Aresta-Dasilva S, Bochenek M, Mendoza-Elias J, Wang Y, Qi M, Lavin DM, Chen M, Dholakia N, Thakrar R, Lacík I, Weir GC, Oberholzer J, Greiner DL, Langer R, Anderson DG. Size-and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat Mater. 2015;14:643–651. doi: 10.1038/nmat4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mundargi RC, Babu VR, Rangaswamy V, Patel P, Aminabhavi TM. Nano/micro technologies for delivering macromolecular therapeutics using poly (D, L-lactide-co-glycolide) and its derivatives. J Control Release. 2008;125:193–209. doi: 10.1016/j.jconrel.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 128.Champion JA, Katare YK, Mitragotri S. Particle shape: a new design parameter for micro-and nanoscale drug delivery carriers. J Control Release. 2007;121:3–9. doi: 10.1016/j.jconrel.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Matlaga BF, Yasenchak LP, Salthouse TN. Tissue response to implanted polymers: the significance of sample shape. J Biomed Mater Res A. 1976;10:391–397. doi: 10.1002/jbm.820100308. [DOI] [PubMed] [Google Scholar]
- 130.Salthouse TN. Some aspects of macrophage behavior at the implant interface. J Biomed Mater Res A. 1984;18:395–401. doi: 10.1002/jbm.820180407. [DOI] [PubMed] [Google Scholar]
- 131.Zandstra J, Hiemstra C, Petersen A, Zuidema J, van Beuge M, Rodriguez S, Lathuile A, Veldhuis G, Steendam R, Bank R, Popa E. Microsphere size influences the foreign body reaction. Eur Cells Mater. 2014;28:335–347. doi: 10.22203/ecm.v028a23. [DOI] [PubMed] [Google Scholar]
- 132.Kusaka T, Nakayama M, Nakamura K, Ishimiya M, Furusawa E, Ogasawara K. Effect of silica particle size on macrophage inflammatory responses. PLoS ONE. 2014;9:e92634. doi: 10.1371/journal.pone.0092634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Citrome L. Aripiprazole long-acting injectable formulations for schizophrenia: aripiprazole monohydrate and aripiprazole lauroxil. Expert Rev Clin Pharmacol. 2016;9:169–186. doi: 10.1586/17512433.2016.1121809. [DOI] [PubMed] [Google Scholar]
- 134.Levy G. Predicting effective drug concentrations for individual patients. Clin Pharmacokinet. 1998;34:323–333. doi: 10.2165/00003088-199834040-00005. [DOI] [PubMed] [Google Scholar]
- 135.Chiou WL. The phenomenon and rationale of marked dependence of drug concentration on blood sampling site. Clin Pharmacokinet. 1989;17:175–199. doi: 10.2165/00003088-198917030-00004. [DOI] [PubMed] [Google Scholar]
- 136.Ravaine V, Ancla C, Catargi B. Chemically controlled closed-loop insulin delivery. J Control Release. 2008;132:2–11. doi: 10.1016/j.jconrel.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 137.Mo R, Jiang T, Di J, Tai W, Gu Z. Emerging micro-and nanotechnology based synthetic approaches for insulin delivery. Chem Soc Rev. 2014;43:3595–3629. doi: 10.1039/c3cs60436e. [DOI] [PubMed] [Google Scholar]
- 138.Wu Q, Wang L, Yu H, Wang J, Chen Z. Organization of glucose-responsive systems and their properties. Chem Rev. 2011;111:7855–7875. doi: 10.1021/cr200027j. [DOI] [PubMed] [Google Scholar]
- 139.Maitz MF, Freudenberg U, Tsurkan MV, Fischer M, Beyrich T, Werner C. Bio-responsive polymer hydrogels homeostatically regulate blood coagulation. Nat Commun. 2013;4:2168. doi: 10.1038/ncomms3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang Y, Yu J, Wang J, Hanne NJ, Cui Z, Qian C, Wang C, Xin H, Cole JH, Gallippi CM, Zhu Y, Gu Z. Thrombin-responsive transcutaneous patch for auto-anticoagulant regulation. Adv Mater. 2017;29 doi: 10.1002/adma.201604043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lee H, Choi TK, Lee YB, Cho HR, Ghaffari R, Wang L, Choi HJ, Chung TD, Lu N, Hyeon T, Choi SH, Kim DH. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat Nanotechnol. 2016;11:566–572. doi: 10.1038/nnano.2016.38. [DOI] [PubMed] [Google Scholar]
- 142.Shao J, Xue S, Yu G, Yu Y, Yang X, Bai Y, Zhu S, Yang L, Yin J, Wang Y, Liao S, Guo S, Xie M, Fussenegger M, Ye H. Smartphone-controlled optogenetically engineered cells enable semiautomatic glucose homeostasis in diabetic mice. Sci Transl Med. 2017;9:eaal2298. doi: 10.1126/scitranslmed.aal2298. [DOI] [PubMed] [Google Scholar]