Abstract
Neurological disorders cause gastrointestinal (GI) symptoms that are debilitating and markedly diminish quality of life in patients. The enteric nervous system (ENS), the intrinsic nervous system of the GI tract that is often referred to as “the second brain”, shares many features with the central nervous system. The ENS plays an essential role in regulating many GI functions including motility and fluid secretion. Enteric neuronal degeneration could therefore be responsible for the GI symptoms commonly observed in neurological conditions. Here we describe the organization and functions of the ENS and then review the evidence for ENS involvement in two common neurodegenerative disorders, Parkinson’s disease (PD) and Alzheimer’s disease (AD). Data from patients as well as animal models suggest that PD affects distinct subsets of neurons and glia in the ENS, and that the ENS may participate in the pathogenesis of this disorder. While there has been great enthusiasm for the possibility of sampling the ENS for diagnosis or therapeutic monitoring of PD, further work is needed to determine which enteric neurons are most affected and how ENS function could be modulated to ameliorate GI symptoms in patients. Although AD is far more common than PD and AD patients also experience GI symptoms, understanding of ENS dysfunction in AD is in its infancy. Much work remains to be done in both of these fields to determine how the ENS contributes to and/or is altered by these disorders, and how to target the ENS for more effective treatment of GI comorbidities.
Keywords: Enteric nervous system, Parkinson’s disease, Alzheimer’s disease, Neurodegeneration
Introduction
The enteric nervous system (ENS) is often referred to as the “second brain” in the bowel because it shares many features with the brain, including the full complement of neurotransmitters utilized for synaptic transmission, ultrastructural features of neuron-glia interactions, similar transcriptional programs for generating neuronal diversity, and a laminar organization1. The ENS is in fact the largest division of the peripheral nervous system (PNS) and is unique in the PNS because it can perform many of its functions independent of input from the central nervous system (CNS). The ENS consists of two ganglionated plexuses composed of neurons and glia that regulate a variety of gastrointestinal functions and are essential for life (Figure 1A). A more detailed description of the structure, function and component cell types of the ENS is beyond the scope of this review, but has been well reviewed in the past2, 3. While the ENS does not require CNS input to perform its duties, in practice, it works in collaboration with vagal, sympathetic and parasympathetic inputs to regulate many GI functions, such as motility. This bi-directional communication between the ENS and other parts of the nervous system has been postulated to serve as a conduit for transmission of pathogenic proteins and infectious particles, such as α-synuclein and prions, from the gut to the brain. Another article in this special issue addresses the latest evidence in support of this possibility, often referred to as Braak’s hypothesis4. Not only is the ENS a potential portal for the pathogenesis of neurodegenerative disorders, there is growing evidence that the ENS is also a target of these disorders. ENS dysfunction can be associated with a number of GI symptoms including severe constipation, anorexia, and delayed gastric emptying (gastroparesis), which are all common in patients with neurodegenerative conditions. This review presents the latest evidence for ENS involvement in two common neurodegenerative disorders, Parkinson’s disease (PD) and Alzheimer’s disease (AD), and discusses the need for sampling the ENS for disease monitoring and therapeutic development.
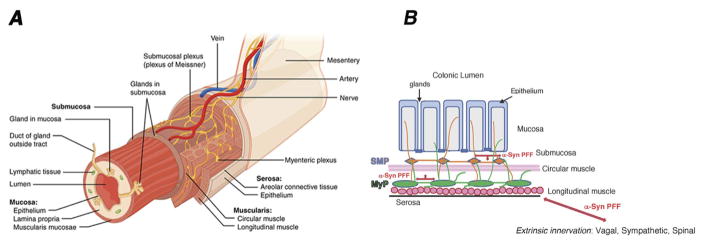
FIGURE 1. Organization of the enteric nervous system and potential pathways for α-synuclein propagation.
A: Schematic illustrating the laminar organization of the bowel in three-dimensions from the mesentery to the lumen. The two major plexuses of the ENS are the myenteric plexus (MyP), located in between the circular and longitudinal muscle layers in the muscularis externa, and the submucosal plexus (SMP), located in the submucosa. Image obtained from Wikimedia and reproduced under the Creative Commons Attribution-Share Alike 3.0 Unported license.
B: Schematic of a cross-section of the colon illustrating the interconnected enteric plexuses (MyP and SMP), which are both unmyelinated and contain neurons projecting to the mucosa. Alpha-synucleopathies (α-Syn preformed fibrils [PFF], red arrows) could propagate from pre-synaptic nerve terminals to vulnerable post-synaptic neurons both within the ENS, and from the ENS to the CNS through extrinsic projections to the gut, which include vagal, sympathetic and spinal afferent fibers. This could be one of the factors in the progression of Parkinson’s disease.
Parkinson’s disease
PD is a chronic, progressive movement disorder characterized by rigidity, resting tremor, postural instability and bradykinesia. While these motor symptoms were defined decades ago, it was only more recently recognized that PD can also affect bowel function, causing constipation that often starts before motor symptoms and correlates with disease severity5. In the CNS, PD is associated with toxic accumulation of α-synuclein protein aggregates in cell bodies (Lewy bodies; LB) and neurites (Lewy neurites), leading to degeneration of nigrostriatal dopaminergic neurons. With the advent of antibodies that made it easier to detect α-synuclein accumulation, several groups reported Lewy-type pathology in enteric neurons from biopsies of PD patients, suggesting that the ENS could be involved in PD6, 7. A series of subsequent studies have tried to determine whether Lewy pathology in the ENS is specific to PD, pre-dates motor functions, and correlates with PD disease severity (summarized in Table 1)8–12. These studies have shown that α-synuclein aggregates can be detected in the ENS more commonly in PD patients than healthy controls, but it remains unclear if this is associated with enteric neurodegeneration or GI symptomatology. In total, Lewy pathology was most often detected in the submucosal plexus and dopaminergic neurons were not necessarily the most affected. A major challenge in conducting and interpreting these studies is that many aspects of enteric neuronal organization remain unknown and it is not clear if PD targets a specific site or neuronal subtype in the ENS the way it targets the substantia nigra in the CNS. Studies of ENS pathology in PD have thus offered inconsistent findings with regard to which plexus and type(s) of neurons are most affected. These inconsistencies prompted an effort to change the methodology for assessment of Lewy type α-synucleinopathies (LTS) to improve sensitivity and specificity13. A consortium of laboratories used a series of defined immunohistochemical methods to detect pathological α-synuclein from full thickness sections of the sigmoid colon at autopsy and found the highest prevalence of pathological LTS staining in neuronal elements of the submucosa14. In PD patients with chronic constipation, colonic submucosal neurons downregulate vasoactive intestinal peptide (VIP), but are not lost15. These data suggest that constipation in PD could be secondary to dysfunction, but not loss, of VIPergic secretomotor neurons in the submucosal plexus. Larger studies with careful standardization of assays and locations sampled will be required to test this hypothesis.
TABLE 1. Pathological studies of the ENS in human patients with PD.
Summary of the studies to date of ENS pathology in human PD. In the CNS column, the clinical diagnosis of the patients studied, presence of motor symptoms, and the sample size is summarized. In the ENS column, the neuropathological marker(s), plexuses examined, and the primary observations of each study are summarized. MyP: myenteric plexus; SMP: submucosal plexus; IR: immunoreactive; DBH: dopamine beta hydroxylase; LB: Lewy Bodies; LN: Lewy neurites; NF: neurofilament; NO: nitric oxide; α-SYN: alpha-synuclein; TH: tyrosine hydroxylase; VIP: vasoactive intestinal peptide; Nd: not determined.
| CNS | ENS | |||
|---|---|---|---|---|
| Motor symptoms? (# of patients) | Affected neuron types, αSYN-IR fibers | Neuropathy marker | GI symptoms | References |
| PD, yes (1) | MyP: colon | LBs | Acquired megacolon | Kupsky et al., 1987 |
| PD, yes (10) | VIP-IR elements MyP: esophagus, entire GI tract SMP: duodenum, colon No cell body loss |
LBs | Achalasia | Wakabayashi et al., 1988, 1990 |
| Advanced PD, yes (11) | VIP-IR, TH-IR MyP: colon Loss of dopamine-IR neurons and dopamine depletion |
LBs | Chronic constipation, megacolon | Singaram et al., 1995 |
| PD, no (5) | Neurofibrillary lesions SMP more affected than MyP in stomach |
αSYN-IR inclusions | Not indicated | Braak et al., 2006 |
| Early, mid- and late stages PD; some with motor symptoms (29) | SMP: colon Loss of NF-IR neurons Nerve fibers-IR |
LB and LN αSYN-IR |
Chronic constipation | Lebouvier et al., 2010 |
| PD, no (9) | SMP: colon Nerve fibers-IR |
αSYN-IR | Not indicated | Shannon et al., 2012 |
| PD, no (129) | Plexus unspecified Nerve fibers-IR |
No correlation with rostral-caudal gradient of αSYN | Defecatory dysfunction | Cersosimo, Benarroch 2012 Cersosimo et al., 2013 |
| Advanced PD, Yes (7) | Myp: no loss of NO-, VIP-, TH- neurons Nerve fibers-IR in stomach (MyP> SMP) TH-IR rare |
αSYN | GI dysfunction unspecified | Annerino et al.,2012 |
| PD, yes (35) | SMP: colon No loss of TH- or DBH- IR neurons |
Nd | GI dysfunction unspecified | Corbillé et al., 2014 |
| PD, yes (5) | Sigmoid Colon, full thickness SMP>Muscularis>Mucosa neuronal -IR structures |
αSYN/LTS | GI dysfunction | Beach et al., 2016 |
Enteric neuropathies in animal models of PD
Animal models of PD are based on either pharmacologic or genetic approaches to simulate nigrostriatal neurodegeneration and PD pathogenesis. The first and most commonly used pharmacologic models of PD have been based on administration of one of two neurotoxins to mice, rats, or more recently, nonhuman primates. Both of these neurotoxins, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and 6-hydroxydopamine (6-OHDA), consistently affect the dopaminergic nigrostriatal pathways16, but their impact on gut function and the ENS is variable, depending on the agent, mode of administration, and assays used (reviewed in Table 2)17–20. Systemic MPTP administration in both mice and non-human primates causes loss of dopaminergic neurons in the myenteric plexus, but no major defects in GI motility17, 18. Interestingly, delivery of 6-OHDA directly into the substantia nigra is sufficient to slow colonic motility, suggesting that the constipation observed in PD patients could reflect CNS pathology alone19, 20. Unfortunately, the submucosal plexus was not analyzed in some of these studies making it difficult to make direct comparisons to the most robust findings from PD patients.
TABLE 2. Comparative analysis of CNS and ENS manifestations in animal models of PD.
Summary of the studies to date on ENS pathology and GI phenotypes in animal models of PD. In the CNS column, the model organism, experimental paradigm (chemical or genetic lesion), and CNS pathology are summarized. In the ENS column, the neuropathological marker(s), plexuses examined, gastrointestinal (GI) deficits and the primary observations of each study are summarized. MyP: myenteric plexus; SMP: submucosal plexus; IR: immunoreactive; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; I.P.: intraperitoneal; I.V.: intravenous; S.Q.: subcutaneous; 6-OHDA: 6-hydroxydopamine; LB: Lewy Bodies; LN: Lewy neurites; α-SYN: alpha-synuclein; NO: nitric oxide; ChAT: choline acetyltransferase; GE: gastric emptying; TGTT: total GI transit time; DAT: dopamine transporter; SN: substantia nigra; TH: tyrosine hydroxylase; nNOS: neuronal nitric oxide synthase; AR: adrenergic receptor; VIP: vasoactive intestinal peptide; DMV: dorsal motor nucleus of the vagus; IML: intermediolateral column of the spinal cord; Nd: not determined
| CNS | ENS | ||||
|---|---|---|---|---|---|
|
| |||||
| PD Model Route of toxin administration |
Motor Symptoms (no, yes) Affected neurons |
Affected Neuron types, αSYN-IR fibers plexuses |
Neuropathy marker |
GI Symptoms | References |
|
| |||||
|
MPTP mice I.P., test after 10 days |
Nd | Loss of TH-neurons and NADPH/NO; ChAT unchanged MyP: Ileum SMP: Nd |
Nd | Colonic motility transiently increased; GE, TGTT unchanged | Anderson et al., 2007 |
|
MPTP rhesus monkeys I.V., test after 5 months |
Yes, Decrease striatal DAT | % Total neurons: Increased NOS, decreased TH No change ChAT, VIP No change glia MyP, colon Decreased TH SMP, colon |
Nd | No change in stool consistency | Chaumette et al., 2009 |
|
| |||||
|
6-OHDA rats SN injection (does not reach the ENS), test after 4 weeks |
Nd Loss of SN TH- neurons |
Increased TH-IR, Decreased nNOS-IR ChAT-IR unchanged Downregulation of TH & nNOS mRNA, ChAT mRNA expression Stomach, colon Plexuses unspecified |
Nd | Slower colon motility GE delayed |
Zhu et al., 2012 |
|
6-OHDA rats SN injected, test after 6 weeks |
Yes, (less time Rotarod test) Loss of SN TH-neurons |
DA content increased (muscularis exerna), β3-AR upregulated 5-HT4R downregul. colon, plexuses unspecified |
Nd | Decreased colonic motility | Zhang et al., 2015 |
|
| |||||
|
Rotenone rats I.P., 5x/week test after 3d, 1 or 6 months |
No, No loss of SN- striatal neurons | Loss of neurons MyP, small intestine SMP, Nd |
Loss αSYN-IR (3 day post inj.) LBs (6 months post inj.) |
Slower GI motility (6 months post inj.) | Drolet et al., 2009 |
|
Rotenone rats Osmotic minipumps S.Q. for 22–28 days |
No, Grossly normal behavior Unaltered TH protein content in midbrain or striatum |
%total neurons ChAT, NOS, VIP, TH unchanged MyP, Ileum, pylori, proximal colon |
Nd | Delayed GE; transient decrease in colonic motility; physiological defect of inhibitory neurons | Greene et al., 2009 |
|
Rotenone mice Intragastric, 5d/week for 1.5 or 3 months |
No, (rotarod test 1.5 mth) Yes, 3 mth αSYN-IR neurons in SN, dmX, IML, Loss TH-IR, SN |
ENS Ganglia , Extra and intra neuronal α-SYN aggreg Gliosis MyP, (Duod ,leum) SMP, Nd |
αSYN-IR aggreg (3mth) p-α-SYN |
Nd | Pan-Montojo et al., 2010 |
|
| |||||
|
Mice transgenic for human α-synuclein (expression driven by Thy1 promoter) Aged 8–10 months |
No loss of striatal dopamine | αSYN varicoses around pChAT neurons; no loss of pChAT-, nNOS-, TH- or VIP- neurons MyP, distal colon SMP, Nd | αSYN-IR | Normal GE Defecatory dysfunction |
Wang et al., 2012 |
| Aged 11–12 months | Nd | Nd | Nd | Slower colonic transit; increased defecation under stress | Wang et al., 2008 |
|
Mice transgenic for mutant A53T or A30P human α-synuclein Age: 3–18 months |
No dopaminergic deficit, No LB A30P, no gross motor dysfunction A53T, reduced activity, discoordination on Rotarod test | In TH-IR In varicose terminals on NOS-IR MyP and SMP |
Mutated αSYN-IR | A53T & A30P Increased GITT. Reduced colonic motility |
Kuo et al., 2010 |
| Mice transgenic for mutant A53T inj. stomach LB from brain extract of PD patient Test after 4 months | PD patient presumed motor symptoms | Neurons-IR MyP SMP, Nd |
Aggregates Endogenous A53T SYN-IR |
Nd | Lee et al., 2011 |
|
Mice transgenic for mutant A53T (expression driven by prion promoter) Test 2–20 months |
Motor symptoms, Nd DMV efferent fibers A53T SYN- IR |
No SYN-IR neurons, only surrounding varicose processes Stomach > Duodenum >colon MyP SMP, Nd |
A53T SYN-IR Age related decline |
Aged related decline defecation and slower Colonic transit | Noorian et al., 2012 |
A breakthrough in the understanding of PD pathogenesis was the demonstration that a single intra-striatal injection of synthetic α-synuclein fibrils in a normal mouse was sufficient to transmit the pathologic form of α-synuclein from cell to cell in anatomically connected brain areas21. This transmission was accompanied by LB formation, a progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc), and motor deficits, effectively modeling the progression of PD21. Intragastric administration of a third neurotoxin, the pesticide rotenone, recapitulates this cell-to-cell transmission of α-synuclein. Pesticide exposure has been linked to PD in epidemiological studies, and chronic intragastric administration of rotenone causes nigrostriatal degeneration preceded by LTS and myenteric neuron loss in mice22–25, suggesting it might be a particularly good rodent model of PD. In rotenone-treated mice, ENS neurodegeneration was associated with slowed GI motility prior to onset of CNS findings, suggesting that in addition to serving as a portal for α-synuclein transmission to the CNS, ENS Lewy pathology could be sufficient to explain the GI symptoms in early PD24.
Genetic models in which transgenic mice over-express either the wildtype or the human mutant forms (A53T or A30P) of theα-synuclein gene have also been used to study PD α-synucleinopathies26. In most of these mouse models, age-dependent aggregation of α-synuclein in either enteric neurons or varicosities around enteric neurons is associated with GI motility dysfunction, particularly slowed colonic transit (Table 2)27–30. In a subset of these models, moreover, pathological aggregation of α-synuclein was shown to both spread from the dorsal motor nucleus of the vagus (DMV) to the ENS via vagal efferent fibers in A53T transgenic mice30, or from the ENS back to the DMV via retrograde transport when human αsynuclein pre-formed fibrils were injected into the wall of the intestine31. Genetic and chemical injury models of PD both support the idea that enteric neurons are vulnerable to α-synucleinopathies.
Relative vulnerability of nigrostriatal and enteric neurons
The diverse risk factors that determine the vulnerability of specific types of neurons in neurodegenerative disorders, such as nigrostriatal dopaminergic neurons in PD, are still being defined32, 33. Nigrostriatal dopaminergic neurons exhibit sustained depolarizing activity patterns that could lead to metabolic stress and mitochondrial dysfunction34, 35. Other suggested risk factors are autonomous activity, limited calcium buffering capability, and long, poorly myelinated axons35. Many enteric neurons, particularly those involved in peristalsis, are autonomous and probably continuously active, making them similarly vulnerable. Guinea-pig myenteric ganglia in organotypic explants exposed to rotenone or sustained high K+ induced depolarization for several days, form and accumulate α-synuclein fibrils in axonal varicosities of cholinergic neurons36. Enteric neurons all have unmyelinated axons and many have multiple synaptic endings, features that could also increase their vulnerability in PD and could facilitate the release and reuptake of α-synuclein and its propagation through connected ganglia (Figure 1B). Consistent with this, cultured embryonic enteric neurons accumulate α-synuclein when treated with forskolin, and release it upon depolarization37. Enteric neurons also have unique vulnerabilities related to their close proximity to the intestinal epithelium and gut mucosal immune system, both of which can become altered in PD and potentially harm enteric neurons38, 39. Further exploration of which subsets of enteric neurons are most affected in PD will help define the characteristics that place them at risk and advance the development of enteric neuroprotective therapies.
Alzheimer’s disease
AD is a neurodegenerative disorder causing progressive cognitive impairment and loss of working memory. Brain pathology is characterized by extracellular plaques containing β-amyloid (Aβ) and intracellular neurofibrillary tangles comprised of hyper-phosphorylated tau protein. AD, like many neurological disorders, is associated with a variety of GI symptoms, raising the possibility that the ENS could also be affected. Supporting this idea, an early report showed that plaques immunoreactive for Aβ could be found in the submucosa of two AD patients40. Amyloid precursor protein (APP), from which Aβ is derived, is normally expressed by enteric neurons and glia41, making it further plausible that AD pathophysiology could involve the ENS. Follow up studies, however, have been limited in number and shown conflicting results. A study of autopsy specimens from esophagus, stomach, small and large intestine from 18 AD patients found that there was no difference in neuronal density or tau pathology between AD patients and elderly controls, or between AD patients and those with non-AD dementias42. Another study reported elevated Aβ immunoreactivity in the colons of 11 patients with AD, but did not include controls43. Further studies of enteric neuronal pathology in AD are clearly needed.
While data from human samples is sparse, translational studies have shown that mutations associated with familial AD in human patients can cause GI pathology in mice. In AβPP/PS1 transgenic mice, the mouse prion protein (PrP) promoter directs expression of mutant forms of mouse APP and human presenilin 1, which act to alter APP processing and lead to brain Aβ deposition44. Enteric neurons in both plexuses of AβPP/PS1 mice accumulate Aβ but there is no resulting dysmotility or change in overall neuronal density43, 45. Gut macrophage density and levels of several pro-inflammatory proteins, however, were markedly increased suggesting that enteric Aβ deposition is not benign. Related mouse models in which either the hamster PrP or Thy1 promoters are used to drive mutant forms of APP within neurons also show amyloid deposits in the bowel, but have divergent effects on neuronal density46, 47. A limitation of these studies is that the methods used to measure neuronal density vary and the promoters used to drive transgene expression have often not been fully characterized in the bowel. Regardless, work to date suggests that the Aβ deposits can form in the bowel and trigger an inflammatory response associated with macrophage recruitment. Given the increasing evidence that microglia, the resident macrophages of the CNS, play an important role in the synapse loss associated with AD48, further investigation of neuroimmune interactions and synaptic deficits in the bowel is warranted.
Conclusions and Future Directions
Given the many commonalities between the CNS and ENS, it is highly likely that disorders that affect CNS function also affect ENS function and that ENS dysfunction contributes to the GI symptoms experienced by patients with neurodegenerative disease. Studies of ENS pathology in PD and AD patients, however, remain limited and often convey conflicting results. Data from animal models is more robust and suggest that ENS involvement in both of these conditions is highly plausible. Enteric ganglia are heterogeneous and it remains unknown to what extent there might be regional specialization or functional “nuclei” along the length of the bowel. To better translate findings from animal models to human disease, it will be important for future studies to carefully standardize the bowel locations sampled and maximize the number of neurons examined from each patient. The submucosal plexus is likely to be of highest yield for further analysis in PD, and therapeutic strategies to protect enteric neurons in the pre-motor phase of PD may be disease modifying, at least for ameliorating GI symptoms. Glial dysfunction is increasingly recognized to play a major role in CNS neurodegeneration but has been little examined in the ENS. Enteric glial involvement in PD and AD remains another important area for future investigation. The GI tract represents a major interface with the outside environment; it is essential to better understand how the neurons and glia embedded within it might serve as conduits for and targets of neurodegenerative disorders.
HIGHLIGHTS.
The ENS and CNS have comparable neuronal diversity and developmental programs.
In PD, α-synuclein accumulates in enteric neurons, prior to motor symptoms.
Genetic and chemical injury models of PD variably affect the ENS
Submucosal enteric neurons are probably most vulnerable to degeneration in PD
ENS pathology in AD is understudied; Aβ plaques have been observed in the bowel.
Acknowledgments
We thank O. Levy and M. Rutlin for reviewing the manuscript.
FUNDING
This work was supported by the National Institutes of Health [DK098903]; the American Gastroenterological Association-Takeda Research Scholar Award [M.R]; and philanthropic support from Ivan and Phyllis Seidenberg.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gershon MD. The Second Brain. New York, N.Y: Harper Collins; 1998. p. 312. [Google Scholar]
- 2.Rao M, Gershon MD. The bowel and beyond: the enteric nervous system in neurological disorders. Nat Rev Gastroenterol Hepatol. 2016;13(9):517–28. doi: 10.1038/nrgastro.2016.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furness JB. The enteric nervous system. Malden, Mass: Blackwell Pub; 2006. p. xiii.p. 274. [Google Scholar]
- 4.Liddle RA. Brain Research Reviews. 2018. Parkinson's Disease from the Gut. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards LL, Pfeiffer RF, Quigley EM, Hofman R, Balluff M. Gastrointestinal symptoms in Parkinson's disease. Mov Disord. 1991;6(2):151–6. doi: 10.1002/mds.870060211. [DOI] [PubMed] [Google Scholar]
- 6.Kupsky WJ, Grimes MM, Sweeting J, Bertsch R, Cote LJ. Parkinson's disease and megacolon: concentric hyaline inclusions (Lewy bodies) in enteric ganglion cells. Neurology. 1987;37(7):1253–5. doi: 10.1212/wnl.37.7.1253. [DOI] [PubMed] [Google Scholar]
- 7.Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F. Parkinson's disease: the presence of Lewy bodies in Auerbach's and Meissner's plexuses. Acta Neuropathol. 1988;76(3):217–221. doi: 10.1007/BF00687767. [DOI] [PubMed] [Google Scholar]
- 8.Wakabayashi K, Takahashi H, Ohama E, Ikuta F. Parkinson's disease: an immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta Neuropathol. 1990;79(6):581–3. doi: 10.1007/BF00294234. [DOI] [PubMed] [Google Scholar]
- 9.Singaram C, Ashraf W, Gaumnitz EA, Torbey C, Sengupta A, Pfeiffer R, Quigley EM. Dopaminergic defect of enteric nervous system in Parkinson's disease patients with chronic constipation. Lancet. 1995;346(8979):861–4. doi: 10.1016/s0140-6736(95)92707-7. [DOI] [PubMed] [Google Scholar]
- 10.Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner's and Auerbach's plexuses in cases staged for Parkinson's disease-related brain pathology. Neurosci Lett. 2006;396(1):67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Lebouvier T, Neunlist M, Bruley des Varannes S, Coron E, Drouard A, N'Guyen JM, Chaumette T, Tasselli M, Paillusson S, Flamand M, Galmiche JP, Damier P, Derkinderen P. Colonic biopsies to assess the neuropathology of Parkinson's disease and its relationship with symptoms. PLoS One. 2010;5(9):e12728. doi: 10.1371/journal.pone.0012728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shannon KM, Keshavarzian A, Mutlu E, Dodiya HB, Daian D, Jaglin JA, Kordower JH. Alpha-synuclein in colonic submucosa in early untreated Parkinson's disease. Mov Disord. 2012;27(6):709–15. doi: 10.1002/mds.23838. [DOI] [PubMed] [Google Scholar]
- 13.Beach TG, White CL, Hamilton RL, Duda JE, Iwatsubo T, Dickson DW, Leverenz JB, Roncaroli F, Buttini M, Hladik CL, Sue LI, Noorigian JV, Adler CH. Evaluation of alpha-synuclein immunohistochemical methods used by invited experts. Acta Neuropathol. 2008;116(3):277–88. doi: 10.1007/s00401-008-0409-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beach TG, Corbille AG, Letournel F, Kordower JH, Kremer T, Munoz DG, Intorcia A, Hentz J, Adler CH, Sue LI, Walker J, Serrano G, Derkinderen P. Multicenter Assessment of Immunohistochemical Methods for Pathological Alpha-Synuclein in Sigmoid Colon of Autopsied Parkinson's Disease and Control Subjects. J Parkinsons Dis. 2016;6(4):761–770. doi: 10.3233/JPD-160888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giancola F, Torresan F, Repossi R, Bianco F, Latorre R, Ioannou A, Guarino M, Volta U, Clavenzani P, Mazzoni M, Chiocchetti R, Bazzoli F, Travagli RA, Sternini C, De Giorgio R. Downregulation of neuronal vasoactive intestinal polypeptide in Parkinson's disease and chronic constipation. Neurogastroenterol Motil. 2017;29(5) doi: 10.1111/nmo.12995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tieu K. A guide to neurotoxic animal models of Parkinson's disease. Cold Spring Harb Perspect Med. 2011;1(1):a009316. doi: 10.1101/cshperspect.a009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson G, Noorian AR, Taylor G, Anitha M, Bernhard D, Srinivasan S, Greene JG. Loss of enteric dopaminergic neurons and associated changes in colon motility in an MPTP mouse model of Parkinson's disease. Exp Neurol. 2007;207(1):4–12. doi: 10.1016/j.expneurol.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaumette T, Lebouvier T, Aubert P, Lardeux B, Qin C, Li Q, Accary D, Bezard E, Bruley des Varannes S, Derkinderen P, Neunlist M. Neurochemical plasticity in the enteric nervous system of a primate animal model of experimental Parkinsonism. Neurogastroenterol Motil. 2009;21(2):215–22. doi: 10.1111/j.1365-2982.2008.01226.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhu HC, Zhao J, Luo CY, Li QQ. Gastrointestinal dysfunction in a Parkinson's disease rat model and the changes of dopaminergic, nitric oxidergic, and cholinergic neurotransmitters in myenteric plexus. J Mol Neurosci. 2012;47(1):15–25. doi: 10.1007/s12031-011-9560-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Li Y, Liu C, Fan R, Wang P, Zheng L, Hong F, Feng X, Zhang Y, Li L, Zhu J. Alteration of enteric monoamines with monoamine receptors and colonic dysmotility in 6-hydroxydopamine-induced Parkinson's disease rats. Transl Res. 2015;166(2):152–62. doi: 10.1016/j.trsl.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Luk KC, Kehm V, Carroll J, Zhang B, O'Brien P, Trojanowski JQ, Lee VM. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338(6109):949–53. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drolet RE, Cannon JR, Montero L, Greenamyre JT. Chronic rotenone exposure reproduces Parkinson's disease gastrointestinal neuropathology. Neurobiol Dis. 2009;36(1):96–102. doi: 10.1016/j.nbd.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 23.Greene JG, Noorian AR, Srinivasan S. Delayed gastric emptying and enteric nervous system dysfunction in the rotenone model of Parkinson's disease. Exp Neurol. 2009;218(1):154–61. doi: 10.1016/j.expneurol.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan-Montojo F, Anichtchik O, Dening Y, Knels L, Pursche S, Jung R, Jackson S, Gille G, Spillantini MG, Reichmann H, Funk RH. Progression of Parkinson's disease pathology is reproduced by intragastric administration of rotenone in mice. PLoS One. 2010;5(1):e8762. doi: 10.1371/journal.pone.0008762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan-Montojo F, Schwarz M, Winkler C, Arnhold M, O'Sullivan GA, Pal A, Said J, Marsico G, Verbavatz JM, Rodrigo-Angulo M, Gille G, Funk RH, Reichmann H. Environmental toxins trigger PD-like progression via increased alpha-synuclein release from enteric neurons in mice. Sci Rep. 2012;2:898. doi: 10.1038/srep00898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koprich JB, Kalia LV, Brotchie JM. Animal models of alpha-synucleinopathy for Parkinson disease drug development. Nat Rev Neurosci. 2017;18(9):515–529. doi: 10.1038/nrn.2017.75. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Fleming SM, Chesselet MF, Tache Y. Abnormal colonic motility in mice overexpressing human wild-type alpha-synuclein. Neuroreport. 2008;19(8):873–6. doi: 10.1097/WNR.0b013e3282ffda5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L, Magen I, Yuan PQ, Subramaniam SR, Richter F, Chesselet MF, Tache Y. Mice overexpressing wild-type human alpha-synuclein display alterations in colonic myenteric ganglia and defecation. Neurogastroenterol Motil. 2012;24(9):e425–36. doi: 10.1111/j.1365-2982.2012.01974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuo YM, Li Z, Jiao Y, Gaborit N, Pani AK, Orrison BM, Bruneau BG, Giasson BI, Smeyne RJ, Gershon MD, Nussbaum RL. Extensive enteric nervous system abnormalities in mice transgenic for artificial chromosomes containing Parkinson disease-associated alpha-synuclein gene mutations precede central nervous system changes. Hum Mol Genet. 2010;19(9):1633–50. doi: 10.1093/hmg/ddq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noorian AR, Rha J, Annerino DM, Bernhard D, Taylor GM, Greene JG. Alpha-synuclein transgenic mice display age-related slowing of gastrointestinal motility associated with transgene expression in the vagal system. Neurobiol Dis. 2012;48(1):9–19. doi: 10.1016/j.nbd.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Holmqvist S, Chutna O, Bousset L, Aldrin-Kirk P, Li W, Bjorklund T, Wang ZY, Roybon L, Melki R, Li JY. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014;128(6):805–20. doi: 10.1007/s00401-014-1343-6. [DOI] [PubMed] [Google Scholar]
- 32.Brichta L, Greengard P. Molecular determinants of selective dopaminergic vulnerability in Parkinson's disease: an update. Front Neuroanat. 2014;8:152. doi: 10.3389/fnana.2014.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michel PP, Hirsch EC, Hunot S. Understanding Dopaminergic Cell Death Pathways in Parkinson Disease. Neuron. 2016;90(4):675–91. doi: 10.1016/j.neuron.2016.03.038. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura K, Bindokas VP, Marks JD, Wright DA, Frim DM, Miller RJ, Kang UJ. The selective toxicity of 1-methyl-4-phenylpyridinium to dopaminergic neurons: the role of mitochondrial complex I and reactive oxygen species revisited. Mol Pharmacol. 2000;58(2):271–8. doi: 10.1124/mol.58.2.271. [DOI] [PubMed] [Google Scholar]
- 35.Sulzer D, Surmeier DJ. Neuronal vulnerability, pathogenesis, and Parkinson's disease. Mov Disord. 2013;28(6):715–24. doi: 10.1002/mds.25187. [DOI] [PubMed] [Google Scholar]
- 36.Sharrad DF, Chen BN, Gai WP, Vaikath N, El-Agnaf OM, Brookes SJ. Rotenone and elevated extracellular potassium concentration induce cell-specific fibrillation of alpha-synuclein in axons of cholinergic enteric neurons in the guinea-pig ileum. Neurogastroenterol Motil. 2017;29(4) doi: 10.1111/nmo.12985. [DOI] [PubMed] [Google Scholar]
- 37.Paillusson S, Clairembault T, Biraud M, Neunlist M, Derkinderen P. Activity-dependent secretion of alpha-synuclein by enteric neurons. J Neurochem. 2013;125(4):512–7. doi: 10.1111/jnc.12131. [DOI] [PubMed] [Google Scholar]
- 38.Salat-Foix D, Tran K, Ranawaya R, Meddings J, Suchowersky O. Increased intestinal permeability and Parkinson disease patients: chicken or egg? Can J Neurol Sci. 2012;39(2):185–8. doi: 10.1017/s0317167100013202. [DOI] [PubMed] [Google Scholar]
- 39.Devos D, Lebouvier T, Lardeux B, Biraud M, Rouaud T, Pouclet H, Coron E, Bruley des Varannes S, Naveilhan P, Nguyen JM, Neunlist M, Derkinderen P. Colonic inflammation in Parkinson's disease. Neurobiol Dis. 2013;50:42–8. doi: 10.1016/j.nbd.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 40.Joachim CL, Mori H, Selkoe DJ. Amyloid beta-protein deposition in tissues other than brain in Alzheimer's disease. Nature. 1989;341(6239):226–30. doi: 10.1038/341226a0. [DOI] [PubMed] [Google Scholar]
- 41.Arai H, Lee VM, Messinger ML, Greenberg BD, Lowery DE, Trojanowski JQ. Expression patterns of beta-amyloid precursor protein (beta-APP) in neural and nonneural human tissues from Alzheimer's disease and control subjects. Ann Neurol. 1991;30(5):686–93. doi: 10.1002/ana.410300509. [DOI] [PubMed] [Google Scholar]
- 42.Shankle WR, Landing BH, Ang SM, Chui H, Villarreal-Engelhardt G, Zarow C. Studies of the enteric nervous system in Alzheimer disease and other dementias of the elderly: enteric neurons in Alzheimer disease. Mod Pathol. 1993;6(1):10–4. [PubMed] [Google Scholar]
- 43.Puig KL, Lutz BM, Urquhart SA, Rebel AA, Zhou X, Manocha GD, Sens M, Tuteja AK, Foster NL, Combs CK. Overexpression of mutant amyloid-beta protein precursor and presenilin 1 modulates enteric nervous system. J Alzheimers Dis. 2015;44(4):1263–78. doi: 10.3233/JAD-142259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, Copeland NG, Lee MK, Younkin LH, Wagner SL, Younkin SG, Borchelt DR. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet. 2004;13(2):159–70. doi: 10.1093/hmg/ddh019. [DOI] [PubMed] [Google Scholar]
- 45.Han X, Tang S, Dong L, Song L, Dong Y, Wang Y, Du Y. Loss of nitrergic and cholinergic neurons in the enteric nervous system of APP/PS1 transgenic mouse model. Neurosci Lett. 2017;642:59–65. doi: 10.1016/j.neulet.2017.01.061. [DOI] [PubMed] [Google Scholar]
- 46.Semar S, Klotz M, Letiembre M, Van Ginneken C, Braun A, Jost V, Bischof M, Lammers WJ, Liu Y, Fassbender K, Wyss-Coray T, Kirchhoff F, Schafer KH. Changes of the enteric nervous system in amyloid-beta protein precursor transgenic mice correlate with disease progression. J Alzheimers Dis. 2013;36(1):7–20. doi: 10.3233/JAD-120511. [DOI] [PubMed] [Google Scholar]
- 47.Van Ginneken C, Schafer KH, Van Dam D, Huygelen V, De Deyn PP. Morphological changes in the enteric nervous system of aging and APP23 transgenic mice. Brain Res. 2011;1378:43–53. doi: 10.1016/j.brainres.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 48.Hong S, V, Beja-Glasser F, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, Merry KM, Shi Q, Rosenthal A, Barres BA, Lemere CA, Selkoe DJ, Stevens B. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352(6286):712–716. doi: 10.1126/science.aad8373. [DOI] [PMC free article] [PubMed] [Google Scholar]