SUMMARY
The ketogenic diet (KD) is used to treat refractory epilepsy, but the mechanisms underlying its neuroprotective effects remain unclear. Here we show that the gut microbiota is altered by the KD and required for protection against acute electrically-induced seizures and spontaneous tonic-clonic seizures in two mouse models. Mice treated with antibiotics or reared germ-free are resistant to KD-mediated seizure protection. Enrichment of, and gnotobiotic co-colonization with, KD-associated Akkermansia and Parabacteroides restores seizure protection. Moreover, transplantation of the KD gut microbiota, and treatment with Akkermansia and Parabacteroides, each confer seizure protection to mice fed a control diet. Alterations in colonic lumenal, serum and hippocampal metabolomic profiles correlate with seizure protection, including reductions in systemic gamma-glutamylated amino acids and elevated hippocampal GABA:glutamate levels. Bacterial cross-feeding decreases gamma-glutamyltranspeptidase activity and inhibiting gamma-glutamylation promotes seizure protection in vivo. Overall, this study reveals that the gut microbiota modulates host metabolism and seizure susceptibility in mice.
Graphical abstract
INTRODUCTION
The low-carbohydrate, high-fat ketogenic diet (KD) is an effective treatment for refractory epilepsy, a condition affecting more than one-third of epileptic individuals and defined by a failure to respond to existing anticonvulsant medications (Kwan and Brodie, 2000). However, despite its value for treating epilepsy and its increasing application to other disorders, including autism spectrum disorder, Alzheimer’s disease, metabolic syndrome and cancer (Stafstrom and Rho, 2012), use of the KD remains low due to difficulties with implementation, dietary compliance and adverse side effects (Freeman and Kossoff, 2010). Molecular targets are needed to develop viable clinical interventions for intractable epilepsy and other disorders for which the KD is beneficial. Many studies have proposed roles for ketone bodies, gamma-aminobutyric acid (GABA) modulation, and mitochondrial anaplerosis in mediating the neurological effects of the KD (Rogawski et al., 2016), but exactly how the KD confers beneficial effects on brain activity and behavior remains unclear.
The gut microbiota is a key intermediary between diet and host physiology; the species composition and function of the gut microbiota is critically shaped by diet, and nutrients made available to the host depend on microbial metabolism (Sonnenburg and Backhed, 2016). Diet-induced changes in the gut microbiota are reproducible and persistent (David et al., 2014), and as such, have lasting impacts on the host. Several diet-induced host pathologies are mediated by changes in the gut microbiota in mouse models, including symptoms of atherosclerosis in response to the carnitine-rich diet, undernutrition in response to the Malawian diet and abnormal social behavior in response to maternal high-fat diet (Buffington et al., 2016; Koeth et al., 2013; Smith et al., 2013).
The gut microbiota modulates several metabolic and neurological pathways in the host that could be relevant to KD-mediated seizure protection. The KD alters the composition of the gut microbiota in mice (Klein et al., 2016a; Newell et al., 2016), and ketosis is associated with altered gut microbiota in humans (David et al., 2014; Duncan et al., 2008). Interestingly, fasted mice that lack microbiota exhibit impaired hepatic ketogenesis and altered myocardial ketone metabolism compared to fasted mice that are conventionally-colonized (Crawford et al., 2009). The microbiota is also increasingly associated with changes in factors relevant to neurotransmission, including neurotransmitter signaling, synaptic protein expression, long termpotentiation and myelination, as well as a variety of complex host behaviors, including stress-induced, social and cognitive behaviors (Vuong et al., 2017). Notably, several clinical studies report that antibiotic treatment increases risk of status epilepticus or symptomatic seizures in epileptic individuals (Sutter et al., 2015), suggesting a possible role for the microbiota in mitigating seizure likelihood.
Based on emerging studies linking the gut microbiota to host responses to diet, metabolism, neural activity and behavior, we hypothesized that the gut microbiota impacts the anti-seizure effects of the KD. We show herein that the gut microbiota is necessary and sufficient for seizure protection in two mouse models of intractable epilepsy, and further identify cooperative interactions between two diet-associated bacteria that regulate levels of circulating dietary metabolites, brain neurotransmitters, and seizure incidence in mice.
RESULTS
The Ketogenic Diet Alters the Gut Microbiota
To test whether the microbiota plays a role in KD-mediated seizure protection, we first utilize the 6-Hz induced seizure model of refractory epilepsy, which involves low-frequency corneal stimulation to induce complex partial seizures reminiscent of human temporal lobe epilepsy. The 6-Hz model is resistant to several anti-epileptic drugs and used as a model of refractory epilepsy for investigational drug screening (Barton et al., 2001). The KD protects against 6-Hz seizures (Hartman et al., 2010; Samala et al., 2008), as indicated by the increased current intensity required to elicit a seizure in 50% of the subjects tested (CC50, seizure threshold).
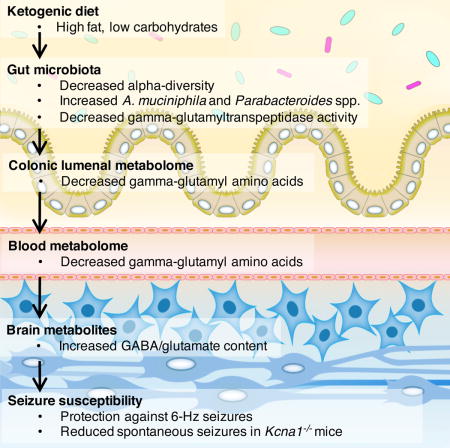
We fed conventionally-colonized (specific pathogen-free, SPF) Swiss Webster mice a 6:1 fat:protein KD or a vitamin- and mineral-matched control diet (CD) (Table S1). Compared to CD controls, mice fed the KD exhibit elevated seizure thresholds in response to 6-Hz stimulation (Figure 1A), decreased serum glucose (Figure 1B) and increased serum β-hydroxybutyrate (BHB; Figure 1C). There were no significant differences in food consumption or weight gain across CD vs. KD groups (Figure S1A).
Figure 1. The Ketogenic Diet Alters the Gut Microbiota and Protects Against 6-Hz Psychomotor Seizures.
(A) Seizure thresholds in response to 6-Hz stimulation in independent cohorts of mice fed the CD or KD for 2, 4, 8, 10 or 14 days (left). n = 8, 6, 9, 20, 6 (CD); 8, 7, 12, 21, 5 (KD). Behavior in representative cohort of seizure-tested mice at 14 days post dietary treatment (right). Yellow line at y=10 seconds represents threshold for scoring seizures, and yellow triangle at 24 mA denotes starting current per experimental cohort. n = 16.
(B) Levels of serum glucose in mice fed CD or KD for 2, 4, 8, 10 or 14 days. Data are normalized to serum glucose levels seen in SPF CD mice for each time point. n = 8, 5, 8, 8, 19 (CD); 8, 8, 8, 7, 19 (KD).
(C) Levels of serum BHB mice fed CD or KD for 2, 4, 8, 10 or 14 days. n = 8, 13, 8, 8, 37 (CD); 8, 16, 8, 7, 38 (KD).
(D) Principal coordinates analysis of weighted (left) and unweighted (right) UniFrac distance based on 16S rDNA profiling of feces from mice fed CD or KD for 0, 4, 8 or 14 days. n = 3 cages/group.
(E) Alpha diversity of fecal 16S rDNA sequencing data from mice fed CD or KD for 14 days. n = 3 cages/group.
(F) Taxonomic distributions of bacteria from fecal 16S rDNA sequencing data (left). n = 3 cages/group. Relative abundances of Akkermansia muciniphila and Parabacteroides (right). n = 3 cages/group.
Data are presented as mean ± SEM. Two-way ANOVA with Bonferroni (A–C, E), Kruskal-Wallis with Bonferroni (F): *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. n.s.=not statistically significant. SPF=specific pathogen-free, CD=control diet, KD=ketogenic diet, CC50=current intensity producing seizures in 50% of mice tested, BHB=beta-hydroxybutyrate, OTUs=operational taxonomic units. See also Figure S1 and Tables S1 and S2.
In addition to raising seizure thresholds, the KD alters the composition of the gut microbiota by 4 days post-dietary treatment (Figure 1D and Table S2). Decreased alpha-diversity is observed at each time point (Figures 1E and S1B) suggesting KD-induced losses of particular bacterial taxa. Notably, the KD substantially increases the relative abundance of Akkermansia muciniphila, from 2.8 ± 0.4% to 36.3 ± 2.8% (mean ± SEM), by 4 days and through 14 days of dietary treatment (Figures 1F, S1C and S1D). Parabacteroides, Sutterella and Erysipelotrichaceae are also significantly increased in KD-fed mice, whereas Allobaculum, Bifidobacterium and Desulfovibrio are increased in CD-fed mice (Figures 1F and S1D). These results reveal that the composition of the gut microbiota is rapidly and substantially altered in response to the KD.
The Gut Microbiota is Necessary and Sufficient for the Anti-Seizure Effects of the KD
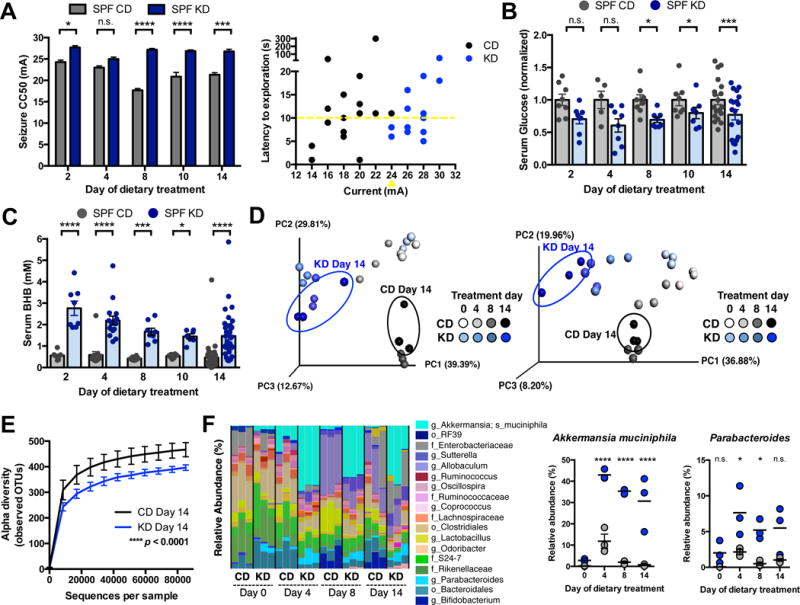
To determine whether the gut microbiota is necessary for the anti-seizure effects of the KD, we measured 6-Hz seizure thresholds in mice reared germ-free (GF) or treated with antibiotics (Abx). Compared to CD controls, SPF mice fed the KD for 14 days exhibit increased seizure thresholds and altered microbiota (Figure 1). These protective effects of the KD are abrogated in GF mice (Figure 2A) and Abx-treated SPF mice (Figure 2C), indicating that the gut microbiota is required for KD-mediated increases in seizure protection. Postnatal conventionalization of GF mice with the SPF gut microbiota restores seizure protection to levels seen in native SPF KD mice (Figure 2A), suggesting that the microbiota actively mediates seizure protection through pathways that are independent of pre-weaning developmental processes. Notably, microbial effects on seizure resistance do not correlate with changes in serum BHB or glucose levels (Figures 2B and 2D), and there are no significant differences between groups in levels of intestinal, liver, or brain BHB (Figure S2A–S2C). Consistent with this, previous studies report that BHB concentrations do not necessarily correlate with seizure protection, and ketosis may be necessary but not sufficient for KD-mediated seizure control (Bough and Rho, 2007). Overall, these data demonstrate that the gut microbiota is required for the anti-seizure effects of the KD in the 6-Hz seizure model and further suggest that gut microbes modulate seizure susceptibility through mechanisms that do not involve alterations in BHB levels.
Figure 2. The Microbiota is Required for the Anti-Seizure Effects of the Ketogenic Diet.
(A) Seizure thresholds in response to 6-Hz stimulation in SPF, GF or conventionalized GF mice fed CD or KD. n = 13, 18, 12, 6. (B) Serum BHB (left) and glucose (right) levels in SPF, GF or conventionalized GF mice fed CD or KD. n = 37, 38, 19, 8.
(C) Seizure thresholds in response to 6-Hz stimulation in SPF mice treated with vehicle or Abx pre-dietary treatment. n = 13, 18, 13.
(D) Serum BHB (left) and glucose (right) levels in SPF mice treated with vehicle or Abx predietary treatment. n = 18, 18, 19 (BHB); n = 12, 11, 11 (glucose).
Data are presented as mean ± SEM. One-way ANOVA with Bonferroni: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. n.s.=not statistically significant. SPF=specific pathogen-free, GF=germ-free, GF-conv=germ-free conventionalized with SPF microbiota, CD=control diet, KD=ketogenic diet, CC50=current intensity producing seizures in 50% of mice tested. BHB=beta-hydroxybutyrate, veh=vehicle, Abx=antibiotics (ampicillin, vancomycin, neomycin, metronidazole). See also Figure S2.
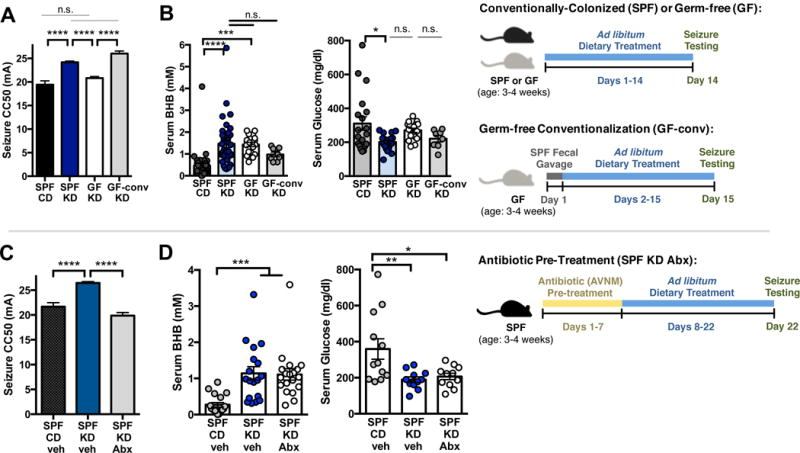
To determine whether specific bacterial taxa mediate seizure protection in response to the KD, Abx-treated SPF mice were colonized with select KD-associated bacteria, fed the KD and then tested for 6-Hz seizures. A. muciniphila and Parabacteroides were selected as the taxa most highly enriched by the KD. Mice were gavaged with 109 cfu bacteria: i) A. muciniphila; ii) 1:1 ratio of Parabacteroides merdae and P. distasonis, as species with highest homology to the Parabacteroides operational taxonomic unit sequences that were enriched by the KD (Figure 1D and Table S2); or iii) 2:1:1 ratio of A. muciniphila, P. merdae and P. distasonis. At 14 days after oral gavage, mice treated with A. muciniphila harbored 43.7 ± 0.4% relative abundance of A. muciniphila (Figure S3A). Mice gavaged with Parabacteroides harbored 70.9 ± 4.0% relative abundance, and mice gavaged with both taxa harbored 49.0 ± 4.1% A. muciniphila and 22.5 ± 5.4% Parabacteroides. Consistent with this, colonic sections from mice treated with A. muciniphila and Parabacteroides exhibit increased hybridization of the A. muciniphila probe MUC1437 (Derrien et al., 2008) and the Bacteroides and Parabacteroides probe BAC303 (Manz et al., 1996) (Figure 3A). There were no significant differences in weight, serum glucose levels, or bacterial enrichment across mice fed CD vs. KD (Figures S3A–S3C). Consistent with our previous observation (Figures 2B, 2D), serum BHB was similarly elevated in KD-fed groups, independent of colonization status (Figure S3C). These data reveal that microbiota depletion by Abx treatment followed by oral gavage of exogenous bacteria results in their persistent intestinal enrichment by 14 days post-inoculation.
Figure 3. KD-Associated Bacteria Sufficiently Mediate the Anti-Seizure Effects of the Ketogenic Diet.
(A) Fluorescence in situ hybridization for A. muciniphila (MUC1437) and select Bacteroides and Parabacteroides, including P. merdae and P. merdae (BAC303) in colonic lumen from SPF mice fed CD, SPF mice fed KD or A. muciniphila and Parabacteroides-enriched mice fed KD. Scale bar = 25 um. Dotted lines indicate the borders of the intestinal epithelium. n = 3.
(B) Seizure thresholds in response to 6-Hz stimulation in SPF mice pre-treated with vehicle or Abx, and colonized with Parabacteroides, Akkermansia muciniphila, both, or Bifidobacterium longum (left). n = 13, 18, 15, 6, 8, 5, 5. Behavior in representative cohort of seizure-tested mice (right). Yellow line at y=10 seconds represents threshold for scoring seizures, and yellow triangle at 24 mA denotes starting current per experimental cohort. n = 12, 16, 8, 25.
(C) Seizure thresholds in response to 6-Hz stimulation in GF mice colonized with Parabacteroides and/or A. muciniphila (top). n = 15, 4, 9, 9. Behavior in seizure-tested mice (bottom). n = 17, 19.
Data are presented as mean ± SEM. One-way ANOVA with Bonferroni: **p < 0.01, ***p < 0.001, ****p < 0.0001. SPF=specific pathogen-free, GF=germ-free, CD=control diet, KD=ketogenic diet, CC50=current intensity producing seizures in 50% of mice tested, veh=vehicle, Abx=pre-treated with antibiotics (ampicillin, vancomycin, neomycin, metronidazole), Pb=Parabacteroides (P. merdae and P. distasonis), Akk=Akkermansia muciniphila, AkkPb=A. muciniphila, P. merdae and P. distasonis, Bf=Bifidobacterium longum. See also Figure S3.
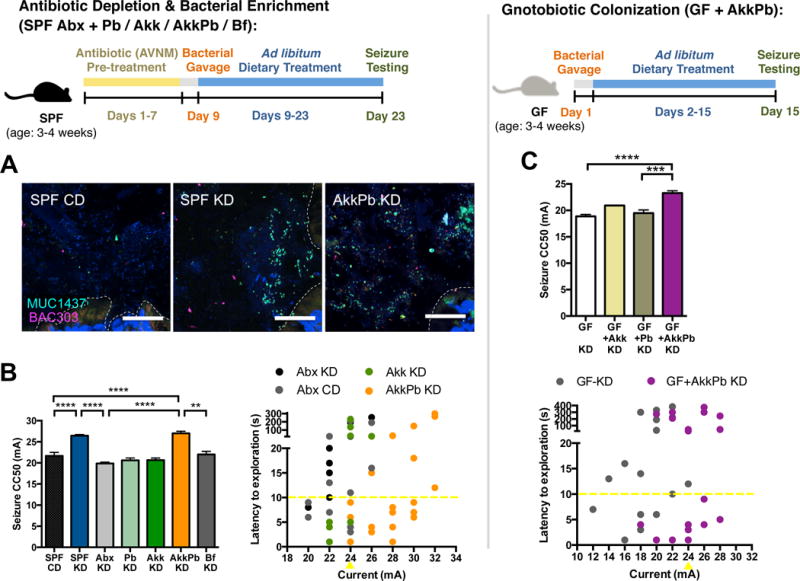
We next examined seizure susceptibility in mice with selective enrichment of KD-associated gut bacteria. Treatment with the KD alone elevated seizure thresholds by 24.5% from 19.4 ± 0.8 mA, in SPF CD mice, to 24.2 ± 0.3 mA, in SPF KD mice, whereas Abx treatment of KD-fed mice prevented this anti-seizure effect (Figure 3B). Co-administration of A. muciniphila and Parabacteroides restores seizure protection in Abx-treated mice fed the KD, raising thresholds by 36.0%, from 19.9 ± 0.3 mA, in Abx KD mice, to 27.0 ± 0.5 mA, in AkkPb KD mice (Figure 3B). This protective effect is specific to A. muciniphila with P. merdae, as mice gavaged with A. muciniphila and P. distasonis exhibit no restoration of seizure protection (Figure S3D). There is no significant increase in seizure threshold after enrichment of either A. muciniphila or Parabacteroides alone (Figure 3B), indicating that both taxa are required for mediating the anti-seizure effects of the diet. There is also no effect of treatment with Bifidobacterium longum (Figure 3B), as a negative control taxon that was enriched in CD-fed mice (Figure S1D). Moreover, co-colonization of A. muciniphila and Parabacteroides in GF mice promotes seizure protection in response to the KD, when compared to GF, Parabacteroides-monocolonized or A. muciniphila-monocolonized mice (Figure 3C), suggesting that A. muciniphila and Parabacteroides together raise seizure thresholds in the absence of other indigenous gut microbes. Overall, these findings reveal that A. muciniphila and Parabacteroides increase in response to the KD and mediate its protective effect in the 6-Hz seizure model.
The Gut Microbiota Confers Seizure Protection to Mice Fed the CD
The previous experiments examine seizure protection in mice fed the KD. To determine whether KD-associated gut microbes also confer anti-seizure effects to mice fed the CD, Abx-treated mice were transplanted with CD vs. KD microbiota from SPF mice, fed the CD and tested for their susceptibility to 6-Hz seizures after 4 days of dietary treatment. Abx-treated mice were used to mimic the GF condition and preclude any confounding developmental effects of GF rearing (Reikvam et al., 2011). Day 4 was selected based on i) the ability of the KD to induce significant microbiota changes by that time (Figures 1D–1F) and ii) evidence that the KD microbiota exhibits incomplete reversion to CD profiles at 4 days after switching from KD to CD (Figure S4A). Mice transplanted with a CD microbiota and fed KD display increased seizure threshold compared to mice transplanted with the same CD microbiota and fed the CD (Figure 4A). This is consistent with the ability of the KD to promote seizure protection. Importantly, transplant of the KD microbiota raises seizure thresholds in mice fed CD, as compared to controls transplanted with the CD microbiota. This suggests that the KD microbiota confers seizure protection even in mice fed the CD. Notably, this seizure protection is abrogated after complete reversion of the KD microbiota to CD profiles on day 28 (Figure S4B), suggesting that persistent interactions between the KD microbiota, diet and neuronal activity are required. Similar anti-seizure effects are seen after enriching A. muciniphila and Parabacteroides in Abx-treated mice fed CD, as compared to controls colonized with Parabacteroides, A. muciniphila or B. longum alone (Figure 4B). However, increases in seizure threshold in SPF CD mice treated with Abx alone and GF CD mice relative to SPF CD controls confound interpretation of these results.
Figure 4. KD-Associated Bacteria Sufficiently Confer Seizure Protection in Mice Fed the Control Diet.
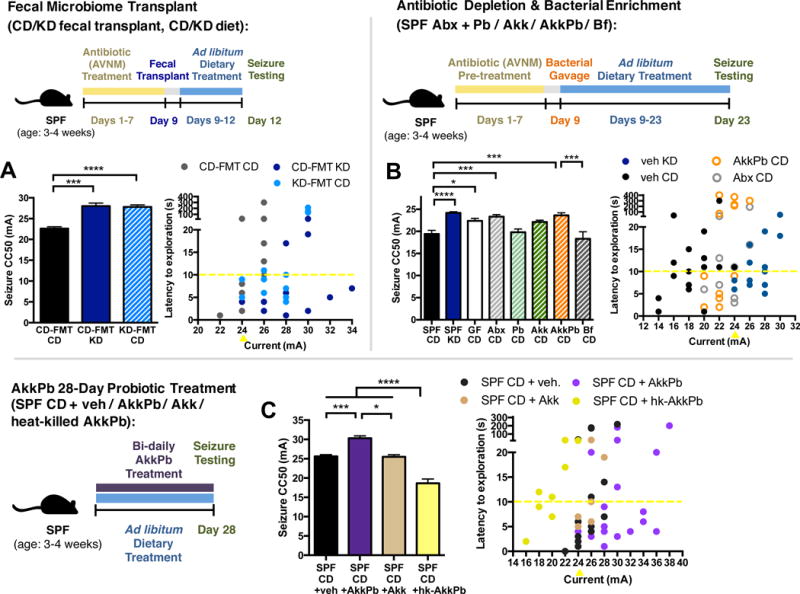
(A) Seizure thresholds in response to 6-Hz stimulation in Abx-treated SPF transplanted with the CD microbiota (CD-FMT) or KD microbiota (KD-FMT) and fed the CD or KD (left). n = 6, 5, 5. Behavior in representative cohort of seizure-tested mice (right). Yellow line at y=10 seconds represents threshold for scoring seizures, and yellow triangle at 24 mA denotes starting current per experimental cohort. n = 12.
(B) Seizure thresholds in response to 6-Hz stimulation in SPF mice pre-treated with vehicle or Abx, and colonized with Parabacteroides, Akkermansia muciniphila, both, or Bifidobacterium longum (left). n = 13, 18, 8, 9, 8, 6, 6. Behavior in representative cohort of seizure-tested mice (right). n = 16, 16, 12, 12.
(C) Seizure thresholds in response to 6-Hz stimulation in SPF mice orally gavaged with A. muciniphila, P. merdae and P. distasonis, A. muciniphila alone, or heat-killed A. muciniphila and Parabacteroides. (left). n = 6, 6, 4, 3. Behavior in seizure-tested mice (right). n = 15, 20, 8, 8. Data are presented as mean ± SEM. One-way ANOVA with Bonferroni: *p < 0.05, ***p < 0.001, ****p < 0.0001. SPF=specific pathogen-free, CD=control diet, KD=ketogenic diet, CC50=current intensity producing seizures in 50% of mice tested, CD-FMT=transplanted with CD microbiota, KD-FMT=transplanted with KD microbiota, veh=vehicle, Abx=pre-treated with antibiotics (ampicillin, vancomycin, neomycin, metronidazole), Pb=Parabacteroides (P. merdae and P. distasonis), Akk=Akkermansia muciniphila, AkkPb=A. muciniphila, P. merdae and P. distasonis, Bf=Bifidobacterium longum, hk-AkkPb=heat-killed A. muciniphila, P. merdae and P. distasonis. See also Figure S4.
To clarify this uncertainty, we used a bacterial treatment approach to investigate whether exogenous treatment with A. muciniphila and Parabacteroides confers anti-seizure effects in mice fed CD. SPF CD mice were gavaged bi-daily for 28 days with 109 cfu A. muciniphila and Parabacteroides, or with vehicle. This bacterial treatment increased seizure thresholds relative to vehicle-gavaged controls (Figure 4C). Consistent with our experiments on mice fed the KD (Figure 3), this seizure protection is not observed in animals treated with A. muciniphila alone, revealing that co-administration of A. muciniphila and Parabacteroides is required for seizure protection (Figure 4C). Moreover, treatment with heat-killed bacteria decreases seizure thresholds compared to vehicle-treated controls, suggesting that viable bacteria are necessary for conferring anti-seizure effects, and release of bacterial cell surface and/or intracellular factors promotes sensitivity to 6-Hz seizures. Persistent exposure to A. muciniphila and Parabacteroides is required, as increases in seizure thresholds were lost after ceasing treatment for 21 days (Figure S4C). In addition, seizure protection was not observed in mice treated for only 4 days (Figure S4D), suggesting that long-term exposure is required. Taken together, these findings reveal that fecal transplant of the KD microbiota and long-term bacterial treatment with the KD-associated taxa A. muciniphila and Parabacteroides confer protection against 6-Hz seizures in mice fed the CD.
KD-Associated Bacteria Reduce Tonic-Clonic Seizures in Kcna1−/− Mice
Epilepsy is a heterogeneous disorder with diverse clinical presentations. The 6-Hz seizure model for pharmacoresistant epilepsy examines acute, electrically-induced seizures and is widely used for testing the efficacy of the KD and new anti-epileptic drugs (Hartman et al., 2010; Samala et al., 2008). While a powerful tool for studying fundamental influences on seizure susceptibility, the model exhibits low construct validity for human epilepsy and conveys limited information on seizure severity and form. To determine whether our findings from the 6-Hz model also apply to different seizure types and etiologies, we further tested roles for the microbiota in modulating generalized tonic-clonic seizures in the Kcna1−/− mouse model for temporal lobe epilepsy and sudden unexpected death in epilepsy (SUDEP). Kcna1−/− mice harbor a null mutation in the voltage-gated potassium channel Kv1.1 alpha subunit, mimicking associations of human KCNA1 gene variants with epilepsy, episodic ataxia and SUDEP (Scheffer et al., 1998; Zuberi et al., 1999). Kcna1−/− mice develop severe spontaneous recurrent seizures, which are reduced 54% by the KD (Fenoglio-Simeone et al., 2009).
Kcna1−/− SPF C3HeB/FeJ mice were treated with Abx or vehicle for 1 week, gavaged with vehicle or A. muciniphila and Parabacteroides, and fed KD or CD for 3 weeks. Seizure frequency and duration were recorded by EEG over 3 days, where electrographic seizures were identified based on characteristic epileptiform spike patterns (Figure 5C). Consistent with findings from the 6-Hz seizure model (Figure 1F), the KD significantly increases A. muciniphila and Parabacteroides in Kcna1−/− mice (Figure 5A and 5B). The degree of diet-induced enrichment of these taxa is less than that seen in the 6-Hz model, which could be due to an effect of host genotype on baseline microbiota composition and responses to KD (Klein et al., 2016b). Nonetheless, we observed decreases in seizure incidence and duration in KD-fed Kcna1−/− mice compared to CD-fed Kcna1−/− controls (Figure 5D). Interestingly, Kcna1−/− mice that were pre-treated with Abx exhibit a significant increase in seizures per day and total seizure duration compared to vehicle-treated, KD-fed Kcna1−/− controls. There is no significant difference in average spike frequency or average duration per seizure (Figure S5A–S5D) suggesting a primary effect of Abx treatment and depletion of the gut microbiota on spontaneous seizure occurrence rather than seizure form (Figure S5A). Moreover, colonization of Abx-treated Kcna1−/− mice with A. muciniphila and Parabacteroides reduces seizure frequency and total duration of seizures toward levels seen in vehicle-treated controls (Figure 5D). There were no significant differences in weight gain or food consumption between mice fed the KD vs. CD (Figure S5E). In contrast to a previous report that used a different KD formulation (Simeone et al., 2016), we observed no differences in survival across groups (Figure S5F). Taken together, these findings support the notion that the gut microbiota mediates the anti-seizure effects of the KD across varied seizure types and mouse models.
Figure 5. KD-Associated Bacteria Mediate Protection Against Tonic-Clonic Seizures in Response to the Ketogenic Diet.
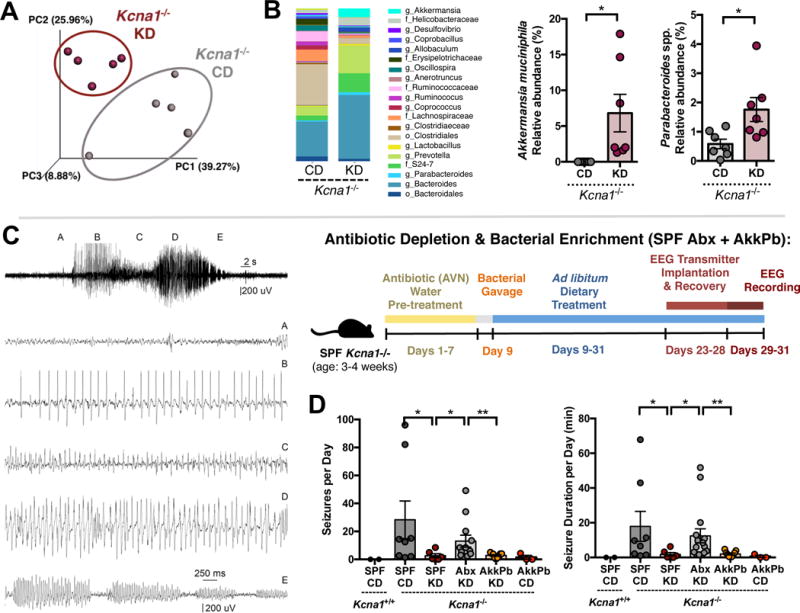
(A) Principal coordinates analysis of weighted UniFrac distances based on 16S rDNA profiling of feces Kcna1−/− mice fed CD or KD for 14 days. n = 7 cages/group.
(B) Average taxonomic distributions of bacteria from fecal 16S rDNA sequencing data (left). Relative abundances of Akkermansia muciniphila and Parabacteroides (right). n = 7 cages/group.
(C) Representative EEG trace showing stages used to define seizures quantified in (D).
(D) Average number of seizures per day (left) and total duration of seizures per day (right) in SPF Kcna1−/− mice treated with vehicle or Abx, colonized with A. muciniphila and Parabacteroides spp. or nothing, and fed CD or KD. n = 2, 8, 6, 12, 9, 3.
Data are presented as mean ± SEM. Kruskal-Wallis with Bonferroni (A,B), Mann-Whitney (D): *p < 0.05. SPF=specific pathogen-free, CD=control diet, KD=ketogenic diet, veh=vehicle, Abx=pre-treated with antibiotics (ampicillin, vancomycin, neomycin, metronidazole), AkkPb=A. muciniphila, P. merdae and P. distasonis. See also Figure S5.
The Microbiota Modulates Gut, Serum and Brain Metabolomes
Based on the role of the gut microbiota in modulating effects of the KD on seizure occurrence, we hypothesized that microbial dietary metabolism regulates secondary metabolites that impact seizure susceptibility. We utilized metabolomic profiling to identify candidate microbiota-dependent molecules in colonic lumenal contents and sera of SPF mice fed CD, SPF mice fed KD, Abx-treated SPF fed KD, and A. muciniphila- and Parabacteroides-enriched mice fed KD (Figures 6A and S6A). 622 metabolites, spanning amino acid, carbohydrate, lipid, nucleotide, peptide and xenobiotic supergroups, were detected in mouse colonic contents, and 670 metabolites were detected in mouse sera (Tables S4 and S5). Metabolomic profiles in colonic contents and sera discriminate seizure-protected groups from seizure-susceptible groups, with a predictive accuracy of 94% for colonic lumenal metabolites and 87.5% for serum metabolites (Figure 6B). Interestingly, the majority of metabolites that contribute highly to group discrimination are relevant to amino acid metabolism, including derivatives of lysine, tyrosine and threonine. In addition, we observed widespread decreases in subsets of ketogenic gamma-glutamylated amino acids—gamma-glutamyl (GG)-leucine, GG-lysine, GG-threonine, GG-tryptophan and GG-tyrosine— in both colonic lumenal contents (Figure 6C) and sera (Figure 6D) from seizure-protected compared to seizure-susceptible groups. Gamma-glutamylated forms of the amino acids were particularly affected (Figures 6C and 6D), as compared to their unmodified counterparts (Figures S6B and S6C). This suggests that the gut microbiota modulates gamma-glutamylation itself or selective metabolism of ketogenic GG-amino acids, and that increased ketogenic GG-amino acids are associated with seizure susceptibility. Overall, these findings indicate that the gut microbiota modulates intestinal and systemic metabolomic responses to the KD, and further reveal an association between KD-induced seizure protection and microbiota-dependent alterations in levels of ketogenic GG-amino acids.
Figure 6. Reductions in Peripheral Gamma-Glutamyl Amino Acids and Increases in Hippocampal GABA/Glutamate Ratios are Associated with Diet- and Microbiota-Dependent Seizure Protection.
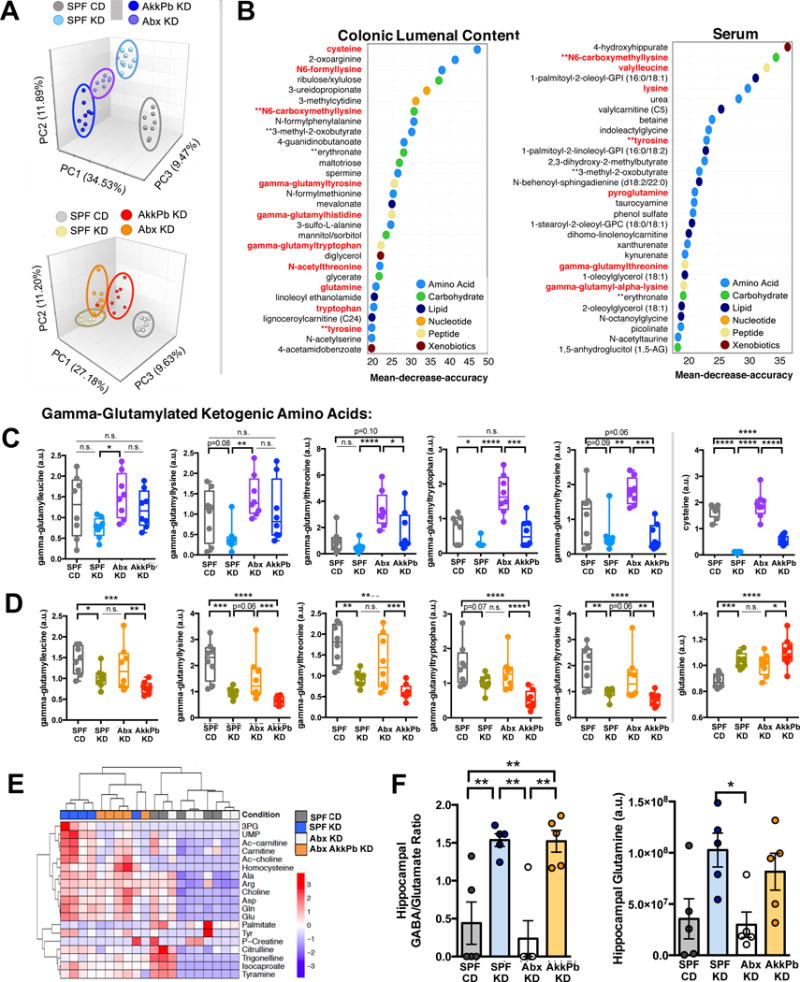
(A) Principal components analysis of colonic lumenal metabolites (top) and serum metabolites (bottom) from SPF mice fed CD, SPF mice fed KD, Abx-treated mice fed KD, and AkkPb-colonized mice fed KD. n = 8 cages/group.
(B) Biochemicals, identified by Random Forests classification of colonic lumenal (left) and serum (right) metabolomes, that contribute most highly to the discrimination of seizure-protected (SPF CD, Abx KD) from seizure-susceptible (SPF KD, AkkPb KD) groups. n = 8 cages/group.
(C) Levels of gamma-glutamylated amino acids and cysteine in colonic lumenal contents from SPF mice fed CD, SPF mice fed KD, Abx-treated mice fed KD, and AkkPb-colonized mice fed KD. n = 8 cages/group.
(D) Levels of gamma-glutamyl amino acids and glutamine in sera from SPF mice fed CD, SPF mice fed KD, Abx-treated mice fed KD, and AkkPb-colonized mice fed KD. n = 8 cages/group.
(E) Heatmap of metabolites identified in hippocampi of SPF mice fed CD, SPF mice fed KD, Abx-treated mice fed KD, and AkkPb-colonized mice fed KD. n = 5.
(F) Levels of GABA/glutamate (left) and glutamine (right) in hippocampi of SPF mice fed CD, SPF mice fed KD, Abx-treated mice fed KD, and AkkPb-colonized mice fed KD. n = 5.
Data are presented as mean ± SEM. Two-way ANOVA contrasts (A-D), One-way ANOVA with Bonferroni (E-F): *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. n.s.=not statistically significant. CD=control diet, KD=ketogenic diet, SPF=specific pathogen-free, veh=vehicle, Abx=pre-treated with antibiotics (ampicillin, vancomycin, neomycin, metronidazole), AkkPb=A muciniphila, P. merdae and P. distasonis, a.u.=arbitrary units. See also Figure S6 and Tables S3–S6.
The brain relies on active import of essential amino acids to fuel neurotransmitter biosynthesis, and as such, is sensitive to fluctuations in peripheral amino acid bioavailability (Smith, 2000). Peripheral amino acids serve as substrates for the synthesis of GABA and glutamate through anaplerotic refilling of Kreb’s cycle intermediates, or indirectly through carbon dioxide fixation stimulated by hyperammonemia (Cooper and Jeitner, 2016). GG-amino acids, in particular, are hypothesized to exhibit increased transport properties compared to non-gamma-glutamylated forms (Castellano and Merlino, 2012). Based on our data revealing diet- and microbiota-dependent alterations in serum ketogenic amino acids, links between amino acid importation and brain GABA levels, and prevailing theories that GABA contributes to the antiseizure effects of the KD (Yudkoff et al., 2001), we examined bulk levels of GABA and glutamate in the hippocampus, a brain region important for the propagation of seizure activity. Hippocampal metabolite profiles distinguished samples from seizure-protected vs. seizure-susceptible mice (Figure 6E). Both GABA and glutamate levels are increased in diet- and microbiome-mediated seizure-protection groups, with GABA more substantially elevated compared to glutamate (Figure S6D). Consistent with this, hippocampal GABA/glutamate ratios are significantly increased in KD-fed SPF mice compared to CD-fed controls (Figure 6F and Table S6). These increases are abrogated in Abx-treated mice fed KD and restored after enrichment of A. muciniphila and Parabacteroides. Similar changes are seen for hippocampal levels of glutamine, a precursor of glutamate and GABA. Overall, these results reveal diet- and microbiota-dependent regulation of hippocampal GABA and glutamate levels in mice.
Bacterial Gamma-Glutamylation Impacts Seizure Susceptibility
Gamma-glutamylated forms of amino acids are generated by transpeptidation of GG moieties from glutathione onto amino acids. To determine whether gamma-glutamylation of amino acids impacts seizure susceptibility, we gavaged SPF CD mice for 3 days with GGsTop, a selective irreversible inhibitor of GGT. SPF CD mice treated with GGsTop exhibit increases in 6-Hz seizure thresholds toward levels seen in SPF KD mice (Figure 7A). Similarly, EEG recordings of CD-fed SPF Kcna1−/− mice treated with GGsTop display a significant decrease in average duration per seizure (Figure S5G). This reveals that peripheral inhibition of gamma-glutamylation and restriction of GG-amino acids promotes seizure protection, consistent with decreases in ketogenic GG-amino acids seen in colonic lumenal content and sera from seizure-protected groups compared to seizure-susceptible controls.
Figure 7. The Ketogenic Diet and Bacterial Cross-Feeding Reduces Gamma Glutamyltranspeptidase (GGT) Activity, and GGT Inhibition Sufficiently Confers Seizure Protection.
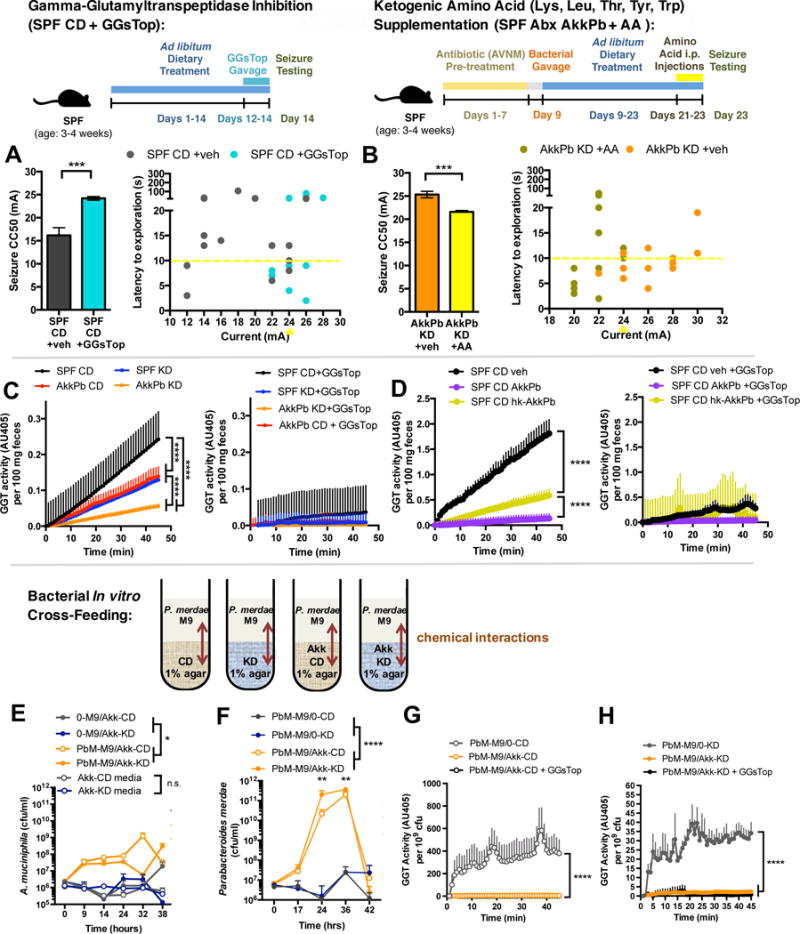
(A) 6-Hz seizure thresholds in response to oral gavage with the GGT inhibitor, GGsTop, in SPF mice fed CD (left). n = 6, 9. Behavior in seizure-tested mice (right). Yellow line at y=10 seconds represents threshold for scoring seizures, and yellow triangle at 24 mA denotes starting current per experimental cohort. n = 16.
(B) 6-Hz seizure thresholds in response to supplementation with ketogenic amino acids in Abx-treated SPF mice enriched for A. muciniphila and Parabacteroides (left). n = 5, 6. Behavior in seizure-tested mice (right). n = 12.
(C) Total GGT activity per 100 mg feces from SPF CD, SPF KD, AkkPb KD, or AkkPb CD mice (left), and inhibition by GGsTop (right). n = 5.
(D) Total GGT activity per 100 mg feces from SPF CD animals treated with vehicle, A. muciniphila and Parabacteroides probiotic, or heat-killed bacteria for bi-daily for 28 days (left), and inhibition by GGsTop (right). n = 5.
(E) Levels of live A. muciniphila (Akk) after incubation in CD vs KD culture media or in CD or KD agar overlaid with M9 minimal media containing live P. merdae (PbM) or no bacteria (0). n = 3.
(F) Levels of live PbM after incubation in M9 minimal media overlaid on CD or KD agar containing Akk or no bacteria (0). n = 5.
(G) GGT activity in P. merdae grown in M9 media overlaid on CD agar containing A. muciniphila or no bacteria at t=24 hrs, and inhibition of GGT activity by GGsTop. n = 5.
(H) GGT activity in P. merdae grown in M9 media overlaid on KD agar containing A. muciniphila or no bacteria at t=24 hrs, and inhibition of GGT activity by GGsTop. n = 5.
Data are presented as mean ± SEM. Students t-test (A,B), Two-way ANOVA with Bonferroni (C, D), One-way ANOVA with Bonferroni (E-H): *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. SPF=specific pathogen-free, CD=control diet, KD=ketogenic diet, CC50=current intensity producing seizures in 50% of mice tested, AA=amino acids, veh=vehicle, Abx=pre-treated with antibiotics (ampicillin, vancomycin, neomycin, metronidazole), AkkPb=A. muciniphila, P. merdae and P. distasonis, GGsTop=GGT inhibitor, PbM=Parabacteroides merdae, Akk=Akkermansia muciniphila, M9=minimal media, GGT=gamma-glutamyltranspeptidase, AU=absorbance units. See also Figure S7.
Importantly, to determine whether restriction of amino acids, rather than catabolism of glutathione, is necessary for the anti-seizure effects of the KD microbiota, we supplemented KD-fed A. muciniphila and Parabacteroides-enriched mice with the ketogenic amino acids leucine, lysine, threonine, tryptophan and tyrosine, and then tested for 6-Hz seizures. Elevating systemic levels of ketogenic amino acids decreases seizure thresholds to levels seen in vehicle-treated SPF CD controls (Figure 7B). This suggests that restriction of peripheral ketogenic amino acids is necessary for mediating microbiota- and KD-dependent increases in seizure resistance.
Both host cells and particular bacterial species exhibit GGT activity (van der Stel et al., 2015). To gain insight into whether the KD and interactions between A. muciniphila and Parabacteroides suppress bacterial gamma-glutamylation in vivo, we measured GGT activity in fecal samples collected from SPF or A. muciniphila and Parabacteroides-enriched mice fed the CD or KD. Feeding SPF mice with KD decreases fecal GGT activity compared to CD controls (Figure 7C). Similar reduction in fecal GGT activity is seen after enriching A. muciniphila and Parabacteroides in CD-fed mice. Moreover, enriching A. muciniphila and Parabacteroides and feeding with KD further decreases fecal GGT activity relative to that seen in SPF KD and SPF CD mice. Exposing all fecal samples to the GGT inhibitor GGsTop eliminates the detected signals, confirming that the measurements reflect GGT activity. Consistent with this, treatment of CD-fed SPF mice with A. muciniphila and Parabacteroides decreases fecal GGT activity relative to vehicle-treated controls and mice treated with heat-killed bacteria (Figure 7D). Overall, these data reveal that enriching for or exogenous treatment with A. muciniphila and Parabacteroides reduces fecal GGT activity, which could explain the low levels of colonic and serum GG-amino acids observed in seizure-protected mice.
To explore whether bacterial gamma-glutamylation is affected by interactions between A. muciniphila and Parabacteroides, we measured GGT activity in bacteria grown in an in vitro cross-feeding system (Flynn et al., 2016). Since A. muciniphila does not exhibit GGT activity, we focused particularly on P. merdae, where GGT activity was ablated by GGsTop (Figure S7A). When A. muciniphila is embedded in a CD- or KD-based agar, and P. merdae is overlaid in M9 minimal media over the agar, both bacteria exhibit enhanced growth (Figures 7E and 7F), suggesting that A. muciniphila liberates soluble factors to enable P. merdae growth and in turn P. merdae enhances A. muciniphila growth. This cooperative interaction could contribute to the endogenous enrichment of both A. muciniphila and Parabacteroides in KD-fed mice (Figure 1F). Pilot experiments revealed no growth of A. muciniphila in M9 media when overlaid on P. merdae embedded in KD or CD agar, suggesting that A. muciniphila cannot rely solely on crossfeeding from P. merdae to persist. Interestingly, P. merdae exhibits high GGT activity that is eliminated by the addition of A. muciniphila embedded in CD or KD agar (Figures 7G and 7H), which aligns with the decreases in fecal GGT activity and GG amino acid levels observed after KD bacterial enrichment in vivo. Findings from these experiments raise the question of whether there is physiological function of restricting GGT activity in abundant P. merdae as opposed to restricting overall growth of P. merdae. To determine whether reduction of GGT activity in P. merdae affects A. muciniphila growth, we pre-treated P. merdae with vehicle or GGsTop to pharmacologically inhibit GGT activity prior to testing in the cross-feeding assay. A. muciniphila exposed to P. merdae that was pre-treated with GGsTop exhibits increased growth at 24 hours after incubation as compared to A. muciniphila exposed to vehicle-treated P. merdae (Figure S7B). Taken together, these findings suggest that A. muciniphila is capable of metabolizing components from the KD and CD diet to support P. merdae growth, and that this cooperative interaction reduces GGT activity. In turn, reductions in GGT activity in P. merdae promote A. muciniphila growth. This is consistent with our finding that the KD increases intestinal relative abundance of both A. muciniphila and Parabacteroides, and that enrichment of A. muciniphila and Parabacteroides reduces fecal GGT activity, colonic lumenal GG-amino acids and serum GG-amino acids in vivo. Overall, results from this study reveal that the KD alters the gut microbiota, promoting select microbial interactions that reduce bacterial gamma-glutamylation activity, decrease peripheral GG-amino acids, elevate bulk hippocampal GABA/glutamate ratios and protect against seizures.
DISCUSSION
The microbiota plays a key role in host digestion, metabolism and behavior, but whether microbial responses to diet also impact neuronal activity is poorly understood. Here we demonstrate that the KD alters the gut microbiota across two seizure mouse models and that changes in the microbiota are necessary and sufficient for conferring seizure protection. Several clinical studies link antibiotic treatment to increased risk of status epilepticus or symptomatic seizures in epileptic individuals (Sutter et al., 2015). Prolonged treatment with metronidazole can provoke convulsions (Beloosesky et al., 2000), and ampicillin exposure is associated with elevated seizure risk (Hornik et al., 2016). Penicillin and other β-lactams are hypothesized to directly reduce GABAergic inhibition, but whether microbiota depletion contributes to increases in seizure frequency is not clear. Results from our study reveal that microbiota depletion via high-dose antibiotic treatment raises seizure susceptibility and incidence in response to the KD in both wildtype and Kcna1−/− mice. These effects of antibiotic treatment are abrogated by recolonization with gut bacteria, suggesting that links between antibiotic use and seizure incidence in humans could be mediated by the microbiota. Future investigation is warranted to determine whether human epilepsy is associated with microbial dysbiosis and whether antibiotic exposure in epileptic individuals impacts response to the KD.
We observe in both Taconic Swiss Webster and Jackson C3HeB/FeJ Kcna1−/− mice that the KD reduces gut bacterial alpha diversity, while elevating relative abundance of A. muciniphila and Parabacteroides. Similar diet-induced increases in A. muciniphila are observed during fasting in humans (Dao et al., 2016a; Remely et al., 2015). A. muciniphila and Parabacteroides are also associated with increased ketosis (David et al., 2014) and metabolic improvement in humans (Everard et al., 2013). One study reports changes in the gut microbiota in response to the KD in BTBRT+tf/ and C57Bl/6J mice, where levels of Akkermansia were correlated with levels of serum glutamate, lactate, taurine and sarcosine (Klein et al., 2016b). However, different taxonomic shifts were observed, highlighting that the KD-induced microbiota likely depends on host genetics and baseline microbiota profiles. Indeed, different species, strains and even cohorts of animals are known to exhibit microbiota profiles that vary in taxonomic membership but are functionally redundant, raising the question of whether there are additional taxa that also perform similarly to A. muciniphila and Parabacteroides in our study. Further research is needed to determine effects of the KD on microbiome profiles in individuals with refractory epilepsy and whether particular taxonomic changes correlate with seizure severity.
Amino acids are transported across the blood-brain barrier (BBB) and serve as nitrogen donors for glutamate and GABA biosynthesis (Yudkoff et al., 2001). GG-amino acids, in particular, are reported to exhibit increased transport properties, where the gamma-glutamyl moiety promotes translocation across lipid barriers (Castellano and Merlino, 2012). Our data suggest that KD- and microbiota-related restrictions in GG-amino acids are important for seizure protection, which aligns with previous studies linking GGT activity to altered seizure severity. In a study of 75 epileptic patients, high serum GGT activity was observed in 84.5% of the patients compared to controls (Ewen and Griffiths, 1973). In a rat seizure model, GGT activity was increased after 5 consecutive daily electroshock deliveries (Eraković et al., 2001). Decreases in various peripheral amino acids are associated with KD-mediated seizure suppression in animals and humans (Sariego-Jamardo et al., 2015). Previous studies also highlight KD-induced increases in brain GABA in animal models (Calderon et al., 2017) and in humans (Dahlin et al., 2005; Wang et al., 2003). Future research is needed to determine whether peripheral amino acid restriction alters brain GABA/glutamate metabolism.
The gut microbiota can impact levels of various neuroactive molecules in the periphery and in the brain itself (Vuong et al., 2017). We find that diet- and microbiota-dependent seizure protection is associated with elevations in bulk GABA relative to glutamate content in the hippocampus (Figure 6F). Future studies are needed to determine whether other brain regions are similarly affected, and whether GABA localized particularly to neuronal synapses or intracellular vesicles are also modulated by the gut microbiota. Consistent with a role for the gut microbiota in modulating brain GABA levels, a previous study reveals that dietary fermentation by the gut microbiota modulates GABA levels in hypothalamic extracts (Frost et al., 2014). In addition, chronic Lactobacillus treatment elevates GABA levels in hippocampal and prefrontal cortex as detected by magnetic resonance spectroscopy (Janik et al., 2016). While our study examines microbial and metabolic mechanisms underlying how the gut microbiota influences seizure outcomes, further interrogation of the precise neurological mechanisms underlying the anti-seizure effects of the KD and KD-associated microbiota is needed. Of particular interest is the question of whether the gut microbiota modulates seizure susceptibility and incidence via alterations in excitatory/inhibitory balance and neurotransmission in particular neural circuits.
Overall, our study reveals a novel role for the gut microbiota in mediating and conferring seizure protection in two mouse models for refractory epilepsy. While the results lend credence to future research examining the gut microbiota in human epilepsy, several additional studies are needed to determine whether microbe-based treatments can be safely and effectively applied for clinical amelioration of seizure severity and incidence.
STAR★METHODS
Detailed methods are provided and include the following:
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and Virus Strains | ||
| Akkermansia muciniphila | ATCC | ATCC BAA845 |
| Parabacteroides merdae | ATCC | ATCC 43184 |
| Parabacteroides distasonis | ATCC | ATCC 8503 |
| Bifidobacterium longum | ATCC | ATCC 15707 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| GGsTop, 3-[[(3-amino-3-carboxypropyl) methoxyphosphinyl]oxy]benzeneacetic acid | Tocris Bioscience | 4452; CAS: 926281-37-0 |
| Mucin from porcine stomach, type III | Sigma-Aldrich | M1778; CAS: 84082-64-4 |
| L-gamma-glutamyl-3-carboxy-4-nitroanilide | Gold Bio | G-380-1; CAS: 63699-78-5 |
| 4 kDa FITC-dextran | Sigma-Aldrich | 46944; CAS: 60842-46-8 |
| Critical Commercial Assays | ||
| Glucose Colorimetric Assay Kit | Cayman Chemical | Cat# 10009582 |
| Beta-hydroxybutyrate Colorimetric Assay Kit | Cayman Chemical | Cat# 700190 |
| MoBio PowerSoil Kit | Mo Bio | Cat# 12888-100 |
| Deposited Data | ||
| 16S rDNA sequences | This paper | https://qiita.ucsd.edu, 11566 |
| Experimental Models: Organisms/Strains | ||
| Mouse: Swiss Webster, germ-free | Taconic | Taconic: SW GF |
| Mouse: Swiss Webster, specific pathogen-free | Taconic | Taconic: SW MPF |
| Mouse: Kcna1−/−: C3HeB.129S7-Kcna1tm1|em/J | Dr. Bruce Tempel | JAX: 003532 |
| Oligonucleotides | ||
| MUC1437: 6FAM-CCTTGCGGTTGGCTTA | Derrien, et al., 2008 | Sigma Aldrich: custom oligo |
| BAC303: Texas Red-CCAATGTGGGGGACCTT | Manz, et al., 1996 | Sigma Aldrich: custom oligo |
| Software and Algorithms | ||
| Ethovision XT 11 | Noldus | Cat # EX11-MAMBP-E |
| QIIME1.8.0 | Caporaso et al., 2010 | http://qiime.org/ |
| Greengenes | Lawrence Berkeley National Labs | http://greengenes.lbl.gov/cgi-bin/nphindex.cgi |
| PICRUSt | Langille et al., 2013 | http://picrust.github.io/picrust/ |
| Ponemah V5.1 | Data Sciences International | https://www.datasci.com/products/software/ponemah |
| Neuroscore | Data Sciences International | https://www.datasci.com/products/software/neuroscore |
| TraceFinder 3.3 | ThermoFisher Scientific | OPTON-30491 |
| ImageJ | National Institutes of Health | https://imagej.nih.gov/ij/ |
| Other | ||
| Ketogenic Diet Mouse Chow | Envigo | TD.07797.PWD |
| Control Diet Mouse Chow | Envigo | TD.150300 |
| Standard Diet Mouse Chow | LabDiet | # 5010 |
| ECT Unit | Ugo Basile | Model # 57800 |
| EEG transmitters | Data Sciences International | PhysioTel ETA-F10 |
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Elaine Hsiao (ehsiao@ucla.edu)
EXPERIMENTAL MODELS AND SUBJECT DETAILS
Mice
3–4 week old SPF wildtype Swiss Webster mice (Taconic Farms), GF wildtype Swiss Webster mice (Taconic Farms) and SPF C3HeB/FeJ KCNA1 KO mice (Jackson Laboratories) were bred in UCLA’s Center for Health Sciences Barrier Facility. Breeding animals were fed “breeder” chow (Lab Diets 5K52). Experimental animals were fed standard chow (Lab Diet 5010), 6:1 ketogenic diet (Harlan Teklad TD.07797.PWD; Supplementary Table 1) or vitamin- and mineral-matched control diet (Harlan Teklad TD.150300; Supplementary Table 1). Juvenile mice were used to i) mimic the typical use of the KD to treat pediatric and adolescent epileptic patients, ii) align the timing of mouse brain development to early human brain development, where neurodevelopmental milestones in 3-week old mice are comparable to those of the 2–3 year old human brain (Semple et al., 2013),, and iii) preclude pre-weaning dietary treatment, where effects of the diet on maternal behavior and physiology would confound direct effects of the diet on offspring. Mice were randomly assigned to an experimental group. Experiments include age- and sex-matched cohorts of males and females. Consistent with prior reports (Dutton et al., 2011), we observed no significant differences in seizure threshold or BHB levels in male vs. female WT mice. All animal experiments were approved by the UCLA Animal Care and Use Committee.
Bacteria
A. muciniphila (ATCC BAA845) was cultured under anaerobic conditions at 37°C in Brain Heart Infusion (BHI) media supplemented with 0.05% hog gastric mucin type III (Sigma Aldrich). P. merdae (ATCC 43184) and P. distasonis (ATCC 8503) were grown in anaerobic conditions at 37°C in Reinforced Clostridial Media (RCM). Cultures were authenticated by full-length 16S rDNA sequencing.
METHOD DETAILS
6-Hz Psychomotor Seizure Assay
The 6-Hz test was conducted as previously described (Samala et al., 2008). Pilot studies revealed no sexual dimorphism in seizure threshold. All subsequent experimental cohorts contained male mice. One drop (~50 ul) of 0.5% tetracaine hydrochloride ophthalmic solution was applied to the corneas of each mouse 10–15 min before stimulation. Corneal electrodes were coated with a thin layer of electrode gel (Parker Signagel). A constant-current current device (ECT Unit 57800, Ugo Basile) was used to deliver current at 3 sec duration, 0.2 ms pulse-width and 6 pulses/s frequency. CC50 (the intensity of current required to elicit seizures in 50% of the experimental group) was measured as a metric for seizure susceptibility. Pilot experiments were conducted to identify 24 mA as the CC50 for SPF wild-type Swiss Webster mice. Each mouse was seizure-tested only once, and thus at least n > 6 mice were used to adequately power each experiment group. To determine CC50s for each experimental group, 24 mA currents were administered to the first mouse per experimental group per cohort, followed by fixed increases or decreases by 2 mA intervals. Mice were restrained manually during stimulation and then released into a new cage for behavioral observation. Locomotor behavior was recorded using Ethovision XT software (Noldus) and quantitative measures for falling, tail dorsiflexion (Straub tail), forelimb clonus, eye/vibrissae twitching and behavioral remission were scored manually. For each behavioral parameter, we observed no correlation between percentage incidence during 24 mA seizures and microbiota status or group seizure susceptibility, suggesting a primary effect of the microbiota on seizure incidence rather than presentation or form. Latency to exploration (time elapsed from when an experimental mouse is released into the observation cage (after corneal stimulation) to its first lateral movement) was scored using Ethovision and manually with an electronic timer. Mice were blindly scored as protected from seizures if they did not show seizure behavior and resumed normal exploratory behavior within 10 s. Seizure threshold (CC50) was determined as previously described (Kimball et al., 1957), using the average log interval of current steps per experimental group, where sample n is defined as the subset of animals displaying the less frequent seizure behavior. Data used to calculate CC50 are also displayed as latency to explore for each current intensity, where n represents the total number of biological replicates per group regardless of seizure outcome.
Glucose Measurements
Blood samples were collected by cardiac puncture and spun through SST vacutainers (Becton Dickinson) for serum separation. Glucose levels were detected in sera by colorimetric assay according to the manufacturer’s instructions (Cayman Chemical). Data compiled across multiple experiments are expressed as glucose concentrations normalized to SPF controls within each experiment.
Beta-hydroxybutyrate (BHB) Measurements
Blood was collected by cardiac puncture and spun through SST vacutainers (Becton Dickinson) for serum separation. The colon was washed and flushed with PBS to remove lumenal contents. Frontal cortex, hippocampus, hypothalamus and cerebellum were microdissected and livers were harvested and washed in PBS. Tissue samples were sonicated on ice in 10 s intervals at 20 mV in RIPA lysis buffer (Thermo Scientific). BHB levels were detected in sera by colorimetric assay according to the manufacturer’s instructions (Cayman Chemical). Data were normalized to total protein content as detected by BCA assay (Thermo Pierce). Data compiled across multiple experiments are expressed as BHB concentrations normalized to SPF controls within each experiment.
16S rDNA Microbiota Profiling
Bacterial genomic DNA was extracted from mouse fecal samples or colonic lumenal contents using the MoBio PowerSoil Kit, where the sample n reflects independent cages containing 3 mice per cage in order to capture biological variation across cages and preclude effects of cohousing on microbiota composition. The library was generated according to methods adapted from (Caporaso et al., 2011). The V4 regions of the 16S rDNA gene were PCR amplified using individually barcoded universal primers and 30 ng of the extracted genomic DNA. The PCR reaction was set up in triplicate, and the PCR product was purified using the Qiaquick PCR purification kit (Qiagen). The purified PCR product was pooled in equal molar concentrations quantified by the Kapa library quantification kit (Kapa Biosystems, KK4824) and sequenced by Laragen, Inc. using the Illumina MiSeq platform and 2 × 250bp reagent kit for paired-end sequencing. Operational taxonomic units (OTUs) were chosen by open reference OTU picking based on 97% sequence similarity to the Greengenes 13_5 database. Taxonomy assignment and rarefaction were performed using QIIME1.8.0 (Caporaso et al., 2010) using 85,134 reads per sample.
Microbiota Conventionalization
Fecal samples were freshly collected from adult SPF Swiss Webster mice and homogenized in pre-reduced PBS at 1 ml per pellet. 100 ul of the settled suspension was administered by oral gavage to recipient GF mice. For mock treatment, mice were gavaged with pre-reduced PBS.
Antibiotic Treatment
SPF mice were gavaged with a solution of vancomycin (50mg/kg), neomycin (100 mg/kg) and metronidazole (100 mg/kg) every 12 hours daily for 7 days, according to methods previously described (Reikvam et al., 2011), using dosages typically greater than those used for clinical therapeutic treatment. Ampicillin (1 mg/ml) was provided ad libitum in drinking water. For mock treatment, mice were gavaged with normal drinking water every 12 hours daily for 7 days. For Kcna1−/− mice, drinking water was supplemented with vancomycin (500 mg/ml), neomycin (1 mg/ml) and ampicillin (1 mg/ml) for 1 week to preclude the stress of oral gavage in seizure-prone mice. Antibiotic-treated mice were maintained in sterile caging with sterile food and water and handled aseptically for the remainder of the experiments.
Gnotobiotic Colonization and Bacterial Enrichment
109 cfu bacteria were suspended in 200 ul pre-reduced PBS and orally gavaged into antibiotic-treated mice or germ-free mice. When co-administered as “A. muciniphila and Parabacteroides”, a ratio of 2:1:1 was used for A. muciniphila: P. merdae: P. distasonis. For mock treatment, mice were gavaged with pre-reduced PBS. Pilot studies revealed no significant differences between colonization groups in fecal DNA concentration or 16S rDNA amplification, as measures relevant to bacterial load. Mice were maintained in microisolator cages and handled aseptically. Mice were seizure tested at 14 days after colonization.
Bacterial Fluorescence In Situ Hybridization (FISH)
Mouse colon was cut into distal, medial and proximal sections and fixed in 4% paraformaldehyde solution overnight at 4°C, washed and passed through 15% and 30% sucrose solutions. Colon tissues were then embedded in optimal cutting temperature (OCT) compound (Tissue-Tek) and cryosectioned into 5 um longitudinal sections (Leica CM1950). Slides were equilibrated in hybridization buffer (0.9 M NaCl, 20 mM Tris-HCl, 0.01% sodium dodecyl sulfate, 10% formamide) and incubated in 10 ng/ul FISH probe (Sigma Aldrich) for 15 hr at 47°C in a humidified chamber. The probes were BAC303 (Manz et al., 1996): 5’ Texas Red-CCAATGTGGGGGACCTT. MUC1437 (Derrien et al., 2008): 5’ 6FAM-CCTTGCGGTTGGCTTA. Slides were then incubated for 20 min in wash buffer (0.9 M NaCl, 20 mM Tris-HCl) pre-heated to 47°C and washed gently three times in PBS. Sampl es were then incubated in the dark with 10mg/ml DAPI in PBS for 1 hour at 4°C, washed three times in PBS and mounted in Vectashield mounting medium (Vector Labs). Images were acquired on a Leica TCS Sp5 confocal microscope using LAS AF software (Leica). A 63× oil-immersion objective was used for imaging. Stacks spanning 10 um thickness were compressed using Fiji software (NIH).
Fecal Microbiota Transplant
Fecal samples were freshly collected from donor mice fed KD or CD for 14 days and suspended at 50 mg/ml in pre-reduced PBS. Antibiotic-treated mice were colonized by oral gavage of 100 ul suspension. For mock treatment, mice were gavaged with pre-reduced PBS. Mice were housed in microisolator cages and handled aseptically. Seizure testing was conducted at 4 days after transplant.
Bacterial Treatment
A. muciniphila, P. merdae and P. distasonis were freshly cultured in anaerobic conditions as described above, and then washed, pelleted and re-suspended at 5 × 109 cfu/ml in pre-reduced PBS. A. muciniphila with Parabacteroides were prepared at a 2:1:1 ratio. For heat-killing, bacteria were placed at 95°C for 10 min. Mice were gavaged every 12 hours for 28 days with 200 ul bacterial suspension or sterile pre-reduced PBS as vehicle control.
Kcna1 Seizure Recordings
EEG Implantation and Recovery
EEGs were recorded from male and female Kcna1−/− mice at 6–7 weeks of age. Kcna1+/+ littermates were used as controls. We observed no significant differences between males and females in seizure frequency and duration. Data presented include both sexes. Mice were anesthetized with isoflurane (5% induction, 2% maintenance) and eye ointment applied to each eye. Fur was removed along the head, and the area was cleaned with three alternative scrubs of chlorohexidine and 70% isopropanol. In a biosafety cabinet, mice were positioned in a stereotaxic apparatus (Harvard Biosciences) and 1 mg/kg lidocaine + 1 mg/kg bupivacaine was applied locally along the incision site. Using sterile surgical tools, a 2 cm incision was made along the dorsal midline from the posterior margin of the eyes to a point midway between the scapulae. A subcutaneous pocket along the dorsal flank was created and the pocket irrigated with sterile saline. A wireless telemetry transmitter was inserted with bi-potential leads oriented cranially. The skull was cleaned with 3% hydrogen peroxide followed by 70% isopropanol. Using a 1.0 mm micro drill bit, the skull was perforated to generate two small holes halfway between the bregma and lambda, and 1–2 mm from the sagittal suture. Bilateral EEG recording electrodes (Data Sciences International (DSI) PhysioTel, ETA-F10) were epidurally implanted over the frontoparietal cortex. Sterile acrylic was applied to the dried area. The incision site was closed with absorbable 5–0 sutures and cleaned with 3% hydrogen peroxide followed by 70% ethanol. Animals were housed individually in autoclaved microisolator cages and allowed to recover for 3–5 days before recordings were initiated.
Data Acquisition and Analysis
During EEG recordings animals were freely moving and maintained on experimental diet. EEG traces were acquired over 3 days using the DSI Ponemah V5.1 data acquisition system. Seizures were identified based on characteristic spike patterns of 5 stages: A) low-frequency background, with low-voltage spiking, B) synchronized high-frequency, high-voltage spiking, C) high-frequency, low-voltage spiking, D) unsynchronized high-frequency, high-voltage spiking, and E) high-frequency, burst spiking. Simultaneous video recordings of behavioral seizures were correlated with EEG recordings and scored based on an adapted Racine scale and defined by 5 stages: 1) myoclonic jerk, 2) head stereotypy and facial clonus, 3) bilateral and alternating forelimb/hindlimb clonus, 4) rearing and falling, and 5) generalized tonic-clonic episodes (Fenoglio-Simeone et al., 2009; Simeone et al., 2016; Supplementary Video 1). Data were analyzed by a blinded researcher using Neuroscore CNS Software (DSI). EEG signals were filtered using a 10 Hz high pass filter, and seizure events were detected by blinded manual scoring. Seizures were defined as patterns of high-frequency, high-voltage synchronized heterogeneous spike wave forms with amplitudes at least 2-fold greater than background with more than 6 s duration (Baraban et al., 2009). The average spike frequency per seizure was determined as number of spikes occurring above baseline in a given seizure per second and averaged per animal (Sato and Woolley, 2016).
Colonic Lumenal and Serum Metabolomics
Samples were collected from mice housed across independent cages, with at least 2 mice per cage. Colonic lumenal contents were collected from terminal mouse dissections, immediately snap frozen in liquid nitrogen and stored at −80°C. Serum samples were collected by cardiac puncture, separated using SST vacutainer tubes and frozen at −80°C. Samples were prepared using the automated MicroLab STAR system (Hamilton Company) and analyzed on GC/MS, LC/MS and LC/MS/MS platforms by Metabolon, Inc. Protein fractions were removed by serial extractions with organic aqueous solvents, concentrated using a TurboVap system (Zymark) and vacuum dried. For LC/MS and LC-MS/MS, samples were reconstituted in acidic or basic LC-compatible solvents containing >11 injection standards and run on a Waters ACQUITY UPLC and Thermo-Finnigan LTQ mass spectrometer, with a linear ion-trap front-end and a Fourier transform ion cyclotron resonance mass spectrometer back-end. For GC/MS, samples were derivatized under dried nitrogen using bistrimethyl-silyl-trifluoroacetamide and analyzed on a Thermo-Finnigan Trace DSQ fast-scanning single-quadrupole mass spectrometer using electron impact ionization. Chemical entities were identified by comparison to metabolomic library entries of purified standards. Following log transformation and imputation with minimum observed values for each compound, data were analyzed using one-way ANOVA to test for group effects. P and q-values were calculated based on two-way ANOVA contrasts. Principal components analysis was used to visualize variance distributions. Supervised Random Forest analysis was conducted to identify metabolomics prediction accuracies.
Hippocampal Metabolomics
Hippocampal tissues were homogenized in 1 ml cold 80% MeOH and vigorously mixed on ice followed by centrifugation (1.3*104 rpm, 4 °C 5 ug supernatant was transferred into a glass vial, supplemented with 5 nmol D/L-norvaline, dried down under vacuum, and finally resuspended in 70% acetonitrile. For the mass spectrometry-based analysis of the sample, 5 l were injected onto a Luna NH2 (150 mm × 2 mm, Phenomenex) column. The samples were analyzed with an UltiMate 3000RSLC (Thermo Scientific) coupled to a Q Exactive mass spectrometer (Thermo Scientific). The Q Exactive was run with polarity switching (+4.00 kV/−4.00 kV) in full scan mode with an m/z range of 70–1050. Separation was achieved using A) 5 mM NH4AcO (pH 9.9) and B) ACN. The gradient started with 15% A) going to 90% A) over 18 min, followed by an isocratic step for 9 min. and reversal to the initial 15% A) for 7 min. Metabolites were quantified with TraceFinder 3.3 using accurate mass measurements (≤ 3 ppm), retention times of pure standards and MS2 fragmentation patterns. Data analysis, including principal component analysis and hierarchical clustering was performed using R.
Amino Acid Supplementation
4-week old Swiss Webster SPF mice were treated with antibiotics, colonized with A. muciniphila and Parabacteroides and fed KD for 14 days, as described in methods above. Beginning on the evening of day 11, mice were injected intraperitoneally every 12 hours for 3 days with ketogenic amino acid cocktail (Sigma Aldrich)– L-leucine (2.0 mg/kg), L-lysine (2.0 mg/kg), L-tyrosine (2.4 mg/kg), L-tryptophan (1.6 mg/kg), and L-threonine (3.1 mg/kg) in sterile PBS. Physiologically-relevant concentrations of amino acids were calculated based on serum metabolomic data (Table S5), such that dosages for each were projected to restore blood levels to concentrations measured in vehicle-treated SPF CD controls. Concentrations are based on physiological levels reported for each amino acid in mouse blood (Smith, 2000) and on fold-changes observed in our metabolomics dataset for each amino acid between control SPF CD and AkkPb KD mice (Table S5). Vehicle-treated mice were injected with PBS (200 ul/30 g mouse). On day 14, mice were tested for 6-Hz seizures 2 hours after the final morning amino acid injection, with a prior 1-hour acclimation period in the behavioral testing room.
GGsTop Treatment
For wildtype mice: 4-week old SPF Swiss Webster mice were fed CD ad libitum for 14 days. Beginning on the evening of day 11, mice were orally gavaged every 12 hours with 13.3 mg/kg 3-[[(3-amino-3-carboxypropyl)methoxyphosphinyl]oxy]benzeneacetic acid (GGsTop, Tocris Bioscience) in sterile water. Vehicle-treated mice were gavaged with sterile water (200 ul/30 g mouse). On day 14, mice were tested for 6-Hz seizures 2 hours after the final morning GGsTop gavage, with a prior 1-hour acclimation period in the behavioral testing room. For Kcna1 mice: 3-4 week old Kcna1−/− mice were fed the CD ad libitum for 23 days. On Day 15, EEG transmitters were implanted as described in the Kcna1 Seizure Recordings section above. On the evening of day 18, mice were orally gavaged every 12 hours with 13.3 mg/kg GGsTop through the morning of day 21. Seizures were recorded over 3 days by EEG beginning 2 hours after the final gavage.
Cross-Feeding in vitro Assay
Cross-feeding was measured as previously described (Flynn et al., 2016). A. muciniphila was embedded at 2×106 cfu/ml in 5 ml pre-reduced CD or KD-based liquid media supplemented with 1% agar at the bottom of an anaerobic tube, and P. merdae was overlaid above it at 6×106 cfu/ml in 5 ml pre-reduced M9 minimal media. Diet-based media were generated by aseptically suspending mouse KD vs. CD diets, described above, to 2 kcal/ml in M9 media. Pilot experiments confirmed no ectopic translocation of embedded A. muciniphila from the agar compartment into the above M9 liquid compartment. For each time point, aliquots were taken from the top and bottom compartments, plated in a dilution series in rich media (RCM for P. merdae and BHI + 0.05% mucins for A. muciniphila), and colonies were counted. For GGsTop pre-treatment experiments, P. merdae was incubated with 500 uM GGsTop vs vehicle in RCM media at 37°C for 2 hours and then washed with ster ile media. Pilot experiments revealed no significant effect of GGsTop pre-treatment on P. merdae viability.
GGT Activity Assay
GGT activity was measured as previously described (van der Stel et al., 2015). For anaerobic cultures, bacteria were seeded at 3 × 105 cfu/ml in CD- and KD-based media. 1 ml bacterial suspension was pelleted and frozen at −80°C for 1 hr. Separate aliquots of the same suspension were plated in BHI mucin agar media or RCM and incubated at 37°C in a Coy anaerobic chamber for later data normalization by bacterial cfu. Pellets were then resuspended in 250 ul lysis buffer (50 mM Tris-HCl with 1 ug/ml lysozyme) and incubated on ice for 30 min. For fecal samples, one pellet was weighed and homogenized in 1 ml lysis buffer. Bacterial and fecal suspensions were then sonicated (QSonica 125) and centrifuged at 12000 xg for 10 min at 4°C. 20 ul supernatant was mixed with 180 ul substrate buffer (2.9 mM L-gamma-glutamyl-3-carboxy-4-nitroanilide (Gold Bio), 100 mM of glycylglycine (Sigma Aldrich), 100 mM Tris-HCl), and 500 uM GGsTop (if noted). Absorbance at 405 nm denoting production of 3-carboxy-4-nitroaniline was measured every minute for 1 hr at 37°C using an automated multimode plate reader (Biotek Synergy H1).
Intestinal Permeability Assay
Mice were fasted for 4 hr beginning at 7am before gavage with 0.6 g/kg 4 kDa FITC-dextran (Sigma Aldrich). 4 hours after gavage, serum samples were collected by cardiac puncture, diluted 3-fold in water and read in duplicate for fluorescence intensity at 521 nm using a Synergy H9 multimode plate reader (Biotek) against a standard dilution series of stock FITC-dextran in 3-fold diluted normal mouse serum in water.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical analysis was performed using Prism software (GraphPad). Data were assessed for normal distribution and plotted in the figures as mean ± SEM. For each figure, n = the number of independent biological replicates. No samples or animals were excluded from the analyses. Differences between two treatment groups were assessed using two-tailed, unpaired Student t test with Welch’s correction. Differences among >2 groups with only one variable were assessed using one-way ANOVA with Bonferroni post hoc test. Taxonomic comparisons from 16S rDNA sequencing analysis were analyzed by Kruskal-Wallis test with Bonferroni post hoc test. Data for Kcna1 mice were analyzed by non-parametric Mann-Whitney test. Two-way ANOVA with Bonferroni post-hoc test was used for ≥ 2 groups with two variables (e.g. seizure time course, BHB time course, metabolomics data, bacterial growth curves). One-way ANOVA with repeated measures and Bonferroni post-hoc test was used for GGT assays. Significant differences emerging from the above tests are indicated in the figures by *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 Notable near-significant differences (0.5 < p < 0.1) are indicated in the figures. Notable non-significant (and non-near significant) differences are indicated in the figures by “n.s.”.
DATA AND SOFTWARE AVAILABILITY
16S rDNA sequencing data and metadata are available online through the QIITA website (https://qiita.ucsd.edu/) with the study accession #11566.
Supplementary Material
(A) Weight gain (top) and food (consumption) over 14 days on CD vs. KD. n = 9, 9, 12, 3 (weight); n = 3 (food).
(B) Alpha diversity based on 16S rDNA sequencing of the fecal gut microbiota on days 0, 4, 8 and 14 after treatment with the control diet (CD, top) vs. ketogenic diet (KD, bottom) n = 3 per time point.
(C) Heatmap of 16S rDNA sequencing data, where columns represent bacterial taxa indicated by their lowest resolved taxonomic levels. Rows represent fecal samples collected from CD or KD-fed mice at Day 0 (pre-treatment), 4, 8 or 14, where gray fields indicate mice fed CD and blue indicate mice fed KD. Phylogenetic classifications are based on weighted UniFrac data. Asterisks denote statistical significance between CD and KD groups (excluding Day 0). Red text highlights A. muciniphila and Parabacteroides. n = 3.
(D) Relative abundances of select bacterial taxa that are enriched in SPF mice fed KD (top row) or CD (bottom row). n = 3.
Data are presented as mean ± SEM. Kruskal-Wallis with Bonferroni: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. n.s.=not statistically significant. CD=control diet, KD=ketogenic diet.
Table S1. Dietary Information, Related to Figure 1
Table S2. Fecal 16S rDNA Sequencing from SPF Mice Fed Ketogenic Diet vs. Control Diet, Related to Figure 1.
Table S3. Select Amino Acid-Related Genes from Inferred Fecal Metagenomes of SPF Mice Fed Ketogenic Diet vs. Control Diet, Related to Figure 6
Table S4. Colonic Lumenal Metabolites in Seizure-Protected (SPF KD, AkkPb KD) vs. Seizure-Susceptible (SPF CD, Abx KD) Mice, Related to Figure 6
Supplemental Video 1. Seizure Behavior in Kcna1−/− Mouse, Related to Figure 5
(A) BHB (left) levels from liver, colon and small intestine. Data are normalized to levels seen in vehicle-treated SPF mice fed CD. n = 5, 7, 4, 3 (liver); n = 15, 15, 4, 6 (colon); n = 9, 9, 4, 5 (small intestine).
(B) Serum FITC-dextran levels after oral gavage in intestinal permeability assay. n = 15, 12, 5, 4.
(C) Brain BHB levels. Data are normalized to levels seen in vehicle-treated SPF mice fed CD. n = 17, 17, 4, 9 (frontal cortex and hippocampus); n = 3, 4, 3, 3 (hypothalamus); n = 3, 4, 4, 3 (cerebellum).
Data are presented as mean ± SEM. One-way ANOVA with Bonferroni: *p < 0.05. SPF=specific pathogen-free (conventionally-colonized), GF=germ-free, veh=vehicle Abx=pre-treatment with antibiotics (ampicillin, vancomycin, neomycin, metronidazole [AVNM]), CD=control diet, KD=ketogenic diet, BHB=betahydroxybutyrate, HMGCS2= 3-hydroxy-3-methylglutaryl-CoA synthase 2, ACAT1=acetyl-CoA acetyltransferase 1, BDH1=3-hydroxybutyrate dehydrogenase 1, CPT1A=carnitine palmitoyltransferase 1A, GAPDH=glyceraldehyde 3-phosphate dehydrogenase.
(A) Relative abundance of Akkermansia muciniphila (left) and Parabacteroides (right), measured by 16S rDNA sequencing, after colonization of Abx-treated SPF mice. n = 3, 3, 3, 3, 3, 3, 4, 7, 4, 3, 3, 3 cages.
(B) Cecal weight (left) and mouse weight (right) at 14 days after dietary treatment in Abx-treated SPF mice colonized with different bacterial taxa. n = 28, 28, 12, 32, 6, 16, 12, 14, 18, 15, 6, 6.
(C) Serum glucose (left) and BHB (right) concentrations at 14 days after dietary treatment in Abx-treated SPF mice colonized with different bacterial taxa. n = 12, 11, 11, 8, 6, 14, 6, 14, 6 (glucose); n = 18, 18, 12, 19, 6, 16, 12, 6, 18, 6, 12, 6 (BHB).
(D) Seizure thresholds in response to 6-Hz stimulation in SPF mice pre-treated with vehicle or Abx, and colonized with A. muciniphila and P. merdae (Pbmerd), P. distasonis (Pbdist), or both (left). n = 8, 5, 6. Behavior in representative cohort of seizure-tested mice (right). Yellow line at y=10 seconds represents threshold for scoring seizures, and yellow triangle at 24 mA denotes starting current per experimental cohort. n = 16, 25.
Data are presented as mean ± SEM. One-way ANOVA with Bonferroni: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. SPF=specific pathogen-free (conventionally-colonized), GF=germ-free, CD=control diet, KD=ketogenic diet, CC50=current intensity producing seizures in 50% of mice tested, veh=vehicle, Abx=pre-treated with antibiotics (ampicillin, vancomycin, neomycin, metronidazole [AVNM]), Pb=Parabacteroides (P. merdae and P. distasonis), Akk=Akkermansia muciniphila, AkkPb=A. muciniphila, P. merdae and P. distasonis, Bf=Bifidobacterium longum.
(A) Principal coordinates analysis of weighted UniFrac distance matrices based on longitudinal 16S rDNA profiling of feces from SPF mice fed CD for 28 days (CD), mice fed KD for 28 days (KD), or mice fed KD for 14 days followed by CD for 14 days (KD-CD). n = 3 cages/group.
(B) Seizure thresholds in response to 6-Hz stimulation in SPF mice fed CD for 28 days (CD), mice fed KD for 28 days (KD), or mice fed KD for 14 days followed by CD for 14 days (KD-CD) (left). n = 4. Behavior in representative cohort of seizure-tested mice (right). Yellow line at y=10 seconds represents threshold for scoring seizures, and yellow triangle at 24 mA denotes starting current per experimental cohort. n = 8, 9, 9.
(C) Seizure thresholds in response to 6-Hz stimulation in SPF mice at 21 days after probiotic treatment with A. muciniphila, P. merdae and P. distasonis (AkkPb), A. muciniphila alone (Akk), or heat-killed A. muciniphila and Parabacteroides spp (hk-AkkPb). (left). n = 8.
(D) Seizure thresholds in response to 6-Hz stimulation in SPF mice orally gavaged for 4 days with vehicle or A. muciniphila, P. merdae and P. distasonis (AkkPb). n = 6, 7, 7, 7.
Data are presented as mean ± SEM. One-way ANOVA with Bonferroni: *p < 0.05, **p < 0.01, ****p < 0.0001, n.s.=not statistically significant. SPF=specific pathogen-free (conventionally-colonized), CD=control diet, KD=ketogenic diet, KD-CD=fed KD for 14 days followed by CD for 14 days. CC50=current intensity producing seizures in 50% of mice tested, veh=vehicle, Akk=Akkermansia muciniphila, AkkPb=A. muciniphila, P. merdae and P. distasonis, hk-AkkPb=heat-killed A. muciniphila, P. merdae and P. distasonis.
(A) Representative EEG traces from SPF Kcna1−/− mice treated with vehicle or Abx, colonized with A. muciniphila and Parabacteroides, or neither, and fed CD or KD. The yellow arrow above each waveform indicates the spikelet enlarged to the right of each trace.
(B) Average duration per seizure (right) in SPF Kcna1−/− mice treated with vehicle or Abx, colonized with A. muciniphila and Parabacteroides, or neither, and fed CD or KD. n = 2, 8, 5, 12, 9, 3.
(C) Average seizure frequency per day for seizures observed during the light phase (0600–1800) (left) and dark phase (1800−0600) (right). n = 6, 4, 12, 9, 3.
(D) Average spike frequency per seizure for seizures observed during the light phase (left), dark phase (middle), or total duration. n = 6, 4, 12, 9, 1.
(E) Weight gain (top) and food consumption (bottom) in Kcna1−/− mice fed CD vs KD Weight gain (top) and food consumption (bottom) in Kcna1−/− mice fed CD vs KD. n = 8 (weight); n = 6 (food).
(F) Survival curves in Kcna1−/− mice fed CD vs KD. n = 79.
(G) Average number of seizures per day (left), average duration per seizure (middle) and total duration of seizures per day (right) in SPF CD Kcna1−/− mice treated with GGsTop. Data for SPF CD mice are as in (d). n = 8, 4.
Data are presented as mean ± SEM. Non-parametric one-way ANOVA with Dunn (B-D), Twoway ANOVA with Bonferroni (E,F), Students t-test with Welch (G): *p < 0.05, **p < 0.01. n.s.=not statistically significant. SPF=specific pathogen-free (conventionally-colonized), CD=control diet, KD=ketogenic diet.
(A) Number of statistically significant alterations in metabolites out of the 622 detected in colonic lumen and 670 detected in serum. Values noted in black text are total number of metabolites altered, with values in green denoting upregulation and values in red denoting downregulation. n = 8 cages/group.
(B) Levels of non-gamma glutamylated amino acids in colonic lumenal contents from SPF mice fed CD, SPF mice fed KD, Abx-treated mice fed KD, and AkkPb-colonized mice fed KD. n = 8 cages/group.
(C) Levels of non-gamma glutamylated amino acids in sera from SPF mice fed CD, SPF mice fed KD, Abx-treated mice fed KD, and AkkPb-colonized mice fed KD. n = 8 cages/group.
(D) Levels of GABA and glutamate in hippocampal lysates from SPF mice fed CD, SPF mice fed KD, Abx-treated mice fed KD and AkkPb-colonized mice fed KD. n=5.
Data are presented as mean ± SEM. Two-way ANOVA contrasts: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. n.s.=not statistically significant. CD=control diet, KD=ketogenic diet, SPF=specific pathogen-free (conventionally-colonized), veh=vehicle, Abx=pre-treated with antibiotics (ampicillin, vancomycin, neomycin, metronidazole [AVNM]), AkkPb=A muciniphila, P. merdae and P. distasonis, a.u.=arbitrary units, BHB=beta-hydroxybutyrate.
(A) GGT activity in conventionally-cultured P. merdae (left) and A. muciniphila (right), treated with GGsTop or vehicle. n = 5.
(B) Levels of A. muciniphila (Akk) after 0, 7, or 24 hours incubation in CD agar overlaid with P. merdae (PbM) that was pre-treated with vehicle or GGsTop in M9 minimal media. n = 3.
Data are presented as mean ± SEM. One-way ANOVA with Bonferroni (A): *p < 0.05, Two-way ANOVA with Bonferroni (B): **p < 0.01, PbM=Parabacteroides merdae, Akk=Akkermansia muciniphila, GGsTop=GGT inhibitor, GGT=gamma-glutamyltranspeptidase, AU=absorbance units.
Table S5. Serum Metabolites in Seizure-Protected (SPF KD, AkkPb KD) vs. Seizure-Susceptible (SPF CD, Abx KD) Mice, Related to Figure 6
Table S6. Hippocampal Metabolites in Seizure-Protected (SPF KD, AkkPb KD) vs. Seizure-Susceptible (SPF CD, Abx KD) Mice, Related to Figure 6
HIGHLIGHT.
Changes in the gut microbiota are required for the anti-seizure effects of the KD
Specific KD-associated bacteria mediate and confer anti-seizure effects of the KD
KD microbiota regulate amino acid γ-glutamylation and hippocampal GABA/glutamate
The beneficial effects of a ketogenic diet on epileptic seizures is mediated by the gut microbiome through their modulation of hippocampal GABA/glutamate ratios.
Acknowledgments
The authors acknowledge Julianne McGinn and Tomiko Rendon for generating and caring for the germ-free animals; Kristie Yu, Sandy Wong and Gauri Shastri for assistance with initial experiments and data analysis; Thomas Fung, Geoffrey Pronovost, Gregory Donaldson, Xia Yang, Carlos Cepeda, and Kim McDowell for helpful advice; Bruce Tempel and Marie Francoise-Chesselet for sharing mice and reagents. This work was supported by funds from UCLA Department of Integrative Biology & Physiology and Division of Life Sciences (EYH), Alfred P. Sloan Foundation Fellowship (EYH), Army Research Office Multidisciplinary University Research Initiative Grant (W911NF-17-1-0402; EYH), Mallinckrodt Foundation Grant (EYH), NIH Ruth L. Kirschstein Award (T32GM065823; CAO), UPLIFT: UCLA Postdoctoral Longitudinal Investment in Faculty Award (K12GM106996 HEV).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
CAO, HEV, JMY, QL, DJN and EYH performed the experiments and analyzed the data, CAO, HEV, JMY and EYH designed the study, CAO, HEV and EYH wrote the manuscript. All authors discussed the results and commented on the manuscript.
DECLARATION OF INTERESTS
The authors declare no competing interests. Findings reported in the manuscript are the subject of UCLA provisional patent application US 2017/67548.
SUPPLEMENTAL INFORMATION
Supplemental Information includes seven figures, six tables and one video, and can be found with this article.
References
- Baraban SC, Southwell DG, Estrada RC, Jones DL, Sebe JY, Alfaro-Cervello C, Garcia-Verdugo JM, Rubenstein JL, Alvarez-Buylla A. Reduction of seizures by transplantation of cortical GABAergic interneuron precursors into Kv1.1 mutant mice. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15472–15477. doi: 10.1073/pnas.0900141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton ME, Klein BD, Wolf HH, White HS. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy research. 2001;47:217–227. doi: 10.1016/s0920-1211(01)00302-3. [DOI] [PubMed] [Google Scholar]
- Beloosesky Y, Grosman B, Marmelstein V, Grinblat J. Convulsions induced by metronidazole treatment for Clostridium difficile-associated disease in chronic renal failure. The American journal of the medical sciences. 2000;319:338–339. doi: 10.1097/00000441-200005000-00012. [DOI] [PubMed] [Google Scholar]
- Bough KJ, Rho JM. Anticonvulsant mechanisms of the ketogenic diet. Epilepsia. 2007;48:43–58. doi: 10.1111/j.1528-1167.2007.00915.x. [DOI] [PubMed] [Google Scholar]
- Buffington SA, Di Prisco GV, Auchtung TA, Ajami NJ, Petrosino JF, Costa-Mattioli M. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell. 2016;165:1762–1775. doi: 10.1016/j.cell.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon N, Betancourt L, Hernandez L, Rada P. A ketogenic diet modifies glutamate, gamma-aminobutyric acid and agmatine levels in the hippocampus of rats: A microdialysis study. Neurosci Lett. 2017;642:158–162. doi: 10.1016/j.neulet.2017.02.014. [DOI] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, et al. QIIME allows analysis of high-throughput community sequencing data. Nature methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(Suppl 1):4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano I, Merlino A. gamma-Glutamyltranspeptidases: sequence, structure, biochemical properties, and biotechnological applications. Cellular and molecular life sciences: CMLS. 2012;69:3381–3394. doi: 10.1007/s00018-012-0988-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AJ, Jeitner TM. Central Role of Glutamate Metabolism in the Maintenance of Nitrogen Homeostasis in Normal and Hyperammonemic Brain. Biomolecules. 2016;6 doi: 10.3390/biom6020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford PA, Crowley JR, Sambandam N, Muegge BD, Costello EK, Hamady M, Knight R, Gordon JI. Regulation of myocardial ketone body metabolism by the gut microbiota during nutrient deprivation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:11276–11281. doi: 10.1073/pnas.0902366106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlin M, Elfving A, Ungerstedt U, Amark P. The ketogenic diet influences the levels of excitatory and inhibitory amino acids in the CSF in children with refractory epilepsy. Epilepsy research. 2005;64:115–125. doi: 10.1016/j.eplepsyres.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016a;65:426–436. doi: 10.1136/gutjnl-2014-308778. [DOI] [PubMed] [Google Scholar]
- Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016b;65:426–436. doi: 10.1136/gutjnl-2014-308778. [DOI] [PubMed] [Google Scholar]
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrien M, Collado MC, Ben-Amor K, Salminen S, de Vos WM. The Mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Applied and environmental microbiology. 2008;74:1646–1648. doi: 10.1128/AEM.01226-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan SH, Lobley GE, Holtrop G, Ince J, Johnstone AM, Louis P, Flint HJ. Human colonic microbiota associated with diet, obesity and weight loss. International journal of obesity. 2008;32:1720–1724. doi: 10.1038/ijo.2008.155. [DOI] [PubMed] [Google Scholar]
- Dutton SB, Sawyer NT, Kalume F, Jumbo-Lucioni P, Borges K, Catterall WA, Escayg A. Protective effect of the ketogenic diet in Scn1a mutant mice. Epilepsia. 2011;52:2050–2056. doi: 10.1111/j.1528-1167.2011.03211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eraković V, Župan G, Varljen J, Laginja J, Simonić A. Altered Activities of Rat Brain Metabolic Enzymes in Electroconvulsive Shock—Induced Seizures. Epilepsia. 2001;42:181–189. doi: 10.1046/j.1528-1157.2001.30499.x. [DOI] [PubMed] [Google Scholar]
- Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proceedings of the National Academy of Sciences. 2013;110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewen LM, Griffiths J. γ-Glutamyl Transpeptidase: Elevated Activities in Certain Neurologic Diseases. American Journal of Clinical Pathology. 1973;59:2–9. doi: 10.1093/ajcp/59.1.2. [DOI] [PubMed] [Google Scholar]
- Fenoglio-Simeone KA, Wilke JC, Milligan HL, Allen CN, Rho JM, Maganti RK. Ketogenic diet treatment abolishes seizure periodicity and improves diurnal rhythmicity in epileptic Kcna1-null mice. Epilepsia. 2009;50:2027–2034. doi: 10.1111/j.1528-1167.2009.02163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JM, Niccum D, Dunitz JM, Hunter RC. Evidence and Role for Bacterial Mucin Degradation in Cystic Fibrosis Airway Disease. PLoS Pathog. 2016;12:e1005846. doi: 10.1371/journal.ppat.1005846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JM, Kossoff EH. Ketosis and the ketogenic diet, 2010: advances in treating epilepsy and other disorders. Advances in pediatrics. 2010;57:315–329. doi: 10.1016/j.yapd.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Frost G, Sleeth ML, Sahuri-Arisoylu M, Lizarbe B, Cerdan S, Brody L, Anastasovska J, Ghourab S, Hankir M, Zhang S, et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. 2014;5:3611. doi: 10.1038/ncomms4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman AL, Zheng X, Bergbower E, Kennedy M, Hardwick JM. Seizure tests distinguish intermittent fasting from the ketogenic diet. Epilepsia. 2010;51:1395–1402. doi: 10.1111/j.1528-1167.2010.02577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornik CP, Benjamin DK, Jr, Smith PB, Pencina MJ, Tremoulet AH, Capparelli EV, Ericson JE, Clark RH, Cohen-Wolkowiez M. Electronic Health Records and Pharmacokinetic Modeling to Assess the Relationship between Ampicillin Exposure and Seizure Risk in Neonates. The Journal of pediatrics. 2016;178:125–129.e121. doi: 10.1016/j.jpeds.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janik R, Thomason LAM, Stanisz AM, Forsythe P, Bienenstock J, Stanisz GJ. Magnetic resonance spectroscopy reveals oral Lactobacillus promotion of increases in brain GABA, N-acetyl aspartate and glutamate. Neuroimage. 2016;125:988–995. doi: 10.1016/j.neuroimage.2015.11.018. [DOI] [PubMed] [Google Scholar]
- Kimball AW, Burnett WT, Jr, Doherty DG. Chemical protection against ionizing radiation. I. Sampling methods for screening compounds in radiation protection studies with mice. Radiation research. 1957;7:1–12. [PubMed] [Google Scholar]
- Klein MS, Newell C, Bomhof MR, Reimer RA, Hittel DS, Rho JM, Vogel HJ, Shearer J. Metabolomic Modeling To Monitor Host Responsiveness to Gut Microbiota Manipulation in the BTBR(T+tf/j) Mouse. Journal of proteome research. 2016a;15:1143–1150. doi: 10.1021/acs.jproteome.5b01025. [DOI] [PubMed] [Google Scholar]
- Klein MS, Newell C, Bomhof MR, Reimer RA, Hittel DS, Rho JM, Vogel HJ, Shearer J. Metabolomic Modeling To Monitor Host Responsiveness to Gut Microbiota Manipulation in the BTBRT+tf/j Mouse. Journal of proteome research. 2016b;15:1143–1150. doi: 10.1021/acs.jproteome.5b01025. [DOI] [PubMed] [Google Scholar]
- Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nature medicine. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. The New England journal of medicine. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- Manz W, Amann R, Ludwig W, Vancanneyt M, Schleifer KH. Application of a suite of 16S rRNA-specific oligonucleotide probes designed to investigate bacteria of the phylum cytophaga-flavobacter-bacteroides in the natural environment. Microbiology. 1996;142(Pt 5):1097–1106. doi: 10.1099/13500872-142-5-1097. [DOI] [PubMed] [Google Scholar]
- Newell C, Bomhof MR, Reimer RA, Hittel DS, Rho JM, Shearer J. Ketogenic diet modifies the gut microbiota in a murine model of autism spectrum disorder. Mol Autism. 2016;7:37. doi: 10.1186/s13229-016-0099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reikvam DH, Erofeev A, Sandvik A, Grcic V, Jahnsen FL, Gaustad P, McCoy KD, Macpherson AJ, Meza-Zepeda LA, Johansen FE. Depletion of murine intestinal microbiota: effects on gut mucosa and epithelial gene expression. PloS one. 2011;6:e17996. doi: 10.1371/journal.pone.0017996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remely M, Hippe B, Geretschlaeger I, Stegmayer S, Hoefinger I, Haslberger A. Increased gut microbiota diversity and abundance of Faecalibacterium prausnitzii and Akkermansia after fasting: a pilot study. Wiener klinische Wochenschrift. 2015;127:394–398. doi: 10.1007/s00508-015-0755-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski MA, Loscher W, Rho JM. Mechanisms of Action of Antiseizure Drugs and the Ketogenic Diet. Cold Spring Harbor perspectives in medicine. 2016;6 doi: 10.1101/cshperspect.a022780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samala R, Willis S, Borges K. Anticonvulsant profile of a balanced ketogenic diet in acute mouse seizure models. Epilepsy research. 2008;81:119–127. doi: 10.1016/j.eplepsyres.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Sariego-Jamardo A, Garcia-Cazorla A, Artuch R, Castejon E, Garcia-Arenas D, Molero-Luis M, Ormazabal A, Sanmarti FX. Efficacy of the Ketogenic Diet for the Treatment of Refractory Childhood Epilepsy: Cerebrospinal Fluid Neurotransmitters and Amino Acid Levels. Pediatric neurology. 2015;53:422–426. doi: 10.1016/j.pediatrneurol.2015.07.013. [DOI] [PubMed] [Google Scholar]
- Sato SM, Woolley CS. Acute inhibition of neurosteroid estrogen synthesis suppresses status epilepticus in an animal model. Elife. 2016;5 doi: 10.7554/eLife.12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer H, Brunt ER, Mol GJ, van der Vlies P, Stulp RP, Verlind E, Mantel G, Averyanov YN, Hofstra RM, Buys CH. Three novel KCNA1 mutations in episodic ataxia type I families. Hum Genet. 1998;102:464–466. doi: 10.1007/s004390050722. [DOI] [PubMed] [Google Scholar]
- Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Progress in neurobiology. 2013;106–107:1–16. doi: 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone KA, Matthews SA, Rho JM, Simeone TA. Ketogenic diet treatment increases longevity in Kcna1-null mice, a model of sudden unexpected death in epilepsy. Epilepsia. 2016;57:e178–182. doi: 10.1111/epi.13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MI, Yatsunenko T, Manary MJ, Trehan I, Mkakosya R, Cheng J, Kau AL, Rich SS, Concannon P, Mychaleckyj JC, et al. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science. 2013;339:548–554. doi: 10.1126/science.1229000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith QR. Transport of glutamate and other amino acids at the blood-brain barrier. The Journal of nutrition. 2000;130:1016S–1022S. doi: 10.1093/jn/130.4.1016S. [DOI] [PubMed] [Google Scholar]
- Sonnenburg JL, Backhed F. Diet-microbiota interactions as moderators of human metabolism. Nature. 2016;535:56–64. doi: 10.1038/nature18846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafstrom CE, Rho JM. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol. 2012;3:59. doi: 10.3389/fphar.2012.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter R, Ruegg S, Tschudin-Sutter S. Seizures as adverse events of antibiotic drugs: A systematic review. Neurology. 2015;85:1332–1341. doi: 10.1212/WNL.0000000000002023. [DOI] [PubMed] [Google Scholar]
- van der Stel AX, van Mourik A, Laniewski P, van Putten JP, Jagusztyn-Krynicka EK, Wosten MM. The Campylobacter jejuni RacRS two-component system activates the glutamate synthesis by directly upregulating gamma-glutamyltranspeptidase (GGT) Frontiers in microbiology. 2015;6:567. doi: 10.3389/fmicb.2015.00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong HE, Yano JM, Fung TC, Hsiao EY. The Microbiome and Host Behavior. Annual review of neuroscience. 2017 doi: 10.1146/annurev-neuro-072116-031347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZJ, Bergqvist C, Hunter JV, Jin D, Wang DJ, Wehrli S, Zimmerman RA. In vivo measurement of brain metabolites using two-dimensional double-quantum MR spectroscopy–exploration of GABA levels in a ketogenic diet. Magn Reson Med. 2003;49:615–619. doi: 10.1002/mrm.10429. [DOI] [PubMed] [Google Scholar]
- Yudkoff M, Daikhin Y, Nissim I, Lazarow A, Nissim I. Ketogenic diet, amino acid metabolism, and seizure control. Journal of neuroscience research. 2001;66:931–940. doi: 10.1002/jnr.10083. [DOI] [PubMed] [Google Scholar]
- Zuberi SM, Eunson LH, Spauschus A, De Silva R, Tolmie J, Wood NW, McWilliam RC, Stephenson JB, Kullmann DM, Hanna MG. A novel mutation in the human voltage-gated potassium channel gene (Kv1.1) associates with episodic ataxia type 1 and sometimes with partial epilepsy. Brain. 1999;122(Pt 5):817–825. doi: 10.1093/brain/122.5.817. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Weight gain (top) and food (consumption) over 14 days on CD vs. KD. n = 9, 9, 12, 3 (weight); n = 3 (food).
(B) Alpha diversity based on 16S rDNA sequencing of the fecal gut microbiota on days 0, 4, 8 and 14 after treatment with the control diet (CD, top) vs. ketogenic diet (KD, bottom) n = 3 per time point.
(C) Heatmap of 16S rDNA sequencing data, where columns represent bacterial taxa indicated by their lowest resolved taxonomic levels. Rows represent fecal samples collected from CD or KD-fed mice at Day 0 (pre-treatment), 4, 8 or 14, where gray fields indicate mice fed CD and blue indicate mice fed KD. Phylogenetic classifications are based on weighted UniFrac data. Asterisks denote statistical significance between CD and KD groups (excluding Day 0). Red text highlights A. muciniphila and Parabacteroides. n = 3.
(D) Relative abundances of select bacterial taxa that are enriched in SPF mice fed KD (top row) or CD (bottom row). n = 3.
Data are presented as mean ± SEM. Kruskal-Wallis with Bonferroni: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. n.s.=not statistically significant. CD=control diet, KD=ketogenic diet.
Table S1. Dietary Information, Related to Figure 1
Table S2. Fecal 16S rDNA Sequencing from SPF Mice Fed Ketogenic Diet vs. Control Diet, Related to Figure 1.
Table S3. Select Amino Acid-Related Genes from Inferred Fecal Metagenomes of SPF Mice Fed Ketogenic Diet vs. Control Diet, Related to Figure 6
Table S4. Colonic Lumenal Metabolites in Seizure-Protected (SPF KD, AkkPb KD) vs. Seizure-Susceptible (SPF CD, Abx KD) Mice, Related to Figure 6
Supplemental Video 1. Seizure Behavior in Kcna1−/− Mouse, Related to Figure 5
(A) BHB (left) levels from liver, colon and small intestine. Data are normalized to levels seen in vehicle-treated SPF mice fed CD. n = 5, 7, 4, 3 (liver); n = 15, 15, 4, 6 (colon); n = 9, 9, 4, 5 (small intestine).
(B) Serum FITC-dextran levels after oral gavage in intestinal permeability assay. n = 15, 12, 5, 4.
(C) Brain BHB levels. Data are normalized to levels seen in vehicle-treated SPF mice fed CD. n = 17, 17, 4, 9 (frontal cortex and hippocampus); n = 3, 4, 3, 3 (hypothalamus); n = 3, 4, 4, 3 (cerebellum).
Data are presented as mean ± SEM. One-way ANOVA with Bonferroni: *p < 0.05. SPF=specific pathogen-free (conventionally-colonized), GF=germ-free, veh=vehicle Abx=pre-treatment with antibiotics (ampicillin, vancomycin, neomycin, metronidazole [AVNM]), CD=control diet, KD=ketogenic diet, BHB=betahydroxybutyrate, HMGCS2= 3-hydroxy-3-methylglutaryl-CoA synthase 2, ACAT1=acetyl-CoA acetyltransferase 1, BDH1=3-hydroxybutyrate dehydrogenase 1, CPT1A=carnitine palmitoyltransferase 1A, GAPDH=glyceraldehyde 3-phosphate dehydrogenase.
(A) Relative abundance of Akkermansia muciniphila (left) and Parabacteroides (right), measured by 16S rDNA sequencing, after colonization of Abx-treated SPF mice. n = 3, 3, 3, 3, 3, 3, 4, 7, 4, 3, 3, 3 cages.
(B) Cecal weight (left) and mouse weight (right) at 14 days after dietary treatment in Abx-treated SPF mice colonized with different bacterial taxa. n = 28, 28, 12, 32, 6, 16, 12, 14, 18, 15, 6, 6.
(C) Serum glucose (left) and BHB (right) concentrations at 14 days after dietary treatment in Abx-treated SPF mice colonized with different bacterial taxa. n = 12, 11, 11, 8, 6, 14, 6, 14, 6 (glucose); n = 18, 18, 12, 19, 6, 16, 12, 6, 18, 6, 12, 6 (BHB).
(D) Seizure thresholds in response to 6-Hz stimulation in SPF mice pre-treated with vehicle or Abx, and colonized with A. muciniphila and P. merdae (Pbmerd), P. distasonis (Pbdist), or both (left). n = 8, 5, 6. Behavior in representative cohort of seizure-tested mice (right). Yellow line at y=10 seconds represents threshold for scoring seizures, and yellow triangle at 24 mA denotes starting current per experimental cohort. n = 16, 25.
Data are presented as mean ± SEM. One-way ANOVA with Bonferroni: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. SPF=specific pathogen-free (conventionally-colonized), GF=germ-free, CD=control diet, KD=ketogenic diet, CC50=current intensity producing seizures in 50% of mice tested, veh=vehicle, Abx=pre-treated with antibiotics (ampicillin, vancomycin, neomycin, metronidazole [AVNM]), Pb=Parabacteroides (P. merdae and P. distasonis), Akk=Akkermansia muciniphila, AkkPb=A. muciniphila, P. merdae and P. distasonis, Bf=Bifidobacterium longum.
(A) Principal coordinates analysis of weighted UniFrac distance matrices based on longitudinal 16S rDNA profiling of feces from SPF mice fed CD for 28 days (CD), mice fed KD for 28 days (KD), or mice fed KD for 14 days followed by CD for 14 days (KD-CD). n = 3 cages/group.
(B) Seizure thresholds in response to 6-Hz stimulation in SPF mice fed CD for 28 days (CD), mice fed KD for 28 days (KD), or mice fed KD for 14 days followed by CD for 14 days (KD-CD) (left). n = 4. Behavior in representative cohort of seizure-tested mice (right). Yellow line at y=10 seconds represents threshold for scoring seizures, and yellow triangle at 24 mA denotes starting current per experimental cohort. n = 8, 9, 9.
(C) Seizure thresholds in response to 6-Hz stimulation in SPF mice at 21 days after probiotic treatment with A. muciniphila, P. merdae and P. distasonis (AkkPb), A. muciniphila alone (Akk), or heat-killed A. muciniphila and Parabacteroides spp (hk-AkkPb). (left). n = 8.
(D) Seizure thresholds in response to 6-Hz stimulation in SPF mice orally gavaged for 4 days with vehicle or A. muciniphila, P. merdae and P. distasonis (AkkPb). n = 6, 7, 7, 7.
Data are presented as mean ± SEM. One-way ANOVA with Bonferroni: *p < 0.05, **p < 0.01, ****p < 0.0001, n.s.=not statistically significant. SPF=specific pathogen-free (conventionally-colonized), CD=control diet, KD=ketogenic diet, KD-CD=fed KD for 14 days followed by CD for 14 days. CC50=current intensity producing seizures in 50% of mice tested, veh=vehicle, Akk=Akkermansia muciniphila, AkkPb=A. muciniphila, P. merdae and P. distasonis, hk-AkkPb=heat-killed A. muciniphila, P. merdae and P. distasonis.
(A) Representative EEG traces from SPF Kcna1−/− mice treated with vehicle or Abx, colonized with A. muciniphila and Parabacteroides, or neither, and fed CD or KD. The yellow arrow above each waveform indicates the spikelet enlarged to the right of each trace.
(B) Average duration per seizure (right) in SPF Kcna1−/− mice treated with vehicle or Abx, colonized with A. muciniphila and Parabacteroides, or neither, and fed CD or KD. n = 2, 8, 5, 12, 9, 3.
(C) Average seizure frequency per day for seizures observed during the light phase (0600–1800) (left) and dark phase (1800−0600) (right). n = 6, 4, 12, 9, 3.
(D) Average spike frequency per seizure for seizures observed during the light phase (left), dark phase (middle), or total duration. n = 6, 4, 12, 9, 1.
(E) Weight gain (top) and food consumption (bottom) in Kcna1−/− mice fed CD vs KD Weight gain (top) and food consumption (bottom) in Kcna1−/− mice fed CD vs KD. n = 8 (weight); n = 6 (food).
(F) Survival curves in Kcna1−/− mice fed CD vs KD. n = 79.
(G) Average number of seizures per day (left), average duration per seizure (middle) and total duration of seizures per day (right) in SPF CD Kcna1−/− mice treated with GGsTop. Data for SPF CD mice are as in (d). n = 8, 4.
Data are presented as mean ± SEM. Non-parametric one-way ANOVA with Dunn (B-D), Twoway ANOVA with Bonferroni (E,F), Students t-test with Welch (G): *p < 0.05, **p < 0.01. n.s.=not statistically significant. SPF=specific pathogen-free (conventionally-colonized), CD=control diet, KD=ketogenic diet.
(A) Number of statistically significant alterations in metabolites out of the 622 detected in colonic lumen and 670 detected in serum. Values noted in black text are total number of metabolites altered, with values in green denoting upregulation and values in red denoting downregulation. n = 8 cages/group.
(B) Levels of non-gamma glutamylated amino acids in colonic lumenal contents from SPF mice fed CD, SPF mice fed KD, Abx-treated mice fed KD, and AkkPb-colonized mice fed KD. n = 8 cages/group.
(C) Levels of non-gamma glutamylated amino acids in sera from SPF mice fed CD, SPF mice fed KD, Abx-treated mice fed KD, and AkkPb-colonized mice fed KD. n = 8 cages/group.
(D) Levels of GABA and glutamate in hippocampal lysates from SPF mice fed CD, SPF mice fed KD, Abx-treated mice fed KD and AkkPb-colonized mice fed KD. n=5.
Data are presented as mean ± SEM. Two-way ANOVA contrasts: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. n.s.=not statistically significant. CD=control diet, KD=ketogenic diet, SPF=specific pathogen-free (conventionally-colonized), veh=vehicle, Abx=pre-treated with antibiotics (ampicillin, vancomycin, neomycin, metronidazole [AVNM]), AkkPb=A muciniphila, P. merdae and P. distasonis, a.u.=arbitrary units, BHB=beta-hydroxybutyrate.
(A) GGT activity in conventionally-cultured P. merdae (left) and A. muciniphila (right), treated with GGsTop or vehicle. n = 5.
(B) Levels of A. muciniphila (Akk) after 0, 7, or 24 hours incubation in CD agar overlaid with P. merdae (PbM) that was pre-treated with vehicle or GGsTop in M9 minimal media. n = 3.
Data are presented as mean ± SEM. One-way ANOVA with Bonferroni (A): *p < 0.05, Two-way ANOVA with Bonferroni (B): **p < 0.01, PbM=Parabacteroides merdae, Akk=Akkermansia muciniphila, GGsTop=GGT inhibitor, GGT=gamma-glutamyltranspeptidase, AU=absorbance units.
Table S5. Serum Metabolites in Seizure-Protected (SPF KD, AkkPb KD) vs. Seizure-Susceptible (SPF CD, Abx KD) Mice, Related to Figure 6
Table S6. Hippocampal Metabolites in Seizure-Protected (SPF KD, AkkPb KD) vs. Seizure-Susceptible (SPF CD, Abx KD) Mice, Related to Figure 6


