Abstract
Since their very beginnings, animals had gut sensory epithelial cells. In one of the first multicellular animals, Trichoplax – a literal wandering gut – food sensing and feeding was coordinated by specialized ventral sensor cells. In mammals, including humans, gut epithelial sensor cells (a.k.a enteroendocrine cells) have been recognized for an array of neuropeptides, like ghrelin and cholecystokinin, that modulate hunger or satiety. Indeed, since first described as “clear cells” by Rudfolf Heidenhain (1868), research efforts increasingly focused on their hormone neuropeptides leading to the alphabetical classification of one cell-one hormone (e.g. I-cell synthesizes only cholecystokinin). A recent explosion of molecular tools to study the biology of single cells is expanding the imagination of studies and unveiling intriguing aspects of gut sensory transduction. To mention a few: multimodal sensing, one cell expressing both ghrelin and cholecystokinin—the yin and yang of appetite—, and synapses with nerves. This brief account examines recent advances on gut sensory transduction to highlight how food and bacteria in the gut alter eating.
Keywords: Enteroendocrine cells, Neuropods, Sensory transduction, Neuroepithelial circuit, Sensory ganglia
GRAPHICAL ABSTRACT
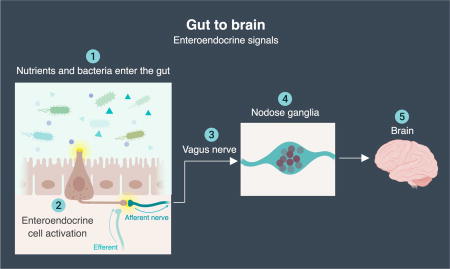
The synaptic path of nutrient signaling from gut to brain.
One of the most rewarding, albeit dangerous, tasks we engage in on a daily basis is eating. The gut transforms food into feces and in the process extracts nourishment for all cells of the body. But food also triggers an array of feelings - from pleasure to pain, to reward, to disgust, to aversion. Extensive efforts have elucidated how those behaviors are modulated by sensory detection and transduction of olfactory and taste cues. However, what happens after nutrients are ingested has only began to emerge.
Up until a couple of years ago, research in the field of gastrointestinal chemosensation was centered around “gut hormones.” This line of inquiry has persisted for the last 115 years since the discovery of secretin by Bayliss and Starling in 1902.1 The dogma is that nutrients entering the intestine stimulate the release of gut hormones.2 It was built around, and largely limited by, the available technologies over time. This idea is reflected in the current name of the sensor cell – enteroendocrine cell or a gut cell that secretes a hormone. That name was proposed in 1938 by Friedrich Feyrter who pioneered the concept of a diffused endocrine organ in the gut based on observations using available tools at the time.3
But molecular biology has taken a giant leap in recent years. Today, it is possible to manipulate the genome of individual cell types, dissect the transcriptome of single cells, and unhinge neural circuits linked by one synapse. This molecular revolution is fueling our ability to uncover the elegant underpinnings of how gut sensory epithelial cells make sense of the gut luminal environment. Besides the well stablished paracrine function of enteroendocrine cells, synaptic neurotransmission is becoming an additional and attractive function of these cellular transducers. Enteroendocrine cells are electrically excitable gut transducers. They serve as the “eyes of the gut” by converting a stimulus into a purposeful electrical signal. Therefore, for the remainder of this review, we refer to them as gut epithelial sensors.
At the beginning of cellular life was food
The gut and the brain are exchanging constantly feelings from food and bacteria. As with anything, this entangled language was not as complex in the beginning. Over 600 million years ago, single cells sensed the need to aggregate as multiple cells to consume larger, more complex meals. They developed cellular junctions to co-exist as multicellular animals. An elegant example of one of the first multicellular organisms is Trichoplax adhaerens. The only existing member of the phylum Placozoa4 and a true “wandering gut.” It is a flat, round, 1-millimeter diameter, gliding creature with 1 dorsal and 1 ventral epithelial layer.
With only six cell types, Trichoplax had already a dedicated cell on its ventral side to sense nutrients.5 Although the division of labor is clear, all cells are dedicated in one way or another to finding and ingesting food, typically algae or bacteria. The ventral layer has three cell types: 1) ciliated cells that beat their cilia to propel the animal towards food; 2) lipophilic cells that secrete enzymes to digest the food; and 3) neurosecretory cells or ventral sensors. These neurosecretory cells have one slender cilium to detect algae. This cilium has voltage-gated calcium channels (Cav2, Cav3), and secretory vesicle-associated SNARE proteins (syntaxin, synaptobrevin, SNAP-25).5 In the presence of algae, they release an endomorphin-like peptide –an ancestor of modern mammalian neuropeptides.6 In humans, endomorphins are endogenous opioids that bind to mu-opiod receptors to mediate several functions. In the brain, endomorphins can mediate broad functions such as appetite driven by reward, and in the gut they can mediate functions like motility7. Trichoplax displays similar behaviors. When it senses algae, the endomorphin-like peptide arrests ciliary beating and slows motility to cease gliding and begin ingestion of the algae.
But the peptide signaling cascade does not stop with the individual animal. The endomorphin-like peptide also serves as a social signal. It triggers a domino effect by stimulating neurosecretory cells from adjacent animals to secrete more neuropeptide. Much like bison on a grassy plain in Yellowstone, an individual Trichoplax animal can initiate a signaling cascade that causes an entire colony to slow down and graze on algae. Algae is then broken down for absorption by digestive enzymes released from lipophilic cells. The sated animal then resumes gliding, and so do its neighbors.4–6 Trichoplax is a remarkable example of a simple animal in which feeding behavior is orchestrated by a sensory neurosecretory cell that transduces information from available food to the rest of the body. This secretory cell has survived throughout evolution and persisted despite species extinction. From wasps to whales, gut epithelial sensors appear in every gut of the animal kingdom.8,9
The alphabet of the bowel
In mammals, the knowledge of gut sensory biology is scattered and recent. Some other sensory epithelial cells, where transduction mechanisms are known with precise detail (olfactory receptor neurons, taste cells, inner ear hair cells), are grouped in specific regions of an organ, facilitating their identification, isolation, and interrogation. However, to survey the luminal contents of the entire bowel, gut epithelial sensors are diffused among a vast number of epithelial cells.
Gut epithelial sensors were first documented by the German Physiologist Rudolph Heidenhain in 1870. Heidenhain reported a unique group of gastrointestinal cells with “yellow” chromate staining properties.10 Thereafter the field of gut sensory biology progressed slowly, stunted by the lack of tools and the challenge of spotting a cell among 1000 other epithelial neighbors. The most popular tools at the time were: heavy-metal histochemistry and deductive speculation. For the first 50 years, their function was unclear but who found them was not: “clear cells of Kultschitzky (1897)”,11 “enterochromaffin cells of Ciaccio (1907)”,12 “argentaffin cells of Mason (1914)”.13
In the subsequent 50 years, there were two main revelations: 1) the term paracrine, coined by Friedrich Feyrter (1938), was used to describe the effect of “clear cells” on neighboring cells and tissues;3 and 2) the APUD concept, proposed by Anthony Pearse (1968), brought up the debate that a group intestinal epithelial cells could be of neural crest origin.14 APUD was used to describe epithelial cells that readily took on amine precursors such as 5-hydroxytryptophan (5-HTP) and dihydroxyphenylalanine (DOPA). Eventually, the hypothesis faded out of the literature, unsubstantiated by lack of direct evidence and limited tools for the study of single cells.
Instead, the field turned its focus to gut peptides. Around the 1960s, cell signaling research was catalyzed by three emerging technologies: 1) transmission electron microscopy, 2) monoclonal antibodies, and 3) solid phase peptide synthesis. Bruce Merrifield (1963)15 invented the latter method enabling the synthesis of just about any peptide. In only 20 years, over 20 gut peptides were identified in the intestinal and colon mucosa.16 Each peptide was assigned its own cell. Referring to the cell types quickly became a cumbersome task. Most had one or more peptide, some had none, and some had a range of morphological features. Therefore, in 1977, at a scientific congress in Lausanne-Switzerland, an alphabetic solution was proposed suitable for what was known about gut sensory epithelial cells at the time.17 Alphabet letters were assigned to different hormone peptides – S for secretin, A for Ghrelin, D for somatostatin – and the S, L, A, N, G cells of the gut were born.
For 40 years, this classification trapped the field under the notion that one cell will only secrete one neuropeptide. For instance, those cells containing cholecystokinin were classified as I cells. Those containing gastric inhibitory polypeptide were called K cells. And when researchers began adding more antibodies to their solutions and finding that one cell could immunoreact with more than one peptide antibody, the issue was solved by referring to the cells as I/K. However, recent advances in single cell molecular technologies are unraveling the sophistication of each one of these tasteful gut epithelial sensors. This past November (2017), a single-cell survey of intestinal epithelial cells, analyzed the transcriptome of 533 individual enteroendocrine cells, and revealed at least 12 different subsets.18 The scientists clustered the cells based on maturity and secretory peptides. All of the clusters expressed secretin. Not surprisingly, regional differences were observed, after all, the rich chyme tasted by the duodenum would have been transformed by the time it arrives in the ileum. Similar reports have been confirmed recently.19 At the peptide level, in our laboratory we found that the same cell type can synthesize up to 11 neuropeptides. What is remarkable is that a single gut sensor can express both satiety-inducing peptides (PYY, CCK, GLP1) and the hunger-inducing ghrelin.19 Which peptide is secreted appears to depend on whether the activated G-protein coupled receptors engages an inhibitory Gi protein or excitatory Gs/Gq protein.20 By recognizing the need for the nomenclature to evolve, it is becoming possible to vocalize the molecular symphony of how gut epithelial sensors transform luminal signals into meaningful chemo-electrical codes.
Tasting calories in chyme
Sensing nutrients is a complex task executed in an elegantly simple manner. A meal is deconstructed down to simple chemicals recognized by dedicated receptors on the surface of an electrically excitable cell. For example, ingested milk. Ten ounces of whole milk offers: water (8.7oz.), carbohydrates (0.49oz.), fat (0.34oz.), proteins (0.33oz.), 10 minerals, 12 vitamins, and numerous microorganisms. In the stomach, it is broken down into nutrient rich chyme.21 At least for macronutrients, the mechanisms of gut sensory transduction have begun to emerge in the last 10 years.22 For micronutrients, as well as mechanical and thermal stimuli, the gut is the last frontier for sensory exploration.
Here is a brief account of how the gut epithelial sensors recognize the most abundant monosaccharide in nature, D-glucose. In 2008, Professor Gribble and colleagues published an article entitled Glucose sensing in L cells: a primary cell study.23 Up until then, the cellular mechanisms of gastrointestinal sensory function had been inferred using indirect methods. GLUTag and STC1 cells, both cell lines isolated from intestinal tumors, were the most popular models. These cells secrete glucagon-like peptide 1 (GLP1) and other peptides, which made them an attractive tool to identify ligands that induce secretion of gut peptides.24 Professor Gribble and colleagues advanced the field by using BAC transgenics to target the yellow fluorescence protein Venus to the coding region of proglucagon. The result was a mouse in which cells that secrete GLP1 (and an array of other peptides) were readily visible under UV light. The cells could now be isolated by fluorescence from other epithelial cells and their sensory receptors identified. The first question was simple – can these cells sense glucose?
The foundation to answer this question was established at least 25 years earlier. By the early 1980s, it was known that pancreatic β cells sensed glucose and released insulin. The response was dependent upon the absorption and catabolism of glucose inside the cell, which in turn reduced the cell’s permeability to potassium. Then, in 1984, Frances Ashcroft, now a Royal Society Research Professor at Oxford University and Gribble’s early mentor, discovered that the glucose sensing mechanism involved in the closing of KATP channels.25 The KATP channel is made of 8 proteins: 4 regulatory SUR1 and 4 pore-forming Kir6.2. As glucose is catabolized, intracellular ATP molecules accumulate. ATP binds to the Kir6.2 subunits facing the cytoplasm, which causes the channel pore to close and the cell to depolarize.26 The Venus GLP1 cells express all components of this path including the glycolysis enzyme glucokinase. As expected, a 10mM glucose stimulus causes colonic GLP1 cells to elicit a burst of action potentials. Activating the KATP channel with its ligand tolbutamide blocks the depolarization of the membrane – indicating that the cell is capable of sensing the energy that glucose provides in the form of ATP.23 But, the Venus GLP1cells also responded to alpha-methyl-glucopyranoside.22 Often referred as αMG, this artificial sugar is transported inside the cell like glucose but, unlike regular glucose, αMG does not enter glycolysis and no ATP is accumulated inside the cell.
Gut epithelial sensors seem to be capable of recognizing both the caloric and taste values of sugars. Metabolic sensing of sugars through the KATP channel mechanism was first described in pancreatic β cells and subsequently in hypothalamic neurons.27 These cells are exposed to pretty narrow fluctuations of glucose. Depending on the time of day, meals, and physiological status, blood glucose can range from 2–5mM. However, gut epithelial sensor cells are exposed to much larger concentrations and ranges of sugars entering the gut lumen. For reference, the concentration of glucose in a regular can of soda is 900mM. Therefore, a sophisticated adaptation to detect the caloric and taste values of sugars entering the gut lumen seems fit for these cells. In fact, a subset of gut epithelial sensors have the Tas1r2 and Tas1r3 proteins initially discovered as sweet receptors in taste cells28 but the molecular details of how the sensor cell separates “taste” from “caloric” value remain unclear. What is clear is that we eat sugars because we need them and because we like them.
The gut-brain situation
Our brain is fed with sensory signals from neural circuits reaching every epithelial surface of the body. In the skin, for example, Merkel cells synapse with somatosensory afferents to allow us to discriminate shapes and textures by light touch.29 Likewise, in the tongue, taste cells synapse with primary sensory neurons to allow for the perception of flavor30. But in the gut, epithelial sensors were thought to lack physical contact with nerves.31,32 As a consequence, the dogma is that the gut and the brain exchange sensory information only through the endocrine or paracrine action of hormones. The concept became popularly known as the gut-brain axis.33
This is a critical gap in how the gut epithelium transduces sensory information from food and bacteria because, as shown for other epithelial sensory cells, it is the connection with neurons that allows for rapid transmission29,30,34 and real-time fine-tuning of the excitability of the sensory cell35. This synaptic connection means that the nervous system could modulate processes like motility and nutrient absorption in real time as opposed to the 10+ minute delay we associate with satiety. Precise synaptic connectivity could also allow for temporal feedback regarding the passage of nutrients from one region of the gut to another. For these reasons, scientists have understood the need for synaptic connections, however, the identification has been elusive.
In the late 1970s, two reports attempted to document the innervation of gut epithelial sensor cells using conventional electron microscopy. Unfortunately, both attempts were unsuccessful. One reported a micrograph in which the closest nerve was within 100 nm of a putative a sensor cell give rise to the term “neurocrine” signaling.36 The second claimed to have identified a synapse, but the image resolution was poor and the structures were indistinguishable,37 so it has been largely ignored. At the time, they were limited by inadequate technology. Electron microscopy, despite its unparalleled resolution in x and y, has a limited field of view and poor resolution in z. Transgenic reporter mice did not exist so distinguishing a gut epithelial sensor cell from 10,000 other epithelial cells was a task of unparalleled difficulty at the time.
But, gut epithelial sensor cells have all the canonical features of innervated epithelial sensors. They are electrically excitable and express functional voltage-gated channels.38 Besides large dense core vesicles, they contain small clear synaptic vesicles and secrete neurotransmitters.39 Some of these cells have basal cytoplasmic processes,40–42 which we have defined as neuropods. In recent years, there has been an explosion of molecular tools for the study of neural circuits. The use of viruses to trace monosynaptic circuits is one to highlight. Aided by this (and other tools), in 2015, our group found synaptic links between gut epithelial sensor cells in the colon and colonic mucosa nerves (Figure 2).43
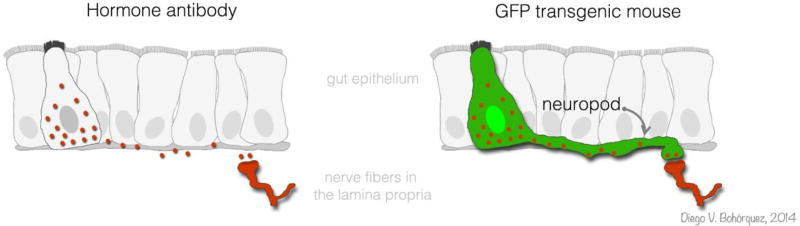
FIGURE 2. The classic view of nutrient-sensing in the gut is rapidly changing.
Gut epithelial sensor cells have been traditionally identified using hormone antibodies. But the development of transgenic mice expressing GFP in these cells has allowed us to reveal the full anatomy of gut epithelial sensor cells and their connections to neurons.
Indeed, by having the ability to identify, isolate, culture, or manipulate their genome, our group has been able to document the following features in gut epithelial sensors:
First
Gut epithelial sensors express synaptic active zone, synaptic adhesion, and postsynaptic density proteins. Almost 9 out of 10 Pyy-GFP gut epithelial sensor cells immunoreact with the pre-synaptic marker Synapsin-1. Indeed they express: Piccolo, Bassoon, Munc13b, Rims2, Latrophilin 1, and the trans-synaptic Neurexins 1 and 2.44 The finding of pre-synaptic proteins has recently been confirmed in enterochromaffin cells – a subset of gut epithelial sensors that secrete serotonin.45 They also contain DOPA decarboxylase and tyrosine hydroxylase, both essential enzymes in dopamine synthesis. These data suggest that gut epithelial sensors can form pre-synaptic terminals in which dopamine could act as a neurotransmitter. In addition, almost 30% of Pyy-GFP cells immunoreact with postsynaptic density 95 – a conserved marker of neuronal post-synapses.46 Pyy-GFP gut epithelial sensors express several genes of the post-synaptic proteins, including: PSD95, Homer 2 and 3, and the Neuroligins 1 and 2.46 Also, previous studies have shown an efferent effect in which vagal stimulation leads to the release of 5-HT from enterochromaffin cells.47,48 More recently, norepinephrine was shown to stimulate enterochromaffin cell activity.45 Therefore, the postsynaptic proteins appear to be a very important part of the puzzle because it opens the possibility to interrogate how neurons modulate sensory excitability of gut epithelial sensors through efferent action.
Second
Even in isolation, gut epithelial sensors and neurons remain capable of forming a neural circuit. We developed a method to co-culture purified Cck-GFP cells and sensory neurons. Cck-GFP cells are isolated by means of fluorescence activated cell sorting,49 and sensory neurons are dissociated from sensory ganglia by enzymatic digestion.50 Both cell types are then plated together and imaged over days using time-lapse fluorescence microscopy. In a dramatic fashion, video footage from these experiments shows clearly how individual gut epithelial sensors seek to connect with the neurons. Connections remain for days after the initial link. These experiments highlight the specific affinity between gut epithelial sensors and neurons in the absence of inputs from other cells.
Third
Gut epithelial sensors are transduced by the neurotropic rabies virus. We discovered that rabies infects gut epithelial sensors in the living animal over other epithelial cells. Using a monosynaptic strain of the neurotrophic rabies virus,51 we unveiled a monosynaptic link between gut epithelial sensors and neurons in the colon of the mouse. The system consists of three parts: 1) a rabies virus that has been stripped of its coating glycoprotein G, which is necessary for synaptic spread; 2) the rabies carries the gene for GFP; 3) a transgenic mouse that expresses G glycoprotein under the Pyy promoter. By delivering the virus into the lumen of the colon via enema, we discovered that rabies transduces gut epithelial sensors. In wild type mice, as expected, the virus does not spread beyond the epithelial layer; but in the Pyy-rabG transgenic mouse, the rabies spreads one synapse and labels nerves.
These findings have two immediate consequences: 1) given the circumstances, a pathogen in the lumen of the gut can access through this circuit the peripheral and central nervous system. Several neurodegenerative diseases, including Parkinson’s, appear to start in the bowel but their source and point of entry are unknown. This neuroepithelial represents a portal with direct access to the nervous system that could circumvent the blood brain barrier. 2) The neural circuit serves as a dedicated path for gastrointestinal sensory transduction.43 Recent evidence shows that a nutrient stimulus can suppress the activity of hypothalamic hunger AgRP neurons within seconds of entering the lumen of the intestine. The signaling involves Cck, Pyy, Serotonin, and Amylin – all found in gut epithelial sensors.52,53 Therefore, this synapse between gut epithelial sensors and nerves represents a temporal and topographical precise point of integration for gut brain signaling. This signifies a point where the gut meets the brain. And a potential node to advice our brain on the need versus desire to eat.
A closing thought:
To eat, or not to eat, that is the question: Whether ‘tis nobler in the mind to consume The fats and sugars of outrageous meals, Or to take Arms against the siren calls of cravings.
It may all be up to the gut.
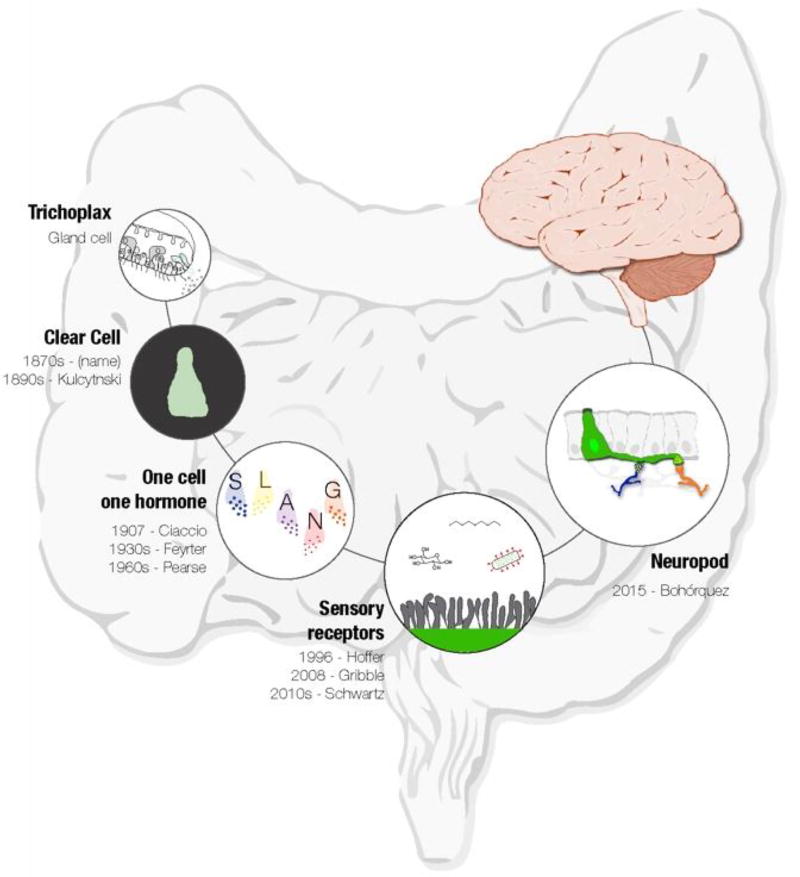
FIGURE 1. Evolution of gut sensory transduction.
Gut epithelial sensors are present throughout the animal kingdom from the first multicellular animals to the gastrointestinal tract of humans. Since first reported in 1870, these cells were deemed as a source of hormones only. However, the recent evolution of molecular tools for the study of neural circuits is rapidly advancing the field of gastrointestinal sensory biology. These are true gut transducers equipped to convert chemical, mechanical, and perhaps thermal stimuli, into meaningful chemo-electrical signals that convey signals from the gut lumen to the brain.
HIGHLIGHTS.
Gut epithelial sensor cells appeared early in evolution
Mechanical, chemical, and perhaps thermal sensory transduction mechanisms are found in gut epithelial sensor cells
An array of neuropeptides is found within the same type of gut epithelial sensor cells
Gut epithelial sensor cells form neural circuits
There is a link between gut sensory transduction and behavioral disorders
Acknowledgments
FUNDING
Funding support provided by the National Institutes of Health grants to DVB K01 DK-103832, R03 DK-114500, OT2 OD023849 (co-I), The Hartwell Foundation and The Dana Foundation.
Abbreviations
- PYY
peptide YY
- CCK
cholecystokinin
- GLP1
glucagon-like peptide 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bayliss WM, Starling EH. The mechanism of pancreatic secretion. J Physiol. 1902;28:325–353. doi: 10.1113/jphysiol.1902.sp000920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Psichas A, Reimann F, Gribble FM. Gut chemosensing mechanisms. The Journal of clinical investigation. 2015;125:908–917. doi: 10.1172/jci76309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feyrter F. In: Uber diffuse endokrine epitheliale Organe. Barth JA, editor. 1938. [Google Scholar]
- 4.Eitel M, Osigus HJ, DeSalle R, Schierwater B. Global diversity of the Placozoa. PLoS One. 2013;8:e57131. doi: 10.1371/journal.pone.0057131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith CL, et al. Novel cell types, neurosecretory cells, and body plan of the early-diverging metazoan Trichoplax adhaerens. Curr Biol. 2014;24:1565–1572. doi: 10.1016/j.cub.2014.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Senatore A, Reese TS, Smith CL. Neuropeptidergic integration of behavior in Trichoplax adhaerens, an animal without synapses. J Exp Biol. 2017;220:3381–3390. doi: 10.1242/jeb.162396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu Y, et al. In vitro characterization of the effects of endomorphin 1 and 2, endogenous ligands for mu-opioid receptors, on mouse colonic motility. Biochem Pharmacol. 2007;73:1384–1393. doi: 10.1016/j.bcp.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Tarpley RJ, Sis RF, Albert TF, Dalton LM, George JC. Observations on the anatomy of the stomach and duodenum of the bowhead whale, Balaena myst icetus. Am J Anat. 1987;180:295–322. doi: 10.1002/aja.1001800310. [DOI] [PubMed] [Google Scholar]
- 9.Hauser F, et al. Genomics and peptidomics of neuropeptides and protein hormones present in the parasitic wasp Nasonia vitripennis. J Proteome Res. 2010;9:5296–5310. doi: 10.1021/pr100570j. [DOI] [PubMed] [Google Scholar]
- 10.Heidenhain R. Untersuchungen über den Bau der Labdrüsen. Arch Mikr Anat. 1870;6:368. [Google Scholar]
- 11.Kulchitsky N. Zur Frage über den Bau des Darmkanals. Arch Mikr Anat. 1897;49:7–35. [Google Scholar]
- 12.Ciaccio C. Sopra speciali cellule granulose della mucosa intestinale. Arch Ital Anat Embriol. 1907;66:12–40. [Google Scholar]
- 13.Gosset A, Masson P. Tumeurs endocrines se lappendice. Presse Méd. 1914;25:237–240. [Google Scholar]
- 14.Pearse AG. Common cytochemical and ultrastructural characteristics of cells producing polypeptide hormones (the APUD series) and their relevance to thyroid and ultimobranchial C cells and calcitonin. Proc R Soc Lond B Biol Sci. 1968;170:71–80. doi: 10.1098/rspb.1968.0025. [DOI] [PubMed] [Google Scholar]
- 15.Merrifield RB. Solid Phase Peptide Synthesis. I. The Synthesis of a Tetrapeptide. Journal of the American Chemical Society. 1963;85:2149–2154. doi: 10.1021/ja00897a025. [DOI] [Google Scholar]
- 16.Rehfeld JF. The new biology of gastrointestinal hormones. Physiol Rev. 1998;78:1087–1108. doi: 10.1152/physrev.1998.78.4.1087. [DOI] [PubMed] [Google Scholar]
- 17.Solcia E, et al. In: Gut Hormones. Bloom SR, editor. Churchill Livingstone; 1978. [Google Scholar]
- 18.Haber AL, et al. A single-cell survey of the small intestinal epithelium. Nature. 2017;551:333–339. doi: 10.1038/nature24489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glass LL, et al. Single-cell RNA-sequencing reveals a distinct population of proglucagon-expressing cells specific to the mouse upper small intestine. Molecular metabolism. 2017;6:1296–1303. doi: 10.1016/j.molmet.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engelstoft MS, Schwartz TW. Opposite Regulation of Ghrelin and Glucagon-like Peptide-1 by Metabolite G-Protein-Coupled Receptors. Trends Endocrinol Metab. 2016;27:665–675. doi: 10.1016/j.tem.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 21.28, N. N. D. f. S. R. R. USA - National Agricultural Library; Washington, D.C.: 2016. [Google Scholar]
- 22.Efeyan A, Comb WC, Sabatini DM. Nutrient-sensing mechanisms and pathways. Nature. 2015;517:302–310. doi: 10.1038/nature14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reimann F, et al. Glucose sensing in L cells: a primary cell study. Cell metabolism. 2008;8:532–539. doi: 10.1016/j.cmet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drucker DJ, Habener JF, Holst JJ. Discovery, characterization, and clinical development of the glucagon-like peptides. J Clin Invest. 2017;127:4217–4227. doi: 10.1172/JCI97233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashcroft FM, Harrison DE, Ashcroft SJ. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature. 1984;312:446–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- 26.Craig TJ, Ashcroft FM, Proks P. How ATP inhibits the open K(ATP) channel. J Gen Physiol. 2008;132:131–144. doi: 10.1085/jgp.200709874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parton LE, et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- 28.Jang HJ, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15069–15074. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lumpkin EA, Marshall KL, Nelson AM. The cell biology of touch. J Cell Biol. 2010;191:237–248. doi: 10.1083/jcb.201006074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaudhari N, Roper SD. The cell biology of taste. The Journal of cell biology. 2010;190:285–296. doi: 10.1083/jcb.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bertrand P. The cornucopia of intestinal chemosensory transduction. Front Neurosci. 2009;3:1–9. doi: 10.3389/neuro.21.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raybould HE. Gut chemosensing: interactions between gut endocrine cells and visceral afferents. Auton Neurosci. 2010;153:41–46. doi: 10.1016/j.autneu.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117:13–23. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeMaria S, Ngai J. The cell biology of smell. The Journal of cell biology. 2010;191:443–452. doi: 10.1083/jcb.201008163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castellano-Munoz M, Israel SH, Hudspeth AJ. Efferent control of the electrical and mechanical properties of hair cells in the bullfrog's sacculus. PloS one. 2010;5:e13777. doi: 10.1371/journal.pone.0013777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lundberg JM, et al. Ultrastructural evidence for an innervation of epithelial enterochromaffine cells in the guinea pig duodenum. Acta physiologica Scandinavica. 1978;104:3–12. doi: 10.1111/j.1748-1716.1978.tb06245.x. [DOI] [PubMed] [Google Scholar]
- 37.Newson B, Ahlman H, Dahlstrom A, Das Gupta TK, Nyhus LM. On the innervation of the ileal mucosa in the rat--a synapse. Acta physiologica Scandinavica. 1979;105:387–389. doi: 10.1111/j.1748-1716.1979.tb06357.x. [DOI] [PubMed] [Google Scholar]
- 38.Rogers GJ, et al. Electrical activity-triggered glucagon-like peptide-1 secretion from primary murine L-cells. The Journal of physiology. 2011;589:1081–1093. doi: 10.1113/jphysiol.2010.198069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nilsson O, et al. Distribution and immunocytochemical colocalization of peptide YY and enteroglucagon in endocrine cells of the rabbit colon. Endocrinology. 1991;129:139–148. doi: 10.1210/endo-129-1-139. [DOI] [PubMed] [Google Scholar]
- 40.Chandra R, Samsa LA, Vigna SR, Liddle RA. Pseudopod-like basal cell processes in intestinal cholecystokinin cells. Cell Tissue Res. 2010;341:289–297. doi: 10.1007/s00441-010-0997-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bohórquez D, Chandra R, Samsa L, Vigna S, Liddle R. Characterization of basal pseudopod-like processes in ileal and colonic PYY cells. J Mol Histol. 2011;42:3–13. doi: 10.1007/s10735-010-9302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gustafsson BI, Bakke I, Tommeras K, Waldum HL. A new method for visualization of gut mucosal cells, describing the enterochromaffin cell in the rat gastrointestinal tract. Scandinavian journal of gastroenterology. 2006;41:390–395. doi: 10.1080/00365520500331281. [DOI] [PubMed] [Google Scholar]
- 43.Bohorquez DV, et al. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. The Journal of clinical investigation. 2015;125:782–786. doi: 10.1172/jci78361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sudhof TC. The presynaptic active zone. Neuron. 2012;75:11–25. doi: 10.1016/j.neuron.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bellono NW, et al. Enterochromaffin Cells Are Gut Chemosensors that Couple to Sensory Neural Pathways. Cell. 2017;170:185–198. e116. doi: 10.1016/j.cell.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen X, et al. Organization of the core structure of the postsynaptic density. Proc Natl Acad Sci U S A. 2008;105:4453–4458. doi: 10.1073/pnas.0800897105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahlman H, et al. On the presence of serotonin in the gut lumen and possible release mechanisms. Acta Physiol Scand. 1981;112:263–269. doi: 10.1111/j.1748-1716.1981.tb06815.x. [DOI] [PubMed] [Google Scholar]
- 48.Pettersson G. The neural control of the serotonin content in mammalian enterochromaffin cells. Acta Physiol Scand Suppl. 1979;470:1–30. [PubMed] [Google Scholar]
- 49.Wang Y, et al. Amino acids stimulate cholecystokinin release through the calcium-sensing receptor. Am J Physiol Gastrointest Liver Physiol. 2010 doi: 10.1152/ajpgi.00387.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malin SA, Davis BM, Molliver DC. Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nat Protoc. 2007;2:152–160. doi: 10.1038/nprot.2006.461. [DOI] [PubMed] [Google Scholar]
- 51.Wall NR, Wickersham IR, Cetin A, De La Parra M, Callaway EM. Monosynaptic circuit tracing in vivo through Cre-dependent targeting and complementation of modified rabies virus. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21848–21853. doi: 10.1073/pnas.1011756107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beutler LR, et al. Dynamics of Gut-Brain Communication Underlying Hunger. Neuron. 2017;96:461–475. e465. doi: 10.1016/j.neuron.2017.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Su Z, Alhadeff AL, Betley JN. Nutritive, Post-ingestive Signals Are the Primary Regulators of AgRP Neuron Activity. Cell Rep. 2017;21:2724–2736. doi: 10.1016/j.celrep.2017.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]