Abstract
Acinetobacter baumannii adapts to different environmental conditions by expressing complex regulatory circuitry. Recent studies revealed that this circuitry includes regulatory factors that control the emergence of distinct bacterial subpopulations, which are critical for the capacity of this pathogen to persist in medical settings and cause infections in compromised hosts.
Keywords: Transcriptional regulation, bacterial subpopulations, resistance, persistence, virulence
The opportunistic pathogen Acinetobacter baumannii persists and thrives within the host and its surrounding environment inside and outside of medical settings. Thus, as a facultative pathogen, A. baumannii senses and responds to a myriad of intra- and extracellular signals that allow it to adapt to distinct environmental and host lifestyles through the action of different transcriptional regulatory systems, some of which are represented in Figure 1. Unexpectedly, light, one of the most ubiquitous environmental signals, is one of the cues to which A. baumannii responds to via the photoreceptor and global regulator BlsA [1, 2], which allows this pathogen to ‘see’ the surrounding world. This response together with the action of the quorum sensing-responsive regulator AbaR, which controls biofilm biogenesis among other cellular functions [3], explain the ability of A. baumannii to adjust its lifestyle to persist in the nosocomial environment, and colonize and cause severe infections in compromised hosts.
Figure 1.
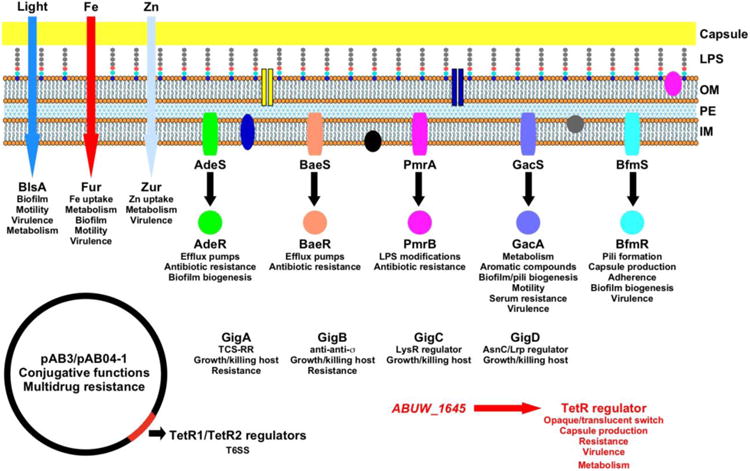
Representation of the Main Transcriptional Regulatory Systems Controlling Gene Expression in A. baumannii and Their Effects on Cellular Functions and Responses. sCircles, ovals and bars represent proteins located in the membranes or the cytoplasm. Additional transcriptional regulatory factors, including sRNA transcripts and Hfq, which control the differential expression of genes coding for persistence and virulence functions, were not included for the sake of simplicity. Abbreviations: OM, outer membrane; PE, periplasmic space; IM, inner/cytoplasmic membrane; LPS, lipopolysaccharides; TCS, two-component regulatory system; RR, response regulator; σ, sigma transcriptional factor.
Adaptation to changes in the abundance of Fe and Zn, critical metals for most living cells, through the action of the cognate metal-binding transcriptional regulators Fur and Zur, respectively, allows A. baumannii to determine its spatial location and trigger a network of cellular responses needed for its persistence and virulence [4, 5]. The A. baumannii arsenal of regulators that respond to extracellular signals also include two-component regulatory systems (TCS), which are composed of a cytoplasmic membrane-embedded sensory protein and its cognate cytoplasmic transcriptional response regulator that effects transcriptional regulation via phosphorylation. These systems are major players in A. baumannii pathophysiology; they regulate genes encoding drug efflux pumps and lipopolysaccharide modification functions involved in antibiotic resistance, as is the case with the AdeRS, BaeSR and PmrAB regulatory systems [6]. BfmRS is another critical regulatory system. It controls the interaction of A. baumannii with abiotic surfaces, cell morphology, antibiotic resistance, capsule production, and is essential for colonization, persistence and pathogenicity in invertebrate and vertebrate hosts [6]. Equally relevant is the role of the GacAS system that controls the expression of genes coding for a wide range of functions involved in metabolism and virulence, including the transcription of the paa operon. This particular operon codes for the catabolism of aromatic compounds that affect host innate immune responses [6].
Recent work by Weber et al. [7] showed that TetR-like regulators, which are encoded by the conjugative resistance plasmids pAB3 and pAB04-1 in the ATCC 17978 and Ab04 strains, respectively, play a key role in the capacity of these isolates to persist in the presence of antibiotics without expressing type 6 secretion system (T6SS) antimicrobial functions needed outcompete other microorganisms. However, the absence of antibiotic pressure, particularly outside the human host, results in a plasmid-cured bacterial subpopulation that outcompetes other microorganisms located in the same niche. This subpopulation could eventually facilitate the reemergence of resistant derivatives after acquisition of the aforementioned plasmids by conjugation.
Analysis of A. baumannii AB5075 using Tn-Seq and the Galleria mellonella infection model further enhanced our understanding of key regulatory functions in this pathogen [8]. This approach identified 32 virulence genes coding for AraC-, AsnC/Lrp-, LysR-, MarR- and TetR-like regulators, TCS and an anti-anti-sigma factor, most of which have not been experimentally characterized. Further analyses resulted in the identification of GigA, GigB, GigC and GigD as critical regulators required for growth within and killing of infected larvae, with the two first regulators also required for antibiotic resistance.
Interestingly, the work by Gebhardt et al. [8] also identified ABUW_1645 as a critical virulence factor, the role of which has remained unknown until the newest report by Chin et al. [9] showing that this TetR-type transcriptional regulator indeed plays role in the emergence of two different subpopulations with distinct phenotypes. This valuable and timely report not only represents a valuable extension of Dr. Rather's original observations on AB5075 phase variability [10], but also provides novel and interesting insights into A. baumannii's pathophysiology. The authors [9] showed that AB5075's virulence and resistance to disinfectants, desiccation and host factors depend on the emergence and prevalence of a subpopulation of bacterial cells expressing the virulent-opaque (VIR-O) phenotype, which is directly associated with capsule production when compared to avirulent-translucent (AV-T) derivatives. The observation that BfmRS also regulates capsule production [6] suggests that a rather complex regulatory circuitry controls this trait by unidentified mechanisms. The fact that all isolates obtained from infected patients were VIR-O type demonstrate that this is a predominant virulence phenotype that is not strain specific [9]. This outcome matched the finding that animal infections resulted in the selection of VIR-O cells, even when the inocula contained equal number of AV-T cells, which converted to and maintained the VIR-O phenotype during infection. Transcriptional analyses showed that ABUW_1645 was one of the highest differentially-expressed genes when VIR-O and AV-T cells were compared. Elegant phenotypic, resistance and virulence studies using an AB5075 derivative overexpressing ABUW_1645 proved that the product of this gene is the only bacterial factor needed to control the high-frequency AV-T/VIR-O phenotypic switch. Furthermore, this low-virulence derivative proved to be an efficient live-attenuated vaccine against A. baumannii.
In summary, ABUW_1645 acts as a key master regulator that allows A. baumannii to adjust its lifestyle either to harsh conditions imposed by the host and medical settings or the nutrient-limiting conditions it encounters in natural environments throughout its poorly understood life cycle. It is also apparent that the emergence of cell subpopulations expressing distinct and critical phenotypes required to persist in different niches is becoming a recurrent theme in the pathobiology of A. baumannii. The study of these cellular and molecular responses, particularly the nature of the regulatory signals and the potential connections among the components of a predictable complex and interconnected regulatory circuitry, as suggested by the effect of Zur and ABUW_1645 on the expression of TetR- and AsnC/Lrp-like regulators [5, 9], should provide a deeper understanding of A. baumannii's physiological and virulence functions. Ultimately, this knowledge could facilitate the identification of alternative bacterial targets required for the development of effective therapeutics critically needed to treat A. baumannii infections, particularly those caused by multi-drug resistant isolates.
Acknowledgments
Current work in the Actis laboratory is supported by National Institutes of Health Public Health grant R15GM117478-01 as well as by Miami University research funds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Muller GL, et al. Light modulates metabolic pathways and other novelphysiological traits in the human pathogen Acinetobacter baumannii. J Bacteriol. 2017;199:e00011–17. doi: 10.1128/JB.00011-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mussi MA, et al. The opportunistic human pathogen Acinetobacter baumannii senses and responds to light. J Bacteriol. 2010;192:6336–6345. doi: 10.1128/JB.00917-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niu C, et al. Isolation and characterization of an autoinducer synthase from Acinetobacter baumannii. J Bacteriol. 2008;190:3386–3392. doi: 10.1128/JB.01929-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eijkelkamp BA, et al. Investigation of the human pathogen Acinetobacter baumannii under iron limiting conditions. BMC Genomics. 2011;12:126. doi: 10.1186/1471-2164-12-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mortensen BL, et al. Acinetobacter baumannii response to host-mediated zinc limitation requires the transcriptional regulator Zur. J Bacteriol. 2014;196:2616–2626. doi: 10.1128/JB.01650-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kröger C, et al. Genetic regulation of virulence and antibiotic resistance in Acinetobacter baumannii. Genes. 2016;8:12. doi: 10.3390/genes8010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weber BS, et al. A multidrug resistance plasmid contains the molecular switch for type VI secretion in Acinetobacter baumannii. Proc Natl Acad Sci USA. 2015;112:9442–9447. doi: 10.1073/pnas.1502966112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gebhardt MJ, et al. Joint transcriptional control of virulence and resistance to antibiotic and environmental stress in Acinetobacter baumannii. MBio. 2015;6:e01660–15. doi: 10.1128/mBio.01660-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chin CY, et al. A high-frequency phenotypic switch links bacterial virulence and environmental survival in Acinetobacter baumannii. Nat Microbiol. 2018;3:563–569. doi: 10.1038/s41564-018-0151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tipton KA, et al. Phase-variable control of multiple phenotypes in Acinetobacter baumannii strain AB5075. J Bacteriol. 2015;197:2593–2599. doi: 10.1128/JB.00188-15. [DOI] [PMC free article] [PubMed] [Google Scholar]


