Abstract
This study examined the effects and mechanisms of strontium ranelate (SrRn)—a drug used to treat osteoporosis—on the proliferation and differentiation/mineralization of cloned dental pulp-like cells (mouse dental papillae cells; MDPs). It also determined whether topical application of SrRn to exposed dental pulp tissue promotes the formation of mineralized tissue in vivo. The MDPs were cultured with or without SrRn, and cell proliferation, odonto-/osteoblastic gene expression, mineralized nodule formation, and Akt phosphorylation were evaluated. The formation of mineralized tissue in SrRn-treated pulp tissue in rat upper first molars was evaluated histologically. The SrRn up-regulated cell proliferation and expression of Alp (alkaline phosphatase), Bsp (bone sialoprotein), Dmp (dentin matrix acidic phosphoprotein)-1, Dspp (dentin sialophosphoprotein), and Oc (osteocalcin) in a dose-dependent manner. Mineralized nodule formation was also enhanced by SrRn. NPS-2143, a calcium-sensing receptor (CaSR) antagonist, and siRNA against the CaSR gene blocked SrRn-induced proliferation, odonto-/osteoblastic gene expression, and mineralized nodule formation. SrRn induced Akt phosphorylation, and this was blocked by NPS-2143. Topical application of SrRn to exposed rat molar pulps induced the formation of osteodentin-like mineralized tissue. Our study revealed for the first time that SrRn promotes proliferation and odonto-/osteogenic differentiation/mineralization of MDPs via PI3K/Akt signaling activated by CaSR in vitro; mineralized tissue forms from the dental pulp in vivo.
Subject terms: Reverse transcription polymerase chain reaction, Calcium signalling, Cell proliferation, Differentiation
Introduction
Vital pulp therapy has been practiced for over 200 years1. This procedure involves the application of a pulp-capping agent onto a remaining thin layer of dentin (indirect pulp capping) or an exposed/excavated pulp tissue (direct capping/pulpotomy)2,3. The physical and biological requirements of an ideal direct pulp capping agent include adherence to tooth substrate, maintenance of a sufficient seal, insolubility in tissue fluids, dimensional stability, nontoxicity, lack of carcinogenicity and genotoxicity, radiopacity, and capacity to stimulate hard tissue (tertiary dentin) formation4–6.
Calcium hydroxide (CH) and mineral trioxide aggregates (MTA) are the two most popular pulp-capping materials7. CH was introduced in the 1930s, and it is the most common agent used in vital pulp therapy2,8. CH induces necrosis when applied directly to the pulp tissue due to its high alkalinity. There can be mild inflammation in the subjacent pulp tissue. Next, tissue repair due to inherent reparative capacity of the pulp progresses to form mineralized tissue called a dentin bridge9. One drawback of CH is that it is soluble, and the CH-applied area will become “dead space” over time10. Furthermore, the first-formed hard tissue of any dentin bridge by CH is irregular with tubular openings or a canalicular lumina containing vessels and cells11. These structural defects are termed “tunnel defects”, and they may induce microleakage and subsequent inflammation of the remaining pulp tissue12. MTA was originally introduced as a root-end filling material13. It has recently been widely investigated as a material for direct pulp capping14,15. MTA has a “gentle” hard tissue-inducing capacity8,16, and its hard tissue-inducing mechanisms are similar to those of CH17. Currently, CH, MTA, and their derivatives are generally used for vital pulp therapy, but more effective pulp capping agents/materials with active dentin-like tissue-inducing capacity might increase the success rate of vital pulp therapy.
Strontium ranelate (SrRn) contains two strontium atoms coupled by ranelic acid. It is used to treat osteoporosis18. Previous clinical trials have shown that SrRn significantly improves bone mass/quality and increases bone strength via changes in the bone matrix properties and bone mineral density in osteoporotic patients18. SrRn is promising in the treatment of symptomatic osteoarthritis19. SrRn has gained attention in osteoporosis therapy because it offers two mechanisms of action, i.e., induction of osteoblastogenesis and suppression of osteoclastogenesis20,21. Furthermore, local application of SrRn with a collagen sponge to artificial bone defects formed in rat calvaria promotes the regeneration of bone tissue22. These findings indicate the potent mineralized tissue-forming capacity of SrRn and suggest that SrRn can be used on dentin (indirect pulp capping) and dental pulp tissue (direct pulp capping) as a pulp-capping agent with a potent capacity to form mineralized tissue. Thus, the aims of this study were (1) to examine the effects and mechanisms of SrRn on the proliferation and differentiation/mineralization of cloned dental pulp-like cells (mouse dental papilla cells; MDPs); and (2) to determine whether topical application of SrRn to exposed dental pulp tissue promotes mineralized tissue formation in vivo.
Materials and Methods
Cell culture and chemicals
The MDPs were derived from the incisor apical buds of ICR mice and were immortalized by transfection of human papillomavirus type 16 E6 gene missing the PDZ domain binding motif 23,24. They were cultured in alpha-modified minimum essential medium (α-MEM, Wako Pure Chemical Industries, Osaka, Japan) with 10% fetal bovine serum (FBS, HyClone/GE Healthcare, UT, USA) and an antibiotic and anti-fungal solution (penicillin-streptomycin-amphotericin B suspension; Wako Pure Chemical Industries) at 37 °C/5% CO2/100% humidity. SrRn (LKT Laboratories, St Paul, MN, USA) and calcium chloride (CaCl2; Wako Pure Chemical Industries) were dissolved in α-MEM directly and sterilized with a syringe filter (Millex-HA filter, Merck Millipore, Darmstadt, Germany). NPS-2143 hydrochloride (dissolved in dimethylsulfoxide, 1.0 µM; Cayman Chemicals, Ann Arbor, MI, USA) and LY294002 (dissolved in dimethylsulfoxide, 1.0 µM; Cayman Chemicals) were used as selective and potent calcium-sensing receptor (CaSR) antagonists25 along with a potent pan-PI3K/Akt inhibitor26, respectively.
Proliferation of MDPs
MDPs (5 × 103 cells/well) were seeded in 96-well plates. After 24 h of culturing, the media was changed to contain the test agents. Cell proliferation was measured with the WST-8 assay (CCK-8, Dojindo Molecular Technologies, Kumamoto, Japan) at 48 and 72 h.
Odonto-/osteoblastic gene expression
MDPs (5 × 104 cells/well) were seeded in 12-well plates. After 24 h of culture, the media was changed to include the test agents, and cells were cultured for 72 h. The total RNA was extracted by QuickGene RNA cultured cell kit S (Wako Pure Chemical Industries). The cDNA was converted from extracted RNA using Primescript (Takara, Shiga, Japan). The qPCR assays were performed with specific primers (Table 1) and GoTaq qPCR Master Mix (Promega, Madison, WI, USA) using a CFX96 Real-Time PCR Detection Systems (Bio-Rad, Hercules, CA, USA). Gyceraldehyde-3-phosphate dehydrogenase (Gapdh) was the internal control.
Table 1.
Primer sequences.
| Genes | Upper Primers | Lower Primers | Gene bank No. | Size |
|---|---|---|---|---|
| Gapdh | 5′-TGACGACTTCAACAGCAACTC-3′ | 5′-ATGTAGGCCAATGAGGTCCAC-3′ | BC096590 | 143 |
| Alp | 5′-GATTACGCTCACAACAACTACCAG-3 | 5′-GGAATGTAGTTCTGCTCATGGAC-3′ | NM_007431 | 140 |
| BSP | 5′-TATGAAGTCTATGACAACGAGAACG-3 | 5′-AGTAATAATTCTGACCCTCGTAGCC-3′ | NM_008318 | 121 |
| CaSR | 5′-AATGACACTTTGAACAGACACCAG-3′ | 5′- ATTTCATCTGGGCTTTCTATTTCTG-3′ | NM_013803.3 | 139 |
| Dmp-1 | 5′-CGTTCTGAGGAAGACAGTGACTC-3′ | 5′-TTAGTTTCCTACTGTCAGCTTCCAT-3′ | NM_016779 | 130 |
| Dspp | 5′-AAGGATAGCAGTTCTGACAGCAG-3′ | 5′-AATCATCACTGGTTGAGTGGTTCCT-3′ | NM_010080 | 136 |
| Oc | 5′-CATACTGGTCTGATAGCTCGTCAC-3′ | 5′-AGGGCAATAAGGTAGTGAACAGAC-3′ | NM_007541 | 129 |
Mineralized nodule formation
MDPs (1 × 104 cells/well) were seeded in 48-well plates and cultured in Opti-MEM (Thermo Fisher Scientific, Waltham, MA, USA) containing 10% FBS. After 24 h of culturing, the media was changed to an osteogenic medium containing L-ascorbic acid (0.2 mM; Wako Pure Chemical Industries) and β-glycerophosphate (5.0 mM; Sigma Aldrich, St. Louis, MO, USA) with or without test agents. Mineralized nodules were stained with alizarin red S (Wako Pure Chemical Industries) after 7 d of culture. The density of the mineralized nodules was measured by ImageJ software v2 (https://imagej.net/ImageJ2).
Western blotting
MDPs (2 × 105 cells/well) were seeded in 24-well plates. Test agents were added to the media after 24 h of culture. Cells were lysed with a RIPA buffer (20 mM Tris/HCl pH 7.4, 150 mM NaCl, 10 mM MgCl2, 5 mM EDTA, 1% NP-40, 5% glycerol) containing cOmplete Ultra (Merck Millipore) and PhosSTOP EASY (Merck Millipore). Cell lysates were applied to SDS-PAGE (10%), and the gel was transferred to a PVDF membrane (Immobilon-P, Merck Millipore) using a semi-dry transfer system (0.15 mA, 1 h; WSE-4040, ATTO, Tokyo, Japan). Transferred membranes were incubated with anti-Akt (1:1000; polyclonal, GTX121936, GeneTex, Los Angeles, CA, USA) and anti-p-Akt (1:500; polyclonal, GTX121936, GeneTex) antibodies overnight at 4 °C followed by horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (1:1000; Jackson Immuno Research Labs, West Grove, PA, USA) for 30 min at room temperature. Gapdh was used as a loading control (HRP-conjugated mouse anti-GAPDH, 1:1000; Clone: 3H12, Medical & Biological Laboratories Co., LTD., Tokyo, Japan). Chemiluminescent detection used an HRP substrate (Immobilon, Merck Millipore) and a digital autoradiograph imaging system (LAS-3000, Fujifilm, Tokyo, Japan). The pixel density was measured with ImageJ software v2.
siRNA transfection of the CaSR in MDPs
MDPs (1 × 105 cells/well) were seeded in 12-well plate, and CaSR siRNA (#s201124; Thermo Fisher Scientific) or negative control (siNC, Ambion Silencer Negative Control #1 siRNA, Thermo Fisher Scientific) were transfected with Lipofectamine RNAiMAX transfection reagent (Thermo Fisher Scientific). After 12 h of culture, the medium was changed to include the test agents, and the cells were cultured for 72 h.
In vivo study
All animal experiments were approved by the Animal Care and Use Committee of TMDU, all surgical methods were performed in accordance with relevant ethical guidelines and regulations (#A2017-155A). Wistar rats (n = 12, male, 5-wk-old; Clea Japan, Tokyo, Japan) were given ad libitum access to food and water prior to the experiment. The rats were anesthetized with an intraperitoneal injection of ketamine (90 mg/kg) and xylazine (10 mg/kg). The cavity preparation and pulp exposure were performed in the upper first molars of both sides with #1/2 round bars using a dental handpiece motor under a stereoscopic microscope (Dental Microscope Z; Mani, Tochigi, Japan). Bleeding in the cavities following the pulp exposure was removed with sterile cotton pellets. The SrRn (mixed with sterile water at 2 mg/µl), mineral trioxide aggregate (ProRoot MTA, Dentsply Sirona, Ballaigues, Switzerland; mixed according to the manufacturer’s instructions), or CaCl2 (mixed with sterile water at 2 mg/µl) was dressed over the exposed pulp (n = 4 in each group). No application of SrRn, MTA, and CaCl2 was used as a control. The samples applied to each cavity were randomly chosen from SrRn, MTA, CaCl2, and no application; there was no rat in which the same samples were applied contra-laterally. The cavities were sealed with glass ionomer cement (Ionosit-Baseliner, DMG, Hamburg, Germany). The rats were sacrificed by CO2 euthanasia after 3 weeks. The upper jaws were dissected from the maxilla and fixed with 4% paraformaldehyde/PBS for 24 hours at 4 °C. Samples were then demineralized using 17% EDTA (Dojindo Molecular Technologies) for 3 weeks. After demineralization, the glass ionomer cement was removed, and the samples were embedded in paraffin. Hematoxylin and eosin staining was performed on 5 µm-thick sections, and the stained sections were observed under a light microscope (Axio Vert.A1, Carl Zeiss, Oberkochen, Germany).
Statistical Analysis
All in vitro experiments were carried out in triplicates. The data were submitted to one-way ANOVA followed by Tukey’s test. The level of significance was established at *P < 0.05 or **p < 0.001 using Prism software v7 (GraphPad, San Diego, CA, USA).
Results
SrRn promoted cell proliferation and differentiation/mineralization of MDPs
First, we examined the effect of SrRn on the growth, odonto-/osteoblastic gene expression and mineralized nodule formation of MDPs. SrRn significantly increased the proliferation of MDPs at 48 and 72 h in a dose-dependent manner (Fig. 1A). Expression of Alp (alkaline phosphatase), Bsp (bone sialoprotein), Dmp (dentin matrix acidic phosphoprotein)-1, Dspp (dentin sialophosphoprotein), and Oc (osteocalcin) was also upregulated by SrRn in a dose-dependent manner (Fig. 1B). Osteogenic medium containing SrRn (0.1 mM) induced mineralized nodule formation (Fig. 1C). The CaCl2 failed to induce cell proliferation and mineralized nodule formation (see Supplemental Fig. 1).
Figure 1.
The effect of SrRn on proliferation, odonto-/osteogenic differentiation, and mineralization of MDPs. (A) Proliferation of MDPs was increased by SrRn at 48 and 72 h. (B) mRNA expression of Alp, Bsp, Dmp-1, Dspp, and Oc in MDPs was up-regulated by SrRn. (C) Mineralized nodule formation increased in MDPs cultured in the osteogenic medium with SrRn (0.1 mM) for 7 d. *P < 0.05 or **p < 0.001 compared to control.
CaSR is involved in the up-regulation of cell proliferation and differentiation/mineralization of MDPs induced by SrRn
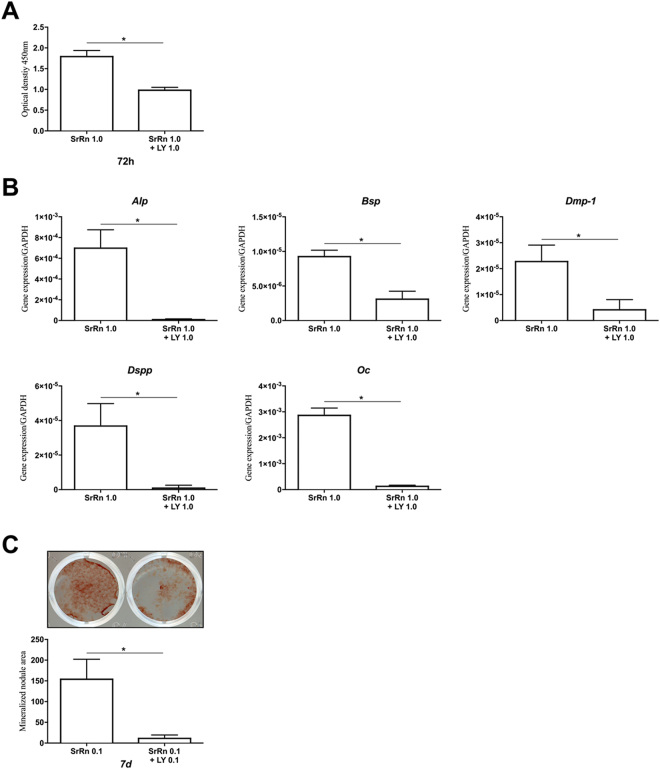
Next, we investigated the possibility that CaSR acts as a targets of SrRn in MDPs, because Sr2+ is known to activate CaSR27,28, which is involved in the control of many important cellular functions such as proliferation and differentiation29. The promoted cell proliferation and expression of Alp, Bsp, Dmp-1, Dspp, and Oc induced by SrRn on MDPs were disrupted by NPS-2143—a selective and potent CaSR antagonist (Fig. 2A,B). The CaSR siRNA down-regulated the mRNA expression of CaSR in MDPs and also suppressed the expression of Alp, Bsp, Dmp-1, Dspp, and Oc induced by SrRn in MDPs (Fig. 2C). Mineralized nodule formation promoted by SrRn in MDPs was blocked by NPS-2143 (Fig. 2D).
Figure 2.
The effect of CaSR inhibition on enhanced proliferation, odonto-/osteoblastic gene expression, and mineralized nodule formation of MDPs induced by SrRn. (A) Proliferation of MDPs enhanced by SrRn (1.0 mM) was blocked by NPS-2143 (1.0 µM). (B) mRNA expression of Alp, Bsp, Dmp-1, Dspp, and Oc in MDPs by SrRn (1.0 mM) was blocked by NPS-2143 (1.0 µM) at 72 h. (C) siRNA CaSR down-regulated the mRNA expression of CaSR in MDPs. Expression of Alp, Bsp, Dmp-1, Dspp, and Oc promoted by SrRn in MDPs was blocked by siCaSR. (D) Mineralized nodule formation promoted by SrRn in MDPs was down-regulated by NPS-2143. NPS: NPS-2143, siNC: negative control of siRNA, and siCaSR: siRNA of CaSR. *P < 0.05 compared with each other.
PI3K/AKT signaling was activated by SrRn via CaSR
We further sought to demonstrate intracellular pathways linked to CaSR in SrRn-stimulated MDPs and investigated PI3K/AKT signaling, which is known as a major signaling pathway of CaSR in osteoblasts27,30. SrRn (0.1 mM) induced upregulation of Akt phosphorylation in MDPs at 30 and 60 minutes, which was blocked by NPS-2143 (Fig. 3). LY294002-a potent pan-PI3K/Akt inhibitor, downregulated cell proliferation (Fig. 4A), expression of Alp, Bsp, Dmp-1, Dspp, and Oc (Fig. 4B) as well as mineralized nodule formation (Fig. 4C) that was promoted by SrRn.
Figure 3.
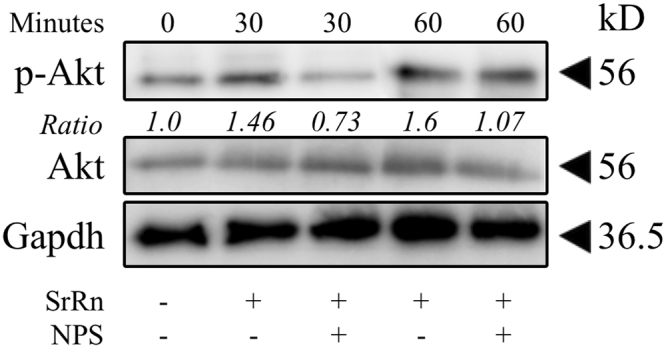
The effect of SrRn on Akt signaling in MDPs. The expression of p-Akt in MDPs was promoted by SrRn, which was down-regulated by NPS-2143. Bands of p-Akt and Akt were quantitated using densitometry, and their ratios were calculated. The experiment was performed in triplicates, and typical results are shown. NPS: NPS-2143.
Figure 4.
The effect of a PI3K/AKT inhibitor on SrRn-induced proliferation, differentiation and mineralized nodule formation in MDPs. (A) Proliferation of MDPs enhanced by SrRn is down-regulated by LY294002. (B) LY294002 down-regulated the expression of Alp, Bsp, Dmp-1, Dspp, and Oc promoted by SrRn in MDPs. (C) Mineralized nodule formation promoted by SrRn in MDPs was down-regulated by LY294002. LY: LY294002, *P < 0.05 compared with each other.
SrRn promoted mineralization of the exposed rat molar pulp in vivo
Finally, the impact of topical application of SrRn on exposed dental pulp tissue including the formation of mineralized tissue was examined in vivo. The application of SrRn to the exposed pulp tissue of rat upper first molars induced mineralized tissue formation in the pulp tissue of all specimens (Fig. 5A,B). The newly formed mineralized tissue exhibited an atubular, osteodentin-like structure with sparse or no cellular inclusions in the inner (pulp side) portion. This new tissue completely separated the pulp tissue from the pulp chamber exposed to the oral cavity. The pulp tissue appeared mostly normal with a few inflammatory cells.
Figure 5.
Mineralized tissue formation by topical application of SrRn to the exposed pulp tissue in rat upper first molars. H&E staining in (A) shows that mineralized tissue was newly formed in the coronal part of the pulp tissue after the application of SrRn. (B) A high-magnification view of the boxed area indicated in A. (C) New mineralized tissue was also formed in the coronal part of the pulp tissue after application of MTA. (D) A high-power view of the boxed area indicated in C. (E) Islets of mineralized tissues—but not continuous mineralized tissue—was formed in the pulp tissue after application of CaCl2. An abscess is formed in the exposed area of pulp tissue. (F) A high-power view of the boxed area indicated in E. (G) No mineralized tissue was formed in non-application control. (H) A high-power view of the boxed area indicated in G. AB; Abscess, BV; Blood vessel, D; Dentin, DP; Dentin pulp, MT; newly formed mineralized tissue.
MTA also induced mineralized tissue formation in the exposed pulp tissue in all specimens; the mineralized tissue had irregularly aligned tubules and fewer cellular inclusions compared to the SrRn-applied specimens. The underlying pulp tissue appeared essentially normal (Fig. 5C,D). In contrast, application of CaCl2 failed to induce continuous mineralized tissue formation; some islets of mineralized tissues were formed in the dental pulp tissue. Abscess formation was detected in the exposed area of the pulp tissue (Fig. 5E,F). Some of the CaCl2-applied samples showed deposition of mineralized tissues on the surface of the root canal wall dentin. Controls without treatment (Fig. 5G,H) failed to form mineralized tissue in the exposed pulp tissue. Four specimens were examined, and the results were reproducible.
Discussion
In this study, SrRn up-regulated the proliferation of MDPs (Fig. 1A), which possess dental pulp cell properties23,24. SrRn also increased the production of human dental pulp cells (see Supplemental Fig. 2). This is consistent with prior findings showing that SrRn up-regulates the proliferation of MC3T3-E1 cells (a mouse calvaria derived osteoblastic cell line28,31) and a primary osteoblast cell line32. However, the opposite results have also been reported for MC3T3-E1 cells33. The reason(s) for such discrepancy remains unclear, but there is a large variation among the sub-clones of MC3T3-E134, and differences in the properties of different MC3T3-E1 sub-clones may result in different results during cell proliferation. The SrRn also promoted the odonto-/osteoblastic gene expression in a dose-dependent manner (Fig. 1B) and induced mineralization in MDPs (Fig. 1C). Osteoblastic differentiation by SrRn is reported in primary murine bone cells21, murine marrow stromal cells35, and MC3T3-E1 cells36. SrRn also induces commitment of osteoblastic differentiation from mesenchymal stem cells37.
One of the targets of Sr2+ is CaSR—a Class C G-protein coupled receptor29 expressed on various cells including osteoblasts and their precursors38. Osteoblasts from the conditional knockouts of CaSR exhibit delayed differentiation, reduced mineralization capacity, and altered expression of regulators of mineralization39. This suggests that CaSR signaling in osteoblasts is essential for their differentiation and mineralization. The enhanced cell proliferation, odonto-/osteogenic differentiation and mineralization induced by SrRn in MDPs were blocked by NPS-2143 (a potent CaSR antagonist) and CaSR siRNA (Fig. 2A–D). The NPS-2143 also inhibited the SrRn-induced proliferation of human dental pulp cells (see Supplemental Fig. 2). Furthermore, SrRn promoted CaSR expression in MDPs (see Supplemental Fig. 3). These results indicate that SrRn may promote the proliferation and differentiation/mineralization of MDPs via CaSR signaling.
Downstream of CaSR, the phospholipase C (PLC)/inositol-1,4,5-trisphosphate (InsP3) signaling pathway40 is widely studied, but Sr2+ is less potent than Ca2+ in stimulating inositol phosphate accumulation41. CaSR has also been linked to several signaling pathways such as the extracellular-signal-regulated kinases (ERKs) 1/242,43 and Jun amino-terminal kinase (JNK)44. The SrRn induced the phosphorylation of the Akt in MDPs (Fig. 3), and this was abolished by NPS-2143 (Fig. 3). The PI3K/Akt inhibitor LY294002 disrupted the enhanced proliferation, differentiation, and mineralization by SrRn (Fig. 4) suggesting that the PI3K/Akt pathway is a major signaling cascades of CaSR in MDPs (Fig. 6).
Figure 6.
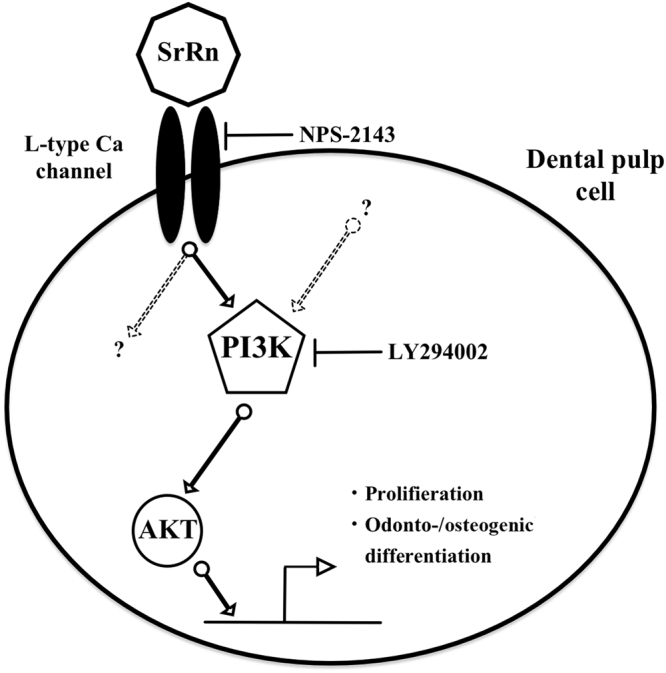
A schematic illustration of the proposed effects of SrRn on MDPs. SrRn releases Sr2+ ions entering the cytoplasm through the CaSR, and then activates the PI3K/AKT signaling pathway to promote proliferation and odonto-/osteogenic differentiation of MDPs.
Involvement of the PI3K/Akt pathway is also reported in SrRn-induced osteogenic differentiation of human osteoblasts30. The PI3K/Akt signaling stimulates canonical Wnt signaling37, and this may be involved in promoted proliferation, differentiation, and mineralization of dental pulp cells due to SrRn. We previously reported that Wnt signaling upregulates odontoblast marker expression in MDPs45, and Wnt signaling activated by SrRn via CaSR/PI3K/Akt signaling may induce odonto-/osteoblastic gene expressions of MDPs. The PI3K/Akt signaling activates the production of BMP246, which may also be involved in the differentiation and mineralization of MDPs. Ca2+ is an agonist of the CaSR with a higher affinity than Sr2+41; CaCl2 (2 mM) promotes the proliferation of MC3T3-E1 cells28. However, CaCl2 (1 mM) did not enhance the proliferation and differentiation/mineralization of MDPs in our experiment (see Supplemental Fig. 1) suggesting that 1 mM of Ca2+ concentration may be too low to induce proliferation and differentiation/mineralization of MDPs.
In vivo application of SrRn to rat upper first molars induced tissue mineralization along the exposed pulp tissue (Fig. 5A,B). Mineralized tissues formed in CH-treated pulp tissue have been reported to be irregular with tubular openings or canalicular lumina containing vessels and cells11. The mineralized tissue induced by SrRn in this study had an atubular, osteodentin-like structure with sparse or no cellular inclusions in its mineralized structure indicating that it might have sufficient capacity to protect the underlying pulp tissue from the oral environment. The MTA induced dentin-like mineralized tissue (Fig. 5C,D) as previously reported14,15, and it offers slow-release of Ca2+47. In contrast, CaCl2 failed to induce homogeneous mineralized tissue did form islets of mineralized bodies in the exposed pulp tissue (Fig. 5E,F). Ca2+ is reported to have mineralized tissue formation capacity28,47, which agrees with these findings. However, CaCl2 did not induce a continuous mineralized barrier to separate the intact pulp tissue from the oral cavity suggesting that an application method resulting in continuous and slow release of Ca2+ might be essential to the formation of a mineralized dentin-like barrier.
Systemic side effects should always be considered in developing new treatment agents. Lithium chloride (LiCl2)—a GSK3b inhibitor and potent activator of Wnt canonical signaling—has recently been reported to induce mineralized tissue formation following direct application to the rat pulp tissue48; however, a high intake of LiCl2 can cause a risk of confusion and speech impairment including a risk of death at 20 mg/L of LiCl249.
The systemic side effects of SrRn are also of note and include cerebrovascular disease, peripheral vascular disease, and prior myocardial infarction. These were reported in patients given a 2,000 mg daily systemic dose treatment of SrRn for a year50. However, it is reasonable to suppose that topical application during vital pulp therapy may pose a much lower risk of side effects compared to systemic application32,37,51,52; in vivo studies using rat models reported that systemic use of SrRn (625 g/kg) is well tolerated and safe with no adverse side effects53,54. However, it is necessary to evaluate the systemic side effects induced by the local application of SrRn before its clinical application.
In conclusion, we revealed for the first time that SrRn increases the proliferation, odonto-/osteoblastic gene expression, and mineralized nodule formation of dental pulp-like cells. This may be partially mediated by CaSR/PI3K/Akt signaling (Fig. 6). Our in vivo study revealed that topical application of SrRn induced a continuous barrier of osteodentin-like mineralized tissue on the rat exposed pulp tissue highlighting the potential utility of SrRn as a new pulp-capping material.
Electronic supplementary material
Acknowledgements
This work was supported by a Grant-in-Aid from the Japan Society for the Promotion of Science (#25293386 and #16K15795 to N.K., #17H04380 to T.O.). We thank Prof. Masahiro Saito for his offer to provide MDPs and Dr. Kayoko Ohnishi for her technical support.
Author Contributions
Dr. Alamuddin Bakhit performed all experiments including cell culture, cell growth, gene expression, mineralization induction and protein expression, in vivo pulp capping application of the materials and prepared the manuscript. Dr. Nobuyuki Kawashima organized the experiment and supervised the manuscript. Dr. Kentaro Hashimoto performed statistical analysis of this study. Dr. Sonoko Noda performed some parts of PI3K/AKT signaling cascade expression. Dr. Keisuke Nara performed some parts of cell culture and cell growth, and helped to organize the figures, Dr. Masashi Kuramoto performed some part of cell culture, Dr. Kento Tazawa performed some parts of the in vivo study and Prof. Takashi Okiji edited the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/15/2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-27461-7.
References
- 1.Dammaschke T. The history of direct pulp capping. J Hist Dent. 2008;56:9–23. [PubMed] [Google Scholar]
- 2.Bergenholtz G, et al. Treatment of pulps in teeth affected by deep caries - A systematic review of the literature. Singapore Dent J. 2013;34:1–12. doi: 10.1016/j.sdj.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Parisay I, Ghoddusi J, Forghani M. A review on vital pulp therapy in primary teeth. Iran Endod J. 2015;10:6–15. [PMC free article] [PubMed] [Google Scholar]
- 4.Camilleri J, Pitt Ford TR. Mineral trioxide aggregate: a review of the constituents and biological properties of the material. Int Endod J. 2006;39:747–754. doi: 10.1111/j.1365-2591.2006.01135.x. [DOI] [PubMed] [Google Scholar]
- 5.Roberts HW, Toth JM, Berzins DW, Charlton DG. Mineral trioxide aggregate material use in endodontic treatment: a review of the literature. Dent Mater. 2008;24:149–164. doi: 10.1016/j.dental.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Torabinejad M, Parirokh M. Mineral trioxide aggregate: a comprehensive literature review–part II: leakage and biocompatibility investigations. J Endod. 2010;36:190–202. doi: 10.1016/j.joen.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 7.da Rosa, W. L. O. et al. Current trends and future perspectives of dental pulp capping materials: A systematic review. J Biomed Mater Res B Appl Biomater, 10.1002/jbm.b.33934 (2017). [DOI] [PubMed]
- 8.Accorinte Mde L, et al. Evaluation of mineral trioxide aggregate and calcium hydroxide cement as pulp-capping agents in human teeth. J Endod. 2008;34:1–6. doi: 10.1016/j.joen.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Fitzgerald, M. Cellular mechanics of dentin bridge repair using 3H-thymidine. J Dent Res58, 2198–2206 (1979). [DOI] [PubMed]
- 10.Komabayashi T, Zhu Q, Eberhart R, Imai Y. Current status of direct pulp-capping materials for permanent teeth. Dent Mater J. 2016;35:1–12. doi: 10.4012/dmj.2015-013. [DOI] [PubMed] [Google Scholar]
- 11.Schroder U, Granath LE. Scanning electron microscopy of hard tissue barrier following experimental pulpotomy of intact human teeth and capping with calcium hydroxide. Odontol Revy. 1972;23:211–220. [PubMed] [Google Scholar]
- 12.Cox CF, Subay RK, Ostro E, Suzuki S, Suzuki SH. Tunnel defects in dentin bridges: their formation following direct pulp capping. Oper Dent. 1996;21:4–11. [PubMed] [Google Scholar]
- 13.Torabinejad M, Rastegar AF, Kettering JD, Pitt Ford TR. Bacterial leakage of mineral trioxide aggregate as a root-end filling material. J Endod. 1995;21:109–112. doi: 10.1016/S0099-2399(06)80433-4. [DOI] [PubMed] [Google Scholar]
- 14.Mente J, et al. Treatment outcome of mineral trioxide aggregate or calcium hydroxide direct pulp capping: long-term results. J Endod. 2014;40:1746–1751. doi: 10.1016/j.joen.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 15.Zhu C, Ju B, Ni R. Clinical outcome of direct pulp capping with MTA or calcium hydroxide: a systematic review and meta-analysis. Int J Clin Exp Med. 2015;8:17055–17060. [PMC free article] [PubMed] [Google Scholar]
- 16.Andelin WE, Shabahang S, Wright K, Torabinejad M. Identification of hard tissue after experimental pulp capping using dentin sialoprotein (DSP) as a marker. J Endod. 2003;29:646–650. doi: 10.1097/00004770-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Okiji T, Yoshiba K. Reparative dentinogenesis induced by mineral trioxide aggregate: a review from the biological and physicochemical points of view. Int J Dent. 2009;2009:464280. doi: 10.1155/2009/464280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilmane M, Salma-Ancane K, Loca D, Locs J, Berzina-Cimdina L. Strontium and strontium ranelate: Historical review of some of their functions. Mater Sci Eng C Mater Biol Appl. 2017;78:1222–1230. doi: 10.1016/j.msec.2017.05.042. [DOI] [PubMed] [Google Scholar]
- 19.Tenti S, Cheleschi S, Guidelli GM, Galeazzi M, Fioravanti A. What about strontium ranelate in osteoarthritis? Doubts and securities. Mod Rheumatol. 2014;24:881–884. doi: 10.3109/14397595.2014.888156. [DOI] [PubMed] [Google Scholar]
- 20.Marie PJ. Strontium ranelate: a dual mode of action rebalancing bone turnover in favour of bone formation. Curr Opin Rheumatol. 2006;18(Suppl 1):S11–15. doi: 10.1097/01.bor.0000229522.89546.7b. [DOI] [PubMed] [Google Scholar]
- 21.Bonnelye E, Chabadel A, Saltel F, Jurdic P. Dual effect of strontium ranelate: stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone. 2008;42:129–138. doi: 10.1016/j.bone.2007.08.043. [DOI] [PubMed] [Google Scholar]
- 22.Masalskas BF, et al. Local delivery of strontium ranelate promotes regeneration of critical size bone defects filled with collagen sponge. J Biomed Mater Res A. 2018;106:333–341. doi: 10.1002/jbm.a.36237. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto M, et al. Three-dimensional spheroid culture promotes odonto/osteoblastic differentiation of dental pulp cells. Arch Oral Biol. 2014;59:310–317. doi: 10.1016/j.archoralbio.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Tsubakimoto T, Kousaka K, Saito M. Immortalization of Dental Papilla Cells Differentiating into Odontoblast in vitro. J Conserv Dent. 2007;50:292–301. [Google Scholar]
- 25.Nemeth EF, et al. Calcilytic compounds: potent and selective Ca2+ receptor antagonists that stimulate secretion of parathyroid hormone. J Pharmacol Exp Ther. 2001;299:323–331. [PubMed] [Google Scholar]
- 26.Akinleye A, Avvaru P, Furqan M, Song Y, Liu D. Phosphatidylinositol 3-kinase (PI3K) inhibitors as cancer therapeutics. J Hematol Oncol. 2013;6:88. doi: 10.1186/1756-8722-6-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fromigue O, et al. Calcium sensing receptor-dependent and receptor-independent activation of osteoblast replication and survival by strontium ranelate. J Cell Mol Med. 2009;13:2189–2199. doi: 10.1111/j.1582-4934.2009.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caverzasio J. Strontium ranelate promotes osteoblastic cell replication through at least two different mechanisms. Bone. 2008;42:1131–1136. doi: 10.1016/j.bone.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Brennan SC, et al. Calcium sensing receptor signalling in physiology and cancer. Biochim Biophys Acta. 2013;1833:1732–1744. doi: 10.1016/j.bbamcr.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Rybchyn MS, Slater M, Conigrave AD, Mason RS. An Akt-dependent increase in canonical Wnt signaling and a decrease in sclerostin protein levels are involved in strontium ranelate-induced osteogenic effects in human osteoblasts. J Biol Chem. 2011;286:23771–23779. doi: 10.1074/jbc.M111.251116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caverzasio J, Thouverey C. Activation of FGF receptors is a new mechanism by which strontium ranelate induces osteoblastic cell growth. Cell Physiol Biochem. 2011;27:243–250. doi: 10.1159/000327950. [DOI] [PubMed] [Google Scholar]
- 32.Silva GA, et al. Effects of strontium ranelate treatment on osteoblasts cultivated onto scaffolds of trabeculae bovine bone. J Bone Miner Metab. 2017 doi: 10.1007/s00774-017-0822-y. [DOI] [PubMed] [Google Scholar]
- 33.Almeida MM, et al. Strontium ranelate increases osteoblast activity. Tissue Cell. 2016;48:183–188. doi: 10.1016/j.tice.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Wang D, et al. Isolation and characterization of MC3T3-E1 preosteoblast subclones with distinct in vitro and in vivo differentiation/mineralization potential. J Bone Miner Res. 1999;14:893–903. doi: 10.1359/jbmr.1999.14.6.893. [DOI] [PubMed] [Google Scholar]
- 35.Choudhary S, Halbout P, Alander C, Raisz L, Pilbeam C. Strontium ranelate promotes osteoblastic differentiation and mineralization of murine bone marrow stromal cells: involvement of prostaglandins. J Bone Miner Res. 2007;22:1002–1010. doi: 10.1359/jbmr.070321. [DOI] [PubMed] [Google Scholar]
- 36.Barbara A, Delannoy P, Denis BG, Marie PJ. Normal matrix mineralization induced by strontium ranelate in MC3T3-E1 osteogenic cells. Metabolism. 2004;53:532–537. doi: 10.1016/j.metabol.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 37.Yang F, et al. Strontium enhances osteogenic differentiation of mesenchymal stem cells and in vivo bone formation by activating Wnt/catenin signaling. Stem Cells. 2011;29:981–991. doi: 10.1002/stem.646. [DOI] [PubMed] [Google Scholar]
- 38.Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81:239–297. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- 39.Dvorak-Ewell MM, et al. Osteoblast extracellular Ca2+ -sensing receptor regulates bone development, mineralization, and turnover. J Bone Miner Res. 2011;26:2935–2947. doi: 10.1002/jbmr.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hofer AM, Brown EM. Extracellular calcium sensing and signalling. Nat Rev Mol Cell Biol. 2003;4:530–538. doi: 10.1038/nrm1154. [DOI] [PubMed] [Google Scholar]
- 41.Chattopadhyay N, Quinn SJ, Kifor O, Ye C, Brown EM. The calcium-sensing receptor (CaR) is involved in strontium ranelate-induced osteoblast proliferation. Biochem Pharmacol. 2007;74:438–447. doi: 10.1016/j.bcp.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 42.Kifor O, et al. Regulation of MAP kinase by calcium-sensing receptor in bovine parathyroid and CaR-transfected HEK293 cells. Am J Physiol Renal Physiol. 2001;280:F291–302. doi: 10.1152/ajprenal.2001.280.2.F291. [DOI] [PubMed] [Google Scholar]
- 43.McNeil SE, Hobson SA, Nipper V, Rodland KD. Functional calcium-sensing receptors in rat fibroblasts are required for activation of SRC kinase and mitogen-activated protein kinase in response to extracellular calcium. J Biol Chem. 1998;273:1114–1120. doi: 10.1074/jbc.273.2.1114. [DOI] [PubMed] [Google Scholar]
- 44.Arthur JM, Lawrence MS, Payne CR, Rane MJ, McLeish KR. The calcium-sensing receptor stimulates JNK in MDCK cells. Biochem Biophys Res Commun. 2000;275:538–541. doi: 10.1006/bbrc.2000.3226. [DOI] [PubMed] [Google Scholar]
- 45.Koizumi Y, et al. Wnt11 expression in rat dental pulp and promotional effects of Wnt signaling on odontoblast differentiation. Congenit Anom (Kyoto) 2013;53:101–108. doi: 10.1111/cga.12011. [DOI] [PubMed] [Google Scholar]
- 46.Mukherjee A, Rotwein P. Akt promotes BMP2-mediated osteoblast differentiation and bone development. J Cell Sci. 2009;122:716–726. doi: 10.1242/jcs.042770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsumoto S, et al. Calcium ions released from mineral trioxide aggregate convert the differentiation pathway of C2C12 cells into osteoblast lineage. J Endod. 2013;39:68–75. doi: 10.1016/j.joen.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 48.Ishimoto K, et al. Topical application of lithium chloride on the pulp induces dentin regeneration. PLoS One. 2015;10:e0121938. doi: 10.1371/journal.pone.0121938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aral H, Vecchio-Sadus A. Toxicity of lithium to humans and the environment–a literature review. Ecotoxicol Environ Saf. 2008;70:349–356. doi: 10.1016/j.ecoenv.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 50.Abrahamsen B, Grove EL, Vestergaard P. Nationwide registry-based analysis of cardiovascular risk factors and adverse outcomes in patients treated with strontium ranelate. Osteoporos Int. 2014;25:757–762. doi: 10.1007/s00198-013-2469-4. [DOI] [PubMed] [Google Scholar]
- 51.Elgali I, et al. Guided bone regeneration using resorbable membrane and different bone substitutes: Early histological and molecular events. Acta Biomater. 2016;29:409–423. doi: 10.1016/j.actbio.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 52.Isaac J, et al. Effects of strontium-doped bioactive glass on the differentiation of cultured osteogenic cells. Eur Cell Mater. 2011;21:130–143. doi: 10.22203/eCM.v021a11. [DOI] [PubMed] [Google Scholar]
- 53.Rosa JA, et al. Strontium Ranelate Effect on the Repair of Bone Defects and Molecular Components of the Cortical Bone of Rats. Braz Dent J. 2016;27:502–507. doi: 10.1590/0103-6440201600693. [DOI] [PubMed] [Google Scholar]
- 54.Bain SD, Jerome C, Shen V, Dupin-Roger I, Ammann P. Strontium ranelate improves bone strength in ovariectomized rat by positively influencing bone resistance determinants. Osteoporos Int. 2009;20:1417–1428. doi: 10.1007/s00198-008-0815-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.