Abstract
Ghrelin is a unique fatty acid-modified peptide hormone produced in the stomach and has important roles in energy homeostasis and gastrointestinal motility. However, the medium-chain fatty acid source for ghrelin acyl-modification is not known. We found that a fat-free diet and the removal of intestinal microbiota did not decrease acyl-ghrelin production in the stomach or plasma acyl-ghrelin levels in mice. RT-PCR analysis showed that genes involving fatty acid synthesis, metabolism, and transport were expressed in pancreas-derived ghrelinoma (PG-1) cells. Treatment with an irreversible inhibitor of carnitine palmitoyltransferase-1 (CPT-1) strongly decreased acylated ghrelin levels but did not affect ghrelin or ghrelin o-acyl transferase (GOAT) mRNA levels in PG-1 cells. Our results suggest that the medium-chain fatty acid used for the acyl-modification of ghrelin is produced in ghrelin-producing cells themselves by β-oxidation of long-chain fatty acids provided from the circulation.
Introduction
Ghrelin is a 28-amino acid peptide hormone, which was identified by Kojima and colleagues in 19991. This hormone is mainly released from the stomach and has many physiological activities such as stimulating food intake2, promoting growth hormone release3, modulating gastrointestinal motility4,5, decreasing blood pressure, and regulating energy metabolism6. In humans and rodents, plasma ghrelin levels increase in the fasting state and decrease immediately after eating7. It has also been reported that plasma ghrelin levels increased before each meal in humans who had a meal at determined time every day8,9, indicating that ghrelin plays an important role as a hunger hormone.
The structure of ghrelin is unique; the third serine residue of ghrelin is modified by medium-chain fatty acids (MCFAs) such as octanoic acid (C8) and decanoic acid (C10)10. Ghrelin is present in the blood and tissues in two forms: fatty acid-modified ghrelin called acylated- or acyl-ghrelin, and the unmodified form of ghrelin called des-acyl ghrelin. It has been well documented that this ghrelin modification is essential for the binding of ghrelin to its receptor (growth hormone secretagogue receptor; GHS-R) and the exertion of its physiological effects11. On the other hand, it has been reported that des-acyl ghrelin evokes some physiological functions, such as regulating body temperature12, increasing cardiac perfusion13, and impairing the orexigenic effects of ghrelin14, even though its receptor has not been identified15. In 2008, ghrelin o-acyl transferase (GOAT), an enzyme that specifically attaches MCFAs to ghrelin, was identified16,17. Mboat4 knockout mice and ghrelin knockout mice showed similar phenotypes regarding body weight and blood glucose metabolism18,19. Moreover, a GOAT-specific inhibitor has been developed and the administration of this GOAT inhibitor decreased body weight gain20, indicating that targeting GOAT is a potential strategy to treat ghrelin-related issues such as energy imbalance or gastrointestinal disorders.
It has been found that feeding mice with chow containing heptanoic acid (C7) leads to the production of C7-modified ghrelin, which is not naturally synthesized in vivo in mammals21. Moreover, we previously found that the oral administration of octanoic acid to hatched chicks increased the amount of acyl-ghrelin in the stomach22. These results suggested that dietary MCFAs were directly used for ghrelin modification. However, the contribution of dietary MCFAs on ghrelin acylation is still unknown and other possible sources of MCFAs remain unclear.
Fatty acids are synthesized in the liver, adipose tissue, kidney, brain, lung, and mammary glands23. Specifically, MCFAs are synthesized in mammary glands, but it is expected that the amount of MCFAs from these organs is low24. On the other hand, fatty acid metabolic pathways that shorten acyl chains of long chain fatty acids (LCFAs) are also present and LCFAs and very long-chain fatty acids (VLCFAs) can be transferred from the cytoplasm into mitochondria or peroxisomes25. Recently, Bando et al. demonstarated that LCFAs are essential for acyl modification of ghrelin in a ghrelinoma cell line (MGN3-1)26. These LCFAs become medium-chain acyl-CoA by β-oxidation, and then medium-chain acyl-CoA is transferred from mitochondria or peroxisomes to the cytoplasm after being subjected to the action of acyl-CoA thioesterase (ACOT) or carnitine octanoyltransferase (CROT)27–29.
The aim of this study was to determine the source of MCFAs that modify ghrelin; thus, we examined the effect of diet, intestinal bacteria removal, and de novo synthesis or utilization of LCFAs in ghrelin-producing cells on acyl-ghrelin production in mice.
Results
Effect of a fat-free diet on acyl-ghrelin production in mice
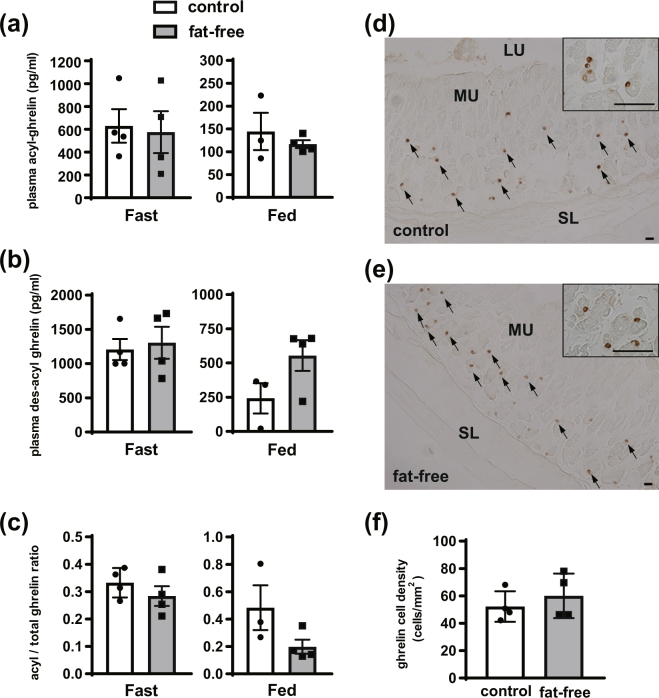
We first examined the effect of dietary fatty acids on ghrelin acylation. A fat-free diet was given to mice for 3 weeks after weaning (Fig. S1a). Although 3 weeks of fat-free diet intake tended to decrease body weight in comparison with control group mice (Fig. S2), plasma acyl-ghrelin concentrations were not altered between fasted and fed states (Fig. 1a). Des-acyl ghrelin concentrations and acyl/total ghrelin ratios also were not significantly different between groups (Fig. 1b,c). Immunohistochemical analysis using an antibody that recognizes acyl-ghrelin showed that the staining intensity of ghrelin-immunopositive cells in the gastric fundus was similar between control and fat-free groups (Fig. 1d,e). In addition, the number of ghrelin-immunopositive cells per unit mucosal area of the fat-free group was also comparable to the number in the control group (Fig. 1f).
Figure 1.
Effect of fat-free diet on ghrelin production in mice. After 3 weeks of feeding with the control or fat-free diet, plasma was collected at the fasting state (0900) and feeding state (1300). Plasma acyl-ghrelin levels (a), des-acyl ghrelin levels (b), and acyl/total ghrelin ratio (c) were not significantly different between control and fat-free groups. Staining intensities of acyl ghrelin-immunopositive cells from the control and fat-free group were similar (d and e). Ghrelin cell density in the gastric mucosa was not significantly different between the two groups (f). This experiment was replicated twice and the results of 1st data set were used in this figure. MU, mucosal layer; SL, smooth muscle layer; LU, lumen. Scale bars = 25 µm (d and e). Each value represents the mean ± SEM. n = 4.
Effect of removing intestinal bacteria on acyl-ghrelin production in mice
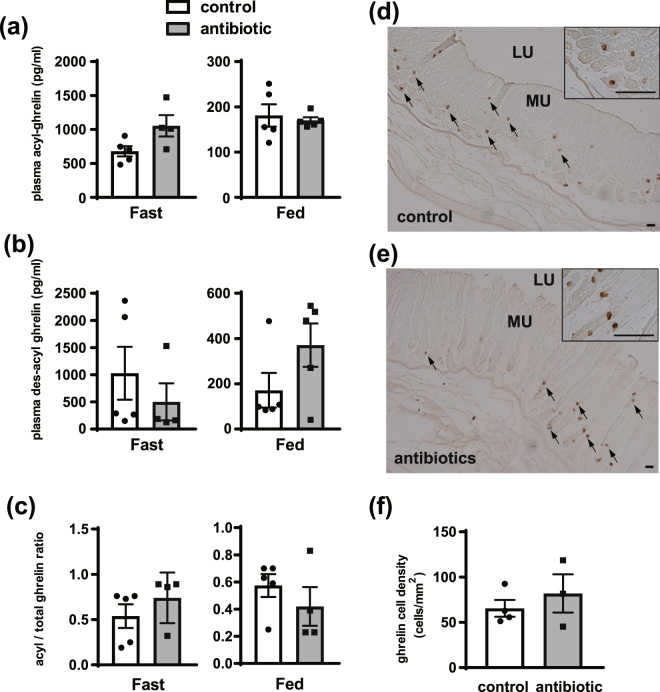
Next, we tested the usage of the fatty acids produced from intestinal bacteria (Fig. S1b). To verify successful removal of intestinal bacteria in mice, feces were incubated on brain-heart infusion plates, and we confirmed that there were no colonies in antibiotic-treated mice (Fig. S3). Plasma acyl-ghrelin concentration was higher in the antibiotic-treated group than the concentration in the control group (Fig. 2a). However, plasma des-acyl ghrelin level and acyl/total ghrelin ratio was not significantly different between groups (Fig. 2b,c). The staining intensity of the ghrelin-immunopositive cells in the gastric fundus and the number of ghrelin-immunopositive cells per unit mucosal area were not significantly different between the antibiotic group and control group (Fig. 2d,e,f).
Figure 2.
Effect of removing intestinal bacteria on ghrelin production in mice. After 5 days with or without administration of antibiotics, plasma was collected at the fasting state (0900) and feeding state (1300). Plasma acyl ghrelin level in the antibiotic group in the fasting state was higher than levels in the control group in the fasting state (a). Plasma des-acyl ghrelin level and acyl/total ghrelin ratio were not significantly different between the control and antibiotic groups (b,c, respectively). Signal intensity and the number of ghrelin-immunopositive cell in the gastric mucosa did not change after antibiotic administration (d–f). This experiment was replicated twice and the results of 2nd data set were used in this figure. MU, mucosal layer; SM, smooth muscle layer; LU, lumen. Scale bars = 25 µm (d,e). Each value represents the mean ± SEM. n = 3–5. *P < 0.05.
Expression of genes involved in fatty acid synthesis and metabolism in PG-1 cells
We then focused on de novo fatty acid synthesis in ghrelin-producing cells themselves, and examined the mRNA expression of fatty acid synthesis- and metabolism-related genes in PG-1 cells by RT-PCR. PG-1 cells expressed Acaca, Fasn, Crot, Cd36, Fabp5, Cpt1a and Acots mRNA but not Acot3 and Acot5 mRNA (Fig. 3). In addition, we identified protein production of CPT1A, FASN, and ACOT7 in PG-1 cell (Fig. S4).
Figure 3.
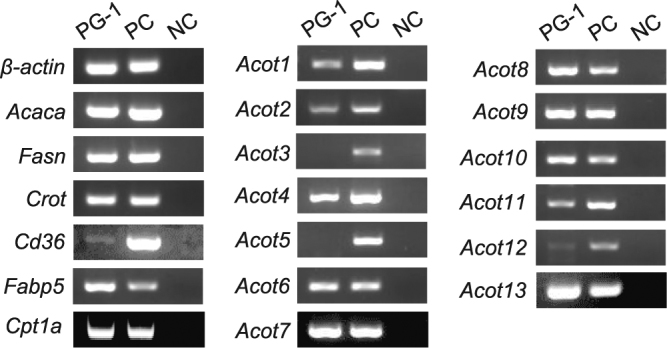
mRNA expression analysis by RT-PCR for genes involved in fatty acid synthesis and metabolism in PG-1 cells. Total RNA was extracted from PG-1 cells, cDNA was synthesized, then PCR was performed. PG-1 cells expressed acetyl-CoA carboxylase (Acaca) and fatty acid synthase (Fasn) from the fatty acid synthesis pathway. The mRNA expression of carnitine octanoyltransferase (Crot), CD36 antigen (Cd36), fatty acid binding protein 5 (Fabp5), and acyl-CoA thioesterase (Acot) 1, Acot2, Acot4, Acot6, Acot7, Acot8, Acot9, Acot10, Acot11, Acot12, and Acot13 were detected in PG-1 cells. PC, positive control: adipose tissue (β-actin, Acaca, Fasn, Crot, Cd36, and Acot1-Acot11) or liver (Fabp5, Acot12, and Acot13); NC, negative control (DW).
Effect of treating PG-1 cells with a FAS inhibitor (C75) and β-oxidation inhibitor (Etomoxir) on acyl-ghrelin production
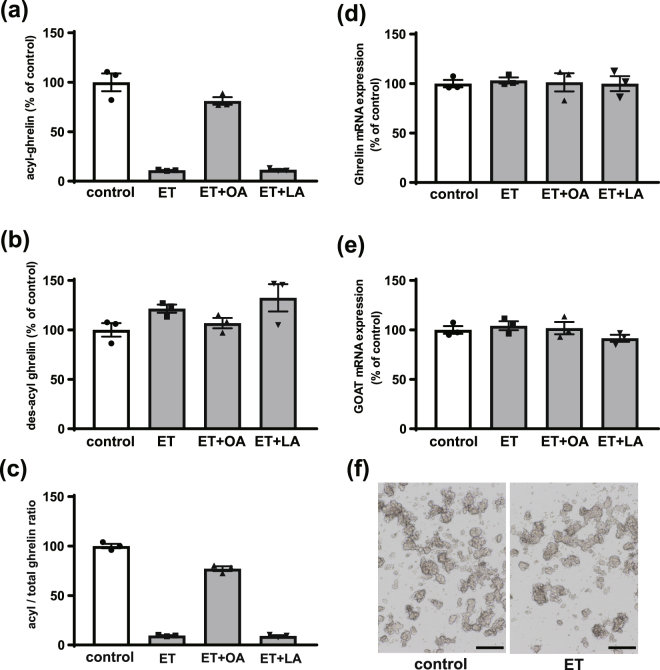
To determine the effect of fatty acid synthesis and β-oxidation on acyl-ghrelin production, we treated PG-1 cells with C75, a FAS inhibitor, and etomoxir, an irreversible carnitine palmitoyltransferase-1 (CPT-1) inhibitor. Although des-acyl ghrelin secretion was unchanged upon C75 treatment, acyl-ghrelin secretion showed a decreasing trend upon FAS inhibition compared to control (P = 0.07 by Kruskal-Wallis test) (Fig. S5a,b). C75 treatment decreased the medium acyl-ghrelin concentration, which is 307 pg/ml, whereas the control is 448 pg/ml. However, most acyl-ghrelin production was maintained (Fig. S5c). On the other hand, acyl-ghrelin production was dramatically decreased upon etomoxir treatment (Fig. 4a), and acyl-ghrelin production was rescued by co-treating with etomoxir and octanoate (Fig. 4a). LCFA treatment was not able to recover acyl-ghrelin production, however (Fig. 4a). Total ghrelin levels in culture medium were similar between all experimental groups (Fig. 4b), and the acyl/total ghrelin ratio showed the same tendency as acyl-ghrelin levels (Fig. 4c). On the other hand, etomoxir treatment did not change Ghrl and Mboat4 mRNA levels in PG-1 cells (Fig. 4d,e) and PG-1 cell morphology was not altered after etomoxir treatment (Fig. 4f).
Figure 4.
Effect of inhibiting β-oxidation on ghrelin production in PG-1 cells. PG-1 cells were plated in 24-well plates and etomoxir (ET, 5 µmol/L), octanoate (OA, 50 µmol/L), or a mixture of stearate and palmitate (LA, 50 µmol/L) was added. After incubation, acyl-ghrelin levels (a), des-acyl ghrelin levels (b), and acyl/total ghrelin ratio (c) in the medium were measured. Des-acyl ghrelin levels were not different between groups. ET treatment decreased acyl-ghrelin levels and the acyl/total ghrelin ratio, and ET + OA rescued acyl-ghrelin and acyl/total ghrelin ratio (a–c). On the other hand, Ghrl and Mboat4 mRNA expression was not changed between all experimental groups (d,e). PG-1 cell morphology did not change after the etomoxir treatment (f). This experiment was replicated twice and the results of 2nd data set were used in this figure. Scale bars=500 μm Each value represents the mean ± SEM. n = 3.
Discussion
The third serine of the ghrelin peptide is modified by MCFAs and this acyl-modification is pivotal for the physiological functions of ghrelin. Since ghrelin plays important physiological roles in maintaining homeostasis, stable production of acyl-ghrelin is necessary. For this, a sustainable MCFA supply into ghrelin-producing cells is also required to produce sufficient amounts of acyl-ghrelin. On the other hand, des-acyl ghrelin inhibits ghrelin by impairing the orexigenic effects of ghrelin14, suppressing gastric emptying30, and disrupting gastric motility31. In addition, it has been reported that des-acyl ghrelin lowers mitochondrial reactive oxygen species production and altered metabolic condition32. Previous studies found that dietary MCFAs are directly utilized for ghrelin acylation21,22. Since the discovery of ghrelin, researchers thought that the source of MCFAs for ghrelin modification originated from the diet; however, the possible contribution of other sources remains unclear.
First, we examined the effect of a fat-free diet on acyl-ghrelin production in mice. After 3 weeks of feeding mice a fat-free diet, plasma acyl-ghrelin level and the number of ghrelin-immunopositive cells in the stomach of the fat-free group were comparable with levels in the control group. This result suggests that the involvement of diet on acyl-ghrelin production is relatively minor. Thus, we next examined the effect of fatty acids produced from intestinal bacteria in mice. It is general accepted that most fatty acids produced by intestinal bacteria are short-chain fatty acids (SCFAs)33. However, some bacteria produce MCFAs. Thus, we measured acyl-ghrelin production after the removal of intestinal bacteria and found that removing intestinal bacterial did not reduce acyl-ghrelin levels in the plasma or stomach. However, in Fig. 2a,the plasma acyl-ghrelin concentration in a fasted state increased with antibiotic treatment, suggesting that intestinal bacteria could modulate plasma acyl-ghrelin levels. Recently, it has been demonstrated that some intestinal bacteria stimulate peptide hormone secretion such as GLP-134 and PYY35. Considering this function of intestinal bacteria, there is a possibility that intestinal bacteria could be associated with acyl-ghrelin secretion, but it is not thought to be the main source of acyl modifications of ghrelin because the acyl/total ghrelin ratio shown in Fig. 2c did not change with antibiotic treatment. We used mice of different ages in fat-free diet and antibiotic experiment, which may cause some differences in body condition, such as lipid metabolism, and the function and number of mitochondria. Although it is needed to take mice ages into account, acyl/total ghrelin ratio did not change between the control and treated groups in both fat-free diet and antibiotic experiment, indicating that feeding and intestinal bacteria are less relevant to acyl modification. Taken together, this finding suggests that MCFAs from de novo synthesis, which involves production from SCFAs or the breakdown of LCFAs, are utilized for ghrelin acyl modification.
In this study, we also used the pancreatic ghrelinoma cell line, PG-1. PG-1 cells express high levels of Ghrl and Mboat4 mRNA, and the addition of octanoate into cell culture medium increases the production of acyl-ghrelin by these cells36. RT-PCR analysis showed that mRNAs for genes involved in fatty acid synthesis, transport, and metabolism were expressed in PG-1 cells, indicating that ghrelin-producing cells have the ability to produce MCFAs by elongation from citrate or breakdown from LCFAs in ghrelin-producing cells themselves. Thus, we examined the effect of de novo synthesis by fatty acid synthase and found that fatty acid synthase inhibition slightly decreased acyl-ghrelin production by PG-1 cells, suggesting the involvement of this enzyme in the ghrelin acylation process. It has been reported that acyl-ghrelin stimulates the expression of fatty acid synthase in several cell types, such as the rat hypothalamic neurons37 or human differentiated adipocytes38, indicating that there is a possibility of positive feedback regulation in PG-1 cells. However, acyl-ghrelin production remained mostly intact despite fatty acid synthase inhibition in this experiment.
LCFAs are shortened by β-oxidation in mitochondria and peroxisomes25, and fatty acids in the cytoplasm are transformed into acyl-CoAs and transported to mitochondria via carnitine palmitoyltransferase-1(CPT-1) or peroxisome directly, where they are finally subjected to β-oxidation39,40. In mitochondrial β-oxidation, LCFAs (C12-C20), MCFAs (C6–12) and SCFAs (<C6) are shortened, whereas LCFAs and VLCFAs (>C22) are shortened in peroxisomal β-oxidation25. To date, 13 types of acetyl-CoA transporters (ACOTs) have been identified, and ACOT2, 9, 10, and 13 are located in the mitochondria41. On the other hand, ACOT3, 4, 5, 6, and 8 are located in the peroxisome41. In this study, we found that Acot2, Acot4, Acot6, Acot8, Acot9, Acot10, and Acot13 mRNA were expressed in PG-1 cells, suggesting that MCFAs are produced by β-oxidation from LCFAs and VLCFAs in ghrelin-producing cells. To test this, we treated PG-1 cells with a CPT-1 inhibitor, etomoxir. Etomoxir treatment dramatically reduced acyl-ghrelin production without affecting Ghrl and Mboat4 mRNA levels. In addition, co-administration of etomoxir and octanoic acid rescued acyl-ghrelin production, however, co-administration of etomoxir and a LCFA did not rescue acyl-ghrelin production. These results strongly suggested that MCFAs from β-oxidation of LCFAs or VLCFAs are predominantly used for ghrelin acyl modification.
In conclusion, we demonstrated that MCFAs resulting from β-oxidation of LCFA are utilized for ghrelin acylation in ghrelin-producing cells in vitro. Although MCFAs from the diet can be used for ghrelin acylation, it is reasonable to consider that ghrelin-producing cells can utilize the supply of stored LCFA or LCFAs produced in the body without relying on food.
Materials and Methods
Animals
Male C57BL/6 J wild-type mice (Nihonikagaku, Tokyo, Japan) were used for these experiments. Animals were housed in plastic cages under controlled conditions (23 ± 2 °C, lights on from 0800 to 2000) with ad libitum access to food and drinking water. All procedures were approved and performed in accordance with the Saitama University Committee on Animal Research.
Fat-free diet experiment
Male, 3-week-old mice were fed with the control diet (CE-2, CLEA Japan) or a fat-free diet (D04112303, Research Diets Inc., New Brunswick, NJ, USA) for 3 weeks. Mice were subjected to ad libitum feeding for the first 2 weeks, then feeding was restricted (fed from 0900 to 1300) for 1 week (Fig. S1a). After the 3 weeks, blood was collected from tail veins in the fasted state (0900) and feeding state (1300) to measure plasma ghrelin concentrations. Blood (100 µl) was collected in EDTA-coated tubes with p-hydroxymercuribenzoic acid (PHMB) at a final concentration of 1 mmol/L. Then blood was centrifuged at 4825 g for 10 min at 4 °C. The plasma was collected in a new tube and HCl was added to a final concentration of 0.1 N, and samples were stored at −80 °C until analysis. Mouse stomachs were fixed in 4% paraformaldehyde in PBS for immunohistochemistry. The experiment is repeated independently at least twice to confirm results.
Removal of intestinal bacteria
Male, 10-week-old mice were fed with a fat-free diet and subjected to restriction feeding for 1 week (from Day 0 to 8). From day 2 to 6, mice were orally administered a mixture of antibiotics: ampicillin (017–10381, Wako, Osaka, Japan), kanamycin sulfate (6169400A1036, Meiji Seika Pharma, Kanagawa, Japan), vancomycin hydrochloride (226–01301, Wako), and metronidazole (132–18061, Wako) (10 mg of each antibiotic per mouse per day). On Day 7, drinking water was changed to water containing antibiotics (ampicillin, kanamycin sulfate, metronidazole: 1 g/L; vancomycin hydrochloride; 500 mg/L) to maintain sterile conditions (Fig. S1B). Blood and stomach tissue were collected as described above on Day 8. Feces of these mice were also collected to verify removal of intestinal bacteria. The experiment is repeated independently at least twice to confirm results.
β-Oxidation inhibitor treatment in cells
PG-1 cells were plated at a concentration of 1 × 106 cells/well in 12-well plates with DMEM/F12 50:50 (Cellgro, Manassas, VA, USA) supplemented with 10% fetal bovine serum, penicillin (100 U/mL), streptomycin (100 μg/mL), and etomoxir (E1905, Sigma-Aldrich, St. Louis, MO, USA) at a final concentration of 5 µmol/L. Simultaneously, sodium octanoate (C5038, Sigma-Aldrich) or mixture of sodium stearate and palmitate (199–03285, Wako) was added at a final concentration of 50 µmol/L and cells were incubated in a 37 °C incubator with 5% CO2 for 24 hours. After incubation, the medium was collected and centrifuged at 4135 g for 5 min at 4 °C. Next, 100 μL of supernatant was transferred to a separate tube and 10 μL of 1 N HCl was immediately added to the tube. Cells were collected in ISOGEN (Nippon Gene, Tokyo, Japan) for quantification of Ghrl and Mboat4 mRNA. The collected samples were stored at −80 °C until analysis.
Measurement of ghrelin concentration
Plasma and medium concentrations of acyl-ghrelin and des-acyl ghrelin were measured by an acylated Ghrelin (mouse, rat) Express EIA Kit (A05117, SPI Bio, Montigny-le-Bretonneux, France) and an Unacylated Ghrelin (mouse, rat) Express EIA Kit (A05118, SPI Bio). Acyl-ghrelin and des-acyl ghrelin concentrations were determined according to the manufacturer’s protocol. For acyl-ghrelin ELISA, the intra-assay coefficient of variation = 5.7%, and the inter-assay coefficient of variation = 6.1%. For des-acyl ghrelin ELISA, the intra-assay coefficient of variation = 5.2%, and the inter-assay coefficient of variation = 5.5%. Absorbance data were collected by using a Bio-Rad iMark Microplate Reader spectrophotometer, using Microplate Manager 6 Software (Bio-Rad, Hercules, CA, USA). Total ghrelin was calculated by adding acyl-ghrelin and des-acyl ghrelin concentrations together.
Immunohistochemistry
After 24 hours of fixation, tissues were dehydrated with an ascending ethanol series, immersed in xylene, and then embedded in paraplast. Sections (10 µm thick) were made using a microtome and were mounted on silane-coated slides (Sigma-Aldrich). The sections were deparaffinized with xylene, rehydrated, treated with 0.5% sodium metaperiodate to block endogenous peroxidase for 10 min, treated with 1% sodium thiosulfate to clear residual iodine from sodium metaperiodate for 10 min, and then were incubated with blocking reagent (1% bovine serum albumin and 0.4% Triton X-100 in PBS) for 1 hour. After washing with PBS, sections were incubated with rabbit anti-ghrelin serum (#603)42 diluted 1:100,000 in blocking reagent for 18 hours. After washing with PBS, a second incubation with biotin-conjugated anti-rabbit IgG (Vectastain ABC kit; Vector, Burlingame, CA, USA) diluted 1:300 in blocking reagent was performed for 1 hour. Finally, the sections were incubated for 30 min with an ABC solution (Vectastain ABC kit). After washing in PBS for 15 min, sections were incubated in 0.02% 3,3-diaminobenzidine-tetrachloride (DAB) mixed with 0.006% H2O2 in 0.05 mol/L Tris-HCl (pH 7.6) for 3–5 min to detect immunostaining. After washing with water, the sections were dehydrated with a graded ethanol series, cleared in xylene, mounted with Entellan (Merck, Darmstadt, Germany), and viewed under a light microscope (BX60, Olympus, Tokyo, Japan). All procedures were performed at room temperature and incubations were carried out in a humidified chamber. After taking digital photographs of gastric fundi under a light microscope with a digital camera (DP70, Olympus), the number of acyl-ghrelin-positive cells in each section was counted and the area of the mucosal layer in each section was measured using Image J software (National Institutes of Health, Bethesda, MD). The ghrelin-producing cell density was calculated as the number of immunopositive cells per unit area. All data are expressed as mean ± SEM.
RT-PCR to analyze the expression of fatty acid synthesis and metabolism genes in PG-1 cells
PG-1 cells were cultured in DMEM/F12 supplemented with 10% fetal bovine serum, penicillin, and streptomycin (Wako) in a 37 °C incubator with 5% CO2.
Total RNA was extracted from PG-1 cells and adipose tissue from 12-week-old male C57BL/6 J mice using ISOGEN (Nippon Gene). Trace DNA contamination was removed by DNase digestion, and cDNA was synthesized from 1 µg total RNA using the reverse transcriptase PrimeScript II (Takara Bio, Shiga, Japan). The primers were designed to amplify the mouse β-actin, Acaca, Fasn, Acots, Crot, Cd36, Cpt1a and Fabp5 (Supplemental Table 1). PCR amplification was performed using Ex Taq polymerase (Takara Bio). Initial template denaturation was performed for 5 min at 94 °C. PCR cycles were programmed as follows: 30 sec at 94 °C (denaturation), 30 sec at 60 °C (annealing), and 30 sec at 72 °C (extension) for 40 cycles, followed by a final extension step for 10 min at 72 °C except for Acaca, Fasn, and Cd36 (40 sec for each cycle step). Amplified cDNAs were run on 2% agarose gels and visualized by ethidium bromide staining using a UV illuminator (ATTO, Tokyo, JAPAN).
Quantitative RT-PCR for Ghrl and Mboat4 mRNA
After the extraction of total RNA, 0.5 µg of total RNA were used for cDNA synthesis using Primescript II Reverse Transcriptase (Takara Bio). Real-time PCR was performed using a LightCycler 96 (Roche Diagnostics, Indianapolis, IN, USA) with SYBR premix Ex Taq (Takara Bio) with the following primers: mouse Ghrl (fragment size: 67 bp): forward, 5′-CCCAGGCATTCCAGGTCAT-3′, reverse, 5′-AACTGCAGATGGTGCCTGAAG-3′; mouse Mboat4 (fragment size: 71 bp): forward, 5′-TCCACAGCCTGGCTCTTTAAAC-3′, reverse, 5′-GCCGCGTGGAGGAGAGA-3′. The initial template denaturation was performed for 30 sec at 95 °C and PCR was performed with 45 cycles of 5 sec at 95 °C and 30 sec at 60 °C.
Statistical analysis
All data are expressed as the mean ± SEM and statistical analyses were performed using GraphPad Prism 5 Software (La Jolla, CA, USA). Data were analyzed using a Mann–Whitney U test or kruskal-wallis test. Differences were considered statistically significant at P < 0.05.
Electronic supplementary material
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number 15K14557.
Author Contributions
The author(s) have made the following declarations about their contributions: conceived and designed the experiments: C.I., S.T., T.S., and I.S. Performed the experiments: C.I., A.K., S.A., S.O., S.T., Z.G., R.C., D.K., and S.T. Analyzed the data: C.I., S.T., Z.G., R.C., R.W., T.T., S.T., I.S., and T.S. Wrote the paper: C.I,. S.T., S.T., T.S., and I.S.
Competing Interests
The authors declare no competing interests.
Footnotes
Chika Ikenoya and Shota Takemi contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-27458-2.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kojima M, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 2.Kojima M, Kangawa K. Ghrelin: more than endogenous growth hormone secretagogue. Ann N Y Acad Sci. 2010;1200:140–148. doi: 10.1111/j.1749-6632.2010.05516.x. [DOI] [PubMed] [Google Scholar]
- 3.Peino R, et al. Ghrelin-induced growth hormone secretion in humans. European journal of endocrinology. 2000;143:R11–14. doi: 10.1530/eje.0.143R011. [DOI] [PubMed] [Google Scholar]
- 4.Mondal A, et al. Coordination of motilin and ghrelin regulates the migrating motor complex of gastrointestinal motility in Suncus murinus. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1207–1215. doi: 10.1152/ajpgi.00379.2011. [DOI] [PubMed] [Google Scholar]
- 5.Sakahara S, et al. Physiological characteristics of gastric contractions and circadian gastric motility in the free-moving conscious house musk shrew (Suncus murinus) Am J Physiol Regul Integr Comp Physiol. 2010;299:R1106–1113. doi: 10.1152/ajpregu.00278.2010. [DOI] [PubMed] [Google Scholar]
- 6.Delporte C. Structure and physiological actions of ghrelin. Scientifica (Cairo) 2013;2013:518909. doi: 10.1155/2013/518909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toshinai K, et al. Upregulation of Ghrelin expression in the stomach upon fasting, insulin-induced hypoglycemia, and leptin administration. Biochemical and biophysical research communications. 2001;281:1220–1225. doi: 10.1006/bbrc.2001.4518. [DOI] [PubMed] [Google Scholar]
- 8.Cummings DE, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 9.Cummings DE, et al. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 10.Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 11.Ariyasu H, et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86:4753–4758. doi: 10.1210/jcem.86.10.7885. [DOI] [PubMed] [Google Scholar]
- 12.Inoue Y, et al. Central and peripheral des-acyl ghrelin regulates body temperature in rats. Biochemical and biophysical research communications. 2013;430:278–283. doi: 10.1016/j.bbrc.2012.10.137. [DOI] [PubMed] [Google Scholar]
- 13.Grossini E, et al. Intracoronary Des-Acyl Ghrelin Acutely Increases Cardiac Perfusion Through a Nitric Oxide-Related Mechanism in Female Anesthetized Pigs. Endocrinology. 2016;157:2403–2415. doi: 10.1210/en.2015-1922. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez G, et al. Des-Acyl Ghrelin Directly Targets the Arcuate Nucleus in a Ghrelin-Receptor Independent Manner and Impairs the Orexigenic Effect of Ghrelin. Journal of neuroendocrinology. 2016;28:12349. doi: 10.1111/jne.12349. [DOI] [PubMed] [Google Scholar]
- 15.Delhanty PJ, Neggers SJ, van der Lely AJ. Mechanisms in endocrinology: Ghrelin: the differences between acyl- and des-acyl ghrelin. European journal of endocrinology. 2012;167:601–608. doi: 10.1530/EJE-12-0456. [DOI] [PubMed] [Google Scholar]
- 16.Gutierrez JA, et al. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci USA. 2008;105:6320–6325. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein JL, et al. Surviving starvation: essential role of the ghrelin-growth hormone axis. Cold Spring Harb Symp Quant Biol. 2011;76:121–127. doi: 10.1101/sqb.2011.76.010447. [DOI] [PubMed] [Google Scholar]
- 19.Zhao TJ, et al. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci USA. 2010;107:7467–7472. doi: 10.1073/pnas.1002271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnett BP, et al. Glucose and weight control in mice with a designed ghrelin O-acyltransferase inhibitor. Science. 2010;330:1689–1692. doi: 10.1126/science.1196154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishi Y, et al. Ingested medium-chain fatty acids are directly utilized for the acyl modification of ghrelin. Endocrinology. 2005;146:2255–2264. doi: 10.1210/en.2004-0695. [DOI] [PubMed] [Google Scholar]
- 22.Yamato M, Sakata I, Wada R, Kaiya H, Sakai T. Exogenous administration of octanoic acid accelerates octanoylated ghrelin production in the proventriculus of neonatal chicks. Biochemical and biophysical research communications. 2005;333:583–589. doi: 10.1016/j.bbrc.2005.05.107. [DOI] [PubMed] [Google Scholar]
- 23.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 24.Mohammad MA, Sunehag AL, Haymond MW. De novo synthesis of milk triglycerides in humans. Am J Physiol Endocrinol Metab. 2014;306:E838–847. doi: 10.1152/ajpendo.00605.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy JK, Hashimoto T. Peroxisomal beta-oxidation and peroxisome proliferator-activated receptor alpha: an adaptive metabolic system. Annu Rev Nutr. 2001;21:193–230. doi: 10.1146/annurev.nutr.21.1.193. [DOI] [PubMed] [Google Scholar]
- 26.Bando M, et al. High incorporation of long-chain fatty acids contributes to the efficient production of acylated ghrelin in ghrelin-producing cells. FEBS Lett. 2016;590:992–1001. doi: 10.1002/1873-3468.12132. [DOI] [PubMed] [Google Scholar]
- 27.Hunt MC, Siponen MI, Alexson SE. The emerging role of acyl-CoA thioesterases and acyltransferases in regulating peroxisomal lipid metabolism. Biochim Biophys Acta. 2012;1822:1397–1410. doi: 10.1016/j.bbadis.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Le Borgne F, Ben Mohamed A, Logerot M, Garnier E, Demarquoy J. Changes in carnitine octanoyltransferase activity induce alteration in fatty acid metabolism. Biochemical and biophysical research communications. 2011;409:699–704. doi: 10.1016/j.bbrc.2011.05.068. [DOI] [PubMed] [Google Scholar]
- 29.Hunt MC, Rautanen A, Westin MA, Svensson LT, Alexson SE. Analysis of the mouse and human acyl-CoA thioesterase (ACOT) gene clusters shows that convergent, functional evolution results in a reduced number of human peroxisomal ACOTs. FASEB J. 2006;20:1855–1864. doi: 10.1096/fj.06-6042com. [DOI] [PubMed] [Google Scholar]
- 30.Asakawa A, et al. Stomach regulates energy balance via acylated ghrelin and desacyl ghrelin. Gut. 2005;54:18–24. doi: 10.1136/gut.2004.038737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen CY, et al. Des-acyl ghrelin acts by CRF type 2 receptors to disrupt fasted stomach motility in conscious rats. Gastroenterology. 2005;129:8–25. doi: 10.1053/j.gastro.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 32.Gortan Cappellari G, et al. Unacylated Ghrelin Reduces Skeletal Muscle Reactive Oxygen Species Generation and Inflammation and Prevents High-Fat Diet-Induced Hyperglycemia and Whole-Body Insulin Resistance in Rodents. Diabetes. 2016;65:874–886. doi: 10.2337/db15-1019. [DOI] [PubMed] [Google Scholar]
- 33.Barczynska R, et al. Intestinal Microbiota, Obesity and Prebiotics. Pol J Microbiol. 2015;64:93–100. doi: 10.1099/jmm.0.077768-0. [DOI] [PubMed] [Google Scholar]
- 34.Yadav H, Lee JH, Lloyd J, Walter P, Rane SG. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. The Journal of biological chemistry. 2013;288:25088–25097. doi: 10.1074/jbc.M113.452516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Psichas A, et al. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J Obes (Lond) 2015;39:424–429. doi: 10.1038/ijo.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao TJ, et al. Ghrelin secretion stimulated by {beta}1-adrenergic receptors in cultured ghrelinoma cells and in fasted mice. Proc Natl Acad Sci USA. 2010;107:15868–15873. doi: 10.1073/pnas.1011116107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez M, et al. Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell metabolism. 2008;7:389–399. doi: 10.1016/j.cmet.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez A, et al. Acylated and desacyl ghrelin stimulate lipid accumulation in human visceral adipocytes. Int J Obes (Lond) 2009;33:541–552. doi: 10.1038/ijo.2009.40. [DOI] [PubMed] [Google Scholar]
- 39.Westin MA, Hunt MC, Alexson SE. Short- and medium-chain carnitine acyltransferases and acyl-CoA thioesterases in mouse provide complementary systems for transport of beta-oxidation products out of peroxisomes. Cell Mol Life Sci. 2008;65:982–990. doi: 10.1007/s00018-008-7576-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bartlett K, Eaton S. Mitochondrial beta-oxidation. Eur J Biochem. 2004;271:462–469. doi: 10.1046/j.1432-1033.2003.03947.x. [DOI] [PubMed] [Google Scholar]
- 41.Kirkby B, Roman N, Kobe B, Kellie S, Forwood JK. Functional and structural properties of mammalian acyl-coenzyme A thioesterases. Prog Lipid Res. 2010;49:366–377. doi: 10.1016/j.plipres.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 42.Hosoda H, Kojima M, Matsuo H, Kangawa K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochemical and biophysical research communications. 2000;279:909–913. doi: 10.1006/bbrc.2000.4039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.