Abstract
Having gained momentum in the last decade, the One Health initiative promotes a holistic approach to address complex global health issues. Before recommending its adoption to stakeholders, however, it is paramount to first compile quantitative evidence of the benefit of such an approach. The aim of this scoping review was to identify and summarize primary research that describes monetary and non-monetary outcomes following adoption of a One Health approach. An extensive literature search yielded a total of 42,167 references, of which 85 were included in the final analysis. The top two biotic health issues addressed in these studies were rabies and malaria; the top abiotic health issue was air pollution. Most studies described collaborations between human and animal (n = 42), or human and environmental disciplines (n = 41); commonly reported interventions included vector control and animal vaccination. Monetary outcomes were commonly expressed as cost–benefit or cost–utility ratios; non-monetary outcomes were described using disease frequency or disease burden measurements. The majority of the studies reported positive or partially positive outcomes. This paper illustrates the variety of health challenges that can be addressed using a One Health approach, and provides tangible quantitative measures that can be used to evaluate future implementations of the One Health approach.
Electronic supplementary material
The online version of this article (10.1007/s10393-017-1310-5) contains supplementary material, which is available to authorized users.
Keywords: One Medicine, Transdisciplinarity, Endemic and emerging infectious diseases, Zoonoses, Non-communicable diseases, Systematic evidence, Scoping review
Introduction
The One Health (OH) approach is based on the notion that human, animal, and environmental health are intimately connected and mutually dependent (Rabinowitz et al. 2008; Dixon et al. 2014). Consequently, advocates of this movement describe the need for a holistic and transdisciplinary approach when tackling complex global health issues with high societal values (American Veterinary Medical Association 2008; Greter et al. 2014).
Despite being considered by some as a novel approach, the concept of OH dates back many centuries (Oura 2014; Woods and Bresalier 2014). Several key figures have played an important role in the promotion of this approach, through recognition of the similarities between human and veterinary medical science, the study of zoonoses and vaccine discovery, and the coining of the terms “One Medicine,” “One Health,” and “Ecohealth” (Day 2010; Zinsstag et al. 2011; Murray et al. 2014; Roberts 2014; Woods and Bresalier 2014). More recent key events in the OH movement include the publication of the Manhattan Principles recognizing the importance of a holistic approach when tackling both epidemic and epizootic diseases (World Conservation Society 2004) and the signing of the Tripartite Concept Note which puts onus on promoting prevention and control of disease at the human–animal–ecosystem interface (The FAO-OIE-WHO Collaboration 2010).
While the benefits of such a holistic and integrative movement may seem intuitive, the OH approach has come under scrutiny for its accountability, particularly since further investment in such collaborative projects will require a change in the way funds are allocated (Cleaveland et al. 2014; Gibbs 2014). Currently, most funds are administered within sectors. Yet, the collaborative approaches and applications encouraged by the OH movement often require a substantial initial investment which may go well beyond the possibilities of independent sectors or institutions. Therefore, to allow for more researchers to embrace this approach, there is a need to create interministerial platforms which allow for more integrated surveillance and disease control programs involving the animal, human, and environmental sectors, or novel funding mechanisms which will provide and accommodate for this transdisciplinary approach (Häsler et al. 2012; Gibbs 2014). For example, to prevent human disease and mitigate agricultural damages, a solution may lie primarily with more effective animal vaccination programs, requiring commitment and cohesion across disciplines. However, for this paradigm shift to occur, funding agencies and policy-makers must be provided with more evidence on the added value and cost-effectiveness of such cross-sectorial approaches (Hodgson and Darling 2011; Häsler et al. 2012; The World Bank 2012; Boden et al. 2014).
Therefore, the aim of this scoping review (SR) was (1) to systematically identify those studies that describe a quantitative outcome when using a OH approach and (2) to review and qualitatively summarize the health issues addressed, the type of OH approaches used, and the nature and value of the quantitative outcomes described. The purpose of this study is to create an evidence base of the types of OH applications, and consequent monetary and non-monetary outcomes accrued.
Methods
Research Question, Definitions, and Protocol
This SR was conducted to identify and summarize studies which describe a quantitative outcome when using a OH approach to address complex global health challenges. The study was performed as a joint project among residents of the European College of Veterinary Public Health. The population of interest within the studies was defined as the human and animal population worldwide. The intervention of interest was the “OH approach,” defined as “the collaborative efforts of multiple disciplines working locally, nationally and globally to attain optimal health for people, animals and our environment” (American Veterinary Medical Association 2008). The outcome of interest was a “quantitative outcome,” measured either in monetary or non-monetary terms (Rusthon 2009; Rushton et al. 2012; Minutes of the expert workshop 2013).
An a priori protocol was developed to define eligibility criteria and procedure after consultation with experts in OH and veterinary economics. Additional references were used to help structure the SR (Higgins and Green 2008; Centre for reviews and dissemination 2009), which is reported according to PRISMA guidelines (Moher et al. 2009). Screening tools (S1 and S2) were pretested before implementation to ensure clarity of questions.
Literature Search Strategy
The outline of the methodological activities undertaken is presented in Fig. 1. The search terms presented in Table 1 were used to systematically search four electronic databases: MEDLINE, CAB Abstracts, Embase, and the National Health Service Economic Evaluation Database (NHS EED; UK). The final search was performed on June 5, 2014, and the search strategies used for each database are presented in S3–S6. In addition to the electronic search, a search verification was performed through expert elicitation to help with the identification of relevant studies within the gray literature, and by manually searching references in recent reviews on the topic (Zinsstag et al. 2007; Zinsstag et al. 2011; Häsler et al. 2012; Min et al. 2013).
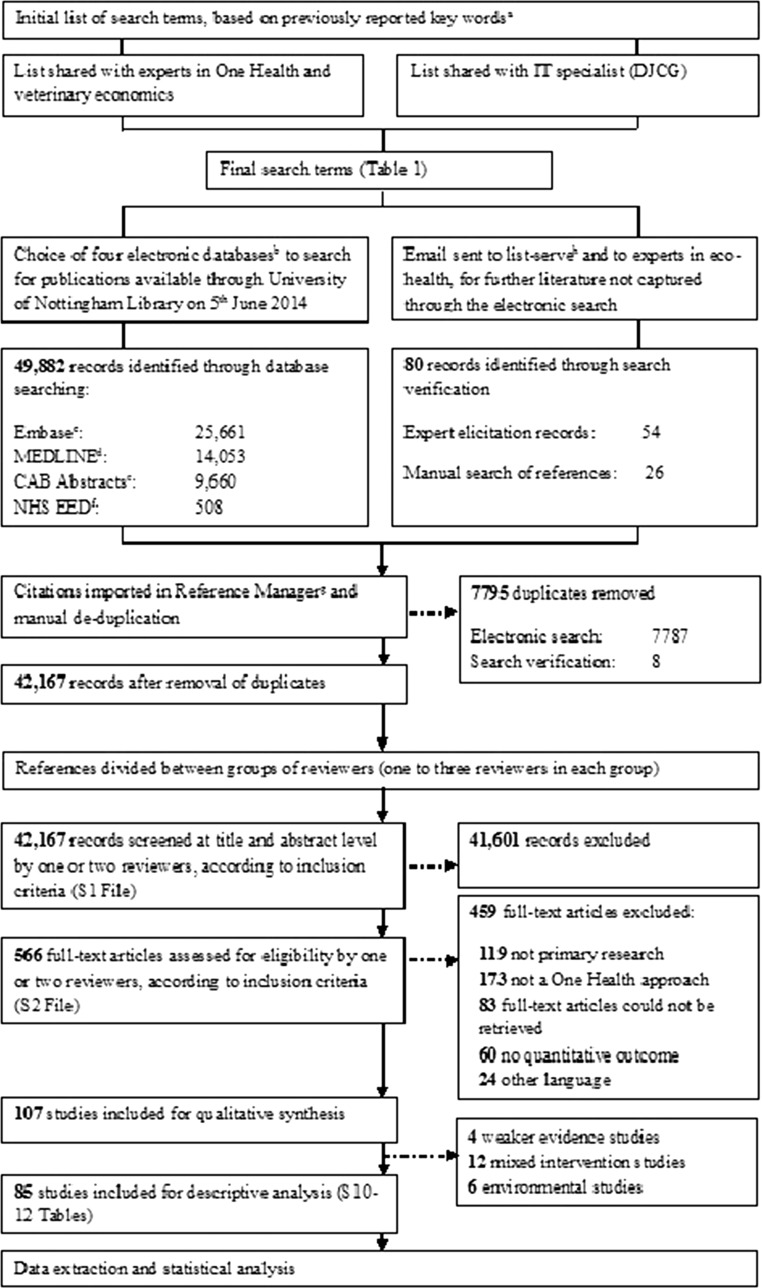
Fig. 1.
Flow of methodological activities and information through the different phases of a scoping review on the quantitative outcome of a One Health approach to address complex global health challenges, as described by the PRISMA guidelines (Moher et al. 2009). aKeywords reported in Rushton 2009; Häsler et al. 2012; Minutes of the Expert Workshop 2013. bBased on a recommendation that three to five databases are considered sufficient (Young et al. 2014). cTexts available between 1980 and 2014. dTexts available between 1946 and 2014. eTexts available between 1910 and 2014. fPart of the National Institute for Health Research Centre for Reviews and Dissemination, UK. gRefworks© (ProQuest, LLC, Cambridge Information Group; Betheseda, MD, USA). hCommunity of Practice in Ecosystem Approaches to Health—Canada.
Table 1.
A List of the Search Terms Used in Four Electronic Databases (MEDLINE, Embase, NHS EED, and CAB Abstracts) to Identify References that Describe a Quantitative Outcome when Using a One Health Approach to Address Complex Global Health Challenges.
| ((animal AND human) OR (animals and human) OR (animal AND humans) OR (animals AND humans) OR (human AND environment) OR (humans AND environment) OR (animal AND environment) OR (animals AND environment) OR “animal to human” OR “human to animal” OR “social-ecological” OR “socio-ecological” OR “One Health” OR “Ecohealth” OR “One World” OR “One Medicine” OR (ecosystem AND health) OR (holistic AND health) OR (veterinary AND human medicine) OR interdisciplinary OR multidisciplinary OR transdisciplinary OR “cross sector” OR “inter sector” OR “trans sector” OR zoonos* OR zoonotic OR “veterinary public health” OR “VPH” OR “farm to fork” OR “stable to table” OR “value chain”) |
| AND |
| (DALY* OR HALY* OR QALY* OR “disability adjusted life year” OR “disability adjusted life years” OR “health adjusted life year” OR “health adjusted life years” OR “quality adjusted life year” OR “quality adjusted life years” OR “expected quality adjusted life year” OR “expected quality adjusted life years” OR “opportunity cost” OR “opportunity costs” OR “cost benefit” OR “cost benefits” OR “cost analys*” OR “cost assessment” OR “cost effectiveness” OR “cost utility” OR “cost utilities” OR profit* OR “cost allocation” OR “cost benefit analys*” OR “cost control” OR “cost controls” OR “cost saving” OR “cost savings” OR “costs savings” OR “cost of illness” OR “costs of illness” OR “cost of disease” OR “costs of disease” OR “cost of intervention” OR “costs of intervention” OR “cost sharing” OR “costs sharing” OR “health care cost” OR “health care costs” OR “health care expenditure” OR “health care expenditures” OR “value of life” OR “societal benefit*” OR “economic evaluation” OR “economic analys*” OR “economic assessment” OR “health economics” OR “resource allocation” OR “cost avoidance” OR “costs avoidance” OR “loss avoidance” OR “losses avoidance”) |
Study Inclusion Criteria and Screening
The predetermined criteria for a publication to be eligible for inclusion are given in S1, while the screening strategy followed is shown in Fig. 1. A publication was considered eligible for inclusion if it reported primary research on a quantitative outcome when using a OH approach, even if not explicitly defined as such, to address complex global health challenges, and was published after 1910. This date was selected based on the setup of the databases, whereby the earliest publication date available was 1910. Primary research was defined as a study where the author(s) collected and/or analyzed data, and included case reports and case series, qualitative studies, observational studies, and experimental studies. Mathematical models and economic studies were included if they were based on field data collected in the same study or elsewhere. References were included if they were in English, German, Italian, Spanish, French, Portuguese, Greek, Dutch, Finnish, Russian, Norwegian, or Swedish; references in other languages were excluded. If no abstract was available, and the title was not sufficiently clear, the publication was included for full-text screening. Discrepancies regarding a publication’s eligibility were first resolved among the smaller group of reviewers and, when necessary, through an online discussion with all reviewers involved in this study.
Qualitative Data Extraction and Analysis
Data extracted from the included publications are shown in S7-S12; these included: (1) bibliographic information and study design characteristics, (2) how the reference was identified, (3) the health issue addressed, (4) the intersectoral approach used (i.e., human–animal vs. human–environment vs. animal–environment vs. human–animal–environment), (5) the quantitative outcome described, and (6) a quality assessment based on the clarity of the methods. All extracted data were checked for consistency by two of the authors (LCF and MLB), and any disagreements were resolved through discussion between all reviewers.
To allow for further exploration and description of the studies, the following parameters were extracted: (1) continent where the study was performed; (2) whether the country was considered developed or developing, and its income status; (3) whether the disease agent was abiotic or biotic and, in case of the latter, whether it was a bacterium, virus, protozoa, helminth, or insect; (4) whether the health issue was considered a neglected tropical disease (NTD) or not; and (5) the type of transmission. The definitions of these parameters are based on references provided in S13. Descriptive statistics of the study characteristics (e.g., health issue described, type of intervention, and outcome) were performed using Stata (version 13, StataCorp LP, College Station, TX, USA). Due to the heterogeneity of the studies and topics involved, quantitative meta-analyses were not undertaken.
This review was approved by the Ethical Review Committee at the University of Nottingham, UK (Ethics Approval Number: 1328141209).
Results
Figure 1 shows the flow of references through the screening process. Of the 107 studies that were included for qualitative synthesis, 4 were excluded because they showed elements of a OH approach, but multiple steps described the link between the OH approach and quantitative outcome, with certain overarching assumptions not explicitly discussed (S7). Twelve studies were excluded as “Mixed Interventions” because, while they described both interdisciplinary and disciplinary interventions, it was not possible to determine the quantitative outcome specifically due to the OH approach (S8). Another six studies that described a OH approach to address environmental health issues were classified separately (S9).
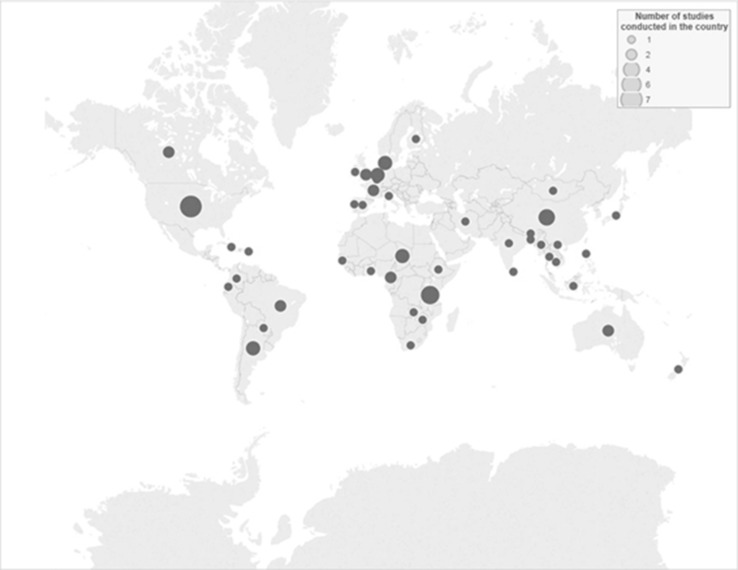
The remaining 85 studies fully met our aim and eligibility criteria (S10–S12); of these, 72 were identified through the electronic search, while 13 were identified through search verification. The studies were performed in all five continents, primarily in Europe (n = 23), Asia (n = 20), and Africa (n = 16). A total of 56 different countries or regions were represented (Fig. 2), most commonly the USA (n = 7), China (n = 4), and Tanzania (n = 4). Thirty-six studies were performed in developed countries, while another 44 were performed in developing countries; the remaining 5 studies either did not specify the country, or were performed in countries (Cambodia and Puerto Rico) that did not appear within the reference document used for the classification of developing/developed status (United Nations 2014; see S13). Similarly, 37, 25, and 14 of these studies were performed in high-, middle-, and low-income countries, respectively.
Fig. 2.
A world map indicating the number of studies conducted in different countries and included in a scoping review on the quantitative outcome of a One Health approach to address complex global health challenges.
The publication date of the included studies ranged between 1984 and 2014; the majority (n = 70) were published after 2000, of which 33 between 2010 and 2014. The majority of the included references described modeling studies such as economic analyses (n = 42), mathematical modeling (n = 12), and risk assessments (n = 4).
Health Issues Addressed
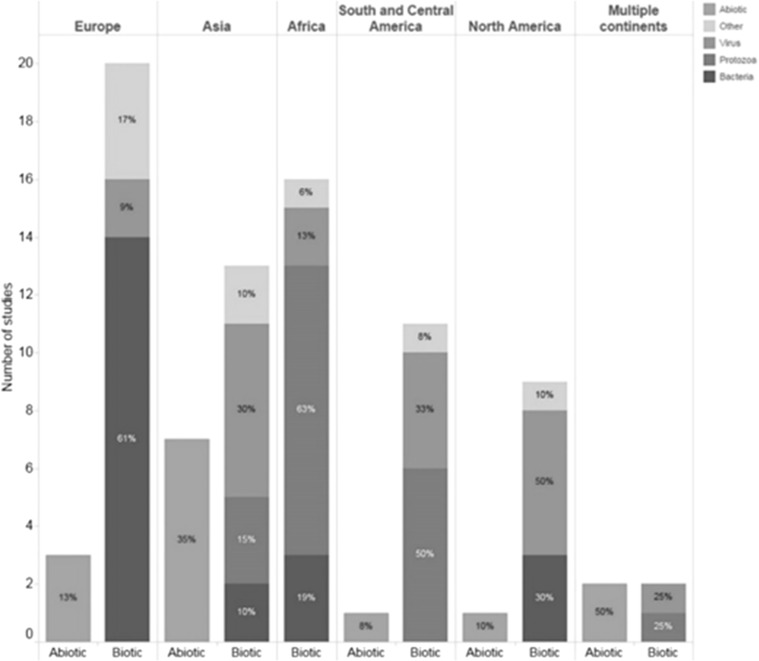
The health issues addressed in the 85 studies were classified as biotic (n = 69), abiotic (n = 14), or both (n = 2; Figs. 3 and 4).
Fig. 3.
Abiotic and biotic health issues described, per continent, in a scoping review on the quantitative outcome of a One Health approach to address complex global health challenges.
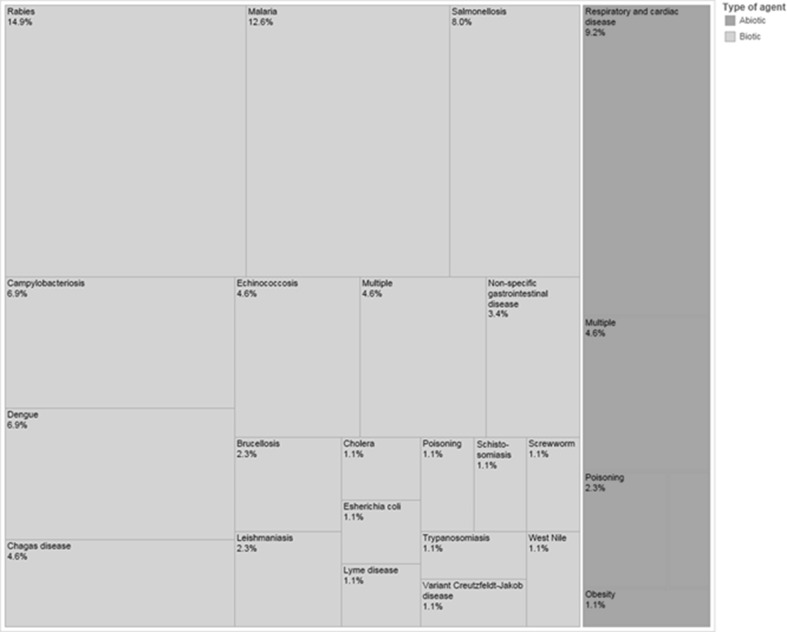
Fig. 4.
Abiotic and biotic health issues described in a scoping review on the quantitative outcome of a One Health approach to study complex health challenges.
Among those studies that included a biotic issue, the top five diseases described were rabies (n = 13), malaria (n = 11), salmonellosis (n = 7), campylobacteriosis (n = 6), and dengue (n = 6). Almost half (n = 32) dealt with a NTD such as rabies, dengue, echinococcosis, and Chagas disease. Most of the bacterial studies were performed in Europe (n = 14), while most protozoal studies were performed in Africa (n = 10).
Air pollution was the most common abiotic health issue addressed (n = 5); other issues included pesticides, micro-pollutants in water, and exposure to heavy metals in water or soil. Most of the 14 studies investigating abiotic health issues were conducted after the year 2000 and were performed in Asia (n = 7) and Europe (n = 3).
One Health Approach
The majority of these 85 studies either described a collaboration between human and animal (n = 42), or between human and environmental (n = 41) disciplines. Of all interventions, environmental interventions were the most commonly described, and these targeted vector control (n = 26), pollution (n = 8), sanitation and water (n = 8), or modified environmental spaces to encourage physical activity (n = 1). More specifically, vector control was achieved primarily through the use of insecticide-treated bed nets, control of breeding sites, and habitat restoration. Pollution and sanitation were largely controlled through policies and structural changes. Other interventions described included vaccination of domestic animals or wildlife (either singly or in combination with other interventions; n = 20), best management practices targeting primary production (n = 12), treatment (n = 6), integrated surveillance (n = 2), and combined human and animal physical activity (n = 2).
Quantitative Outcomes
Of the studies included, some described both monetary and non-monetary outcomes (n = 31), while others described only monetary (n = 33) or non-monetary (n = 21) outcomes (S10-S12).
Most monetary outcomes were described as cost–benefit ratios (n = 26), cost–utility ratios (n = 18), or cost savings (n = 15). The majority of the studies had positive (n = 40) or partially positive (n = 18) monetary outcomes expressed as positive benefit–cost ratios and net present values, increased cost–utility ratios, or marked cost savings. Only four of the studies had a negative monetary outcome, expressed as negative benefit–to–cost ratios or imbalanced costs.
Among the non-monetary outcomes, measures of disease frequency were the most commonly reported outcome (n = 40), followed by measures of disease burden (n = 15). Other reported outcomes included vaccination coverage, disease transmission rates, case detection rates, animal and human productivity traits, weight loss, and animal welfare scores. Most studies described positive (n = 43) or partially positive (n = 6) non-monetary outcomes, such as reduced number of deaths, decreased prevalence, or increased disability-adjusted life years (DALYs) saved. Three studies reported no significant difference in outcome between the OH intervention and control groups.
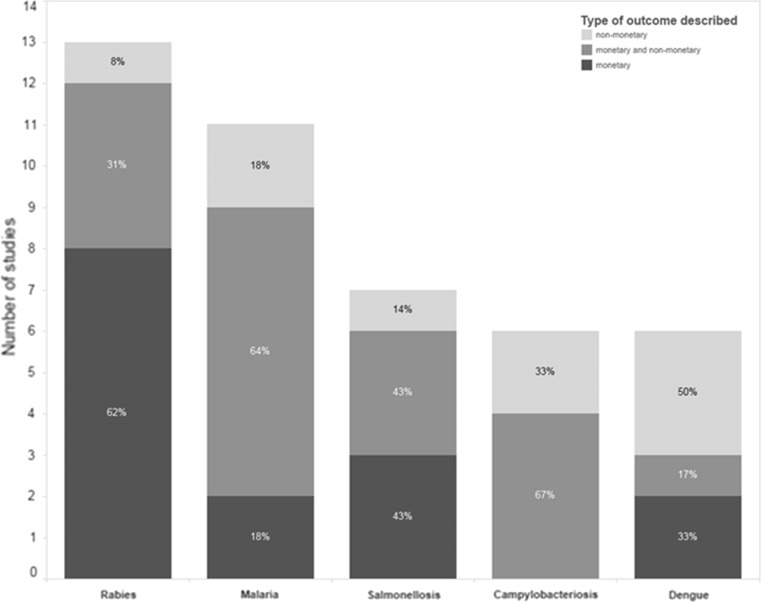
The quantitative outcomes reported in studies pertaining to the top five diseases were examined in further detail (Fig. 5). The majority of the rabies studies included in this review showed the benefits, in terms of cost savings or deaths averted, that could be accrued through either dog or wildlife vaccination campaigns (Table 2). The food-borne zoonoses’ studies illustrated the potential reduction in disease primarily via best management practices at the farm and slaughterhouse level (Tables 3 and 4), while the vector-borne studies illustrated benefits in terms of the interventions’ cost-effectiveness or their impact on disease transmission (Tables 5 and 6).
Fig. 5.
Proportion of studies that described monetary, non-monetary, or both outcomes to assess the top five diseases included in a scoping review on the quantitative outcome of a One Health approach to address complex global health challenges.
Table 2.
An Overview of the Type and Value of Quantitative Outcomes Featuring in Those Studies that Described One Health Interventions to Address Rabies Included in this Scoping Review.
| References | Geographical location | Intervention | Type of quantitative outcome described | Outcome reported |
|---|---|---|---|---|
| Dogs | ||||
| Bögel and Meslin (1990) | Developing countries | Combined dog vaccination and human PEPa | Cost efficiency | Cost-efficient in 5 years |
| Fishbein et al. (1991) | Philippines | One-year dog vaccination campaign | Time to recoup costs | 4.1–11.0 years |
| Fitzpatrick et al. (2014) | Tanzania | Annual dog vaccination campaigns (at different vaccination coverage) | Number of deaths averted | 0.6–2.0 |
| Percentage of deaths averted | 8.3–39.3% | |||
| Cost-effectiveness | Cost-effective to very cost-effective | |||
| Häsler et al. (2014b) | Sri Lanka | Dog vaccination and other control interventions | DALYsb averted | 738 |
| Animal welfare impact score | Improved | |||
| Program costs | US$ 1.03 million | |||
| Pinto et al. (2011) | Brazil | Dog vaccination (vs. human PEPa) | Cost comparison | Costs 9.2–20.2 lower (in Brazilian Real) |
| Tenzin and Ward (2012) | Bhutan | Combined dog vaccination and human PEPa (vs. human PEPa only) | Cost savings | US$ 0.09 million saved after 6 years |
| Townsend et al. (2013) | Bali | Comprehensive high coverage dog vaccination | Human lives saved over 10 years | 550 |
| Money saved over 10 years | US$ 15 million | |||
| Zinsstag et al. (2009) | Chad | One-year dog vaccination campaign | Cost per death averted | US$ 596 by 10th year |
| Time to recoup costs | 5.9 years | |||
| Wildlife | ||||
| Aubert (1999) | France | Wildlife vaccination (vs. fox depopulation) | Cost–benefit analysis | Beneficial after 4th year |
| Ministère de la Santé et de la Protection Sociale Française (1989) | France | Evaluation of oral vaccination programs in wildlife | Cost–benefit analysis | Beneficial in 10–12 years (less for some departments) |
| Shwiff et al. (2011) | Canada | Rabies control program including fox vaccination | Benefit–cost ratio | 0.49–1.36 |
| Cost savings | US$ 35.48–98.41 million | |||
| Shwiff et al. (2012) | Canada | Rabies control programs including raccoon vaccination | Benefit–cost ratio | 0.96–1.55 |
| Cost savings | US$ 46.70–52.93 million | |||
| Uhaa et al. (1992) | USA | Administration of oral vaccines to raccoons | Benefit–cost ratio | 2.21–6.80 |
| Cost savings | US $1.95 million |
aPEP post-exposure prophylaxis.
bDALYs disability-adjusted life years.
Table 3.
An Overview of the Type and Value of Quantitative Outcomes Featured in Those Studies that Described One Health Interventions to Address Salmonellosis Included in this Scoping Review.
| References | Geographical location | Intervention | Type of quantitative outcome described | Outcome reported |
|---|---|---|---|---|
| Goldbach and Alban (2006) | Denmark | Hot water decontamination of pig carcasses | Net present value | 3.5 million Euro over 15 years |
| Kangas et al. (2007) | Finland | Salmonella control policies in broiler production | Benefit–cost ratio | 0.04–21.25 |
| Korsgaard et al. (2009) | Denmark | Salmonella control programs in egg production | Number of human cases averted | 10,200 (95% CI: 8100–12,400) |
| Societal costs saved | 23.3 million Euro (95% CI: 16.3– 34.9) | |||
| Cost–benefit ratio | 0.5 | |||
| Miller et al. (2005) | USA | Pig vaccination | Reduction in human cases | 60% |
| Benefit–cost ratio | Less than 1 | |||
| Pig carcass rinsing at various water temperatures | Benefit–cost ratio | Greater than 1 | ||
| Persson and Jendteg (1992) | England, Wales and Sweden | Use of competitive exclusion in poultry production | Costs of illness saved | Up to 12.6 million GBP |
| Romero-Barrios et al. (2013) | European Union | Interventions on pig farms and during pig slaughter | Risk reduction | Up to 90% risk reduction |
| Wegener et al. (2003) | Denmark | Salmonella control programs in pig and poultry production | Costs saved | US $25.5 million |
Table 4.
An Overview of the Type and Value of Quantitative Outcomes Featured in Those Studies that Described One Health Interventions to Address Campylobacteriosis Included in this Scoping Review.
| References | Geographical location | Intervention | Type of quantitative outcome described | Outcome reported |
|---|---|---|---|---|
| Gellynck et al. (2008) | Belgium | Decontamination of poultry carcasses with electrolyzed oxidizing water | Cost–benefit ratio | 17.66 |
| Decontamination of poultry carcasses with lactic acid | 4.06 | |||
| Phage therapy used on chicken farms | 2.54 | |||
| Havelaar et al. (2007) | The Netherlands | Strict hygienic measures on chicken farms | Cost-effectiveness based on a cost–utility ratioa ≤ Euro 50,000/DALYsb | Cost-effective |
| Reduced fecal leakage during carcass processing | Cost-effective | |||
| Chemical decontamination of poultry carcasses | Cost-effective | |||
| Jensen and Jensen (2013) | European Union | Vaccination of chicks | Cost neutralization | 1.65 Euro per vaccine dose |
| Lake et al. (2013) | New Zealand | Poultry slaughterhouse improvements (e.g., new evisceration machines) | Cost per DALYsb saved | NZ$ 1200 |
| Continuous chemical treatment of poultry carcass | NZ$ 1700 | |||
| Phage-based controls on chicken farms | NZ$ 3000 | |||
| Mangen et al. (2007) | The Netherlands | Phage therapy used on chicken farms | Cost-effectiveness based on a cost–utility ratioa ≤ 100,000 Euro/DALYsb | Cost-effective |
| Romero-Barrios et al. (2013) | European Union | Application of fly screens in indoor poultry flocks | Risk reduction | 60% |
| Treating or freezing broiler carcasses | 87–98% |
aCost–utility ratio is described as the ratio of the net cost of intervention to averted disease burden in DALYs.
bDALYs disability-adjusted life years.
Table 5.
An Overview of the Type and Value of Quantitative Outcomes Featuring in Those Studies that Described One Health Interventions to Address Malaria Included in this Scoping Review.
| References | Geographical location | Intervention | Type of quantitative outcome described | Outcome reported |
|---|---|---|---|---|
| Aikins et al. (1998) | Gambia | Use of ITNa | Cost-effectiveness per death averted | US$ 471 |
| Cost-effectiveness per discounted life years gained | US$ 31.53 | |||
| Akhavan et al. (1999) | Brazil | National malaria control program including vector control | Cost-effectiveness per life saved | US$ 2672 |
| Cost-effectiveness per DALYsb averted | US$ 69 | |||
| Gatton and Cheng (2010) | Australia | ITNa and chemotherapy | Disease transmission | No transmission possible |
| Goodman et al. (1999) | Low-income country in sub-Saharan Africa | Provision of bed nets | Cost-effectiveness per DALYsb averted | US$ 19–85 |
| Insecticide treatment of existing bed nets | US$ 4–10 | |||
| Goodman et al. (2001) | South Africa | ITNa (vs. residual house spraying) | Effectiveness (adjusted rate ratio based on number of cases) | 0.69 |
| Cost per case averted | US$ 16 | |||
| Cost per death averted | US$ 1696 | |||
| Mueller et al. (2008) | Togo | Three-year ITNa campaign | Number of deaths averted | 6285 |
| Number of cases averted | 1.2 million | |||
| Cost per death averted | US$ 635 | |||
| Cost per DALYsb averted | US$ 16.39 | |||
| Mulligan et al. (2008) | Tanzania | ITNa voucher program | Number of child deaths averted | 12,039 |
| Cost per child death averted | US$ 873 | |||
| Pulkki-Brännström et al. (2012) | Not specified | Long-lasting ITNa (vs. conventional ITNa) | Child deaths averted | 30,800 |
| DALYsb averted | 1.02 million | |||
| Cost per DALYsb averted | US$ 16.8 | |||
| Cost-effectiveness | Cost-effective if priced at no more than US$ 1.5 above conventional ITNa | |||
| Riedel et al. (2010) | Zambia | Bed nets | Odds of parasitaemia | 40% less (12–60%) |
| Smithuis et al. (2013) | Myanmar | ITNa (vs. early diagnosis and effective treatment) | Cost per DALYsb averted | US$ 51 |
| Yhdego and Majura (1988) | Tanzania | Comparison of two vector control programs: engineering vs. use of larvicides and insecticides | Program effectiveness | 97 vs. 75% |
| Cost-effectiveness | Tshs 2.8 million vs. Tshs 10.5 million |
aITN insecticide-treated bed nets.
bDALYs disability-adjusted life years.
Table 6.
An Overview of the Type and Value of Quantitative Outcomes Featured in Those Studies that Described One Health Interventions to Address Dengue Included in this Scoping Review.
| References | Geographical location | Intervention | Quantitative outcome described | Values reported |
|---|---|---|---|---|
| Díaz (2012) | Cuba | Integrated surveillance system | Detection of febrile cases | Increased |
| McConnell and Gubler (2003) | Puerto Rico | Control of vector breeding sites | Cost-effectiveness | Cost-effective if dengue transmission is reduced by 50% and intervention costs less than US$ 2.50 per person |
| Ocampoa et al. (2014) | Colombia | Identification and spraying of vector breeding sites | Rate ratio of human incidence | 0.19 (95% CI 0.12–0.30) compared to control area |
| Orellano and Pedroni (2008) | Argentina | Fumigation of vectors | Net present value | I$ 196,879 |
| Cost–benefit analysis | Beneficial when more than 1363 cases of dengue and at least 1 case of dengue hemorrhagic fever are averted | |||
| Suaya et al. (2007) | Cambodia | Annual targeted larvicidal campaigns | Cost per DALYsa saved (public perspective) | US$ 313 |
| Cost per DALYsa saved (societal perspective) | US$ 37 | |||
| Tsunoda et al. (2013) | Vietnam | Use of insecticide-treated nets to cover water reservoirs | Human seroprevalence | 62.2% (vs. 74.6% in control area) |
| Addition of insecticide to other water containers |
aDALYs disability-adjusted life years.
Quality Assessment
To perform a quality assessment on the included studies, judgement was made as to whether the methods were explicitly stated. The majority of the studies (n = 69) were determined to have clearly explained and reproducible methods, while six studies lacked certain information and were therefore considered as partly reproducible. For the remaining ten studies, the methods were considered insufficiently described; there were no recognizable similarities between these studies as they were conducted in different regions and described different health issues (S7–S12).
Discussion
This study provides an extensive evidence base for research highlighting the quantitative outcomes, both monetary and non-monetary, of an OH approach. Moreover, it adds to recently published reviews (Häsler et al. 2014a; Baum et al. 2017) by also including research that may not have explicitly included definitions or terminology relating to “One Health” but employed a OH approach. This work is of substantial importance in relation to decision-making at the policy or governmental level and provides some proof that financing OH projects can be beneficial in a number of ways. Additionally, this review showcases the approaches used by a number of researchers and organizations that could be utilized in a number of global economic settings to improve human and animal health and welfare.
Most of the included studies dealt with biotic health issues, and the top five diseases were rabies, malaria, salmonellosis, campylobacteriosis, and dengue; this could be driven by funding priorities which are often focused on large global health challenges. Three of these are zoonoses, while the other two are vector-borne diseases. It is not surprising that zoonoses would be among the most commonly addressed OH topics as they are suited for a collaborative approach between human and veterinary medicine, such as through joint human–animal vaccination programs, integrated surveillance, and increased investment in cost-effective animal-level interventions with consequent human health benefits (Roth et al. 2003; Schelling et al. 2007; Zinsstag et al. 2009; Tschopp et al. 2013, Stärk et al. 2015).
Rabies is a clear example where OH approaches can be beneficial. Thirteen of the included studies described rabies, and all investigated vaccination as an option of controlling rabies in either dogs or wildlife. Most of these studies showed that those control programs that include vaccination are often cost-effective over a long time span, ranging from 4.1 to 11.0 years in the Philippines (Fishbein et al. 1991), 5.9 years in N’Djaména (Zinsstag et al. 2009), and 6 years in Bhutan (Tenzin and Ward 2012).
Our review also identified several OH interventions targeting food-borne zoonoses, a growing concern due to the increased demand for livestock products and consequent intensification and globalization of the food market (Karesh et al. 2012; Wall 2014). The importance of food safety for the general public and policy-makers was emphasized in a recent document by the European Union Scientific Steering Committee (European Union Scientific Steering Committee 2015) and was reiterated in the choice of Food Safety as the topic for the 2015 World Health Day (Chan 2014). Seven studies described interventions to control salmonellosis in either poultry or pig production systems, and considered the effect of these interventions on the number of human cases and overall costs incurred. Competitive exclusion (Persson and Jendteg 1992), control programs (Kangas et al. 2007; Korsgaard et al. 2009), and management practices such as hot water decontamination of carcasses (Miller et al. 2005; Goldbach and Alban 2006) were all found to be economically effective interventions. Similarly, the other benefits listed for Salmonella and other food-borne diseases such as Campylobacter could be utilized by policy-makers to keep these diseases to a minimum.
Vector-borne diseases, such as malaria and dengue, also featured prominently in our list of included studies. All the malaria studies assessed control programs which included vector control, mostly through the use of insecticide-treated bed nets (ITNs). In several African countries, ITNs (and long-lasting ITNs) proved to be effective in reducing the disease (Goodman et al. 1999; Riedel et al. 2010), though these benefits were sometimes outweighed by the costs incurred (Goodman et al. 2001; Pulkki-Brännström et al. 2012). The WHO recommends only distributing long-lasting ITNs (World Health Organization 2007); the findings in the current study are valuable in identifying those interventions that are superior to others when a number are available. These studies also emphasize the importance of environmental interventions, such as vector control, improved sanitation and hygiene, and integrated surveillance programs, to control the human impact of such diseases (World Health Organization 2014). Increased trade and globalization, together with climate change, habitat encroachment, and forest fragmentation, have augmented the possibility of vector-borne disease transmission (Sherman 2010), and this was exemplified by the recent emergence of Chikungunya and Zika virus in Latin America and the Caribbean (World Health Organization 2016a). Cross-sectorial approaches identified in this review could therefore set an example for future endeavors focusing on emerging vector-borne diseases. Ultimately it appears that the magnitude of benefit and the timescale over which control programs must be in place for the realization of benefit is disease and environment dependent. There is value in policy-makers identifying diseases and contexts similar to their own within this review to use as framework for designing programs specific to their own situations.
While the top biotic health issues described in our included studies may reflect funding priorities, they also mirror to a large extent recent findings on the global burden of disease (GBD). Infectious diseases such as rabies, malaria, and dengue are ranked among the top six WHO parasitic and vector-borne diseases (World Health Organization 2016b), and among the top ten NTD by the Lancet (Global Burden Disease 2015 DALYs and HALE Collaborators, 2016). Similarly, among all food-borne hazards, campylobacteriosis and salmonellosis, together with enteropathogenic Escherichia coli, were found to be the most relevant contributors to DALYs (World Health Organization Global Burden of Foodborne Diseases 2015). Noticeably, other zoonotic diseases with a high GBD, such as leishmaniasis or schistosomiasis, rarely featured in our findings. Reasons for this might be either that the OH interventions have not yet been used for their control, or that the study outcome was not assessed in a quantitative manner or it could not be attributed clearly to the OH intervention. Recent guidelines for OH studies, which also encourage authors to mention how they think the OH approach added value to the study, should help by clarifying whether a OH approach was used in the study and how it contributed to the final outcome (Davis et al. 2017).
In our review, abiotic health issues, such as respiratory disease due to air pollution or metal intoxication, were only described in 16.5% of the included studies. The importance of considering the environmental component of public health was recently reiterated in the Hanoi Declaration (Hanoi Declaration 2015) and subsequent Sustainable Development Goals [particularly non-communicable conditions such as cardiac disease, cancer, and obesity (United Nations 2015)]. Therefore, these cross-sectorial studies that tackle abiotic health issues, such as the impact of air and water pollution on human health, bring to light opportunities and avenues for a collaborative OH approach which need not be limited to communicable diseases. Two studies included in this review investigated the positive health benefits accrued through dog walking (Bauman et al. 2001; Kushner et al. 2006). Dog ownership encourages owner physical activity and has been described as a cost-effective and socially acceptable preventive measure for the current obesity epidemic (Mills and Hall 2014). This highlights the opportunity for improved disease prevention and control through OH approaches, by investigating the pivotal human–animal companionship relationship to combat not only obesity, but also depression and cognitive disorders.
Antimicrobial resistance (AMR) did not feature in any of our included studies. This was surprising given both the attention it has received in recent years, and its complex and multifaceted nature which makes it amenable to cross-sectorial approaches (Queenan et al. 2016; Singh 2017; World Health Organization 2017). Since our literature search was conducted in 2014, it is likely the more recent focus on AMR in published research in the last few years would not have been captured. Similarly, we may have missed studies that describe a OH approach when dealing with other health issues, such as salmonellosis and trypanosomiasis, but were published after our final literature search was conducted (Sundström et al. 2014; Shaw et al. 2015).
The majority of the 85 studies included for qualitative synthesis were performed after 2000. This is not surprising as the OH initiative has been gaining momentum over the past decade, and the amount of interdisciplinary research has been shown to be increasing (Stärk et al. 2015; Van Noorden 2015). Nonetheless, segregation between disciplines still persists, particularly between the veterinary and ecological sciences (Manlove et al. 2016), and future interdisciplinary studies should ensure that the ecosystem component is properly represented (Barrett and Bouley 2015). Most identified studies described modeling approaches, either as mathematical modeling of infectious diseases or economic analyses. We realize that this may have been biased both by our search terms which targeted such studies and by our inclusion criteria which selected only for those studies that had a quantitative outcome. However, we think that this could also be partly due to the fact that some of the topics addressed may be hard to implement in the field given their underlying complexity. Moreover, funding for such interdisciplinary endeavors may be hard to obtain, thus making modeling approaches a more feasible and economically viable option.
One of the greatest challenges of this review lay with the definition of OH. The definition provided by the American Veterinary Medical Association (2008) was chosen to inform the review, and several examples were provided within the screening forms to ensure consistency in the interpretation of OH. Despite this, the interpretation of some references was difficult. Therefore, it is possible that studies may have been excluded which according to other definitions may be considered OH or, conversely, included studies which may not be considered OH. The recently published COHERE checklist for OH studies (Davis et al. 2017) should help with such future endeavors by setting a benchmark as to what should be considered a OH approach.
The final list of studies only included around 0.0025% of all screened references. This was expected given the broad search terms used. It was agreed that given the objective to identify those studies that described a OH approach (without necessarily containing the term OH), the sensitivity of the search should be prioritized over the specificity. Despite the broad search terms, a certain publication bias is to be expected based on the selection of literature databases, although they were selected pertinent to the type of studies that were sought in the review. An information specialist who specializes in objective, structured reviews of the literature (DG) was consulted and involved in the process of this review to ensure that the most appropriate databases were searched. Furthermore, we attempted to identify relevant studies in the gray literature through our search verification, which included expert elicitation and review of relevant textbooks. Future work should prioritize investigating these alternative sources further, as it is possible that the expected positive publication bias could have affected the results obtained.
As our review question focused on quantitative outcomes, we excluded those studies which described qualitative outcomes of a OH approach, such as improved knowledge on health topics, changes in attitude or practices, or improved participation, which are a necessary preceding step to ensure uptake and implementation of interventions and practices (World Health Organization 2014). These outcomes may be harder to evaluate as they are often intangible and incommensurable. Yet they are important components of the overall societal benefit and should therefore be taken into consideration when making decisions regarding fund allocation for disease control programs or other interventions.
We note that during the full-text screening process we excluded 60 references which described a OH approach but not a quantitative outcome. This lack of reported outcomes is similar to findings reported by other recently published reviews (Häsler et al. 2014a; Baum et al. 2017) and underlines a gap in current published research, where missing quantification of the evidence may hinder the uptake of research findings. Additionally, while this review identified a numerous diversity of monetary and non-monetary terms, this diversity in itself may impede comparisons between studies. We therefore encourage harmonization of metrics to ensure that future research is both outcome-based and comparable, thus facilitating interpretation and implementation of findings based on OH approaches. It is important for a number of stakeholders to be involved in the decision-making process in relation to the prioritization of which outcomes should be consistently measured in studies employing a OH approach. All levels of decision-makers should be included in the process, from those in the field to those at the policy-making level. This will ensure that the most appropriate outcomes, and therefore the most likely to be successfully captured, are identified. It is suggested that structured objective frameworks such as the Delphi methodology (Okoli and Pawlowski 2004) and those employed by the James Lind Alliance (http://www.jla.nihr.ac.uk/) be utilized for this purpose.
This review identifies a number of studies that may not have included terminology relating to OH but have employed a OH approach. Additionally, this is the first time that the quantitative outcomes of OH studies have been collectively reported, and therefore could provide an additional resource for policy-makers to utilize for similar OH research studies in the future. Future work should focus on investigating further the gray literature for other similar studies and the harmonization of metrics employed to determine the success of approaches across all OH studies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Many thanks to Prof. Jakob Zinsstag (who helped to sow the idea for the review, and helped with defining the research question, search terms and protocol); Dr. Barbara Häsler (who helped with search terms and search verification); Ulrich Sperling (for his assistance with the preparation of the figures); and the European College of Veterinary Public Health for allowing us to embark on this project. This work has partially been supported by work from the COST Action TD1404 (Network for Evaluation of One Health) and supported by COST (European Cooperation in Science and Technology).
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s10393-017-1310-5) contains supplementary material, which is available to authorized users.
References
- Aikins MK, Fox-Rushby J, D’Alessandro U, Langerock P, Cham K, New L, Bennett S, Greenwood B, Mills A. The Gambian national impregnated bednet programme: costs, consequences and net cost-effectiveness. Social Science and Medicine. 1998;46:181–191. doi: 10.1016/S0277-9536(97)00145-7. [DOI] [PubMed] [Google Scholar]
- Akhavan D, Musgrove P, Abrantes A, Gusmão RDA. Cost-effective malaria control in Brazil. Cost-effectiveness of a malaria control program in the Amazon Basin of Brazil, 1988–1996. Social Science and Medicine. 1999;49:1385–1399. doi: 10.1016/S0277-9536(99)00214-2. [DOI] [PubMed] [Google Scholar]
- American Veterinary Medical Association (2008) One Health: a new professional imperative. Final Report. Available: https://www.avma.org/KB/Resources/Reports/Documents/onehealth_final.pdf. Accessed 5 December 2014
- Aubert MFA. Costs and benefits of rabies control in wildlife in France. Revue Scientifique et Technique. 1999;18:533–543. doi: 10.20506/rst.18.2.1174. [DOI] [PubMed] [Google Scholar]
- Barrett MA, Bouley TA. Need for enhanced environmental representation in the implementation of One Health. Ecohealth. 2015;12:212–219. doi: 10.1007/s10393-014-0964-5. [DOI] [PubMed] [Google Scholar]
- Baum SE, Machalaba C, Daszak P, Salerno RH, Karesh WB. Evaluating One Health: are we demonstrating effectiveness? One Health. 2017;3:5–10. doi: 10.1016/j.onehlt.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman AE, Russell SJ, Furber SE, Dobson AJ. The epidemiology of dog walking: an unmet need for human and canine health. Medical Journal of Australia. 2001;175:632–634. doi: 10.5694/j.1326-5377.2001.tb143757.x. [DOI] [PubMed] [Google Scholar]
- Boden L, Auty H, Goddard P, Stott A, Ball N, Mellor D. Working at the science-policy interface. Veterinary Record. 2014;174:165–167. doi: 10.1136/vr.g1430. [DOI] [PubMed] [Google Scholar]
- Bögel K, Meslin FX. Economics of human and canine rabies elimination: guidelines for programme orientation. Bulletin of the World Health Organization. 1990;68:281–291. [PMC free article] [PubMed] [Google Scholar]
- Centre for Reviews and Dissemination (2009) Systematic reviews: CRD’s guidance for undertaking reviews in health care. Available: https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf. Accessed 12 December 2016
- Chan M. Food safety must accompany food and nutrition security. Lancet. 2014;384:1910–1911. doi: 10.1016/S0140-6736(14)62037-7. [DOI] [PubMed] [Google Scholar]
- Cleaveland S, Lankester F, Townsend S, Lembo S, Lembo T, Hampson K. Rabies control and elimination: a test case for One Health. Veterinary Record. 2014;175:188–193. doi: 10.1136/vr.g4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MF, Rankin SC, Schurer JM, Cole S, Conti L, Rabinowitz P, COHERE Expert Review Group (2017) Checklist for One Health epidemiological reporting of evidence (COHERE). One Health 17:14–21 [DOI] [PMC free article] [PubMed]
- Day MJ. One Health: the small animal dimension. Veterinary Record. 2010;167:847–849. doi: 10.1136/vr.c6492. [DOI] [PubMed] [Google Scholar]
- Díaz C (2012) Preventing dengue at the local level in Havana city. In: Ecohealth Research in Practice: Innovative Applications of an Ecosystem Approach to Health, Charron DF (editor), International Development Research Centre, pp 163–171
- Dixon MA, Dar OA, Heymann DL. Emerging infectious diseases: opportunities at the human–animal–environment interface. Veterinary Record. 2014;174:546–551. doi: 10.1136/vr.g3263. [DOI] [PubMed] [Google Scholar]
- European Union Scientific Steering Committee (2015) The role of research in global food and nutrition security. Available: http://europa.eu/expo2015/sites/default/files/files/FINAL_Expo-Discussion-paper_lowQ(1).pdf. Accessed 12 December 2016
- Fishbein DB, Miranda NJ, Merrill P, Camba RA, Meltzer M, Carlos ET, Bautista CF, Sopungco PV, Mangahas LC, Hernandez LM. Rabies control in the Republic of the Philippines: benefits and costs of elimination. Vaccine. 1991;9:581–587. doi: 10.1016/0264-410X(91)90246-3. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick MC, Hampson K, Cleaveland S, Mzimbiri I, Lankester F, Lembo T, Meyers LA, Paltiel AD, Galvani AP. Cost-effectiveness of canine vaccination to prevent human rabies in rural Tanzania. Annals of Internal Medicine. 2014;160:91–100. doi: 10.7326/M13-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatton ML, Cheng Q (2010) Interrupting malaria transmission: quantifying the impact of interventions in regions of low to moderate transmission. PLoS One 5: 10.1371/journal.pone.0015149 [DOI] [PMC free article] [PubMed]
- Gellynck X, Messens W, Halet D, Grijspeerdt K, Hartnett E, Viaene J. Economics of reducing Campylobacter at different levels within the Belgian poultry meat chain. Journal of Food Protection. 2008;71:479–485. doi: 10.4315/0362-028X-71.3.479. [DOI] [PubMed] [Google Scholar]
- Gibbs EP. The evolution of One Health: a decade of progress and challenges for the future. Veterinary Record. 2014;174:85–91. doi: 10.1136/vr.g143. [DOI] [PubMed] [Google Scholar]
- Global Burden Disease DALYs and HALE Collaborators (2016) Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and health life expectancy (HALE), 1990–2015: a systematic analysis for the Global Burden of Disease Study. Lancet. 2015;388:1603–1658. doi: 10.1016/S0140-6736(16)31460-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbach SG, Alban L. A cost-benefit analysis of Salmonella-control strategies in Danish pork production. Preventive Veterinary Medicine. 2006;17:1–14. doi: 10.1016/j.prevetmed.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Goodman CA, Coleman PG, Mills AJ. Cost-effectiveness of malaria control in sub-Saharan Africa. Lancet. 1999;354:378–385. doi: 10.1016/S0140-6736(99)02141-8. [DOI] [PubMed] [Google Scholar]
- Goodman CA, Mnzava AE, Dlamini SS, Sharp BL, Mthembu DJ, Gumede JK. Comparison of the cost and cost-effectiveness of insecticide-treated bednets and residual house-spraying in KwaZulu-natal South Africa. Tropical Medicine and International Health. 2001;6:280–295. doi: 10.1046/j.1365-3156.2001.00700.x. [DOI] [PubMed] [Google Scholar]
- Greter H, Jean-Richard V, Crump L, Béchir M, Alfaroukh IO, Schelling E, Bonfoh B, Zinsstag J. The benefits of One Health for pastoralists in Africa. Onderstepoort Journal of Veterinary Research. 2014;81:1–9. doi: 10.4102/ojvr.v81i2.726. [DOI] [PubMed] [Google Scholar]
- Hanoi Declaration (2015) The sustainable development goals: turning words into action. Available: http://www.ipu.org/conf-e/132/rpt-gendebate.htm. Accessed 29 March 2016
- Häsler B, Gilbert W, Jones BA, Pfeiffer DU, Rushton J, Otte MJ. The economic value of One Health in relation to the mitigation of zoonotic disease risks. Current Topics in Microbiology and Immunology. 2012;365:127–151. doi: 10.1007/82_2012_239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häsler B, Cornelsen L, Bennani H, Rushton J (2014a) A review of the metrics for One Health benefits. Revue scientifique et technique 33:453–464 [DOI] [PubMed]
- Häsler B, Hiby E, Gilbert W, Obeyesekere N, Bennani H, Rushton J (2014b) A One Health framework for the evaluation of rabies control programmes—a case study from Colombo city Sri Lanka. PLOS Neglected Tropical Diseases 8:e3270. 10.1371/journal.pntd.0003270 [DOI] [PMC free article] [PubMed]
- Havelaar AH, Mangen MJ, de Koeijer AA, Bogaardt MJ, Evers EG, Jacobs-Reitsma WF, van Pelt W, Wagenaar JA, de Wit GA, van der Zee H, Nauta MJ. Effectiveness and efficiency of controlling Campylobacter on broiler chicken meat. Risk Analysis. 2007;27:831–844. doi: 10.1111/j.1539-6924.2007.00926.x. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Chichester: Wiley Blackwell Press; 2008. [Google Scholar]
- Hodgson K, Darling M. Zooeyia: an essential component of “One Health”. Canadian Veterinary Journal. 2011;52:189–191. [PMC free article] [PubMed] [Google Scholar]
- Jensen HG, Jensen JD (2013) Vaccination of poultry against Campylobacter in the EU—what are the benefits? Food Economics.http://dx.doi.org/10.1080/2164828X.2013.859142
- Kangas S, Lyytikäinen T, Peltola J, Ranta J, Maijala R (2007) Costs of two alternative Salmonella control policies in Finnish broiler production. Acta Veterinaira Scandinavica 49. 10.1186/1751-0147-49-35 [DOI] [PMC free article] [PubMed]
- Karesh WB, Dobson A, Lloyd-Smith JO, Lubroth J, Dixon MA, Bennett M, Aldrich S, Harrington T, Formenty P, Loh EH, Machalaba C, Thomas MJ, Heymann DL. Ecology of zoonoses: natural and unnatural histories. Lancet. 2012;380:1936–1945. doi: 10.1016/S0140-6736(12)61678-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsgaard H, Madsen M, Feld NC, Mygind J, Hald T. The effects, costs and benefits of Salmonella control in the Danish table-egg sector. Epidemiology and Infection. 2009;137:828–836. doi: 10.1017/S0950268808000903. [DOI] [PubMed] [Google Scholar]
- Kushner RF, Blatner DJ, Jewell DE, Rudloff K. The PPET study: people and pets exercising together. Obesity. 2006;14:1762–1770. doi: 10.1038/oby.2006.203. [DOI] [PubMed] [Google Scholar]
- Lake RJ, Horn BJ, Dunn AH, Parris R, Green FT, McNickle DC. Cost-effectiveness of interventions to control Campylobacter in the New Zealand poultry meat food supply. Journal of Food Protection. 2013;76:1161–1167. doi: 10.4315/0362-028X.JFP-12-481. [DOI] [PubMed] [Google Scholar]
- Mangen M-JJ, Havelaar AH, Poppe KP, de Wit GA, CARMA Project Team Cost-utility analysis to control Campylobacter on chicken meat - dealing with data limitations. Risk Analysis. 2007;27:815–830. doi: 10.1111/j.1539-6924.2007.00925.x. [DOI] [PubMed] [Google Scholar]
- Manlove KR, Walker JG, Craft ME, Huyvaert KP, Joseph MB, Miller RS, Nol P, Patyk KA, O’Brien D, Walsh DP, Cross PC (2016) “One Health” or three? Publication silos among the One Health disciplines. PLoS Biology 14(4):e1002448. 10.1371/journal.pbio.1002448 [DOI] [PMC free article] [PubMed]
- McConnell KJ, Gubler DJ. Guidelines on the cost-effectiveness of larval control programs to reduce dengue transmission in Puerto Rico. Revista Panamericana de Salud Pública. 2003;14:9–16. doi: 10.1590/S1020-49892003000600003. [DOI] [PubMed] [Google Scholar]
- Miller GY, Liu X, McNamara PE, Barber DA. Influence of Salmonella in pigs preharvest and during pork processing on human health costs and risks from pork. Journal of Food Protection. 2005;68:1788–1798. doi: 10.4315/0362-028X-68.9.1788. [DOI] [PubMed] [Google Scholar]
- Mills D, Hall S. Animal-assisted interventions: making better use of the human-animal bond. Veterinary Record. 2014;174:269–273. doi: 10.1136/vr.g1929. [DOI] [PubMed] [Google Scholar]
- Min B, Allen-Scott LK, Buntain B. Transdisciplinary research for complex One Health issues: a scoping review of key concepts. Preventive Veterinary Medicine. 2013;112:222–229. doi: 10.1016/j.prevetmed.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Ministère de la Santé et de la Protection Sociale Française Étude coût-bénéfice de la prophylaxie médicale de la rage vulpine. Bulletin Epidemiologique Hebdomadaire. 1989;36:145–148. [Google Scholar]
- Minutes of the expert workshop (Leverhulme Centre for Integrative Research on Agriculture and Health, Royal Veterinary College, SOAS University of London, London School of Hygiene and Tropical Medicine) (2013) One Health benefits: key inputs to create an economic evidence base. Available: http://www.lcirah.ac.uk/sites/default/files/Metrics%20for%20One%20Health%20benefits%20workshop%20report%20final.pdf. Accessed 5 December 2014
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7): e1000097. 10.1371/journal.pmed000097. Accessed 12 December 2016 [DOI] [PMC free article] [PubMed]
- Mueller DH, Wiseman V, Bakusa D, Morgah K, Daré A, Tchamdja P (2008) Cost effectiveness analysis of insecticide-treated net distribution as part of Togo integrated child health campaign. Malaria Journal 7. 10.1186/1475-2875-7-73 [DOI] [PMC free article] [PubMed]
- Mulligan J-A, Yukich J, Hanson K (2008) Costs and effects of the Tanzanian national voucher scheme for insecticide-treated nets. Malaria Journal 7. 10.1186/1475-2875-7-32 [DOI] [PMC free article] [PubMed]
- Murray M, Holmes P, Wright N, Jarrett O, Kennedy P. History of One Health and One Medicine. Veterinary Record. 2014;174:227. doi: 10.1136/vr.g1801. [DOI] [PubMed] [Google Scholar]
- Ocampoa CB, Minaa NJ, Carabalía M, Alexandera N, Osorio L (2014) Reduction in dengue cases observed during mass control of Aedes (Stegomyia) in street catch basins in an endemic urban area in Colombia. Acta Tropica 132. 10.1016/j.actatropica.2013.12.019 [DOI] [PMC free article] [PubMed]
- Okoli C, Pawlowski SD. The Delphi method as a research tool: an example, design considerations and applications. Information & management. 2004;42:15–29. doi: 10.1016/j.im.2003.11.002. [DOI] [Google Scholar]
- Orellano PW, Pedroni E. Cost-benefit analysis of vector control in areas of potential dengue transmission. Revista Panamericana de Salud Pública. 2008;24:113–119. doi: 10.1590/S1020-49892008000800005. [DOI] [PubMed] [Google Scholar]
- Oura C. A One Health approach to the control of zoonotic vectorborne pathogens. Veterinary Record. 2014;174:398–402. doi: 10.1136/vr.g2539. [DOI] [PubMed] [Google Scholar]
- Persson U, Jendteg S. The economic impact of poultry-borne salmonellosis: how much should be spent on prophylaxis? International Journal of Food Microbiology. 1992;15:207–213. doi: 10.1016/0168-1605(92)90050-D. [DOI] [PubMed] [Google Scholar]
- Pinto HDBF, Assis A, Pinto RM, Monteiro SLP, Pinheiro SR. Avaliacao do custo-beneficio das atividades de prevencao da raiva humana e das atividades de controle da raiva caninca no municipio de mogi guacu, estado de Sao Paulo, no periodo de 2000 a 2004. Arquivo Brasileiro de Medicina Veterinária e Zootecnia. 2011;18:112–122. [Google Scholar]
- Pulkki-Brännström A, Wolff C, Brännström N, Skordis-Worrall J (2012) Cost and cost effectiveness of long-lasting insecticide-treated bed nets—a model-based analysis. Cost Effectiveness and Resource Allocation 10. 10.1186/1478-7547-10-5 [DOI] [PMC free article] [PubMed]
- Queenan K, Häsler B, Rushton J. A One Health approach to antimicrobial resistance surveillance: is there a business case for it? International Journal of Antimicrobial Agents. 2016;48:422–427. doi: 10.1016/j.ijantimicag.2016.06.014. [DOI] [PubMed] [Google Scholar]
- Rabinowitz PM, Odofin L, Dein FJ. From “us vs. them” to “shared risk”: can animals help link environmental factors to human health? Ecohealth. 2008;5:224–229. doi: 10.1007/s10393-008-0170-4. [DOI] [PubMed] [Google Scholar]
- Riedel N, Vounatsou P, Miller JM, Gosoniu L, Chizema-Kawesha K, Mukonka V, Steketee RW (2010) Geographical patterns and predictors of malaria risk in Zambia: Bayesian geostatistical modelling of the 2006 Zambia national malaria indicator survey (ZMIS). Malaria Journal 9(37). 10.1186/1475-2875-9-37 [DOI] [PMC free article] [PubMed]
- Roberts RJ. History of One Health and One Medicine. Veterinary Record. 2014;174:283. doi: 10.1136/vr.g2064. [DOI] [PubMed] [Google Scholar]
- Romero-Barrios P, Hempen M, Messens W, Stella P, Hugas M. Quantitative microbiological risk assessment (QMRA) of food-borne zoonoses at the European level. Food Control. 2013;29:343–349. doi: 10.1016/j.foodcont.2012.05.043. [DOI] [Google Scholar]
- Roth F, Zinsstag J, Orkhon D, Chimed-Ochir G, Hutton G, Cosivi O, Carrin G, Otte J. Human health benefits from livestock vaccination for brucellosis: case study. Bulletin of the World Health Organization. 2003;81:867–876. [PMC free article] [PubMed] [Google Scholar]
- Rushton J, Häsler B, De Haan N, Rushton R (2012) Economic benefits or drivers of a “One Health” approach: why should anyone invest? Onderstepoort Journal of Veterinary Research 79. 10.4102/ojvr.v79i2.461 [DOI] [PubMed]
- Rusthon J. The economics of animal health and production. Oxfordshire: CABI editions; 2009. [Google Scholar]
- Schelling E, Bechir M, Ahmed MA, Wyss K, Randolph TF, Zinsstag J. Human and animal vaccination delivery to remote nomadic families, Chad. Emerging Infectious Diseases. 2007;13:373–379. doi: 10.3201/eid1303.060391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw APM, Wint GRW, Cecchi G, Torr SJ, Mattioli RC, Robinson TP. Mapping the benefit-cost ratios of interventions against bovine trypanosomosis in Eastern Africa. Preventive Veterinary Medicine. 2015;4:406–416. doi: 10.1016/j.prevetmed.2015.06.013. [DOI] [PubMed] [Google Scholar]
- Shwiff SA, Nunan CP, Kirkpatrick KN, Shwiff SS. A retrospective economic analysis of the Ontario red fox oral rabies vaccination programme. Zoonoses Public Health. 2011;58:169–177. doi: 10.1111/j.1863-2378.2010.01335.x. [DOI] [PubMed] [Google Scholar]
- Shwiff S, Aenishaenslin C, Ludwig A, Berthiaume P, Bigras-Poulin M, Kirkpatrick K, Lambert L, Bélanger D. Bioeconomic Modelling of Raccoon Rabies Spread Management Impacts in Quebec, Canada. Transboundary and Emerging Diseases. 2012;60:330–337. doi: 10.1111/j.1865-1682.2012.01351.x. [DOI] [PubMed] [Google Scholar]
- Sherman DM. A global veterinary medical perspective on the concept of One Health: focus on livestock. Institute for Laboratory Animal Research Journal. 2010;51:281–287. doi: 10.1093/ilar.51.3.281. [DOI] [PubMed] [Google Scholar]
- Singh P (2017) One Health approach to tackle antimicrobial resistance in South East Asia. BMJ. 10.1136/bmj.j3625 [DOI] [PMC free article] [PubMed]
- Smithuis FM, Kyaw MK, Phe UO, van der Broek I, Katterman N (2013) The effect of insecticide-treated bed nets on the incidence and prevalence of malaria in children in an area of unstable seasonal transmission in western Myanmar. Malaria Journal 12. 10.1186/1475-2875-12-363 [DOI] [PMC free article] [PubMed]
- Stärk KD, Kuribreña MA, Dauphin G, Vokaty S, Ward MP, Wieland B, Lindberg A. One Health surveillance–more than a buzz word? Preventive Veterinary Medicine. 2015;120:124–130. doi: 10.1016/j.prevetmed.2015.01.019. [DOI] [PubMed] [Google Scholar]
- Suaya JA, Shepard DS, Chang MS, Caram M, Hoyer S, Socheat D, Chantha N, Nathan MB. Cost-effectiveness of annual targeted larviciding campaigns in Cambodia against the dengue vector Aedes aegypti. Tropical Medicine and International Health. 2007;12:1026–1036. doi: 10.1111/j.1365-3156.2007.01889.x. [DOI] [PubMed] [Google Scholar]
- Sundström K, Wahlström H, Ivarsson S, Lewerin SS (2014) Economic effects of introducing alternative Salmonella control strategies in Sweden. PLoS One 15(9):e96446. 10.1371/journal.pone.0096446 [DOI] [PMC free article] [PubMed]
- Tenzin Wangdi K, Ward MP. Human and animal rabies prevention and control cost in Bhutan, 2001–2008: the cost-benefit of dog rabies elimination. Vaccine. 2012;31:260–270. doi: 10.1016/j.vaccine.2012.05.023. [DOI] [PubMed] [Google Scholar]
- The FAO-OIE-WHO Collaboration (2010) Sharing responsibilities and coordinating global activities to address health risks at the animal-human-ecosystems interfaces. A tripartite concept note. Available: http://www.who.int/influenza/resources/documents/tripartite_concept_note_hanoi_042011_en.pdf. Accessed 22 December 2014
- The World Bank (2012) People, pathogens and our planet. Volume 2. The economics of One Health. Available: https://openknowledge.worldbank.org/bitstream/handle/10986/11892/691450ESW0whit0D0ESW120PPPvol120web.pdf. Accessed 7 November 2017
- Townsend SE, Sumantra IP, Pudjiatmoko, Bagus GN, Brum E (2013) Designing programs for eliminating canine rabies from islands: Bali, Indonesia as a case study. PLOS Neglected Tropical Diseases 7:e2372. 10.1371/journal.pntd.0002372 [DOI] [PMC free article] [PubMed]
- Tschopp R, Hattendorf J, Roth F, Choudhury AA, Shaw A, Aseffa A, Zinsstag J. Cost estimate of bovine tuberculosis to Ethiopia. Current Topics in Microbiology and Immunology. 2013;365:249–268. doi: 10.1007/82_2012_245. [DOI] [PubMed] [Google Scholar]
- Tsunoda T, Kawada H, Huynh TT, Luu LL, Le SH, Tran HN, Vu HT, Le HM, Hasebe F, Tsuzuki A, Tagaki, M (2013) Field trial on a novel control method for the dengue vector, Aedes aegypti by the systematic use of Olyset Net and pyriproxyfen in Southern Vietnam. Parasites and Vectors 6. 10.1186/1756-3305-6-6 [DOI] [PMC free article] [PubMed]
- Uhaa IJ, Dato VM, Sorhage FE, Beckley JW, Roscoe DE, Gorsky RD, Fishbein DB. Benefits and costs of using an orally absorbed vaccine to control rabies in raccoons. Journal of the American Veterinary Medical Association. 1992;201:1873–1882. [PubMed] [Google Scholar]
- United Nations (2014) World Economic Situation and Prospects 2014. Available: http://www.un.org/en/development/desa/policy/wesp/wesp_current/2014wesp_country_classification.pdf. Accessed 17 November 2017
- United Nations (2015) Sustainable Development Goals. Available: http://www.un.org/sustainabledevelopment/sustainable-development-goals/. Accessed 29 March 2016
- Van Noorden R. Interdisciplinary research by the numbers. Nature. 2015;525:306–307. doi: 10.1038/525306a. [DOI] [PubMed] [Google Scholar]
- Wall P. One Health and the food chain: maintaining safety in a globalised industry. Veterinary Record. 2014;174:189–192. doi: 10.1136/vr.g1512. [DOI] [PubMed] [Google Scholar]
- Wegener HC, Hald T, Lo Fo Wong D, Madsen M, Korsgaard H, Bager F, Gerner-Smidt P, Mølbak K. Salmonella control programs in Denmark. Emerging Infectious Diseases. 2003;9:774–780. doi: 10.3201/eid0907.030024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Bresalier M. One health, many histories. Veterinary Record. 2014;174:650–654. doi: 10.1136/vr.g3678. [DOI] [PubMed] [Google Scholar]
- World Conservation Society (2004) The Manhattan Principles. Available: http://www.cdc.gov/onehealth/pdf/manhattan/twelve_manhattan_principles.pdf. Accessed 22 December 2014
- World Health Organization (2007) WHO releases new guidance on insecticide-treated mosquito nets. Available at: http://www.who.int/mediacentre/news/releases/2007/pr43/en/. Accessed 7 November 2017
- World Health Organization (2014) The Control of Neglected Zoonotic Diseases: from advocacy to action. Available: http://www.who.int/neglected_diseases/ISBN9789241508568_ok.pdf. Accessed 29 March 2016
- World Health Organization Global Burden of Foodborne Diseases (2015) Available: http://www.who.int/foodsafety/areas_work/foodborne-diseases/ferg/en/. Accessed 11 December 2016
- World Health Organization (2016a) Estimates for 2000–2012. Available: http://www.who.int/healthinfo/global_burden_disease/estimates/en/index2.html. Accessed 11 December 2016
- World Health Organization (2016b) WHO Director-General summarizes the outcome of the Emergency Committee regarding clusters of microcephaly and Guillain-Barré syndrome. Available: http://www.who.int/mediacentre/news/statements/2016/emergency-committee-zika-microcephaly/en/. Accessed 29 March 2016
- World Health Organization (2017) Fact sheets on sustainable development goals: health targets. Antimicrobial Resistance. Available at: http://www.euro.who.int/__data/assets/pdf_file/0005/348224/Fact-sheet-SDG-AMR-FINAL-07-09-2017.pdf?ua=1. Accessed 8 November 2017
- Yhdego M, Majura P. Malaria control in Tanzania. Environment International. 1988;14:479–483. doi: 10.1016/0160-4120(88)90408-4. [DOI] [Google Scholar]
- Young I, Waddell L, Sanchez J, Wilhelm B, McEwen SA, Rajić A. The application of knowledge synthesis methods in agri-food public health: recent advancements, challenges and opportunities. Preventive Veterinary Medicine. 2014;113:339–355. doi: 10.1016/j.prevetmed.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Zinsstag J, Dürr S, Penny MA, Mindekem R, Roth F, Menendez Gonzalez S, Naissengar S, Hattendorf J. Transmission dynamics and economics of rabies control in dogs and humans in an African city. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14996–15001. doi: 10.1073/pnas.0904740106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinsstag J, Schelling E, Roth F, Bonfoh B, de Savigny D, Tanner M. Human benefits of animal interventions for zoonosis control. Emerging Infectious Diseases. 2007;13:527–531. doi: 10.3201/eid1304.060381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinsstag J, Schelling E, Waltner-Toews D, Tanner M. From “One Medicine” to “One Health” and systemic approaches to health and well-being. Preventive Veterinary Medicine. 2011;101:148–156. doi: 10.1016/j.prevetmed.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.