Benign postsurgical anastomotic colon strictures can be challenging to manage because of their refractory nature and the potential need for reoperation. Limited data are available on the endoscopic management of such lesions with the use of fully covered self-expanding metal stents or lumen-apposing metal stents (LAMSs).1, 2
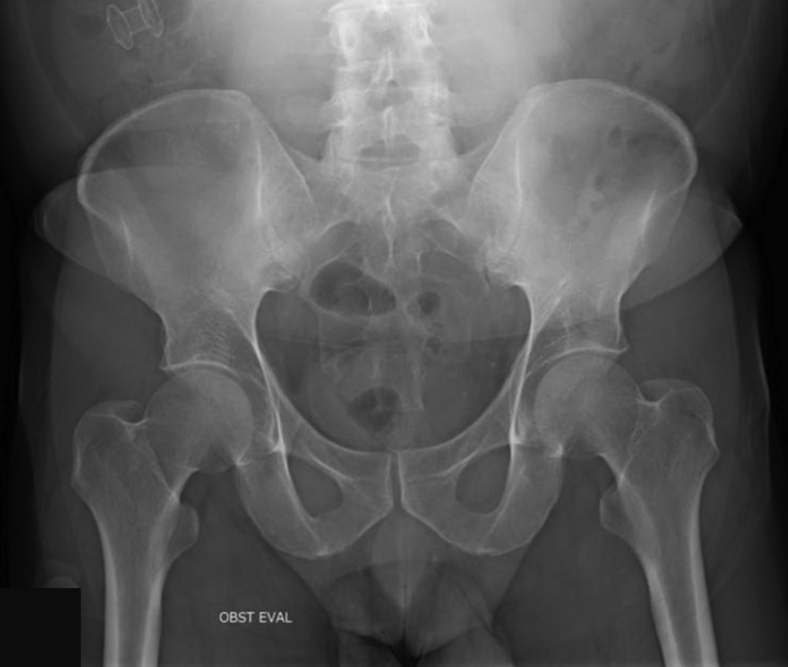
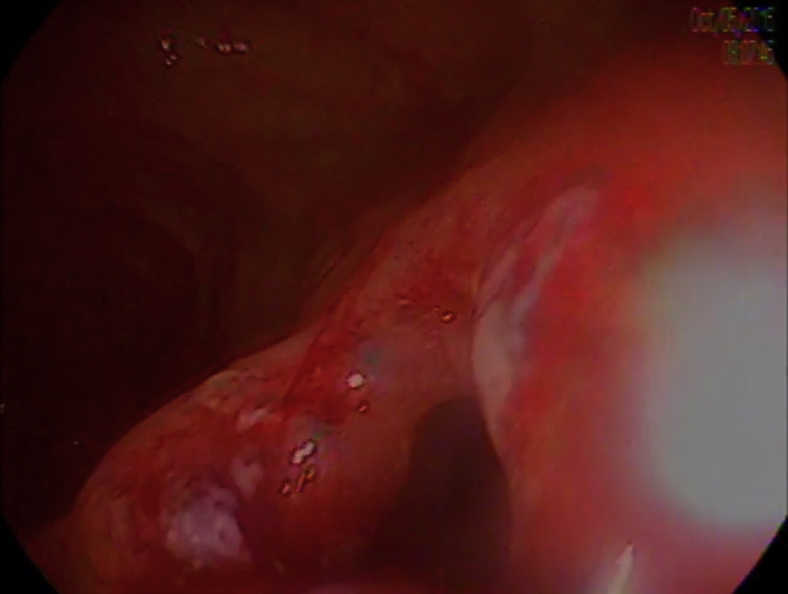
This case report describes the successful use of a 10-mm-lumen LAMS to treat and resolve a refractory ileocolonic anastomotic stricture (Video 1, available online at www.VideoGIE.org). A 54-year-old man with previously uncontrolled HIV complicated by Mycobacterium avium complex (MAC) and cytomegalovirus (CMV) infection requiring ileocolonic resection resulting from stricture formation presented several years after his operation to our clinic because of recurrent symptomatic small-bowel obstructions (SBOs) necessitating repeated hospitalizations. Imaging revealed findings consistent with partial SBO at the level of the ileocolonic anastomosis (Fig. 1), colonoscopy demonstrated a tight benign anastomotic stricture that was not traversable with the colonoscope (Fig. 2).
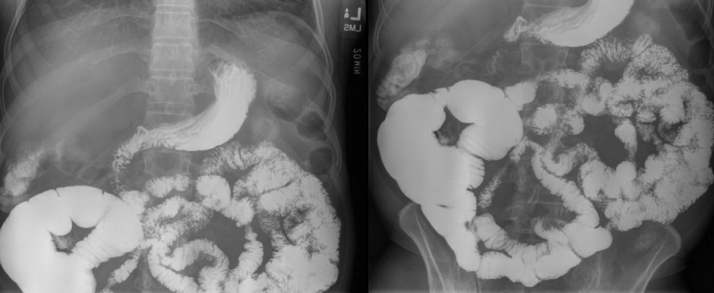
Figure 1.
Small-bowel follow-through imaging with evidence of partial small-bowel obstruction at level of ileocolonic anastomosis with proximal bowel dilatation.
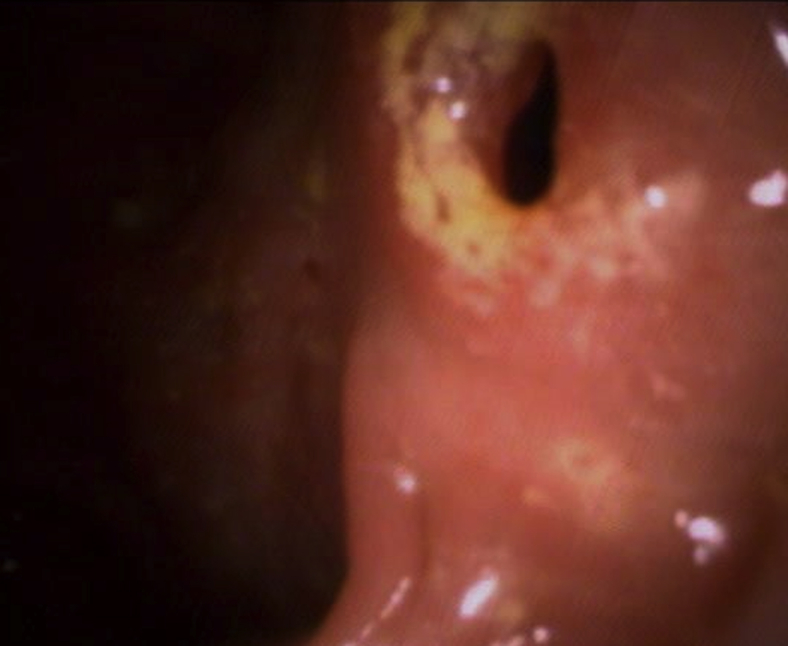
Figure 2.
Colonoscopic finding of anastomotic stricture at level of anastomosis.
Under fluoroscopic and direct endoscopic guidance, a 10-mm-lumen LAMS was placed across the strictured area. Proximal stent migration occurred during initial deployment because of incompatibility of the catheter delivery system with a therapeutic forward-viewing endoscope. The stent was successfully repositioned with a rat-tooth forceps (Fig. 3), and the position was confirmed by fluoroscopic imaging (Fig. 4). After deployment, copious liquid and solid stool was seen flowing through the stent.
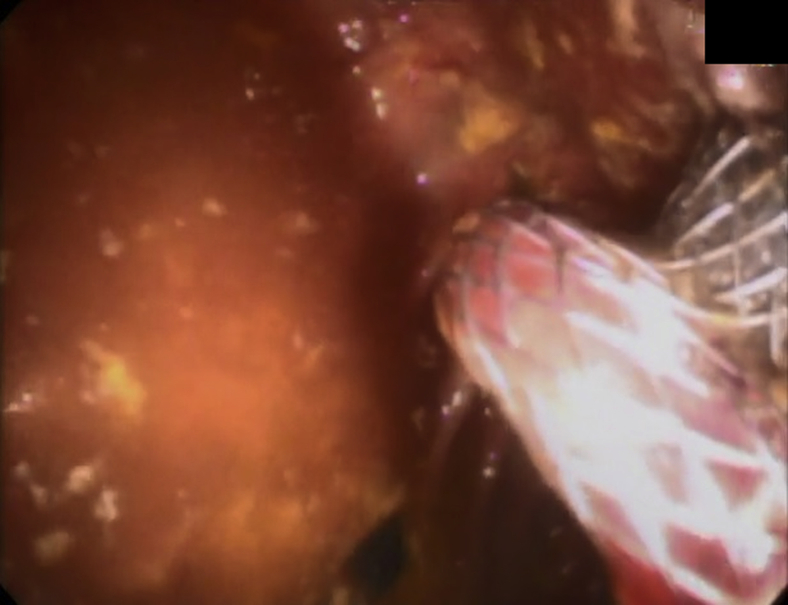
Figure 3.
Colonoscopic imaging after successful repositioning of lumen-apposing metal stent through stricture.
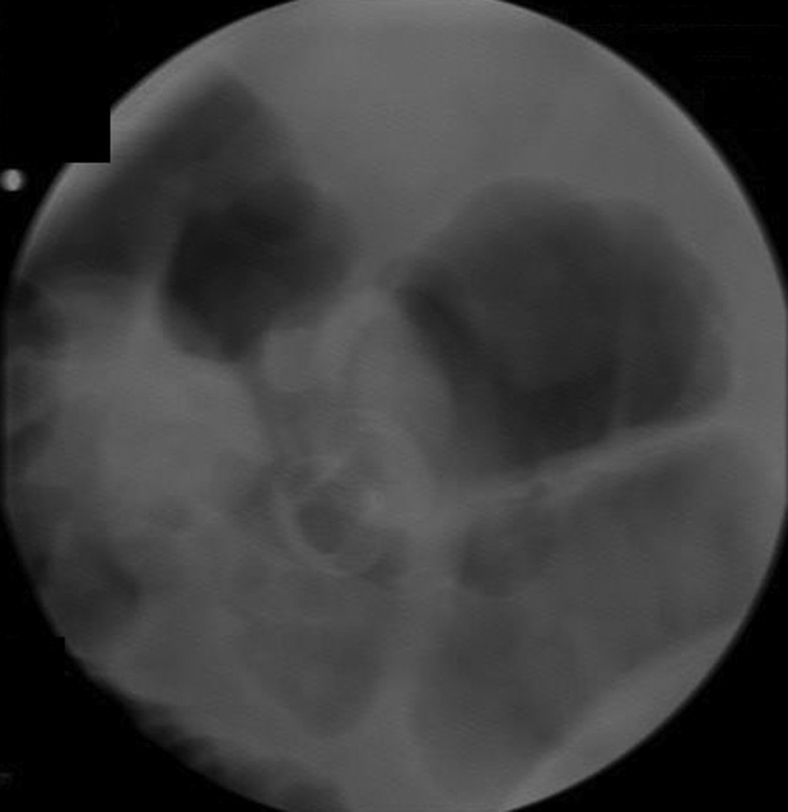
Figure 4.
Fluoroscopic confirmation of lumen-apposing metal stent placement.
Follow-up imaging 1 month later showed good positioning of the stent without further evidence of obstruction (Fig. 5). Repeated colonoscopy 6 weeks later for stent extraction revealed a patent anastomosis with an inner luminal diameter of 10 mm, easily facilitating passage of the colonoscope into the small bowel (Fig. 6).
Figure 5.
Follow-up radiographic imaging taken 1 month after placement of lumen-apposing metal stent.
Figure 6.
Colonoscopic view of patent ileocolonic anastomosis after extraction of lumen-apposing metal stent.
Lumen-apposing stent placement is a minimally invasive solution for management of short benign anastomotic strictures of the GI tract and has the potential to delay or ultimately prevent the necessity for recurrent endoscopic dilations or surgical intervention. A LAMS was chosen rather than a colonic stent approved by the U.S. Food and Drug Administration because it is covered and removable. Alternatively, a short, fully covered esophageal stent could have been used; however, the risk of migration and possible stent adverse event is higher. Thus, despite the additional cost of the stent, a LAMS was chosen.
Disclosure
Drs Kedia and Tarnasky are consultants and speakers for Boston Scientific. All other authors disclosed no financial relationships relevant to this publication.
Footnotes
Written transcript of the video audio is available online at www.VideoGIE.org.
Supplementary data
Lumen-apposing stent placement for management of a short benign colonic anastomotic stricture.
References
- 1.Clinic B. Safety and efficacy of coaxial lumen-apposing metal stents in the management of refractory gastrointestinal luminal strictures: a multicenter study. Endosc Int Open. 2017;5:861–867. doi: 10.1055/s-0043-114665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamazza A., Fiori E., Sterpetti A.V. Self-expandable metal stents in the treatment of benign anastomotic stricture after rectal resection for cancer. Colorectal Dis. 2014;16:O150–O153. doi: 10.1111/codi.12488. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lumen-apposing stent placement for management of a short benign colonic anastomotic stricture.