Abstract
Primary biliary cholangitis (PBC) is an autoimmune inflammatory liver disease of the interlobular bile ducts that can lead to cirrhosis and liver failure. Until recently, the only effective treatment was ursodeoxycholic acid (UDCA). However, up to 40% of PBC patients have an inadequate response to UDCA and may continue to have disease progression. Several models have been developed, including the UK-PBC and GLOBE scores, to assist in identifying patients who may benefit from second-line therapies, such as the farnesoid X receptor (FXR) agonist obeticholic acid (OCA). The addition of OCA can significantly improve serum alkaline phosphatase and total bilirubin, which are strong surrogate markers of clinical outcomes in PBC. Other alternatives, including the peroxisome proliferator-activated receptor (PPAR)-α agonists fenofibrate and bezafibrate, may also improve liver biochemistries in PBC patients with an inadequate response to UDCA, but further study is needed to demonstrate their safety and long-term efficacy. Other novel agents, including those targeting the FXR pathway and PPAR-δ agonists, have shown promising results and may alter the therapeutic landscape of PBC in the near future. For now, OCA remains the only approved second-line agent for PBC patients with an inadequate response to UDCA while results of long-term studies of its safety and clinical benefit are awaited.
Keywords: Primary biliary cholangitis, therapy, farnesoid X receptor, fibrate, FGF19 analog, peroxisome proliferator-activated receptor agonist
Primary biliary cholangitis (PBC), previously known as primary biliary cirrhosis, is a progressive and rare autoimmune inflammatory disease of the interlobular bile ducts, leading to cholestasis and secondary damage of hepatocytes that may ultimately progress to cirrhosis and liver failure.1 A prototypical autoimmune disease, PBC predominantly affects middle-aged women in whom loss of tolerance to mitochondrial antigens leads to the development of antimitochondrial antibodies (AMAs) and immunologic destruction of biliary epithelial cells. The diagnosis of PBC is typically made when there is persistent elevation of alkaline phosphatase (ALP) with normal imaging of the biliary tract and serologic reactivity to AMAs. PBC-specific antinuclear antibodies, such as anti-Sp100 and anti-Gp210, can be used to identify some AMA-negative cases of PBC. Very few cases of PBC require histologic diagnosis with the classic features of nonsuppurative obstructive cholangitis or florid duct lesions.2,3
In the absence of treatment, PBC is a slowly progressive disease, with the majority of patients advancing 1 histologic stage every 2 years4 and the median survival of symptomatic and asymptomatic patients being 7.5 and 16 years, respectively.5 Notably, most asymptomatic patients will develop symptoms over the course of 4.5 to 17.8 years,5-10 and survival among PBC patients without symptoms is still worse than that of a similar control population.11 Unlike in autoimmune hepatitis, immune-based therapies have failed to show effectiveness in PBC. Instead, bile acid–based treatments, namely ursodeoxycholic acid (UDCA) and obeticholic acid (OCA), have emerged as first- and second-line treatments, respectively.
In addition to the risk of cirrhosis and its inherent complications, approximately 50% of PBC patients experience the symptoms of fatigue and pruritus. Thus, the optimal management of PBC should address not only progression of liver disease but also symptoms (fatigue and pruritus) as well as associated conditions such as keratoconjunctivitis sicca, xerostomia, osteoporosis, and hypothyroidism. However, this article focuses on PBC treatment options that target the liver disease.
Ursodeoxycholic Acid Treatment in Patients With Primary Biliary Cholangitis
Approval of UDCA by the US Food and Drug Administration (FDA) for the treatment of PBC required overcoming several barriers to drug development, and these barriers continue to pose challenges for current and future drug development. Because PBC is a rare disease, recruitment of adequate numbers of PBC patients to power clinical trials requires many participating sites. In addition, clinical outcomes, including liver transplantation and survival, occur at a low rate, particularly in patients with early-stage disease. Thus, the time frame for a clinical trial to demonstrate clinical benefits is not practical, especially for therapies that may be most effective in early-stage disease. With the exception of UDCA, the efficacy of PBC treatments has been based solely upon surrogate clinical endpoints, which, although strongly associated with clinical outcomes, do not themselves prove clinical benefit.
In the clinical trials in which UDCA was given at adequate doses for at least 2 years, biochemical and histologic benefits have been firmly established.12-17 In the longest randomized, placebo-controlled trial, which included 180 patients treated for 4 years, UDCA therapy was associated with a delayed time to treatment failure, which was defined as any of the following outcomes: death; liver transplantation; histologic progression by 2 stages or to cirrhosis; development of varices, ascites, or encephalopathy; doubling of bilirubin; marked worsening of fatigue or pruritus; inability to tolerate the drug; or voluntary withdrawal for any reason.17 In addition, long-term observation of UDCA-treated PBC patients has demonstrated a lower rate of progression to cirrhosis18 and a reduction in the number and percentage of liver transplants performed for PBC despite a trend toward increasing incidence.19-21
However, despite the benefits of UDCA, there remains a group of PBC patients who continue to progress to cirrhosis and its complications. New models to identify predictors of long-term transplant-free survival based upon changes in biochemistries after 1 year of UDCA treatment have been developed and extensively validated in large cohorts of PBC patients. The initial models, which included the Rotterdam criteria, Barcelona criteria, Paris I and II criteria, and Toronto criteria, introduced the concept of biochemical response to UDCA treatment and demonstrated that patients who met these response criteria had better clinical outcomes. In fact, in many studies, PBC patients who met criteria for a biochemical response had a transplant-free survival that was not significantly different from a matched control population.2,22 More recently, models such as the UK-PBC and GLOBE scores have provided a continuous risk estimate and have demonstrated excellent performance for predicting transplant-free survival up to 10 to 15 years.23,24 Several differences distinguish each model in terms of algorithms and variables, but the primary drivers of transplant-free survival have been identified as serum total bilirubin and serum ALP. Interestingly, a total bilirubin within the normal range but greater than 0.7 times the upper limit of normal (ULN; equivalent to a total bilirubin of 0.9 mg/dL) has been associated with a reduction in transplant-free survival.25
Second-Line Treatment Options in Patients With Primary Biliary Cholangitis
Obeticholic Acid
Depending upon the patient population and the criteria used, 30% to 40% of UDCA-treated PBC patients will have an inadequate biochemical response after 6 to 12 months of therapy. In addition, there is a small subset of PBC patients who cannot tolerate UDCA. These 2 patient groups have been in need of additional therapies. Nearly 2 decades after the approval of UDCA for the treatment of PBC, OCA received conditional approval by the FDA in 2016, making it the second approved drug for PBC. OCA is a potent farnesoid X receptor (FXR) agonist. FXR is a nuclear hormone receptor, which, when bound by its natural occurring ligand (chenodeoxycholic acid), regulates the synthesis and enterohepatic circulation of bile acids. Within hepatocytes, FXR activation inhibits conversion of cholesterol to bile acids while enhancing their excretion. Increased fecal excretion of bile acids by FXR activation is mediated in the ileum; there, FXR decreases bile acid reabsorption and increases expression of FGF19, which circulates to the liver, where it decreases bile acid synthesis.
The initial development of OCA involved phase 2 studies of the drug as monotherapy and as add-on therapy to UDCA in PBC patients with an inadequate response to UDCA (Table 1).26-28 In the monotherapy trial, 59 PBC patients who had not received UDCA for at least 6 months were randomized to placebo, OCA 10 mg daily, or OCA 50 mg daily for 3 months.26 Patients who received OCA 10 or 50 mg had a significant reduction from baseline in ALP (-53.9%; 95% CI, -62.6 to -29.3 and -37.2%; 95% CI, -54.8 to 24.6, respectively) compared to placebo (-0.8%; 95% CI, -6.4 to 8.7). Pruritus was the most common adverse event leading to discontinuation, occurring in 15% and 38% of patients receiving 10 and 50 mg of OCA, respectively. In the second phase 2 trial, 165 UDCA-treated PBC patients with a serum ALP 1.5 to 10 times the ULN were randomized to 10, 25, or 50 mg daily of OCA or placebo while continuing UDCA.27 All OCA-treated groups had significantly greater reductions from baseline in serum ALP compared to the placebo group, with a mean relative decrease of 24% (95% CI, -30% to -18%), 25% (95% CI, -30% to -20%), and 21% (95% CI, -30% to -12%) for the 10-, 25-, and 50-mg OCA groups, respectively, compared to 3% (95% CI, -7% to 2%) in the placebo group. The groups that received OCA also had significant reductions in γ-glutamyl transferase and alanine aminotransferase (ALT), but only 7% of OCA-treated patients experienced complete normalization of ALP. Pruritus was again a notable adverse event that was more frequent and severe in the OCA groups with a dose-response effect.
Table 1.
Studies of OCA in PBC Patients
| Study | Study Design | Inclusion Criteria | Study Duration and Number of Patients | Dose | Main Results |
|---|---|---|---|---|---|
| Kowdley et al26 | Randomized, double-blind, placebo-controlled trial | PBC patients with ALP 1.5-10 × ULN off UDCA ≥6 months | 3 months; 59 patients | OCA 10, 50 mg/d | Decrease in ALP, ALT, bilirubin, and IgM, and dose-dependent increase in pruritus; 38% of patients in the 50-mg group discontinued OCA due to pruritis |
| Hirschfield et al27 | Randomized, double-blind, placebo-controlled trial | PBC patients with ALP 1.5-10 × ULN while still on UDCA | 3 months; 165 patients | OCA 10, 25, 50 mg/d | Decrease in ALP, GGT, and ALT; only 7% of patients in OCA groups had normalization of ALP |
| Hirschfield et al27 | Open-label study | Long-term safety extension | 12 months; 78 patients | OCA 10, 25, 50 mg/d | Further decrease in ALP in 10- and 25-mg groups after 12 months; 87% of patients reported pruritis. Pruritis worsened following titration to 25 mg. |
| Nevens et al28 | Randomized, double-blind, placebo-controlled trial | PBC patients with ALP 1.5-10 × ULN while still on UDCA | 12 months; 216 patients | OCA 5, 10 mg/d | Primary endpoint (ALP < 1.67 × ULN with a reduction of ≥15% from baseline, normal total bilirubin) was achieved by 47% in the 10-mg OCA group and 46% in the 5-mg titrated to 10-mg OCA group compared to 10% in the placebo group. Pruritis was the most common adverse effect in the OCA groups. |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; GGT, γ-glutamyl transferase; Ig, immunoglobulin; OCA, obeticholic acid; PBC, primary biliary cholangitis; UDCA, ursodeoxycholic acid; ULN, upper limit of normal.
The pivotal phase 3 POISE (PBC OCA International Study of Efficacy) trial evaluated the effects of 12 months of treatment with OCA in PBC patients who had an inadequate response to UDCA—defined by a serum ALP of 1.67 times the ULN, or a bilirubin greater than 1 times the ULN but less than 2 times the ULN—or who were intolerant of UDCA (Table 1).28 Two hundred sixteen patients were randomized to placebo, OCA 10 mg daily, or an initial dose of OCA 5 mg daily titrated to 10 mg after 6 months based upon response and tolerability. The primary endpoint after 12 months of treatment was both a serum ALP less than 1.67 times the ULN with a reduction of at least 15% from baseline as well as a normal total bilirubin. More than 90% of the patients received UDCA as background therapy. The primary endpoint was met by 47% and 46% of patients in the 10-mg and the 5-mg titrated to 10-mg OCA groups, respectively, compared to 10% in the placebo group (P<.001). In addition, significant reductions in ALP and total bilirubin occurred in the OCA-treated groups compared to the placebo group. Pruritus was again more common in the OCA groups; it was reported by 68% and 56% of patients in the 10-mg and the 5-mg titrated to 10-mg OCA treatment arms compared to 38% in the placebo arm. Notably, while discontinuation due to pruritus occurred in 10% of the 10-mg OCA-treated patients, only 1% discontinued due to pruritus in the 5-mg titrated to 10-mg OCA group and none in the placebo group. The majority of patients (91%) entered an open-label, long-term extension of the POISE trial, with results to date supporting the ongoing efficacy of OCA through 2 years.
Based upon the effects of OCA on biochemical markers associated with improved clinical outcomes, the FDA granted conditional approval of OCA for the treatment of PBC patients with an inadequate response or intolerance to UDCA. However, the definition of inadequate response is left open to clinical judgment. The recommended starting dose is 5 mg with titration to 10 mg after 3 months based upon tolerability.
Although no studies had been performed on patients with decompensated cirrhosis, the FDA-approved prescribing guidance advised that patients with Child-Pugh class B and C cirrhosis be initiated at OCA 5 mg once weekly, which can be increased to the maximum approved dose of OCA 10 mg twice weekly. Subsequently, the FDA has issued a safety announcement related to 19 PBC patients with Child-Pugh class B or C cirrhosis who had received an incorrect dose of OCA, leading to severe liver injury and/or death.29 Whether these cases were due to OCA toxicity or the natural progression of advanced liver disease remains to be determined. This has led to a new boxed warning for OCA cautioning health care providers against incorrect dosing.30
Hypercholesterolemia, including increases in both high-density lipoprotein (HDL) and low-density lipoprotein cholesterol, is found in 75% to 95% of patients with PBC, although the clinical significance of this finding is uncertain.31 Studies in nonalcoholic fatty liver disease have consistently demonstrated that OCA alters serum lipids.32-36 In PBC, OCA resulted in a decrease in serum HDL and triglyceride levels.28 The long-term clinical significance of these dyslipidemias due to OCA remains to be determined, but an increase in cardiovascular events has not been identified.
In summary, OCA has demonstrated that it improves liver biochemistries, including serum ALP and total bilirubin (which are associated with improved clinical outcomes), in PBC patients with an inadequate response to UDCA. However, additional long-term trials, some of which are currently underway, as well as real-world experience are needed to confirm that these effects translate into clinically meaningful outcomes. Given the limited options for PBC patients with an incomplete response to UDCA, OCA should be considered, particularly in those without significant pruritus.
Fibrates
Fibrates have anticholestatic effects mediated through the peroxisome proliferator-activated receptor (PPAR)-α UDP-glucuronosyltransferases signaling axis. Thus, fibrates have been studied as therapeutic agents because of their potential ability to reduce bile acid synthesis and bile acid–related hepatic inflammation.37,38 Numerous small pilot studies and case reports have reported that fibrates, including fenofibrate in the United States and bezafibrate in Europe and Japan, improve liver biochemistries, liver stiffness measurements, and pruritus in PBC patients (Tables 2 and 3).39-61 Meta-analyses have demonstrated that fibrates improve liver biochemistries without an increase in adverse events.62,63 These data have been further supported by a recently completed phase 3, randomized, placebo-controlled trial of bezafibrate (BEZURSO, Phase 3 Study of Bezafibrate in Combination With Ursodeoxycholic Acid in Primary Biliary Cirrhosis) in which PBC patients who did not meet Paris II criteria were randomized to receive bezafibrate 400 mg daily or placebo for 2 years. The primary endpoint—normal total bilirubin, ALP, aspartate aminotransferase (AST), ALT, albumin, and prothrombin time—was reached more frequently in the bezafibrate group than in the placebo group (30% vs 0%, respectively).61 There were also significant beneficial changes from baseline to 2 years in serum ALP, ALT, total bilirubin, and albumin. Although these results are promising, serious adverse effects, including hepatotoxicity and elevations of serum creatinine and creatinine kinase, have been observed with fibrate therapy, and should give caution to its use outside of clinical trials.27,64 If fibrates are used, patients should be closely monitored for toxicities. As with OCA, longer-term studies are needed to substantiate any significant improvements in liver-related death or the need for liver transplantation.65
Table 2.
Studies of Fenofibrate in PBC Patients
| Study | Study Design | Inclusion Criteria | Study Duration and Number of Patients | Dose | Main Results |
|---|---|---|---|---|---|
| Han et al41 | Prospective case series | PBC patients treated with UDCA for at least 1 year | 3-6 months; 22 patients | Fenofibrate 200 mg/d | Decrease in ALP, GGT, AST, cholesterol, and TG; no significant effect on serum bilirubin |
| Levy et al43 | Pilot study | PBC patients with ALP > 2 × ULN | 12 months; 20 patients | Fenofibrate 160 mg/d | Decrease in ALP, AST, IgM, IL-1, and IL-6; no significant decrease in bilirubin |
| Liberopoulos et al45 | Pilot study | PBC patients with incomplete biochemical response to UDCA for ≥8 months | 2 months; 10 patients | Fenofibrate 200 mg/d | Decrease in ALP, GGT, ALT, cholesterol, TG, and HDL; fenofibrate was well tolerated |
| Walker et al46 | Retrospective analysis of UK cohort | PBC patients with lack of response to UDCA | 23 months; 16 patients | Fenofibrate 134-200 mg/d | Decrease in ALP (89% of patients had normalization of ALP) and IgM |
| Dohmen et al53 | Prospective case series | PBC patients with lack of response to UDCA | 3 months; 9 patients | Fenofibrate 100 or 150 mg/d | Decrease in ALP, GGT, and IgM; AMAs decreased in 4 patients; no adverse events noted |
| Ohira et al55 | Prospective case series | Biopsy-proven PBC with lack of response to UDCA | 6 months; 7 patients | Fenofibrate 150-200 mg/d | Decrease in ALP, GGT, and IgM |
| Nakamuta et al50 | Prospective case series | Biopsy-proven PBC with prior UDCA treatment | 25-53 months; 5 patients | Bezafibrate 400 mg/d, fenofibrate 150 mg/d | Decrease in ALP, GGT, ALT, IgM, and TG |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AMAs, antimitochondrial antibodies; AST, aspartate aminotransferase; GGT, γ-glutamyl transferase; HDL, high-density lipoprotein; Ig, immunoglobulin; IL, interleukin; PBC, primary biliary cholangitis; TG, triglyceride; UDCA, ursodeoxycholic acid; UK, United Kingdom; ULN, upper limit of normal.
Table 3.
Studies of Bezafibrate in PBC Patients
| Study | Study Design | Inclusion Criteria | Study Duration and Number of Patients | Dose | Main Results |
|---|---|---|---|---|---|
| Lens et al39 | Prospective case series | PBC patients treated with UDCA and abnormal ALP | 12 months; 30 patients | Bezafibrate 400 mg/d | Decrease in ALP (13 patients had normalization of ALP); decrease in GGT, cholesterol, TG, and pruritis; no adverse events noted |
| Honda et al40 | Prospective case series | Early-stage PBC with incomplete response to UDCA | 3 months; 19 patients | Bezafibrate 400 mg/d | Decrease in ALP, GGT, ALT, IgM, cholesterol, TG, and C4 (a marker of bile acid synthesis) |
| Takeuchi et al42 | Prospective case series | PBC patients with incomplete biochemical response to UDCA within 6 months of treatment | 12 months; 15 patients | Bezafibrate 400 mg/d | Decrease in ALP (12 patients had normalization of ALP) and IgM |
| Hazzan et al44 | Prospective case series | PBC patients with incomplete biochemical response to UDCA | 4-12 months; 8 patients | Bezafibrate 400 mg/d | Decrease in ALP (6 patients had normalization of ALP) and GGT |
| Iwasaki et al47 | Two prospective, randomized, controlled trials | Noncirrhotic PBC patients | 52 weeks; Study 1: 45 patients on UDCA or bezafibrate monotherapy; study 2: 21 patients on UDCA and bezafibrate therapy | Bezafibrate 400 mg/d | Study 1: bezafibrate shown to be as effective as UDCA; study 2: bezafibrate with UDCA improved liver enzymes in patients who had an inadequate response to UDCA |
| Kita et al48 | Prospective case series | PBC patients | 6 months; 22 patients | Bezafibrate 400 mg/d | Decrease in ALP, GGT, ALT, and IgM |
| Ohmoto et al49 | Prospective case series | Biopsy-proven PBC | 24-88 months; 17 patients | Bezafibrate 400 mg/d | Decrease in markers of fibrosis |
| Nakamuta et al50 | Prospective case series | Biopsy-proven PBC with prior UDCA treatment | 25-53 months; 5 patients | Bezafibrate 400 mg/d, fenofibrate 150 mg/d | Decrease in ALP, GGT, ALT, IgM, and TG |
| Akbar et al51 | Prospective case series | Biopsy-proven PBC | 12 months; 16 patients | Bezafibrate 400 mg/d | Decrease in ALP, GGT, cholesterol, and IgM; decreased nitrite production by dendritic cells, suggesting an anti-inflammatory effect |
| Itakura et al52 | Randomized trial | Compensated PBC | 12 months; 16 patients | Bezafibrate 400 mg/d | Decrease in ALP, GGT, IgM, and TG |
| Kanda et al54 | Randomized trial | PBC patients with elevated ALP while on UDCA | 6 months; 22 patients | Bezafibrate 400 mg/d | Decrease in ALP and pruritis; no significant decrease in bilirubin |
| Yano et al56 | Prospective case series | Asymptomatic PBC patients | 72-78 months; 2 patients | Bezafibrate 400 mg/d | Decrease in ALP; however, no significant histologic improvement |
| Kurihara et al57 | Prospective case series | PBC patients | 36-60 months; 3 patients | Bezafibrate 400 mg/d | Stabilization or improvement in liver histology |
| Ohmoto et al58 | Pilot study | PBC patients with inadequate response to UDCA | 12 months; 10 patients | Bezafibrate 400 mg/d | Decrease in ALP (10 patients had normalization of ALP), GGT, ALT, IgM, pruritis, and fatigue |
| Kurihara et al59 | Randomized trial | Biopsy-proven PBC | 12 months; 24 patients | Bezafibrate 400 mg/d | Decrease in ALP, GGT, ALT, and IgM; no adverse events noted |
| Corpechot et al61 | Randomized, placebo-controlled trial | PBC patients with inadequate response to UDCA by Paris II criteria | 24 months; 100 patients | Bezafibrate 400 mg/d | Primary endpoint (normalization of bilirubin, ALP, AST, ALT, albumin, and PT) was achieved by 30% in the bezafibrate group compared to 0% in the placebo group |
| Reig et al60 | Prospective study | PBC patients treated with UDCA with ALP > 1.5 × ULN | 22-50 months; 48 patients | Bezafibrate 400 mg/d | 54% of patients with normalized ALP; improvement in jaundice and liver stiffness; all but 1 case reported improvement in pruritus |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ-glutamyl transferase; Ig, immunoglobulin; PBC, primary biliary cholangitis; PT, prothrombin time; TG, triglyceride; UDCA, ursodeoxycholic acid; ULN, upper limit of normal.
Novel Therapeutic Targets for Patients With Primary Biliary Cholangitis
Although it took nearly 2 decades from the approval of UDCA for a second drug to be approved for the treatment of PBC, it is unlikely that the wait for more therapies will be as long. Approaches that build along the same therapeutic pathways as OCA and fibrates are likely to result in potent agents with improved tolerability and safety. Non–bile acid FXR agonists, including GS 9674 (Gilead Sciences) and LJN452 (Novartis), are currently being evaluated in phase 2 trials with the hope of having similar effects on liver biochemistries as OCA with better tolerability, especially in terms of reduced pruritus.66,67 A novel approach includes the targeting of nicotinamide adenine dinucleotide phosphate oxidase (NOX). NOX 1, 2, and 4 are expressed in hepatic myofibroblasts producing reactive oxygen species and regulate liver fibrosis through hepatic stellate cell activation and hepatocyte apoptosis. The NOX 1/4 inhibitor GKT137831 (Genkyotex SA) is currently being evaluated in a phase 2 study in PBC patients who are on a stable dose of UDCA and have persistently elevated ALP levels.68
Despite the lack of success with past immunomodulatory approaches to PBC treatment, there remains the potential that this quintessentially autoimmune disease can be treated at its underlying cause. Fractalkine is a chemokine that promotes the migration and adhesion of leukocytes and is upregulated in biliary epithelial cells derived from PBC patients.69 Currently, 2 agents are in clinical trials targeting lymphocyte homing, one with an antifractalkine (CX3CL1) monoclonal antibody (E6011, EA Pharma Co) and the other with etrasimod (APD334, Arena Pharmaceuticals), a selective S1P receptor modulator.70,71
Below are details regarding the development of 2 advanced agents.
NGM282 (Synthetic FGF19 Analog)
NGM282 (NGM Biopharmaceuticals) is a synthetic protein analog of FGF19, a protein secreted by the intestinal epithelium in response to activation of FXR and which acts in a hormonal fashion in the liver to regulate bile acid synthesis. FGF19 downregulates the expression of CYP7A1, an enzymatic catalyst in the rate-limiting step in bile acid synthesis.72-75 In animal models, FGF19 decreases bile acids and liver enzymes to a significantly greater degree when compared with OCA or bezafibrate.76 Although prolonged exposure to FGF19 has led to the development of hepatocellular carcinoma in some mouse models, similar effects have not occurred with NGM282.77
In a phase 2, randomized, placebo-controlled, double-blind study, 45 PBC patients with an incomplete response to UDCA received NGM282 0.3 mg or 3 mg subcutaneously or placebo for 28 days.78 NGM282 demonstrated a significant dose-dependent reduction in AST, ALT, and ALP. In addition, NGM282 suppressed serum levels of C4, an intermediate in bile acid synthesis, demonstrating its role in regulation of bile acid synthesis. Unlike with OCA, pruritus was uncommon and reported by only 3 patients treated with NGM282. However, diarrhea was reported by 21% of patients receiving NGM282 0.3 mg and 43% of patients receiving NGM282 3 mg, compared to only 13% of patients who received placebo. A phase 2b study evaluating the safety, tolerability, and activity of extended treatment with NGM282 for 24 weeks in PBC patients has been completed, and results are pending.79
Seladelpar
Seladelpar (MBX-8025, CymaBay Therapeutics) is an oral, once-daily, selective PPAR-δ agonist.80 PPAR-δ controls genes involved in bile acid homeostasis and is expressed in hepatocytes, cholangiocytes, Kupffer cells, and hepatic stellate cells.81 Seladelpar downregulates the expression of CYP7A1, similar to FGF19 and fibrates.82 In mouse studies, PPAR-δ agonists are hepatoprotective and antifibrotic, and have a choleretic effect.81,83
In a phase 2, double-blind, placebo-controlled, 12-week clinical trial of seladelpar in PBC patients, 13 patients received seladelpar 50 mg, 10 patients received seladelpar 200 mg, and 12 patients received placebo. The mean decrease in ALP from baseline was -53% (standard deviation [SD], + 14%) in the seladelpar 50-mg group, -63% (SD, + 8%) in the seladelpar 200-mg group, and -2% (SD, + 16%) in the placebo group. The decrease in ALP in the seladelpar groups when compared to placebo was statistically significant, whereas there was no statistically significant decrease in ALP between the 2 seladelpar groups. Side effects noted in both seladelpar groups included grade 3 elevations of ALT that were fully reversible when the drug was discontinued.82 A second phase 2 clinical trial with lower doses of seladelpar is ongoing. If the drug maintains its efficacy and is not associated with ALT elevations at lower doses, seladelpar has the potential to become a second-line agent for the treatment of patients with PBC.
Summary
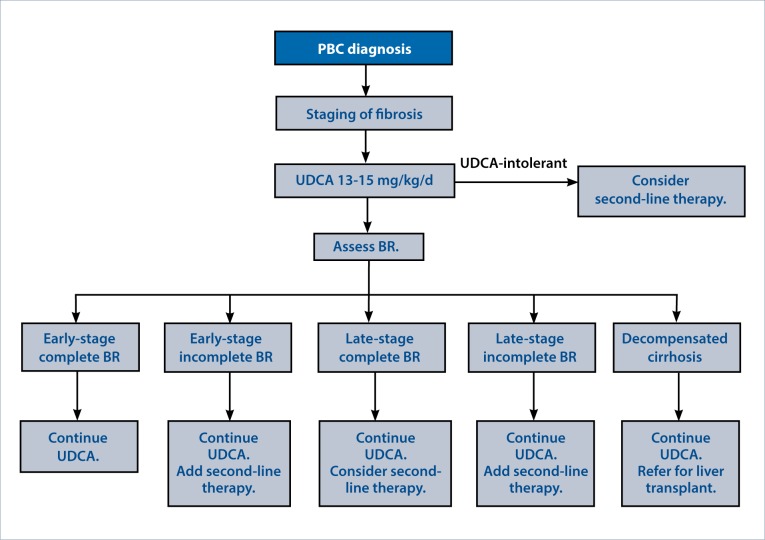
All patients with PBC should be treated with UDCA at 13 to 15 mg/kg/d and monitored for a biochemical response using any of the available criteria. If there is an incomplete response to UDCA, then second-line therapies should be considered, namely OCA. If OCA is not tolerated or is contraindicated, growing evidence supports the use of fibrates, but their safety has not been firmly established, and caution should be used (Figure). Alternatively, patients may be referred for one of the many current clinical trials in PBC. For the small number of patients intolerant of UDCA, these second-line agents may also be considered. Other factors such as advanced fibrosis should also be used to determine appropriate candidates for second-line treatment even if biochemical response is achieved. However, in PBC patients with decompensated cirrhosis, medical treatment is unlikely to significantly impact the course of disease, and the unknown risk of second-line therapies in these patients should give caution. Instead, liver transplantation should be considered for these patients.
Figure.
A treatment algorithm for primary biliary cholangitis (PBC). Following the diagnosis of PBC, staging of fibrosis can be achieved by liver biopsy or noninvasive methods such as vibration-controlled transient elastography. First-line treatment with ursodeoxycholic acid (UDCA) should be given to all PBC patients. After 12 months, biochemical response (BR) should be assessed by the GLOBE score or other criteria. Second-line therapy should be considered according to the BR and the stage of fibrosis. Obeticholic acid should be considered unless there is severe pruritus or other contraindications to its use. A fibrate may be an acceptable alternative second-line agent, but is not currently approved by the US Food and Drug Administration for this indication.
References
- 1.Selmi C, Bowlus CL, Gershwin ME, Coppel RL. Primary biliary cirrhosis. Lancet. 2011;377(9777):1600–1609. doi: 10.1016/S0140-6736(10)61965-4. [DOI] [PubMed] [Google Scholar]
- 2.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67(1):145–172. doi: 10.1016/j.jhep.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 3.Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ. American Association for Study of Liver Diseases. Primary biliary cirrhosis. Hepatology. 2009;50(1):291–308. doi: 10.1002/hep.22906. [DOI] [PubMed] [Google Scholar]
- 4.Locke GR, III, Therneau TM, Ludwig J, Dickson ER, Lindor KD. Time course of histological progression in primary biliary cirrhosis. Hepatology. 1996;23(1):52–56. doi: 10.1002/hep.510230108. [DOI] [PubMed] [Google Scholar]
- 5.Mahl TC, Shockcor W, Boyer JL. Primary biliary cirrhosis: survival of a large cohort of symptomatic and asymptomatic patients followed for 24 years. J Hepatol. 1994;20(6):707–713. doi: 10.1016/s0168-8278(05)80139-4. [DOI] [PubMed] [Google Scholar]
- 6.Long RG, Scheuer PJ, Sherlock S. Presentation and course of asymptomatic primary biliary cirrhosis. Gastroenterology. 1977;72(6):1204–1207. [PubMed] [Google Scholar]
- 7.Mitchison HC, Lucey MR, Kelly PJ, Neuberger JM, Williams R, James OF. Symptom development and prognosis in primary biliary cirrhosis: a study in two centers. Gastroenterology. 1990;99(3):778–784. doi: 10.1016/0016-5085(90)90968-7. [DOI] [PubMed] [Google Scholar]
- 8.Nyberg A, Lööf L. Primary biliary cirrhosis: clinical features and outcome, with special reference to asymptomatic disease. Scand J Gastroenterol. 1989;24(1):57–64. doi: 10.3109/00365528909092240. [DOI] [PubMed] [Google Scholar]
- 9.Springer J, Cauch-Dudek K, O’Rourke K, Wanless IR, Heathcote EJ. Asymptomatic primary biliary cirrhosis: a study of its natural history and prognosis. Am J Gastroenterol. 1999;94(1):47–53. doi: 10.1111/j.1572-0241.1999.00770.x. [DOI] [PubMed] [Google Scholar]
- 10.Prince M, Chetwynd A, Newman W, Metcalf JV, James OF. Survival and symptom progression in a geographically based cohort of patients with primary biliary cirrhosis: follow-up for up to 28 years. Gastroenterology. 2002;123(4):1044–1051. doi: 10.1053/gast.2002.36027. [DOI] [PubMed] [Google Scholar]
- 11.Balasubramaniam K, Grambsch PM, Wiesner RH, Lindor KD, Dickson ER. Diminished survival in asymptomatic primary biliary cirrhosis. A prospective study. Gastroenterology. 1990;98(6):1567–1571. doi: 10.1016/0016-5085(90)91091-j. [DOI] [PubMed] [Google Scholar]
- 12.Poupon RE, Balkau B, Eschwège E, Poupon R. UDCA-PBC Study Group. A multicenter, controlled trial of ursodiol for the treatment of primary biliary cirrhosis. N Engl J Med. 1991;324(22):1548–1554. doi: 10.1056/NEJM199105303242204. [DOI] [PubMed] [Google Scholar]
- 13.Poupon RE, Poupon R, Balkau B. The UDCA-PBC Study Group. Ursodiol for the long-term treatment of primary biliary cirrhosis. N Engl J Med. 1994;330(19):1342–1347. doi: 10.1056/NEJM199405123301903. [DOI] [PubMed] [Google Scholar]
- 14.Heathcote EJ, Cauch-Dudek K, Walker V, et al. The Canadian multicenter double-blind randomized controlled trial of ursodeoxycholic acid in primary biliary cirrhosis. Hepatology. 1994;19(5):1149–1156. [PubMed] [Google Scholar]
- 15.Parés A, Caballería L, Rodés J, et al. UDCA-Cooperative Group from the Spanish Association for the Study of the Liver. Long-term effects of ursodeoxycholic acid in primary biliary cirrhosis: results of a double-blind controlled multicentric trial. J Hepatol. 2000;32(4):561–566. doi: 10.1016/s0168-8278(00)80216-0. [DOI] [PubMed] [Google Scholar]
- 16.Combes B, Carithers RL, Jr, Maddrey WC, et al. A randomized, double-blind, placebo-controlled trial of ursodeoxycholic acid in primary biliary cirrhosis. Hepatology. 1995;22(3):759–766. [PubMed] [Google Scholar]
- 17.Lindor KD, Dickson ER, Baldus WP, et al. Ursodeoxycholic acid in the treatment of primary biliary cirrhosis. Gastroenterology. 1994;106(5):1284–1290. doi: 10.1016/0016-5085(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 18.Angulo P, Batts KP, Therneau TM, Jorgensen RA, Dickson ER, Lindor KD. Long-term ursodeoxycholic acid delays histological progression in primary biliary cirrhosis. Hepatology. 1999;29(3):644–647. doi: 10.1002/hep.510290301. [DOI] [PubMed] [Google Scholar]
- 19.Kuiper EM, Hansen BE, Metselaar HJ, et al. Trends in liver transplantation for primary biliary cirrhosis in the Netherlands 1988-2008. BMC Gastroenterol. 2010;10:144. doi: 10.1186/1471-230X-10-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J, Belanger A, Doucette JT, Stanca C, Friedman S, Bach N. Transplantation trends in primary biliary cirrhosis. Clin Gastroenterol Hepatol. 2007;5(11):1313–1315. doi: 10.1016/j.cgh.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Lu M, Li J, Haller IV, et al. FOLD Investigators. Factors associated with prevalence and treatment of primary biliary cholangitis in United States health systems. Clin Gastroenterol Hepatol. doi: 10.1016/j.cgh.2017.10.018. published online October 21, 2017. doi:10.1016/j.cgh.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Parés A, Caballería L, Rodés J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic acid. Gastroenterology. 2006;130(3):715–720. doi: 10.1053/j.gastro.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 23.Lammers WJ, Hirschfield GM, Corpechot C, et al. Global PBC Study Group. Development and validation of a scoring system to predict outcomes of patients with primary biliary cirrhosis receiving ursodeoxycholic acid therapy. Gastroenterology. 2015;149(7):1804–1812.e4. doi: 10.1053/j.gastro.2015.07.061. [DOI] [PubMed] [Google Scholar]
- 24.Cheung KS, Seto WK, Fung J, Lai CL, Yuen MF. Prognostic factors for transplant-free survival and validation of prognostic models in Chinese patients with primary biliary cholangitis receiving ursodeoxycholic acid. Clin Transl Gastroenterol. 2017;8(6):e100. doi: 10.1038/ctg.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lammers WJ, van Buuren HR, Hirschfield GM, et al. Global PBC Study Group. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow-up study. Gastroenterology. 2014;147(6):1338–1349.e5. doi: 10.1053/j.gastro.2014.08.029. quiz e15. [DOI] [PubMed] [Google Scholar]
- 26.Kowdley KV, Luketic V, Chapman R, et al. Obeticholic Acid PBC Monotherapy Study Group. A randomized trial of obeticholic acid monotherapy in patients with primary biliary cholangitis. Hepatology. doi: 10.1002/hep.29569. published online October 10, 2017. doi:10.1002/hep.29569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirschfield GM, Mason A, Luketic V, et al. Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology. 2015;148(4):751–761.e8. doi: 10.1053/j.gastro.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Nevens F, Andreone P, Mazzella G, et al. POISE Study Group. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N Engl J Med. 2016;375(7):631–643. doi: 10.1056/NEJMoa1509840. [DOI] [PubMed] [Google Scholar]
- 29.US Food and Drug Administration. FDA Drug Safety Communication: FDA warns about serious liver injury with Ocaliva (obeticholic acid) for rare chronic liver disease. [Accessed February 14, 2018]. https://www.fda.gov/Drugs/DrugSafety/ucm576656.htm. Published September 21, 2017.
- 30.US Food and Drug Administration. FDA adds boxed warning to highlight correct dosing of Ocaliva (obeticholic acid) for patients with a rare chronic liver disease. [Accessed February 14, 2018]. https://www.fda.gov/Drugs/DrugSafety/ucm594941.htm Published February 1, 2018.
- 31.Longo M, Crosignani A, Battezzati PM, et al. Hyperlipidaemic state and cardiovascular risk in primary biliary cirrhosis. Gut. 2002;51(2):265–269. doi: 10.1136/gut.51.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. NASH Clinical Research Network. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385(9972):956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Makri E, Cholongitas E, Tziomalos K. Emerging role of obeticholic acid in the management of nonalcoholic fatty liver disease. World J Gastroenterol. 2016;22(41):9039–9043. doi: 10.3748/wjg.v22.i41.9039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pencek R, Marmon T, Roth JD, Liberman A, Hooshmand-Rad R, Young MA. Effects of obeticholic acid on lipoprotein metabolism in healthy volunteers. Diabetes Obes Metab. 2016;18(9):936–940. doi: 10.1111/dom.12681. [DOI] [PubMed] [Google Scholar]
- 35.Hameed B, Terrault NA, Gill RM, et al. NASH CRN. Clinical and metabolic effects associated with weight changes and obeticholic acid in non-alcoholic steato-hepatitis. Aliment Pharmacol Ther. 2018;47(5):645–656. doi: 10.1111/apt.14492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mudaliar S, Henry RR, Sanyal AJ, et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145(3):574–582.e1. doi: 10.1053/j.gastro.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 37.Suraweera D, Rahal H, Jimenez M, Viramontes M, Choi G, Saab S. Treatment of primary biliary cholangitis ursodeoxycholic acid non-responders: a systematic review. Liver Int. 2017;37(12):1877–1886. doi: 10.1111/liv.13477. [DOI] [PubMed] [Google Scholar]
- 38.Hegade VS, Khanna A, Walker LJ, Wong LL, Dyson JK, Jones DEJ. Long-term fenofibrate treatment in primary biliary cholangitis improves biochemistry but not the UK-PBC risk score. Dig Dis Sci. 2016;61(10):3037–3044. doi: 10.1007/s10620-016-4250-y. [DOI] [PubMed] [Google Scholar]
- 39.Lens S, Leoz M, Nazal L, Bruguera M, Parés A. Bezafibrate normalizes alkaline phosphatase in primary biliary cirrhosis patients with incomplete response to ursodeoxycholic acid. Liver Int. 2014;34(2):197–203. doi: 10.1111/liv.12290. [DOI] [PubMed] [Google Scholar]
- 40.Honda A, Ikegami T, Nakamuta M, et al. Anticholestatic effects of bezafibrate in patients with primary biliary cirrhosis treated with ursodeoxycholic acid. Hepatology. 2013;57(5):1931–1941. doi: 10.1002/hep.26018. [DOI] [PubMed] [Google Scholar]
- 41.Han XF, Wang QX, Liu Y, et al. Efficacy of fenofibrate in Chinese patients with primary biliary cirrhosis partially responding to ursodeoxycholic acid therapy. J Dig Dis. 2012;13(4):219–224. doi: 10.1111/j.1751-2980.2012.00574.x. [DOI] [PubMed] [Google Scholar]
- 42.Takeuchi Y, Ikeda F, Fujioka S, et al. Additive improvement induced by bezafibrate in patients with primary biliary cirrhosis showing refractory response to ursodeoxycholic acid. J Gastroenterol Hepatol. 2011;26(9):1395–1401. doi: 10.1111/j.1440-1746.2011.06737.x. [DOI] [PubMed] [Google Scholar]
- 43.Levy C, Peter JA, Nelson DR, et al. Pilot study: fenofibrate for patients with primary biliary cirrhosis and an incomplete response to ursodeoxycholic acid. Aliment Pharmacol Ther. 2011;33(2):235–242. doi: 10.1111/j.1365-2036.2010.04512.x. [DOI] [PubMed] [Google Scholar]
- 44.Hazzan R, Tur-Kaspa R. Bezafibrate treatment of primary biliary cirrhosis following incomplete response to ursodeoxycholic acid. J Clin Gastroenterol. 2010;44(5):371–373. doi: 10.1097/MCG.0b013e3181c115b3. [DOI] [PubMed] [Google Scholar]
- 45.Liberopoulos EN, Florentin M, Elisaf MS, Mikhailidis DP, Tsianos E. Fenofibrate in primary biliary cirrhosis: a pilot study. Open Cardiovasc Med J. 2010;4:120–126. doi: 10.2174/1874192401004010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walker LJ, Newton J, Jones DE, Bassendine MF. Comment on biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology. 2009;49(1):337–338. doi: 10.1002/hep.22670. [DOI] [PubMed] [Google Scholar]
- 47.Iwasaki S, Ohira H, Nishiguchi S, et al. Study Group of Intractable Liver Diseases for Research on a Specific Disease, Health Science Research Grant, Ministry of Health, Labour and Welfare of Japan. The efficacy of ursodeoxycholic acid and bezafibrate combination therapy for primary biliary cirrhosis: a prospective, multicenter study. Hepatol Res. 2008;38(6):557–564. doi: 10.1111/j.1872-034X.2007.00305.x. [DOI] [PubMed] [Google Scholar]
- 48.Kita R, Takamatsu S, Kimura T, Kokuryu H, Osaki Y, Tomono N. Bezafibrate may attenuate biliary damage associated with chronic liver diseases accompanied by high serum biliary enzyme levels. J Gastroenterol. 2006;41(7):686–692. doi: 10.1007/s00535-006-1831-0. [DOI] [PubMed] [Google Scholar]
- 49.Ohmoto K, Yoshioka N, Yamamoto S. Long-term effect of bezafibrate on parameters of hepatic fibrosis in primary biliary cirrhosis. J Gastroenterol. 2006;41(5):502–503. doi: 10.1007/s00535-006-1778-1. [DOI] [PubMed] [Google Scholar]
- 50.Nakamuta M, Enjoji M, Kotoh K, Shimohashi N, Tanabe Y. Long-term fibrate treatment for PBC. J Gastroenterol. 2005;40(5):546–547. doi: 10.1007/s00535-004-1583-7. [DOI] [PubMed] [Google Scholar]
- 51.Akbar SM, Furukawa S, Nakanishi S, Abe M, Horiike N, Onji M. Therapeutic efficacy of decreased nitrite production by bezafibrate in patients with primary biliary cirrhosis. J Gastroenterol. 2005;40(2):157–163. doi: 10.1007/s00535-004-1518-3. [DOI] [PubMed] [Google Scholar]
- 52.Itakura J, Izumi N, Nishimura Y, et al. Prospective randomized crossover trial of combination therapy with bezafibrate and UDCA for primary biliary cirrhosis. Hepatol Res. 2004;29(4):216–222. doi: 10.1016/j.hepres.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 53.Dohmen K, Mizuta T, Nakamuta M, Shimohashi N, Ishibashi H, Yamamoto K. Fenofibrate for patients with asymptomatic primary biliary cirrhosis. World J Gastroenterol. 2004;10(6):894–898. doi: 10.3748/wjg.v10.i6.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kanda T, Yokosuka O, Imazeki F, Saisho H. Bezafibrate treatment: a new medical approach for PBC patients? J Gastroenterol. 2003;38(6):573–578. doi: 10.1007/s00535-002-1102-7. [DOI] [PubMed] [Google Scholar]
- 55.Ohira H, Sato Y, Ueno T, Sata M. Fenofibrate treatment in patients with primary biliary cirrhosis. Am J Gastroenterol. 2002;97(8):2147–2149. doi: 10.1111/j.1572-0241.2002.05944.x. [DOI] [PubMed] [Google Scholar]
- 56.Yano K, Kato H, Morita S, Takahara O, Ishibashi H, Furukawa R. Is bezafibrate histologically effective for primary biliary cirrhosis? Am J Gastroenterol. 2002;97(4):1075–1077. doi: 10.1111/j.1572-0241.2002.05645.x. [DOI] [PubMed] [Google Scholar]
- 57.Kurihara T, Maeda A, Shigemoto M, Yamashita K, Hashimoto E. Investigation into the efficacy of bezafibrate against primary biliary cirrhosis, with histological references from cases receiving long term monotherapy. Am J Gastroenterol. 2002;97(1):212–214. doi: 10.1111/j.1572-0241.2002.05413.x. [DOI] [PubMed] [Google Scholar]
- 58.Ohmoto K, Mitsui Y, Yamamoto S. Effect of bezafibrate in primary biliary cirrhosis: a pilot study. Liver. 2001;21(3):223–224. doi: 10.1034/j.1600-0676.2001.021003223.x. [DOI] [PubMed] [Google Scholar]
- 59.Kurihara T, Niimi A, Maeda A, Shigemoto M, Yamashita K. Bezafibrate in the treatment of primary biliary cirrhosis: comparison with ursodeoxycholic acid. Am J Gastroenterol. 2000;95(10):2990–2992. doi: 10.1111/j.1572-0241.2000.03220.x. [DOI] [PubMed] [Google Scholar]
- 60.Reig A, Sese P, Pares A. Effects of bezafibrate on outcome and pruritus in primary biliary cholangitis with suboptimal ursodeoxycholic acid response. Am J Gastroenterol. 2018;113(1):49–55. doi: 10.1038/ajg.2017.287. [DOI] [PubMed] [Google Scholar]
- 61.Corpechot C, Chazouillères O, Rousseau A, et al. A 2-year multicenter, double-blind, randomized, placebo-controlled study of bezafibrate for the treatment of primary biliary cholangitis in patients with inadequate biochemical response to ursodeoxycholic acid therapy (Bezurso) J Hepatol. 2017;66(1):S89. [Google Scholar]
- 62.Zhang Y, Chen K, Dai W, et al. Combination therapy of bezafibrate and ursodeoxycholic acid for primary biliary cirrhosis: a meta-analysis. Hepatol Res. 2015;45(1):48–58. doi: 10.1111/hepr.12373. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Y, Li S, He L, et al. Combination therapy of fenofibrate and ursodeoxycholic acid in patients with primary biliary cirrhosis who respond incompletely to UDCA monotherapy: a meta-analysis. Drug Des Devel Ther. 2015;9:2757–2766. doi: 10.2147/DDDT.S79837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davidson MH, Armani A, McKenney JM, Jacobson TA. Safety considerations with fibrate therapy. Am J Cardiol. 2007;99(6A):3C–18C. doi: 10.1016/j.amjcard.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 65.Bowlus CL, Kenney JT, Rice G, Navarro R. Primary biliary cholangitis: medical and specialty pharmacy management update. J Manag Care Spec Pharm. 2016;22(10-a-s suppl):S3–S15. doi: 10.18553/jmcp.2016.22.10-a-s.s3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.ClinicalTrials.gov. Safety, tolerability, and efficacy of GS 9674 in adults with primary biliary cholangitis without cirrhosis (PBC-Phase 2) [Accessed December 15, 2017]. https://clinicaltrials.gov/ct2/show/NCT02943447.Identifier:NCT02943447
- 67.ClinicalTrials.gov. A multi-part, double blind study to assess safety, tolerability and efficacy of LJN452 in PBC patients. [Accessed December 15, 2017]. https://clinicaltrials.gov/ct2/show/NCT02516605.Identifier:NCT02516605
- 68.ClinicalTrials.gov. Study to assess safety and efficacy of GKT137831 in patients with primary biliary cholangitis receiving ursodeoxycholic acid. [Accessed December 15, 2017]. https://clinicaltrials.gov/ct2/show/NCT03226067.Identifier:NCT03226067
- 69.Shimoda S, Harada K, Niiro H, et al. CX3CL1 (fractalkine): a signpost for biliary inflammation in primary biliary cirrhosis. Hepatology. 2010;51(2):567–575. doi: 10.1002/hep.23318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.ClinicalTrials.gov. Study of E6011 in Japanese subjects with primary biliary cholangitis inadequately responding to ursodeoxycholic acid. [Accessed December 15, 2017]. https://clinicaltrials.gov/ct2/show/NCT03092765.Identifier:NCT03092765
- 71.ClinicalTrials.gov. Safety, tolerability, and efficacy of etrasimod (APD334) in patients with primary biliary cholangitis. [Accessed December 15, 2017]. https://clinicaltrials.gov/ct2/show/NCT03155932.Identifier:NCT03155932
- 72.Inagaki T, Choi M, Moschetta A, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2(4):217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 73.Xie MH, Holcomb I, Deuel B, et al. FGF-19, a novel fibroblast growth factor with unique specificity for FGFR4. Cytokine. 1999;11(10):729–735. doi: 10.1006/cyto.1999.0485. [DOI] [PubMed] [Google Scholar]
- 74.Zhang JH, Nolan JD, Kennie SL, et al. Potent stimulation of fibroblast growth factor 19 expression in the human ileum by bile acids. Am J Physiol Gastrointest Liver Physiol. 2013;304(10):G940–G948. doi: 10.1152/ajpgi.00398.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schaap FG, Trauner M, Jansen PL. Bile acid receptors as targets for drug development. Nat Rev Gastroenterol Hepatol. 2014;11(1):55–67. doi: 10.1038/nrgastro.2013.151. [DOI] [PubMed] [Google Scholar]
- 76.Luo J, Ko B, To C, et al. Hepatoprotective effects of NGM282 compared to obeticholic acid and bezafibrate in mouse models of cholestasis. J Hepatol. 2014;60(1):S531. [Google Scholar]
- 77.Ling L. Human FGF19 but not NGM282, an engineered variant of FGF19, causes hepatocellular carcinoma (HCC) in a diet-induced mouse model of nonalcoholic steatohepatitis (NASH). Paper presented at: The Liver Meeting 2016; November 11-15, 2016; Boston, Massachusetts. Abstract LB-19.
- 78.Mayo MJ, Wigg AJ, Roberts SK, et al. NGM282, a novel variant of FGF-19, demonstrates biologic activity in primary biliary cirrhosis patients with an incomplete response to ursodeoxycholic acid: results of a phase 2 multicenter, randomized, double blinded, placebo controlled trial. Hepatology. 2015;62(suppl 1):263A–264A. [Google Scholar]
- 79.ClinicalTrials.gov. Phase 2b study of NGM282 extended treatment in patients with primary biliary cirrhosis. [Accessed December 15, 2017]. https://clinicaltrials.gov/ct2/show/NCT02135536.Identifier:NCT02135536
- 80.Bays HE, Schwartz S, Littlejohn T, III, et al. MBX-8025, a novel peroxisome proliferator receptor-delta agonist: lipid and other metabolic effects in dyslipidemic overweight patients treated with and without atorvastatin. J Clin Endocrinol Metab. 2011;96(9):2889–2897. doi: 10.1210/jc.2011-1061. [DOI] [PubMed] [Google Scholar]
- 81.Iwaisako K, Haimerl M, Paik YH, et al. Protection from liver fibrosis by a peroxisome proliferator-activated receptor δ agonist. Proc Natl Acad Sci U S A. 2012;109(21):E1369–E1376. doi: 10.1073/pnas.1202464109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jones D, Boudes PF, Swain MG, et al. Seladelpar (MBX-8025), a selective PPAR-δ agonist, in patients with primary biliary cholangitis with an inadequate response to ursodeoxycholic acid: a double-blind, randomised, placebo-controlled, phase 2, proof-of-concept study. Lancet Gastroenterol Hepatol. 2017;2(10):716–726. doi: 10.1016/S2468-1253(17)30246-7. [DOI] [PubMed] [Google Scholar]
- 83.Vrins CL, van der Velde AE, van den Oever K, et al. Peroxisome proliferator-activated receptor delta activation leads to increased transintestinal cholesterol efflux. J Lipid Res. 2009;50(10):2046–2054. doi: 10.1194/jlr.M800579-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]