Abstract
Here, we report that treatment of syngeneic mouse tumors transduced to overexpress human epidermal growth factor receptor-2 (HER2) with the anti-human HER2 antibody trastuzumab upregulated the level of programmed death-ligand 1 (PD-L1), an important negative regulator of T-cell response, in a transgenic mouse model immune-tolerant to human HER2. We further found that trastuzumab alone had no detectable effect on the level of PD-L1 expression in monocultures of HER2-overexpressing human breast cancer cells but upregulated PD-L1 in the same panel of HER2-overexpressing breast cancer cells when they were co-cultured with human peripheral blood mononuclear cells, and the upregulation of PD-L1 could be blocked by an IFNγ-neutralizing antibody. Inhibition of HER2 intrinsic signaling via HER2 expression knockdown or kinase inhibition had variable and cell-context-specific effects on downregulating the PD-L1 level. Analysis of The Cancer Genome Atlas database showed no direct correlation between HER2 and PD-L1 at the messenger RNA level. Trastuzumab-mediated upregulation of PD-L1 through engagement of immune effector cells may function as a potential mechanism of trastuzumab resistance. Our data justify further investigation of the value of adding anti-PD-1 or anti-PD-L1 therapy to trastuzumab-based treatment.
Keywords: Breast cancer, HER2, PD-L1, IFNγ, trastuzumab
1. Introduction
The anti-human epidermal growth factor receptor-2 (HER2) antibody trastuzumab is the current gold standard for treating patients with HER2-overexpressing breast cancer, but it is effective only in approximately 30%–50% of patients [1–4]. Two well-documented mechanisms underlying trastuzumab’s antitumor activity are inhibition of cell signaling downstream of HER2 that regulates cell survival, proliferation, invasion and metastasis and induction of antibody-dependent cell-mediated cytotoxicity (ADCC). Aberrations or deficiencies in either of these mechanisms may lead to tumor resistance to trastuzumab treatment [5,6]. Emerging evidence indicates that HER2 antibody-mediated antitumor activity may also involve adaptive immune responses. Preclinical studies using an antibody (7.16.4) targeting the rodent counterpart of HER2 (HER2/neu) in immunocompetent mouse models indicated that the antitumor activity of the antibody required participation of CD8+ and CD4+ T cells through secretion of interferon gamma (IFNγ) [7–9]. Accumulating clinical evidence also shows that breast tumors with a high level of tumor-infiltrating lymphocytes are more responsive to trastuzumab than are tumors with a low level of tumor-infiltrating lymphocytes [10–13]. However, few mechanistic insights are available into the role of adaptive immunity in resistance to trastuzumab, in part because trastuzumab recognizes only human HER2 and immunocompromised mice are therefore often used to host xenografts of HER2-overexpressing human breast cancer cells. In addition, mouse IFNγ secreted by host immune cells does not seem to activate IFNγ receptor signaling in human cells [14].
Programmed death-1 (PD-1; also known as CD279), a member of the CD28 family, is an inhibitory receptor expressed on various immune cells, including T and B lymphocytes, dendritic cells, monocytes, and macrophages [15–18]. Ligation to PD-1 by programmed death-ligand 1 (PD-L1; also known as CD274 and B7-H1), a member of the B7 family [19], induces anergy or apoptosis of immune cells, leading to their exhaustion or depletion [19–22]. Like PD-1, PD-L1 is expressed on various immune cells. PD-L1 is also expressed in several nonhematopoietic tissues and on cancerous cells [20,23–25]. It is now clear that cancer cells can exploit this immune checkpoint mechanism to escape from host immune surveillance [20]. Recent advances in understanding of the role of the immune checkpoint pathways in cancer initiation, progression, and metastasis have led to US Food and Drug Administration (FDA) approval of several drugs targeting the immune checkpoint pathways, including pembrolizumab and nivolumab (anti-PD-1 antibodies) atezolizumab (anti-PD-L1 antibody), and ipilimumab (anti-CTLA-4 antibody) [26,27].
Expression of PD-L1 on immune cells is regulated by several cytokines, including IFNγ, granulocyte-macrophage colony-stimulating factor (GM-CSF), and interleukin-4 (IL-4) [28]. Activation of signaling pathways downstream of IFNγ receptor leads to binding of interferon regulatory factor-1 (IRF-1) to the PD-L1 gene promoter [29]. In contrast, expression of PD-L1 on cancer cells is regulated not only by the cytokines secreted by immune cells in the tumor microenvironment (extrinsic pathway) but also by intracellular cell signaling within the cancer cells (intrinsic pathway) [30]. Successful therapeutic targeting of intracellular cell signaling may lead to downregulation of PD-L1 in cancer cells; however, the signaling pathway in cancer cells is frequently dysregulated, for example, due to oncogenic mutations of key proto-oncogenes, including EGFR, PI3KCA, and BRAF [31–35], and mutational inactivation of tumor suppressors, including PTEN, in human cancer [36,37], leading to constitutively high expression of PD-L1 in cancer cells.
Mutations of PI3KCA and PTEN are common in breast cancer and are a major mechanism of resistance to trastuzumab [1,38]. This information, taken together with the link between mutations in these genes and the intrinsic pathway regulating PD-L1 expression on cancer cells, raised an expectation that co-targeting the PD-1/PD-L1 pathway might potentiate the therapeutic activity of trastuzumab and has prompted clinical trials testing combinations of trastuzumab with an immune checkpoint inhibitor (anti-PD-1 or anti-PD-L1 antibody) (see ClinicalTrials.gov).
In contrast with HER2 tyrosine kinase inhibitors, such as lapatinib, trastuzumab not only can inhibit HER2-mediated cell signaling but also can engage immune cells to secret IFNγ via trastuzumab-mediated ADCC [39–42]. In the present study, we attempted to address two seemingly opposite questions related to regulation of PD-L1 upon trastuzumab treatment, both of which are linked to therapeutic activities of trastuzumab against human HER2-overexpressing cancer cells. First, can trastuzumab-mediated ADCC lead to upregulation of PD-L1 as a result of release of cytokines following trastuzumab treatment (i.e., via the extrinsic pathway)? Second, can trastuzumab-mediated inhibition of cell signaling downstream of HER2 lead to downregulation of PD-L1 as a result of inhibition of the intrinsic pathway regulating PD-L1? To attempt to answer the first question, we analyzed the level of PD-L1 after treatment with trastuzumab or an isotype-matched control antibody (i) in a transgenic mouse model immune-tolerant to human HER2 in which syngeneic mouse tumors transduced to overexpress human HER2 were transplanted and (ii) in human HER2-overexpressing breast cancer cells co-cultured or not with human peripheral blood mononuclear cells (PBMC). To attempt to answer the second question, we investigated the effects of HER2 overexpression and knockdown and HER2 kinase inhibition on the PD-L1 level in HER2-overexpressing breast cancer cells in monoculture. Finally, we analyzed whether there is a correlation between HER2 and PD-L1 at the messenger RNA (mRNA) level in The Cancer Genome Atlas (TCGA) database.
2. Materials and Methods
2.1 Mouse model
The transgenic mouse line in C57BL/6J background developed to be immune-tolerant to human HER2 was previously described [43]. Syngeneic B16-BL6 melanoma cells were transplanted into the mice following retroviral infection of the cells with a human HER2 cDNA construct. The mice were treated intraperitoneally with trastuzumab or bevacizumab (control antibody). Tumor specimens were collected and subjected to multicolor flow cytometry analysis.
2.2 Cell lines and cell culture
HER2-overexpressing breast cancer cell lines (BT474, SKBR3, SUM190, and HCC1954) and HER2-low-expressing MCF7 breast cancer cells, including their pooled HER2-overexpressing transfectants (MCF7-HER2) and clonal HER2-overexpressing MCF7 cells (HER18), were grown in Dulbecco’s modified Eagle’s medium/F12 medium (50/50, v/v) supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin in a 5% CO2 atmosphere at 37°C [44–46].
2.3 Conditioned media from co-culture of breast cancer cells with PBMC
PBMC from healthy donors were isolated from buffy coats of healthy donors using Ficoll-Paque PLUS (GE Healthcare Life Sciences) gradient centrifugation as described in the literature [47]. Cell-free conditioned media were collected after 48-h co-culture of each type of HER2-overexpressing breast cancer cell (BT474, SKBR3, SUM190, and HCC1954) with PBMC at a ratio of 1:5 (cancer cells: PBMC) in the presence of trastuzumab or rituximab (isotype-matched control antibody). The conditioned media were either used fresh or kept at −80°C until next use. For treatment of cells, the conditioned media were added into culture at a 1:1 ratio with fresh medium. An IFNγ-neutralizing antibody (clone NIB42, eBioscience) was used to neutralize IFNγ in the conditioned media from co-culture of breast cancer cells with PBMC.
2.4 Multicolor flow cytometry analysis
Cells cultured in plates or dishes were harvested by incubation with TrypLE reagent (Life Technologies) at 37°C until cells detached as we recently described [14]. Cells from mouse tumor tissues were prepared by mincing tumor tissues into small pieces and passing the samples into a conical tube through a 70-μm mesh cell strainer. Single cell suspensions (0.5–1×106 cells) were prepared in 100 μl of fluorescence-activated cell sorting buffer (0.5% BSA in PBS) and then stained with various fluorescently conjugated primary antibodies (3–5 μl in 100 μl) for 30 min at 4°C. An isotype-matched antibody was used as a control. Excess antibodies were washed away twice with FACS buffer prior to analysis with a BD Biosciences flow cytometer. The flow cytometry data were analyzed using FlowJo 10 software.
For mouse studies, the following antibodies were ordered from BioLegend: PE/Cy7-conjugated H-2Db antibody (clone KH95), PE-conjugated H-2Kb antibody (clone AF6-88.5), PE/Cy5-conjugated CD80 (B7-1) antibody (clone 16-10A1), allophycocyanin (APC)-conjugated PD-L1 (CD274) antibody (clone 10F.9G2), and brilliant violet-conjugated human HER2 antibody (clone 24D2). Fluorescein isothiocyanate (FITC)-conjugated CD86 (B7-2) antibody (clone GL-1) was ordered from TONBO Biosciences. For human cell line studies, the following antibodies were ordered from BioLegend: APC-conjugated HLA-ABC antibody (clone W6/32) and APC- or PE-conjugated PD-L1 antibody (clone 29E.2A3). FITC-conjugated anti-human IgG antibody was ordered from Jackson ImmunoResearch Laboratories.
2.5 Quantification of IFNγ in conditioned media by ELISA
IFNγ produced in conditioned media after co-culture of various breast cancer cells with PBMC in the presence of trastuzumab or control IgG was measured in a 96-well plate using a human IFNγ ELISA kit from BioLegend as we recently described [14]. In brief, properly diluted conditioned media were added onto the wells coated with anti-IFNγ capture antibodies and then incubated for 2 h, followed by additional sequential incubations with an avidin-labeled IFNγ detection antibody and horseradish peroxidase-conjugated biotin. TMB (3,3′,5,5′-tetramethylbenzidine) was used as the substrate of horseradish peroxidase for color development.
2.6 HER2 kinase inhibition and HER2 knockdown by RNA interference
HER2 kinase inhibition was performed by treatment of HER2-overexpressing breast cancer cells with lapatinib (Calbiochem), followed by analysis with Western blotting. HER2 knockdown was performed by transfection using HER2-targeted siRNA (target DNA sequence #1, CATCAAAGTGTTGAGGGAA; #2, GCAGTTACCAGTGCCAATA; and #3, CAGAATGGCTCAGTGACCT) or control siRNA (Sigma-Aldrich). The siRNAs were transfected into cells using a protocol we previously described [48,49]. At 72 h after siRNA transfection, the cells were harvested for analysis.
2.7 Western blot analysis
Western blot analysis was performed as we recently described [50,51]. After transfer of lysate proteins separated by SDS gel electrophoresis onto nitrocellulose membrane, the membrane was subjected to immunoblotting with various primary antibodies of interest as follows: anti-HER2 (Calbiochem) and anti-tyrosine 1248-phosphorylated HER2 antibodies (Cell Signaling Technology); anti-STAT1 and anti-Y701-phosphorylated STAT1 antibodies (Cell Signaling Technology); anti-STAT3 and anti-Y705-phosphorylated STAT3 antibodies (Cell Signaling Technology); anti-Akt and anti-S473-phosphorylated Akt antibodies (Cell Signaling Technology); anti-Erk (Santa Cruz Biotechnology) and anti-T202/Y204-phosphorylated Erk antibodies (Cell Signaling Technology); and anti-β-actin antibody (Sigma-Aldrich). The signals were visualized using the enhanced chemiluminescence detection kit (GE Healthcare).
2.8 TCGA data retrieval and analysis
RNAseq data from the two queried genes, CD274 (PD-L1) and ERBB2 (HER2), were downloaded from the cbioportal (http://www.cbioportal.org/public-portal/). Correlation between CD274 and ERBB2 mRNA expression in various conditions was analyzed with Spearman correlation coefficients calculated using GraphPad Prism7 software.
2.9 Statistical analysis
Each experiment was repeated at least two to three times. Data represent the mean values with standard error of the mean. A two-tailed unpaired Student’s t-test was used to compare two groups of independent samples using GraphPad Prism 7 software. p<0.05 was considered statistically significant.
3. Results
3.1 Trastuzumab upregulates MHC-I, T-cell co-stimulatory molecules, and PD-L1 and downregulates HER2 in immunocompetent mice immune-tolerant to human HER2
Humanized antibodies are known to bind to mouse immune effector cells with binding affinities similar to those of mouse antibodies [52–54]. We first conducted an in vivo study in a transgenic mouse line in C57BL/6J background (hmHER2) that was developed to be immune tolerant to human HER2 [43]. hmHER2 transgenic mice were transplanted subcutaneously with syngeneic B16-BL6 melanoma cells transduced to overexpress human HER2. When tumors were well established, the mice were given a single peritoneal injection of trastuzumab or an isotype-matched control antibody (bevacizumab, an anti-human VEGFA antibody that does not cross-react with mouse VEGFA [55,56]). Forty-eight hours after the injection, the tumors were collected, and single-tumor-cell suspensions were prepared and stained with various antibodies for multicolor flow cytometry analysis (Fig. 1). The level of HER2 detected by a fluorescence-labeled anti-HER2 antibody was significantly lower in the trastuzumab-treated B16-BL6/HER2 tumor cells than in the control antibody-treated tumor cells (measured as both medium fluorescence intensity [MFI]) and the percentage of HER2-positive cells) (Fig. 1A). Anti-human IgG antibody detected significant more binding of trastuzumab than of the control antibody to B16-BL6/HER2 cells (Fig. 1B). Similar to our recently reported finding from a nude mouse study of 4T1/HER2 tumor [14], the expression of MHC-I (H-2Db) (Fig. 1C) and CD80 and CD86 (Fig. 1D) in B16-BL6/HER2 tumor cells was significantly higher after trastuzumab treatment than after control antibody treatment; expression of MHC-I (H-2Kb) was higher but not significantly higher. Moreover, the expression of PD-L1 in B16-BL6/HER2 tumor cells was also significantly higher after trastuzumab treatment than after control antibody treatment (Fig. 1E). This experiment provides important in vivo evidence indicating a potential double-edged-sword role of trastuzumab in regulating adaptive immune responses—i.e., trastuzumab not only upregulates the expression of MHC-I and CD80 and CD86 T-cell co-stimulatory molecules but also upregulates the expression of PD-L1 in HER2-overexpressing cancer cells in an immune-competent host.
Fig. 1.
Upregulation of MHC-I, T-cell co-stimulatory molecules, and PD-L1 and downregulation of HER2 by trastuzumab in HER2-overexpressing tumors in vivo. Syngeneic B16-BL6 melanoma cells transduced to overexpress human HER2 were transplanted in hmHER2 transgenic mice. When the tumors became palpable, the mice were treated with 100 μg/mouse of trastuzumab (n=10) or control antibody bevacizumab (n=9) via intraperitoneal injection. The tumors were harvested 48 h after the treatment, and single tumor cell suspensions were prepared and subjected to multicolor flow cytometry analysis after staining or not with fluorescence-labeled anti-human HER2 antibody (A), anti-human IgG antibody (B), anti-mouse H-2Kb or H-2Db antibody (C), anti-mouse CD80 or CD86 antibody (D), or anti-mouse PD-L1 antibody (E). Analyses of the MFI values in (C), (D), and (E) were gated for HER2-positive cells only.
Trastuzumab does not upregulate or downregulate PD-L1 in monocultures of HER2-overexpressing breast cancer cells but does upregulate PD-L1 in HER2-overexpressing breast cancer cells co-cultured with human PBMC
We recently reported that trastuzumab upregulated HLA-ABC expression in HER2-overexpressing cells co-cultured with PBMC via stimulation of IFNγ secretion by engaging Fc receptor-expressing immune cells, mainly natural killer (NK) cells, in PBMC [14]. Because IFNγ is a potent transcriptional regulator of PD-L1 [57], we sought to determine if trastuzumab treatment of HER2-overexpressing breast cancer cells co-cultured with human PBMC would result in PD-L1 upregulation via trastuzumab-mediated engagement of immune cells, a mechanism similar to that we recently reported [14]. As shown in Fig. 2A, treatment of HER2-overexpressing breast cancer cells with conditioned media from trastuzumab-treated monocultures of the same types of breast cancer cells produced no detectable change in PD-L1 expression. However, treatment of cells with conditioned media from control antibody-treated or trastuzumab-treated co-cultures of breast cancer cells with PBMC significantly increased PD-L1 expression, and the increase in PD-L1 expression was greater in the trastuzumab-treated co-cultures. The increases in PD-L1 level following treatment with conditioned media correlated with the basal level of PD-L1 in the cell lines: basal PD-L1 MFI values and PD-L1 MFI values after treatment with conditioned media from control antibody-treated and trastuzumab-treated co-cultures, respectively, were 647, 840, and 1,350 in BT474 cells; 677, 1,796, and 2,368 in SKBR3 cells; 1,417, 5,068, and 6,104 in SUM190 cells; and 10,340, 77,988, and 101,662 in HCC1954 cells. The PD-L1 upregulation by the conditioned media from the cells treated with control antibody, which was not capable of engaging immune cells, was likely due to HLA mismatch between the PBMC donors and the patients from whom the breast cancer cells were originally derived, leading to release of cytokines into the conditioned media in the co-culture, with the level of cytokine release depending on the extent of HLA isotype mismatch between the PBMC donors and the patients. Such cytokine release would not occur in patients because NK cells and breast cancer cells in the same individual are autologous.
Fig. 2.
Effect of conditioned media from breast cancer cells in monoculture or co-culture with PBMC and treated with trastuzumab or control antibody on PD-L1 level in HER2-overexpressing breast cancer cells. A, The indicated HER2-overexpressing breast cancer cells were treated with trastuzumab (5 μg/ml, equivalent to ~30 nM) or a control IgG (rituximab) in monoculture or co-culture with PBMC (at a cancer cell: PBMC ratio of 1:5) for 48 h. After treatment, cell-free conditioned media from mono-cultures and co-cultures were collected and added into new cultures of the breast cancer cells by mixing the conditioned medium with fresh medium at a 1:1 ratio. After culture for another 48 h, the cells were harvested and stained with APC-conjugated anti-PD-L1 antibody and then subjected to flow cytometry analysis. The MFI values of PD-L1 are shown in bar graphs. The data represent results from two different experiments and donors. * p<0.05. NS, not significant. B, The conditioned media from co-culture with PBMC in (A) were measured for the level of IFNγ using a human IFNγ ELISA kit. *p<0.05 compared with corresponding control.
To confirm the production of IFNγ as a result of engagement of immune cells in PBMC by trastuzumab, we measured the amount of IFNγ released into the conditioned media after co-culture of the established breast cancer cells with donor PBMC in the presence of a control antibody and trastuzumab. As shown in Fig. 2B, the level of IFNγ was significantly higher in the conditioned media from trastuzumab-treated co-cultures than in the conditioned media from control antibody-treated co-cultures. The level of IFNγ production in conditioned media from trastuzumab-treated co-cultures was similar in BT474, SUM190, and HCC1954 cells and significantly higher in SKBR3 cells, likely because of a high degree of HLA isotype mismatch between the PBMC donor and the patient from whom SKBR3 cells were originally derived.
To further confirm the role of IFNγ in the upregulation of PD-L1 by the conditioned media, we added an IFNγ-neutralizing antibody in the conditioned media. We found that addition of this antibody substantially mitigated or abolished the PD-L1 upregulation by the conditioned media from trastuzumab-treated co-cultures (Fig. 3).
Fig. 3.
Role of IFNγ in upregulation of PD-L1 by conditioned medium from co-culture of PBMC and HER2-overexpressing breast cancer cells treated with trastuzumab. The indicated HER2-overexpressing breast cancer cells were treated for 48 h with conditioned medium (mixed with fresh medium at a 1:1 ratio) from mono-culture or co-culture as described in Fig. 2 or conditioned medium from co-culture plus an IFNγ-neutralizing antibody (10 μg/ml). The cells were harvested and stained with APC-conjugated anti-PD-L1 antibody and then subjected to flow cytometry analysis. The MFI values of PD-L1 are shown in bar graphs. *p<0.05.
Together, these data supported a role of trastuzumab in upregulating PD-L1 in responsive breast cancer cells through engaging immune cells in PBMC and stimulating IFNγ production.
3.3 Differences in recombinant IFNγ–induced upregulation of PD-L1 and HLA-ABC in HER2-overexpressing breast cancer cells with low and high basal levels of PD-L1
As shown in Fig. 2B, the level of IFNγ upregulation after treatment with conditioned medium from trastuzumab-treated co-culture of BT474 cells with PBMC was similar to the level of IFNγ upregulation after treatment with conditioned medium from trastuzumab-treated co-culture of SUM190 or HCC1954 cells with PBMC; however, as shown in Fig. 2A, the level of PD-L1 upregulation (compared with the basal PD-L1 level in monoculture) after treatment with conditioned medium from trastuzumab-treated co-culture of BT474 cells with PBMC was significantly lower than the level of PD-L1 upregulation after treatment with conditioned medium from trastuzumab-treated co-culture of SUM190 or HCC1954 cells with PBMC. In the case of SKBR3 cells, the conditioned medium from trastuzumab-treated co-culture of SKBR3 cells with PBMC significantly increased the amount of IFNγ (Fig. 2B) but only modestly increased the PD-L1 level. Therefore, the observed difference in conditioned medium-induced upregulation of PD-L1 level, from moderate upregulation in BT474 cells to strong upregulation in HCC1954 cells, was most likely not due to insufficient IFNγ secreted by PBMC in the conditioned medium.
Multiple cytokines can be produced in the conditioned medium after co-culture of breast cancer cells with PBMC. Therefore, to focus our study on the impact of signaling generated only by IFNγ, we further investigated the different responses after treatment of the breast cancer cells with recombinant IFNγ, which was added at an equal amount in culture medium alone and in combination with trastuzumab (Fig. 4). Similar to our results obtained with the use of conditioned medium, exposure to recombinant IFNγ at an equal amount upregulated PD-L1 level substantially in SUM190 and HCC1954 cells, moderately in SKBR3 cells, and barely at all in BT474 cells. By contrast, exposure to the same amount of recombinant IFNγ led to a marked increase in the level of HLA-ABC in all four cell lines examined. The cells’ responsiveness to IFNγ-induced upregulation of HLA-ABC indicated that the minimal upregulation of PD-L1 following exposure to IFNγ in BT474 and SKBR3 cells was not due to a lack of or a poor response of the cells to IFNγ.
Fig. 4.
Differential effects of IFNγ on PD-L1 and HLA-ABC expression in HER2-overexpressing breast cancer cells. The indicated HER2-overexpressing breast cancer cells were treated for 48 h with recombinant IFNγ (500 units/ml), trastuzumab (5 μg/ml, equivalent to ~30 nM), or both for 48 h. Cells were then double-stained with PE-conjugated anti-PD-L1 antibody and APC-conjugated anti-HLA-ABC antibody and then subjected to multicolor flow cytometry analysis. Left, Contour plots of flow cytometry data of PD-L1 and HLA-ABC expression in the indicated HER2-overexpressing breast cancer cells. Right, MFI values of PD-L1 and HLA-ABC expression from flow cytometry analysis.* p<0.05 compared with corresponding untreated control.
3.4 Effects of HER2 knockdown or overexpression and HER2 kinase inhibition on the level of PD-L1 protein in breast cancer cells
Our data in Fig. 1 as well as an earlier study in the literature [58] showed that the level of HER2 can be downregulated in vivo after trastuzumab treatment. HER2 activates downstream cell signaling pathways, such as the PI3K/mTOR/Akt pathway, that not only promote cell proliferation and survival but also help cancer cells escape from T-cell recognition and attack through mechanisms such as upregulation of PD-L1 [31–35]. To determine if there is a link between the levels of PD-L1 and HER2 in HER2-overexpressing breast cancer, we examined if knockdown of HER2 by siRNA in HER2-overexpressing breast cancer cells or overexpression of HER2 in HER2-low-expressing breast cancer cells had any direct effect on PD-L1 expression.
For the experiments involving HER2 knockdown, we used three different HER2-specific siRNAs, each of which successfully knocked down HER2 in the four breast cancer cell lines used in Fig. 5. We found variable impacts of HER2 knockdown on the level of PD-L1 (Fig. 5A). In breast cancer cells with a low basal level of PD-L1 (BT474 and SKBR3), the level of PD-L1 was significantly lower after HER2 knockdown than after control siRNA treatment. In SUM190 cells, which have a medium basal level of PD-L1, the level of PD-L1 was also significantly lower after HER2 knockdown than after control siRNA treatment. However, in HCC1954 cells, which have a very high basal level of PD-L1 (at least 10 times the level in BT474 and SKBR3 cells), the level of PD-L1 was only slightly lower after HER2 knockdown than after control siRNA treatment.
Fig. 5.
Effects of HER2 knockdown and overexpression on PD-L1 expression in respective HER2-overexpressing and low expressing breast cancer cells. A, The indicated HER2-overexpressing breast cancer cells were transfected with one of three different HER2-specific siRNAs or control siRNA. At 72 h after siRNA transfection, the cells were double-stained with APC-conjugated anti-PD-L1 antibody and trastuzumab plus FITC-conjugated anti-human IgG antibody and then subjected to multicolor flow cytometry analysis. Left, Contour plots of flow cytometry data on PD-L1 and HER2 expression in the indicated HER2-overexpressing breast cancer cells with and without HER2 knockdown. Right, MFI values of PD-L1 and HER2 expression from flow cytometry analysis. * p<0.05 compared with corresponding control siRNA-treated cells. B, Left, Contour plots of flow cytometry data of PD-L1 and HER2 expression in MCF7, MCF7-HER2, and HER18 cells. The cells were double-stained with antibodies and subjected to flow cytometry analysis as described in (A). Right, MFI values of PD-L1 and HER2 expression from flow cytometry analysis. * p<0.05 compared with parental MCF7 cells.
By contrast, overexpression of HER2 in MCF7 cells, which have a low basal level of PD-L1, led to a modest but clear increase in the level of PD-L1 (Fig. 5B). Of note, MCF7 cells bear a gain-of-function mutation at exon 9 (E545K) of the PIK3CA gene [59].
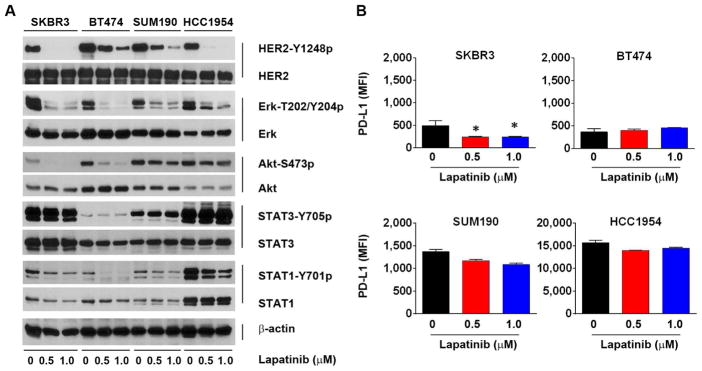
We next examined whether inhibition of HER2 kinase activity by lapatinib, a HER2 kinase inhibitor approved by the FDA for treating breast cancer patients, had any effect on PD-L1 level in the same panel of four breast cancer cell lines. As shown in Fig. 6A, HER2 kinase activity (represented by the level of HER2 activation-specific phosphorylation on Y1248) was strongly inhibited by lapatinib at 0.5 or 1 μM for 24 h in all four cell lines. The treatment led to a significant decrease in the level of PD-L1 in SKBR3 cells, but only a modest decrease in the level of PD-L1 in SUM190 and HCC1954 cells (Fig. 6B). In BT474 cells, there was no noticeable change in PD-L1 level after the treatment, most likely because the very low basal level of PD-L1 in those cells limited detection of a decrease in PD-L1 level after lapatinib treatment in this particular experiment.
Fig. 6.
Effect of HER2 kinase inhibition on PD-L1 expression in HER2-overexpressing breast cancer cells. A, The indicated HER2-overexpressing breast cancer cells were treated with lapatinib (0.5 or 1.0 μM) or vehicle control (DMSO) for 24 h. After treatment, the cells were lysed and subjected to Western blot analysis with the indicated antibodies. B, Cells treated as described in (A) were stained with APC-conjugated anti-PD-L1 antibody and then subjected to flow cytometry analysis. The MFI values of PD-L1 are shown in bar graphs. * p<0.05 compared with vehicle control-treated cells.
Further analysis of the cell signaling results in Fig. 6A shows that HER2 kinase activity was inhibited by lapatinib more prominently in SKBR3 and HCC1954 cells than in BT474 and SUM190 cells; however, the cell lines did not differ with respect to inhibition of several well-known substrates downstream of HER2 after lapatinib treatment. For example, activation-specific phosphorylation (on T202 and Y204) of Erk was potently inhibited by lapatinib in all four cell lines. In contrast, activation-specific phosphorylation (on S473) of Akt was inhibited in SKBR3 and BT474 cells but not in SUM190 and HCC1954 cells, likely because of a known PIK3CA mutation that leads to constitutive activation of PI3K in these cells [59–62]. A notable observation was a higher basal level of Y701 phosphorylation of STAT1 in HCC1954 cells than in the other three cell lines, which may explain the high basal level of PD-L1 in HCC1954 cells given that PD-L1 is known to be regulated by STAT1 at the transcription level [57]. Whereas lapatinib treatment decreased STAT1 phosphorylation in all four cell lines, lapatinib treatment barely affected STAT3 phosphorylation in the four cell lines.
Taken together, these results suggested that the impact of HER2 overexpression on PD-L1 expression in HER2-overexpressing breast cancer cells is cell-context-specific—i.e., while inhibition of HER2 expression or kinase activity can downregulate the level of PD-L1 expression, this relationship is modest and variable and is likely affected by other mechanisms, such as activation status of some key signaling components in the signaling pathway downstream of HER2.
3.5 Analysis of TCGA database for potential correlation between HER2 and PD-L1 expression
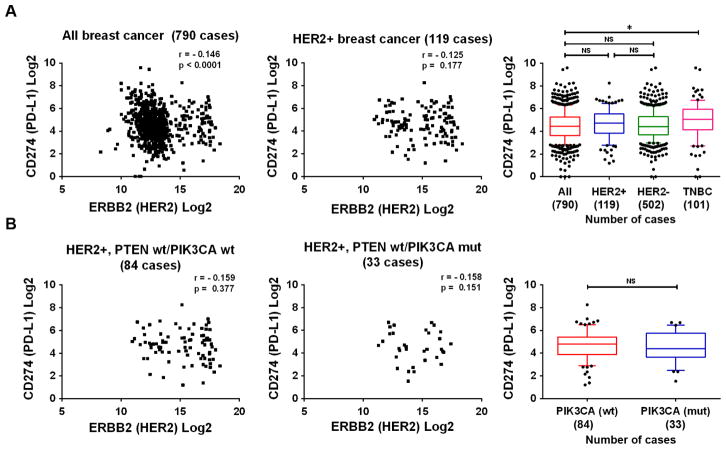
Finally, we analyzed RNAseq data from breast cancer samples available in the TCGA database to determine if the mRNA level of HER2 expression correlates with the mRNA level of PD-L1 expression in breast cancer. We found no significant correlation between the mRNA expression levels of HER2 and PD-L1 in 790 available cases of breast cancer (Fig. 7A, left panel) or in a subgroup of 119 cases of HER2-positive breast cancer (score >2+ on immunohistochemical staining) (Fig. 7A, middle panel). The mean PD-L1 mRNA values (in log2) for all 790 breast cancers and for the HER2-positive and HER2-negative subgroups were 4.51, 4.62, and 4.54, respectively (Fig. 7A, right panel). In contrast, the mean PD-L1 mRNA value for TNBC was 4.97, significantly higher than the mean PD-L1 mRNA value for all breast cancers, consistent with the finding in a recent study that the level of PD-L1 was higher in TNBC than in non-TNBC tumors [37]. In that recent study, the high expression of PD-L1 in TNBC was associated with PIK3CA mutation. Thus, we next examined the correlation between mRNA levels of HER2 and PD-L1 in HER2-positive breast cancer samples stratified by PIK3CA and PTEN mutation status. We found only one case with PTEN deletion, and this case was removed from analysis because it would not have been meaningful to compare results between patients with and without PTEN mutation. Interestingly, we found no significant correlation between the mRNA levels of HER2 and PD-L1 (Fig. 7B).
Fig. 7.
Correlation analysis of CD274 (PD-L1) and ERBB2 (HER2) expression in breast cancer patient samples available in the TCGA database. A, Left and middle, Spearman correlation analyses of the RNAseq data from TCGA queried for CD274 and ERBB2 in all breast cancer specimens (left panel) and in HER2-overexpressing (HER2 immunohistochemistry score >2+) breast cancer specimens (middle panel). Right, Box-and-whisker plots (ends of whiskers at 10th and 90th percentiles) of CD274 RNAseq data in indicated subgroups. The subgroups were compared using Student’s t-test. *p<0.05, NS, not significant. B, Left and middle, Spearman correlation analyses of the RNAseq data from TCGA queried for CD274 and ERBB2 in HER2-overexpressing (HER2 immunohistochemistry score >2+) breast cancer specimens with wild-type PTEN and wild-type PIK3CA (left panel) and with wild-type PTEN and mutated PIK3CA (middle panel). Right, Box-and-whisker plots (ends of whiskers at 10th and 90th percentiles) of CD274 RNAseq data in indicated subgroups. The subgroups were compared using Student’s t-test. NS, not significant.
4. Discussion
Our findings in this study suggest that trastuzumab may upregulate PD-L1 level in targeted HER2-overexpressing cancer cells through activating the extrinsic pathway—i.e., by engaging immune effector cells to release IFNγ. We observed that inhibition of HER2 intrinsic signaling by means of HER2 knockdown or kinase inhibition downregulated PD-L1 expression in a cell-context-specific manner. We also observed differential responses to IFNγ-induced regulation of PD-L1 and MHC-I and T-cell co-stimulatory molecules in the panel of HER2-overexpressing breast cancer cells.
Recent advances in understanding of the biology of PD-L1 have substantially enhanced our knowledge of the function of PD-L1 and how it is regulated. PD-L1 is known to be regulated through the extrinsic pathway via the JAK/STAT pathway by the cytokines released by NK cells and other immune cells during an immune response in the tumor microenvironment. IFNγ is a key cytokine released during trastuzumab-mediated engagement of immune cells [39–42]. PD-L1 is also known to be regulated through the intrinsic pathway in cancer cells via canonical growth factor receptor- or oncogene-mediated signaling pathways. IFNγ has been shown to downregulate HER2 level through inhibiting HER2 transcription and thereby inhibit HER2-regulated cell signaling [58]. We thus propose that the extrinsic and intrinsic pathways may interact at the level of HER2 in regulating PD-L1 expression in HER2-overexpressing breast cancer cells after trastuzumab treatment in the presence of immune effector cells. It is noteworthy, however, that the intrinsic signaling pathway is often dysregulated owing to concurrent presence of oncogenic mutations of several key components in the pathways downstream of HER2 [16]. In the absence of such mutations, HER2 downregulation by IFNγ could inhibit PD-L1 expression via inhibiting the intrinsic pathway, thereby counterbalancing an increase in PD-L1 expression induced by IFNγ via stimulation of the JAK/STAT extrinsic pathway. On the other hand, in the breast cancer cells with constitutive activation of key components in the pathways downstream of HER2, HER2 downregulation by IFNγ may not lead to PD-L1 downregulation. Of the cells used in the current study, HCC1954 and SUM190 cells both contain a gain-of-function mutation at exon 20 (H1047R) of the PIK3CA gene; SKBR3 cells have wild-type PIK3CA; and BT474 cells harbor a rare mutation at exon 2 (K111N) of PIK3CA that lacks transforming ability [59–62]. This complex landscape in terms of cancer genetic background may explain some of the cell-context-specific responses to treatment with trastuzumab and/or IFNγ.
Another interesting finding in our study was the difference in upregulation of HLA-ABC and PD-L1 in our cell-line panel in response to both the IFNγ released following engagement of immune cells by trastuzumab and the recombinant IFNγ added in culture. This interesting finding warrants further study. Release of IFNγ in the tumor microenvironment following trastuzumab-mediated engagement of immune cells can increase expression of MHC-I and T-cell co-stimulatory molecules in cancer cells, which can enhance T-cell-mediated immune response; the release of IFNγ can also increase expression of PD-L1 in cancer cells, which can dampen the immune response. Although both MHC-I and PD-L1 are regulated by IFNγ, the response of cell signaling pathways, from activation of IFNγ receptor-associated JAK1 or JAK2 to transcriptional activation of MHC-I components and PD-L1, may be different in different cell lines, and such differences may lead to differential responses of MHC-I and PD-L1 to IFNγ stimulation.
Our current knowledge of the requirement for an adaptive immune response in HER2 antibody-mediated antitumor activity comes mainly from studies in preclinical models using 7.16.4 antibody, which targets HER2/neu [7,8]. In particular, an early preclinical study reported that the combination of an anti-PD-1 antibody and 7.16.4 anti-HER2/neu antibody produced a significantly greater response than 7.16.4 anti-HER2/neu antibody alone in immune-competent mice transplanted with HER2/neu-overexpressing mouse mammary tumors [9]. Our current findings justify further work to determine whether combination of trastuzumab with anti-PD-1 or anti-PD-L1 will achieve therapeutic benefits in the human HER2-tolerant transgenic mouse model. Clinical trials are already under way to test the therapeutic strategy of combining trastuzumab with an anti-PD-1 or anti-PD-L1 antibody in HER2-overexpressing breast cancer (www.ClinicalTrials.gov).
Recent pilot clinical studies with several other types of cancer showed that patients with tumors expressing a high basal level of PD-L1 generally responded better to anti-PD-L1 antibody than did patients with tumors expressing a low level of PD-L1 [63]. In our current study, we found that HER2-overexpressing breast cancer cells with a low basal level of PD-L1 responded poorly to IFNγ-induced upregulation of PD-L1 but remained responsive to IFNγ-induced upregulation of HLA-ABC. This observation raises the interesting question whether or not it will be productive to combine trastuzumab with an anti-PD-1 or anti-PD-L1 therapy to improve the efficacy of trastuzumab treatment in patients with HER2-overexpressing tumors with a low basal level of PD-L1 expression.
The results from our analysis of the TCGA data should be interpreted with caution. Our analysis showed no significant correlation between PD-L1 and HER2 levels in HER2-overexpressing breast cancer with either wild-type or mutated PIK3CA. These findings seem in notable contrast to the findings from other reports on head and neck cancer with EGFR overexpression [64] and on non–small cell lung cancer with activating mutations of EGFR [31], in which PD-L1 level was upregulated owing to high-level activation of several signal transduction pathways downstream of EGFR. An important caveat is that our conclusion is based on analysis of mRNA levels, which may not accurately reflect protein levels. Unfortunately, although HER2 immunohistochemical staining results are available in the TCGA database, PD-L1 staining results are not. In addition, the frequent coexistence of various genetic aberrations in these samples creates another layer of complexity in the data analysis. From our analysis with a limited number of cell lines, we found that HCC1954, a cell line carrying a H1047R mutation of the PIK3CA gene, exhibited a very high basal level of PD-L1, much higher than the basal levels in SKBR3 and BT474 cells, which have wild-type PI3KCA (SKBR3) or a non-transforming mutation of PI3KCA (BT474). SUM190, which bears the same PIK3CA gene mutation, also exhibits a higher basal level of PD-L1 than the basal levels in SKBR3 and BT474 cells.
In summary, given that adaptive immune responses are involved in cancer cell response to anti-HER2 antibodies and that PD-L1 is a negative regulator of T-cell immune response, upregulation of PD-L1 following trastuzumab treatment may function as a mechanism of resistance to trastuzumab treatment. However, we must interpret our findings with caution. This is because upregulation of PD-L1 by trastuzumab in the presence of immune cells appears to be limited to HER2-overexpressing breast cancer cells with a high basal level of PD-L1 and successful inhibition of cell signaling downstream of HER2 may also downregulate PD-L1 depending on the genetic background of the cancer cells. Our findings justify further investigation of the value of adding anti-PD-1 or anti-PD-L1 therapy to trastuzumab-based treatment, especially in patients whose tumors have low basal PD-L1 expression.
Highlights.
Trastuzumab upregulates PD-L1 in a syngeneic tumor model expressing human HER2
Trastuzumab engages immune cells and upregulates PD-L1 via the extrinsic pathway
Inhibiting HER2 signaling downregulates PD-L1 in a cell-context-specific manner
IFNγ differentially upregulates PD-L1 and MHC-I in HER2+ breast cancer cells
Acknowledgments
This work was supported in part by the Breast Cancer Research Foundation (New York, USA) and by US National Institutes of Health (NIH) through R01 awards (CA129036 and CA179015). The work was also supported in part by the NIH through MD Anderson’s Cancer Center Support Grant, CA016672. We thank the staff of the Flow Cytometry and Cellular Imaging Core Facility for technical assistance and thank Stephanie Deming of the Department of Scientific Publications at MD Anderson Cancer Center for editorial assistance.
Abbreviations
- ADCC
antibody-dependent cell-mediated cytotoxicity
- APC
allophycocyanin
- ELISA
enzyme-linked immunosorbent assay
- FDA
US Food and Drug Administration
- FITC
fluorescein isothiocyanate
- HER2
human epidermal growth factor receptor 2
- PD-1
programmed death-1
- PD-L1
programmed death-ligand 1
- MHC
major histocompatibility complex
- HLA-ABC
HLA-A, HLA-B, and HLA-C
- IFNγ
interferon gamma
- MFI
medium fluorescence intensity
- mRNA
messenger RNA
- NK
natural killer
- PBMC
peripheral blood mononuclear cells
- TCGA
The Cancer Genome Atlas
- TNBC
triple-negative breast cancer
Footnotes
Conflict of Interest Statement
The authors declare no conflict of interest related to the contents of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hortobagyi GN. Trastuzumab in the treatment of breast cancer. N Engl J Med. 2005;353:1734–1736. doi: 10.1056/NEJMe058196. [DOI] [PubMed] [Google Scholar]
- 2.Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 3.Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A, Heeson S, Clark E, Ross G, Benyunes MC, Cortes J. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fabi A, Malaguti P, Vari S, Cognetti F. First-line therapy in HER2 positive metastatic breast cancer: is the mosaic fully completed or are we missing additional pieces? J Exp Clin Cancer Res. 2016;35:104. doi: 10.1186/s13046-016-0380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muntasell A, Cabo M, Servitja S, Tusquets I, Martinez-Garcia M, Rovira A, Rojo F, Albanell J, Lopez-Botet M. Interplay between natural killer cells and anti-HER2 antibodies: perspectives for breast cancer immunotherapy. Front Immunol. 2017;8:1544. doi: 10.3389/fimmu.2017.01544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang K, Esteva FJ, Albarracin C, Stemke-Hale K, Lu Y, Bianchini G, Yang CY, Li Y, Li X, Chen CT, Mills GB, Hortobagyi GN, Mendelsohn J, Hung MC, Fan Z. Recombinant human erythropoietin antagonizes trastuzumab treatment of breast cancer cells via Jak2-mediated Src activation and PTEN inactivation. Cancer Cell. 2010;18:423–435. doi: 10.1016/j.ccr.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park S, Jiang Z, Mortenson ED, Deng L, Radkevich-Brown O, Yang X, Sattar H, Wang Y, Brown NK, Greene M, Liu Y, Tang J, Wang S, Fu YX. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell. 2010;18:160–170. doi: 10.1016/j.ccr.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mortenson ED, Park S, Jiang Z, Wang S, Fu YX. Effective anti-neu-initiated antitumor responses require the complex role of CD4+ T cells. Clin Cancer Res. 2013;19:1476–1486. doi: 10.1158/1078-0432.CCR-12-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stagg J, Loi S, Divisekera U, Ngiow SF, Duret H, Yagita H, Teng MW, Smyth MJ. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc Natl Acad Sci U S A. 2011;108:7142–7147. doi: 10.1073/pnas.1016569108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loi S. Tumor-infiltrating lymphocytes, breast cancer subtypes and therapeutic efficacy. Oncoimmunology. 2013;2:e24720. doi: 10.4161/onci.24720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savas P, Loi S. Investigating the positive relationship between tumor-infiltrating lymphocytes and trastuzumab therapy. Immunotherapy. 2014;6:803–805. doi: 10.2217/imt.14.60. [DOI] [PubMed] [Google Scholar]
- 12.Beano A, Signorino E, Evangelista A, Brusa D, Mistrangelo M, Polimeni MA, Spadi R, Donadio M, Ciuffreda L, Matera L. Correlation between NK function and response to trastuzumab in metastatic breast cancer patients. J Transl Med. 2008;6:25. doi: 10.1186/1479-5876-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ladoire S, Arnould L, Mignot G, Apetoh L, Rebe C, Martin F, Fumoleau P, Coudert B, Ghiringhelli F. T-bet expression in intratumoral lymphoid structures after neoadjuvant trastuzumab plus docetaxel for HER2-overexpressing breast carcinoma predicts survival. Br J Cancer. 2011;105:366–371. doi: 10.1038/bjc.2011.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaganty BK, Lu Y, Qiu S, Somanchi SS, Lee DA, Fan Z. Trastuzumab upregulates expression of HLA-ABC and T cell costimulatory molecules through engagement of natural killer cells and stimulation of IFNgamma secretion. Oncoimmunology. 2016;5:e1100790. doi: 10.1080/2162402X.2015.1100790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chinai JM, Janakiram M, Chen F, Chen W, Kaplan M, Zang X. New immunotherapies targeting the PD-1 pathway. Trends Pharmacol Sci. 2015;36:587–595. doi: 10.1016/j.tips.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 18.Ceeraz S, Nowak EC, Noelle RJ. B7 family checkpoint regulators in immune regulation and disease. Trends Immunol. 2013;34:556–563. doi: 10.1016/j.it.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 20.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 21.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Selenko-Gebauer N, Majdic O, Szekeres A, Hofler G, Guthann E, Korthauer U, Zlabinger G, Steinberger P, Pickl WF, Stockinger H, Knapp W, Stockl J. B7-H1 (programmed death-1 ligand) on dendritic cells is involved in the induction and maintenance of T cell anergy. J Immunol. 2003;170:3637–3644. doi: 10.4049/jimmunol.170.7.3637. [DOI] [PubMed] [Google Scholar]
- 23.Feng M, Xiong G, Cao Z, Yang G, Zheng S, Song X, You L, Zheng L, Zhang T, Zhao Y. PD-1/PD-L1 and immunotherapy for pancreatic cancer. Cancer Lett. 2017;407:57–65. doi: 10.1016/j.canlet.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Meng X, Liu Y, Zhang J, Teng F, Xing L, Yu J. PD-1/PD-L1 checkpoint blockades in non-small cell lung cancer: New development and challenges. Cancer Lett. 2017;405:29–37. doi: 10.1016/j.canlet.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 25.Shi X, Zhang X, Li J, Zhao H, Mo L, Shi X, Hu Z, Gao J, Tan W. PD-1/PD-L1 blockade enhances the efficacy of SA-GM-CSF surface-modified tumor vaccine in prostate cancer. Cancer Lett. 2017;406:27–35. doi: 10.1016/j.canlet.2017.07.029. [DOI] [PubMed] [Google Scholar]
- 26.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pardoll DM. Immunology beats cancer: a blueprint for successful translation. Nat Immunol. 2012;13:1129–1132. doi: 10.1038/ni.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, Azuma M, Yagita H. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 29.Lee SJ, Jang BC, Lee SW, Yang YI, Suh SI, Park YM, Oh S, Shin JG, Yao S, Chen L, Choi IH. Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-gamma-induced upregulation of B7-H1 (CD274) FEBS Lett. 2006;580:755–762. doi: 10.1016/j.febslet.2005.12.093. [DOI] [PubMed] [Google Scholar]
- 30.Ritprajak P, Azuma M. Intrinsic and extrinsic control of expression of the immunoregulatory molecule PD-L1 in epithelial cells and squamous cell carcinoma. Oral Oncol. 2015;51:221–228. doi: 10.1016/j.oraloncology.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 31.Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, Mikse OR, Cherniack AD, Beauchamp EM, Pugh TJ, Wilkerson MD, Fecci PE, Butaney M, Reibel JB, Soucheray M, Cohoon TJ, Janne PA, Meyerson M, Hayes DN, Shapiro GI, Shimamura T, Sholl LM, Rodig SJ, Freeman GJ, Hammerman PS, Dranoff G, Wong KK. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3:1355–1363. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atefi M, Avramis E, Lassen A, Wong DJ, Robert L, Foulad D, Cerniglia M, Titz B, Chodon T, Graeber TG, Comin-Anduix B, Ribas A. Effects of MAPK and PI3K pathways on PD-L1 expression in melanoma. Clin Cancer Res. 2014;20:3446–3457. doi: 10.1158/1078-0432.CCR-13-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loi S, Dushyanthen S, Beavis PA, Salgado R, Denkert C, Savas P, Combs S, Rimm DL, Giltnane JM, Estrada MV, Sanchez V, Sanders ME, Cook RS, Pilkinton MA, Mallal SA, Wang K, Miller VA, Stephens PJ, Yelensky R, Doimi FD, Gomez H, Ryzhov SV, Darcy PK, Arteaga CL, Balko JM. RAS/MAPK Activation Is Associated with Reduced Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer: Therapeutic Cooperation Between MEK and PD-1/PD-L1 Immune Checkpoint Inhibitors. Clin Cancer Res. 2016;22:1499–1509. doi: 10.1158/1078-0432.CCR-15-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lastwika KJ, Wilson W, III, Li QK, Norris J, Xu H, Ghazarian SR, Kitagawa H, Kawabata S, Taube JM, Yao S, Liu LN, Gills JJ, Dennis PA. Control of PD-L1 expression by oncogenic activation of the AKT-mTOR pathway in non-small cell lung cancer. Cancer Res. 2016;76:227–238. doi: 10.1158/0008-5472.CAN-14-3362. [DOI] [PubMed] [Google Scholar]
- 35.Crane CA, Panner A, Murray JC, Wilson SP, Xu H, Chen L, Simko JP, Waldman FM, Pieper RO, Parsa AT. PI(3) kinase is associated with a mechanism of immunoresistance in breast and prostate cancer. Oncogene. 2009;28:306–312. doi: 10.1038/onc.2008.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, Mischel PS, Stokoe D, Pieper RO. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 37.Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, Su X, Wang Y, Gonzalez-Angulo AM, Akcakanat A, Chawla A, Curran M, Hwu P, Sharma P, Litton JK, Molldrem JJ, Alatrash G. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2:361–370. doi: 10.1158/2326-6066.CIR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 39.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 40.Arnould L, Gelly M, Penault-Llorca F, Benoit L, Bonnetain F, Migeon C, Cabaret V, Fermeaux V, Bertheau P, Garnier J, Jeannin JF, Coudert B. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br J Cancer. 2006;94:259–267. doi: 10.1038/sj.bjc.6602930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barok M, Isola J, Palyi-Krekk Z, Nagy P, Juhasz I, Vereb G, Kauraniemi P, Kapanen A, Tanner M, Vereb G, Szollosi J. Trastuzumab causes antibody-dependent cellular cytotoxicity-mediated growth inhibition of submacroscopic JIMT-1 breast cancer xenografts despite intrinsic drug resistance. Mol Cancer Ther. 2007;6:2065–2072. doi: 10.1158/1535-7163.MCT-06-0766. [DOI] [PubMed] [Google Scholar]
- 42.Wang W, Erbe AK, Hank JA, Morris ZS, Sondel PM. NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front Immunol. 2015;6:368. doi: 10.3389/fimmu.2015.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang S, Astsaturov IA, Bingham CA, McCarthy KM, von MM, Xu W, Alpaugh RK, Tang Y, Littlefield BA, Hawkins LD, Ishizaka ST, Weiner LM. Effective antibody therapy induces host-protective antitumor immunity that is augmented by TLR4 agonist treatment. Cancer Immunol Immunother. 2012;61:49–61. doi: 10.1007/s00262-011-1090-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu J, Lu Y, Qiu S, Chen ZN, Fan Z. A novel role of EMMPRIN/CD147 in transformation of quiescent fibroblasts to cancer-associated fibroblasts by breast cancer cells. Cancer Lett. 2013;335:380–386. doi: 10.1016/j.canlet.2013.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang H, Sun W. CRISPR-mediated targeting of HER2 inhibits cell proliferation through a dominant negative mutation. Cancer Lett. 2017;385:137–143. doi: 10.1016/j.canlet.2016.10.033. [DOI] [PubMed] [Google Scholar]
- 46.Kim JY, Cho Y, Oh E, Lee N, An H, Sung D, Cho TM, Seo JH. Disulfiram targets cancer stem-like properties and the HER2/Akt signaling pathway in HER2-positive breast cancer. Cancer Lett. 2016;379:39–48. doi: 10.1016/j.canlet.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 47.Somanchi SS, Senyukov VV, Denman CJ, Lee DA. Expansion, purification, and functional assessment of human peripheral blood NK cells. J Vis Exp. 2011;48:e2540. doi: 10.3791/2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ai Z, Lu Y, Qiu S, Fan Z. Overcoming cisplatin resistance of ovarian cancer cells by targeting HIF-1-regulated cancer metabolism. Cancer Lett. 2016;373:36–44. doi: 10.1016/j.canlet.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu H, Li X, Lu Y, Qiu S, Fan Z. ASCT2 (SLC1A5) is an EGFR-associated protein that can be co-targeted by cetuximab to sensitize cancer cells to ROS-induced apoptosis. Cancer Lett. 2016;381:23–30. doi: 10.1016/j.canlet.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tao X, Lu Y, Qiu S, Wang Y, Qin J, Fan Z. AP1G1 is involved in cetuximab-mediated downregulation of ASCT2-EGFR complex and sensitization of human head and neck squamous cell carcinoma cells to ROS-induced apoptosis. Cancer Lett. 2017;408:33–42. doi: 10.1016/j.canlet.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo J, Hong Y, Lu Y, Qiu S, Chaganty BK, Zhang L, Wang X, Li Q, Fan Z. Acetyl-CoA carboxylase rewires cancer metabolism to allow cancer cells to survive inhibition of the Warburg effect by cetuximab. Cancer Lett. 2017;384:39–49. doi: 10.1016/j.canlet.2016.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Overdijk MB, Verploegen S, Ortiz BA, Vink T, Leusen JH, Bleeker WK, Parren PW. Crosstalk between human IgG isotypes and murine effector cells. J Immunol. 2012;189:3430–3438. doi: 10.4049/jimmunol.1200356. [DOI] [PubMed] [Google Scholar]
- 53.Ober RJ, Radu CG, Ghetie V, Ward ES. Differences in promiscuity for antibody-FcRn interactions across species: implications for therapeutic antibodies. Int Immunol. 2001;13:1551–1559. doi: 10.1093/intimm/13.12.1551. [DOI] [PubMed] [Google Scholar]
- 54.Dekkers G, Bentlage AEH, Stegmann TC, Howie HL, Lissenberg-Thunnissen S, Zimring J, Rispens T, Vidarsson G. Affinity of human IgG subclasses to mouse Fc gamma receptors. MAbs. 2017;9:767–773. doi: 10.1080/19420862.2017.1323159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu L, Wu X, Cheng Z, Lee CV, LeCouter J, Campa C, Fuh G, Lowman H, Ferrara N. Interaction between bevacizumab and murine VEGF-A: a reassessment. Invest Ophthalmol Vis Sci. 2008;49:522–527. doi: 10.1167/iovs.07-1175. [DOI] [PubMed] [Google Scholar]
- 56.Gerber HP, Wu X, Yu L, Wiesmann C, Liang XH, Lee CV, Fuh G, Olsson C, Damico L, Xie D, Meng YG, Gutierrez J, Corpuz R, Li B, Hall L, Rangell L, Ferrando R, Lowman H, Peale F, Ferrara N. Mice expressing a humanized form of VEGF-A may provide insights into the safety and efficacy of anti-VEGF antibodies. Proc Natl Acad Sci U S A. 2007;104:3478–3483. doi: 10.1073/pnas.0611492104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Loke P, Allison JP. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc Natl Acad Sci U S A. 2003;100:5336–5341. doi: 10.1073/pnas.0931259100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shi Y, Fan X, Meng W, Deng H, Zhang N, An Z. Engagement of immune effector cells by trastuzumab induces HER2/ERBB2 downregulation in cancer cells through STAT1 activation. Breast Cancer Res. 2014;16:R33. doi: 10.1186/bcr3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weigelt B, Warne PH, Downward J. PIK3CA mutation, but not PTEN loss of function, determines the sensitivity of breast cancer cells to mTOR inhibitory drugs. Oncogene. 2011;30:3222–3233. doi: 10.1038/onc.2011.42. [DOI] [PubMed] [Google Scholar]
- 60.O’Brien NA, Browne BC, Chow L, Wang Y, Ginther C, Arboleda J, Duffy MJ, Crown J, O’Donovan N, Slamon DJ. Activated phosphoinositide 3-kinase/AKT signaling confers resistance to trastuzumab but not lapatinib. Mol Cancer Ther. 2010;9:1489–1502. doi: 10.1158/1535-7163.MCT-09-1171. [DOI] [PubMed] [Google Scholar]
- 61.Zhang H, Liu G, Dziubinski M, Yang Z, Ethier SP, Wu G. Comprehensive analysis of oncogenic effects of PIK3CA mutations in human mammary epithelial cells. Breast Cancer Res Treat. 2008;112:217–227. doi: 10.1007/s10549-007-9847-6. [DOI] [PubMed] [Google Scholar]
- 62.Kataoka Y, Mukohara T, Shimada H, Saijo N, Hirai M, Minami H. Association between gain-of-function mutations in PIK3CA and resistance to HER2-targeted agents in HER2-amplified breast cancer cell lines. Ann Oncol. 2010;21:255–262. doi: 10.1093/annonc/mdp304. [DOI] [PubMed] [Google Scholar]
- 63.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Concha-Benavente F, Srivastava RM, Trivedi S, Lei Y, Chandran U, Seethala RR, Freeman GJ, Ferris RL. Identification of the cell-intrinsic and -extrinsic pathways downstream of EGFR and IFNgamma that induce PD-L1 expression in head and neck cancer. Cancer Res. 2016;76:1031–1043. doi: 10.1158/0008-5472.CAN-15-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]