Abstract
Background
Ganoderma lucidum, a valuable medicinal fungus, is widely distributed in China. It grows alongside with a complex microbial ecosystem in the substrate. As sequencing technology advances, it is possible to reveal the composition and functions of substrate-associated bacterial communities.
Methods
We analyzed the bacterial community dynamics in the substrate during the four typical growth stages of G. lucidum using next-generation sequencing.
Results
The physicochemical properties of the substrate (e.g. acidity, moisture, total nitrogen, total phosphorus and total potassium) changed between different growth stages. A total of 598,771 sequences from 12 samples were obtained and assigned to 22 bacterial phyla. Proteobacteria and Firmicutes were the dominant phyla. Bacterial community composition and diversity significantly differed between the elongation stage and the other three growth stages. LEfSe analysis revealed a large number of bacterial taxa (e.g. Bacteroidetes, Acidobacteria and Nitrospirae) with significantly higher abundance at the elongation stage. Functional pathway prediction uncovered significant abundance changes of a number of bacterial functional pathways between the elongation stage and other growth stages. At the elongation stage, the abundance of the environmental information processing pathway (mainly membrane transport) decreased, whereas that of the metabolism-related pathways increased.
Discussion
The changes in bacterial community composition, diversity and predicted functions were most likely related to the changes in the moisture and nutrient conditions in the substrate with the growth of G. lucidum, particularly at the elongation stage. Our findings shed light on the G. lucidum-bacteria-substrate relationships, which should facilitate the industrial cultivation of G. lucidum.
Keywords: Ganoderma lucidum, Dynamic change, NGS, G. lucidum–bacteria-substrate, Functional pathways, Bacterial composition
Introduction
Ganoderma lucidum belongs to the phylum Basidiomycota, and its growth mainly depends on lignin as a carbon source (Mizuno et al., 1995). The fruiting bodies and spores of G. lucidum are highly appreciated as health products in China for their richness in polysaccharides and triterpenoids, which are demonstrated to strengthen the immune system and inhibit tumor formation (Wang et al., 2002; Sakamoto et al., 2016). Because of its high medicinal value, the planting area of G. lucidum is expanding. G. lucidum has become the main economic pillar in some places due to the advantages of its cost-effective production management, e.g., requiring a small investment, having a short life cycle and yielding benefits fast (Boh et al., 2007). Like other edible fungi, the growth of G. lucidum depends on many environmental factors (e.g., temperature, enzyme activity and microbial community), which likely induce changes in the content of nutrients such as polysaccharides and microelements in its fruiting bodies (Stajic et al., 2002; Tanaka et al., 2016). Li et al. (2014) demonstrated that the nutrient content in different tissues of G. lucidum changed with its growth and that it attributed to the extracellular enzyme activities. Significant growth-related differences were present in crude polysaccharides and triterpenes in the fruiting bodies of most of the G. lucidum strains that were tested (Fu et al., 2008). A greater number of trace elements and heavy metals were found in the mycelia than in the fruiting bodies or spores of G. lucidum (Xing et al., 2001). Studies have characterized the subtle changes of various substances in the body of G. lucidum during its growth; however, changes in microbial community in the surrounding cultivating environment or in the substrate have seldom been studied.
As previously reported, bacteria in the surrounding soil or the culture media were likely to change into endophytic bacteria, and play an important role in the growth of edible fungi (Gagne et al., 1987; Elvira & Van, 2000). The endophytic bacteria assist their host with nitrogen fixation, growth promotion and disease resistance (Mano & Morisaki, 2008; Zhang, 2010; Wang, Chen & Peng, 2016). Qiu et al. (2011) revealed that a variety of microorganisms existed in the mushroom substrate and significantly affected the host development. In particular, tiny changes in microbial communities in the culture substrates may impact the growth and development of edible fungi (Cho et al., 2003). Ma, Chen & Chen (2016) characterized the significant allelopathic effects of the dominant microbes (mainly molds and bacteria) on the growth of G. lucidum in a continuous cropping soil using culture-based methods and demonstrated that bacteria such as Clostridium, Alkaligenes and Bacillus had stronger allelopathic effects on G. lucidum. In addition, pollution rates were associated with changes in microbial communities in the industrial production of Pleurotus eryngii (Lin et al., 2010).
The DNA-based community-fingerprinting methods, such as DGGE and T-RFLP (Székely et al., 2009), are cost-effective ways to explore the changes in microbial community structure in the environment. Nevertheless, these methods lack a clear description of the microbial taxonomy and tend to underestimate microbial diversity with a relatively low resolution. Currently, next-generation sequencing technology has overcome these issues and is widely utilized to explore the distribution of microorganisms in diverse ecological conditions, including freshwater lakes, marine water, agriculture soil, forest soil, thermal vents and even in some valuable herbs (Patel & Jain, 2015; Gigliotti et al., 2015; Xia et al., 2016; Meier, 2016; Ávila et al., 2016; Su et al., 2017).
Previous studies on G. lucidum were mainly related to the cultivation technology and the active components in fruiting bodies. There have been few reports on the dynamic changes in microbial communities in the substrate at different growth stages of G. lucidum. In this study, next-generation sequencing of the V3–V4 region of bacterial 16S rRNA gene was used to determine the composition and diversity of bacterial communities and to predict the potential functions of the dominant microorganisms in the substrate during the four growth stages of G. lucidum (hyphal stage, budding stage, elongation stage and mature stage).
Materials and Methods
Cultivation of Ganoderma lucidum
The Ganoderma lucidum cultivar Chuan Yuanzhi No. 1, provided by the Soil and Fertilizer Institute at the Sichuan Academy of Agricultural Sciences, has been deposited in the China General Microbiological Culture Collection Center (CGMCC) with the strain number of CGMCC 13174 on October 21st, 2016. The substrate was composed of cottonseed hull (90%), wheat bran (5%), corn flour (4%) and gypsum (1%) (Fig. S1), all of which were fresh, dry and unspoiled. The substrate was put into polypropylene cultivation bags (size: 17 cm × 33 cm × 0.005 cm). One side of the polypropylene bag was covered with breathable paper for air entry during the cultivation of G. lucidum. The cultivation bags were then autoclaved at 121 °C for 2 h. The purpose of autoclaving was to create a relatively aseptic environment for mycelium germination. The existence of microbes in the substrate would likely acidify the substrate, affecting the germination and growth of G. lucidum. Therefore, it was a good cultivation practice to sterilize the substrate before inoculation of the fungus G. lucidum. After sterilization, the bags were cooled to room temperature and placed in a laminar flow cabinet for inoculation of G. lucidum (Fig. S2). A small piece of colonised grain (approximately 80 g) that was filled with mycelium of G. lucidum with great vitality was inoculated into each cultivation bag. After inoculation, the cultivation bags were placed in a greenhouse in the cultivation site at Zhaojia, Jintang, China (N 30°48′16.45″, E 104°35′48.79″). The space of the cultivation site had been previously ventilated, cleaned and simply disinfected with lime before the experiment was carried out. The cultivation bags were arranged in parallel in two rows and two layers with seven to eight bags in each on the ground in the greenhouse and the edge of each group was reinforced with wooden piles (Fig. S3).
The sampling of G. lucidum was done at the four growth stages: hyphal stage, budding stage, elongation stage and mature stage. After inoculation, the mycelia of G. lucidum began to germinate. The first sampling was done at the hyphal stage (approximately 35 days after the inoculation) when the mycelia of G. lucidum spread and subsequently filled the whole culture medium. The mycelia twisted together. The second sampling was done at the budding stage (approximately 46 days after the inoculation) when the primordia formed and started to differentiate. The third sampling was done at the elongation stage (approximately 56 days after inoculation) when the promordia grew longer and the stipe was formed. Cap differentiation began after elongation. The last sampling was done at the mature stage (approximately 66 days after the inoculation) when the spores appeared on the pileus surface and gradually covered the yellow edges. Disposable disinfected gloves, sterilized tweezers and knives were used for sampling. At each growth stage, three cultivation bags were brought to the lab and the substrate materials taken from different parts of each cultivation bag were pooled together and homogenized (Fig. S4). Finally, a total of twelve samples were collected in the four growth stages. The fresh samples were stored at −20 °C in 2 mL Eppendorf tubes prior to DNA extraction.
Chemical analysis of substrate materials
Substrate materials at different growing stages of G. lucidum were collected and the chemical properties were determined including pH value, moisture, total nitrogen, total phosphorus and total potassium. The samples were first digested with sulfuric acid hydrogen peroxide. Then the treatment solution of each sample was analyzed with the conventional method according to Thomas, Sheard & Moyer (1967).
DNA extraction, PCR amplification and MiSeq sequencing
No less than 500 mg of substrate materials per sample were collected for DNA extraction. Three biological replicates of samples taken at each growth stage were treated independently to ensure the methodological reproducibility. The E.Z.N.A.® Soil DNA kit (OMEGA Bio-Tek, Norcross, GA, USA) was used to isolate DNA from the substrate following the manufacturer’s protocol. DNA concentration was measured using a UV spectrophotometer (Bio Photometer; Eppendorf, Hamburg, Germany). The quality and size of the extracted DNA was checked by 0.8% agarose gel electrophoresis.
The PCR amplification was performed by Shanghai Personal Biotechnology Co., Ltd (Shanghai, China). Both the primer information and the PCR protocol have been described in detail in Srinivasan et al. (2012). PCR amplification was performed using the bacterial 16S rRNA gene-specific primers 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) with the following conditions: 98 °C for 2 min (initial denaturation), 25 cycles of 98 °C for 15 s (denaturation), 55 °C for 30 s (annealing) and 72 °C for 30 s (extension), and 72 °C for 5 min (final extension) (Langenheder & Székely, 2011). The PCR products were purified with Agencourt AMPure Beads (Beckman Coulter, Indianapolis, IN) and quantified using a Quant-iT Pico Green dsDNA Assay Kit with a microplate reader (FLx800; Bio-Tek, Norcross, GA, USA) and were mixed based on the concentration of each sample. Amplicon sequencing was performed on Illumina’s MiSeq platform (Personalbio, Shanghai, China). The samples were barcoded before pooling. The barcodes and adapters were trimmed with FASTX Toolkit. All raw data were submitted to the Sequence Read Archive (SRA) database with the accession numbers SRR5801759–SRR5801768 and SRR5801783–SRR5801784.
Sequence and statistical analysis
Reads containing ambiguous ‘N’ or with length <120 nt or >140 nt were discarded. High-quality sequences with 97% or greater similarity were clustered into OTUs using UCLUST (Edgar, 2010), a sequence alignment tool, using QIIME pipeline version 1.7.0 (Caporaso et al., 2010). All non-bacterial sequences were removed after classification. The most abundant sequence of each OTU was selected as the representative sequence of this OTU. The relative abundances of the OTUs were calculated. The OTUs with relative abundance lower than 0.001% of the total sequences across all samples were removed (Bokulich & Mills, 2013). The multivariate statistical analyses were done using the OTU relative abundance data in R environment (R Core Team, 2016). An unconstrained ordination (non-metric multidimensional scaling NMDS) was used to visualize the broad pattern of the distribution of bacterial communities. PERMANOVA was used to test the significance of the difference in bacterial communities between different growth stages of G. lucidum based on 999 permutations. Both NMDS and PERMANOVA analysis were performed based on weighted UniFrac distance using the R vegan package (McArdle & Anderson, 2001; Oksanen et al., 2008). The pairwise PERMANOVA was used to test the difference in the bacterial community composition between two growth stages at each time, when the growth stage was found significant to affect the bacterial community composition in the overall term PERMANOVA test. The numbers of shared OTUs were presented in a Venn diagram using the R VennDiagram package (Chen & Boutros, 2011). Bacterial alpha diversity indices including observed OTUs, Chao1, ACE, Shannon and Simpson were rarefied and calculated based on the smallest library size of the samples. LEfSe analysis (Segata et al., 2011) was used to reveal the bacterial taxa that showed differential abundance between different growth stages of G. lucidum at all taxonomic levels. PICRUSt software (Langille et al., 2013) was used to predict the metabolic functions of bacterial communities based on the microbial metabolic function categories in the KEGG database. All significant differences were concluded at P < 0.05.
Results
Chemical analysis of the substrate materials
The physicochemical properties of the substrate materials (e.g., acidity, moisture content, total nitrogen content, total phosphorus content and total potassium content) changed between the four growth stages of G. lucidum (Table 1). The substrate was acidic throughout the growth of G. lucidum. Substrate pH was lowest at the hyphal stage (4.34). After reaching a peak at the budding stage (5.29), the substrate pH decreased at the later growth stages. Substrate moisture content declined along with growth of G. lucidum. Particularly, the moisture content dropped sharply at the mature stage. We observed a lower total nitrogen content in the substrate at the budding and the elongation stages than at the hyphal and mature growth stages of G. lucidum. Total phosphorus and potassium in the substrate displayed the identical fluctuation pattern with the growth of G. lucidum. Both of them showed the highest content at the budding stage and the lowest content at the elongation stage.
Table 1. Chemical properties of substrate.
Mean ± standard deviation. Statistical analysis was carried out by ANOVA using SPSS 19.0 software. The Post Hoc tests were done with LSD method. Different lower-case letters showed significant difference (P < 0.05) in the substrate chemical properties between the different growth stages of G. lucidum.
| Growth stage | pH | Moisture (%) | Total nitrogen (g/kg) | Total phosphorus (g/kg) | Total potassium (g/kg) |
|---|---|---|---|---|---|
| Hyphal stage | 4.34 ± 0.11 c | 64.60 ± 1.08 a | 13.53 ± 0.30 a | 2.74 ± 0.13 b | 13.31 ± 0.34 b |
| Budding stage | 5.29 ± 0.15 a | 58.01 ± 1.22 b | 10.75 ± 0.29 b | 3.36 ± 0.08 a | 15.45 ± 0.13 a |
| Elongation stage | 5.05 ± 0.01 b | 54.48 ± 2.02 c | 11.25 ± 0.59 b | 1.64 ± 0.07 d | 8.80 ± 0.19 d |
| Mature stage | 4.89 ± 0.06 b | 38.56 ± 1.54 d | 12.60 ± 0.04 a | 2.43 ± 0.03 c | 11.47 ± 0.68 c |
Taxonomy-based analysis of bacterial community
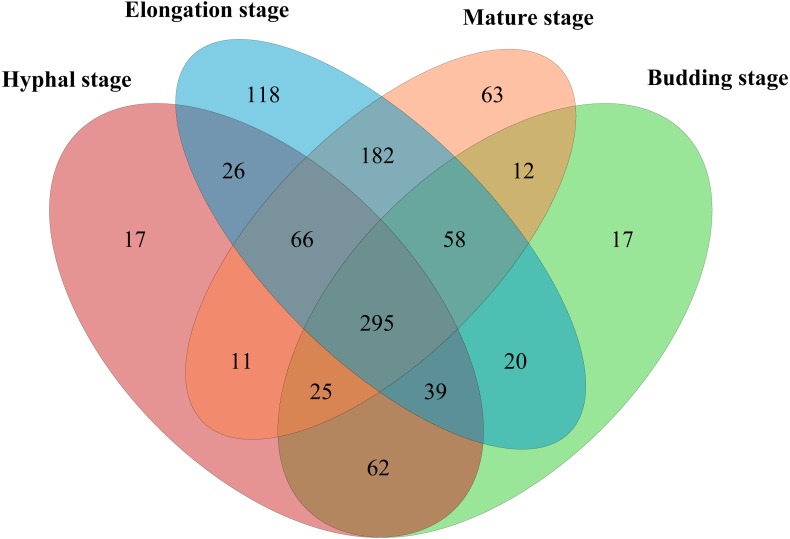
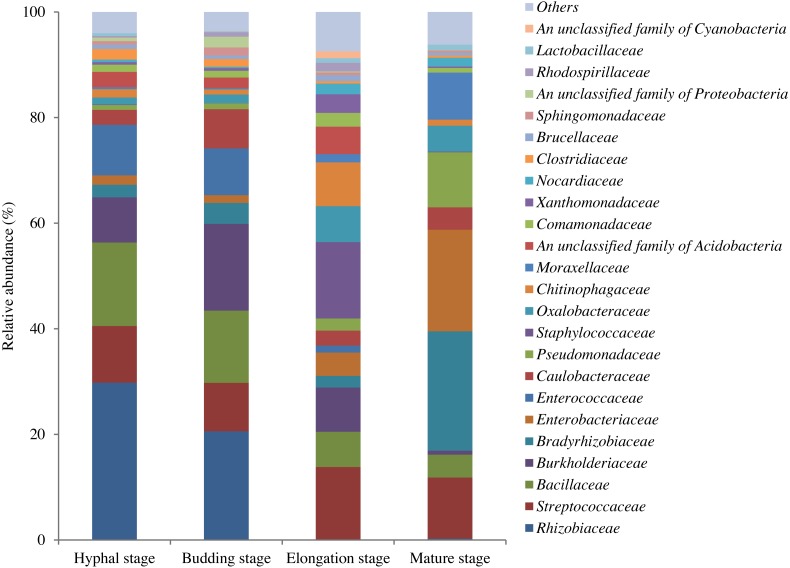
In total, 598,771 sequences from the 12 samples were clustered into 1200 OTUs at 97% similarity. A total of 15.7% of OTUs were unclassified at the phylum level. The high quality reads ranged from 25,996 to 69,618 OTUs between samples (Table S1). A total of 295 shared bacterial OTUs were found between the four growth stages of G. lucidum in the substrate (Fig. 1). A total of 22 phyla were detected in the substrate at the four growth stages of G. lucidum. As shown in Table 2, the most abundant phylum was Proteobacteria, which accounted for 41.47%–72.86% (average 57.23%) of all the bacterial sequences, followed by Firmicutes (22.50%–40.33%, average 34.12%). Together, these two phyla represented 80.12%–95.93% of the bacterial species. The less dominant phyla (average abundance >1%) included Bacteroidetes (3.30%), Acidobacteria (2.75%) and Actinobacteria (2.03%). A total of 195 genera were identified in the samples. Most of the identified genera belonged to the phyla of Proteobacteria, Firmicutes, Bacteroidetes and Actinobacteria (Table S2). Of the 24 major bacterial families (relative abundance >1%) showed in Fig. 2, 20 belonged to Proteobacteria and Firmicutes. Among these major families, two families of Firmicutes (e.g., Streptococcaceae and Bacillaceae) showed high relative abundance across all the growth stages. The relative abundance of proteobacterial families such as Rhizobiaceae, Bradyrhizobiaceae and Enterobacteriaceae changed greatly between the different growth stages.
Figure 1. Venn diagram showing the number of shared OTUs between different growth stages of G. lucidum.
Table 2. The average relative abundance of different bacterial phyla in the substrate during the four growth stages of G. lucidum.
Others are all unclassified phyla.
| Phylum | Abu. (%) at hyphal stage | Abu. (%) at budding stage | Abu. (%) at elongation stage | Abu. (%) at mature stage |
|---|---|---|---|---|
| [Thermi] | 0.01 | 0.01 | 0.02 | 0.01 |
| Acidobacteria | 3.08 | 2.11 | 5.64 | 0.04 |
| Actinobacteria | 1.11 | 0.87 | 3.37 | 2.58 |
| Bacteroidetes | 1.65 | 1.00 | 8.66 | 1.48 |
| Chloroflexi | 0.00 | 0.00 | 0.03 | 0.01 |
| Cyanobacteria | 0.09 | 0.02 | 1.54 | 0.03 |
| Elusimicrobia | 0.01 | 0.00 | 0.02 | 0.01 |
| Firmicutes | 40.37 | 34.96 | 40.06 | 20.70 |
| Fusobacteria | 0.04 | 0.03 | 0.07 | 0.04 |
| Gemmatimonadetes | 0.01 | 0.01 | 0.04 | 0.00 |
| Nitrospirae | 0.00 | 0.00 | 0.03 | 0.00 |
| Planctomycetes | 0.01 | 0.00 | 0.03 | 0.00 |
| Proteobacteria | 53.6 | 60.99 | 40.43 | 74.93 |
| Tenericutes | 0.00 | 0.00 | 0.00 | 0.02 |
| Verrucomicrobia | 0.00 | 0.00 | 0.01 | 0.01 |
| Others | 0.01 | 0.00 | 0.06 | 0.14 |
Figure 2. OTU average relative abundances of the major bacterial families in the substrate of G. lucidum during all growth stages.
Others: the families with relative abundances lower than 1% at each growth stage of G. lucidum.
Bacterial alpha diversity
Bacterial alpha diversity indices significantly differed between the four growth stages of G. lucidum (Table 3). The richness indices (e.g., observed OTUs, Chao1 and ACE) were significantly higher at the late growth stages (e.g., elongation and mature stages) than at the early growth stages (e.g., hyphal and budding stages). The Shannon diversity was significantly higher at the elongation stage than at the hyphal stage. There was no difference in the Simpson index between the different growth stages of G. lucidum.
Table 3. Bacterial alpha diversity indices.
Mean ± standard deviation. Statistical analysis was carried out by ANOVA using SPSS 19.0 software. The Post Hoc tests were done with LSD method. The index of the observed OTUs, was used to evaluate the observed OTU richness, whereas the Chao1 and ACE were used to estimate the total (observed and unobserved) OTU richness of the bacterial community. The indices of Shannon and Simpson were used to access the richness and evenness of bacterial community, respectively. Different lower-case letters showed significant difference (P < 0.05) in the diversity indices between the different growth stages of G. lucidum.
| Sample | Observed OTUs | Chao1 | ACE | Simpson | Shannon |
|---|---|---|---|---|---|
| Hyphal stage | 363 ± 21 b | 197.00 ± 21.66 b | 260.22 ± 40.72 b | 0.85 ± 0.10 a | 4.02 ± 0.62 b |
| Budding stage | 357 ± 13 b | 189.00 ± 24.58 b | 270.17 ± 35.29 b | 0.88 ± 0.00 a | 4.08 ± 0.09 ab |
| Elongation stage | 505 ± 50 a | 372.00 ± 70.55 a | 479.66 ± 99.77 a | 0.90 ± 0.06 a | 4.77 ± 0.33 a |
| Mature stage | 491 ± 33 a | 336.67 ± 53.46 a | 422.42 ± 56.42 a | 0.87 ± 0.03 a | 4.21 ± 0.13 ab |
Bacterial beta diversity
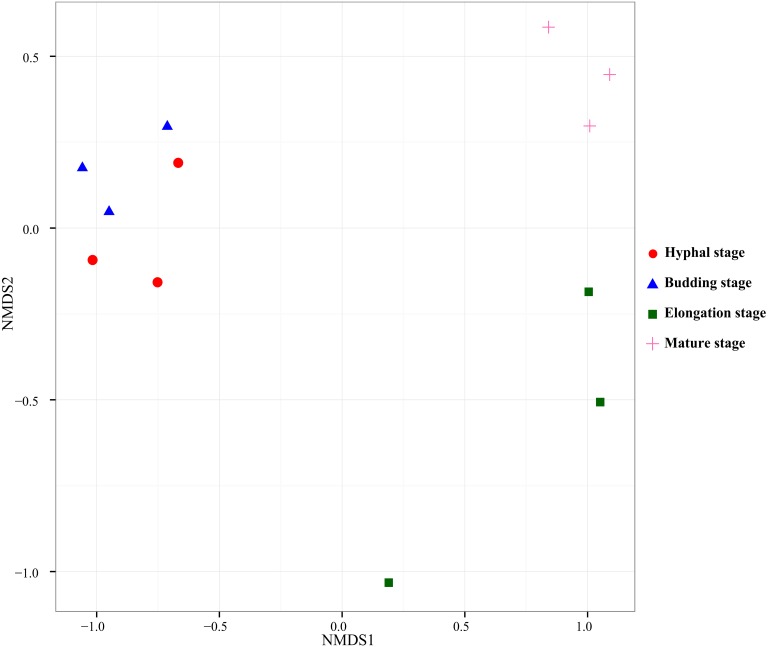
PERMANOVA test was used to test the effect of the growth stage of G. lucidum on the bacterial community composition in the substrate. According to the pairwise PERMANOVA test (Table 4), bacterial community composition significantly differed between the elongation stage and the other three growth stages. NMDS, an unconstrained ordination, was used to visualize the patterns of bacterial community distribution. The separation of the bacterial community samples at the elongation stage from the other three stages were clearly displayed in the NMDS ordination (Fig. 3). The bacterial community samples taken at the elongation stage were separated from the hyphal and budding stages on the first axis, whereas they were separated from the mature stage on the second axis in the NMDS ordination plot.
Table 4. PEMANOVA analysis of bacterial community between different growth stages based on weighted UniFrac distance.
Significance of the differences in bacterial communities between the different growth stages were tested using 999 permutations.
| Significance | ||
|---|---|---|
| Hyphal stage | Budding stage | 0.5014 |
| Elongation stage | 0.0014 | |
| Mature stage | 0.1000 | |
| Budding stage | Elongation stage | 0.0014 |
| Mature stage | 0.1000 | |
| Elongation stage | Mature stage | 0.0014 |
| Hyphal stage, Budding stage, Elongation stage, Mature stage | 0.0014 | |
Figure 3. Nonmetric Multidimensional Scaling ordination of bacterial communities based on weighted UniFrac distance.
Biomarker discovery
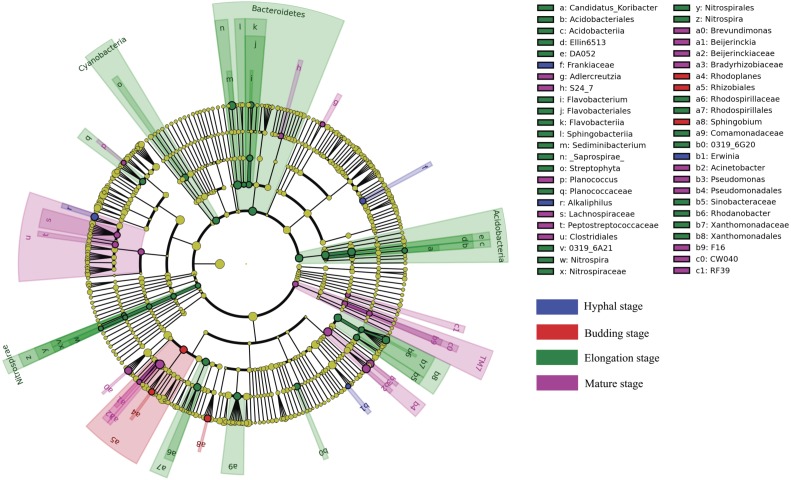
LEfSe analysis was used to reveal the bacterial taxa that showed differential abundances between the four different growth stages of G. lucidum at all taxonomic levels (Fig. 4). LEfSe analysis revealed a significantly higher abundance of the order Rhizobiales (e.g., genus Rhodoplanes) and the genus Sphingobium at the budding stage than at the other three growth stages of G. lucidum. The family Frankiaceae and the genera of Alkaliphilus and Erwinia were significantly enriched in the substrate at the hyphal stage, with regard to other growth stages. A large number of bacterial taxa exhibited a significantly higher abundance at the elongation stage. Those taxa included the phyla of Bacteroidetes (e.g., genera Flavobacterium and Sediminibacterium), Acidobacteria (e.g., genus Candidatus Koribacter), Nitrospirae (e.g., genus Nitrospira), Cyanobacteria (e.g., order Streptophyta) and other taxa such as the orders of Xanthomonadales and Rhodospirillales and the family of Comamonadaceae. The phylum of TM7 and the orders of Clostridiales (e.g., families Peptostreptococcaceae and Lachnospiraceae) and Pseudomonadales (e.g., genera Acinetobacter and Pseudomonas) were significantly more abundant at the mature stage than at other growth stages.
Figure 4. A cladogram showing the differentially abundant bacterial taxa at each of the four growing stages of G. lucidum based on LEfSe analysis (P < 0.05, LDA score > 2).
Functional prediction of substrate bacteria community
A total of 39 KEGG pathways were identified in the study (Fig. S5). Of these KEGG pathways, 48.93% were related to metabolism, 17.24% to environmental information processing and 14.23% to genetic information processing. The ten most prevalent pathways were related to four function types: metabolism, environmental information processing, genetic information processing and unclassified. Membrane transport was the predominant KEGG pathway predicted by PICRUSt, accounting for an average of 15.93% during the growth of G. lucidum. Carbohydrate metabolism (on average 10.03%) and amino acid metabolism (on average 9.81%) were the second and the third most abundant pathways during the growth of G. lucidum.
The predicted pathways that had relative abundances over 1% at different growth stages of G. lucidum were presented in Table 5. A number of predicted bacterial functional pathways in the substrate were different between the elongation stage and the other three growth stages of G. lucidum. At the elongation stage, the relative abundance of membrane transport, and xenobiotics biodegradation and metabolism were decreased; other predicted pathways such as folding, sorting and degradation, replication and repair, translation, carbohydrate metabolism, energy metabolism, enzyme families, glycan biosynthesis and metabolism, and metabolism of cofactors and vitamins were enriched in the substrate bacterial community. Other pathways were not significantly different in abundance between different growth stages (e.g., cell motility, signal transduction, amino acid metabolism, and cellular processes and signaling).
Table 5. PICRUSt predicted KEGG pathways of bacterial community in the substrate (relative abundance > 1% in at least one growth stage) at different growth stages of G. lucidum.
Mean ± standard deviation. Statistical analysis was carried out by ANOVA using SPSS 19.0 software. The Post Hoc tests were done with LSD method. Different lower-case letters showed significant difference (P < 0.05) in the relative abundance of the pathways between the different growth stages of G. lucidum.
| Function type | KEGG pathway | Relative abundance (%) | |||
|---|---|---|---|---|---|
| Hyphal stage | Budding stage | Elongation stage | Mature stage | ||
| Cellular processes | Cell motility | 2.77 ± 0.05 a | 2.97 ± 0.32 a | 2.75 ± 0.32 a | 2.95 ± 0.32 a |
| Environmental information processing | Membrane transport | 17.40 ± 1.96 a | 16.31 ± 1.80 a | 11.36 ± 0.81 b | 14.08 ± 0.72 ab |
| Signal transduction | 2.14 ± 0.05 a | 2.20 ± 0.09 a | 2.27 ± 0.05 a | 2.35 ± 0.16 a | |
| Genetic information processing | Folding, sorting and degradation | 1.77 ± 0.10 c | 1.79 ± 0.04 bc | 2.18 ± 0.04 a | 1.98 ± 0.11 ab |
| Replication and repair | 6.06 ± 0.27 b | 5.99 ± 0.14 b | 6.91 ± 0.15 a | 6.06 ± 0.12 b | |
| Transcription | 2.44 ± 0.10 ab | 2.41 ± 0.02 ab | 2.54 ± 0.09 a | 2.26 ± 0.05 b | |
| Translation | 3.46 ± 0.23 b | 3.42 ± 0.12 b | 4.11 ± 0.16 a | 3.58 ± 0.07 ab | |
| Metabolism | Amino acid metabolism | 9.81 ± 0.16 a | 10.11 ± 0.46 a | 10.38 ± 0.12 a | 10.07 ± 0.27 a |
| Biosynthesis of other secondary metabolites | 0.93 ± 0.02 ab | 0.92 ± 0.04 ab | 1.03 ± 0.11 a | 0.80 ± 0.04 b | |
| Carbohydrate metabolism | 10.07 ± 0.26 b | 10.09 ± 0.11 b | 10.68 ± 0.05 a | 9.94 ± 0.28 b | |
| Energy metabolism | 4.85 ± 0.07 c | 4.80 ± 0.04 c | 5.42 ± 0.05 a | 5.05 ± 0.01 b | |
| Enzyme families | 1.80 ± 0.09 b | 1.77 ± 0.02 b | 2.01 ± 0.05 a | 1.67 ± 0.06 b | |
| Glycan biosynthesis and metabolism | 1.76 ± 0.09 b | 1.73 ± 0.04 b | 2.18 ± 0.25 a | 1.74 ± 0.15 b | |
| Lipid metabolism | 3.75 ± 0.01 a | 3.86 ± 0.19 a | 3.89 ± 0.10 a | 3.93 ± 0.10 a | |
| Metabolism of cofactors and vitamins | 3.58 ± 0.01 b | 3.58 ± 0.03 b | 3.94 ± 0.14 a | 3.74 ± 0.11 ab | |
| Metabolism of other amino acids | 1.89 ± 0.04 b | 2.02 ± 0.15 ab | 1.94 ± 0.05 ab | 2.13 ± 0.07 a | |
| Metabolism of terpenoids and polyketides | 1.98 ± 0.03 b | 2.06 ± 0.10 b | 2.04 ± 0.04 ab | 2.22 ± 0.07 a | |
| Nucleotide metabolism | 2.97 ± 0.09 a | 2.90 ± 0.14 a | 3.16 ± 0.10 a | 2.98 ± 0.05 a | |
| Xenobiotics biodegradation and metabolism | 4.37 ± 0.20 ab | 4.61 ± 0.28 a | 3.73 ± 0.23 b | 4.86 ± 0.42 a | |
| Unclassified | Cellular processes and signaling | 3.71 ± 0.13 a | 3.73 ± 0.03 a | 3.99 ± 0.09 a | 3.89 ± 0.34 a |
| Genetic information processing | 1.98 ± 0.16 b | 2.05 ± 0.08 b | 2.45 ± 0.06 a | 2.48 ± 0.18 a | |
| metabolism | 2.72 ± 0.08 a | 2.76 ± 0.04 a | 2.72 ± 0.16 a | 2.96 ± 0.12 a | |
| Poorly characterized | 4.90 ± 0.12 a | 4.91 ± 0.03 a | 5.29 ± 0.07 a | 5.13 ± 0.27 a | |
Discussion
In this study, we observed significant changes in the physicochemical properties of the substrate materials during the growth of G. lucidum. The substrate materials together with the aeration and temperature used in our study provided an optimum condition for the cultivation of G. lucidum. For cultivation of edible and/or medicinal mushrooms, the substrate rich in essential bio-available nutrients with optimum pH, moisture, and air content is preferred (Chang & Wasser, 2018). Cottonseed hulls provide fine water holding capacity and nitrogen to ensure the water and nutrient supply for the cultivation of edible mushrooms such as Pleurotus ostreatus (Lu & Qu, 1984) and aerobic bacteria (Slifkin & Pouchet, 1975). The mixture of cottonseed hulls with other materials such as wheat bran, corn flour and gypsum was used for industrial production of G. lucidum in China. The growth of G. lucidum slightly increased substrate pH, especially at the budding stage. However, the substrate still remained acidic with pH values ranging between 4 and 5.5 at different growth stages. The moisture content continued declining with the vegetative growth of G. lucidum. The observed sharp decline of moisture content was likely associated with the development of the fruiting bodies at the mature stage, when the water demand was the largest. The lowered total nitrogen content in the substrate likely indicated the higher uptake of nitrogen of G. lucidum at the budding and elongation stages than at the hyphal and mature stages. This phenomenon might be related to the rapid mycelia growth and protein synthesis processes of G. lucidum at the later growth stages. Nitrogen content is one limiting factor that should be seriously taken into consideration during mushroom cultivation. Nitrogen supplementation improves the nutritional content in substrates (Lelley & Janssen, 1993), contributing to a higher yield of mushrooms (Yang, Chienyan & Chen, 2003). Dependent on the fungus physiology and the medium composition, nitrogen source was demonstrated to affect enzyme synthesis (Couto et al., 2004; Kapich et al., 2004). In addition, the C: N ratio can greatly impact the mycelia growth and fruiting body formation of G. lucidum (Hsieh & Yang, 2004). It has been previously stated that more enzymes are involved in cell wall synthesis during fruiting body formation than at the mycelium and primordial stages. However, enzymes related to cell wall component degradation were higher expressed at the earlier stages of mushroom growth (Rahmad et al., 2014). Total phosphorus and potassium content in the substrate was lowest at the elongation stage, likely attributable to a high nutrient demand of G. lucidum at this stage. G. lucidum grows most prosperously at the elongation stage, a critical period for biomass and nutrient production. Minerals such as phosphorus and potassium were strongly correlated with the yield and biological efficiency of some edible fungi (e.g., Ganoderma lucidum and Lentinula edodes) (Ozcelik & Peksen, 2007; Peksen & Yakupoglu, 2009).
Proteobacteria and Firmicutes were the dominating phyla observed in the bacterial community in the substrate of G. lucidum, with variations in relative abundance between different growth stages of G. lucidum. Proteobacteria widely exist in high abundance in soils with diverse morphologies, physiologies and metabolisms and is considered to be advantageous in global carbon, nitrogen and sulfur cycling (Janssen, 2006; Kersters et al., 2006; Spain, Krumholz & Elshahed, 2009). A recent study suggested that the members of the phylum Firmicutes play important roles in the conversion of wheat straw into bio-utilizable sugars throughout the Agaricus bisporus mushroom cropping process (Mcgee et al., 2017). Durrer et al. (2016) characterized the role of biological connections in agricultural soils and revealed that competition among various bacteria may cause a decline in the abundance of some taxa. The relative abundance of Firmicutes decreased with time in our study, likely due to the reduced bioavailability of the wheat straw and the resource competition between other organisms in the substrate at the late growth stage of G. lucidum. Besides Firmicutes, other lignocellulose degrading bacteria belonging to Actinobacteria, Proteobacteria and Bacteroidetes (Ntougias et al., 2004; Zhang et al., 2014; Liang et al., 2015) were also present with relatively high abundance in the mushroom substrate, ensuring an effective simultaneous degradation system of lignocellulose in collaboration with the mushroom G. lucidum.
In our study, bacterial alpha diversity in the substrate increased significantly during the growth of G. lucidum. Bacterial alpha diversity indices (e.g., observed OTUs, Chao1, ACE and Shannon) peaked at the elongation stage. Vajna et al. (2010) attributed the higher diversity of bacterial community around the mushrooms to the growth of the mushrooms. Nevertheless, the evenness of bacterial community (Simpson index) remained unchanged with time. Bacterial community composition in the substrate changed significantly during the growth of G. lucidum. Particularly, bacterial community composition significantly differed between the elongation stage and the other three growth stages of G. lucidum, which might be linked to the changes in the substrate environment and the growth of G. lucidum.
LEfSe analysis revealed that a large number of bacterial taxa were enriched in the substrate at the elongation stage than at other growth stages of G. lucidum, including Bacteroidetes (e.g., genera Flavobacterium and Sediminibacterium), Acidobacteria (e.g., genus Candidatus Koribacter), Nitrospirae (e.g., genus Nitrospira), Cyanobacteria (e.g., order Streptophyta) and other taxa such as the orders of Xanthomonadales and Rhodospirillales and the family of Comamonadaceae. The increased relative abundance of these taxa were likely attributable to the colonization of these bacteria and the changes in the physicochemical condition of the substrate at the elongation stage of G. lucidum. These bacteria were characterized by their capacity in the biogeochemical cycling of major biogenic elements such as carbon, nitrogen, phosphorus and micronutrients. So the nitrogen, phosphorus and potassium and other nutrients in the substrate were likely more demanded by these bacteria at the elongation stage than at other stages. The reduction in substrate total nitrogen, phosphorus and potassium at the elongation stage highly supported this assumption. The activities of the bacterial community in the cultivation substrate significantly affect growth and quality of the edible mushrooms (Miller et al., 1990; Stölzer & Grabbe, 1991). Nitrogen fixation genes were detected in the genus of Burkholderia (Minerdi et al., 2001). Nitrospirae plays important roles in nitrification during the mushroom cropping process (Mcgee et al., 2017). Other bacteria may provide essential growth factors such as thiamine (Henningsson, 1967). The enrichment of these bacteria and the increased bacterial diversity at the elongation stage might be important to trigger the development of the fruiting bodies of G. lucidum.
G. lucidum produces various enzymes to degrade lignin and synthesize substances to accomplish its growth as other white rot fungi (Coelho et al., 2010). Accordingly, the changes in the physicochemical properties of the substrate materials by G. lucidum during its growth might be an important driver of the successional changes in bacterial community composition in the substrate. As the main ingredient of the substrate material, cottonseed hull can affect the growth of G. lucidum to a great extent. Previous studies reported compositional changes in a substrate with cottonseed hull, which included cellulose, hemicelluloses and lignin, during the growth period of mushrooms (Li, Pang & Zhang, 2001; Ni et al., 2002). The abundances of most bacterial groups were dependent on organic C and total N in the habitat (Zhang, 2010). In our study, the moisture content of substrate continued decreasing with the growth of G. lucidum, likely to be another key factor to affect the bacterial community composition and diversity. Therefore, the qualitative and quantitative change in the substrate physicochemical properties might have driven the dynamic shifts in the composition and functions of bacterial community during the growth of G. lucidum. In addition, bacterial communities can be directly affected by organic substances that fungi secrete (Frey-Klett, Pierrat & Garbaye, 1997). Metabolites of mushrooms such as carbohydrates, amino acids, thiols and all kinds of enzymes were secreted to change the physicochemical conditions in the substrate (Rangel-Castro, Danell & Pfeffer, 2002; Zhu et al., 2013), likely creating a suitable environment for bacterial colonization and growth at the later stage. In this study, differentially abundant bacterial taxa were uncovered in the substrate at different growth stages, most likely associated with the differed metabolic functions in the community.
The reconstruction of metagenome based on marker genes allows inference of the potential functional profile of the bacterial community in the substrate of G. lucidum. Of the identified KEGG pathways, 17.24% were related to environmental information processing, in which membrane transport dominated. Membrane transport of the bacterial community was predicted more abundant at the budding and hyphal stages than at the elongation stage in the substrate. The changes in the predicted functional pathways in the bacterial communities were likely attributable to the changes in the substrate physicochemical conditions. The pathway of membrane transport showed high abundance when the moisture content was high at the early stage. The extracellular enzymes and other organic substances that microbes produce are transported by liquid phase diffusion (Yang et al., 2011). High moisture content facilitates the transport of nutrients and mobility of bacteria, increasing the availability of nutrients to bacteria and intercommunication between microorganisms in the substrate. For bacteria, outer membrane proteins play essential roles in bacterial adaptation (Lin, Huang & Zhang, 2002). The membrane compositions differ from bacterial species (Sohlenkamp & Geiger, 2016). The outer membranes are important for bacterial adaption, as they are associated with the uptake of water, energy and nutrient, microbial interaction regulations and stress-resistance (Lin, Huang & Zhang, 2002; Schwechheimer & Kuehn, 2015). Outer-membrane vesicles make bacteria interact with the environment and survive under stress conditions (Schwechheimer & Kuehn, 2015). Therefore, in addition to the effect of G. lucidum, the high abundance of predicted environmental information processing pathway (particularly membrane transport) in the bacterial community at the early stage of G. lucidum was also likely related to the adaptation and colonization mechanisms of the bacterial settlers that entered into the substrate from air.
At the elongation stage, the abundance of the predicted membrane transport and xenobiotics biodegradation and metabolism pathways reduced whereas the predicted genetic information processing pathways (e.g., replication and repair, and translation), metabolism pathways (e.g., carbohydrate metabolism, energy metabolism, enzyme families, glycan biosynthesis and metabolism, and metabolism of cofactors and vitamins) increased, suggesting a functional change in the bacterial community from environmental adaptation and survival strategies to self-development with the growth of G. lucidum. The increase of predicted translation pathway at the elongation stage might be a result of rapid protein synthesis in bacterial cells in response to the rapid growth of G. lucidum. At this stage, bacterial richness and diversity increased, supported by the evidence of the reduction of the nutrients (e.g., total nitrogen, phosphorus and potassium) and moisture content in the substrate. Thus, the sufficient provision of nutrients and water to the substrate at the elongation stage might be important to sustain the growth and metabolic activities of the fungus and the substrate -associated microbiome.
Microbes are ubiquitous and participate in the life activities of other organisms (Singh, 2015). It was noteworthy that those bacterial OTUs that were present throughout the whole life cycle of G. lucidum accounted for more than 94% of the bacterial community, indicating that they were the first settlers and continued to be present in the substrate. These bacterial OTUs might have great capacity to cope with the acidity of the substrate environment and the resource competition with G. lucidum and other organisms. Some of these OTUs might also be beneficial to the growth of G. lucidum. Bacteria were involved in cell membrane permeability, metabolic activity, exudates absorption and nutrient bioavailability of mycelia, affecting the fruiting body growth (Fitter & Garbaye, 1994; Frey-Klett, Pierrat & Garbaye, 1997). For instance, bacterial metabolites were found related to fruiting body formation of Agaricus bisporus and help the mushroom resist pathogens (Urayama, 1967; Park & Agnihotri, 1969; Zhang, 1999). Bacteria such as fluorescent pseudomonads were particularly beneficial to Pleurotus ostreatus budding, which brings up a potential research direction for improving the yield and quality of Ganoderma lucidum by inoculating beneficial bacteria (Ouhdouch, Barakate & Finance, 2001; Cho et al., 2003). The phyla Actinobacteria and Firmicutes were suggested to carry out the conversion of wheat straw into utilisable sugars (Mcgee et al., 2017). In our study, a higher abundance of Bacillus was observed at the early growth stages (e.g., hyphal stage and budding stage) of G. lucidum. Bacillus spp. isolated from the cultivation substrate can optimize the growth of Pleurotus ostreatus by inhibiting Trichoderma harzianum growth through laccase induction (Velázquez-Cedeño et al., 2008). Bacillus provided relative protection mechanism against other harmful fungi because mycelia of cultivated mushroom were easily contaminated by other harmful fungi before primordia formation. Hence, the potential enhancement of bacterial activities on the growth of G. lucidum is worth further investigation.
Conclusions
The study revealed a significant effect of G. lucidum growth on the physicochemical properties and bacterial community composition of the substrate materials (e.g., pH, moisture and nutrient conditions). Changes in the substrate physicochemical properties associate with the growth of G. lucidum were likely the direct factor that drove the changes in bacterial community composition. The diversity, structure and the predicted functional pathways of the substrate bacterial community greatly differed between the elongation stage and other growth stages. The decrease of nutrient contents and moisture indicated a rigorous growth of G. lucidum at the elongation stage. We therefore suggest that the nutrient supply especially at the elongation stage is crucial to the growth of G. lucidum, the diversity and the metabolic activities of the bacteria in the substrate. Hence, this study increased the understanding of the G. lucidum-bacteria-substrate interactions to hopefully facilitate the industrial cultivation of G. lucidum.
Supplemental Information
Photo by Xiaolin Li.
Photo by Xiaolin Li.
Photo by Xiaolin Li.
Photo by Xiaolin Li.
Different microbial metabolic functions were arranged vertically in accordance with their respective modules, in which the length of the graph revealed the abundance of bacterial genes related to the corresponding functions in the sample.
Acknowledgments
We would like to thank American Journal Experts (http://www.aje.cn/#) for providing linguistic assistance during the preparation of this manuscript.
Funding Statement
This work was supported by the Chinese Agricultural Research System (CARS-20), as well as edible fungi breeding and cultivation project of the innovation team in Sichuan province. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Xiaolin Li, Email: kerrylee_tw@sina.com.
Xiaoping Zhang, Email: zhangxiaopingphd@126.com.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Bo Zhang conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables.
Lijuan Yan performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables.
Qiang Li analyzed the data.
Jie Zou performed the experiments.
Hao Tan and Weihong Peng performed the experiments, authored or reviewed drafts of the paper.
Wei Tan conceived and designed the experiments.
Xiaolin Li conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Xiaoping Zhang conceived and designed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
NCBI: accession numbers are SRR5801759–SRR5801768 and SRR5801783, SRR5801784 in BioProject ID PRJNA392763.
References
- Ávila et al. (2016).Ávila MP, Staehr PA, Barbosa FA, Chartone SE, Nascimento AM. Seasonality of freshwater bacterioplankton diversity in two tropical shallow lakes from the Brazilian Atlantic Forest. FEMS Microbiology Ecology. 2016;93:fiw218. doi: 10.1093/femsec/fiw218. [DOI] [PubMed] [Google Scholar]
- Boh et al. (2007).Boh B, Berovic M, Zhang J, Lin ZB. Ganoderma lucidum and its pharmaceutically active compounds. Biotechnology Annual Review. 2007;13:265–301. doi: 10.1016/S1387-2656(07)13010-6. [DOI] [PubMed] [Google Scholar]
- Bokulich & Mills (2013).Bokulich NA, Mills DA. Improved selection of internal transcribed spacer-specific primers enables quantitative, ultra-high-throughput profiling of fungal communities. Applied & Environmental Microbiology. 2013;79:2519–2526. doi: 10.1128/AEM.03870-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso et al. (2010).Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nature Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang & Wasser (2018).Chang S, Wasser S. The cultivation and environmental impact of mushrooms. Oxford Research Encyclopedia of Environmental Science. 2018 doi: 10.1093/acrefore/9780199389414.013.231. Epub ahead of print Mar 2017. [DOI] [Google Scholar]
- Chen & Boutros (2011).Chen H, Boutros PC. VennDiagram: a package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinformatics. 2011;12(1):35. doi: 10.1186/1471-2105-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho et al. (2003).Cho YS, Kim JS, Crowley DE, Cho BG. Growth promotion of the edible fungus Pleurotus ostreatus, by fluorescent pseudomonads. FEMS Microbiology Letter. 2003;218:271–276. doi: 10.1016/S0378-1097(02)01144-8. [DOI] [PubMed] [Google Scholar]
- Coelho et al. (2010).Coelho JDS, Oliveira ALD, Souza CGMD, Bracht A, Peralta RM. Effect of the herbicides bentazon and diuron on the production of ligninolytic enzymes by Ganoderma lucidum. International Biodeterioration & Biodegradation. 2010;64:156–161. doi: 10.1016/j.ibiod.2009.12.006. [DOI] [Google Scholar]
- Couto et al. (2004).Couto SR, Rosales E, Gundin M, Sanroman MA. Exploitation of wastes from the brewing industry for laccase production by two Trametes species. Journal of Food Engineering. 2004;64(4):423–428. doi: 10.1016/j.jfoodeng.2003.11.009. [DOI] [Google Scholar]
- Durrer et al. (2016).Durrer A, Gumiere T, Taketani RG, Costa DPD, Andreote FD. The drivers underlying biogeographical patterns of bacterial communities in soils under sugarcane cultivation. Applied Soil Ecology. 2016;110:12–20. doi: 10.1016/j.apsoil.2016.11.005. [DOI] [Google Scholar]
- Edgar (2010).Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Elvira & Van (2000).Elvira RM, Van VJ. Natural incidence of endophytic bacteria in pea cultivars under field conditions. Canadian Journal of Microbiology. 2000;46:1036–1041. doi: 10.1139/cjm-46-11-1036. [DOI] [PubMed] [Google Scholar]
- Fitter & Garbaye (1994).Fitter AH, Garbaye J. Interactions between mycorrhizal fungi and other soil organisms. Plant and Soil. 1994;159(1):123–132. doi: 10.1007/BF00000101. [DOI] [Google Scholar]
- Frey-Klett, Pierrat & Garbaye (1997).Frey-Klett P, Pierrat JC, Garbaye J. Location and survival of mycorrhiza helper Pseudomonas fluorescens during establishment of ectomycorrhizal symbiosis between Laccaria bicolor and Douglas Fir. Applied and Environmental Microbiology. 1997;63(1):139–144. doi: 10.1128/aem.63.1.139-144.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu et al. (2008).Fu LZ, Wu XQ, Li MY, Li HB, Wei HL, Wu QQ, Chen JW, He L. Crude polysaccharide and triterpene content of Ganoderma fruit bodies at different stages of development. Acta Edulis Fungi. 2008;15:47–50. doi: 10.3969/j.issn.1005-9873.2008.03.010. [In Chinese] [DOI] [Google Scholar]
- Gagne et al. (1987).Gagne S, Richard C, Rousseau H, Antoun H. Xylem-residing bacteria in alfafa roots. Canadian Journal of Microbiology. 1987;33:996–1000. doi: 10.1139/m87-175. [DOI] [Google Scholar]
- Gigliotti et al. (2015).Gigliotti G, Pezzolla D, Zadra C, Albertini E, Marconi G, Turchetti B, Buzzini P. Dynamics of organic matter and microbial populations in amended soil: a multidisciplinary approach. Soil Biology & Biochemistry. 2015;15:9–20. [Google Scholar]
- Henningsson (1967).Henningsson B. Interactions between micro-organisms found in birch and aspen pulpwood. Studia Forestalia Suecica. 1967;53:1–32. [Google Scholar]
- Hsieh & Yang (2004).Hsieh C, Yang F. Reusing soy residue for the solid-state fermentation of Ganoderma lucidum. Bioresource Technology. 2004;91(1):105–109. doi: 10.1016/S0960-8524(03)00157-3. [DOI] [PubMed] [Google Scholar]
- Janssen (2006).Janssen PH. Identifying the dominant soil bacterial taxa in libraries of 16S rRNA and 16S rRNA genes. Applied & Environmental Microbiology. 2006;72:1719–1728. doi: 10.1128/AEM.72.3.1719-1728.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapich et al. (2004).Kapich AN, Prior BA, Botha A, Galkin S, Lundell T, Hatakka A. Effect of lignocellulose-containing substrate on production of ligninolytic peroxidases in submerged cultures of Phanerochaete chrysosporium ME-446. Enzyme and Microbial technology. 2004;34(2):187–195. doi: 10.1016/j.enzmictec.2003.10.004. [DOI] [Google Scholar]
- Kersters et al. (2006).Kersters K, De Vos P, Gillis M, Swings J, Vandamme P, Stackebrandt E. Introduction to the Proteobacteria. In: Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E, editors. The Prokaryotes. Springer; New York: 2006. pp. 3–37. [DOI] [Google Scholar]
- Langenheder & Székely (2011).Langenheder S, Székely AJ. Species sorting and neutral processes are both important during the initial assembly of bacterial communities. The ISME Journal. 2011;5:1086–1094. doi: 10.1038/ismej.2010.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langille et al. (2013).Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, Beiko RG, Huttenhower C. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nature Biotechnology. 2013;31:814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelley & Janssen (1993).Lelley JI, Janssen A. Productivity improvement of oyster mushroom substrate with a controlled release of nutrient. Mushroom News. 1993;41(2):6–13. [Google Scholar]
- Li, Pang & Zhang (2001).Li X, Pang Y, Zhang R. Compositional changes of cottonseed hull substrate during P. ostreatus growth and the effects on the feeding value of the spent substrate. Bioresource Technology. 2001;80:157–161. doi: 10.1016/S0960-8524(00)00170-X. [DOI] [PubMed] [Google Scholar]
- Li et al. (2014).Li WT, Yu MY, Wei W, Xu XY, Jiang N, Zheng LY, Yang ZR, Luo X. Variation of substance content during growth cycle of cultured Ganoderma lucidum and its mechanism. Chinese Traditional & Herbal Drugs. 2014;45:552–557. doi: 10.7501/j.issn.0253-2670.2014.04.019. [In Chinese] [DOI] [Google Scholar]
- Liang et al. (2015).Liang J, Peng X, Yin D, Li B, Wang D, Lin Y. Screening of a microbial consortium for highly simultaneous degradation of lignocellulose and chlorophenols. Bioresource Technology. 2015;190:381–387. doi: 10.1016/j.biortech.2015.04.105. [DOI] [PubMed] [Google Scholar]
- Lin, Huang & Zhang (2002).Lin J, Huang S, Zhang Q. Outer membrane proteins: key players for bacterial adaptation in host niches. Microbes and Infection. 2002;4(3):325–331. doi: 10.1016/S1286-4579(02)01545-9. [DOI] [PubMed] [Google Scholar]
- Lin et al. (2010).Lin WW, Wu XJ, Guo Q, Zhao MW, Wang RJ, Zhou F. Evaluation of air cleanliness and microbial loads in designated clean areas within a pleurotus eryngii commercial cultivation facility. Acta Edulis Fungi. 2010;17:34–39. [In Chinese] [Google Scholar]
- Lu & Qu (1984).Lu Y, Qu L. Comparisons of several kinds of substrate materials for P. ostreatus cultivation. Proceedings of Guangzhou edible fungi symposium; Guangzhou. 1984. pp. 108–112. [Google Scholar]
- Ma, Chen & Chen (2016).Ma HM, Chen DX, Chen YG. Alelopathic effect of dominant microflora on its mycelium of continuous cropping obstacles Ganoderma lucidum in field cultivation. Chinese Journal of Tropical Crops. 2016;37:372–375. doi: 10.3969/j.issn.1000-2561.2016.02.025. [In Chinese] [DOI] [Google Scholar]
- Mano & Morisaki (2008).Mano H, Morisaki H. Endophytic bacteria in the rice plant. Microbes Environment. 2008;23:109–117. doi: 10.1264/jsme2.23.109. [DOI] [PubMed] [Google Scholar]
- McArdle & Anderson (2001).McArdle BH, Anderson MJ. Fitting multivariate models to community data: a comment on distance-based redundancy analysis. Ecology. 2001;82(1):290–297. doi: 10.1890/0012-9658(2001)082[0290:FMMTCD]2.0.CO;2. [DOI] [Google Scholar]
- Mcgee et al. (2017).Mcgee CF, Byrne H, Irvine A, Wilson J. Diversity and dynamics of the DNA and cDNA-derived bacterial compost communities throughout the Agaricus bisporus mushroom cropping process. Annals of Microbiology. 2017;67(11):751–761. doi: 10.1007/s13213-017-1303-1. [DOI] [Google Scholar]
- Meier (2016).Meier R. From Malaise traps to phylogenetic diversity: developing rapid biodiversity assessment techniques based on NGS. 2016 international congress of entomology; Annapolis. 2016. [DOI] [Google Scholar]
- Miller et al. (1990).Miller FC, Harper ER, Macauley BJ, Gulliver A. Composting based on moderately thermophilic and aerobic conditions for the production of commercial mushroom growing compost. Australian Journal of Experimental Agriculture. 1990;30(2):287–296. doi: 10.1071/EA9900287. [DOI] [Google Scholar]
- Minerdi et al. (2001).Minerdi D, Fani R, Gallo R, Boarino A, Bonfante P. Nitrogen fixation genes in an endosymbiotic Burkholderia strain. Applied and Environmental Microbiology. 2001;67(2):725–732. doi: 10.1128/AEM.67.2.725-732.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno et al. (1995).Mizuno T, Wang G, Zhang J, Kawagishi H, Nishitoba T, Li JX. Reishi, Ganoderma lucidum and Ganoderma tsugae: bioactive substances and medicinal effects. Food Reviews International. 1995;11:151–166. doi: 10.1080/87559129509541025. [DOI] [Google Scholar]
- Ni et al. (2002).Ni X, Feng Z, Liang L, You C, Pan Y. Degradation and transformation of cotton seed hulls by Coprinus comatus. Microbiology. 2002;29:1–4. [Google Scholar]
- Ntougias et al. (2004).Ntougias S, Zervakis GI, Kavroulakis N, Ehaliotis C, Papadopoulou KK. Bacterial diversity in spent mushroom compost assessed by amplified rDNA restriction analysis and sequencing of cultivated isolates. Systematic and Applied Microbiology. 2004;27(6):746–754. doi: 10.1078/0723202042369857. [DOI] [PubMed] [Google Scholar]
- Oksanen et al. (2008).Oksanen J, Kindt R, Legendre P, O’Hara B, Simpson GL, Stevens MHH, Wagner H. The VEGAN Package: community ecology package. http://www.r-project.org. [04 September 2013];2008
- Ouhdouch, Barakate & Finance (2001).Ouhdouch Y, Barakate M, Finance C. Actinomycetes of Moroccan habitats: isolation and screening for antifungal activities. European Journal of Soil Biology. 2001;37(2):69–74. doi: 10.1016/S1164-5563(01)01069-X. [DOI] [Google Scholar]
- Ozcelik & Peksen (2007).Ozcelik E, Peksen A. Hazelnut husk as a substrate for the cultivation of shiitake mushroom (Lentinula edodes) Bioresource Technology. 2007;98(14):2652–2658. doi: 10.1016/j.biortech.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Park & Agnihotri (1969).Park JY, Agnihotri VP. Bacterial metabolites trigger sporophore formation in Agaricus bisporus. Abstract 984Nature. 1969;222(5197) doi: 10.1038/222984a0. [DOI] [PubMed] [Google Scholar]
- Patel & Jain (2015).Patel RK, Jain M. NGS QC toolkit: a platform for quality control of next-generation sequencing data. Encyclopedia of Metagenomics. 2015;7:1–5. doi: 10.1007/978-1-4899-7478-5_348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peksen & Yakupoglu (2009).Peksen A, Yakupoglu G. Tea waste as a supplement for the cultivation of Ganoderma lucidum. World Journal of Microbiology & Biotechnology. 2009;25:611–618. doi: 10.1007/s11274-008-9931-z. [DOI] [Google Scholar]
- Qiu et al. (2011).Qiu LY, Shen JW, Zhang JY, Zhu HX, Zhang Y. Study on the interaction between edible fungi and bacteria. 2011 annual conference of mycological society in China; Beijing. 2011. [In Chinese] [Google Scholar]
- R Core Team (2016).R Core Team . Vienna: 2016. [Google Scholar]
- Rahmad et al. (2014).Rahmad N, Al-Obaidi JR, Rashid NMN, Zean NB, Yusoff MHYM, Shaharuddin NS, Jamil NAM, Saleh NM. Comparative proteomic analysis of different developmental stages of the edible mushroom Termitomyces heimii. Biological Research. 2014;47(1):30–37. doi: 10.1186/0717-6287-47-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel-Castro, Danell & Pfeffer (2002).Rangel-Castro JI, Danell E, Pfeffer PE. A 13C-NMR study of exudation and storage of carbohydrates and amino acids in the ectomycorrhizal edible mushroom Cantharellus cibarius. Mycologia. 2002;94(2):190–199. doi: 10.1080/15572536.2003.11833224. [DOI] [PubMed] [Google Scholar]
- Sakamoto et al. (2016).Sakamoto S, Kikkawa N, Kohno T, Shimizu K, Tanaka H, Morimoto S. Immunochromatographic strip assay for detection of bioactive Ganoderma triterpenoid, ganoderic acid A in Ganoderma lingzhi. Fitoterapia. 2016;114:51–55. doi: 10.1016/j.fitote.2016.08.016. [DOI] [PubMed] [Google Scholar]
- Schwechheimer & Kuehn (2015).Schwechheimer C, Kuehn MJ. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nature Reviews Microbiology. 2015;13(10):605–619. doi: 10.1038/nrmicro3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segata et al. (2011).Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biology. 2011;12 doi: 10.1186/gb-2011-12-6-r60. Article R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh (2015).Singh JS. Microbes: the chief ecological engineers in reinstating equilibrium in degraded ecosystems. Agriculture Ecosystems & Environment. 2015;203:80–82. doi: 10.1016/j.agee.2015.01.026. [DOI] [Google Scholar]
- Slifkin & Pouchet (1975).Slifkin M, Pouchet G. Growth potential of cottonseed culture media for various clinically significant aerobic bacteria. Journal of Clinical Microbiology. 1975;1:495–499. doi: 10.1128/jcm.1.6.495-499.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohlenkamp & Geiger (2016).Sohlenkamp C, Geiger O. Bacterial membrane lipids: diversity in structures and pathways. FEMS Microbiology Reviews. 2016;40(1):133–159. doi: 10.1093/femsre/fuv008. [DOI] [PubMed] [Google Scholar]
- Spain, Krumholz & Elshahed (2009).Spain AM, Krumholz LR, Elshahed MS. Abundance, composition, diversity and novelty of soil Proteobacteria. Isme Journal. 2009;3:992–1000. doi: 10.1038/ismej.2009.43. [DOI] [PubMed] [Google Scholar]
- Srinivasan et al. (2012).Srinivasan S, Hoffman NG, Morgan MT, Matsen FA, Fiedler TL, Hall RW, Ross FJ, McCoy CO, Bumgarner R, Marrazzo JM, Fredricks DN. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLOS ONE. 2012;7(6):e37818. doi: 10.1371/journal.pone.0037818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stajic et al. (2002).Stajic M, Milenkovic I, Brceski I, Vukojevic J, Duletic LS. Mycelial growth of edible and medicinal oyster mushroom [Pleurotus ostreatus (Jacq.: Fr.) Kumm.] on selenium-enriched media. International Journal of Medicinal Mushrooms. 2002;4:4–7. doi: 10.1615/IntJMedMushr.v4.i3.70. [DOI] [Google Scholar]
- Stölzer & Grabbe (1991).Stölzer S, Grabbe K. Mechanisms of substrate selectivity in the cultivation of edible fungi. Mushroom Science. 1991;13:141–146. [Google Scholar]
- Su et al. (2017).Su P, Lou J, Brookes PC, Luo Y, He Y, Xu J. Taxon-specific responses of soil microbial communities to different soil priming effects induced by addition of plant residues and their biochars. Journal of Soils & Sediments. 2017;17:674–684. doi: 10.1007/s11368-015-1238-8. [DOI] [Google Scholar]
- Székely et al. (2009).Székely AJ, Sipos R, Berta B, Vajna B, Hajdú C, Márialigeti K. DGGE and T-RFLP analysis of bacterial succession during mushroom compost production and sequence-aided T-RFLP profile of mature compost. Microbial Ecology. 2009;57:522–533. doi: 10.1007/s00248-008-9424-5. [DOI] [PubMed] [Google Scholar]
- Tanaka et al. (2016).Tanaka M, Knowles W, Brown R, Hondow N, Arakaki A, Baldwin S, Staniland S, Matsunaga T. Biomagnetic recovery of selenium: bioaccumulating of selenium granules in magnetotactic bacteria. Applied & Environmental Microbiology. 2016;82:3886–3891. doi: 10.1128/AEM.00508-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, Sheard & Moyer (1967).Thomas RL, Sheard RW, Moyer JR. Comparison of conventional and automated procedures for nitrogen, phosphorus, and potassium analysis of plant material using a single digestion. Agronomy Journal. 1967;59:240–243. doi: 10.2134/agronj1967.00021962005900030010x. [DOI] [Google Scholar]
- Urayama (1967).Urayama T. Initiation of pinheads in Psilocybe panaeliformis caused by certain bacteria. Mushroom Sciences. 1967;6:141–156. [Google Scholar]
- Vajna et al. (2010).Vajna B, Nagy A, Sajben E, Manczinger L, Szijártó N, Kádár Z, Bordás D, Márialigeti K. Microbial community structure changes during oyster mushroom substrate preparation. Applied Microbiology & Biotechnology. 2010;86:367–375. doi: 10.1007/s00253-009-2371-3. [DOI] [PubMed] [Google Scholar]
- Velázquez-Cedeño et al. (2008).Velázquez-Cedeño M, Farnet AM, Mata G, Savoie JM. Role of Bacillus spp. n antagonism between Pleurotus ostreatus and Trichoderma harzianum in heat-treated wheat-straw substrates. Bioresource technology. 2008;99(15):6966–6973. doi: 10.1016/j.biortech.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Wang, Chen & Peng (2016).Wang ME, Chen WP, Peng C. Risk assessment of Cd polluted paddy soils in the industrial and township areas in Hunan, Southern China. Chemosphere. 2016;144:346–351. doi: 10.1016/j.chemosphere.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2002).Wang YY, Khoo KH, Chen ST, Lin CC, Wong CH, Lin CH. Studies on the immuno-modulating and antitumor activities of Ganoderma lucidum (Reishi) polysaccharides: functional and proteomic analyses of a fucose-containing glycoprotein fraction responsible for the activities. Bioorganic & Medicinal Chemistry. 2002;10:1057–1062. doi: 10.1016/S0968-0896(01)00377-7. [DOI] [PubMed] [Google Scholar]
- Xia et al. (2016).Xia F, Liu Y, Guo MY, Shen GR, Lin J, Zhou XW. Pyrosequencing analysis revealed complex endogenetic microorganism community from natural DongChong XiaCao and its microhabitat. BMC Microbiology. 2016;16:196. doi: 10.1186/s12866-016-0813-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing et al. (2001).Xing ZT, Zhou CY, Zhang JS, Tang QJ, Pan YJ. Assay of trace elements and heavy metals in different growth stages of Ganoderma lucidum. Journal of Chinese Medicinal Materials. 2001;24:469–470. doi: 10.3321/j.issn:1001-4454.2001.07.001. [In Chinese] [DOI] [PubMed] [Google Scholar]
- Yang, Chienyan & Chen (2003).Yang F, Chienyan H, Chen H. Use of stillage grain from a rice-spirit distillery in the solid state fermentation of Ganoderma lucidum. Process Biochemistry. 2003;39(1):21–26. doi: 10.1016/S0032-9592(02)00255-8. [DOI] [Google Scholar]
- Yang et al. (2011).Yang Y, Huang M, Liu HS, Liu HJ. The interrelation between temperature sensitivity and adaptability of soil respiration. Journal of Natural Resources. 2011;26(10):1811–1820. [Google Scholar]
- Zhang (1999).Zhang ZZ. Microflora analysis of Morchella rhizosphere and its root. Edible fungi of China. 1999;18(2):25–26. [Google Scholar]
- Zhang (2010).Zhang JL. Isolation and identification of mushroom endophytes and the preliminary study of metabolites. Dissertation, Fujian Agriculture and Forestry University. 2010 doi: 10.7666/d.y1748461. [in Chinese] [DOI] [Google Scholar]
- Zhang et al. (2014).Zhang X, Zhong YH, Yang SD, Zhang WX, Xu MQ, Ma AZ, Zhuang GQ, Chen GJ, Liu WF. Diversity and dynamics of the microbial community on decomposing wheat straw during mushroom compost production. Bioresource Technology. 2014;170:183–195. doi: 10.1016/j.biortech.2014.07.093. [DOI] [PubMed] [Google Scholar]
- Zhu et al. (2013).Zhu M, Du F, Zhang G, Wang H, Ng T. Purification a laccase exhibiting dye decolorizing ability from an edible mushroom Russula virescens. International Biodeterioration & Biodegradation. 2013;82:33–39. doi: 10.1016/j.ibiod.2013.02.010. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Photo by Xiaolin Li.
Photo by Xiaolin Li.
Photo by Xiaolin Li.
Photo by Xiaolin Li.
Different microbial metabolic functions were arranged vertically in accordance with their respective modules, in which the length of the graph revealed the abundance of bacterial genes related to the corresponding functions in the sample.
Data Availability Statement
The following information was supplied regarding data availability:
NCBI: accession numbers are SRR5801759–SRR5801768 and SRR5801783, SRR5801784 in BioProject ID PRJNA392763.