Abstract
Vascular Nox-derived ROS and ER stress have been implicated in hypertension. However relationships between these processes is unclear. We hypothesized that Nox isoforms localize in a sub-cellular compartment-specific manner, contributing to oxidative and ER stress, which influence the oxidative proteome and vascular function in hypertension. Nox compartmentalization (cell fractionation), O2- (lucigenin), H2O2 (amplex red), reversible protein oxidation (sulfenylation), irreversible protein oxidation (protein tyrosine phosphatase (PTP), peroxiredoxin oxidation) and ER stress (PERK, IRE1α, phosphorylation/oxidation) were studied in SHR VSMCs. VSMC proliferation was measured by FACS and vascular reactivity assessed in SHRSP arteries by myography. Noxs were downregulated by siRNA and pharmacologically. In SHR, Noxs were localized in specific sub-cellular regions: Nox1 in plasma-membrane and Nox4 in ER. In SHR, oxidative stress was associated with increased protein sulfenylation and hyperoxidation of PTPs and peroxiredoxins. Inhibition of Nox1 (NoxA1ds), Nox1/4 (GKT 137831) and ER stress (4-PBA/Tudca), normalized SHR vascular ROS generation. GKT137831 reduced IRE1α sulfenylation and XBP1 splicing in SHR. Increased VSMC proliferation in SHR was normalized by GKT137831,4-PBA and STF083010, (IRE1-XBP1 disruptor). Hypercontractility in SHRSP was attenuated by 4-PBA. We demonstrate that protein hyperoxidation in hypertension is associated with oxidative and ER stress through upregulation of plasmalemmal-Nox1 and ER-Nox4. The IRE1-XBP1 pathway of the ER stress response is regulated by Nox4/ROS and plays a role in the hyperproliferative VSMC phenotype in SHR. Our study highlights the importance of Nox sub-cellular compartmentalization and interplay between cytoplasmic ROS and ER stress response, which contribute to the VSMC oxidative proteome and vascular dysfunction in hypertension.
Keywords: Reactive oxygen species, vascular smooth muscle, compartmentalization
Introduction
NADPH oxidase (Nox)-derived reactive oxygen species (ROS) are important signaling molecules in vascular smooth muscle cells (VSMC) with pleiotropic actions participating in diverse processes such as cell growth, migration, inflammation, fibrosis and contraction1. Mechanisms regulating these distinct functions are unclear but may relate to specific Nox isoforms, sub-cellular compartmentalization of Nox/ROS, post-translational oxidative modification of signaling proteins and interaction with stress response processes, such as endoplasmic reticulum (ER) stress2, 3.
In hypertension, increased vascular Nox expression and activity are associated with oxidative stress and aberrant redox signaling leading to dysregulated endothelial cell and VSMC function and consequent vascular injury4, 5. Nox-derived ROS is a tightly controlled process involving multiple Nox isoforms (Nox 1-5 and Duox 1-2), of which Nox1, Nox2 and Nox4 are functionally present in the rodent vasculature6. Once generated, ROS influence signalling molecules through post-translational oxidative modification of proteins7. Oxidation can be reversible or irreversible, with the oxidation status influencing cellular functional responses. Major forms of reversible oxidation include modification of cysteine to sulfenic acid (sulfenylation, SOH), reaction with glutathione (glutathionylation, GSH), and formation of disulfide bonds among others8, 9. Reversible cysteine oxidation is key to redox signalling providing a mechanism of redox switch for protein function and cell function. In VSMCs, oxidation of protein tyrosine phosphatases (PTPs) is especially important because oxidation inactivates the enzyme, leading to increased phosphorylation of downstream kinases critically involved in the regulation of vascular function10. In pathological conditions, high concentrations of ROS can result in irreversible oxidation, such as protein carbonylation, (modification of amino acid side chains to carbonyl derivatives) and formation of sulfinic and sulfonic acid on cysteine residues (SO2H, SO3H), leading to protein damage, degradation and cell death11.
Oxidative stress can also influence the function of organelles such as the ER, a major site of protein synthesis, lipid biosynthesis and Ca2+ storage and signalling12. Hence alterations in the ER can have an impact on cell function and fate. The ER responds to accumulation of misfolded proteins by activating the unfolded protein response (UPR). This complex signalling network can either restore ER homeostasis and promote cell survival or apoptosis13. ROS are produced in the ER as byproducts of protein folding and certain ER stress conditions can increase ROS production in the ER3. ER stress has been implicated in experimental hypertension14, 15. However, the relationship between oxidative stress and ER stress and the interaction between these processes in vascular dysfunction in hypertension is unknown.
We hypothesize that compartmentalisation of Nox-derived ROS contributes to oxidative and ER stress through oxidation of proteins that influence vascular function in hypertension. To address this we examined in VSMCs the subcellular compartmentalization and ROS-generating function of Nox isoforms and investigated how oxidative stress impacts the oxidative proteome focusing on reversible and irreversible oxido-reductive modifications and explored the role of ER stress in these processes. Further studies investigating the potential functional significance of ROS/ER stress in isolated vessels were assessed by myography.
Methods
The authors declare that all supporting data are available within the article and its online supplementary files.
Isolation and culture of VSMCs
Primary VSMCs were isolated from mesenteric arteries from WKY and SHR by enzymatic digestion, as we described16.
Cell Fractionation
Plasma membrane proteins and nuclear/ER fraction were obtained using a plasma membrane isolation and nuclear extraction kit, respectively. ER and mitochondria were obtained by differential centrifugation.
ROS measurements
ROS levels were measured in VSMCs that had been pre-treated with NoxA1ds (Nox1 inhibitor), GKT137831 (Nox1/4 inhibitor) and 4-Phenylbutyric acid (4-PBA) (ER stress inhibitor) and stimulated with Ang II. Lucigenin-enhanced chemiluminescence was used to detect NADPH-dependent superoxide anion (O2-) production in VSMC16. Hydrogen peroxide (H2O2) levels were assessed by Amplex Red Hydrogen Peroxide/Peroxidase Assay Kit.
Immunoblotting
VSMC proteins were resolved by SDS-PAGE, transferred onto a nitrocellulose membrane and probed with primary antibodies. Secondary fluorescence-coupled antibodies were visualized by an infrared laser scanner. Immunoimages were quantified using the software Image Studio™ Lite.
Assessment of protein sulfenylation
To assess sulfenylation, we used two different probes that specifically bind sulfenic acid groups in proteins; BCN-E-BCN17 and biotin-tagged dimedone-based probe (DCP-Bio)18.
Affinity capture of sulfenylated proteins
VSMCs were labelled with DCP-Bio1 and submitted to the same procedures described for sulfenylation assessment. Affinity capture of sulfenylated proteins was performed using high capacity streptavidin beads.
Determination of irreversible PTP and Peroxiredoxin (Prx) oxidation
VSMCs were lysed and analysed by immunoblotting using specific antibodies, which recognize the hyperoxidized catalytic centre (-SO3H) on PTPs and Prx.
Proliferation assay
The fluorescein-based dye 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE) was used to assess VSMC proliferation in cells treated with NoxA1ds, GKT137831, 4-PBA and STF-083010 (disruptor of Ire1-XBP1 pathway). Flow cytometry analysis with Ex/Em 492/517 filters was performed using the FACS CANTO II system.
Vascular function assessed by wire myography
Segments of mesenteric arteries from WKY and SHRSP were mounted on a wire myograph. Contraction was assessed with KCl. Endothelial integrity was verified by relaxation induced by acetylcholine (Ach) in vessels pre-contracted with phenylephrine (PE). Endothelium-dependent relaxation was assessed by concentration-response curves to ACh and noradrenaline (NA). In some experiments, vessels were pre-treated with 4-PBA.
Statistical Analysis
All results are mean±SEM. For comparisons between two groups, Student’s t-test was used. For multiple comparisons one-way analysis of variance followed by Bonferroni’s post-test was conducted, as appropriate. p<0.05 was considered significant.
Results
Increased protein sulfenylation and irreversible oxidation in VSMCs from hypertensive rats
A major mechanism whereby ROS influence cell function is through post-translational oxidative modification of downstream protein targets. Of the numerous redox-sensitive processes, modification of cysteine residues within proteins are particularly important in the context of oxidative stress, such as in hypertension. Cysteine thiols can be oxidized initially to sulfenic acid (Cys-SOH). The resultant cysteine S-sulfenylation, can be further oxidized into reversible (disulfides, glutathionylated species) or irreversible (sulfinic (–SO2H) or sulfonic acid (–SO3H)) oxidative modifications, depending on the ROS bioavailability. We investigated whether increased ROS bioavailability in hypertension is associated with increased protein sulfenylation and irreversible cysteine oxidation in proteins.
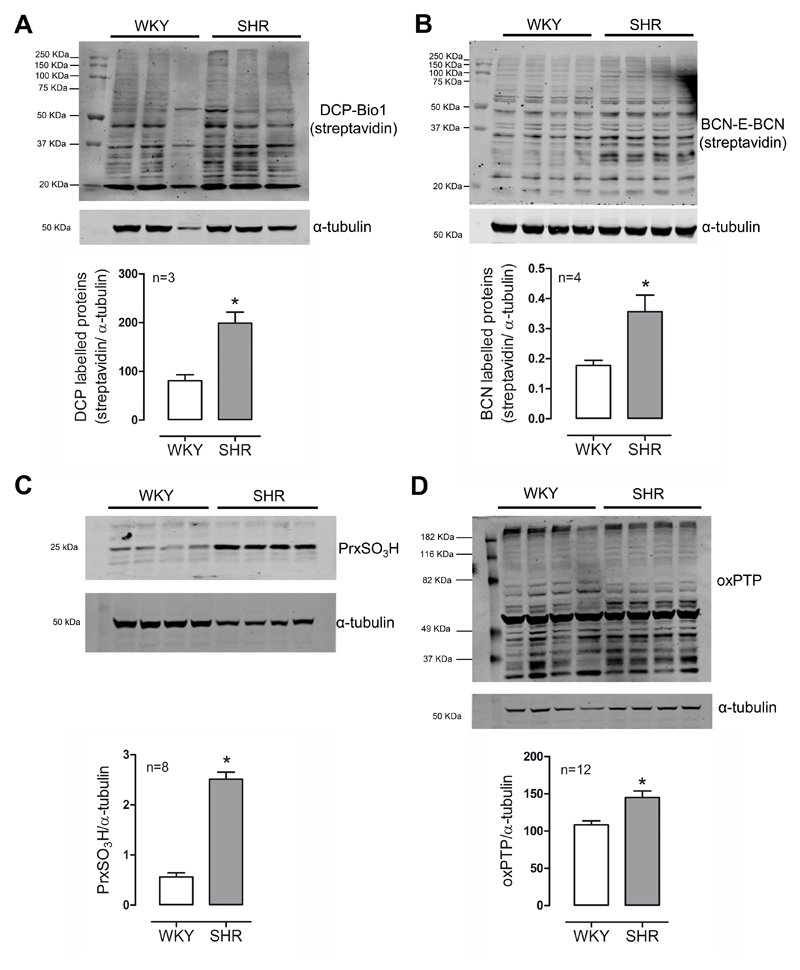
Using two different probes, a biotin-tagged dimedone-based probe (DCP-Bio) and a cyclooctyne probe (BCN-E-BCN), that binds specifically to sulfenylated proteins we showed an increase in protein sulfenylation in SHR cells (Figures 1A and 1B). Irreversible protein oxidation levels of Prx (Prx-SO3) and PTP (–SO2H and –SO3H), which translates into deactivation of these enzymes, was higher in VSMCs from hypertensive rats (Figures 1C and 1D). Such differences suggest that the increased ROS levels in SHR cells are favouring irreversible oxidative modifications.
Figure 1. Protein oxidation is increased in VSMCs from hypertensive rats (SHR).
Reversible cysteine oxidation (sulfenylation) was assessed by biotinylated probes DCP-Bio1 (A) and BCN-E-BCN (B). Irreversible protein oxidation was assessed by western blot of peroxiredoxin hyperoxidation (C) and oxidized PTPs (D). α-tubulin was used as loading control. Data represent mean±SE of 3-12 independent experiments. *p<0.05 SHR vs WKY.x
Nox1 and Nox4 are involved in ROS generation and irreversible protein oxidation in hypertension
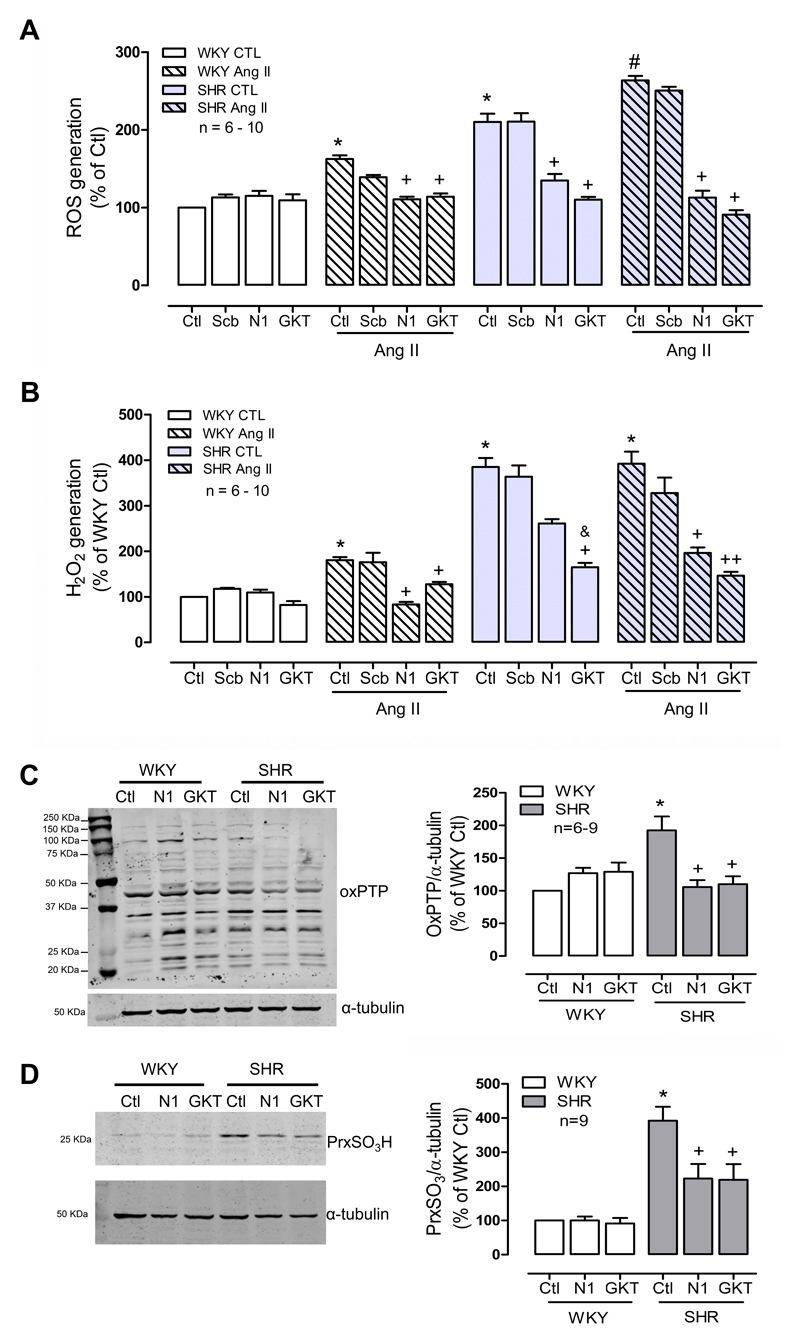
As NADPH oxidases are recognized as the major enzymatic source of ROS in vascular cells, we investigated the role of Nox isoforms in ROS generation and protein oxidation. Nox1, Nox2 and Nox4 are present in total cell homogenates in VSMCs from normotensive and hypertensive groups. Expression of Nox1 and Nox4 was greater in VSMCs from hypertensive versus normotensive rats (Supp Figure S1). Therefore, we investigated the role of Nox1 and Nox4 in ROS generation and oxidative modification of proteins. Both, the selective Nox1 inhibitor NoxA1ds, as well as the Nox1/4 inhibitor GKT137831, decreased basal O2- levels in the hypertensive group (Figure 2A). Basal H2O2 generation was significantly reduced by Nox1/4 inhibition in SHR cells. (Figure 2B). Basal levels were unaffected by Nox inhibitors in WKY. The scrambled peptide control for NoxA1ds had no effect on ROS generation. In addition, Nox1 and Nox4 downregulation with siRNA decreased basal ROS levels in SHR cells in both lucigenin and amplex red assay (Supp Figure S2). These findings suggest that in SHR, Nox1 and Nox4 are constitutively active in resting conditions and are responsible for increased basal ROS generation in VSMCs in hypertensive rats.
Figure 2. Nox1 and Nox4 are involved in ROS generation and protein oxidation in hypertension.
Superoxide anion and hydrogen peroxide were measured by lucigenin-derived chemiluminescence (A) and amplex red (B) in VSMCs stimulated with angiotensin II (Ang II, 100nmol/L) for 5 minutes in the presence of NoxA1ds (N1) (10µmol/L, Nox1 inhibitor), NoxA1ds scrambled control (Scb) or GKT137831 (GKT) (10µmol/L, Nox1/4 inhibitor). Irreversible protein oxidation was assessed by western blot of PTP oxidation (C) and peroxiredoxin hyperoxidation (D) in the presence of Nox inhibitors. Results are expressed as mean±SEM of 6-10 separate experiments.*p<0.05 vs WKY control (Ctl), +p<0.05 and ++p<0.01 vs respective Ctl, #p<0.05 vs SHR Ctl and &p<0.05 vs SHR N1.
Ang II increased O2- and H2O2 generation in WKY cells, an effect that was blocked by NoxA1ds (Figures 2A and B). In SHR cells, NoxA1ds and GKT137831 inhibited Ang II-induced O2- production (Figure 2A). H2O2 generation was not significantly altered by Ang II in SHR VSMCs (Figure2B). Together these data indicate that Ang II, a pro-hypertensive factor, increases ROS generation in WKY cells through Nox1-sensitive processes, similar to findings observed in SHR in basal conditions. Ang II also increased O2- generation in SHR VSMCs, an effect likely involving Nox1 and Nox4.
Next, we assessed the role of Nox1 and Nox4 in irreversible protein oxidation. NoxA1ds and GKT137831 significantly decreased oxidation of PTPs and Prx in SHR VSMCs (Figure 2C and D, respectively). Inhibition of both Nox1 and Nox4 with GKT137831 had no additional effect on irreversible oxidation levels.
Nox isoforms are expressed in an organelle-specific manner
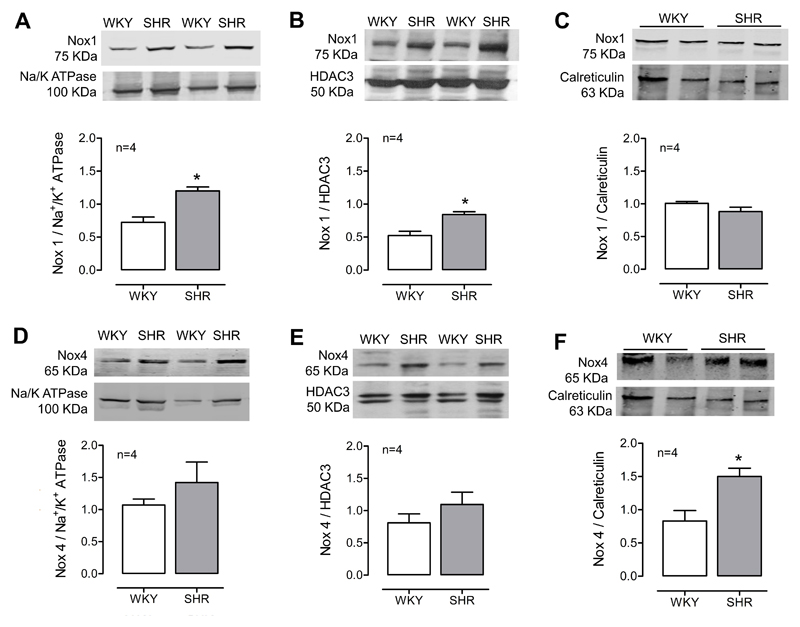
To better characterize redox-signaling in VSMCs and to evaluate whether Nox/ROS regulation is compartment-specific and differentially regulated in hypertension, we investigated the subcellular localization of Nox isoforms. Expression of Nox1, Nox2 and Nox4 was assessed by immunoblotting after cell fractionation (Figure 3). All Nox isoforms were detected in plasma membrane, nuclear/ER fraction and in isolated ER in both strains. Housekeeping proteins used for cell membrane was Na/K ATPase, for ER was calreticulin, and HDAC3 for the nuclear/ER fraction. Expression of Nox1 was significantly increased in plasma membrane and nuclear/ER fraction of SHR compared to WKY cells (Figures 3A and 3B). On the other hand, Nox4 levels were significantly increased only in the ER of VSMCs from SHR rats (Figure 3F). Nox2 levels were detected in all fraction, however no differences were found between WKY and SHR (Supp Figure S3). Noxs were not detected in isolated mitochondria. However, we observed a decrease in basal ROS levels in SHR cells in the presence of mitoTEMPO19, an antioxidant targeted to mitochondria (Supp Figure S4).
Figure 3. Subcellular localization of Nox1 and Nox4 in VSMCs from WKY and SHR rats.
Expression of Nox1 and Nox4 in isolated plasma membrane (A and D), nuclear/endoplasmic reticulum fraction (B and E) and isolated endoplasmic reticulum (C and F). Results are expressed as mean±SEM of 4 separate experiments and were normalized by Na/K ATPase (plasma membrane marker), HDAC3 (Histone deacetylase 3; nuclear marker) or Calreticulin (ER marker).*p<0.05 vs WKY.
These findings indicate that Noxs are expressed in subcellular compartments, especially ER and nucleus. This is a highly regulated process, because not all organelles in which ROS are generated possess Noxs, as indicated by the absence of Nox1, 2 and 4 in isolated mitochondria. In addition, Nox isoforms can affect different protein targets by different subcellular locations: Nox1 in the plasma membrane and nucleus and Nox4 in the ER. The ER is a site of protein synthesis and a platform of stress signaling pathways that may be particularly important in oxidative stress in hypertension and was further investigated in our studies.
ER stress plays a role in increased basal ROS levels in SHR
Based on our findings of increased Nox4 content in the ER in hypertension, we probed the potential interaction between Nox, ROS and ER and the possibility that Nox4-derived ROS in hypertension may influence ER function and ER stress. This is relevant because phosphorylation of the UPR mediators PERK (protein kinase RNA-like endoplasmic reticulum kinase) and IRE1α (inositol-requiring enzyme 1α), as well as expression of the chaperone BiP (immunoglobulin heavy-chain-binding protein) involved in the ER stress response was increased in SHR VSMCs compared with WKY cells (Supp Figure S5), indicating vascular ER stress in hypertension.
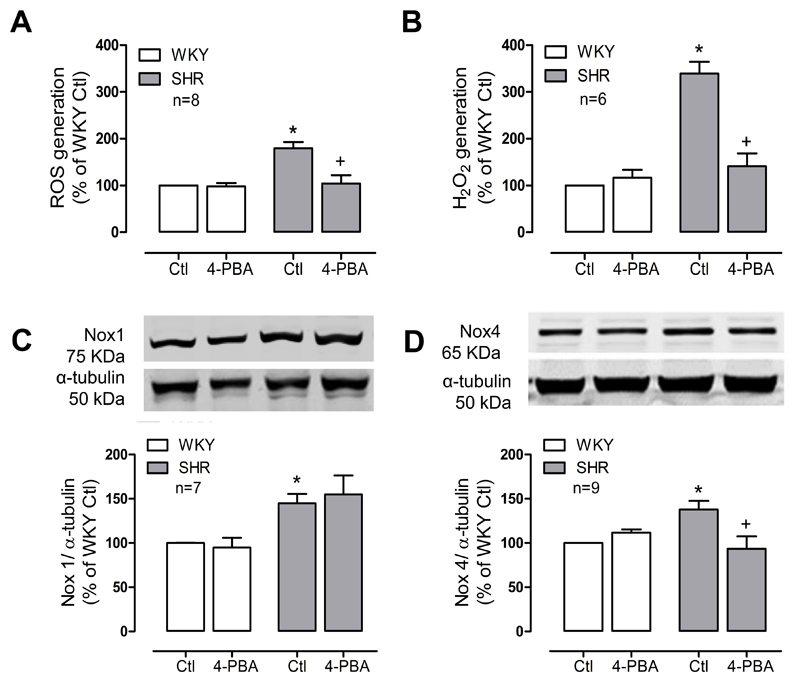
To investigate if ER stress is associated with vascular oxidative stress, cells were treated with the chemical chaperone 4-PBA known to decrease ER stress levels. As shown in figures 4A and 4B, basal levels of O2- and H2O2 were lowered by 4-PBA in SHR cells. In Ang II-treated cells, ROS levels were not significantly reduced by 4-PBA. In addition, tauroursodeoxycholic acid (Tudca), another ER stress inhibitor, decreased basal levels of ROS in SHR cells (Supp Figure S6).
Figure 4. ER stress is involved in ROS generation in SHR VSMCs.
ROS generation was measured by chemiluminescence (A) and amplex red (B) in the presence of the chemical chaperone 4-PBA (1mmol/L, 24h). Nox1 (C) and Nox4 (D) expression in the presence of 4-PBA was assessed by western blot. Protein quantification was normalized by α- tubulin. Results are expressed as mean±SEM of 6-9 separate experiments. *p<0.05 vs WKY Ctl, and +p<0.05 vs SHR Ctl.
Only expression of Nox4 was attenuated by 4-PBA treatment in SHR VSMCs, with no effect observed in Nox1 expression (Figure 4C and 4D). These data suggest that in SHR VSMCs, Nox4 is involved in ER stress induced ROS generation.
Nox-dependent ROS is involved in ER stress
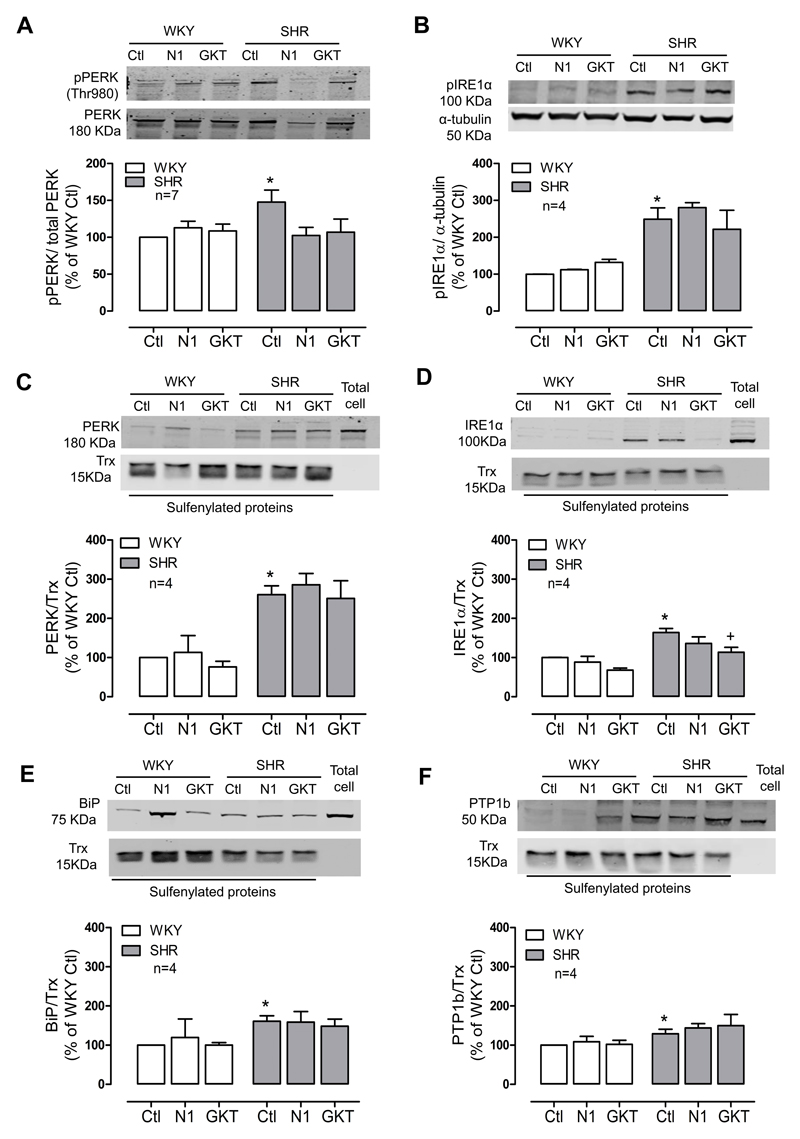
Following our observation of a relationship between ER stress and Nox4-derived ROS in SHR, we next investigated if Noxs are involved in activation of the ER stress response. Nox-derived-ROS through oxidation of cysteine thiols could inactivate PTPs altering phosphorylation of the UPR sensors IRE1α and PERK. To assess this, cells were treated with inhibitors of Nox1 and Nox4. Inhibition of Nox1 significantly decreased phosphorylation of PERK. A similar effect was observed with inhibition of both Nox1 and Nox4 (Figure 5A). However, phosphorylation of IRE1α was not influenced by any of the Nox inhibitors (Figure 5B). siRNA downregulation of Nox1 or Nox4 resulted in decreased levels of phosphorylated PERK and IRE1 α, suggesting involvement of Nox-derived ROS in the activation of the UPR (Supp Figure S7).
Figure 5. Differential activation of UPR activators by Nox1 and Nox4.
(A) Phosphorylation of PERK and (B) IRE1α in the presence of NoxA1ds (N1) (10µmol/L) and GKT137831 (GKT) (10µmol/L) was assessed by western blot. Sulfenylated PERK (C), IRE1 α (D), BiP (E) and PTP1B (F) were pulled down by affinity capture using the biotin-tagged dimedone-based probe (DCP-Bio) in cells treated with NoxA1ds or GKT137831. Protein quantification was normalized by total PERK, α- tubulin or biotinylated thioredoxin from E.coli (Trx) for the sulfenylation assay. Results are expressed as mean±SEM of 4-7 separate experiments. *p<0.05 vs WKY Ctl and +p<0.05 vs SHR Ctl.
The UPR sensors IRE1α and PERK have critical cysteine residues in their structures that could be potential ROS targets. Using the byotinylated probe DCP-Bio1 to trap sulfenylated proteins and performing affinity capture assay using streptavidin beads, we were able to pull down and identify sulfenylated proteins in cells from WKY and SHR rats (Figure 5C-F). Biotinylated thioredoxin (Trx) from E. coli was added to the samples before affinity capture and used as elution and loading control. Our results demonstrated that IRE1α and PERK were more oxidized in SHR VSMCs compared with WKY. The Nox1/4 inhibitor reduced oxidation (sulfenylation) of IRE1α, however no effect was observed in PERK oxidation (Figure 5C and 5D). Other potential ER targets are the chaperone BiP and the phosphatase PTP1B. Both proteins were also more oxidized in SHR cells, however Nox inhibition failed to decrease oxidation levels of BiP and PTP1B (Figure 5E and 5F). These results reveal different roles for Nox1 and Nox4 in the activation of ER stress response. Nox4 seems to be involved specifically on IRE1α oxidation, with no effects on phosphorylation of this protein. On the other hand, both Nox1 and Nox4 are involved in phosphorylation of PERK without affecting its oxidation.
Nox4 and ER stress are involved in increased VSMC proliferation in hypertension
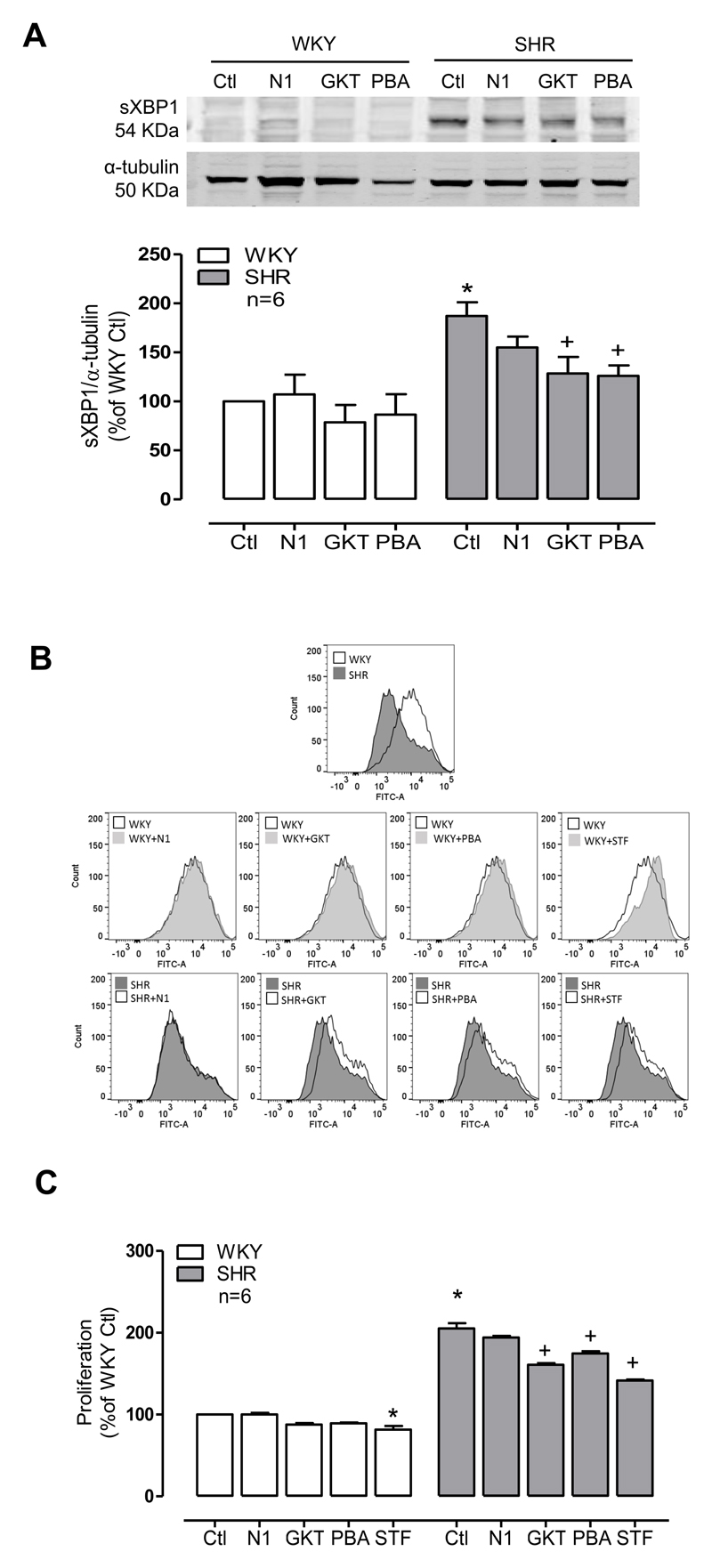
Next we investigated the effects of oxidation on IRE1downstream signalling and cellular function. One of the downstream targets of IRE1 is the transcription factor X-box binding protein 1 (XBP1). Through its RNase activity, activation of IRE1 promotes XBP1 alternative splicing and generation of its active form sXBP1 (spliced XBP1), which we measured in our experiemnatl models. Expression of sXBP1 was assessed by western blot in VSMCs treated with NoxA1ds, GKT137831 and 4-PBA for 24h. As shown in Figure 6A, sXBP1 expression is increased in SHR in basal conditions compared with cells from WKY rats. Nox1/4 and ER stress inhibitors significantly decreased sXBP1 expression in SHR, while Nox1 inhibitor had no effect.
Figure 6. ER stress is involved in increased proliferation in VSMCs from SHR.
(A) Spliced XBP1 expression (sXBP1) was detected by western blot in VSMCs from WKY and SHR in the presence of Nox inhibitors (NoxA1ds or GKT137831) and ER stress inhibitors (4-PBA). Protein quantification was normalized by α- tubulin. Proliferation was assessed using the CSFE assay (B and C) in the presence of Nox inhibitors, ER stress inhibitor and IRE1 inhibitor STF-083010 (STF). Results are expressed as mean±SEM of 5 separate experiments. *p<0.05 vs WKY Ctl and and +p<0.05 vs SHR Ctl.
The IRE1-sXBP1 arm of the ER stress response is involved in prosurvival signalling pathways. Therefore we investigated the functional role of Nox isoforms and ER stress, on VSMC proliferation. Expression of PCNA (Proliferating Cell Nuclear Antigen) as a molecular readout of cell proliferation was assessed by western blot. In basal conditions PCNA expression was increased in SHR rats, this phenomena was inhibited by Nox1/4 and ER stress inhibitors (Supp Figure S8). Furthermore, using the CSFE proliferation assay we demonstrated that SHR cells are more proliferative than cells from WKY rats (Figure 6B and 6C). Nox1/4 inhibitor (GKT137831) and the ER stress inhibitor (4-PBA) normalized SHR VSMC proliferation. In addition, disruption of the IRE1-sXBP1 pathway using the compound STF083010, also decreased proliferation in SHR VSMCs, supporting a role for this arm of the ER stress response in the hyperproliferative phenotype of VSMC from SHR rats. Additional experiments using Nox1 siRNA, Nox4 siRNA and the ER stress inhibitor, Tudca, showed a role for Nox1 in basal growth conditions, while Nox4 and ER stress were involved in SHR proliferation (Supp Fig S9).
ER stress is involved in vascular dysfunction in hypertensive rats
To further investigate the functional impact of redox signaling and ER stress, vascular reactivity was evaluated in isolated mesenteric arteries of WKY and SHRSP rats. SHRSP arteries are especially hyperreactive and accordingly were studied here as a robust model to examine vascular function. Arteries from hypertensive rats showed significantly increased contraction in response to noradrenaline and decreased vascular relaxation to acetylcholine compared to normotensive rats (Supp Figure S10). Treatment with 4-PBA significantly reduced vascular contraction and improved vascular relaxation in the SHRSP group, suggesting a role for redox-regulated ER stress in vascular function.
Discussion
While extensive evidence indicates that Nox-derived ROS generation and vascular oxidative stress are important elements in the pathophysiology of hypertension1, 2, 4, 5, mechanisms linking these phenomena still remain elusive. Here we examined the role of Nox isoforms and ER stress response in these processes and demonstrate that in VSMCs from hypertensive rats: i) cysteine oxidation in proteins is increased, ii) Nox1 and Nox4, which localize primarily in the plasma membrane and ER respectively, are responsible for increased basal ROS generation; iii) Nox1 is associated with irreversible protein oxidation and phosphorylation of the UPR sensor PERK, whereas Nox4 is involved in a feedforward relationship with ER stress response and oxidation of IRE1α, and iv) Nox4-induced hyperoxidation and ER stress promote vascular hyperproliferation hyperreactivity. Our findings identify interplay between oxidative stress and ER stress response as a novel molecular mechanism underlying protein hyperoxidation and vascular dysfunction in hypertension. This process involves differential upstream modulation of ER stress proteins, with PERK being Nox1-regulated and IRE1α Nox4-regulated. Such highly controlled systems may relate, at least in part, to the distinct subcellular compartmentalization of Nox isoforms in VSMCs in hypertension (Supp Figure 11).
Oxidative modifications of cysteine residues within proteins are key components of redox signaling to modulate protein function. The first step in Cys oxidation is formation of sulfenic acid (Cys-SOH), or protein sulfenylation. Due to the unstable nature of Cys-SOH we used a direct approach to trap sulfenic acid in proteins. Dimedone-based probes, such as DCP-Bio1, have been successfuly used to detect protein sulfenylation in various biological samples18, 20, 21. However, a limitation of dimedone-based probes are poor cell permeability, slow rates of reaction and may detect sulfenylamides in addition to sulfenic acid22, 23. To overcome these limitations we used a cyclooctyne probe (BCN-E-BCN), which is cell membrane permeable, that reacts with sulfenic acid with higher constant rates than other sulfenylation probes17. Using both probes, we demonstrated that general protein sulfenylation was increased in hypertension, suggesting an active oxidation process in hypertensive VSMCs.
If exposed to high levels of ROS, further oxidation of cysteine S-sulfenylation can occur leading to formation of sulfinic (–SO2H) or sulfonic acid (–SO3H), irreversible types of oxidation. Of the protein groups that are particularly susceptible to oxidation are PTPs and Prxs. PTPs are important modulators of growth factor- induced signalling10. Irreversible PTP oxidation can result in sustained kinase activity, enhancing growth factor signalling. Our findings showing increased irreversible oxidation of PTPs in VSMCs from hypertensive rats are in line with our previous studies that Ang II –induced oxidation of the phosphatase SHP-2 is increased in hypertension and is associated with enhanced Ang II-dependent AKT signalling24.
Prx, a thiol-dependent peroxide-eliminating enzyme, was also hyperoxidised in SHR. Under physiological conditions, Prx catalyzes the reduction of H2O2 and acts as an anti-oxidant, through reversible Cys oxidation. However in pathological conditions associated with oxidative stress, hyperoxidation of Prxs results in a switch to a redox sensor and chaperone molecule involved in cell signalling25. Prx2 knock-out mice showed higher levels of hydrogen peroxide, increased activation of PDGFR and PDGF-induced cell proliferation and migration26. This is of pathophysiological significance because irreversible protein oxidation causes aberrant cell signalling. formation of protein aggregates, disruption of proteolytic pathways, deregulated cell homeostasis and impaired function11, processes that likely contribute to vascular injury in hypertension.
Noxs, as major enzymatic sources of ROS in the vasculature, play an important role in regulating oxidation of downstream protein targets as we demonstrated with Nox1/4 inhibitors, which normalized ROS production and protein oxidation. Nox-derived ROS production is tightly controlled, and regulatory mechanisms may involve isoform specificity and compartmentalization27–29. We demonstrated that in VSMCs from hypertensive rats and in VSMCs from WKY stimulated with Ang II, a pro-hypertensive peptide, generation of O2- and H2O2 was increased, processes associated with upregulation of Nox1 and Nox4 in a compartment-specific manner. In SHR VSMCs expression of Nox1 was increased in the plasma membrane and nuclear/ER fractions while expression of Nox4 was only increased in the ER fraction. These findings suggest specific roles for different Nox isoforms in hypertension, and highlight a potentially important role for the ER.
Given the observed plasmalemmal localization of Nox1, we speculated that Nox1-derived ROS is involved mainly in protein oxidation in the cell membrane and cytosol, rather than the ER. In support of this, we demonstrated a role for Nox1 in oxidation of PTPs and Prxs but not in ER-resident proteins. Although PERK oxidation was not influenced by Nox1, post translation phosphorylation of PERK and IRE1α was Nox1-ROS-sensitive. The mechanisms involved could be related to altered phosphorylation and dephosphorylation of upstream kinases and phosphatases or an indirect effect of oxidative stress. These changes may reflect a protective mechanism of the cell, because phosphorylation of PERK has been suggested to be an alternative pathway for activation of the antioxidant Nrf230. However, in VSMC from hypertensive rats this mechanism may be dysregulated, as we previously demonstrated31.
Nox4 is constitutively activated and produces primarily H2O2. Unlike previous studies demonstrating an association between Nox4 and mitochondria32, we did not find localization of Nox4 in mitochondria. However, Nox4 was closely associated with the ER, with increased expression in SHR VSMCs. The functional significance of Nox4-ER may highlight cross-talk between oxidative stress and ER stress as previously suggested33, 34. ER stress, which is redox-sensitive, is triggered by altered ER function and activation of the UPR, leading to abnormal cell growth, apoptosis and inflammation13. ER stress also promotes oxidative stress, suggesting that ROS production is both upstream and downstream of UPR targets. Our findings support this feed-forward system, because ER stress inhibition with 2 different agents, 4-PBA and Tudca, reduced H2O2 production, while Nox4 inhibition and silencing in turn attenuated activity of UPR signalling molecules. Activators of ER stress 7-ketocholesterol35 and tunicamycin36 have been shown to stimulate Nox4 activity.
The functional significance of Nox4-ER interaction awaits clarification, but Nox4 association with ER stress induces activation of Ras/ERK signalling promoting autophagy and cell survival36. On the other hand, in cardiac ischemia-reperfusion injury and acute kidney injury, Nox4 inhibited the phosphatase PP1 resulting in increased phosphorylation of eIF2a and enhanced cell survival37. In our study, we showed that Nox4 specifically oxidizes the ER membrane-associated sensor IRE1α but no other ER resident proteins such as PERK, BiP or PTP1B, suggesting that Nox-induced oxidation of ER stress proteins is Nox isoform-specific and highly regulated.
Oxidation of proteins can have different outcomes depending on the state of protein oxidation. For example Cys oxidation of PTPs leads to its inactivation, while sulfenylation of Cys residues in EGFR enhance its kinase activity38. Many proteins undergo both phosphorylation and oxidation, with some studies suggesting that the oxidative modification primes the protein for activation upon phosphorylation21. We demonstrated that PERK is both phosphorylated and sulfenylated in VSMCs from hypertensive rats, likely influencing the activation of this ER stress-related kinase.
IRE1 is composed of an endoribonuclease and a kinase domain. Upon activation of the endoribonuclease domain, IRE1 cleaves XBP1 resulting in the spliced active transcription factor sXBP1 involved in ER homeostasis and cell survival. IRE1 kinase domain is involved in activation of ASK1-JNK pathway resulting in apoptosis39. Sulfenylation of IRE1 in yeast has been reported to block activation of the classic UPR signalling pathway and initiates the p38/Nrf2 antioxidant response promoting cell survival and growth40. Therefore, we investigated if IRE1 oxidation could favour activation of XBP1 and cell survival in SHR cells. We demonstrated that in SHR sXBP1 expression is increased and this effect is reversed by GKT137831 and 4-PBA, but not by NoxA1ds, suggesting a role for Nox4 in modulation of IRE1 signalling. Furthermore SHR proliferation was reduced by GKT137831, 4-PBA and STF 083010, a disruptor of IRE1-XBP1 pathway, supporting a role for IRE1-XBP1 in the hyperproliferative phenotype in SHR cells. Altogether, Nox4-induced oxidation of IRE1α may be a counterregulatory mechanism against oxidative stress. In addition BiP sulfenylation is involved in ER protection in oxidative conditions, since oxidized BiP inhibits its ATPase activity, while enhancing the binding to unfolded proteins, increasing cell survival and proliferation41. Therefore, increased oxidative stress and consequent oxidation of BiP and IRE1 could result in dysregulation of these mechanisms contributing to the hyperproliferative phenotype we observed in SHR VSMCs.
Our studies also demonstrate an important role for redox-regulated ER stress response in the control of vascular function. Reducing ER stress with the chemical chaperone 4-PBA attenuated hypercontractility and improved vasodilatory responses in vessels from hypertensive rats. Supporting our findings, acute induction of ER stress impaired vasorelaxation in isolated mesenteric arteries in a Nox-dependent manner15. In addition, 4-PBA protected against renal injury in hypertension and diabetes and had beneficial effects in experimental models of pulmonary hypertension42–44. Taken together there seems to be important interplay between oxidative and ER stress, that influence vascular function in hypertension.
Perspectives
We demonstrate that in hypertension, vascular redox signalling is related to Nox isoform specificity, distinct subcellular localization of Nox1 and Nox4 and differential post-translational oxidative modification of proteins. Increased ROS generation in hypertension was associated with an altered redox profile characterized by protein hyperoxidation, primarily due to increased irreversible oxidation of proteins, including PTPs, Prx and ER stress-related proteins PERK and IRE1. Our data identify novel Nox/ROS-regulated mechanisms involving the IRE1-XBP1 pathway of the ER stress response in the VSMC proliferative phenotype in SHR, and highlight the important interplay between oxidative stress and redox-regulated ER response in vascular dysfunction in hypertension These findings advance the understanding of vascular redox biology in hypertension.
Supplementary Material
Novelty and Significance.
What is New?
Nox isoforms are upregulated in a compartment-specific manner in SHR VSMCs
Plasma membrane-associated Nox1 and ER-associated Nox4 may contribute to differential vascular redox events in hypertension
Oxidative stress is associated with protein hyperoxidation and ER stress in hypertension
What is relevant?
The vascular oxidative proteome in hypertension is characterised by increased reversible and irreversible protein oxidation.
Complex interplay between oxidative stress and the ER stress response control VSMC proliferation and vascular dysfunction in hypertension
Summary
We provide insights into novel molecular pathways linking Nox compartmentalization, post-translational oxidative modification of proteins and ER stress, that play a role in the vascular phenotype in hypertension.
Acknowledgements
The authors thank Alan Scott and Carol Jenkins for their technical support.
Sources of Funding: This work was funded by grants from the British Heart Foundation (BHF) (RG/13/7/30099, RE/13/5/30177) and the MRC (MC-PC-15076). RMT is supported through a BHF Chair award (CH/4/29762). LLC was supported by the Science without borders Program (Proc 1815-14-8) and RDNOS was recipient of a PNPD fellowship (Proc 88881.1321188/2016-01), both from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior), Brazil.
Non-standard abbreviations
- 4-PBA
4-Phenylbutyric acid
- Ang II
angiotensin II
- BiP
immunoglobulin heavy-chain-binding protein
- CHOP
CCAAT-enhancer-binding protein homologous protein
- Cys
cysteine
- DiGE
differential gel electrophoresis
- DTT
dithiothreitol
- eIF2a
eukaryotic initiation factor 2a
- ER
endoplasmic reticulum
- Grp78
glucose-regulated protein 78
- H2O2
hydrogen peroxide
- HDAC3
histone deacetylase 3
- IRE1α
inositol-requiring enzyme 1
- NEM
N-ethyl-maleimide
- Nox
NADPH oxidases
- Nrf2
nuclear factor erythroid 2-related factor 2
- O2-
superoxide
- PCNA
Proliferating Cell Nuclear Antigen
- PERK
protein kinase RNA-like endoplasmic reticulum kinase
- PP1
protein phosphatase 1
- Prx
peroxiredoxin
- PTP
protein tyrosine phosphatase
- PTP1B
protein tyrosine phosphatase 1B
- ROS
reactive oxygen species
- SHP-2
Src-homology 2 domain (SH2)-containing PTP
- SHR
spontaneously hypertensive rat
- SHRSP
stroke-prone spontaneously hypertensive rat
- UPR
unfolded protein response
- VSMC
vascular smooth muscle cells
- WKY
wistar-kyoto rat
Footnotes
Conflicts.
There are no conflicts to declare
References
- 1.Brown DI, Griendling KK. Regulation of signal transduction by reactive oxygen species in the cardiovascular system. Circ Res. 2015;116:531–549. doi: 10.1161/CIRCRESAHA.116.303584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montezano AC, Tsiropoulou S, Dulak-Lis M, et al. Redox signaling, nox5 and vascular remodeling in hypertension. Curr Opin Nephrol Hypertens. 2015;24:425–433. doi: 10.1097/MNH.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laurindo FR, Araujo TL, Abrahao TB. Nox nadph oxidases and the endoplasmic reticulum. Antioxidants & Redox Signaling. 2014;20:2755–2775. doi: 10.1089/ars.2013.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee MY, Griendling KK. Redox signaling, vascular function, and hypertension. Antioxid Redox Signal. 2008;10:1045–1059. doi: 10.1089/ars.2007.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montezano AC, Touyz RM. Oxidative stress, noxs, and hypertension: Experimental evidence and clinical controversies. Ann Med. 2012;44(Suppl 1):S2–16. doi: 10.3109/07853890.2011.653393. [DOI] [PubMed] [Google Scholar]
- 6.Bedard K, Krause KH. The nox family of ros-generating nadph oxidases: Physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 7.Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic Biol Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Poole LB, Nelson KJ. Discovering mechanisms of signaling-mediated cysteine oxidation. Curr Opin Chem Biol. 2008;12:18–24. doi: 10.1016/j.cbpa.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ostman A, Frijhoff J, Sandin A, Bohmer FD. Regulation of protein tyrosine phosphatases by reversible oxidation. J Biochem. 2011;150:345–356. doi: 10.1093/jb/mvr104. [DOI] [PubMed] [Google Scholar]
- 10.Kappert K, Sparwel J, Sandin A, et al. Antioxidants relieve phosphatase inhibition and reduce pdgf signaling in cultured vsmcs and in restenosis. Arterioscler Thromb Vasc Biol. 2006;26:2644–2651. doi: 10.1161/01.ATV.0000246777.30819.85. [DOI] [PubMed] [Google Scholar]
- 11.Niforou K, Cheimonidou C, Trougakos IP. Molecular chaperones and proteostasis regulation during redox imbalance. Redox Biol. 2014;2:323–332. doi: 10.1016/j.redox.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong J, Kim K, Kim JH, Park Y. The role of endoplasmic reticulum stress in cardiovascular disease and exercise. Int J Vasc Med. 2017;2017:2049217. doi: 10.1155/2017/2049217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bohnert KR, McMillan JD, Kumar A. Emerging roles of er stress and unfolded protein response pathways in skeletal muscle health and disease. Journal of Cellular Physiology. 2018;233:67–78. doi: 10.1002/jcp.25852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spitler KM, Webb RC. Endoplasmic reticulum stress contributes to aortic stiffening via proapoptotic and fibrotic signaling mechanisms. Hypertension. 2014;63:e40–45. doi: 10.1161/HYPERTENSIONAHA.113.02558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kassan M, Galan M, Partyka M, et al. Endoplasmic reticulum stress is involved in cardiac damage and vascular endothelial dysfunction in hypertensive mice. Arterioscler Thromb Vasc Biol. 2012;32:1652–1661. doi: 10.1161/ATVBAHA.112.249318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Callera GE, Touyz RM, Tostes RC, et al. Aldosterone activates vascular p38map kinase and nadph oxidase via c-src. Hypertension. 2005;45:773–779. doi: 10.1161/01.HYP.0000154365.30593.d3. [DOI] [PubMed] [Google Scholar]
- 17.McGarry DJ, Shchepinova MM, Lilla S, Hartley RC, Olson MF. A cell-permeable biscyclooctyne as a novel probe for the identification of protein sulfenic acids. ACS Chem Biol. 2016;11:3300–3304. doi: 10.1021/acschembio.6b00742. [DOI] [PubMed] [Google Scholar]
- 18.Nelson KJ, Klomsiri C, Codreanu SG, et al. Use of dimedone-based chemical probes for sulfenic acid detection methods to visualize and identify labeled proteins. Methods Enzymol. 2010;473:95–115. doi: 10.1016/S0076-6879(10)73004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dikalova AE, Bikineyeva AT, Budzyn K, et al. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res. 2010;107:106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins JA, Wood ST, Nelson KJ, et al. Oxidative stress promotes peroxiredoxin hyperoxidation and attenuates pro-survival signaling in aging chondrocytes. J Biol Chem. 2016;291:6641–6654. doi: 10.1074/jbc.M115.693523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keyes JD, Parsonage D, Yammani RD, et al. Endogenous, regulatory cysteine sulfenylation of erk kinases in response to proliferative signals. Free Radic Biol Med. 2017;112:534–543. doi: 10.1016/j.freeradbiomed.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furdui CM, Poole LB. Chemical approaches to detect and analyze protein sulfenic acids. Mass Spectrom Rev. 2014;33:126–146. doi: 10.1002/mas.21384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang J, Gupta V, Tallman KA, et al. Global, in situ, site-specific analysis of protein s-sulfenylation. Nat Protoc. 2015;10:1022–1037. doi: 10.1038/nprot.2015.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tabet F, Schiffrin EL, Callera GE, et al. Redox-sensitive signaling by angiotensin ii involves oxidative inactivation and blunted phosphorylation of protein tyrosine phosphatase shp-2 in vascular smooth muscle cells from shr. Circ Res. 2008;103:149–158. doi: 10.1161/CIRCRESAHA.108.178608. [DOI] [PubMed] [Google Scholar]
- 25.Veal EA, Underwood ZE, Tomalin LE, Morgan BA, Pillay CS. Hyperoxidation of peroxiredoxins: Gain or loss of function? Antioxid Redox Signal. 2018;28:574–590. doi: 10.1089/ars.2017.7214. [DOI] [PubMed] [Google Scholar]
- 26.Choi MH, Lee IK, Kim GW, et al. Regulation of pdgf signalling and vascular remodelling by peroxiredoxin ii. Nature. 2005;435:347–353. doi: 10.1038/nature03587. [DOI] [PubMed] [Google Scholar]
- 27.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of nox1 and nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 28.Van Buul JD, Fernandez-Borja M, Anthony EC, Hordijk PL. Expression and localization of nox2 and nox4 in primary human endothelial cells. Antioxid Redox Signal. 2005;7:308–317. doi: 10.1089/ars.2005.7.308. [DOI] [PubMed] [Google Scholar]
- 29.Griendling KK, Touyz RM, Zweier JL, et al. Measurement of reactive oxygen species, reactive nitrogen species, and redox-dependent signaling in the cardiovascular system: A scientific statement from the american heart association. Circ Res. 2016;119:e39–75. doi: 10.1161/RES.0000000000000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cullinan SB, Zhang D, Hannink M, et al. Nrf2 is a direct perk substrate and effector of perk-dependent cell survival. Mol Cell Biol. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alves-Lopes R, Neves KB, Montezano AC, et al. Internal pudental artery dysfunction in diabetes mellitus is mediated by nox1-derived ros-, nrf2-, and rho kinase-dependent mechanisms. Hypertension. 2016;68:1056–1064. doi: 10.1161/HYPERTENSIONAHA.116.07518. [DOI] [PubMed] [Google Scholar]
- 32.Ago T, Kuroda J, Pain J, et al. Upregulation of nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res. 2010;106:1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eletto D, Chevet E, Argon Y, Appenzeller-Herzog C. Redox controls upr to control redox. J Cell Sci. 2014;127:3649–3658. doi: 10.1242/jcs.153643. [DOI] [PubMed] [Google Scholar]
- 34.Santos CX, Nabeebaccus AA, Shah AM, et al. Endoplasmic reticulum stress and nox-mediated reactive oxygen species signaling in the peripheral vasculature: Potential role in hypertension. Antioxid Redox Signal. 2014;20:121–134. doi: 10.1089/ars.2013.5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pedruzzi E, Guichard C, Ollivier V, et al. Nad(p)h oxidase nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol Cell Biol. 2004;24:10703–10717. doi: 10.1128/MCB.24.24.10703-10717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu RF, Ma Z, Liu Z, Terada LS. Nox4-derived h2o2 mediates endoplasmic reticulum signaling through local ras activation. Mol Cell Biol. 2010;30:3553–3568. doi: 10.1128/MCB.01445-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santos CX, Hafstad AD, Beretta M, et al. Targeted redox inhibition of protein phosphatase 1 by nox4 regulates eif2alpha-mediated stress signaling. EMBO J. 2016;35:319–334. doi: 10.15252/embj.201592394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paulsen CE, Truong TH, Garcia FJ, et al. Peroxide-dependent sulfenylation of the egfr catalytic site enhances kinase activity. Nat Chem Biol. 2011;8:57–64. doi: 10.1038/nchembio.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao SS, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal. 2014;21:396–413. doi: 10.1089/ars.2014.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hourihan JM, Moronetti Mazzeo LE, Fernandez-Cardenas LP, Blackwell TK. Cysteine sulfenylation directs ire-1 to activate the skn-1/nrf2 antioxidant response. Mol Cell. 2016;63:553–566. doi: 10.1016/j.molcel.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J, Pareja KA, Kaiser CA, Sevier CS. Redox signaling via the molecular chaperone bip protects cells against endoplasmic reticulum-derived oxidative stress. Elife. 2014;3:e03496. doi: 10.7554/eLife.03496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohammed-Ali Z, Lu C, Marway MK, et al. Endoplasmic reticulum stress inhibition attenuates hypertensive chronic kidney disease through reduction in proteinuria. Sci Rep. 2017;7:41572. doi: 10.1038/srep41572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carlisle RE, Werner KE, Yum V, et al. Endoplasmic reticulum stress inhibition reduces hypertension through the preservation of resistance blood vessel structure and function. J Hypertens. 2016;34:1556–1569. doi: 10.1097/HJH.0000000000000943. [DOI] [PubMed] [Google Scholar]
- 44.Wu Y, Adi D, Long M, et al. 4-phenylbutyric acid induces protection against pulmonary arterial hypertension in rats. PLoS One. 2016;11:e0157538. doi: 10.1371/journal.pone.0157538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.