Abstract
To determine whether baseline clinical characteristics and the duration and intensity of ambulation during our step-monitored home-based exercise program were predictive of changes in ambulatory outcomes at completion of the program in symptomatic patients with peripheral artery disease (PAD). Twenty-two men (ankle-brachial index = 0.71 ± 0.19) and 24 women (ankle-brachial index = 0.66 ± 0.23) completed the home exercise program, consisting of intermittent walking to mild-to-moderate claudication pain for three months. Ambulatory outcome measures were peak walking time (PWT) and claudication onset time (COT) during a treadmill test, and distance recorded during a 6-minute walk test (6MWD). Men experienced significant increases (p<0.01) in COT, PWT, and 6MWD following the home exercise program, and women had significant increases in 6MWD (p<0.01) and PWT (p<0.05). In women, average exercise cadence during the home exercise sessions was the only predictor that entered the model for the change in COT (p=0.082), and was the first predictor in the model for the change in PWT (p=0.029) and in 6MWD (p=0.006). In men, ankle-brachial index (ABI) was the only predictor that entered the model for the change in 6MWD (p=0.002) and ABI was a predictor along with metabolic syndrome in the model for change in COT (p=0.003). No variables entered the model for change in PWT. Faster ambulatory cadence during the step-monitored home-based exercise program may predict greater improvements in ambulatory function in women, whereas having less severe PAD and comorbid burden at baseline may predict greater improvements in ambulatory function in men.
Keywords: Claudication, Mobility, Peripheral Artery Disease, Women, 6-Minute Walk
INTRODUCTION
Peripheral artery disease (PAD) is a significant medical concern because it is a highly prevalent,1–3 costly,4–7 and deadly condition.8, 9 The global prevalence of PAD increased by 23.5% from 2000 to 2010,1 resulting in a prevalence of 202 million people worldwide, affecting 15–20% of individuals over 70 years of age.1 PAD is associated with elevated cardiovascular risk, as the combined event rate of myocardial infarction, stroke, revascularization, and vascular death is 6% per year.2 Furthermore, the annual cost paid by Medicare related to PAD is $3.9 billion in the United States alone,4 and this cost is expected to increase dramatically in the future. Development of leg symptoms further complicates the clinical course of PAD, resulting in ambulatory dysfunction,10–12
Supervised exercise therapy is highly efficacious in treating symptomatic PAD.13 Recently, we adopted a new model of exercise therapy for patients with PAD by exporting the exercise program to the home and community environment, while rigorously quantifying the amount of ambulation achieved during the training sessions.14 Our step-monitored home-based exercise program has low attrition, high adherence, and is efficacious for improving exercise and vascular outcome measures,14 such as claudication onset time (COT), peak walking time (PWT), 6-minute walk distance (6MWD), community-based daily ambulatory activity, vascular function, inflammation, and calf muscle hemoglobin oxygen saturation.14, 15
Because a home exercise program is less standardized than a center-based, supervised exercise program, it is important to determine whether more responsive patients can be identified at baseline prior to enrollment, and whether the amount of exercise completed is associated with efficacy. These issues are likely to be sex-specific, given our previous findings that women have greater impairment in baseline ambulation16, 17 vascular function,18, 19 and inflammation,20 and that women, particularly those with diabetes, have less increase in COT and PWT following exercise training.21
To address these issues, we performed a follow-up analysis to our recent prospective, randomized controlled exercise trial in symptomatic patients with PAD.15 Our specific aim was to determine whether baseline clinical characteristics and the duration and intensity of ambulation during our step-monitored home-based exercise program were predictive of the changes in ambulatory outcome measures of COT, PWT, and 6MWD at completion of the program in symptomatic patients with PAD.
METHODS
Patients
Approval and Informed Consent
The institutional review board at the University of Oklahoma Health Sciences Center (HSC), and the Research and Development committee at the Oklahoma City VA Medical Center approved the procedures of this study. Written informed consent was obtained from each patient at the beginning of investigation.
Recruitment
Patients were recruited from vascular labs and vascular clinics from the University of Oklahoma HSC and the Oklahoma City VA Medical Center.
Baseline Clinical Characteristics Obtained from a Medical History and Physical Examination
Patients were evaluated in the morning at the Clinical Research Center, at the University of Oklahoma HSC. Patients arrived fasted, but were permitted to take their usual medications. To begin the study visit, patients were evaluated with a medical history and physical examination in which demographic information, height, mass, waist circumference, cardiovascular risk factors, co-morbid conditions, claudication history, ankle/brachial index (ABI), blood samples, and a list of current medications were obtained.
Inclusion and Exclusion Criteria
Patients with symptomatic PAD were included in this study if they met the following criteria: (a) a history of ambulatory leg pain, (b) ambulatory leg pain confirmed by treadmill exercise,10 and (c) an ABI ≤ 0.903 at rest or ≤ 0.73 after exercise.22 Patients were excluded for the following conditions: (a) absence of PAD (ABI > 0.90 at rest and ABI > 0.73 after exercise), (b) non-compressible vessels (ABI ≥ 1.40), (c) asymptomatic PAD, (d) use of medications indicated for the treatment of claudication (cilostazol or pentoxifylline) initiated within three months prior to investigation, (e) exercise limited by other diseases or conditions, (f) active cancer, (g) end stage renal disease defined as stage 5 chronic kidney disease, (h) abnormal liver function, (i) failure to complete all of the baseline tests in a 3-week baseline run-in phase, and (j) failure to complete more than one-third (>12) of the exercise intervention sessions. A total of 60 patients were enrolled in the home exercise program, of which seven patients did not complete the study and seven others completed only 12 or fewer of the prescribed 36 exercise sessions. Thus, 46 patients who successfully completed the study were included in the analyses.
Step-Monitored Home-Based Exercise Rehabilitation Program
We used a small (75 × 50 × 20 mm), lightweight (38 g) step activity monitor (StepWatch3™, Orthoinnovations, Inc., Oklahoma City, OK) to accurately record the duration and cadence of ambulation during each exercise session.14, 15 The step activity monitor is programmed by placing the unit on a USB docking station connected to a computer with StepWatch3™ Analysis Software. Once programmed, the monitor records the number of steps taken on a minute-to-minute basis for up to two months. Data is downloaded by placing the monitor on the USB docking station, and saving the patient file containing the number of minutes spent walking and the number of steps taken during each minute of walking. Once data is saved as a patient file, the step activity monitor is re-programmed while on the docking station to begin a new recording cycle.
This home-based exercise program consisted of three months of intermittent walking. Patients were instructed to walk to mild-to-moderate claudication pain at least three days per week at a self-selected pace, in which exercise duration was progressively increased from 20 to 45 minutes per session. Patients were given a step activity monitor with an attached elastic Velcro strap, and were instructed to wear the monitor around their right ankle above the lateral malleolus during each exercise session, and then remove the monitor at the completion of each session. Additionally, they received an exercise logbook to record their walking sessions. Patients returned their monitors and logbooks to the research staff at the end of week 1, 4, 8, and 12. During these brief 15-minute meetings, monitor data was downloaded, results were reviewed, and feedback was provided for the upcoming month of training. The total contact time that staff spent with each patient during the four study visits in the home exercise program was approximately one hour, with only several additional minutes being required at the end of the final baseline test visit prior to beginning the program to instruct patients how to properly use and wear the step activity monitor. The accuracy of the step activity monitor exceeds 99% ± 1% compared to hand-tallied step counts in patients with claudication, and the test-retest intraclass reliability coefficient for the daily ambulatory activity measures range from R=0.83 to R=0.94.23
Primary Outcome Measures
COT and PWT Obtained from a Graded Maximal Treadmill Test
Patients performed a graded treadmill test to determine study eligibility, and then repeated the test on a following visit within one week to obtain the primary outcome measures of COT and PWT as previously described.10, 14 Using our procedures, the test-retest intraclass reliability coefficient is R = 0.89 for COT,10 and R = 0.93 for PWT.10
Total Walk Distance Obtained from a 6-Minute Walk Test
On a separate day, typically within one week from the final treadmill test, patients performed an over-ground, 6-minute walk test supervised by trained exercise technicians, as previously described from our laboratory.24 The total distance walked during the test was recorded. The test-retest intraclass reliability coefficient is R = 0.94 for total 6-minute walking distance.24
Statistical Analyses
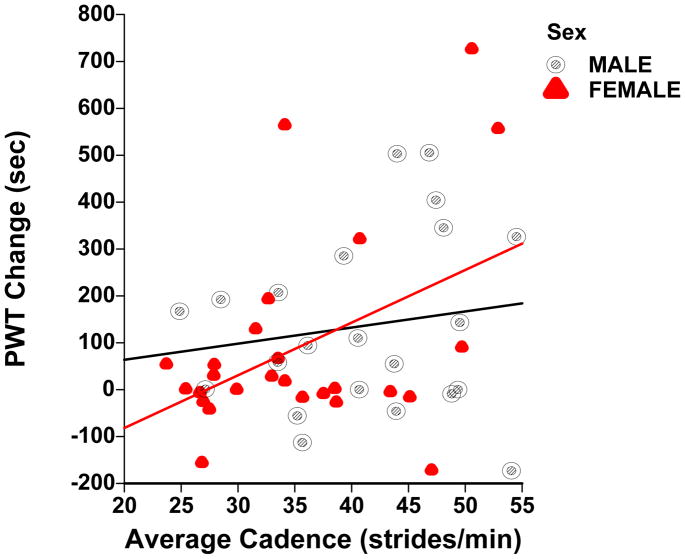
In initial analysis, large differences between men and women were observed in the linear associations among the baseline variables and change scores (see figure 1). Hence, each sex was examined separately. While estimates obtained in within-sex analyses are valid irrespective of any interactions with sex, it was of interest to examine the interaction of sex and all predictor variables in multivariate models. Multiple regression models with interaction terms of sex and predictor variables for both sexes were used to detect significant interactions between sexes. Presence of interactions indicated the inappropriateness of combining data from both sexes.
Figure 1.
Scatterplot of change in peak walking time (PWT) and average cadence during exercise training sessions with corresponding regression lines in men (slope = 3.4326 sec/stride, p = 0.4993) and in women (slope = 12.6274 sec/stride, p = 0.0192) with symptomatic peripheral artery disease.
The baseline treadmill variables exhibited significant skewing and were summarized as medians and Inter Quartile Ranges. Wilcoxon tests were used for testing these variables. Other measurement variables at baseline were summarized within each sex as means and standard deviations, and independent t-tests were used for testing among pairs of means. Dichotomous variables were summarized as percent with attribute present and were compared between sexes using a single degree of freedom Chi Square test. Similar test of baseline variables between evaluable subjects and others revealed no significant differences. Exercise intervention measures were compared between men and women within each sex group as the time simple effects of a 2 time by 2 group ANOVA with repeated measure on time and the interaction term used to compare differences in simple effects across groups Note that these tests assume normality only for the change scores and not for the baseline and time two measures as would be required for main effects tests.
Multivariate regression predictor models for values of outcome variables after completion of the home exercise program were obtained using an iterative least square procedure with baseline variables and exercise training variables as possible predictors. The variable producing the largest R-Square value was entered first and variable producing the largest change in R-Square entered in each subsequent steps. To avoid over parameterization, additional variables were restricted to only those with p-value <0.10. Associations among pairs of variables were measured using correlations. Correlations, (unlike test of means which are very robust relative to normal distribution assumptions) are often sensitive to departure from normal. The distributions of treadmill variables for females exhibited some departures from normal and, therefore, Spearman correlations were reported for these variables, and are presented in italics in tables. Pearson correlations were used for all other variables. All computations were made with the NCSS computer package.
RESULTS
Baseline Clinical Characteristics
The clinical characteristics of the men and women are shown in Table 1. The groups had mean ABI, COT, and PWT values typical for symptomatic PAD, and consisted of a mix of older, overweight Caucasians and African-Americans with a high prevalence of cardiovascular risk factors. The women had significantly lower 6MWD (p < 0.01) and lower body mass (p < 0.01) than the men.
Table 1.
Baseline clinical characteristics of men and women with symptomatic peripheral artery disease who completed the step monitored home exercise program.
| Variables | Men (N = 22) | Women (N = 24) |
|---|---|---|
| Means (SD) | ||
| Age (y) | 66 (11) | 68 (11) |
| Ankle/Brachial Index | 0.71 (0.19) | 0.66 (0.23) |
| Mass (kg) | 93.2 (14.4) | 73.2 (14.3) * |
| Height (cm) | 178.0 (4.8) | 160.9 (5.6) † |
| Body Mass Index (kg/m2) | 29.4 (4.3) | 28.3 (5.6) |
| Percentage with Characteristics Present | ||
| Race (% Caucasian) | 59 | 63 |
| Current Smoking (% yes) | 23 | 42 |
| Hypertension (% yes) | 86 | 92 |
| Dyslipidemia (% yes) | 82 | 88 |
| Diabetes (% yes) | 36 | 46 |
| Obesity (% yes) | 41 | 42 |
| Abdominal Obesity (% yes) | 55 | 58 |
| Metabolic Syndrome (% yes) | 82 | 92 |
| Lower Extremity Revascularization (% yes) | 27 | 50 |
| Angina (% yes) | 27 | 21 |
| Cerebral Vascular Accident (% yes) | 18 | 33 |
| Chronic Obstructive Pulmonary Disease (% yes) | 14 | 33 |
Different than men (p < 0.01),
(p < 0.001).
Exercise Intervention and Changes in Ambulatory Outcome Measures
Exercise training measures and ambulatory outcome change scores are shown in Table 2. The total number of exercise sessions completed, the average exercise time per session, and the average strides taken per session were not significantly different between the men and women, but the women performed their exercise sessions at a slower ambulatory cadence than the men (p < 0.01). Men experienced significant increases (p < 0.01) in COT, PWT, and 6MWD following the home exercise program. Women had significant increases in 6MWD (p < 0.01) and PWT (p < 0.05), but not for the change in COT (p > 0.05). The change scores for COT, PWT, and 6MWD were not significantly different between men and women.
Table 2.
Exercise measures and ambulatory outcome change scores.
| Variables | Men (N = 22) | Women (N = 24) |
|---|---|---|
| Exercise Training Measures: Values are means (SD) | ||
| Exercise Sessions Completed (n) | 35 (10) | 29 (9) |
| Average Exercise Time (min/exercise session) | 41.0 (19.9) | 40.2 (19.1) |
| Average Exercise Strides (strides/exercise session) | 1660 (641) | 1411 (656) |
| Average Exercise Cadence (strides/min) | 41.2 (8.5) | 35.4 (8.4) * |
| Baseline Ambulatory Measures: Values are Medians (Interquartile Range) | ||
| Claudication Onset Time (s) | 158 (172) | 136 (199) |
| Peak Walking Time (s) | 392 (406) | 255 (342) |
| 6-Minute Walk Distance (m) | 416 (125) | 267 (141) * |
| Change Scores of Ambulatory Outcome Measures: Values are Means (SD) | ||
| Change in Claudication Onset Time (s) | 142 (162) ‡ | 80 (196) |
| Change in Peak Walking Time (s) | 136 (192) ‡ | 99 (225) † |
| Change in 6-Minute Walk Distance (m) | 42 (64) ‡ | 56 (47) ‡ |
Different than men (p < 0.01).
Change different from zero (p< 0.05),
(p < 0.01).
Correlates of the Changes in Ambulatory Outcome Measures
The strongest univariate correlates of the change in ambulatory outcomes are displayed in Table 3. In women, the average exercise cadence during the home exercise training sessions was the highest univariate correlate (positive) with the change in 6MWD (p < 0.05) and in COT, whereas race was the highest correlate with the change in PWT (p < 0.05), with caucasian women having more favorable improvements. In men, ABI was the highest univariate correlate (positive) with the change in 6MWD (p < 0.01), whereas metabolic syndrome and age were the highest correlates (negative) with changes in COT (p < 0.05) and PWT, respectively.
Table 3.
Four strongest univariate correlates of the change in ambulatory outcomes with baseline characteristics and exercise training measures in symptomatic patients with peripheral artery disease.
| Group | Δ COT | Δ PWT | Δ 6-MWD |
|---|---|---|---|
| Women | |||
| Average Exercise Cadence (r = 0.36) | Race (r = 0.43) * | Average_Exercise Cadence (r = 0.48) * | |
| Age (r = 0.35) | Age (r=0.39) | Lower Extremity Revascularization (r = 0.39) | |
| Current Smoking (r = −0.26) | Current Smoking (r = −0.32) | Chronic Obstructive Pulmonary Diesease (r = −0.39) | |
| Ankle Brachial Index (r = 0.20) | Average Exercise Cadence (r = 0.27) | Total Exercise Time (r = 0.33) | |
| Men | |||
| Metabolic Syndrome (r = −0.48) * | Age (r = −0.24) | Ankle Brachial Index (r = 0.63) † | |
| Total Exercise Time (r = 0.41) * | Current Smoking (r = 0.23) | Metabolic Syndrome (r = 0.37) | |
| Ankle Brachial Index (r = 0.36) | Average Exercise Cadence (r = 0.15) | Average Exercise Cadence (r = 0.27) | |
| Current Smoking (r = 0.25) | Ankle Brachial Index (r = 0.12) | Lower Extremity Revascularization (r = −0.23) | |
COT = claudication onset time, PWT = peak walking time, 6-MWD = 6-minute walk distance.
Values are Pearson partial correlation coefficients or Spearman partial correlation coefficients (indicated in italicized print). Race was coded as 0 = African-American and 1 = Caucasian.
p < 0.05,
p < 0.01
Multivariate Regression Predictors of Changes in Ambulatory Outcome Measures
In the entire sample of patients, two significant interactions were detected for the change in COT predictors and sex, consisting of sex by average exercise cadence (p = 0.024) and sex by metabolic syndrome (p = 0.007). For the change in PWT, sex by average exercise cadence approached significance (p = 0.075). For the change in 6MWD, both sex by ABI (p = 0.001) and sex by average exercise cadence (p = 0.001) interactions were significant.
In women, the multivariate regression models identifying predictors of the changes in ambulatory outcomes are presented in Table 4. The average exercise cadence during the home exercise training sessions was the only predictor that entered the model for the change in COT (p = 0.082), and was the first predictor in the model for the change in PWT (p = 0.029) and in 6MWD (p = 0.006). In contrast to these results in women, for men the average exercise cadence during the home exercise sessions was not a significant predictor of change in PWT (Figure 1), COT, or 6MWD. In men (Table 5), ABI was the only predictor that entered the model for the change in 6MWD (p = 0.002), and was a predictor along with metabolic syndrome and body mass in the model for change in COT (p = 0.003). No variables entered the model for change in PWT.
Table 4.
Multivariate regression models identifying predictors of the changes in ambulatory outcomes in women with symptomatic peripheral artery disease.
| Dependent Variables | Predictors | Regression Coefficient | Partial Correlations | P Values |
|---|---|---|---|---|
| Δ COT (s) | Average Exercise Cadence | 10.73 | 0.36 | 0.082 |
| Intercept | −299.16 | |||
| Δ PWT (s) | Average Exercise Cadence | 16.78 | 0.46 | 0.029 |
| Lower Extremity Revascularization | −176.12 | −0.45 | 0.032 | |
| Intercept | −406.48 | |||
| Δ 6-MWD (m) | Average Exercise Cadence | 3.25 | 0.59 | 0.006 |
| Ankle Brachial Index | −82.90 | −0.44 | 0.055 | |
| Intercept | −2.87 |
COT = claudication onset time, PWT = peak walking time, 6-MWD = 6-minute walk distance.
Table 5.
Multivariate regression models identifying predictors of the change in ambulatory outcomes in men with symptomatic peripheral artery disease.
| Dependent Variables | Predictors | Regression Coefficient | PartialCorrelations | P Values |
|---|---|---|---|---|
| Δ COT (s) | Metabolic Syndrome | −293.12 | −0.70 | 0.001 |
| Ankle Brachial Index | 503.98 | 0.63 | 0.003 | |
| Mass | 3.78 | 0.42 | 0.067 | |
| Intercept | −330.51 | |||
| Δ PWT (s) | None | |||
| Δ 6-MWD (m) | Ankle Brachial Index | 215.66 | 0.63 | 0.002 |
| Intercept | −111.93 |
COT = claudication onset time, PWT = peak walking time, 6-MWD = 6-minute walk distance.
DISCUSSION
A primary novel finding was that for women with symptomatic PAD, performing the step-monitored home-based exercise training sessions at faster ambulatory cadence was predictive of greater increases in COT, PWT, and 6MWD at completion of the program. In men, higher baseline ABI was predictive of greater increases in COT and 6MWD, whereas having metabolic syndrome at baseline was predictive of less improvement in COT.
Exercise Adherence and Efficacy
Adherence to the step-monitored home-based exercise program was high in the patients, as more than 86% of the men and 79% of the women completed at least two-thirds of the prescribed total of 36 exercise sessions in the program (3 sessions per week for 12 weeks). One particular advantage of the home exercise program, compared to the traditional approach using an on-site supervised exercise program, is that patients have the freedom to over-achieve by completing more than the 36 prescribed sessions. In fact, 36% of the men and 29% of the women completed more than 36 sessions, with the maximum number of completed exercise sessions being 57 for men and 49 for women. Another way for patients to over-achieve in the home exercise program is to walk for longer than the prescribed amount of time during each training session. The prescribed walking duration progressively increased from 20 minutes in the first week to 45 minutes by the end, with an average of 40 minutes. Both the men and women walked for an average of 8 additional minutes per session beyond the prescribed duration. The primary difference between men and women in performing their home exercise sessions was that the women walked at a cadence that was 5.8 strides/min (10.6 steps/min) slower than men. Collectively, these results indicate that individualized monthly feedback is adequate for the majority of patients to adhere to the home exercise program. The high adherence rate agrees with previous randomized controlled trials using a step activity monitor,14, 15, 25 and a group-mediated cognitive behavioral intervention.26, 27
The step-monitored home-based exercise program resulted in significant improvements in COT, PWT, and 6MWD in men, and in PWT and 6MWD in women. There was a non-significant trend for the COT change score to also increase in women. These findings support previous work reporting improvements in COT, PWT, and 6MWD following home-based exercise.14, 15, 25, 26 Interestingly, we recently found that there is a specificity to training such that supervised treadmill training elicits greater improvements in treadmill outcomes (COT and PWT) than over-ground home exercise training, whereas the home exercise program elicits greater improvements in 6MWD.15 However, it is important to note that there are considerable cross-over effects, as both programs resulted in improvements in COT, PWT, and 6MWD. The current study highlights this cross-over effect by demonstrating that over-ground walking performed in the step-monitored home-based exercise program results in significant increases in PWT during treadmill tests in men and women with PAD.
Predictors of Changes in Ambulatory Outcomes
This is the first study to report the association between the ambulatory cadences performed during exercise sessions in a home exercise program with the change scores in ambulatory outcome measures. Ambulatory cadence is important because it represents the intensity, or pace, at which the exercise was accomplished during the home exercise program. The primary finding was that ambulatory cadence was a predictor of the changes in COT, PWT, and 6MWD in women with symptomatic PAD. This finding indicates that women who walked at a relatively fast cadence experienced the greatest improvements in function, and women who walked slower experienced less improvement, perhaps because they were not training at sufficient intensity. It is therefore possible that the instructions given to women participating in a home exercise program should be modified by having them perform their exercise sessions at a pace that is equal to or faster than their self-selected pace.
Men experienced improvements in all three ambulatory outcomes (COT, PWT, and 6MWD) following the home exercise program. However, in contrast to the results of the women, baseline characteristics such as ABI, metabolic syndrome, and body mass were more predictive of the improvements in the outcome measures rather than the ambulatory cadence during exercise training sessions. For example, men who had relatively low ABI and metabolic syndrome at baseline were less responsive to the home exercise program. This finding suggests that men who have more severe PAD and comorbid burden at baseline may either need a greater dose of exercise in the home exercise program, or additional medical intervention to supplement with exercise, or both to elicit more optimal improvements in ambulatory outcomes.
Limitations
Although this trial supports the efficacy of the step-monitored home-based exercise program for men and women with PAD, several limitations exist. One limitation is that patients volunteered to participate in this study. Thus, a self-selection bias may exist because they may represent those who had greater interest in participating, better access to transportation to the research center, and better health than PAD patients who did not volunteer. A second limitation is that the results of this study are only generalizable to patients with symptomatic PAD, and may not be applicable to patients with less severe or more severe PAD. Another limitation is that, by definition, we chose to study only those who successfully adhered to and completed the exercise program (46 out of 60) because we were interested in describing the true potential of the home exercise program to change ambulatory outcome measures if successfully performed. We have already reported the efficacy of this exercise program using several methods to rigorously account for bias due to dropping out of the study or poorly attending the program.14, 15 These methods included using an intent-to-treat analytic strategy, including into the analyses those patients who completed the study but who had low adherence rates to the intervention, and comparing the baseline characteristics of patients who completed with those who did not complete. We believe there is minimal bias associated with failing to complete or poorly attending the program because similar results were obtained using intent-to-treat versus completer analyses, and because the baseline characteristics of drop outs were not different than those who completed the study.14, 15 Another potential study limitation is that although the comorbid conditions were not statistically different between men and women, the women tended to have more clustering of comorbid conditions, which in aggregate could have contributed to their slower cadence during training sessions and relatively smaller improvement. One approach may have been to adjust for baseline covariates in an ANCOVA model, but this could not be done because the assumptions of ANCOVA were not met. A final limitation is that the sample size was small, which limits the ability to identify significant correlates of the exercise change scores, and it provides less precise estimates of change scores than if a larger sample were available. However, it should be noted that even with a small sample size, all of the ambulatory change scores in men and women, except for COT in women, were significant due to the large effect sizes associated with the home exercise program, and that predictors of the change scores were identified in multivariate models.
Conclusions and Clinical Significance
We conclude that faster ambulatory cadence during the step-monitored home-based exercise program may predict greater improvements in ambulatory function in women, whereas having less severe PAD and comorbid burden at baseline may predict greater improvements in ambulatory function in men. However, since this study was a follow-up analysis on a subset of patients from our recent prospective, randomized controlled exercise trial on a larger number of symptomatic patients with PAD,15 the current findings are preliminary which require confirmation in future investigations. The clinical significance may be that the pace of ambulating during the exercise sessions of the home exercise program should be carefully monitored at regular intervals to ensure that the exercise is performed at sufficient intensity to elicit more optimal ambulatory improvements in symptomatic patients with PAD.
Acknowledgments
Funding Sources: This research was supported by grants from the National Institute on Aging (R01-AG-24296), Oklahoma Center for the Advancement of Science and Technology grant (HR09-035), and OUHSC General Clinical Research Center grant (M01-RR-14467) sponsored by National Center for Research Resources.
Footnotes
Clinical Trial Registration Information – URL: http://www.ClinicalTrial.Gov. Unique Identifier: NCT00618670.
Disclosures: None.
References
- 1.Fowkes FG, Rudan D, Rudan I, et al. Comparison of Global Estimates of Prevalence and Risk Factors for Peripheral Artery Disease in 2000 and 2010: A Systematic Review and Analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch AT, Haskal ZJ, Hertzer NR, et al. Acc/Aha 2005 Practice Guidelines for the Management of Patients with Peripheral Arterial Disease (Lower Extremity, Renal, Mesenteric, and Abdominal Aortic): A Collaborative Report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the Acc/Aha Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients with Peripheral Arterial Disease): Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; Transatlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 3.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (Tasc Ii) J Vasc Surg. 2007;45(Suppl S):S5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch AT, Hartman L, Town RJ, Virnig BA. National Health Care Costs of Peripheral Arterial Disease in the Medicare Population. Vasc Med. 2008;13:209–215. doi: 10.1177/1358863X08089277. [DOI] [PubMed] [Google Scholar]
- 5.Jaff MR, Cahill KE, Yu AP, Birnbaum HG, Engelhart LM. Clinical Outcomes and Medical Care Costs among Medicare Beneficiaries Receiving Therapy for Peripheral Arterial Disease. Ann Vasc Surg. 2010;24:577–587. doi: 10.1016/j.avsg.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Mahoney EM, Wang K, Keo HH, et al. Vascular Hospitalization Rates and Costs in Patients with Peripheral Artery Disease in the United States. Circ Cardiovasc Qual Outcomes. 2010;3:642–651. doi: 10.1161/CIRCOUTCOMES.109.930735. [DOI] [PubMed] [Google Scholar]
- 7.Sachs T, Pomposelli F, Hamdan A, Wyers M, Schermerhorn M. Trends in the National Outcomes and Costs for Claudication and Limb Threatening Ischemia: Angioplasty Vs Bypass Graft. J Vasc Surg. 2011;54:1021–1031. e1021. doi: 10.1016/j.jvs.2011.03.281. [DOI] [PubMed] [Google Scholar]
- 8.Criqui MH, Langer RD, Fronek A, et al. Mortality over a Period of 10 Years in Patients with Peripheral Arterial Disease. N Engl J Med. 1992;326:381–386. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 9.Brass EP, Hiatt WR. Review of Mortality and Cardiovascular Event Rates in Patients Enrolled in Clinical Trials for Claudication Therapies. Vasc Med. 2006;11:141–145. doi: 10.1177/1358863x06069513. [DOI] [PubMed] [Google Scholar]
- 10.Gardner AW, Skinner JS, Cantwell BW, Smith LK. Progressive Vs Single-Stage Treadmill Tests for Evaluation of Claudication. Med Sci Sports Exerc. 1991;23:402–408. [PubMed] [Google Scholar]
- 11.McDermott MM, Greenland P, Liu K, et al. Leg Symptoms in Peripheral Arterial Disease: Associated Clinical Characteristics and Functional Impairment. JAMA. 2001;286:1599–1606. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- 12.Hiatt WR, Nawaz D, Regensteiner JG, Hossack KF. The Evaluation of Exercise Performance in Patients with Peripheral Vascular Disease. J Cardiopulmonary Rehabil. 1988;12:525–532. [Google Scholar]
- 13.Gardner AW, Poehlman ET. Exercise Rehabilitation Programs for the Treatment of Claudication Pain. A Meta-Analysis. JAMA. 1995;274:975–980. [PubMed] [Google Scholar]
- 14.Gardner AW, Parker DE, Montgomery PS, Scott KJ, Blevins SM. Efficacy of Quantified Home-Based Exercise and Supervised Exercise in Patients with Intermittent Claudication: A Randomized Controlled Trial. Circulation. 2011;123:491–498. doi: 10.1161/CIRCULATIONAHA.110.963066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gardner AW, Parker DE, Montgomery PS, Blevins SM. Step-Monitored Home Exercise Improves Ambulation, Vascular Function, and Inflammation in Symptomatic Patients with Peripheral Artery Disease: A Randomized Controlled Trial. J Am Heart Assoc. 2014;3:e001107. doi: 10.1161/JAHA.114.001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardner AW. Sex Differences in Claudication Pain in Subjects with Peripheral Arterial Disease. Med Sci Sports Exerc. 2002;34:1695–1698. doi: 10.1097/00005768-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Gardner AW, Parker DE, Montgomery PS, Khurana A, Ritti-Dias RM, Blevins SM. Gender Differences in Daily Ambulatory Activity Patterns in Patients with Intermittent Claudication. J Vasc Surg. 2010;52:1204–1210. doi: 10.1016/j.jvs.2010.05.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardner AW, Parker DE, Montgomery PS, Blevins SM, Nael R, Afaq A. Sex Differences in Calf Muscle Hemoglobin Oxygen Saturation in Patients with Intermittent Claudication. J Vasc Surg. 2009;50:77–82. doi: 10.1016/j.jvs.2008.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardner AW, Montgomery PS, Blevins SM, Parker DE. Gender and Ethnic Differences in Arterial Compliance in Patients with Intermittent Claudication. J Vasc Surg. 2010;51:610–615. doi: 10.1016/j.jvs.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gardner AW, Parker DE, Montgomery PS, et al. Gender and Racial Differences in Endothelial Oxidative Stress and Inflammation in Patients with Symptomatic Peripheral Artery Disease. J Vasc Surg. 2015 doi: 10.1016/j.jvs.2014.02.045. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gardner AW, Parker DE, Montgomery PS, Blevins SM. Diabetic Women Are Poor Responders to Exercise Rehabilitation in the Treatment of Claudication. J Vasc Surg. 2014;59:1036–1043. doi: 10.1016/j.jvs.2013.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiatt WR, Marshall JA, Baxter J, et al. Diagnostic Methods for Peripheral Arterial Disease in the San Luis Valley Diabetes Study. J Clin Epidemiol. 1990;43:597–606. doi: 10.1016/0895-4356(90)90164-k. [DOI] [PubMed] [Google Scholar]
- 23.Gardner AW, Montgomery PS, Scott KJ, Afaq A, Blevins SM. Patterns of Ambulatory Activity in Subjects with and without Intermittent Claudication. J VascSurg. 2007;46:1208–1214. doi: 10.1016/j.jvs.2007.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montgomery PS, Gardner AW. The Clinical Utility of a Six-Minute Walk Test in Peripheral Arterial Occlusive Disease Patients. J Am Geriatr Soc. 1998;46:706–711. doi: 10.1111/j.1532-5415.1998.tb03804.x. [DOI] [PubMed] [Google Scholar]
- 25.Hiatt WR, Creager MA, Amato A, Brass EP. Effect of Propionyl-L-Carnitine on a Background of Monitored Exercise in Patients with Claudication Secondary to Peripheral Artery Disease. J Cardiopulm Rehabil Prev. 2011;31:125–132. doi: 10.1097/HCR.0b013e3181f1fd65. [DOI] [PubMed] [Google Scholar]
- 26.McDermott MM, Liu K, Guralnik JM, et al. Home-Based Walking Exercise Intervention in Peripheral Artery Disease: A Randomized Clinical Trial. JAMA. 2013;310:57–65. doi: 10.1001/jama.2013.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDermott MM, Guralnik JM, Criqui MH, et al. Home-Based Walking Exercise in Peripheral Artery Disease: 12-Month Follow-up of the Goals Randomized Trial. J Am Heart Assoc. 2014;3:e000711. doi: 10.1161/JAHA.113.000711. [DOI] [PMC free article] [PubMed] [Google Scholar]