Abstract
Over the past few years, multiple immune checkpoint blockers (ICBs) have achieved unprecedented clinical success and have been approved by regulatory agencies for the treatment of an increasing number of malignancies. However, only a limited fraction of patients responds to ICBs employed as a standalone intervention, calling for the development of combinatorial regimens. Radiation therapy (RT) stands out as a very promising candidate for this purpose. Indeed, RT mediates antineoplastic effects not only by cytotoxic and cytostatic mechanisms, but also by modulating immunological functions, both locally (within the irradiated field) and systemically. As combinatorial regimens involving RT and ICBs are being developed and clinically tested at an accelerating pace, it is paramount to identify biomarkers that reliably predict the likelihood of individual patients to respond. Here, we discuss emerging biomarkers that may potentially predict the response of cancer patients to RT plus ICBs.
Keywords: DNA damage response, mutational load, natural killer cells, PD-L1, type I interferon
Introduction
Over the past decade, cancer immunotherapy has gone all the way from a promising preclinical application to a clinical reality [1, 2]. In particular, immune checkpoint blockers (ICBs) have been shown to induce durable clinical responses in individuals affected by a variety tumors, resulting in the approval of six ICBs for use in cancer patients by the US Food and Drug Administration (FDA) and other regulatory agencies worldwide [3, 4]. However, the percentage of patients obtaining clinical benefits from any ICBs employed as single immunotherapeutic interventions is relatively low (15–30%), calling for the development of novel combinatorial regimens. Combining distinct ICBs has been shown to ameliorate overall response rates in some oncological indications, but this is associated with increased toxicity [5, 6]. Similarly, chemotherapy and targeted anticancer agents can synergize with ICBs in some settings, but side effects can also be considerable [7]. Radiation therapy (RT) is a particularly promising candidate for combination with ICB, for at least three reasons [8]. First, RT has been universally used to treat patients for more than a century and its effectiveness is well established. Second, RT is an accessible and relatively economical procedure associated with limited and manageable side effects, reflecting the extensive knowledge about its use. Third, it is now clear that – besides mediating cytotoxic and cytostatic effects on malignant cells – RT has multipronged immunomodulatory effects. Such effects, which emerge locally (within the irradiated field) but may have systemic impact, can be harnessed to boost the therapeutic activity of ICBs [9] (Figure 1).
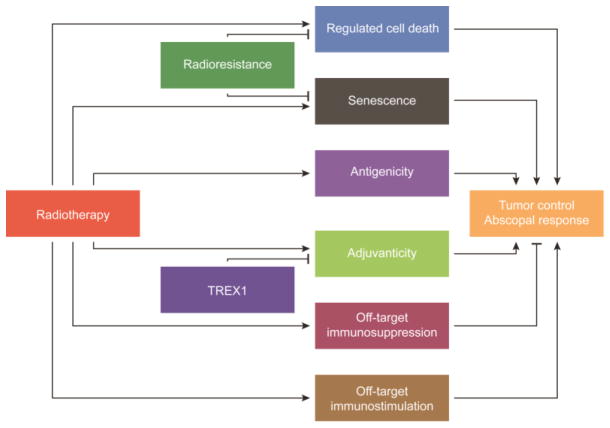
Figure 1. From radiotherapy to abscopal responses: the keys steps.
Radiotherapy (RT) can mediate local tumor control and abscopal responses via three critical steps: (1) by inducing regulated cell death and/or senescence amongst malignant cells; (2) by increasing their antigenicity and/or adjuvanticity; and (3) by mediating local or systemic immunostimulatory effects that do not originate from malignant cells (off-target immunostimulation). Three major resistance mechanisms can counteract such effects and therefore compromise the therapeutic activity of RT: (1) the intrinsic radioresistance of malignant cells; (2) off-target immunosuppression; and (3) the induction of the DNA exonuclease three prime repair exonuclease 1 (TREX1).
RT increases the antigenicity of malignant cells by promoting the upregulation of MHC class I molecules on the cell surface [10, 11], by favoring the expression of tumor-associated antigens [12], and (at least potentially) by enhancing genetic instability or reactivating endogenous retroviruses [13, 14]. Moreover, RT boosts the adjuvanticity of cancer cells by at least two mechanisms. On the one hand, cancer cells surviving irradiation expose increased amounts of (1) immunostimulatory ligands for killer cell lectin like receptor K1 (KLRK1; best known as NKG2D), hence favoring natural killer (NK) cell activation [15, 16]; (2) co-stimulatory molecules such as TNF receptor superfamily member 9 (TNFSF4; best known as OX40L) and TNFSF9 (best known as CD137 or 4-1BB) [17]; and (3) death receptors such as Fas cell surface death receptor (FAS), resulting in increased susceptibility to lysis by immune effector cells [18]. On the other hand, neoplastic cells succumbing to irradiation emit several danger-associated molecular patterns that favor the activation of a tumor-specific immune response [19]. Such an immunogenic cell death modality relies, amongst multiple factors, on the timely release of type I interferon (IFN) [20, 21], which enables clinically relevant abscopal responses [22, 23]. Finally, RT can directly affect stromal cells, endothelial cells as well as multiple components of the immunological tumor infiltrate [24]. A detailed description of the immunobiology of RT goes beyond the scope of the current review, and can be accessed in other publications [9, 24]. However, it is important to mention that cytosolic nucleic acid sensing has recently been shown to play a major role in the immunogenicity of RT as it connects the DNA damage response (DDR) to innate and adaptive immunity [21, 25–27]. Moreover, dose and fractionation seem to have a major impact on the ability of RT to drive immunological tumor rejection. For instance, hypofractionated RT appears to mediate superior immunostimulatory effects when compared to RT administered in single ablative doses, at least in part owing to robust type I IFN responses [21, 28]. Similarly, the effects of radiation on the vascular endothelium are dose-dependent, with doses above 8 Gy resulting in endothelial apoptosis that can contribute to clinical responses [29] Conversely, endothelial activation, which enables tumor infiltration by immune cells, has been observed at low radiation doses (0.5–2 Gy) [30]. Thus, considerable attention should be given to radiation dose and schedule when combinatorial regimens are conceived.
It has been proposed that RT can be harnessed as an in situ vaccine to generate a systemic antitumor response that sensitizes ICB-resistant tumors to treatment [8]. Although several clinical trials are ongoing to investigate the potential synergy between RT and ICBs (as well as other forms of immunotherapy) [31, 32], the understanding of the molecular, cellular and systemic effects of these treatments when used in combination is still evolving. Similarly, accurate predictions of the likelihood of individual patients to respond to RT plus immunotherapy remain elusive. Here, we discuss preclinical and clinical data on emerging biomarkers that could be prospectively evaluated for their potential to predict efficacy when RT is combined with ICBs or other forms of immunotherapy.
Predictive biomarkers of response to RT
Over the past century, accrued scientific understanding of the mechanisms underlying the effects of radiotherapy on cancer and normal tissues has resulted in well established protocols of treatment that both optimize tumor control and limit normal tissue toxicitities. While most fatal recurrences are systemic, some patients recur at the primary tumor site, in the absence of detectable systemic recurrence. These isolated “in field” recurrences have elicited extensive interest among radiation biologists.
Preclinical models have demonstrated that radioresistant neoplastic cells are indeed less susceptible to the induction of cellular senescence and regulated cell death (RCD) by RT than their radiosensitive counterparts. Similar to chemoresistance [33], radioresistance can be innate or acquired, and can originate from molecular alterations in a plethora of cellular processes involved in the biological response to radiation [34]. For a comprehensive discussion of biomarkers for predicting clinical responses to RT, we refer the reader to a special issue of Seminars in Radiation Oncology devoted to this topic [35]. Here, we will briefly discuss four main types of biomarkers that have been associated with predictive value for response to RT: (1) components of the DDR machinery, (2) genetic and (3) epigenetic signatures of radioresistance; and (4) microenvironmental biomarkers, focusing on factors that have been also shown to affect responses to imunotherapy.
DDR-related biomarkers
Although numerous DNA-damaging agents are routinely used in the clinical management of a variety of tumors, few biomarkers are available to identify potential responders to RT within patient populations and/or to guide decision making with respect to dose and administration schedule [35]. ATM serine/threonine kinase (ATM) plays a basic role in the DDR to double-strand breaks (DSBs) [36]. Levels of MRE11 homolog, double strand break repair nuclease (MRE11), a component of the heterotrimeric complex that initiates ATM signaling at DSBs caused by RT [37], regulate the initiation of DDR-driven apoptosis when DNA damage is irreparable [38]. Consistently, individuals with muscle-invasive bladder carcinoma expressing high levels of MRE11 exhibited superior cancer-specific survival following RT as compared to patients bearing MRE11low tumors [39, 40]. Along similar lines, variations in the copy number of nibrin (NBN, which encodes another component of the MRE11-containing complex that recognizes DSBs) have been shown to influence the likelihood of response to RT. In particular, NBN copy gains were a reliable predictor of biochemical relapse-free survival rates at 5 years among 139 localized prostate cancer patients treated with image-guided RT, while the same marker had no predictive value among men treated by radical prostatectomy [41]. Moreover, a combined assessment of the phosphorylation status of checkpoint kinase 1 (CHEK1), the expression level of tumor protein p53 (TP53; best known as p53), and their subcellular localization (CHEK1 and p53 are critical transducers of the DDR initiated by single-strand breaks) was associated with early local recurrence in a large cohort of >900 breast cancer patients receiving adjuvant RT [42]. Interestingly, markers of an ongoing DDR such as the phosphorylation of H2A histone family member X (H2AFX; best known as H2AX) have been detected in circulating tumor cells (CTCs) from non-small cell lung carcinoma (NSCLC) patients undergoing RT [43]. However, whether DDR in CTCs has any prognostic or predictive value remains to be elucidated. Furthermore, it has recently been proposed that the well-established link between human papillomavirus (HPV) positivity and improved responses to RT amongst head and neck squamous cell carcinoma patients [44–46] may reflect the ability of cyclin dependent kinase inhibitor 2A (CDKN2A) – which is upregulated upon HPV infection – to inhibit the DDR [47]. That said, the abundance of MRE11 had no predictive value in a cohort of patients with squamous cell carcinomas of the anus treated with RT in combination with chemotherapy and did not add to the prognostic value of p16 (a marker for HPV) and tumour-infiltrating lymphocyte scores [48]. Moreover, even though the molecular pathways linking RT-driven DNA damage to cellular senescence or RCD have been extensively characterized in preclinical models [49, 50] and at least in part confirmed in the clinical, the actual predictive value of other components of the DDR machinery for cancer patients remains unknown.
Genetic signatures
A signature encompassing 31 genes involved in cell cycle regulation, DNA replication, and cell-to-cell interaction identified by analyzing the radiosensitivity of the NCI-60 cancer cell panel [51] was associated with prognostic and predictive value in two distinct cohorts of 276 and 463 irradiated glioma patients, from the Gene Expression Omnibus (GEO) and The Cancer Genome Atlas (TCGA), respectively [52]. However, the actual predictive (rather than prognostic) value of this signature remains to be validated in prospective randomized clinical trials. An IFN-related DNA damage resistance signature (IRDS) involving the analysis of seven gene pairs has been proposed as a predictive biomarker for radiosensitivity, based on data from 295 breast cancer patients receiving adjuvant chemoradiation [53, 54]. Individual with IRDS+ lesions exhibited inferior locoregional control after adjuvant chemotherapy and RT as compared to patients bearing IRDS− lesions [54]. Similar observations were obtained in cohorts of breast patients receiving either adjuvant chemotherapy or RT [54]. Conversely, IRDS had no prognostic value amongst patients undergoing endocrine therapy or no additional therapy [54]. Importantly, although this signature partially assesses the radiosensitivity of malignant cells (three of the seven genes are directly involved in the DDR), it also interrogates IFN pathways, which are intimately involved in anticancer immunity [55]. Finally, a gene-expression-based radiosensitivity index and the linear quadratic model have been harnessed to generate a genomic-adjusted radiation dose (GARD) based on 8271 tissue samples from the Total Cancer Care cohort [56]. In multivariable analysis, GARD was independently associated with disease outcome in five distinct cohorts of breast (n=263 and n=77), lung (n=60) and pancreas (n=40) and glioblastoma (n=98) patients analyzed retrospectively [56]. Specifically, breast cancer patients with high GARD values exhibited significantly improved metastasis-free survival at 5-year follow-up when compared to patients with low GARD values [56]. Whether the IRDS or GARD has predictive value for patients prospectively treated with RT plus immunotherapy remains to be defined (see below).
Epigenetic signatures
microRNAs regulate a plethora of cellular processes potentially involved in radiosensitivity, including (but not limited to) cell growth and metabolism, differentiation, cell cycle control, autophagy, RCD and the DDR [57–60]. Preclinical investigation in this area unveiled the ability of multiple microRNAs including (but not limited to) miR-17–92 [61], miR-34 [62, 63], miR-145 [64, 65], miR-205 [66], miR-300 [67], miR-338-5p [68] to influence the radiosensitivity of malignant cells of various origin, in vitro and/or in vivo. For instance, low plasma circulating levels of miR-145 were significantly associated with poor differentiation, lymph node invasion and poor clinical outcome in a cohort of 120 cervical cancer patients [69]. A logistic regression model based on the levels of miR-9 and miR-200a generated on a training cohort of 60 cervical cancer patients receiving standard chemoradiation had robust predictive value when validated in a cohort of 42 similar patients [70]. Likewise, specific epigenetic profiles simultaneously assessing the expression of multiple (up to several dozens) different microRNA have been associated with improved responses to adjuvant chemoradiation in cohorts of individuals affected by glioblastoma [71] and colorectal carcinoma [72–74]. However, the actual predictive (over prognostic) value of these signatures cannot be discerned from most of these studies. In addition, standard-of-care regimens often combine chemotherapy with RT, making it impossible to isolate the effects of radiation.
Microenvironmental biomarkers
Multiple variables of the tumor microenvironment have been shown to influence the likelihood of cancer patients to respond to RT. Hypoxia is perhaps the best-characterized of these biomarkers [75]. Indicators of hypoxia such as the expression levels of hypoxia inducible factor 1 alpha subunit (HIF1A) or solute carrier family 2 member 1 (SLC2A1; best known as GLUT1) as well as pimonidazole reactivity [76] have been retrospectively associated with poor disease outcome in multiple cohorts of patients receiving RT [77–80]. Indeed, hypoxic malignant cells are known to exhibit reduced radiosensitivity, reflecting (at least in part) the fact that the damage inflicted to macromolecules by RT involves the formation of reactive oxygen species. Most importantly, chronic hypoxia leads to major changes in the metabolism and phenotype of cancer and stromal cells, promoting metastatic dissemination and immunosuppression [81, 82]. Some of the immunosuppressive effects of hypoxia are mediated by HIF1A-dependent transactivation of vascular endothelial growth factor A (VEGFA), which induces CD4+CD25+FOXP3+ regulatory T (Treg) cells and myeloid-derived suppressor cells (MDSCs) in the tumor microenvironment [83]. Conversly, growing evidence indicates that a favorable immunological infiltrate, with high intratumoral levels of CD8+ cytotoxic T lymphocytes (CTLs) and/or limited infiltration by Treg cells predicts clinical responses to multiple therapeutic interventions, including radiotherapy [19, 84, 85]. For instance, an abundant lymphocytic tumor infiltrate has been associated with improved disease outcome amongst patients with triple negative breast cancer treated in studies of adjuvant doxorubicin-based chemotherapy or carboplatin plus accelerated radiotherapy [86, 87].
The so-called immunoscore has been linked to superior disease outcome amongst 55 colorectal cancer patients receiving neoadjuvant chemoradiation [88], as well as in a cohort of 116 patients with brain metastases including 81 individuals receiving whole-brain RT [89]. However, no data is available on the potential predictive value of the immunoscore in patients prospectively allocated to RT alone. Presently, neither the degree of hypoxia nor the abundance of one or more tumor-infiltrating immune cell populations is routinely used in the clinics to inform decision-making.
In summary, although the preclinical literature on the radiobiological determinants of response to RT is abundant, the current individualization of RT mainly reflects subjective anatomical differences that inform the geometric and physical alignment of the radiation beams. Radiation field configuration, dose and fractionation often have empirically developed as a compromise between optimal local disease control and recoverable toxicity of irradiated normal tissue, with little inclusion of biological diversity across a specific tumor site, with the notable exception of HPV status for head and neck cancers [90], and 1p/19q co-deletion in glioma, which defines tumors with a favorable prognosis and improved responses to chemotherapy that do not need RT [91, 92].
Predictive biomarkers of response to ICBs
ICBs can be associated with significant toxicity, which in some cases calls for treatment discontinuation, and are considerably more expensive than most standard treatments [5, 93]. Moeover reponses to ICBs often develop slowly, and many patients with an initial reponse eventually progress. Thus, over the past few years extraordinary efforts have been devoted to the identification of predictive markers of response, some of which are already employed in clinical settings for decision making [94].
Tumor biomarkers
The expression of the immunosuppressive molecule CD274 (best known as PD-L1) on the surface of malignant cells has been investigated as a potential biomarker of responses to ICBs targeting PD-L1 itself or its receptor programmed cell death 1 (PDCD1; best known as PD-1) since the early development of these agents. Malignant cells from different origin (and associated stromal cells) express indeed high levels of PD-L1, which turns off effector T cells and NK cells in a PD-1-dependent manner [95]. Thus, high PD-L1 expression is often responsible for resistance to immunological tumor rejcetion. Loss of phosphatase and tensin homolog (PTEN) – one of the most common oncogenic events across all human neoplasms – has been shown to favor PD-L1 expression and hence resistance to ICBs [96]. Topalian et al. were the first to show a relationship between clinical responses to a PD-1-targeting ICB and PD-L1 expression levels assessed by immunohistochemistry (IHC) [97]. In particular, 9 out of 25 patients (36%) with PD-L1+ tumors responded to the anti-PD-1 agent nivolumab (which is currently approved for the treatment of multiple tumors) [4], while 0 out of 17 patients with PD-L1− tumors did so [97]. A number of clinical trials reported thereafter confirmed a trend for superior response rates to PD-1 blockers amongst patients with PD-L1+ tumors [98–101]. However, the thresholds employed in these studies to define PD-L1 positivity exhibited considerable degree of variation (ranging from 1% to 50% malignant cells with membranous PD-L1 staining). Moreover: (1) some patients with low or even absent PD-L1 expression appear to benefit from immunotherapy with ICBs [99]; and (2) PD-L1 expression levels vary over a wide dynamic range [102]. Thus, the dichotomous stratification of cancer patients based on PD-L1 expression levels may not be the most appropriate approach to predict clinical responses to PD-1-targeting ICBs. Irrespective of this possibility, one immunohistochemical test to assess PD-L1 expression in bioptic material (PD-L1 22C3 pharmDx, from Dako) is currently approved by FDA as a companion diagnostic to assign NSCLC patients to treatment with the anti-PD-1 agent pembrolizumab [103]. Three additional immunohistochemical assays are approved as complementary diagnostics to inform on risk versus benefit of treatment with nivolumab in patients with non-squamous NSCLC and melanoma (PD-L1 28-8 pharmDx, from Dako) or treatment with atezolizumab or durvalumab (two FDA-approved ICB targeting PD-L1) in patients with metastatic urothelial cancer (PD-L1 SP142 and SP263 respectively, from Roche) [104]. As nowadays no less than 5 ICBs targeting PD-1 or PD-L1 are licensed by FDA and equivalent regulatory agencies for use in cancer patients [4], additional assays testing PD-L1 expression are expected to be available soon.
Another extensively investigated biomarker of clinical responses to ICBs is the tumor mutational load (i.e., the number of non-germline, non-synonymous mutations per exome) [105]. These mutations have the capacity to generate tumor neoantigens, hence considerably boosting the antigenicity of malignant cells [105]. Several studies have demonstrated that a high mutational load is generally associated with an improved sensitivity of cancer patients to immunotherapy with ICBs [106–109]. That said, an increased burden of non-synonymous mutations (which can be assessed by whole-exome DNA sequencing of neoplastic material versus peripheral blood mononuclear cells) augments the likelihood, but does not necessarily result in the accumulation, of neoantigens. Nonetheless, the upstream mechanisms responsible for an increase in mutational burden currently represent the most promising biomarker to predict clinical responses to ICBs [110]. In particular, defects in the molecular machinery that ensures DNA integrity and high-fidelity transmission confer exquisite sendsitivity to ICB therapy. Mismatch repair (MMR) status predicted the likelihood of patients with a range of tumors to respond to immune checkpoint blockade with pembrolizumab [111, 112], confirming that MMR deficient (MMR-D) tumors are more prone to accumulate mutations than their proficient (MMR-P) counterparts, especially the mutational profile commonly associated with microsatellite instability (MSI) [113]. In 2017, the FDA licensed the use of pembrolizumab for the treatment of MMR-D or MSI high (MSI-H) malignancies irrespective of tissue of origin [112], representing the first tissue-agnostic approval in the history of the agency. Similarly, nivolumab has recently been approved for use in patients with MMR-D or MSI-H colorectal carcinoma [114]. Several diagnostic tests are currently available to determine MMR/MSI status from biopic material, mostly based on the PCR-assisted assessment of microsatellite markers or the immunohistochemical quantification of MMR-related proteins [115]. Importantly, some tumors display a relatively high mutational burden but are not MSI-H, suggesting that mechanisms other than MSI can compromise genomic stability [116]. It is currently unclear which of these two biomarkers have superior predictive value [117].
Of note, several other tumor biomarkers have been associated with poor clinical responses to ICBs [118]. These include (but may not be limited to): (1) mutations in beta-2-microglobulin (B2M), a critical component of the MHC class I system for antigen presentation [119]; (2) mutations in genes encoding several components of the interferon gamma receptor 1 (IFNGR1) signaling pathway [120, 121]; (3) activation of WNT signaling, which limits tumor infiltration by immune effectors cells, at least in melanoma [122]; and (4) activation of a multicomponent resistance pathway driven by low-intensity, chronic type I IFN signaling [123]. However, these biomarkers have mostly (if not only) been investigated in melanoma patients (reflecting the prevalence of melanoma studies in the clinical development of ICBs), and are not universally associated with disease outcome [112, 124], which explains why they are not (yet) used in clinical settings for decision-making.
Other biomarkers
The composition of the tumor microenvironment as well as the systemic configuration of the immune system also play an important role in the susceptibility of cancer patients to respond to immunotherapy with ICB. Several studies have demonstrated that elevated levels of tumor-infiltrating lymphocytes are associated with improved clinical responses to ICBs in patients affected by a variety of malignancies [84]. For instance, a study involving 46 melanoma patients treated with pembrolizumab documented higher numbers of CD8+, PD-1+ and PD-L1+ cells at the invasive tumor margin as well as in the tumor core in pretreatment samples from responders (as compared to non responders) [125]. As discussed above, an abundant infiltration with CD8+ CTLs and/or a limited infiltration with CD4+ CD25+ FOXP3+ Treg cells have been attributed robust prognostic and predictive value in patients affected by a large panel of tumor types and treated with a wide array of therapeutic interventions [19, 84, 85], suggesting that these potential biomarkers are not specific for immunotherapy with ICBs. Likewise, genetic signatures indicative of tumor infiltration with effector (as opposed to suppressive) cells and activation of innate and adaptive immunity appear to convey prognostic, if not predictive, value in several oncological settings, including ICB-based immunotherapy [20, 102, 126]. The Immunoscore also represents a promising biomarker in some oncological settings, notably colorectal carcinoma [88, 127]. However, its prognostic versus predictive value remains to be defined, and so far there are no indications that it may specifically predict clinical responses to ICBs. Systemic parameters including the circulating levels of MDSCs have also been proposed as potential predictors of ICB efficacy in cancer patients [128]. The specific predictive value of such biomarkers, however, remains to be elucidated, and none of these factors is currently assessed in the clinic as part of the decision-making process for immunotherapy with ICBs.
Given the complexity of tumor-host interactions and the dynamic nature of most immunological biomarkers, it is not surprising that each of them has shown limited value when used individually [118]. Efforts are ongoing to develop integrative models that take into account multiple parameters and features of the tumor and of the host, such as the so-called “cancer immunogram” [129], to reliably assist clinical decision making in the clinic.
Predictive biomarkers of response to RT plus ICBs
The potential predictive value of the biomarkers discussed above needs to be studied in patients receiving RT plus ICBs. Indeed, some of the biomarkers that are associated with improved clinical responses to RT or ICB-based immunotherapy may have limited predictive value in the context of combinatorial regimens. As an example, while high PD-L1 expression levels are robustly associated with the response of melanoma and NSCLC patients to nivolumab or pembrolizumab (see above), a benefit on progression-free survival was observed irrespective of baseline PD-L1 expression in NSCLC patients treated with chemoradiation followed by durvalumab versus placebo [130]. This result is consistent with the hypothesis that RT can induce PD-L1 expression in the tumor microenvironment, as demonstrated in preclinical settings [131]. It has also been suggested that PD-L1 expression levels at baseline might predict the response of metastatic melanoma patients to RT plus CTLA4-targeting ICBs, based on the PD-L1 positivity in 4 out of 9 non-responding and in 0 out of 2 responding patients [132], but this has not been confirmed. Here, we will focus our discussion on mechanism-based biomarkers that are emerging as candidates to specifically predict the response to RT plus ICBs (not necessarily to either of these treatments administered alone).
NKG2D ligands
NKG2D not only operates as a major NK-cell activatory receptor (NKAR), hence driving innate lymphoid immunity, but also stabilizes the immunological synapsis between CD8+ CTLs and their targets, hence supporting adaptive immunity [16]. Several DNA-damaging agents including RT promote the exposure of NKG2D ligands on the surface of malignant cells, hence rendering them potentially susceptible to NK cell-dependent lysis or improved recognition by CTLs [133]. However, cancer cells often express increased levels of metalloproteases that shed NKG2D ligands from the cell surface and create decoys with immunosuppressive activity [16]. Accordingly, high circulating levels of a soluble NKG2D ligands, including MHC class I polypeptide-related sequence A (MICA), MHC class I polypeptide-related sequence B (MICB), UL16 binding protein 1 (ULBP1) and UL16 binding protein 2 (ULBP2) have been attributed negative predictive value in melanoma patients treated with various ICBs including nivolumab, pembrolizumab and ipilimumab (which are currently approved for the treatment of melanoma patients) [134–137]. These observations suggest that soluble NKG2D ligands and antibodies that neutralize their activity [138, 139] may constitute easily accessible predictive biomarkers for patients receiving RT plus ICBs. Further investigation is warranted to properly assess this possibility.
Type I IFN responses
An abundant preclinical and clinical literature demonstrates that type I IFN release by neoplastic cells and dendritic cells (DCs) is paramount for the initiation of therapeutically relevant anticancer immune responses by chemotherapy, RT and immunotherapy [20, 21, 25, 140–143]. However, many tumors are resistant to ICBs because they are poorly infiltrated by basic leucine zipper ATF-like transcription factor 3 (BATF3)-dependent DCs [122], a DC subset specialized in antigen cross-presentation to CD8+ T cells [144]. As it favors the release of type I IFN in the tumor microenvironment, RT has the potential to create a tumor microenvironment that is permissive to the recruitment and activation of BATF3-dependent DCs and CTLs [21, 142, 143]. Such an activity critically relies on the sequential activation of Mab-21 domain containing 1 (MB21D1; best known as cGAS) and transmembrane protein 173 (TMEM173; best known as STING) by tumor-derived double-stranded DNA (dsDNA) accessing the cytosolic compartment of cancer cells and/or DCs [21, 25, 26, 145]. In some circumstances, cGAS-STING signaling is impaired in cancer cells, generally as a consequence of epigenetic inactivation [146, 147], and this may impair the ability of radiation to enhance recruitment of BATF3-dependent DCs to poorly immunogenic tumors [148]. Thus, the expression levels of cGAS and STING at baseline represent a promising biomarker for the identification of patients who may achieve durable benefits from combinatorial regimens involving RT and ICBs. Of note, the DNA exonuclease three prime repair exonuclease 1 (TREX1) has been demonstrated to counteract the ability of RT to drive type I IFN secretion in cancer cells by degrading cytosolic dsDNA [21]. Importantly, TREX1 is upregulated by RT, but only at single doses above 12–18 Gy in most carcinoma cells, and this dose range is amenable to change across different malignancies [21]. This implies that TREX1 levels need to be measured in the tumor after RT to identify RT doses with optimal immunostimulatory effects. While this is not feasible in most patients, using bioptic material obtained at diagnosis to perform ex vivo irradiation and/or to establish patient-derived tumor xenografts (PDTXs) in immunodeficient mice (which can subsequently be tested for RT-driven TREX1 upregulation) stands out as a promising approach to guide the selection of an RT dose and schedule that are synergistic with ICBs [148].
These examples emphasize the need for well-designed biomarker-driven clinical trials to determine not only which patient and perhaps which lesion, in the metastatic setting, should be irradiated, but also how RT should be delivered to generate an in situ vaccine and increase responses to ICBs. At this stage in the development of combinations of RT and immunotherapy, interrogating the tissue is of paramount importance. Circulating biomarkers, including cytokines, exosomes and tumor-derived DNA [149] should be investigated in parallel and may, in time, be proven to provide safer and cheaper alternatives, at least in some situations. Oncological settings in which ICBs are poorly effective as standalone therapies, like breast carcinoma, may be best suited to this aim, especially in situations in which biopsies can be collected longitudinally. One example is the TONIC trial, recently presented at ESMO, in which patients with metastatic triple negative breast cancer were randomly assigned to one of five 2-week induction treatments: (1) RT given in 3 fractions of 8 Gy to one metastatic lesion, (2) low dose doxorubicin, (3) low dose cyclophosphamide, (4) cisplatin given two times, (5) no induction treatment, followed by nivolumab until progression. The trial is designed to evaluate 10 patients with paired pre and post-treatment biopsies in each arm in order to select the best induction treatment, giving consideration to both clinical response and degree of immunological tumor infiltration [150]. Similar trials should be conducted in patients with tumors amenable to serial biopsing in order to compare the efficacy of different RT regimens.
Concluding remarks
While local disease control by RT is generally achievable, occasional patients develop isolated recurrences and their prospective identification remains elusive. A century of radiation biology has identified several mechanisms that explain radioresistance, but approaches to identify patients at risk for such isolated recurrences (and hence prevent them) have generally failed in the clinic. With the renaissance of cancer immunotherapy, considerable efforts have been dedicated at the identification of predictive biomarkers of clinical responses to ICBs, with promising results. Indeed, two of these biomarkers (PD-L1 expression levels by malignant cells and MMR/MSI status) have already been implemented in the clinic to assist decision-making.
It is now clear that the response to RT is also dependent on its immunomodulatory effects (Figure 1), and upcoming efforts towards the identification of novel biomarkers will have to take this notion into attentive consideration. Since RT stands out as a promising partner for immunotherapy, especially for the treatment of neoplasms that exhibit limited immune infiltrate (so-called cold tumors) validated biomarkers that prospectively predict clinical responses to RT plus immunotherapy are warranted. Importantly, recent evidence about the mechanisms that regulate the immunogenicity of RT supports the notion that dose and fractionation may need to be optimized for each individual tumor [21]. The development of innovative methods such as the irradiation of bioptic specimens ex vivo or the establishment of PDTXs in immunodeficient mice may enable the identification of optimal RT dose and fractionation for each patient, hence overcoming the limitations of biomarkers developed on tumor type rather than on individual lesions. In this era of precision medicine, predictive biomarkers become essential to treatment decisions, but this critical research component is not sufficiently incorporated in most clinical trials. Nonetheless, recent advances in large-scale data-rich biological analyses (so called “omics”) and in functional imaging provide considrebale new opportunities for rapid progress in this field.
Acknowledgments
Grant Support: S.D. is supported by NIH R01CA201246 and R01CA198533, and the Chemotherapy Foundation. S.C.F. and S.D are supported by Breast Cancer Research Foundation (BCRF-17-053). L.G. is supported by startup funds from the Department of Radiation Oncology, WCMC (New York, NY, USA), and a grant from Sotio a.c. (Prague, Czech Republic). C.V.-B. is supported by a U.S. Department of Defense BCRP postdoctoral fellowship award (W81XWH-13-1-0012) and the 2017 Kellen Junior Faculty Fellowship from the Anna-Maria and Stephen Kellen Foundation (USA).
Abbreviations
- CTC
circulating tumor cell
- CTL
cytotoxic T lymphocyte
- DC
dendritic cell
- DDR
DNA damage response
- DSB
double-stand break
- dsDNA
double-stranded DNA
- FDA
Food and Drug Administration
- ICB
immune checkpoint blocker
- IFN
interferon
- IHC
immunohistochemistry
- IRDS
IFN-related DNA damage resistance signature
- MMR
mismatch repair
- MSI
microsatellite instability
- NK
natural killer
- NSCLC
non-small cell lung carcinoma
- RCD
regulated cell death
- RT
radiation therapy
Glossary
- Abscopal response
Immunological response whereby the irradiation of a malignant lesion results in the regression or stabilization of a distant, non-irradiated lesion.
- Adjuvanticity
Property of a specific molecule to enhance the immune response to an antigen.
- Antigen cross-presentation
Ability of some antigen-presenting cells to present exogenous antigens via the route normally employed for endogenous antigens.
- Antigenicity
Property of a chemical structure to be recognized as a foreign substance by the immune system.
- Cellular senescence
Irreversible arrest of cell proliferation (growth) associated with specific morphological and secretory alterations, occurring in the context of specific stress responses.
- Cytotoxic T lymphocyte (CTL)
Effector cell of the immune system that can mediate the lysis of target cells.
- Danger-associated molecular pattern
Endogenous molecule that are normally invisible to the host immune system but, once emitted by stressed or dying cells, operate as endogenous adjuvants.
- Dendritic cell (DC)
Professional antigen-presenting cells that play a key role to link innate and adaptive immunity.
- Hypofractionation
The delivery of radiation therapy in a few fractions, each with a larger dose than standard 1.8 or 2 Gy.
- Immune checkpoint blocker (ICB)
Monoclonal antibody that (re)instates anticancer immunosurveillance by inhibiting immunosuppressive receptors on CTLs and NK cells.
- Immunological synapsis
Dynamic interface formed between an effector cell (T cell or NK cell) and an antigen-presenting cell (e.g., dendritic cell) or a target cell (e.g., tumor cell).
- Immunoscore
Automated image analysis and quantification of effector and memory T cells at specific areas of neoplastic lesions.
- Myeloid-derived suppressor cell (MDSC)
Member of a heterogeneous population of cells that are defined by their myeloid origin, immature state and ability to potently suppress T cell responses.
- Natural killer (NK) cell
Innate lymphoid cell that function as both cytotoxic effector and regulator of immune responses.
- Patient-derived tumor xenografts (PDTX)
Established from the transplantation of a fresh human tumor fragment from a cancer patient directly into a mouse, PDTX models usually preserve key features of a specific cancer.
- Regulated cell death (RCD)
Type of cell death that occurs in the context of failing adaptation to stress and is controlled by a dedicated molecular machinery.
- Regulatory T (Treg) cell
T cell that prevents other immune cells (including CTLs) from attacking the host tissues, hence preventing autoimmune diseases.
- Tumor-associated antigen
Antigen that are preferentially (but not uniquely) expressed by malignant cells.
- Tumor neoantigen
Antigen encoded by tumor-specific mutated genes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–9. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palucka AK, Coussens LM. The Basis of Oncoimmunology. Cell. 2016;164(6):1233–1247. doi: 10.1016/j.cell.2016.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205–14. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanpouille-Box C, Lhuillier C, Bezu L, Aranda F, Yamazaki T, Kepp O, Fucikova J, Spisek R, Demaria S, Fomenti SC, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: Immune checkpoint blockers for cancer therapy. Oncoimmunology. 2017:e1373237. doi: 10.1080/2162402X.2017.1373237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gangadhar TC, Vonderheide RH. Mitigating the toxic effects of anticancer immunotherapy. Nat Rev Clin Oncol. 2014;11(2):91–9. doi: 10.1038/nrclinonc.2013.245. [DOI] [PubMed] [Google Scholar]
- 6.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ribas A, Hodi FS, Callahan M, Konto C, Wolchok J. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med. 2013;368(14):1365–6. doi: 10.1056/NEJMc1302338. [DOI] [PubMed] [Google Scholar]
- 8.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105(4):256–65. doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spiotto M, Fu YX, Weichselbaum RR. The intersection of radiotherapy and immunotherapy: mechanisms and clinical implications. Sci Immunol. 2016;1(3) doi: 10.1126/sciimmunol.aag1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Son CH, Lee HR, Koh EK, Shin DY, Bae JH, Yang K, Park YS. Combination treatment with decitabine and ionizing radiation enhances tumor cells susceptibility of T cells. Sci Rep. 2016;6:32470. doi: 10.1038/srep32470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, Camphausen K, Luiten RM, de Ru AH, Neijssen J, Griekspoor A, Mesman E, Verreck FA, Spits H, Schlom J, van Veelen P, Neefjes JJ. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203(5):1259–71. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004;64(21):7985–94. doi: 10.1158/0008-5472.CAN-04-1525. [DOI] [PubMed] [Google Scholar]
- 13.Galluzzi L, Vitale I. Driving to Cancer on a Four-Lane Expressway. Trends Genet. 2017;33(8):491–492. doi: 10.1016/j.tig.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Lee JR, Ahn K, Kim YJ, Jung YD, Kim HS. Radiation-induced human endogenous retrovirus (HERV)-R env gene expression by epigenetic control. Radiat Res. 2012;178(5):379–84. doi: 10.1667/RR2888.1. [DOI] [PubMed] [Google Scholar]
- 15.Kim JY, Son YO, Park SW, Bae JH, Chung JS, Kim HH, Chung BS, Kim SH, Kang CD. Increase of NKG2D ligands and sensitivity to NK cell-mediated cytotoxicity of tumor cells by heat shock and ionizing radiation. Exp Mol Med. 2006;38(5):474–84. doi: 10.1038/emm.2006.56. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Soto A, Gonzalez S, Smyth MJ, Galluzzi L. Control of Metastasis by NK Cells. Cancer Cell. 2017;32(2):135–154. doi: 10.1016/j.ccell.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Kumari A, Cacan E, Greer SF, Garnett-Benson C. Turning T cells on: epigenetically enhanced expression of effector T-cell costimulatory molecules on irradiated human tumor cells. J Immunother Cancer. 2013;1:17. doi: 10.1186/2051-1426-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chakraborty M, Abrams SI, Camphausen K, Liu K, Scott T, Coleman CN, Hodge JW. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol. 2003;170(12):6338–47. doi: 10.4049/jimmunol.170.12.6338. [DOI] [PubMed] [Google Scholar]
- 19.Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17(2):97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 20.Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, Vitale I, Goubar A, Baracco EE, Remedios C, Fend L, Hannani D, Aymeric L, Ma Y, Niso-Santano M, Kepp O, Schultze JL, Tuting T, Belardelli F, Bracci L, La Sorsa V, Ziccheddu G, Sestili P, Urbani F, Delorenzi M, Lacroix-Triki M, Quidville V, Conforti R, Spano JP, Pusztai L, Poirier-Colame V, Delaloge S, Penault-Llorca F, Ladoire S, Arnould L, Cyrta J, Dessoliers MC, Eggermont A, Bianchi ME, Pittet M, Engblom C, Pfirschke C, Preville X, Uze G, Schreiber RD, Chow MT, Smyth MJ, Proietti E, Andre F, Kroemer G, Zitvogel L. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med. 2014;20(11):1301–9. doi: 10.1038/nm.3708. [DOI] [PubMed] [Google Scholar]
- 21.Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, Inghirami G, Coleman CN, Formenti SC, Demaria S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618. doi: 10.1038/ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res. 2013;1(6):365–72. doi: 10.1158/2326-6066.CIR-13-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, Sedrak C, Jungbluth AA, Chua R, Yang AS, Roman RA, Rosner S, Benson B, Allison JP, Lesokhin AM, Gnjatic S, Wolchok JD. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366(10):925–31. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demaria S, Golden EB, Formenti SC. Role of Local Radiation Therapy in Cancer Immunotherapy. JAMA Oncol. 2015;1(9):1325–32. doi: 10.1001/jamaoncol.2015.2756. [DOI] [PubMed] [Google Scholar]
- 25.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, Li XD, Mauceri H, Beckett M, Darga T, Huang X, Gajewski TF, Chen ZJ, Fu YX, Weichselbaum RR. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity. 2014;41(5):843–52. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harding SM, Benci JL, Irianto J, Discher DE, Minn AJ, Greenberg RA. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature. 2017;548(7668):466–470. doi: 10.1038/nature23470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mackenzie KJ, Carroll P, Martin CA, Murina O, Fluteau A, Simpson DJ, Olova N, Sutcliffe H, Rainger JK, Leitch A, Osborn RT, Wheeler AP, Nowotny M, Gilbert N, Chandra T, Reijns MAM, Jackson AP. cGAS surveillance of micronuclei links genome instability to innate immunity. Nature. 2017;548(7668):461–465. doi: 10.1038/nature23449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanpouille-Box C, Formenti SC, Demaria S. TREX1 dictates the immune fate of irradiated cancer cells. Oncoimmunology. 2017;6(9):e1339857. doi: 10.1080/2162402X.2017.1339857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, Haimovitz-Friedman A, Cordon-Cardo C, Kolesnick R. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293(5528):293–7. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 30.Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, Pfirschke C, Voss RH, Timke C, Umansky L, Klapproth K, Schakel K, Garbi N, Jager D, Weitz J, Schmitz-Winnenthal H, Hammerling GJ, Beckhove P. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24(5):589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Kang J, Demaria S, Formenti S. Current clinical trials testing the combination of immunotherapy with radiotherapy. J Immunother Cancer. 2016;4:51. doi: 10.1186/s40425-016-0156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vacchelli E, Bloy N, Aranda F, Buque A, Cremer I, Demaria S, Eggermont A, Formenti SC, Fridman WH, Fucikova J, Galon J, Spisek R, Tartour E, Zitvogel L, Kroemer G, Galluzzi L. Trial Watch: Immunotherapy plus radiation therapy for oncological indications. Oncoimmunology. 2016;5(9):e1214790. doi: 10.1080/2162402X.2016.1214790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, Castedo M, Kroemer G. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31(15):1869–83. doi: 10.1038/onc.2011.384. [DOI] [PubMed] [Google Scholar]
- 34.Begg AC, Stewart FA, Vens C. Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer. 2011;11(4):239–53. doi: 10.1038/nrc3007. [DOI] [PubMed] [Google Scholar]
- 35.Kirsch DG. Biomarkers for Predicting Radiation Response. Semin Radiat Oncol. 2015;25(4):225–6. doi: 10.1016/j.semradonc.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 36.Hu H, Nahas S, Gatti RA. Assaying Radiosensitivity of Ataxia-Telangiectasia. Methods Mol Biol. 2017;1599:1–11. doi: 10.1007/978-1-4939-6955-5_1. [DOI] [PubMed] [Google Scholar]
- 37.Vitale I, Manic G, De Maria R, Kroemer G, Galluzzi L. DNA Damage in Stem Cells. Mol Cell. 2017;66(3):306–319. doi: 10.1016/j.molcel.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 38.Galluzzi L, Bravo-San Pedro JM, Kroemer G. Organelle-specific initiation of cell death. Nat Cell Biol. 2014;16(8):728–36. doi: 10.1038/ncb3005. [DOI] [PubMed] [Google Scholar]
- 39.Choudhury A, Nelson LD, Teo MT, Chilka S, Bhattarai S, Johnston CF, Elliott F, Lowery J, Taylor CF, Churchman M, Bentley J, Knowles MA, Harnden P, Bristow RG, Bishop DT, Kiltie AE. MRE11 expression is predictive of cause-specific survival following radical radiotherapy for muscle-invasive bladder cancer. Cancer Res. 2010;70(18):7017–26. doi: 10.1158/0008-5472.CAN-10-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laurberg JR, Brems-Eskildsen AS, Nordentoft I, Fristrup N, Schepeler T, Ulhoi BP, Agerbaek M, Hartmann A, Bertz S, Wittlinger M, Fietkau R, Rodel C, Borre M, Jensen JB, Orntoft T, Dyrskjot L. Expression of TIP60 (tat-interactive protein) and MRE11 (meiotic recombination 11 homolog) predict treatment-specific outcome of localised invasive bladder cancer. BJU Int. 2012;110(11 Pt C):E1228–36. doi: 10.1111/j.1464-410X.2012.11564.x. [DOI] [PubMed] [Google Scholar]
- 41.Berlin A, Lalonde E, Sykes J, Zafarana G, Chu KC, Ramnarine VR, Ishkanian A, Sendorek DH, Pasic I, Lam WL, Jurisica I, van der Kwast T, Milosevic M, Boutros PC, Bristow RG. NBN gain is predictive for adverse outcome following image-guided radiotherapy for localized prostate cancer. Oncotarget. 2014;5(22):11081–90. doi: 10.18632/oncotarget.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alsubhi N, Middleton F, Abdel-Fatah TM, Stephens P, Doherty R, Arora A, Moseley PM, Chan SY, Aleskandarany MA, Green AR, Rakha EA, Ellis IO, Martin SG, Curtin NJ, Madhusudan S. Chk1 phosphorylated at serine345 is a predictor of early local recurrence and radio-resistance in breast cancer. Mol Oncol. 2016;10(2):213–23. doi: 10.1016/j.molonc.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin OA, Anderson RL, Russell PA, Cox RA, Ivashkevich A, Swierczak A, Doherty JP, Jacobs DH, Smith J, Siva S, Daly PE, Ball DL, Martin RF, MacManus MP. Mobilization of viable tumor cells into the circulation during radiation therapy. Int J Radiat Oncol Biol Phys. 2014;88(2):395–403. doi: 10.1016/j.ijrobp.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 44.Lassen P, Eriksen JG, Hamilton-Dutoit S, Tramm T, Alsner J, Overgaard J. Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27(12):1992–8. doi: 10.1200/JCO.2008.20.2853. [DOI] [PubMed] [Google Scholar]
- 45.Lassen P, Eriksen JG, Hamilton-Dutoit S, Tramm T, Alsner J, Overgaard J, Danish H G. Neck Cancer. HPV-associated p16-expression and response to hypoxic modification of radiotherapy in head and neck cancer. Radiother Oncol. 2010;94(1):30–5. doi: 10.1016/j.radonc.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 46.Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, Forastiere A, Gillison ML. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261–9. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 47.Wang L, Zhang P, Molkentine DP, Chen C, Molkentine JM, Piao H, Raju U, Zhang J, Valdecanas DR, Tailor RC, Thames HD, Buchholz TA, Chen J, Ma L, Mason KA, Ang KK, Meyn RE, Skinner HD. TRIP12 as a mediator of human papillomavirus/p16-related radiation enhancement effects. Oncogene. 2017;36(6):820–828. doi: 10.1038/onc.2016.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walker AK, Kartsonaki C, Collantes E, Nicholson J, Gilbert DC, Kiltie AE. No additional prognostic value for MRE11 in squamous cell carcinomas of the anus treated with chemo-radiotherapy. Br J Cancer. 2017;117(3):322–325. doi: 10.1038/bjc.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Canman CE, Lim DS, Cimprich KA, Taya Y, Tamai K, Sakaguchi K, Appella E, Kastan MB, Siliciano JD. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281(5383):1677–9. doi: 10.1126/science.281.5383.1677. [DOI] [PubMed] [Google Scholar]
- 50.Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 1995;377(6549):552–7. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- 51.Kim HS, Kim SC, Kim SJ, Park CH, Jeung HC, Kim YB, Ahn JB, Chung HC, Rha SY. Identification of a radiosensitivity signature using integrative metaanalysis of published microarray data for NCI-60 cancer cells. BMC Genomics. 2012;13:348. doi: 10.1186/1471-2164-13-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meng J, Li P, Zhang Q, Yang Z, Fu S. A radiosensitivity gene signature in predicting glioma prognostic via EMT pathway. Oncotarget. 2014;5(13):4683–93. doi: 10.18632/oncotarget.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khodarev NN, Beckett M, Labay E, Darga T, Roizman B, Weichselbaum RR. STAT1 is overexpressed in tumors selected for radioresistance and confers protection from radiation in transduced sensitive cells. Proc Natl Acad Sci U S A. 2004;101(6):1714–9. doi: 10.1073/pnas.0308102100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weichselbaum RR, Ishwaran H, Yoon T, Nuyten DS, Baker SW, Khodarev N, Su AW, Shaikh AY, Roach P, Kreike B, Roizman B, Bergh J, Pawitan Y, van de Vijver MJ, Minn AJ. An interferon-related gene signature for DNA damage resistance is a predictive marker for chemotherapy and radiation for breast cancer. Proc Natl Acad Sci U S A. 2008;105(47):18490–5. doi: 10.1073/pnas.0809242105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zitvogel L, Galluzzi L, Kepp O, Smyth MJ, Kroemer G. Type I interferons in anticancer immunity. Nat Rev Immunol. 2015;15(7):405–14. doi: 10.1038/nri3845. [DOI] [PubMed] [Google Scholar]
- 56.Scott JG, Berglund A, Schell MJ, Mihaylov I, Fulp WJ, Yue B, Welsh E, Caudell JJ, Ahmed K, Strom TS, Mellon E, Venkat P, Johnstone P, Foekens J, Lee J, Moros E, Dalton WS, Eschrich SA, McLeod H, Harrison LB, Torres-Roca JF. A genome-based model for adjusting radiotherapy dose (GARD): a retrospective, cohort-based study. Lancet Oncol. 2017;18(2):202–211. doi: 10.1016/S1470-2045(16)30648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bracken CP, Scott HS, Goodall GJ. A network-biology perspective of microRNA function and dysfunction in cancer. Nat Rev Genet. 2016;17(12):719–732. doi: 10.1038/nrg.2016.134. [DOI] [PubMed] [Google Scholar]
- 58.Czochor JR, Glazer PM. microRNAs in cancer cell response to ionizing radiation. Antioxid Redox Signal. 2014;21(2):293–312. doi: 10.1089/ars.2013.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Korpela E, Vesprini D, Liu SK. MicroRNA in radiotherapy: miRage or miRador? Br J Cancer. 2015;112(5):777–82. doi: 10.1038/bjc.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Metheetrairut C, Slack FJ. MicroRNAs in the ionizing radiation response and in radiotherapy. Curr Opin Genet Dev. 2013;23(1):12–9. doi: 10.1016/j.gde.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang P, Rao EY, Meng N, Zhao Y, Wang JJ. MicroRNA-17-92 significantly enhances radioresistance in human mantle cell lymphoma cells. Radiat Oncol. 2010;5:100. doi: 10.1186/1748-717X-5-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kato M, Paranjape T, Muller RU, Nallur S, Gillespie E, Keane K, Esquela-Kerscher A, Weidhaas JB, Slack FJ. The mir-34 microRNA is required for the DNA damage response in vivo in C. elegans and in vitro in human breast cancer cells. Oncogene. 2009;28(25):2419–24. doi: 10.1038/onc.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zenz T, Mohr J, Eldering E, Kater AP, Buhler A, Kienle D, Winkler D, Durig J, van Oers MH, Mertens D, Dohner H, Stilgenbauer S. miR-34a as part of the resistance network in chronic lymphocytic leukemia. Blood. 2009;113(16):3801–8. doi: 10.1182/blood-2008-08-172254. [DOI] [PubMed] [Google Scholar]
- 64.Gong P, Zhang T, He D, Hsieh JT. MicroRNA-145 Modulates Tumor Sensitivity to Radiation in Prostate Cancer. Radiat Res. 2015;184(6):630–8. doi: 10.1667/RR14185.1. [DOI] [PubMed] [Google Scholar]
- 65.Yan S, Li X, Jin Q, Yuan J. MicroRNA-145 sensitizes cervical cancer cells to low-dose irradiation by downregulating OCT4 expression. Exp Ther Med. 2016;12(5):3130–3136. doi: 10.3892/etm.2016.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang P, Wang L, Rodriguez-Aguayo C, Yuan Y, Debeb BG, Chen D, Sun Y, You MJ, Liu Y, Dean DC, Woodward WA, Liang H, Yang X, Lopez-Berestein G, Sood AK, Hu Y, Ang KK, Chen J, Ma L. miR-205 acts as a tumour radiosensitizer by targeting ZEB1 and Ubc13. Nat Commun. 2014;5:5671. doi: 10.1038/ncomms6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.He J, Feng X, Hua J, Wei L, Lu Z, Wei W, Cai H, Wang B, Shi W, Ding N, Li H, Zhang Y, Wang J. miR-300 regulates cellular radiosensitivity through targeting p53 and apaf1 in human lung cancer cells. Cell Cycle. 2017:1–11. doi: 10.1080/15384101.2017.1367070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Park M, Yoon HJ, Kang MC, Kwon J, Lee HW. MiR-338-5p enhances the radiosensitivity of esophageal squamous cell carcinoma by inducing apoptosis through targeting survivin. Sci Rep. 2017;7(1):10932. doi: 10.1038/s41598-017-10977-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wei H, Wen-Ming C, Jun-Bo J. Plasma miR-145 as a novel biomarker for the diagnosis and radiosensitivity prediction of human cervical cancer. J Int Med Res. 2017;45(3):1054–1060. doi: 10.1177/0300060517709614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hu X, Schwarz JK, Lewis JS, Jr, Huettner PC, Rader JS, Deasy JO, Grigsby PW, Wang X. A microRNA expression signature for cervical cancer prognosis. Cancer Res. 2010;70(4):1441–8. doi: 10.1158/0008-5472.CAN-09-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Niyazi M, Zehentmayr F, Niemoller OM, Eigenbrod S, Kretzschmar H, Schulze-Osthoff K, Tonn JC, Atkinson M, Mortl S, Belka C. MiRNA expression patterns predict survival in glioblastoma. Radiat Oncol. 2011;6:153. doi: 10.1186/1748-717X-6-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Svoboda M, Sana J, Fabian P, Kocakova I, Gombosova J, Nekvindova J, Radova L, Vyzula R, Slaby O. MicroRNA expression profile associated with response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer patients. Radiat Oncol. 2012;7:195. doi: 10.1186/1748-717X-7-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Millino C, Maretto I, Pacchioni B, Digito M, De Paoli A, Canzonieri V, D'Angelo E, Agostini M, Rizzolio F, Giordano A, Barina A, Rajendran S, Esposito G, Lanfranchi G, Nitti D, Pucciarelli S. Gene and MicroRNA Expression Are Predictive of Tumor Response in Rectal Adenocarcinoma Patients Treated With Preoperative Chemoradiotherapy. J Cell Physiol. 2017;232(2):426–435. doi: 10.1002/jcp.25441. [DOI] [PubMed] [Google Scholar]
- 74.Zhu Y, Peng Q, Lin Y, Zou L, Shen P, Chen F, Min M, Shen L, Chen J, Shen B. Identification of biomarker microRNAs for predicting the response of colorectal cancer to neoadjuvant chemoradiotherapy based on microRNA regulatory network. Oncotarget. 2017;8(2):2233–2248. doi: 10.18632/oncotarget.13659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hill RP, Bristow RG, Fyles A, Koritzinsky M, Milosevic M, Wouters BG. Hypoxia and Predicting Radiation Response. Semin Radiat Oncol. 2015;25(4):260–72. doi: 10.1016/j.semradonc.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 76.Young RJ, Moller A. Immunohistochemical detection of tumour hypoxia. Methods Mol Biol. 2010;611:151–9. doi: 10.1007/978-1-60327-345-9_12. [DOI] [PubMed] [Google Scholar]
- 77.Moreno-Acosta P, Vallard A, Carrillo S, Gamboa O, Romero-Rojas A, Molano M, Acosta J, Mayorga D, Rancoule C, Garcia MA, Cotes Mestre M, Magne N. Biomarkers of resistance to radiation therapy: a prospective study in cervical carcinoma. Radiat Oncol. 2017;12(1):120. doi: 10.1186/s13014-017-0856-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Salem A, Asselin MC, Reymen B, Jackson A, Lambin P, West CML, O'Connor JPB, Faivre-Finn C. Targeting Hypoxia to Improve Non-Small Cell Lung Cancer Outcome. J Natl Cancer Inst. 2018;110(1) doi: 10.1093/jnci/djx160. [DOI] [PubMed] [Google Scholar]
- 79.Aebersold DM, Burri P, Beer KT, Laissue J, Djonov V, Greiner RH, Semenza GL. Expression of hypoxia-inducible factor-1alpha: a novel predictive and prognostic parameter in the radiotherapy of oropharyngeal cancer. Cancer Res. 2001;61(7):2911–6. [PubMed] [Google Scholar]
- 80.Ishikawa H, Sakurai H, Hasegawa M, Mitsuhashi N, Takahashi M, Masuda N, Nakajima M, Kitamoto Y, Saitoh J, Nakano T. Expression of hypoxic-inducible factor 1alpha predicts metastasis-free survival after radiation therapy alone in stage IIIB cervical squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2004;60(2):513–21. doi: 10.1016/j.ijrobp.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 81.Braunstein S, Karpisheva K, Pola C, Goldberg J, Hochman T, Yee H, Cangiarella J, Arju R, Formenti SC, Schneider RJ. A hypoxia-controlled cap-dependent to cap-independent translation switch in breast cancer. Mol Cell. 2007;28(3):501–12. doi: 10.1016/j.molcel.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 82.Huber V, Camisaschi C, Berzi A, Ferro S, Lugini L, Triulzi T, Tuccitto A, Tagliabue E, Castelli C, Rivoltini L. Cancer acidity: An ultimate frontier of tumor immune escape and a novel target of immunomodulation. Semin Cancer Biol. 2017;43:74–89. doi: 10.1016/j.semcancer.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 83.Wennerberg E, Lhuillier C, Vanpouille-Box C, Pilones KA, Garcia-Martinez E, Rudqvist NP, Formenti SC, Demaria S. Barriers to Radiation-Induced In Situ Tumor Vaccination. Front Immunol. 2017;8:229. doi: 10.3389/fimmu.2017.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fridman WH, Zitvogel L, Sautes-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017 doi: 10.1038/nrclinonc.2017.101. [DOI] [PubMed] [Google Scholar]
- 85.Semeraro M, Adam J, Stoll G, Louvet E, Chaba K, Poirier-Colame V, Sauvat A, Senovilla L, Vacchelli E, Bloy N, Humeau J, Buque A, Kepp O, Zitvogel L, Andre F, Mathieu MC, Delaloge S, Kroemer G. The ratio of CD8+/FOXP3 T lymphocytes infiltrating breast tissues predicts the relapse of ductal carcinoma in situ. Oncoimmunology. 2016;5(10):e1218106. doi: 10.1080/2162402X.2016.1218106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, Rouas G, Francis P, Crown JP, Hitre E, de Azambuja E, Quinaux E, Di Leo A, Michiels S, Piccart MJ, Sotiriou C. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31(7):860–7. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 87.Formenti SC, Golden EB, Goldberg JD, Li X, Taff J, Fenton-Kerimian MB, Chandrasekhar S, Demaria S, Novik Y. Results of a phase I-II study of adjuvant concurrent carboplatin and accelerated radiotherapy for triple negative breast cancer. Oncoimmunology. 2017;6(3):e1274479. doi: 10.1080/2162402X.2016.1274479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Anitei MG, Zeitoun G, Mlecnik B, Marliot F, Haicheur N, Todosi AM, Kirilovsky A, Lagorce C, Bindea G, Ferariu D, Danciu M, Bruneval P, Scripcariu V, Chevallier JM, Zinzindohoue F, Berger A, Galon J, Pages F. Prognostic and predictive values of the immunoscore in patients with rectal cancer. Clin Cancer Res. 2014;20(7):1891–9. doi: 10.1158/1078-0432.CCR-13-2830. [DOI] [PubMed] [Google Scholar]
- 89.Berghoff AS, Fuchs E, Ricken G, Mlecnik B, Bindea G, Spanberger T, Hackl M, Widhalm G, Dieckmann K, Prayer D, Bilocq A, Heinzl H, Zielinski C, Bartsch R, Birner P, Galon J, Preusser M. Density of tumor-infiltrating lymphocytes correlates with extent of brain edema and overall survival time in patients with brain metastases. Oncoimmunology. 2016;5(1):e1057388. doi: 10.1080/2162402X.2015.1057388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.O'Sullivan B, Huang SH, Siu LL, Waldron J, Zhao H, Perez-Ordonez B, Weinreb I, Kim J, Ringash J, Bayley A, Dawson LA, Hope A, Cho J, Irish J, Gilbert R, Gullane P, Hui A, Liu FF, Chen E, Xu W. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol. 2013;31(5):543–50. doi: 10.1200/JCO.2012.44.0164. [DOI] [PubMed] [Google Scholar]
- 91.Bush NA, Butowski N. The Effect of Molecular Diagnostics on the Treatment of Glioma. Curr Oncol Rep. 2017;19(4):26. doi: 10.1007/s11912-017-0585-6. [DOI] [PubMed] [Google Scholar]
- 92.Jhaveri J, Liu Y, Chowdhary M, Buchwald ZS, Gillespie TW, Olson JJ, Voloschin AD, Eaton BR, Shu HG, Crocker IR, Curran WJ, Patel KR. Is less more? Comparing chemotherapy alone with chemotherapy and radiation for high-risk grade 2 glioma: An analysis of the National Cancer Data Base. Cancer. 2017 doi: 10.1002/cncr.31158. [DOI] [PubMed] [Google Scholar]
- 93.Tartari F, Santoni M, Burattini L, Mazzanti P, Onofri A, Berardi R. Economic sustainability of anti-PD-1 agents nivolumab and pembrolizumab in cancer patients: Recent insights and future challenges. Cancer Treat Rev. 2016;48:20–4. doi: 10.1016/j.ctrv.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 94.Nishino M, Ramaiya NH, Hatabu H, Hodi FS. Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat Rev Clin Oncol. 2017 doi: 10.1038/nrclinonc.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541(7637):321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 96.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, Mischel PS, Stokoe D, Pieper RO. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13(1):84–8. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 97.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhaufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crino L, Blumenschein GR, Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(17):1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, Savage KJ, Hernberg MM, Lebbe C, Charles J, Mihalcioiu C, Chiarion-Sileni V, Mauch C, Cognetti F, Arance A, Schmidt H, Schadendorf D, Gogas H, Lundgren-Eriksson L, Horak C, Sharkey B, Waxman IM, Atkinson V, Ascierto PA. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–30. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 101.Weber JS, Kudchadkar RR, Yu B, Gallenstein D, Horak CE, Inzunza HD, Zhao X, Martinez AJ, Wang W, Gibney G, Kroeger J, Eysmans C, Sarnaik AA, Chen YA. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013;31(34):4311–8. doi: 10.1200/JCO.2013.51.4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Paluch BE, Glenn ST, Conroy JM, Papanicolau-Sengos A, Bshara W, Omilian AR, Brese E, Nesline M, Burgher B, Andreas J, Odunsi K, Eng K, He J, Qin M, Gardner M, Galluzzi L, Morrison CD. Robust detection of immune transcripts in FFPE samples using targeted RNA sequencing. Oncotarget. 2017;8(2):3197–3205. doi: 10.18632/oncotarget.13691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rimm DL, Han G, Taube JM, Yi ES, Bridge JA, Flieder DB, Homer R, West WW, Wu H, Roden AC, Fujimoto J, Yu H, Anders R, Kowalewski A, Rivard C, Rehman J, Batenchuk C, Burns V, Hirsch FR, Wistuba A Prospective, Multi-institutional, Pathologist-Based Assessment of 4 Immunohistochemistry Assays for PD-L1 Expression in Non-Small Cell Lung Cancer. JAMA Oncol. 2017;3(8):1051–1058. doi: 10.1001/jamaoncol.2017.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nods for Atezolizumab and Nivolumab from FDA. Cancer Discov. 2016;6(8):811. doi: 10.1158/2159-8290.CD-NB2016-080. [DOI] [PubMed] [Google Scholar]
- 105.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 106.Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, Ivanova Y, Hundal J, Arthur CD, Krebber WJ, Mulder GE, Toebes M, Vesely MD, Lam SS, Korman AJ, Allison JP, Freeman GJ, Sharpe AH, Pearce EL, Schumacher TN, Aebersold R, Rammensee HG, Melief CJ, Mardis ER, Gillanders WE, Artyomov MN, Schreiber RD. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515(7528):577–81. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, Jamal-Hanjani M, Wilson GA, Birkbak NJ, Hiley CT, Watkins TB, Shafi S, Murugaesu N, Mitter R, Akarca AU, Linares J, Marafioti T, Henry JY, Van Allen EM, Miao D, Schilling B, Schadendorf D, Garraway LA, Makarov V, Rizvi NA, Snyder A, Hellmann MD, Merghoub T, Wolchok JD, Shukla SA, Wu CJ, Peggs KS, Chan TA, Hadrup SR, Quezada SA, Swanton C. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351(6280):1463–9. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, Rekhtman N, Moreira AL, Ibrahim F, Bruggeman C, Gasmi B, Zappasodi R, Maeda Y, Sander C, Garon EB, Merghoub T, Wolchok JD, Schumacher TN, Chan TA. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–8. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, Sucker A, Hillen U, Foppen MHG, Goldinger SM, Utikal J, Hassel JC, Weide B, Kaehler KC, Loquai C, Mohr P, Gutzmer R, Dummer R, Gabriel S, Wu CJ, Schadendorf D, Garraway LA. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350(6257):207–211. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, Stephens PJ, Daniels GA, Kurzrock R. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol Cancer Ther. 2017;16(11):2598–2608. doi: 10.1158/1535-7163.MCT-17-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA., Jr PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372(26):2509–20. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA., Jr Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hewish M, Lord CJ, Martin SA, Cunningham D, Ashworth A. Mismatch repair deficient colorectal cancer in the era of personalized treatment. Nat Rev Clin Oncol. 2010;7(4):197–208. doi: 10.1038/nrclinonc.2010.18. [DOI] [PubMed] [Google Scholar]
- 114.Nivolumab Has Antitumor Activity in dMMR/MSI-H Colorectal Cancer. Cancer Discov. 2017;7(9):930. [Google Scholar]
- 115.Kawakami H, Zaanan A, Sinicrope FA. Microsatellite instability testing and its role in the management of colorectal cancer. Curr Treat Options Oncol. 2015;16(7):30. doi: 10.1007/s11864-015-0348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vitale I, Manic G, Senovilla L, Kroemer G, Galluzzi L. Karyotypic Aberrations in Oncogenesis and Cancer Therapy. Trends Cancer. 2015;1(2):124–135. doi: 10.1016/j.trecan.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 117.Jenkins RW, Thummalapalli R, Carter J, Canadas I, Barbie DA. Molecular and Genomic Determinants of Response to Immune Checkpoint Inhibition in Cancer. Annu Rev Med. 2017 doi: 10.1146/annurev-med-060116-022926. [DOI] [PubMed] [Google Scholar]
- 118.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell. 2017;168(4):707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY, Abril-Rodriguez G, Sandoval S, Barthly L, Saco J, Homet Moreno B, Mezzadra R, Chmielowski B, Ruchalski K, Shintaku IP, Sanchez PJ, Puig-Saus C, Cherry G, Seja E, Kong X, Pang J, Berent-Maoz B, Comin-Anduix B, Graeber TG, Tumeh PC, Schumacher TN, Lo RS, Ribas A. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N Engl J Med. 2016;375(9):819–29. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gao J, Shi LZ, Zhao H, Chen J, Xiong L, He Q, Chen T, Roszik J, Bernatchez C, Woodman SE, Chen PL, Hwu P, Allison JP, Futreal A, Wargo JA, Sharma P. Loss of IFN-gamma Pathway Genes in Tumor Cells as a Mechanism of Resistance to Anti-CTLA-4 Therapy. Cell. 2016;167(2):397–404 e9. doi: 10.1016/j.cell.2016.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Patel SJ, Sanjana NE, Kishton RJ, Eidizadeh A, Vodnala SK, Cam M, Gartner JJ, Jia L, Steinberg SM, Yamamoto TN, Merchant AS, Mehta GU, Chichura A, Shalem O, Tran E, Eil R, Sukumar M, Guijarro EP, Day CP, Robbins P, Feldman S, Merlino G, Zhang F, Restifo NP. Identification of essential genes for cancer immunotherapy. Nature. 2017;548(7669):537–542. doi: 10.1038/nature23477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature. 2015;523(7559):231–5. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 123.Benci JL, Xu B, Qiu Y, Wu TJ, Dada H, Twyman-Saint Victor C, Cucolo L, Lee DS, Pauken KE, Huang AC, Gangadhar TC, Amaravadi RK, Schuchter LM, Feldman MD, Ishwaran H, Vonderheide RH, Maity A, Wherry EJ, Minn AJ. Tumor Interferon Signaling Regulates a Multigenic Resistance Program to Immune Checkpoint Blockade. Cell. 2016;167(6):1540–1554 e12. doi: 10.1016/j.cell.2016.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Riaz N, Havel JJ, Makarov V, Desrichard A, Urba WJ, Sims JS, Hodi FS, Martin-Algarra S, Mandal R, Sharfman WH, Bhatia S, Hwu WJ, Gajewski TF, Slingluff CL, Jr, Chowell D, Kendall SM, Chang H, Shah R, Kuo F, Morris LGT, Sidhom JW, Schneck JP, Horak CE, Weinhold N, Chan TA. Tumor and Microenvironment Evolution during Immunotherapy with Nivolumab. Cell. 2017;171(4):934–949 e15. doi: 10.1016/j.cell.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–71. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stoll G, Zitvogel L, Kroemer G. Differences in the composition of the immune infiltrate in breast cancer, colorectal carcinoma, melanoma and non-small cell lung cancer: A microarray-based meta-analysis. Oncoimmunology. 2016;5(2):e1067746. doi: 10.1080/2162402X.2015.1067746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mlecnik B, Bindea G, Angell HK, Maby P, Angelova M, Tougeron D, Church SE, Lafontaine L, Fischer M, Fredriksen T, Sasso M, Bilocq AM, Kirilovsky A, Obenauf AC, Hamieh M, Berger A, Bruneval P, Tuech JJ, Sabourin JC, Le Pessot F, Mauillon J, Rafii A, Laurent-Puig P, Speicher MR, Trajanoski Z, Michel P, Sesboue R, Frebourg T, Pages F, Valge-Archer V, Latouche JB, Galon J. Integrative Analyses of Colorectal Cancer Show Immunoscore Is a Stronger Predictor of Patient Survival Than Microsatellite Instability. Immunity. 2016;44(3):698–711. doi: 10.1016/j.immuni.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 128.Sade-Feldman M, Kanterman J, Klieger Y, Ish-Shalom E, Olga M, Saragovi A, Shtainberg H, Lotem M, Baniyash M. Clinical Significance of Circulating CD33+CD11b+HLA-DR- Myeloid Cells in Patients with Stage IV Melanoma Treated with Ipilimumab. Clin Cancer Res. 2016;22(23):5661–5672. doi: 10.1158/1078-0432.CCR-15-3104. [DOI] [PubMed] [Google Scholar]
- 129.Blank CU, Haanen JB, Ribas A, Schumacher TN. CANCER IMMUNOLOGY. The "cancer immunogram". Science. 2016;352(6286):658–60. doi: 10.1126/science.aaf2834. [DOI] [PubMed] [Google Scholar]
- 130.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeno J, Wadsworth C, Melillo G, Jiang H, Huang Y, Dennis PA, Ozguroglu M P. Investigators. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377(20):1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 131.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124(2):687–95. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, Herati RS, Mansfield KD, Patsch D, Amaravadi RK, Schuchter LM, Ishwaran H, Mick R, Pryma DA, Xu X, Feldman MD, Gangadhar TC, Hahn SM, Wherry EJ, Vonderheide RH, Minn AJ. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–7. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ruocco MG, Pilones KA, Kawashima N, Cammer M, Huang J, Babb JS, Liu M, Formenti SC, Dustin ML, Demaria S. Suppressing T cell motility induced by anti-CTLA-4 monotherapy improves antitumor effects. J Clin Invest. 2012;122(10):3718–30. doi: 10.1172/JCI61931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lopez-Soto A, Gonzalez S, Galluzzi L. Soluble NKG2D ligands limit the efficacy of immune checkpoint blockade. Oncoimmunology. 2017 doi: 10.1080/2162402X.2017.1346766. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Maccalli C, Giannarelli D, Capocefalo F, Pilla L, Fonsatti E, Di Giacomo AM, Parmiani G, Maio M. Immunological markers and clinical outcome of advanced melanoma patients receiving ipilimumab plus fotemustine in the NIBIT-M1 study. Oncoimmunology. 2016;5(2):e1071007. doi: 10.1080/2162402X.2015.1071007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Maccalli C, Giannarelli D, Chiarucci C, Cutaia O, Giacobini G, Hendrickx W, Amato G, Annesi D, Bedognetti D, Altomonte M, Danielli R, Calabro L, Di Giacomo AM, Marincola FM, Parmiani G, Maio M. Soluble NKG2D ligands are biomarkers associated with the clinical outcome to immune checkpoint blockade therapy of metastatic melanoma patients. Oncoimmunology. 2017;6(7):e1323618. doi: 10.1080/2162402X.2017.1323618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Koguchi Y, Hoen HM, Bambina SA, Rynning MD, Fuerstenberg RK, Curti BD, Urba WJ, Milburn C, Bahjat FR, Korman AJ, Bahjat KS. Serum Immunoregulatory Proteins as Predictors of Overall Survival of Metastatic Melanoma Patients Treated with Ipilimumab. Cancer Res. 2015;75(23):5084–92. doi: 10.1158/0008-5472.CAN-15-2303. [DOI] [PubMed] [Google Scholar]
- 138.Jinushi M, Hodi FS, Dranoff G. Therapy-induced antibodies to MHC class I chain-related protein A antagonize immune suppression and stimulate antitumor cytotoxicity. Proc Natl Acad Sci U S A. 2006;103(24):9190–5. doi: 10.1073/pnas.0603503103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wu J. Antibody targeting soluble NKG2D ligand sMIC refuels and invigorates the endogenous immune system to fight cancer. Oncoimmunology. 2016;5(3):e1095434. doi: 10.1080/2162402X.2015.1095434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bald T, Landsberg J, Lopez-Ramos D, Renn M, Glodde N, Jansen P, Gaffal E, Steitz J, Tolba R, Kalinke U, Limmer A, Jonsson G, Holzel M, Tuting T. Immune cell-poor melanomas benefit from PD-1 blockade after targeted type I IFN activation. Cancer Discov. 2014;4(6):674–87. doi: 10.1158/2159-8290.CD-13-0458. [DOI] [PubMed] [Google Scholar]
- 141.Dunn GP, Bruce AT, Sheehan KC, Shankaran V, Uppaluri R, Bui JD, Diamond MS, Koebel CM, Arthur C, White JM, Schreiber RD. A critical function for type I interferons in cancer immunoediting. Nat Immunol. 2005;6(7):722–9. doi: 10.1038/ni1213. [DOI] [PubMed] [Google Scholar]
- 142.Lim JY, Gerber SA, Murphy SP, Lord EM. Type I interferons induced by radiation therapy mediate recruitment and effector function of CD8(+) T cells. Cancer Immunol Immunother. 2014;63(3):259–71. doi: 10.1007/s00262-013-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, Fu YX, Auh SL. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71(7):2488–96. doi: 10.1158/0008-5472.CAN-10-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, Schreiber RD, Murphy TL, Murphy KM. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322(5904):1097–100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MY, Duggan R, Wang Y, Barber GN, Fitzgerald KA, Alegre ML, Gajewski TF. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41(5):830–42. doi: 10.1016/j.immuni.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xia T, Konno H, Ahn J, Barber GN. Deregulation of STING Signaling in Colorectal Carcinoma Constrains DNA Damage Responses and Correlates With Tumorigenesis. Cell Rep. 2016;14(2):282–97. doi: 10.1016/j.celrep.2015.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xia T, Konno H, Barber GN. Recurrent Loss of STING Signaling in Melanoma Correlates with Susceptibility to Viral Oncolysis. Cancer Res. 2016;76(22):6747–6759. doi: 10.1158/0008-5472.CAN-16-1404. [DOI] [PubMed] [Google Scholar]
- 148.Vanpouille-Box C, Formenti SC, Demaria S. Towards precision radiotherapy for use with immune checkpoint blockers. Clin Cancer Res. 2017 doi: 10.1158/1078-0432.CCR-16-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chaudhuri AA, Binkley MS, Osmundson EC, Alizadeh AA, Diehn M. Predicting Radiotherapy Responses and Treatment Outcomes Through Analysis of Circulating Tumor DNA. Semin Radiat Oncol. 2015;25(4):305–12. doi: 10.1016/j.semradonc.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kok M, Horlings HM, van de Vijver K, Wiersma T, Russel N, Voorwerk L, Sikorska K, van Werkhoven E, Mandjes IA, Kemper I, Foekema J, Wilgenhof S, Chalabi M, Stouthard J, Sonke GS, Cullen D, Salgado R, Schumacher TNM, Blank CU, Linn SC. Adaptive phase II randomized non-comparative trial of nivolumab after induction treatment in triple negative breast cancer: TONIC-trial. Annals of Oncology. 2017;28(suppl_5):v605–v629. [Google Scholar]