Abstract
Several bioactive compounds and nutrients in foods have physiological properties that are beneficial for human health. While nutrients typically have clear definitions with established levels of recommended intakes, bioactive compounds often lack such a definition. Although a food-based approach is often the optimal approach to ensure adequate intake of bioactives and nutrients, these components are also often produced as dietary supplements. However, many of these supplements are not sufficiently studied and have an unclear role in chronic disease prevention. Randomized trials are considered the gold standard of study designs, but have not been fully applied to understand the effects of bioactives and nutrients. We review the specific role of large-scale trials to test whether bioactives and nutrients have an effect on health outcomes through several crucial components of trial design, including selection of intervention, recruitment, compliance, outcome selection, and interpretation and generalizability of study findings. We will discuss these components in the context of two randomized clinical trials, the VITamin D and OmegA-3 TriaL (VITAL) and the COcoa Supplement and Multivitamin Outcomes Study (COSMOS). We will mainly focus on dietary supplements of bioactives and nutrients while also emphasizing the need for translation and integration with food-based trials that are of vital importance in within nutritional research.
1 INTRODUCTION
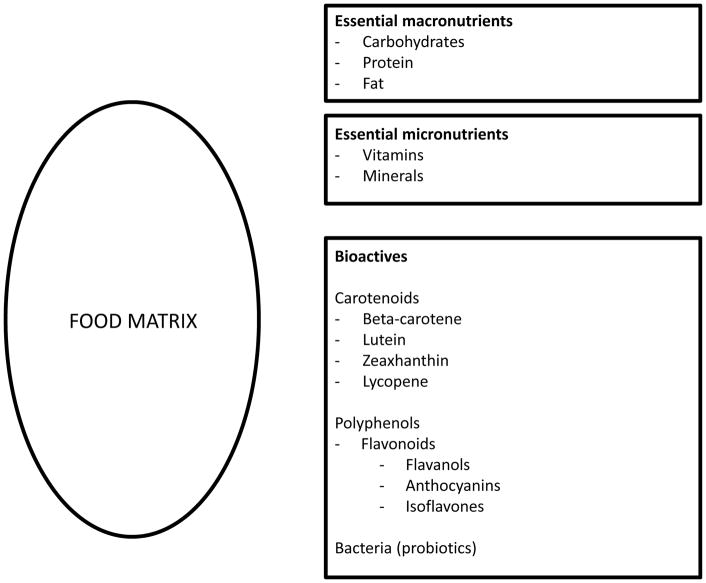
Essential macronutrients and micronutrients are defined as compounds in food that contribute to growth and health by providing energy, regulation of metabolism, cell building or structural materials that regulate body chemistry in living organims.1 Essential nutrients include vitamins, minerals, and trace elements with established dietary intake recommendations, frequently having a recommended dietary allowance (RDA) indicating the required intake needed to meet nutritional requirements and tolerable upper intake level indicating maximum level unlikely to cause adverse health effects. Absence of these nutrients leads to deficiency-related diseases. Food bioactives refer to an expanded array of components that have physiological effects and may modulate health risks, but their absence is not linked to distinct deficiency-related diseases. However, food bioactives currently lack a universally accepted definition and are commonly defined as “constituents in foods or dietary supplements, other than those needed to meet basic human needs, which are responsible for changes in health status” (Figure 1).2 In this review, we will refer bioactives as food constituents that have physiological effects but are not considered essential macro- or micronutrients.
Figure 1.
Examples of bioactives and nutrients in food
Research on food bioactives represents an emerging strategy through which to evaluate the role of functional foods and supplements in health and disease prevention. Bioactives are predominantly found in plant-based foods. Observational studies have suggested that foods such as fruits and vegetables, nuts, chocolate, and fatty fish, as well as beverages such as tea, wine, and coffee, are associated with a wide range of health benefits.3 As a result, many have postulated that various bioactives and/or nutrients in these foods may be responsible for the observed health-related effects. Promising bioactive components of foods have often been isolated and produced as supplements available in many forms (tablets, capsules, gelatin capsules, soft gels, liquids, chewable preparations and powders). Many supplements containing bioactives and nutrients are not well studied and have an unclear role in chronic disease prevention.4 Yet annual sales of dietary supplements continue to rise in the US, Europe, and Asia.5 This may be explained in part by the perception that supplements containing bioactives and nutrients help ensure an adequate intake not only to prevent deficiency of essential vitamins and minerals, but also to potentially reduce the risk of major chronic diseases.6
Randomized trials are considered the gold standard of study designs to provide definitive answers on the effects of food bioactives and nutrients for disease prevention. Randomized trials have already improved our understanding of the role of several vitamin and mineral supplements in health and disease.4 However, the randomized trial design has not yet been fully applied in testing bioactives, particularly when considering large-scale trials with long-term health outcomes.
Therefore, we describe the role of large-scale clinical trials to test whether food bioactives and nutrients have an effect on health outcomes, with special emphasis on two clinical trials, the VITamin D and OmegA-3 TriaL (VITAL) and the COcoa Supplement and Multivitamin Outcomes Study (COSMOS), designed to test the effect of food bioactive and nutrient supplements on cancer and cardiovascular disease (CVD) prevention.
2 WHY ARE LARGE-SCALE RANDOMIZED TRIALS NEEDED?
Several large-scale, long-term observational studies and small-scale, short-term randomized controlled trials have investigated the potential health effects of food bioactives and nutrients. Their interpretation is often complicated by heterogeneity in selected interventions, outcome definitions, findings, study design, and susceptibility to confounding. Randomized trials have a unique role in causal inference for evidence-based medicine and can provide more definitive answers on the effectiveness of interventions for disease prevention. Well-conducted large-scale, long-term randomized trials can overcome many limitations of observational studies, but may not always be feasible due the high costs of recruiting a large study population, logistics of delivering the intervention, and the need to maintain high rates of long-term compliance to rigorously address the scientific hypotheses.
Randomized trials are critically important in nutritional research as they can provide more definitive answers on many of the hypotheses generated from observational studies. Trials testing either food-based or dietary supplement interventions can provide important evidence on that the effects of food bioactives and nutrients on various health outcomes. Food-based and dietary supplement interventions also differ in some aspects. Food-based trials may provide directly generalizable advice on specific foods and/or dietary patterns, but the selection of an appropriate well-defined and masked control group can be challenging. On the other hand, supplement trials with a placebo control often have the advantage of facilitating proper blinding for the trial and dose-response patterns. They can also provide important insights on biological pathways for which a specific food bioactive or nutrient acts on in disease prevention. However, many times the compositions and amounts in supplements of food bioactives and nutrients differ from what are found in foods, which can limit the generalizability of study findings to other contexts than the usage of the particular supplement tested.
2.1 VITamin D and OmegA-3 TriaL (VITAL)
VITAL is a 2x2 factorial, randomized, double-blind, placebo-controlled trial testing the benefits and risks of vitamin D (vitamin D3 [cholecalciferol], 2000 IU/d) and marine omega-3 fatty acids (Omacor fish oil, 1 g/d) in the primary prevention of cancer and CVD among 25,874 men aged ≥50 years and women aged ≥55 years, respectively. The rationale for testing vitamin D and omega-3 supplementation arises from promising observational research and favorable results from some secondary prevention trials testing omega-3 fatty acids.ref In addition, these supplements are taken by a significant proportion of adults for a perceived role in disease prevention despite inconclusive evidence and an absence of nutritional recommendations.7
Vitamin D is a unique micronutrient because it has both endogenous (skin synthesis following sun exposure) and exogenous (food and supplements) sources.8 Vitamin D is found naturally in a limited number of foods (fish, sun-dried mushrooms, eggs), but a variety of foods, particularly milk and other dairy products, are fortified with vitamin D in the U.S. Vitamin D has long been recognized as important for bone health.9 However, more recently studies indicate that vitamin D may also reduce the risk of nonskeletal conditions such as cancer, CVD, and type 2 diabetes due to its effects on cell differentiation, proliferation, apoptosis, and angiogenesis, as well as vascular calcification, blood pressure regulation, and glucose metabolism.10
Dietary marine omega-3 fatty acids such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are derived largely from fish intake. They are considered essential nutrients by some, and bioactives in fish by others. The effect of marine omega-3 fatty acid supplements in the primary prevention of cancer and CVD remains uncertain. There are no large randomized trials testing marine omega-3 fatty acid supplements in a population at usual cardiovascular risk.7 Given the increasing prevalence of fish oil supplement use in the U.S.,11 as well as data suggesting either benefits or risks for CVD, cancer, diabetes, and other conditions, a definitive determination of the balance between benefits and risks for fish oil supplementation in the general U.S. population is paramount.
2.2 COcoa Supplement and Multivitamin Outcomes Study (COSMOS)
COSMOS is a large-scale, long-term randomized clinical trial testing a cocoa extract supplement (Mars Symbioscience) and a commonly used multivitamin (Centrum Silver, Pfizer Inc) in the prevention of CVD and cancer among women aged ≥65 years and men aged ≥60 years in the US. The COSMOS trial has leveraged existing epidemiologic studies for its recruitment, including women in the ongoing Women’s Health Initiative (WHI) Extension Study, women and men contacted for but not randomized into the VITAL trial, and wider mass mailings to the US population.
Cocoa products have demonstrated favorable cardiovascular properties attributable to their high polyphenol content, including flavan-3-ols such as catechins and epicatechins.12–17 These flavanols are considered bioactives of the cocoa bean. Small-scale, short-term randomized trials testing cocoa products and extracts have demonstrated potential benefits on intermediate outcomes, including blood pressure, lipids, insulin sensitivity, and flow-mediated vasodilation, but there are no large-scale trials confirming whether these benefits extend to a reduction in major CVD events.18–21 Thus, the COSMOS trial will allow us to more comprehensively understand the potential cardiovascular effects of cocoa flavanols.
Inclusion of the multivitamin component for COSMOS is based upon their high prevalence of use, by at least one-third of US adults,22,23 and an attempt to replicate a previous randomized clinical trial in men, the Physicians’ Health Study (PHS) II, which tested a Centrum Silver multivitamin in 14,641 men aged ≥50 years, that showed a significant 8% reduction in total cancer24 but no effect on major CVD events.25 Notably, in men aged ≥70 years, a stronger 18% reduction in total cancer (P, interaction by age=0.06) and a nonsignificant 9% reduction in CVD (P, interaction by age =0.04) were found, with weaker effects in men aged <70 years.24,25 Further, among 1,312 men with a baseline history of cancer in PHS II, a daily multivitamin significantly reduced incident total cancer by 27%. Thus, COSMOS was designed as a cost-effective 2x2 factorial design to determine whether a common multivitamin prevents cancer, particularly among women, older adults, and those with a history of cancer.
3 SELECTION OF FOOD BIOACTIVE OR NUTRIENT
When deciding upon the type and amount of a specific food bioactive or nutrient to test in a trial, the right balance between efficacy and safety is essential. This can be done through a careful review of the literature to determine an amount for a target population with a sufficiently large difference between intervention groups to detect a significant, clinically relevant change for the selected primary endpoints. To this end, collaboration across multiple sectors including academia, hospitals, government, non-profit organizations, and industry may be beneficial.
Another important consideration when deciding the amount to test in a long-term clinical trial is the participant burden. A food bioactive or nutrient tested in a short-term trial may not be feasible in a long-term trial based upon its impact on compliance and dropout rates. Both VITAL and COSMOS faced unique challenges when selecting the appropriate composition and quantity of each intervention.
VITAL was designed when the recommended dietary allowances (RDA) set by the Institute of Medicine (IOM) were 400 IU/day for adults aged 50–70 years and 600 IU/day for adults aged ≥70 years.7 However, at that time, accumulating data suggested that vitamin D intake above RDA levels may be necessary for maximal health benefits.7,10 Few safety issues were expected with the selected amount of vitamin D tested in VITAL, 2000 IU/day, which was also projected to lead to serum 25(OH)D levels associated with health benefits in observational studies. Women and men who reported supplemental vitamin D intakes above 800 IU/day (the updated RDA for older adults, as of the 2011 IOM report26) were excluded. As dietary intake of vitamin D is approximately 200 IU/day, very few participants assigned to the active vitamin D group would be expected to consume an amount above 3000 IU/day, which is well below the tolerable upper intake level of 4000 IU/day set by the IOM in 2011.27
The total amount of marine omega-3 fatty acids of 1 g/day selected for VITAL was based on the recommendations by the American Heart Association and previous large secondary prevention trials reporting a beneficial effect on CVD, with minimal side effects.28 Moreover, the FDA had concluded that marine omega-3 fatty acid amounts of up to 3 g/day are “Generally Recognized as Safe”.29 However, as the optimal ratio of EPA-to-DHA ratio is unclear, a ratio of 1.3-to-1, closer to 1-to-1 was chosen for VITAL.
In COSMOS, the amount of bioactive cocoa flavanols to be tested in the cocoa extract supplement was based on previous small-scale, short-term clinical trials. However, a lower amount of cocoa flavanols was selected than previously tested in some of the short-term trials because it was considered important to limit the number of capsules consumed per day to 2 to ensure good compliance and because the long-term safety of high-dose flavanol supplementation had not been previously assessed. Moreover, the 600 mg/d of cocoa flavanols provided in the cocoa extract supplement is in the middle range of amounts previously tested with consistent beneficial effects on intermediate cardiovascular endpoints30–34 and is higher than the amount usually consumed by dietary sources.12,16
The Centrum Silver multivitamin formulation tested in COSMOS is already widely used in the US consumer market, with high quality-control standards in place. It contains RDA or daily intake levels of essential vitamins and minerals that are generally considered safe. In PHS II, daily Centrum silver was associated with some potential side effects, including skin rashes (HR, 1.08; P=0.016). That daily multivitamin was also inconsistently linked with minor bleeding, including a reduction in hematuria (HR, 0.89; P=0.006), an increase in epistaxis (HR, 1.11; P=0.016), and no effect on easy bruising/other bleeding (HR, 1.02; P=0.59).24
4 RECRUITMENT
The design and recruitment process in any randomized trial can be challenging and costly, but gets even more complicated in large-scale, long-term designs. Fortunately, a key advantage of randomized clinical trials of food bioactives and nutrients is that they are conducive to either clinic-based or mail-based designs. Both VITAL and COSMOS have utilized a cost-efficient hybrid design that includes both clinic- and mail-based components. This includes recruitment of a large trial population across the US with mailed calendar packs containing study pills and follow-up primarily by mail, combined with in-clinic visits among a targeted subcohort to allow for more in-depth phenotyping and optimal processing of biospecimens. A large-scale trial with a hybrid design allows for the accrual of sufficient clinical events to optimize trial power for the main hypotheses, while providing detailed and comprehensive information, including changes in nutrient status, clinical measurements, and cardiometabolic biomarkers, on a subset of participants to permit mechanistic studies.
5 COMPLIANCE
Compliance can be challenging when testing bioactives and nutrients that have already received extensive media attention for potential health effects. People do not want to be randomized into the placebo arm because they may already be convinced of the potential health benefits associated with the intervention to be tested. To minimize these concerns for the interventions in VITAL and COSMOS, both trials have strictly defined eligibility criteria regarding out-of-study supplement use. In a trial, it is important to monitor compliance throughout follow-up. VITAL and COSMOS assess compliance through self-reports on annual follow-up questionnaires as well as through plasma biomarker measures at baseline and follow-up in a subset of participants in the active and placebo groups.
6 OUTCOME SELECTION
Short-term clinical trials typically select surrogate endpoints such as biomarkers, imaging studies, or clinical assessments as proxies for the disease of interest to be prevented. This can be a cost-effective approach if the “surrogates” have clear and established relationships with the clinical outcomes of interest, but still does not provide direct evidence of a causal effect on clinical events. Studying the effect of a bioactive on changes in a continuous biomarker allows for smaller sample sizes and shorter follow-up. However, results from studies evaluating changes in biomarkers may not always be predictive of the disease of interest, or the intervention may affect multiple pathways with divergent effects on disease. Regardless, these smaller clinical trials still provide invaluable mechanistic insights about the effects of a bioactive or nutrient to generate hypotheses for subsequent large-scale randomized trials with clinical endpoints.
VITAL and COSMOS are primarily designed to investigate the incidence of cancer and CVD events based on clinical endpoints confirmed by medical record review; however, both studies have added in-clinic visits and collection of other data to allow for in-depth phenotyping in a cost-efficient manner within subsets of participants. In addition, both COSMOS and VITAL have collected biospecimens in a large number of participants to allow for assessments of whether the interventions’ effects are modified by baseline nutritional status, genomic factors, or metabolic footprints.
7 CHALLENGES IN TRANSLATING RESULTS INTO RECOMMENDATIONS AND POLICY-MAKING
When interpreting results on bioactives and nutrients from randomized trials to develop new public health polices and clinical recommendations, the study’s aims and hypotheses should be prespecified and clearly stated. Randomized trials testing a food bioactive or nutrient often begin by either investigating the secondary prevention of the outcome of interest or selecting the participants based upon an elevated risk of the outcome. This affects the interpretation and generalizability of study findings, and results from an unhealthy or high-risk population may not apply for a healthy or usual risk population. VITAL was designed to test vitamin D in the primary prevention of cancer and omega-3 fatty acids in the primary prevention of CVD; COSMOS was designed to test cocoa flavanols in the primary prevention of CVD and a multivitamin in the primary and secondary prevention of cancer. Such large-scale randomized clinical trials are challenging due to the large study populations needed to generate enough incident cases for sufficient statistical power.
Reproducibility of study findings is crucial for causal inference and public health recommendations. However, replication requires considerable patience; large-scale, long-term randomized clinical trial take a long time to design, conduct, and analyze. In addition, an amount of a food bioactive or nutrient as part of an intervention that reflects current thinking at the start of a long-term clinical trial may change over time during the trial. As a result, heterogeneity across clinical trials testing a particular food bioactive or nutrient may exist due to differences in the amounts and combinations of bioactives and nutrients studied, differences in characteristics across study populations, and/or variation in follow-up times. These challenges all affect the translation of findings into clinical guidelines and recommendations. For example, several other clinical trials are running in parallel with VITAL testing vitamin D supplements. However, most other trials are substantially smaller (fewer than 5000 participants), many test bolus doses of vitamin D (often given monthly), and many lack racial/ethnic diversity of their study populations. The multivitamin component of COSMOS, which is the first large-scale trial of multivitamins to include women, will test whether the PHS II findings for men can be replicated in women. In 2013, the US Preventive Services Task Force (USPSTF) concluded that evidence is insufficient to evaluate the potential role of multivitamins on cancer and CVD prevention.35 Studies on multivitamins can be problematic to interpret and translate into recommendations because multivitamins lack a clear definition with regard to composition and amounts of included nutrients or bioactives. The COSMOS trial is testing a multivitamin supplement that is very similar in composition to that tested in PHS II.
8 FUTURE DIRECTIONS
Future clinical trials testing bioactives and nutrients should carefully consider the role of baseline nutritional status to understand the potential health effects of these compounds. Moreover, the efficacy of these supplements may vary between individuals as a result of complex interactions among microbiome, genomics, and metabolomic responses. Trials that apply a personalized medicine-approach to investigate potential inter-individual responses will also provide greater insights. VITAL and COSMOS have in-clinic visits in a subcohort to collect biospecimen and clinical data that will allow for these more comprehensive assessments, with more clinical trials needed that similarly expand their scope.
9 CONCLUSIONS
Large-scale randomized trials are crucial in understanding how bioactives and nutrients, considered to be critical food constituents, may be involved in health and disease prevention or progression. VITAL and COSMOS represent two important examples of large-scale, long-term randomized clinical trials to evaluate the role of food bioactives and nutrients in the prevention of major chronic diseases such as cancer and CVD. Food-based and dietary supplement trials complement each other and are of great importance in nutritional research as they can provide a more holistic view on food bioactives and nutrients as part of dietary patterns. However, enthusiasm for food bioactives and nutrients as functional foods or dietary supplements outpaces available evidence on their potential effectiveness. Thus, it remains of critical public health importance to rigorously test the benefit to risk profiles of these approaches via randomized clinical trials for evidence-based clinical and health policy recommendations to improve human health.
Table 1.
Characteristics of a well-designed, population-based, randomized controlled trial testing food bioactives and nutrients.
| Selection of study population |
|
| Selection of intervention |
|
| Compliance |
|
| Selection of outcome |
|
Acknowledgments
S.R. has received funding from COFAS 2 Marie Curie Fellowship, Stockholm, Sweden. H.D.S. has received grant support from the National Institutes of Health (NIH) (R01 HL102122) related to work on the VITamin D and OmegA-3 TriaL (VITAL). J.E.M. has received grant support from the NIH for VITAL (CA138962) and for other research (HL34594 and HHSN268201100001C).
Footnotes
Conflicts of interest
S.R. declares no conflicts of interest. H.D.S. declares that he has received investigator-initiated grant support from the Council for Responsible Nutrition Foundation and from Mars Symbioscience and Pfizer Inc (including donations of study pills) for the COcoa Supplement and Multivitamin Outcomes Study (COSMOS). J.E.M. declares that she has received investigator-initiated grant support from Mars Symbioscience for COSMOS and donation of study pills from PharmaVite and ProNova/BASF (for VITAL) and from Pfizer Inc and Mars Symbioscience (for COSMOS).
References
- 1.Ross C, Caballero B, Cousins RJ, Tucker KL, Ziegler TR. Modern Nutrition in Health and Disease. 11. Wolters Kluwer Health/Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 2.Lupton JR, Atkinson SA, Chang N, et al. Exploring the benefits and challenges of establishing a DRI-like process for bioactives. Eur J Nutr. 2014;53(Suppl 1):1–9. doi: 10.1007/s00394-014-0666-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mozaffarian D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity: A Comprehensive Review. Circulation. 2016;133(2):187–225. doi: 10.1161/CIRCULATIONAHA.115.018585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rautiainen S, Manson JE, Lichtenstein AH, Sesso HD. Dietary supplements and disease prevention - a global overview. Nat Rev Endocrinol. 2016;12(7):407–420. doi: 10.1038/nrendo.2016.54. [DOI] [PubMed] [Google Scholar]
- 5.Dietary Supplements Market by Ingredients (Botanicals, Vitamins, Minerals, Amino Acids, Enzymes) for Additional Supplements, Medicinal Supplements and Sports Nutrition Applications - Global Industry Perspective, Comprehensive Analysis and Forecast, 2016 – 2022.
- 6.Neuhouser ML, Patterson RE, Levy L. Motivations for using vitamin and mineral supplements. Journal of the American Dietetic Association. 1999;99(7):851–854. doi: 10.1016/S0002-8223(99)00202-3. [DOI] [PubMed] [Google Scholar]
- 7.Manson JE, Bassuk SS, Lee IM, et al. The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012;33(1):159–171. doi: 10.1016/j.cct.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosen CJ, Adams JS, Bikle DD, et al. The nonskeletal effects of vitamin D: an Endocrine Society scientific statement. Endocr Rev. 2012;33(3):456–492. doi: 10.1210/er.2012-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LeFevre ML Force USPST. Screening for vitamin D deficiency in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;162(2):133–140. doi: 10.7326/M14-2450. [DOI] [PubMed] [Google Scholar]
- 10.Manson JE, Bassuk SS. Vitamin D research and clinical practice: at a crossroads. JAMA. 2015;313(13):1311–1312. doi: 10.1001/jama.2015.1353. [DOI] [PubMed] [Google Scholar]
- 11.Kantor ED, Rehm CD, Du M, White E, Giovannucci EL. Trends in Dietary Supplement Use Among US Adults From 1999–2012. JAMA. 2016;316(14):1464–1474. doi: 10.1001/jama.2016.14403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buijsse B, Feskens EJ, Kok FJ, Kromhout D. Cocoa intake, blood pressure, and cardiovascular mortality: the Zutphen Elderly Study. Archives of internal medicine. 2006;166(4):411–417. doi: 10.1001/archinte.166.4.411. [DOI] [PubMed] [Google Scholar]
- 13.Janszky I, Mukamal KJ, Ljung R, Ahnve S, Ahlbom A, Hallqvist J. Chocolate consumption and mortality following a first acute myocardial infarction: the Stockholm Heart Epidemiology Program. Journal of internal medicine. 2009;266(3):248–257. doi: 10.1111/j.1365-2796.2009.02088.x. [DOI] [PubMed] [Google Scholar]
- 14.Mink PJ, Scrafford CG, Barraj LM, et al. Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr. 2007;85(3):895–909. doi: 10.1093/ajcn/85.3.895. [DOI] [PubMed] [Google Scholar]
- 15.Larsson SC, Virtamo J, Wolk A. Chocolate consumption and risk of stroke in women. Journal of the American College of Cardiology. 2011;58(17):1828–1829. doi: 10.1016/j.jacc.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 16.Buijsse B, Weikert C, Drogan D, Bergmann M, Boeing H. Chocolate consumption in relation to blood pressure and risk of cardiovascular disease in German adults. European heart journal. 2010;31(13):1616–1623. doi: 10.1093/eurheartj/ehq068. [DOI] [PubMed] [Google Scholar]
- 17.Aron PM, Kennedy JA. Flavan-3-ols: nature, occurrence and biological activity. Molecular nutrition & food research. 2008;52(1):79–104. doi: 10.1002/mnfr.200700137. [DOI] [PubMed] [Google Scholar]
- 18.Tokede OA, Gaziano JM, Djousse L. Effects of cocoa products/dark chocolate on serum lipids: a meta-analysis. European journal of clinical nutrition. 2011;65(8):879–886. doi: 10.1038/ejcn.2011.64. [DOI] [PubMed] [Google Scholar]
- 19.Jia L, Liu X, Bai YY, et al. Short-term effect of cocoa product consumption on lipid profile: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2010;92(1):218–225. doi: 10.3945/ajcn.2009.28202. [DOI] [PubMed] [Google Scholar]
- 20.Taubert D, Roesen R, Schomig E. Effect of cocoa and tea intake on blood pressure: a meta-analysis. Archives of internal medicine. 2007;167(7):626–634. doi: 10.1001/archinte.167.7.626. [DOI] [PubMed] [Google Scholar]
- 21.Desch S, Schmidt J, Kobler D, et al. Effect of cocoa products on blood pressure: systematic review and meta-analysis. American journal of hypertension. 2010;23(1):97–103. doi: 10.1038/ajh.2009.213. [DOI] [PubMed] [Google Scholar]
- 22.Bailey RL, Gahche JJ, Lentino CV, et al. Dietary supplement use in the United States, 2003–2006. J Nutr. 2011;141(2):261–266. doi: 10.3945/jn.110.133025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gahche J, Bailey R, Burt V, et al. Dietary supplement use among U.S. adults has increased since NHANES III (1988–1994) NCHS Data Brief. 2011;(61):1–8. [PubMed] [Google Scholar]
- 24.Gaziano JM, Sesso HD, Christen WG, et al. Multivitamins in the prevention of cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2012;308(18):1871–1880. doi: 10.1001/jama.2012.14641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sesso HD, Christen WG, Bubes V, et al. Multivitamins in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2012;308(17):1751–1760. doi: 10.1001/jama.2012.14805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moyer VA Force USPST. Vitamin, mineral, and multivitamin supplements for the primary prevention of cardiovascular disease and cancer: U.S. Preventive services Task Force recommendation statement. Ann Intern Med. 2014;160(8):558–564. doi: 10.7326/M14-0198. [DOI] [PubMed] [Google Scholar]
- 27.Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 28.American Heart Association Nutrition C. Lichtenstein AH, Appel LJ, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114(1):82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 29.Department of Health and Human Services, U.S. Food and Drug Administration. Federal Register. 1997. Substances affirmed as generally recognized as safe: menhaden oil. [Google Scholar]
- 30.Desideri G, Kwik-Uribe C, Grassi D, et al. Benefits in cognitive function, blood pressure, and insulin resistance through cocoa flavanol consumption in elderly subjects with mild cognitive impairment: the Cocoa, Cognition, and Aging (CoCoA) study. Hypertension. 2012;60(3):794–801. doi: 10.1161/HYPERTENSIONAHA.112.193060. [DOI] [PubMed] [Google Scholar]
- 31.Davison K, Berry NM, Misan G, Coates AM, Buckley JD, Howe PR. Dose-related effects of flavanol-rich cocoa on blood pressure. J Hum Hypertens. 2010;24(9):568–576. doi: 10.1038/jhh.2009.105. [DOI] [PubMed] [Google Scholar]
- 32.Heiss C, Jahn S, Taylor M, et al. Improvement of endothelial function with dietary flavanols is associated with mobilization of circulating angiogenic cells in patients with coronary artery disease. Journal of the American College of Cardiology. 2010;56(3):218–224. doi: 10.1016/j.jacc.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 33.Davison K, Coates AM, Buckley JD, Howe PR. Effect of cocoa flavanols and exercise on cardiometabolic risk factors in overweight and obese subjects. Int J Obes (Lond) 2008;32(8):1289–1296. doi: 10.1038/ijo.2008.66. [DOI] [PubMed] [Google Scholar]
- 34.Balzer J, Rassaf T, Heiss C, et al. Sustained benefits in vascular function through flavanol-containing cocoa in medicated diabetic patients a double-masked, randomized, controlled trial. Journal of the American College of Cardiology. 2008;51(22):2141–2149. doi: 10.1016/j.jacc.2008.01.059. [DOI] [PubMed] [Google Scholar]
- 35.Fortmann SP, Burda BU, Senger CA, Lin JS, Whitlock EP. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: An updated systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;159(12):824–834. doi: 10.7326/0003-4819-159-12-201312170-00729. [DOI] [PubMed] [Google Scholar]