Abstract
Background
Urgency urinary incontinence (UUI) is a chronic condition for which sacral neuromodulation (SNM) (InterStim/Medtronic) and onabotulinumtoxinA (BTX) (BotoxA/Allergan) are utilized. These therapies have not been compared over extended time.
Objective
To compare UUI episodes (UUIE) over 24 mo following SNM or BTX.
Design, setting, and participants
Multicenter, open-label, randomized, extension trial (February 2012–July 2016) at nine US medical centers involving 386 women with ≥6 UUIE over 3 d inadequately managed by medications. Participants were clinical responders to treatment: ≥50% reduction in UUIEs after SNM placement or 1 mo post BTX.
Intervention
SNM (n = 194) versus 200 U BTX (n = 192). SNM reprogrammings occurred throughout the 24 mo. After 6 mo, two additional BTX injections were allowed.
Outcome measurements and statistical analysis
Primary outcome: change in mean daily UUIE over 24 mo. Secondary outcomes: no UUIE, ≥ 75% and ≥50% UUIE reduction; Overactive Bladder Questionnaire Short Form; Urinary Distress Inventory short form; Incontinence Impact Questionnaire; Patient Global Impression of Improvement; Overactive Bladder Satisfaction of Treatment Questionnaire; and adverse events (AEs). Primary analysis used a linear mixed model.
Results and limitations
Outcome data were available for 260/298 (87%) clinical responders. No difference in decreased mean UUIE was found over 24 mo (−3.88 vs −3.50 episodes/d,95% confidence interval [CI] = −0.14–0.89; p = 0.15), with no differences in UUI resolution, ≥ 75% or ≥50% UUIE reduction. BTX group maintained higher satisfaction (mean difference = −9.14, 95% CI= −14.38– −3.90; p < 0.001), treatment endorsement (mean difference = −12.16, 95% CI = −17.7– −6.63; p < 0.001) through 24 mo. Other secondary measures did not differ. Recurrent urinary tract infections (UTIs) were higher after BTX (24% vs 10%; p < 0.01), 6% required intermittent catheterization post second injection. SNM revision and removals occurred in 3% and 9% patients, respectively.
Conclusions
Both treatments offered sustainable UUI improvement, and higher BTX dosing had low clean intermittent catheterization rates, but with UTI risk. SNM revision/removal rates were low due to standardized lead placement with strict treatment response definitions.
Patient summary
We compared a large group of US women with severe urgency urinary incontinence (UUI) who received sacral neuromodulation (InterStim) or onabotulinumtoxinA (Botox A) therapy during a 2-yr period. We found that both therapies had similar success in reducing UUI symptoms, and adverse events were low. However, women in the BotoxA group had higher satisfaction and endorsement with their treatment, but with a higher chance of a urinary tract infection. We conclude that both therapies offer sustained reduction in daily incontinence over 2 yr.
Introduction
Urgency urinary incontinence (UUI) is a chronic condition, markedly affecting the quality of life (QOL) [1]. Treatments such as medication and behavioral therapy with pelvic muscle exercises require continued adherence and may not remain effective over time [2,3]. Sacral neuromodulation (SNM) and intradetrusor onabotulinumtoxinA (BTX) are third-line therapies that are increasingly being utilized. Furthermore, 6-mo efficacy and adverse events (AEs) were reported in the Refractory Overactive Bladder: Sacral Neuromodulation versus Botulinum Toxin Assessment (ROSETTA) trial [4].
Comparisons between these therapies over an extended time have not been reported. This planned 24-mo extension trial compared efficacy, AEs, and satisfaction with therapy in women randomized to either SNM or BTX 200 units (U).
Materials and methods
This comparative, effectiveness trial compared SNM to BTX in women with idiopathic, refractory UUI over 24 mo and was conducted (July 2012–February 2016) at nine sites participating in the National Institutes of Health (NIH)-sponsored Pelvic Floor Disorders Network. This planned analysis was published in the methods paper [5].
The ROSETTA trial enrolled women who experienced ≥6 UUIE on a 3-d diary and failed (poor efficacy or AE) behavioral interventions/physical therapy and two medications. Participants did not have neurologic disease, post void residual (PVR) >150 ml, previous treatment with either study intervention, or ≥Stage 3 prolapse. Participants were randomized 1:1 to either BTX or SNM and were stratified by clinical site and age group (< 65 vs ≥ 65 yr) using a secure web-based application system to implement the random allocation sequence generated by the data center previously described [5].
SNM participants who demonstrated ≥50% reduction in UUIEs on a bladder diary following Stage I lead (models 3093/3889) placement (SNM “clinical responders” [CRs]) proceeded to Stage II pulse generator placement (models 3023/3058). SNM responders were not to use concomitant UUI medication; however, non-responders were allowed medication, and they could receive BTX therapy after 6 mo. Participants were offered reprogramming for pain or if their Patient Global Symptom Control (PGSC) scores were 1 or 2. Surgical revision was offered if reprogramming proved ineffective, and device removal was performed if symptoms continued.
BTX “CRs,” participants with ≥50% reduction in UUIEs on a bladder diary 1 mo following injection, were not to use concomitant UUI medication; however, non-responders were allowed medication, and they could receive SNM therapy after 6 mo. Between 6 and 24 mo, BTX responders were allowed two additional injections, performed a minimum of 4 mo apart, if PGSC scores were 1 or 2. Voiding assessments were performed after all BTX injections. Participants who required clean intermittent catheterization (CIC) for >6 wk following their initial injection were dose-reduced to 100 U for the second injection. If eligible for the third injection, those previously dose-reduced could either receive 100 U or 200 U. All others eligible for a second injection received 200 U. Participants requiring CIC for >6 wk after the second 200 U injection had their BTX dose-reduced to 100 U for their third injection.
The primary outcome was change from baseline in mean daily UUIE over 24 mo based on bladder diaries collected at baseline and months 1–6, 9, 12, 18, and 24. Secondary outcomes were generated from diary results, QOL measures, and AE results. Bladder diary outcomes included change from baseline in mean daily UUIEs, ≥ 50% UUIE reduction, ≥75% UUIE reduction, and no UUIE. QOL instruments measured at baseline, 6, 12, and 24 mo included the Overactive Bladder Questionnaire Short Form (OABq-SF), Urinary Distress Inventory short form (UDI-SF), Incontinence Impact Questionnaire, and the Sandvik Incontinence Severity Index. The Overactive Bladder Satisfaction of Treatment Questionnaire (OAB-SATq), PGSC, and the Patient Global Impression of Improvement were obtained at 6, 12 and 24 mo. AEs, PGSC, and OABq-SF were assessed by phone at 9 and 18 mo.
Information about AEs collected throughout the 24 mo included: (1) treatment for urinary tract infections (UTIs; defined as symptomatic and culture positive or only symptomatic), (2) proportion of participants who developed recurrent UTIs, (3) need for reprogramming or revision/removal following SNM, and (4) need for CIC following BTX. Participants initiated CIC following BTX for a PVR of either >300 ml or >200 ml with associated moderate to severe voiding bother noted on the UDI-SF. Recurrent UTIs were defined as two symptomatic UTIs treated within 6 mo or three UTIs treated within 1 yr following study treatment. The study also tracked women who received treatment for an early UTI, defined as a UTI within a month of BTX injection. SNM reprogramming data were collected at 1, 3, 6, 12, and 24 mo post-implantation. Investigators recorded each reprogramming event during the 24-mo follow-up as well as reasons for SNM revisions and removals. Investigators collected information on additional therapies (such as medications) and/or alternative study therapy (ie, BTX participants who received SNM or SNM participants who received BTX).
Analyses of continuous UI measure (mean UUIE/d) used a linear mixed model with participant diary month as the unit of analysis and monthly change from baseline in mean UUIE per day as the outcome, with terms for treatment group, diary month, treatment by month interaction, age group, and site; baseline UUIE was not included in the model. Participant was treated as a random effect to account for within-person correlation. The model generated adjusted estimates of change in UUIE from baseline by treatment group over four aggregate time periods (mo 1–6, mo 9 and 12, mo 18, and mo 24); F-test p values assessed the hypothesis that the average mean change from baseline differed between treatment groups. Similar model analyses, modified for planned measurement times, were performed to evaluate treatment difference in continuous QOL measures assessed over time. Analogous generalized linear models based on Poisson regression, with variance estimates described by Zou [6] were used to evaluate treatment difference in binary diary and QOL outcomes with generalized estimating equations used to account for correlation within individuals. No adjustments were made for off-protocol treatment in any of the models. Fisher’s exact tests compared AE measures across treatment arms.
Because all 24-mo analyses were characterized as secondary analyses in the protocol and no interim analyses were done, no adjustments were made for multiple comparisons. All efficacy analyses were based on the CR population and safety analyses on all treated women. All analyses used SAS software version 9.4 (SAS Institute); inferences and descriptive p values are based on two-sided tests.
Results
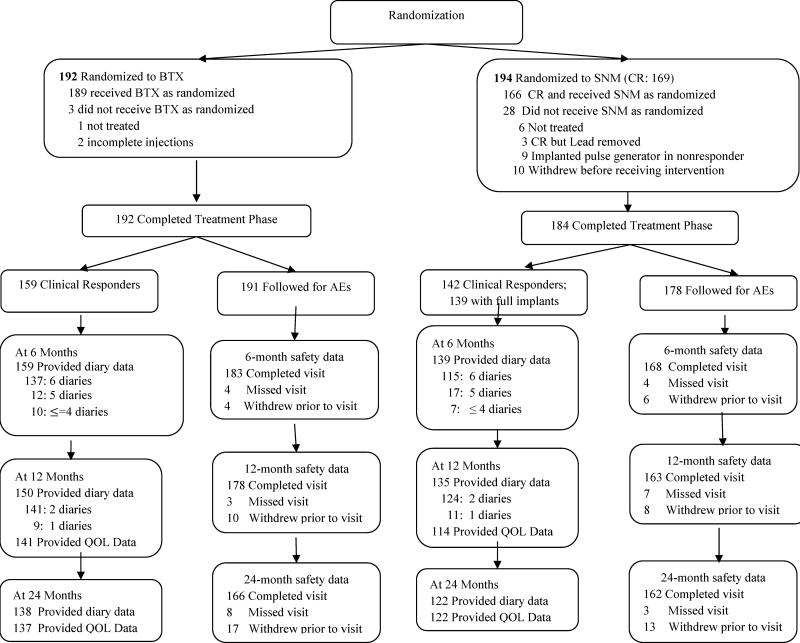
As previously reported, 386 women, compared to the planned sample size of 380, were randomized to either SNM (n = 194) or BTX 200 U (n = 192) [4,5]. At 24 mo, outcome data were available for 260 of 298 (87%) clinical responders. The CR population included 159 of 192 (83%) in the BTX group and 142 of 169 (84%) in the SNM group. Of 142, 139 participants received the full implant and are included in the CR population. A total of 369 participants who were randomized and received any intervention were followed to assess AEs (Fig. 1). Demographic or clinical characteristics showed no meaningful differences between the two treatment arms (Table 1).
Fig. 1. Flow diagram of clinical responders and adverse event population.
AEs = adverse events; BTX = onabotulinumtoxinA; CR = clinical responder; QOL = quality of life; SNM = sacral neuromodulation.
Table 1.
Baseline demographics for clinical responder population
| Characteristic | Treatment group | |
|---|---|---|
| BTX | SNM | |
| (n = 159) | (n = 139) | |
| Age (yr), mean (SD) | 62.4 (11.0) | 62.7 (11.8) |
| History recurrent UTIs, n (%) | 19 (12) | 20 (14) |
| Mean UUIE/d, mean (SD) | 5.3 (2.6) | 5.0 (2.1) |
| Mean UIE/d, mean (SD) | 5.9 (2.9) | 5.6 (2.3) |
| OABq-SF** Symptom Bother, mean (SD) | 75.7 (18.9) | 77.2 (16.5) |
| OABq-SF* HRQL, mean(SD) | 37.8 (23.3) | 35.7 (20.7) |
| Sandvikπ, n (%) Slight | 2 (1) | 1 (1) |
| Moderate | 23 (14) | 20 (14) |
| Severe | 49 (31) | 29 (21) |
| Very severe | 80 (50) | 85 (61) |
BTX = onabotulinumtoxinA; HRQL = health-related quality of life; OAB-SF = Overactive Bladder Questionnaire Short Form; SNM = sacral neuromodulation; UTI = urinary tract infection; UUI = urgency urinary incontinence; UUIE = urgency urinary incontinence episodes.
Values for the Overactive Bladder Questionnaire Short Form (OAB-SF) range from 0 to 100, with higher scores on the symptom severity scale indicating greater severity of symptoms and higher scores on the quality-of-life scale indicating better quality of life.
Sandvik questionnaire is a patient reported measure of incontinence severity as assessed on a scale of slight (1–2) to very severe (10–12) using the standard scoring algorithm.
No difference in mean in UUIE decrease between the groups for the CR population was found over 24 mo (−3.88 vs −3.50 episodes/d; mean difference = 0.38; 95% confidence interval [CI] = −0.14–0.89; p = 0.2; Fig. 2). At 6 mo, the BTX group was more likely to experience complete resolution of UUI (treatment difference = −18%; 95% CI = −29– −6; p < 0.001) and ≥75% reduction (treatment difference = −20%; 95% CI = −31– −8; p = 0.001); however, the differences between the groups decreased over time, and at 24 mo, the groups showed comparable rates of complete resolution (5% each) and >75% reduction (22% for BTX and 21% for SNM; Fig. 3). The BTX group maintained higher treatment satisfaction (mean difference = −9.1, 95% CI= −14.4, −3.9; p<0.001) and treatment endorsement (mean difference = −12.2, 95% CI =−17.7–−6.6; p < 0.001) across 24 mo and had greater treatment preference (mean difference = −9.9, 95% CI = −18.5– −1.2; p = 0.02) at 24 mo based on OAB-SATq subscales. Other QOL measures showed no difference between the treatments (Table 2). No differences were seen in the proportions of participants requesting additional medication (BTX 21% [34/159], SNM 21% [29/139], p = 0.7) or alternative trial therapy off protocol (BTX 6% [10/159], SNM 5% [7/139], p = 0.7).
Fig. 2. Change from baseline in urgency urinary incontinence episode per day in clinical responder population.
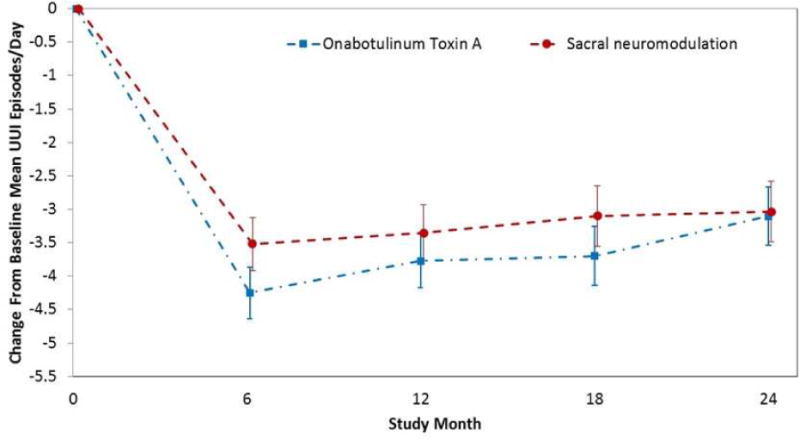
UUI = urgency urinary incontinence.
Values in the graph include adjusted mean estimates and associated 95% confidence intervals (indicated by error bars).
Fig. 3. Urgency urinary incontinence episode (UUIE) reduction post treatment in the clinical responder population.
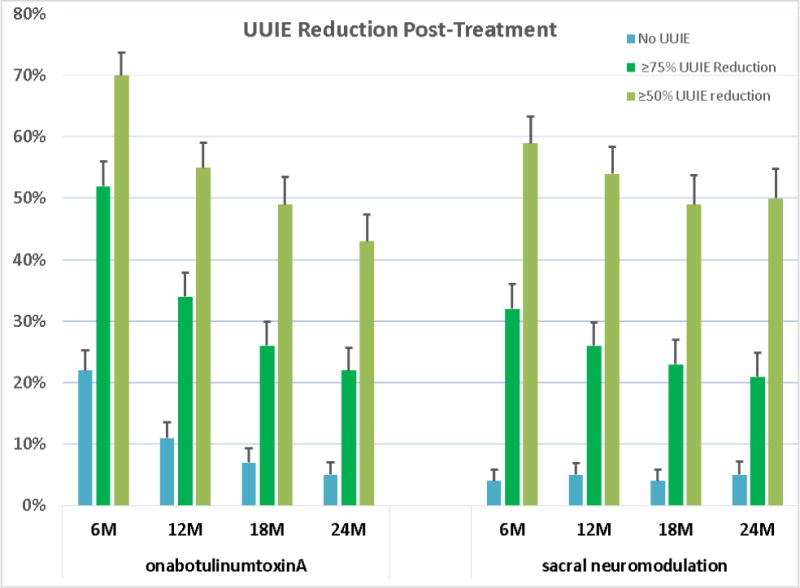
Proportion of UUIE reduction over time is demonstrated for three nested levels of improvement. Dark blue bars on the left represent No UUIE (complete resolution); the green bars in the center (which include those individuals in the blue bars) represent 75% or more reduction, and the gray bars on the right represent 50% or more improvement from baseline. The bars at the top of the graphs represent the standard error of the estimate of the probability of patients achieving that level of improvement. Tests for treatment differences for each of these binary measures between the two treatment groups based on robust Poisson models show evidence of differences at 6 mo for No UUIE and >75% reduction (p < 0.001 and p = 0.001, respectively) but no evidence for >50% reduction (p = 0.1). At 12 mo, analyses provided no evidence of treatment group differences for No UUIE, >75% reduction, and >50% reduction (p = 0.06, p = 0.2, and p = 0.9, respectively). Similarly, no evidence of treatment group differences was found at 18 mo for No UUIE, >75% reduction, and >50% reduction (p = 0.3, p = 0.6, and p = 0.8, respectively), or at 24 mo for No UUIE, >75% reduction, and >50% reduction (p = 0.6, p = 0.8, and p = 0.2, respectively).
Table 2.
Quality of life outcomes of the clinical responder population
| Table 2A – Quantitative quality of life outcomes over 6–24 mo | ||||
|---|---|---|---|---|
| Outcome | BTX (n = 159) | SNM (n = 139) | Treatment difference (95% CI) | p value |
| Overactive Bladder Short Form Questionnaire change from baseline, adjusted mean (Std Err) | ||||
| OABq SFa | ||||
| Symptom bother | ||||
| 6 mo | −51.9(1.9) | −44.5(2.1) | 7.4(2.3–12.6) | 0.005 |
| 12 mo | −39.1(2.1) | −39.5(2.2) | −0.4(−6.1–5.3) | 0.9 |
| 24 mo | −33.9(2.1) | −36.2(2.2) | −2.3(−8.0–3.4) | 0.4 |
| Overall | −41.6(1.9) | −40.1(2.1) | 1.6(−3.5–6.7) | 0.5 |
| Quality of life | ||||
| 6 mo | 46.4(1.9) | 44.8(2.1) | −1.6(−6.8–3.6) | 0.5 |
| 12 mo | 36.9(2.0) | 41.5(2.2) | 4.6(−0.9–10.1) | 0.1 |
| 24 mo | 34.3(2.0) | 38.9(2.2) | 4.6(−0.9–10.2) | 0.1 |
| Overall | 39.2(1.9) | 41.7(2.1) | 2.5(−2.6–7.7) | 0.3 |
| Overactive Bladder Satisfaction Short Form Questionnaireb, adjusted mean (Std Err) | ||||
| Treatment satisfaction | ||||
| 6 mo | 73.5(2.3) | 64.9(2.5) | −8.7(−15.0– −2.4) | 0.007 |
| 12 mo | 67.8(2.2) | 58.3(2.5) | −9.6(−15.7– −3.4) | 0.002 |
| 24 mo | 62.8(2.2) | 53.6(2.4) | −9.2(−15.4– −3.0) | 0.004 |
| Overall | 68.1(1.9) | 58.9(2.1) | −9.1(−14.4– −3.9) | <0.001 |
| Side effects | ||||
| 6 mo | 89.5(2.0) | 85.0(2.1) | −4.5(−10.0–1.0) | 0.1 |
| 12 mo | 85.8(1.9) | 84.2(2.1) | −1.6(−7.0–3.7) | 0.5 |
| 24 mo | 85.9(1.9) | 80.9(2.1) | −5.0(−10.3–0.3) | 0.07 |
| Overall | 87.1(1.5) | 83.4(1.7) | −3.7(−7.8–0.4) | 0.08 |
| Endorsement | ||||
| 6 mo | 84.1(2.4) | 74.0(2.6) | −10.2(−16.8– −3.6) | 0.003 |
| 12 mo | 79.0(2.3) | 64.0(2.6) | −15.1(−21.5– −8.6) | <0.001 |
| 24 mo | 71.8(2.3) | 60.6(2.6) | −11.3(−17.7– −4.8) | <0.001 |
| Overall | 78.3(2.0) | 66.2(2.2) | −12.2(−17.7– −6.6) | <0.001 |
| Convenience | ||||
| 6 mo | 70.4(2.2) | 72.5(2.4) | 2.1(−4.0–8.2) | 0.5 |
| 12 mo | 68.0(2.1) | 65.2(2.3) | −2.8(−8.6–3.1) | 0.4 |
| 24 mo | 65.5(2.1) | 66.8(2.3) | 1.3(−4.5–7.2) | 0.7 |
| Overall | 68.0(1.6) | 68.2(1.8) | 0.2(−4.2–4.6) | 0.9 |
| Urinary Distress Inventory and Incontinence Impact Questionnaires change from baseline, adjusted mean (Std Err) | ||||
| UDI SFc total score | ||||
| 6 mo | −26.3(2.1) | −23.6(2.3) | 2.7(−3.2–8.5) | 0.4 |
| 12 mo | −20.1(2.1) | −22.8(2.3) | −2.6(−8.4–3.1) | 0.4 |
| 24 mo | −18.9(2.1) | −20.0(2.3) | −1.1(−6.9–4.6) | 0.7 |
| Overall | −21.8(1.8) | −22.1(2.0) | −0.4(−5.2–4.5) | 0.9 |
| IIQ SFd Total Score | ||||
| 6 mo | −31.2(2.3) | −29.6(2.6) | 1.6(−4.8–8.1) | 0.6 |
| 12 mo | −28.4(2.3) | −33.1(2.5) | −4.7(−11.0–1.6) | 0.1 |
| 24 mo | −26.3(2.3) | −28.8(2.5) | −2.5(−8.8–3.8) | 0.4 |
| Overall | −28.6(2.1) | −30.5(2.3) | −1.9(7.5–3.7) | 0.5 |
| Table 2B – Categorical quality of life outcomes over 6–24 mo | ||||
| Patient Global Impression of Improvement and Treatment Preferencee at each time point, n (%) | ||||
| PGI-I | ||||
| Urinary leakage | ||||
| 6 mo | 93 (77) | 81 (75) | −1.0(−12.6–10.5) | 0.9 |
| 12 mo | 96 (70) | 76 (69) | 0.5(−11.0–12.0) | 0.9 |
| 24 mo | 83 (62) | 70 (61) | −4.7(−16.9–7.4) | 0.4 |
| Bladder function | ||||
| 6 mo | 93 (76) | 83 (78) | 2.9(−8.5–14.3) | 0.6 |
| 12 mo | 94 (68) | 76 (68) | 0.4(−11.2–12.0) | 0.9 |
| 24 mo | 88 (67) | 68 (59) | −10.5(−22.5–1.4) | 0.08 |
| OAB SATq SF | ||||
| Treatment preference | ||||
| 6 mo | 103 (95) | 80 (93) | −2.7(−10.1–4.6) | 0.5 |
| 12 mo | 109 (94) | 81 (93) | −2.0(−9.2–5.2) | 0.6 |
| 24 mo | 93 (95) | 77 (87) | −9.9(−18.5– −1.2) | 0.02 |
| Patient Global Symptom Controlf value of 3 or greater of five-point agreement scale, n (%) | ||||
| Adequate leakage control | ||||
| 6 mo | 113 (72) | 106 (78) | 0.2 | |
| 12 mo | 101 (66) | 98 (73) | 0.2 | |
| 24 mo | 89 (62) | 88 (67) | 0.2 | |
| Sandvik four-level severity index at each time point, n (%) | ||||
| 6 mo | 0.5 | |||
| Slight | 29 (28) | 21 (21) | ||
| Moderate | 30 (29) | 31 (31) | ||
| Severe | 20 (19) | 17 (17) | ||
| Very severe | 26 (25) | 30 (30) | ||
| 12 mo | 0.1 | |||
| Slight | 25 (19) | 17 (16) | ||
| Moderate | 52 (39) | 38 (35) | ||
| Severe | 23 (17) | 22 (20) | ||
| Very severe | 32 (24) | 32 (29) | ||
| 24 mo | 0.2 | |||
| Slight | 25 (20) | 18 (16) | ||
| Moderate | 45 (35) | 32 (29) | ||
| Severe | 21 (17) | 24 (21) | ||
| Very severe | 36 (28) | 38 (34) | ||
BTX = onabotulinumtoxinA; CI = confidence interval; IIQ-SF = Incontinence Impact Questionnaire short form; OAB-SATq = Overactive Bladder Satisfaction questionnaire; OAB-SF = Overactive Bladder Questionnaire Short Form; PGI-I = Patient Global Impression of Improvement; PGSC = Patient Global Symptom Control; SNM = sacral neuromodulation; UDI-SF = Urinary Distress Inventory short form.
Values for the Overactive Bladder Questionnaire Short Form (OAB-SF) range from 0 to 100, with higher scores on the symptom severity scale indicating greater severity of symptoms and higher scores on the quality-of-life scale indicating better quality of life.
Values for the Overactive Bladder Satisfaction questionnaire (OAB-SATq) range from 0 to 100 and includes five subscales; treatment satisfaction, side effects, treatment endorsement, convenience, and preference, with higher scores reflecting better satisfaction.
The Urinary Distress Inventory short form (UDI-SF) scale has a range of 0 to 100, with higher scores representing a worse outcome.
The Incontinence Impact Questionnaire short form (IIQ-SF) scale has a range of 0 to 100, with higher scores representing a worse outcome.
The Patient Global Impression of Improvement (PGI-I) is a patient reported measure of perceived improvement with treatment on a scale of 1 (very much better) to 7 (very much worse). Included here are participants who had adequate improvement, defined as a rating of 1, 2 or 3 (better).
The Patient Global Symptom Control (PGSC) is a patient reported measure of perceived symptom control with treatment on a scale of 1 (disagree strongly) and 5 (agree strongly). Included here are participants who had adequate symptom control, defined as a rating of 3,4,5
Over 24 months, 72% (115/159) of the BTX participants requested a second injection (median interval from first and second injection was 350 d; interquartile range [IQR] = 242–465). In this group,101/115 (88%) had 200 U of which 6 (6%) required CIC. Per protocol, participants requiring CIC for >6 wk after initial BTX were dose-reduced. Specifically, 14/115 (12%) were dose-reduced from 200 U to 100 U, and 3/14 (21%) required CIC after 100 U. Median CIC duration across the nine participants was 29 d [IQR = 17–56]. A third injection was requested by 48% (55/115), with median interval between second and third injections of 273 d [IQR = 224–350]. The rate of CIC was 2% (1/55) after the third 200 U BTX injection. Only one participant chose to dose-reduce for the third injection. Of the 189 participants treated with BTX, 45 (24%) required CIC at any point over the 2 yr.
In the SNM group, 58% (81/139) required reprogramming with only 17% (14/81) requiring ≥3 reprogrammings. The most common reason for reprogramming was decreased efficacy, as reported on PGSC. Device revisions occurred in 4/139 (3%) because of decreased efficacy. Device removal occurred in 12/139 (8.6%) (infection [n = 4], decreased efficacy [n = 4], subject desire [n = 2], and pain [n = 2]). One participant was re-implanted after a resolved surgical site infection. No revisions or removals were due to “end of service” neurostimulators.
AE data were available for 91% (328/369), and only UTI rates were clinically different between groups (Table 3). Although UTIs decreased over time in both groups, at each time interval, the BTX group had more UTIs. In those without a history of recurrent UTIs at baseline, 15% (25/167) developed a UTI within 1 mo of BTX and 24% (40/167) developed recurrent UTIs after BTX versus 10.4% (16/154) after SNM, p < 0.01.
Table 3.
Adverse eventsa
| Months 1–6 | Months 7–12 | Months 13–24 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Adverse event | BTX Subj (%) |
SNM Subj (%) |
p value | BTX Subj (%) |
SNM Subj (%) |
p value | BTX Subj (%) |
SNM Subj (%) |
p value |
| Urinary tract infection (per protocol) | 69 (36) | 27 (15) | <0.001 | 42 (22) | 21 (12) | 0.012 | 35 (18) | 15 (8) | 0.006 |
| Dysuria | 11 (6) | 2 (1) | 0.021 | 2 (1) | 1 (1) | 1.0 | 6 (3) | 2 (1) | 0.3 |
| GI issues (abdominal pain, diarrhea, constipation, nausea, vomiting) | 21 (11) | 17 (10) | 0.73 | 10 (5) | 10 (6) | 1.0 | 15 (8) | 5 (3) | 0.04 |
| Falls | 7 (4) | 11 (6) | 0.34 | 5 (3) | 8 (4) | 0.40 | 10 (5) | 14 (8) | 0.4 |
| Back pain | 10 (5) | 13 (7) | 0.52 | 4 (2) | 5 (3) | 0.74 | 3 (2) | 6 (3) | 0.3 |
| Arthralgia | 8 (4) | 8 (4) | 1.0 | 6 (3) | 6 (3) | 1.0 | 3 (2) | 7 (4) | 0.2 |
| Procedural pain | 14 (7) | 10 (6) | 0.53 | 3 (2%) | 0 (0) | 0.25 | 5 (3) | 0 (0) | 0.06 |
AE = adverse events; BTX = onabotulinumtoxinA; GI = gastrointestinal; SNM = sacral neuromodulation.
AE information was collected continuously over the study on a log form based on participant self-reported information. Analyses for these tables were based on the study safety population (all participants who received any study treatment grouped by actual treatment received, irrespective of amount or duration of treatment received) and included 191 participants on the BTX arm and 178 participants on the SNM arm. To partition AEs across time periods, the AE was classified as an incident event on the date that the event started.
Discussion
In this randomized trial evaluating BTX and SNM for the treatment of refractory UUI over a 24-mo period, both treatments resulted in sustained and similar reductions in mean daily UUIE. BTX participants were more likely to have complete resolution of UUIE in the first 6 mo, higher satisfaction and treatment endorsement throughout the 24 mo, and greater treatment preference at 24 mo. However, there were no significant difference in symptom-specific QOL measures, global assessment of improvement, or adverse effects subscales. Furthermore, the use of UUI medications or the alternate trial therapy was comparable between the groups, and AEs were low.
Enrollment for the study began in 2012 when published data suggested that 200 U BTX may provide long term durability. Then, little was known regarding efficacy of repeat injections or CIC requirements. Therefore, this study provides safety and efficacy data for repeat 200 U BTX injections and information about dose reduction after prolonged CIC.
This study found similar mean reductions in UUIE/d (−3.9) at 24 mo with 200 U BTX as compared to the 3.5-yr voluntary extension study using 100 U BTX (−3.8) [7]. The current study did find a longer median reinjection interval with a smaller proportion of participants requiring >2 injections over 24 mo without loss of efficacy, QOL, or treatment satisfaction. In contrast, the 100 U extension study reported a duration of effect < 6 months in 33% and <1 yr in over 70%. The longer duration of effect in the present study may be attributed to the higher BTX dose. Other studies using 200 U have found similar durations of effect with median inter-injection intervals between 12 and 15 mo [8–10]. Similar to other BTX studies, we found that the reinjection intervals remained similar after the first and second injections. Also, participant symptoms at reinjection were less severe than those at baseline, even though the majority of participants did not seek additional medications or alternate therapies, unlike other studies [8–10].
The study’s SNM group had a greater reduction (−3.5 vs −2.3) in UUIE/d, a similar clinical responder rate following lead placement (84% vs 80%), and higher baseline UUIE/d (5.2 +/− 2.7) vs (3.1 +/− 2.7) than previously reported [11]. Greater reduction in UUIE/d has been associated with higher baseline incontinence episodes when evaluating predictors for success at 6 mo [12]. Over the 24-mo period, 58% of the SNM cohort required reprogramming, and 17% required three or more reprogrammings. Cameron et al [13] previously reported an average of 2.15 reprogrammings during the first year of the device with decreasing requirements over the next 5 yr. SNM efficacy was maintained in our study with few reprogrammings and few participants requesting additional UUI medicine or alternate trial therapy.
The CIC and UTI rates reported in ROSETTA through 6 mo[4] were lower than the 200 U dose-finding study [14]. This extension study supports the continued low rates of both after reinjections using 200 U with no participant experiencing chronic retention. Also demonstrated were decreased UTIs over the 24 mo although still higher than the SNM group. Given the higher sustained satisfaction rate in the BTX cohort compared with the SNM group and the 1% of BTX participants that chose to dose-reduce, it is likely that performing CIC does not greatly impact QOL. This has been confirmed in a previous study [15].
The tined lead and smaller neurostimulator have reduced SNM reoperations. The revision rate in our 2-yr study was 3%. All surgical revisions were due to decreased efficacy. In contrast, Siegel et al [11] reported a 3-yr revision rate of 32%, and 33% of those revisions were due to battery replacement. Differences in SNM AEs may be due to the study protocol’s standardized procedure that optimized lead placement and increased battery longevity. The permanent removal rate of 8.6% is similar to the 13% reported in a previous study [11].
Study strengths included excellent follow-up with robust quality data. Participants were assessed for a wide spectrum of outcomes at regular intervals to measure efficacy, treatment satisfaction, and AEs. The design allowed participants to receive therapy when they perceived decreased efficacy, which aligns with accepted clinical care. As only 5% in each CR cohort requested alternate trial therapy, this limits any conclusion as to the effects of having both therapies. In addition, we cannot extend our conclusions to the use of 100 U BTX. Furthermore, this trial compared two active treatments, eliminating any determination of a placebo effect.
Conclusions
This is the first randomized trial comparing 200 U BTX and SNM over an extended time in women with refractory UUI. Our data supports the benefits of both therapies with differing risks. Higher BTX dosing affords sustainable UUI improvement with low CIC rates but with UTI risk. SNM revision/removal rates were low due to standardized lead placement with strict definitions for treatment response. Further review of optimal BTX dosing tailored to individual patient needs and continued investigation of OAB pathophysiology and phenotyping are all essential to further enhance the care of women with OAB. In addition, analyses of cost-effectiveness are a critical next step for determining whether one UUI treatment should be recommended over the other.
Sacral neuromodulation and 200 U onabotulinumtoxinA resulted in similar sustained improvements in urgency urinary incontinence episodes throughout 24 mo. OnabotulinumtoxinA provided higher treatment satisfaction and endorsement, and greater treatment preference. Adverse events were low; however, urinary tract infection rates were higher following onbotulinumtoxinA.
Acknowledgments
Funding Support and Role of the Sponsor:
The Eunice Kennedy Shriver National Institute of Child Health and Human Development and the NIH Office of Research on Women’s Health at National Institutes of Health.
UAB: Julie E. Burge, BS, Kathy S. Carter, RN, BSN, Patricia S. Goode, MD, Robert L. Holley, MS, MD, Ryanne R. Johnson, BSN, RNC,WHNP-BC, CRNP, L. Keith Lloyd, MD, Alayne D. Markland, DO, R. Jeannine McCormick, RN, BSN, MSN, CRNP, Nancy B. Saxon, RN, BSN, Robert E. Varner, MS, MD, Velria B. Willis, RN, BSN, CCRC, Robin R. Willingham, RN, BSN, Holly E. Richter, PhD, MD; Brown University: Alexandra Lynch, Ann Meers, Allegra Parrillo, Erika Spearin, Vivian W. Sung, MPH, MD, Charles R. Rardin MD, B. Star Hampton, MD, Kyle J. Wohlrab, MD, Cassandra L. Carberry MD; UCSD: JoAnn Columbo; Kaiser Downey: Nancy Flores; Kaiser San Diego: Gisselle Zazueta-Damian; Cleveland Clinic: Andrea Aaby, Bradley Gill, MD, Ly Pung; Duke: Akira Hayes, MS, Amie Kawasaki, MD, Nicole Longoria, PA-C, Shantae McLean, MPH,CCRC, Nazema Y. Siddiqui, MD, Anthony G. Visco, MD, Alison C. Weidner, MD; University of New Mexico: Julia Middendorf, Karen Taylor; Oregon Health and Science University: S Renee Edwards, MD, Mary Anna Denman, MD, Kamran Sajadi MD, Amanda Holland MS; University of Pennsylvania: Michelle Kinglee, Lorraine Flick, Lily Arya, MD, Ariana Smith MD; University of Pittsburgh: Michael Bonidie MD, Halina M. Zycynski, MD, Judy Gruss, Karen Mislanovich, Pamela Moalli,PhD, MD, Jonathan Shepherd MD, Gary Sutkin MD; RTI International: Katrina Burson, Allyson Drew, Marie Gantz, Kendra Glass, Carolyn Huitema, Amy Kendrick, Tracy Nolen, James Pickett, Amanda Shaffer, Yan Tang, Ryan Whitworth, Kevin Wilson; Data Safety Monitoring Board: Ben Carper, Meg Crawford, Marie Gantz, Lisa Halvorson, Lan Kong, Donna McClish, Leslie Rickey, Lori Schwarze, Dave Shade, Paul Tulikangas, Askok Tuteja, Amanda Shafffer, Taylor Swankle, Susan Yount; NICHD: Donna Mazloomdoost
Supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the NIH Office of Research on Women’s Health at National Institutes of Health 3316 (U01 HD41249U10, HD41261U10 HD41267, U10 HD54215U10-HD41261-11, U10-HD069013, U10-HD054214-06, U10-HD054215-06, U10-HD041267-12, U10-HD069025-01, U10-HD069010, U10-HD054136, U10-HD054241, U10-HD041250-11)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions:
Study concept and design: Cindy L. Amundsen, Yuko M. Komesu, W. Thomas Gregory, Deborah L. Myers, Sandip P. Vasavada, Dennis Wallace
Acquisition of data: Cindy L. Amundsen, Yuko M. Komesu, Christopher Chermansky, W. Thomas Gregory, Deborah L. Myers, Sandip P. Vasavada, John N. Nguyen, Tracey S. Wilson, Heidi S. Harvie
Analysis and interpretation of data: Cindy L. Amundsen, Christopher Chermansky, W. Thomas Gregory, Emily F. Honeycutt, Dennis Wallace
Drafting of the manuscript: Cindy L. Amundsen, Yuko M. Komesu, Christopher Chermansky, W. Thomas Gregory, Deborah L. Myers, Sandip P. Vasavada, Emily F. Honeycutt, Dennis Wallace
Critical revision of the manuscript for important intellectual content: Cindy L. Amundsen, Yuko M. Komesu, Christopher Chermansky, W. Thomas Gregory, Deborah L. Myers, Emily F. Honeycutt, Sandip P. Vasavada, John N. Nguyen, Tracey S. Wilson, Heidi S. Harvie, Dennis Wallace
Statistical analysis: Emily F. Honeycutt, Dennis Wallace
Obtaining funding: Cindy L. Amundsen, Yuko M. Komesu, Christopher Chermansky, W. Thomas Gregory, Deborah L. Myers, Sandip P. Vasavada, John N. Nguyen, Tracey S. Wilson, Heidi S. Harvie, Dennis Wallace
Administrative, technical, or material support: Cindy L. Amundsen, W. Thomas Gregory, Emily F. Honeycutt, Dennis Wallace
Supervision: Cindy L. Amundsen, Yuko M. Komesu, Christopher Chermansky, W. Thomas Gregory, Deborah L. Myers, Sandip P. Vasavada, John N. Nguyen, Tracey S. Wilson, Heidi S. Harvie, Dennis Wallace
Other (specify): None
Financial Disclosure:
Sandip P. Vasavada: consultant for Medtronic, Allergan, and Axonics
References
- 1.Abrams P, Kelleher C, Kerr LA, Rogers RG. Overactive bladder significantly affects quality of life. Am J Manag Care. 2000;6:S580–90. [PubMed] [Google Scholar]
- 2.D’Souza AO, Smith MJ, Miller LA, Doyle J, Ariely R. Persistence, adherence, and switch rates among extended-release and immediate-release overactive bladder medications in a regional managed care plan. J Mana Care Pharm. 2008;14:291–301. doi: 10.18553/jmcp.2008.14.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borello-France D, Burgio KL, Goode PS, et al. Adherence to behavioral interventions for urge incontinence when combined with drug therapy: adherence rates, barriers and predictors. Phys Ther. 2010;90:1493–505. doi: 10.2522/ptj.20080387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amundsen CL, Richter HE, Menefee SA, et al. OnabotulinumtoxinA vs sacral neuromodulation on refractory urgency urinary incontinence in women. A Randomized Clinical Trial. JAMA. 2016;316:1366–74. doi: 10.1001/jama.2016.14617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amundsen CL, Richter HE, Menefee S, et al. The refractory overactive bladder: sacral neuroodulation vs. botulinum toxin assessment: ROSETTA trial. Contemp Clin Trials. 2014;37:272–83. doi: 10.1016/j.cct.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 7.Nitti VW, Ginsberg D, Sievert KD, et al. Durable efficacy and safety of long-term onabotulinumtoxinA treatment in patients with overactive bladder syndrome: final results of a 3.5-year study. J Urology. 2016;196:791–800. doi: 10.1016/j.juro.2016.03.146. [DOI] [PubMed] [Google Scholar]
- 8.Dowson C, Watkins J, Khan MS, Dasgupta P, Sahai A. Repeated botulinum toxin type A injections for refractory overactive bladder: medium-term outcomes, safety profile, and discontinuation rates. Eur Urol. 2012;61:834–9. doi: 10.1016/j.eururo.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Veeratterapillay R, Hardling C, Teo L, et al. Discontinuation rates and inter-injection interval for repeated intravesical botulinum toxin type A injection for detrusor over activity. Int J Urol. 2014;21:175–8. doi: 10.1111/iju.12205. [DOI] [PubMed] [Google Scholar]
- 10.Khan S, Kessler TM, Apostolidis A, et al. What a patient with refractory idiopathic detrusor over activity should know about botulinum neurotoxin type A injection. J Urol. 2009;181:1773–8. doi: 10.1016/j.juro.2008.11.110. [DOI] [PubMed] [Google Scholar]
- 11.Siegel S, Noblett K, Mangel J, et al. Three-year follow up results of a prospective, multicenter study in overactive bladder subjects treated with sacral neuromodulation. Urology. 2016;94:57–63. doi: 10.1016/j.urology.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 12.Richter HE, Amundsen CL, Erickson SW, et al. Characteristics associated with treatment response and satisfaction in women undergoing onabotulinumtoxinA and sacral neuromodulation for refractory urgency urinary incontinence. J Urol. 2017;198:SOO22–5347. doi: 10.1016/j.juro.2017.04.103. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cameron AP, Anger JT, Madison R, Saigal CS, Clemens JQ. Battery explantation after sacral neuromodulation in the medicare population. Neurourol Urodyn. 2013;32:238–41. doi: 10.1002/nau.22294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dmochowski R, Chapple C, Nitti VW, et al. Efficacy and safety of onabotulinumtoxinA for idiopathic overactive bladder: a double-blind, placebo controlled, randomized, dose ranging trial. J Urol. 2010;184:2416–22. doi: 10.1016/j.juro.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 15.Kessler TM, Khan S, Panicker J, Roosen A, Elneil S, Fowler CJ. Clean intermittent self-catheterization after botulinum neurotoxin type A injection: short term effect on QoL. Obstet Gynecol. 2009;113:1046–51. doi: 10.1097/AOG.0b013e3181a1f5ea. [DOI] [PubMed] [Google Scholar]